 Open Access
Open Access
ARTICLE
Codelivery of anti-CD47 antibody and chlorin e6 using a dual pH-sensitive nanodrug for photodynamic immunotherapy of osteosarcoma
1 Department of Radiology, The Fifth Affiliated Hospital of Sun Yat-Sen University, Zhuhai, 510900, China
2 Department of Ultrasound, The Third Affiliated Hospital of Sun Yat-Sen University, Guangzhou, 528405, China
3 PCFM Lab of Ministry of Education, School of Materials Science and Engineering, Guangzhou, 510275, China
* Corresponding Author: SHAOLIN LI. Email:
(This article belongs to the Special Issue: Role of Reactive Oxygen Species and DNA Damage in Tumor Immunological Responses)
Oncology Research 2024, 32(4), 691-702. https://doi.org/10.32604/or.2023.030767
Received 21 April 2023; Accepted 31 July 2023; Issue published 20 March 2024
Abstract
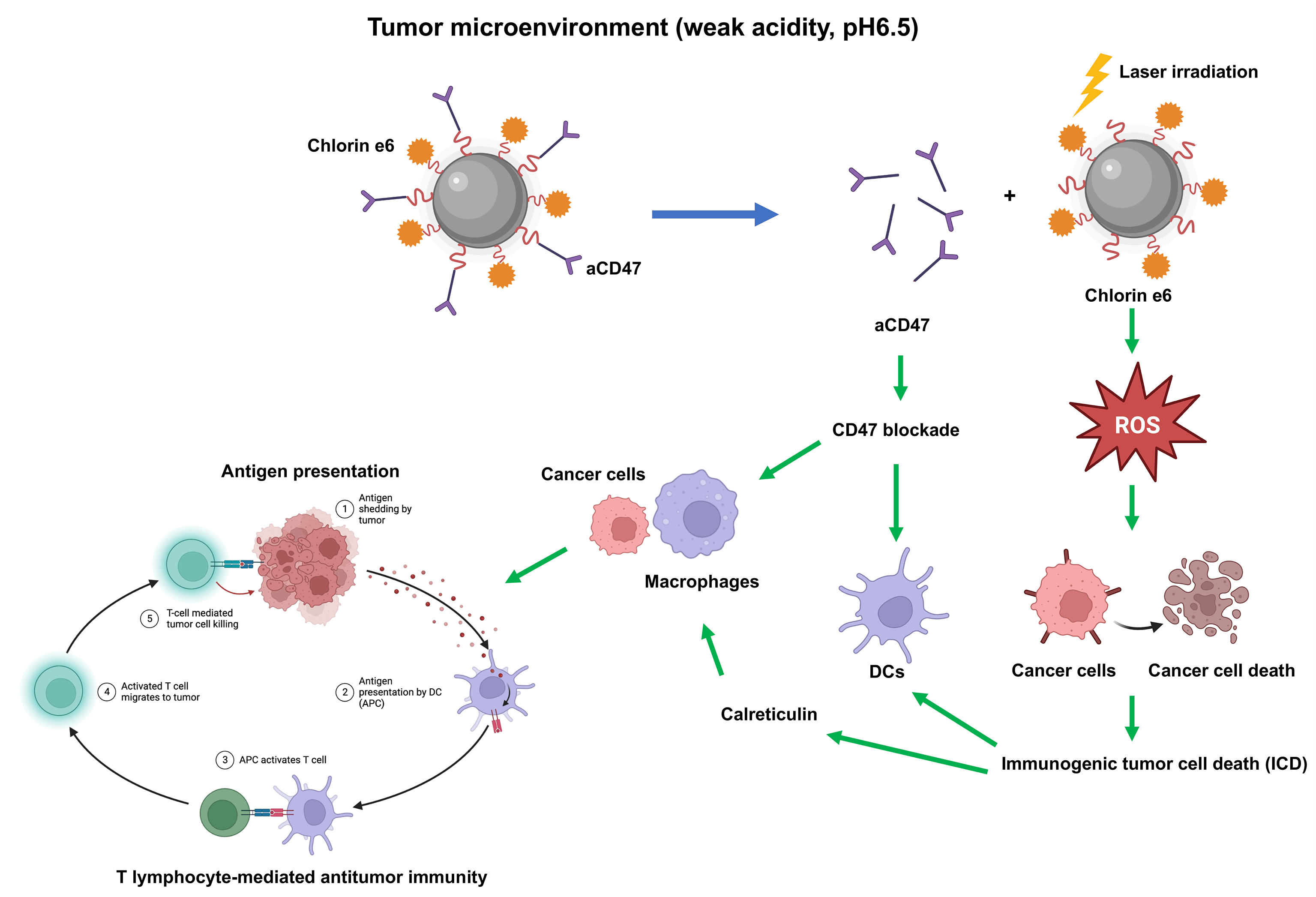
Osteosarcoma is a malignant tumor originating from bone tissue that progresses rapidly and has a poor patient prognosis. Immunotherapy has shown great potential in the treatment of osteosarcoma. However, the immunosuppressive microenvironment severely limits the efficacy of osteosarcoma treatment. The dual pH-sensitive nanocarrier has emerged as an effective antitumor drug delivery system that can selectively release drugs into the acidic tumor microenvironment. Here, we prepared a dual pH-sensitive nanocarrier, loaded with the photosensitizer Chlorin e6 (Ce6) and CD47 monoclonal antibodies (aCD47), to deliver synergistic photodynamic and immunotherapy of osteosarcoma. On laser irradiation, Ce6 can generate reactive oxygen species (ROS) to kill cancer cells directly and induces immunogenic tumor cell death (ICD), which further facilitates the dendritic cell maturation induced by blockade of CD47 by aCD47. Moreover, both calreticulin released during ICD and CD47 blockade can accelerate phagocytosis of tumor cells by macrophages, promote antigen presentation, and eventually induce T lymphocyte-mediated antitumor immunity. Overall, the dual pH-sensitive nanodrug loaded with Ce6 and aCD47 showed excellent immune-activating and anti-tumor effects in osteosarcoma, which may lay the theoretical foundation for a novel combination model of osteosarcoma treatment.Graphic Abstract
Keywords
Supplementary Material
Supplementary Material FileCite This Article
 Copyright © 2024 The Author(s). Published by Tech Science Press.
Copyright © 2024 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools