 Open Access
Open Access
ARTICLE
Isolation and characterization of β-transducin repeat-containing protein ligands screened using a high-throughput screening system
1 Chemical Resource Development Research Unit, RIKEN CSRS, Wako, Saitama, 351-0198, Japan
2 Department of RIKEN Molecular and Chemical Somatology, Graduate School of Medical and Dental Sciences, Tokyo Medical and Dental University, Bunkyo-ku, Tokyo, 113-8510, Japan
3 Bioprobe Application Research Unit, RIKEN CSRS, Wako, Saitama, 351-0198, Japan
4 Chemical Biology Research Group, RIKEN CSRS, Wako, Saitama, 351-0198, Japan
5 Guangdong Engineering Research Center of Oral Restoration and Reconstruction, Affiliated Stomatology Hospital of Guangzhou Medical University, Guangzhou, 510180, China
6 KingMed School of Laboratory Medicine, Guangzhou Medical University, Guangzhou, 510182, China
7 Department of Pharmaceutical Sciences, University of Shizuoka, Suruga-ku, Shizuoka, 422-8526, Japan
* Corresponding Authors: HIROYUKI OSADA. Email: ; NOBUMOTO WATANABE. Email:
(This article belongs to the Special Issue: Approach from Chemical Biology for Cancer Research)
Oncology Research 2023, 31(5), 645-654. https://doi.org/10.32604/or.2023.030240
Received 28 March 2023; Accepted 19 May 2023; Issue published 21 July 2023
Abstract
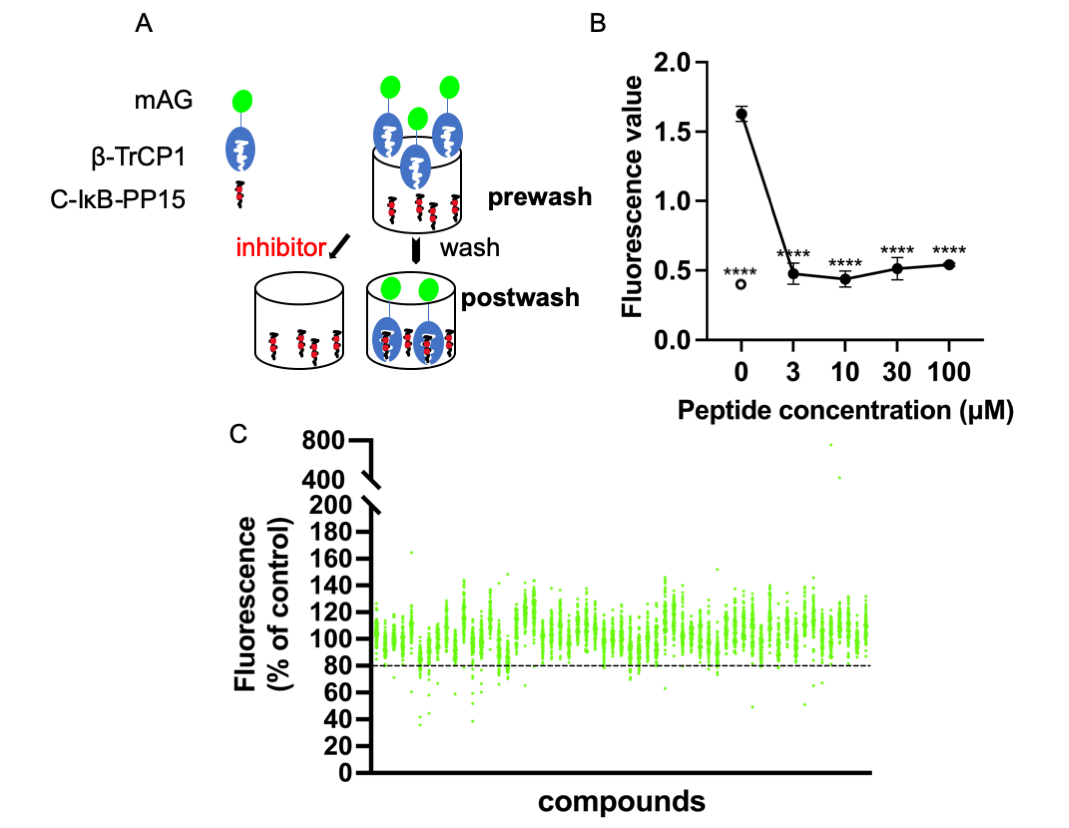
β-transducin repeat-containing protein (β-TrCP) is an F-box protein subunit of the E3 Skp1-Cullin-F box (SCF) type ubiquitin-ligase complex, and provides the substrate specificity for the ligase. To find potent ligands of β-TrCP useful for the proteolysis targeting chimera (PROTAC) system using β-TrCP in the future, we developed a high-throughput screening system for small molecule β-TrCP ligands. We screened the chemical library utilizing the system and obtained several hit compounds. The effects of the hit compounds on in vitro ubiquitination activity of SCFβ-TrCP1 and on downstream signaling pathways were examined. Hit compounds NPD5943, NPL62020-01, and NPL42040-01 inhibited the TNFα-induced degradation of IκBα and its phosphorylated form. Hence, they inhibited the activation of the transcription activity of NF-κB, indicating the effective inhibition of β-TrCP by the hit compounds in cells. Next, we performed an in silico analysis of the hit compounds to determine the important moieties of the hit compounds. Carboxyl groups of NPL62020-01 and NPL42040-01 and hydroxyl groups of NPD5943 created hydrogen bonds with β-TrCP similar to those created by intrinsic target phosphopeptides of β-TrCP. Our findings enhance our knowledge of useful small molecule ligands of β-TrCP and the importance of residues that can be ligands of β-TrCP.Graphic Abstract
Keywords
Cite This Article
 Copyright © 2023 The Author(s). Published by Tech Science Press.
Copyright © 2023 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools