 Open Access
Open Access
ARTICLE
Neural Wiskott-Aldrich syndrome protein (N-WASP) promotes distant metastasis in pancreatic ductal adenocarcinoma via activation of LOXL2
1 Department of Surgery, Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, Korea
2 Department of Surgery, Graduate School of Medical Science, Brain Korea 21 Project, Yonsei University College of Medicine, Seoul, Korea
3 Gibiome Co., Ltd., Gyeonggi-do, Korea
* Corresponding Author: JOON SEONG PARK. Email:
# These authors have contributed equally to this work and shared first authorship
(This article belongs to the Special Issue: Cancer Metastasis)
Oncology Research 2024, 32(4), 615-624. https://doi.org/10.32604/or.2024.044029
Received 19 July 2023; Accepted 04 December 2023; Issue published 20 March 2024
Abstract
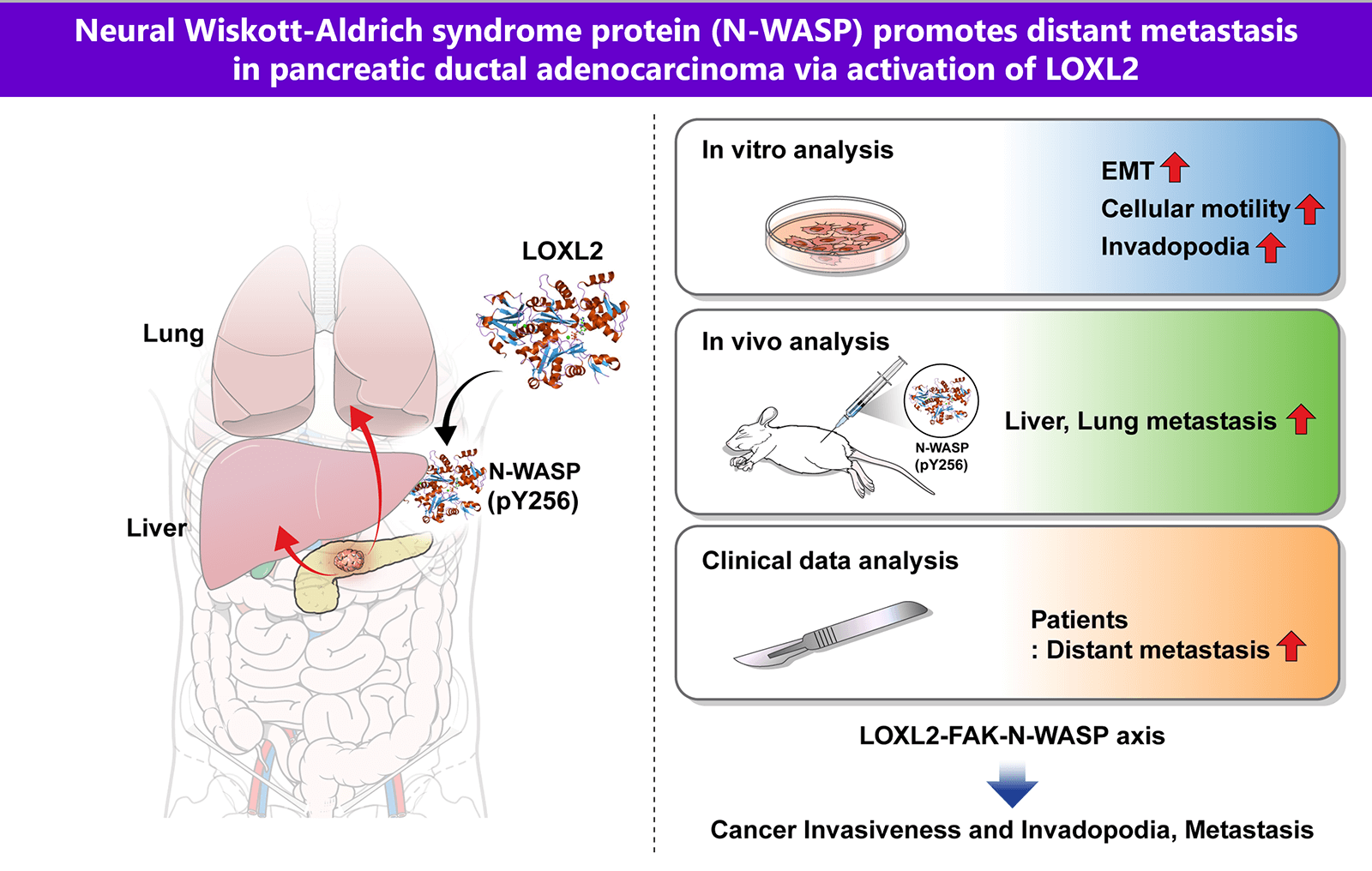
Pancreatic ductal adenocarcinoma (PDAC) is one of the most aggressive solid malignancies. A specific mechanism of its metastasis has not been established. In this study, we investigated whether Neural Wiskott-Aldrich syndrome protein (N-WASP) plays a role in distant metastasis of PDAC. We found that N-WASP is markedly expressed in clinical patients with PDAC. Clinical analysis showed a notably more distant metastatic pattern in the N-WASP-high group compared to the N-WASP-low group. N-WASP was noted to be a novel mediator of epithelial-mesenchymal transition (EMT) via gene expression profile studies. Knockdown of N-WASP in pancreatic cancer cells significantly inhibited cell invasion, migration, and EMT. We also observed positive association of lysyl oxidase-like 2 (LOXL2) and focal adhesion kinase (FAK) with the N-WASP-mediated response, wherein EMT and invadopodia function were modulated. Both N-WASP and LOXL2 depletion significantly reduced the incidence of liver and lung metastatic lesions in orthotopic mouse models of pancreatic cancer. These results elucidate a novel role for N-WASP signaling associated with LOXL2 in EMT and invadopodia function, with respect to regulation of intercellular communication in tumor cells for promoting pancreatic cancer metastasis. These findings may aid in the development of therapeutic strategies against pancreatic cancer.Graphic Abstract
Keywords
Supplementary Material
Supplementary Material FileCite This Article
 Copyright © 2024 The Author(s). Published by Tech Science Press.
Copyright © 2024 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools