 Open Access
Open Access
ARTICLE
IL-17 induces NSCLC cell migration and invasion by elevating MMP19 gene transcription and expression through the interaction of p300-dependent STAT3-K631 acetylation and its Y705-phosphorylation
1 Department of Immunology, Nanjing Medical University, Nanjing, 210000, China
2 Key Laboratory of Immunological Environment and Disease, Nanjing Medical University, Nanjing, 210000, China
3 Department of Oncology, The First Affiliated Hospital of Nanjing Medical University, Nanjing, 210000, China
4 Jiangsu Key Laboratory of Cancer Biomarkers, Prevention and Treatment, Collaborative Innovation Center for Cancer Personalized Medicine, Nanjing Medical University, Nanjing, 210000, China
* Corresponding Author: CHENHUI ZHAO. Email:
# These authors contributed equally to this work
Oncology Research 2024, 32(4), 625-641. https://doi.org/10.32604/or.2023.031053
Received 11 May 2023; Accepted 17 July 2023; Issue published 20 March 2024
Abstract
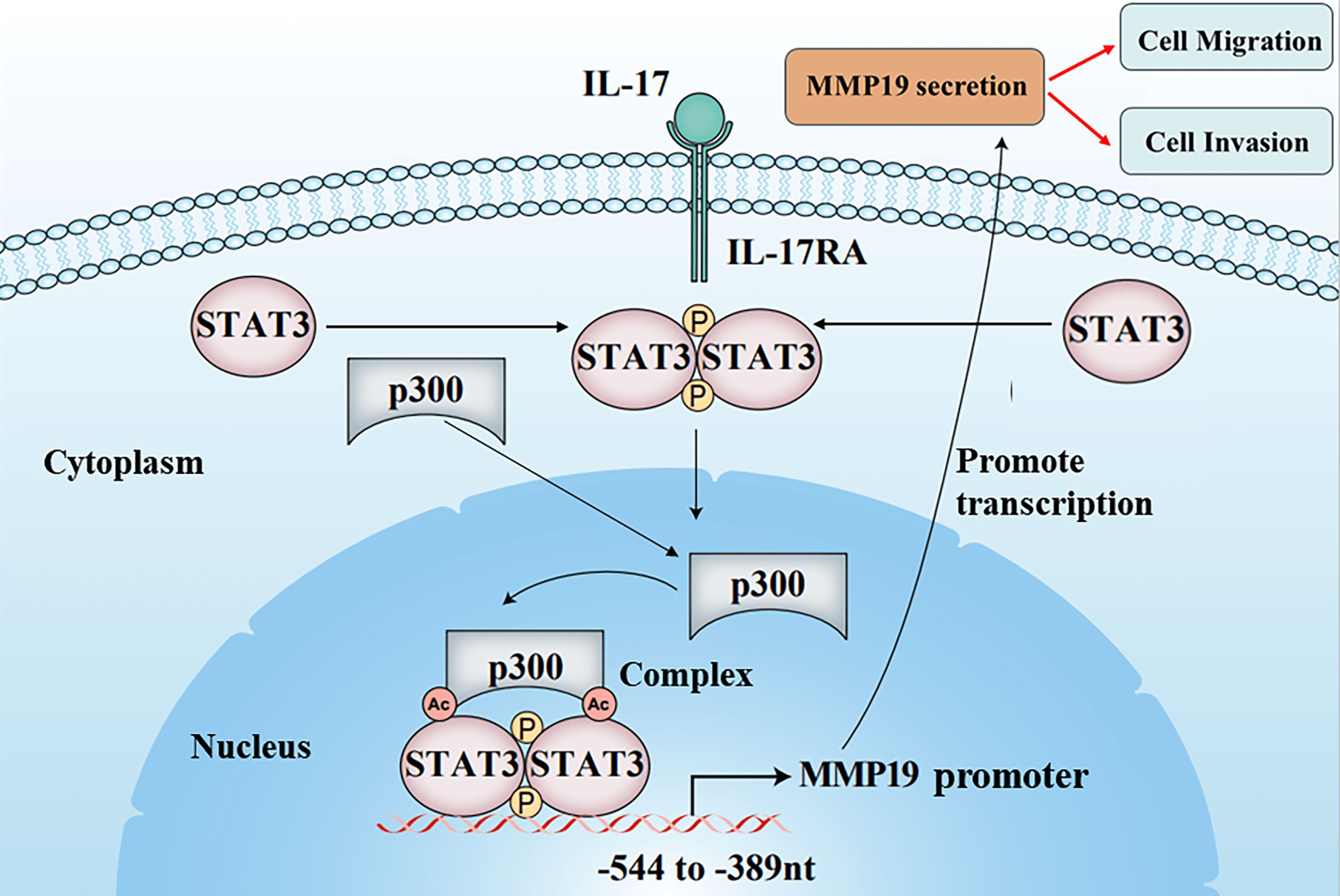
The cancer cell metastasis is a major death reason for patients with non-small cell lung cancer (NSCLC). Although researchers have disclosed that interleukin 17 (IL-17) can increase matrix metalloproteinases (MMPs) induction causing NSCLC cell metastasis, the underlying mechanism remains unclear. In the study, we found that IL-17 receptor A (IL-17RA), p300, p-STAT3, Ack-STAT3, and MMP19 were up-regulated both in NSCLC tissues and NSCLC cells stimulated with IL-17. p300, STAT3 and MMP19 overexpression or knockdown could raise or reduce IL-17-induced p-STAT3, Ack-STAT3 and MMP19 level as well as the cell migration and invasion. Mechanism investigation revealed that STAT3 and p300 bound to the same region (−544 to −389 nt) of MMP19 promoter, and p300 could acetylate STAT3-K631 elevating STAT3 transcriptional activity, p-STAT3 or MMP19 expression and the cell mobility exposed to IL-17. Meanwhile, p300-mediated STAT3-K631 acetylation and its Y705-phosphorylation could interact, synergistically facilitating MMP19 gene transcription and enhancing cell migration and invasion. Besides, the animal experiments exhibited that the nude mice inoculated with NSCLC cells by silencing p300, STAT3 or MMP19 gene plus IL-17 treatment, the nodule number, and MMP19, Ack-STAT3, or p-STAT3 production in the lung metastatic nodules were all alleviated. Collectively, these outcomes uncover that IL-17-triggered NSCLC metastasis involves up-regulating MMP19 expression via the interaction of STAT3-K631 acetylation by p300 and its Y705-phosphorylation, which provides a new mechanistic insight and potential strategy for NSCLC metastasis and therapy.Graphic Abstract
Keywords
Supplementary Material
Supplementary Material FileCite This Article
 Copyright © 2024 The Author(s). Published by Tech Science Press.
Copyright © 2024 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools