 Open Access
Open Access
ARTICLE
Antitumor effects of a novel photosensitizer-mediated photodynamic therapy and its influence on the cell transcriptome
1 Department of Pharmacology, School of Pharmacy, Changzhi Medical College, Changzhi, China
2 Department of Pharmacology, School of Basic Medical Sciences, Health Science Center, Peking University, Beijing, China
3 Beijing Key Laboratory of Tumor Systems Biology, Peking University, Beijing, China
4 Department of Pharmacology, Xinjiang Medical University, Urumqi, China
5 School of Pharmaceutical Sciences, Peking University, Beijing, China
* Corresponding Author: YAN PAN. Email:
Oncology Research 2024, 32(5), 911-923. https://doi.org/10.32604/or.2023.042384
Received 28 May 2023; Accepted 11 October 2023; Issue published 23 April 2024
Abstract
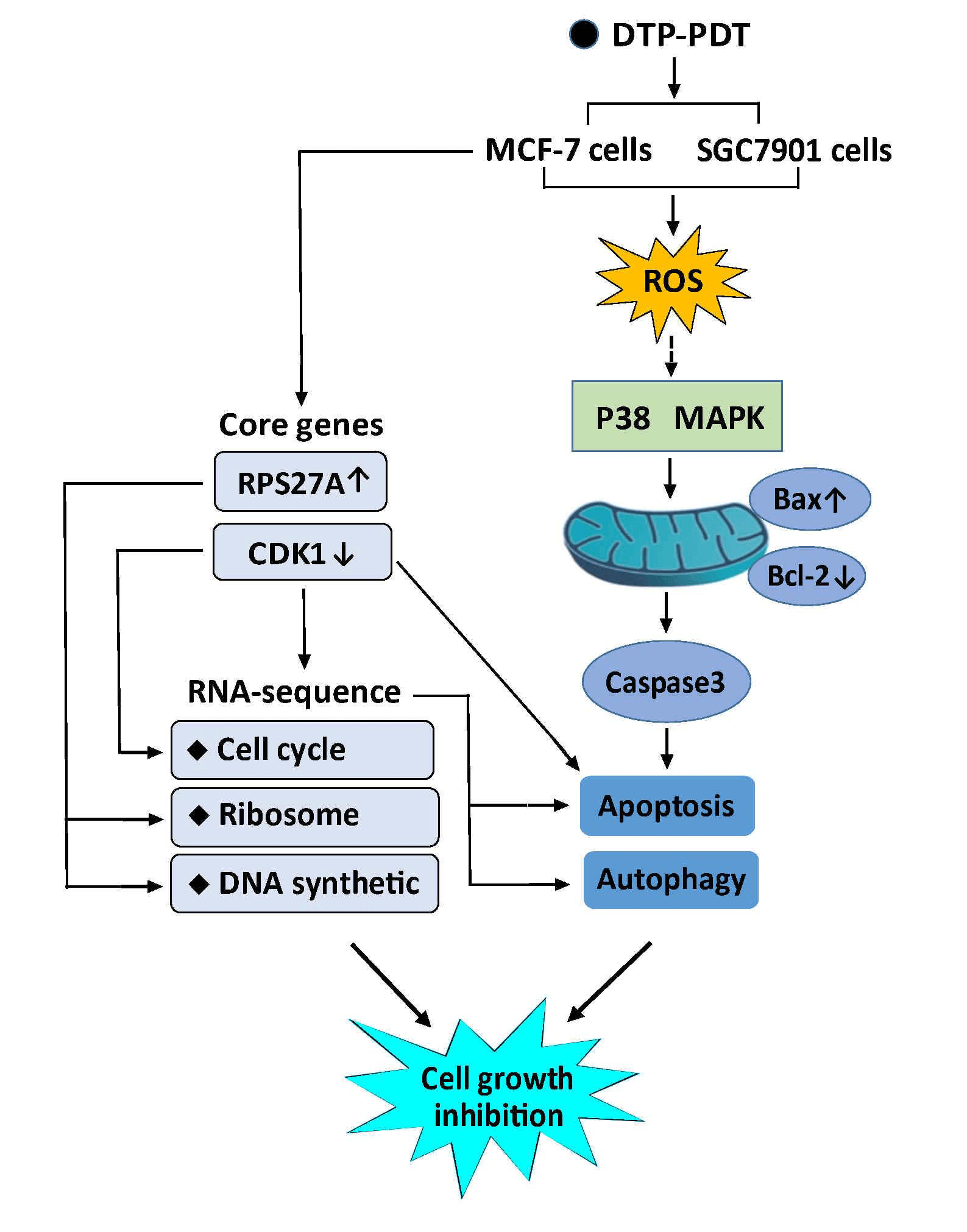
Photodynamic therapy (PDT) is a promising cancer treatment. This study investigated the antitumor effects and mechanisms of a novel photosensitizer meso-5-[ρ-diethylene triamine pentaacetic acid-aminophenyl]−10,15,20-triphenyl-porphyrin (DTP) mediated PDT (DTP-PDT). Cell viability, reactive oxygen species (ROS), and apoptosis were measured with a Cell Counting Kit-8 assay, DCFH-DA fluorescent probe, and Hoechst staining, respectively. Cell apoptosis- and autophagy-related proteins were examined using western blotting. RNA sequencing was used to screen differentially expressed mRNAs (DERs), and bioinformatic analysis was performed to identify the major biological events after DTP-PDT. Our results show that DTP-PDT inhibited cell growth and induced ROS generation in MCF-7 and SGC7901 cells. The ROS scavenger N-acetyl-L-cysteine (NAC) and the P38 MAPK inhibitor SB203580 alleviated DTP-PDT-induced cytotoxicity. DTP-PDT induced cell apoptosis together with upregulated Bax and downregulated Bcl-2, which could also be inhibited by NAC or SB203580. The level of LC3B-II, a marker of autophagy, was increased by DTP-PDT. A total of 3496 DERs were obtained after DTP-PDT. Gene Ontology and Kyoto Encyclopedia of Genes and Genomes analyses indicated that DERs included those involved in cytosolic ribosomes, the nuclear lumen, protein binding, cell cycle, protein targeting to the endoplasmic reticulum, and ribosomal DNA replication. Disease Ontology and Reactome enrichment analyses indicated that DERs were associated with a variety of cancers and cell cycle checkpoints. Protein-protein interaction results demonstrated that cdk1 and rps27a ranked in the top 10 interacting genes. Therefore, DTP-PDT could inhibit cell growth and induce cell apoptosis and autophagy, partly through ROS and the P38 MAPK signaling pathway. Genes associated with the cell cycle, ribosomes, DNA replication, and protein binding may be the key changes in DTP-PDT-mediated cytotoxicity.Graphic Abstract
Keywords
Cite This Article
 Copyright © 2024 The Author(s). Published by Tech Science Press.
Copyright © 2024 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools