 Open Access
Open Access
ARTICLE
Long non-coding RNA H19 promotes proliferation in hepatocellular carcinoma cells via H19/miR-107/CDK6 axis
1 Department of Biochemistry, Faculty of Medicine, Chulalongkorn University, Bangkok, 10330, Thailand
2 Center of Excellence in Hepatitis and Liver Cancer, Faculty of Medicine, Chulalongkorn University, Bangkok, 10330, Thailand
3 Department of Biochemistry, Medical Biochemistry Program, Faculty of Medicine, Chulalongkorn University, Bangkok, 10330, Thailand
4 Center of Excellence in Systems Biology, Faculty of Medicine, Chulalongkorn University, Bangkok, 10330, Thailand
* Corresponding Authors: PISIT TANGKIJVANICH. Email: ; CHAIYABOOT ARIYACHET. Email:
,
# These authors contributed equally to this work
Oncology Research 2023, 31(6), 989-1005. https://doi.org/10.32604/or.2023.030395
Received 03 April 2023; Accepted 12 July 2023; Issue published 15 September 2023
Abstract
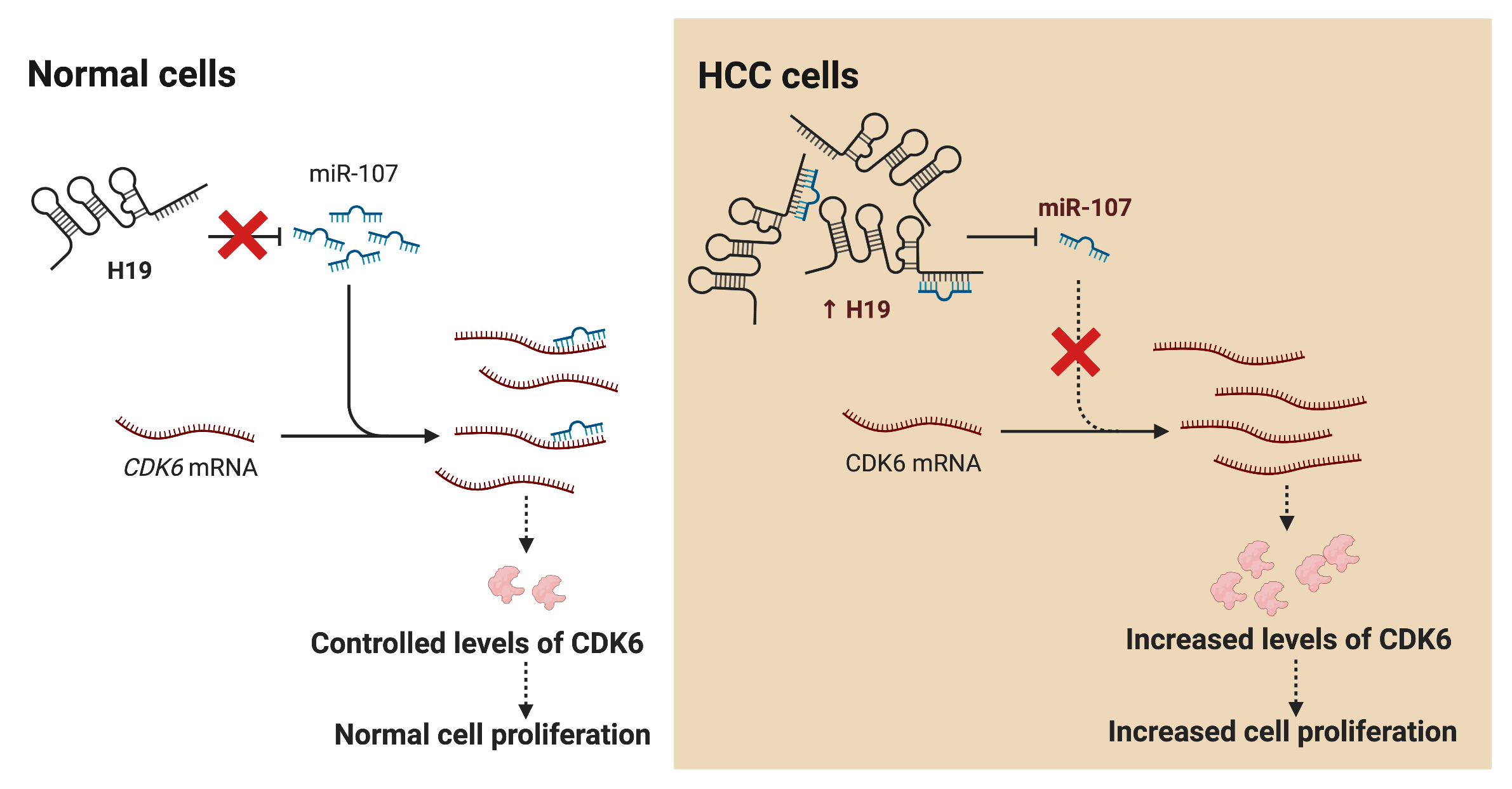
Hepatocellular carcinoma (HCC) is the leading cause of cancer death worldwide; nevertheless, current therapeutic options are limited or ineffective for many patients. Therefore, elucidation of molecular mechanisms in HCC biology could yield important insights for the intervention of novel therapies. Recently, various studies have reported dysregulation of long non-coding RNAs (lncRNAs) in the initiation and progression of HCC, including H19; however, the biological function of H19 in HCC remains unclear. Here, we show that knockdown of H19 disrupted HCC cell growth, impaired the G1-to-S phase transition, and promoted apoptosis, while overexpression of H19 yielded the opposite results. Screening for expression of cell cycle-related genes revealed a significant downregulation of CDK6 at both RNA and protein levels upon H19 suppression. Bioinformatic analysis of the H19 sequence and the 3′ untranslated region (3′ UTR) of CDK6 transcripts showed several binding sites for microRNA-107 (miR-107), and the dual luciferase reporter assay confirmed their direct interaction with miR-107. Consistently, blockage of miR-107 activity alleviated the growth suppression phenotypes induced by H19 downregulation, suggesting that H19 serves as a molecular sponge for miR-107 to promote CDK6 expression and cell cycle progression. Together, this study demonstrates a mechanistic function of H19 in driving the proliferation of HCC cells and suggests H19 suppression as a novel approach for HCC treatment.Graphic Abstract
Keywords
Cite This Article
 Copyright © 2023 The Author(s). Published by Tech Science Press.
Copyright © 2023 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools