 Open Access
Open Access
ARTICLE
H1-antihistamine use and head and neck cancer risk in type 2 diabetes mellitus
1 Department of Otorhinolaryngology, Lo-Hsu Medical Foundation, Lotung Poh-Ai Hospital, Yilan, Taiwan
2 Graduate Institute of Business Administration, College of Management, Fu Jen Catholic University, Taipei, Taiwan
3 Artificial Intelligence Development Center, Fu Jen Catholic University, Taipei, Taiwan
4 Department of Otolaryngology, National Taiwan University Hospital and National Taiwan University College of Medicine, Taipei, Taiwan
5 Department of Food Nutrition and Health Biotechnology, College of Medical and Health Science, Asia University, Taichung, Taiwan
6 Division of Radiation Oncology, Lo-Hsu Medical Foundation, Lotung Poh-Ai Hospital, Yilan, Taiwan
7 Big Data Center, Lo-Hsu Medical Foundation, Lotung Poh-Ai Hospital, Yilan, Taiwan
8 Department of Healthcare Administration, College of Medical and Health Science, Asia University, Taichung, Taiwan
9 Cancer Center, Lo-Hsu Medical Foundation, Lotung Poh-Ai Hospital, Yilan, Taiwan
10 Centers for Regional Anesthesia and Pain Medicine, Taipei Municipal Wan Fang Hospital, Taipei Medical University, Taipei, Taiwan
11 Department of Management, College of Management, Fo Guang University, Yilan, Taiwan
* Corresponding Author: Szu-Yuan Wu,
# These authors have contributed equally to this study (joint primary authors)
Oncology Research 2023, 31(1), 23-34. https://doi.org/10.32604/or.2022.028449
Received 18 December 2022; Accepted 02 February 2023; Issue published 01 March 2023
Abstract
This study aimed to examine the association between the use of H1-antihistamines (AHs) and head and neck cancer (HNC) risk in patients with type 2 diabetes mellitus (T2DM). Data from the National Health Insurance Research Database of Taiwan were analyzed for the period from 2008 to 2018. A propensity-score-matched cohort of 54,384 patients each in the AH user and nonuser groups was created and analyzed using Kaplan-Meier method and Cox proportional hazards regression. The results showed that the risk of HNC was significantly lower in AH users (adjusted hazard ratio: 0.55, 95% CI: 0.48 to 0.64) and the incidence rate was also lower (5.16 vs. 8.10 per 100,000 person-years). The lower HNC incidence rate in AH users (95% CI: 0.63; 0.55 to 0.73) suggests that AH use may reduce the risk of HNC in T2DM patients.Keywords
Abbreviations
| aHR | Adjusted hazard ratio |
| CI | Confidence interval |
| aDCSI | Adapted Diabetes Complications Severity Index |
| AH | Antihistamine |
| cDDD | Cumulative defined daily dose |
| DDD | Defined daily dose |
| IQR | Interquartile range |
| SD | Standard deviation |
| N | Number |
| ASMD | Absolute standardized mean difference |
| HR | Hazard ratio |
| IR | Incidence rate |
| IRR | Incidence rate ratio |
| HNC | Head and neck cancer |
| T2DM | Type 2 diabetes |
| PSM | Propensity score matching |
| NHI | National Health Insurance |
| NHIRD | National Health Insurance Research Database |
| IGF-1 | Insulin-like growth factor-1 |
| DM | Diabetes mellitus |
| ICD-9-CM | International Classification of Diseases, Ninth Revision, Clinical Modification |
| ICD-10-CM | International Classification of Diseases, Tenth Revision, Clinical Modification |
| CAD | Cationic amphiphilic drug |
| RCT | Randomized controlled trial |
In 2020, head and neck cancer (HNC) was a significant global health issue, ranking as the seventh most common cancer and causing the seventh highest number of cancer deaths. The estimated number of new HNC cases in 2020 was 932,000, with 467,000 new HNC deaths [1]. The incidence and mortality of HNC continue to rise, and both are predicted to increase by approximately 25% by 2030 [1]. In 2020, head and neck cancer (HNC) was a prevalent concern in Taiwan, ranking as the third most common cancer among men and the fifth leading cause of cancer death [2–5]. HNC mostly occurs in economically active adult men; the median ages at diagnosis and death are 57 and 60 years, respectively, for men in Taiwan [2–5]. Consequently, HNC places a tremendous burden not only on public finances [6] but also on family finances because those diagnosed with HNC tend to be key providers for their families [2–4].
An estimated 537 million adults worldwide have diabetes mellitus (DM)—more than 1 in 10 adults [7]. The incidence and prevalence of DM are still increasing, with 783 million people globally predicted to be affected in 2045 [7]. Type 2 diabetes mellitus (T2DM) is the most prevalent form of diabetes, affecting hundreds of millions of people worldwide and accounting for over 90% of all diabetes cases [8]. T2DM is marked by hyperglycemia, insulin resistance, decreased insulin secretion, and dyslipidemia, including elevated triglyceride levels and low high-density lipoprotein cholesterol [9–12]. Studies have suggested an association between DM and HNC [13–16]. Tseng et al. [13] found that individuals newly diagnosed with DM had a 1.47-fold higher overall incidence of HNC compared to those without DM, regardless of factors such as age, sex, geographic location, income, and coexisting conditions. A systematic review and meta-analysis study involving more than 51,496 individuals with HNC cancer in East Asia revealed an association between DM and elevated HNC risk [14]. A South Korean national cohort study found that diabetes mellitus (DM) increases the risk of head and neck cancer (HNC) in both genders, with a higher risk compared to moderate alcohol consumption and equal to the risk from cigarette smoking [16]. Therefore, identifying a medication that could mitigate or eliminate the risk of HNC in patients with T2DM is imperative.
H1-antihistamine (AH) use has been linked to a reduction in the risk for various cancers, including glioma, ovarian cancer, and hepatocellular carcinoma [17–19]. One multinational study found that antihistamine (AH) use was linked to a 30% decrease in the risk of adult glioma [17]. AHs were also found to reduce ovarian cancer risk among premenopausal women [18,20]. A retrospective cohort study from 2022 showed that AH use may decrease the risk of hepatocellular carcinoma in a dose-dependent manner among patients infected with hepatitis B virus, hepatitis C virus, or both [19]. Another study found that AH use can lower the risk of HCC in patients with T2DM who are not infected with hepatitis B or C, and the effect is dose-dependent [21]. Despite a growing body of evidence on the anticancer properties of AHs [17–21], the impact of their use on HNC risk in patients with T2DM has not been fully explored. To fill this gap, we performed a nationwide, population-based cohort study using propensity score matching (PSM) to assess the association between AH use and HNC risk in T2DM patients.
This study was based on data from the National Health Insurance Research Database (NHIRD) of Taiwan’s National Health Insurance (NHI) system, which covers over 99.6% of the country’s population. The NHIRD provided detailed medical records of NHI enrollees, after anonymizing them to protect patients’ privacy. Information on patient diagnoses, treatments, and drug usage was extracted for analysis. The study was approved by the Institutional Review Board of Tzu-Chi Medical Foundation and conducted following the Strengthening the Reporting of Observational Studies in Epidemiology guidelines and the principles of the Declaration of Helsinki, with a waiver of informed consent obtained.
Our T2DM cohort initially consisted of 480,000 patients with at least three ambulatory care claims or one inpatient claim from 2008 to 2020 in the NHIRD. The patients were monitored from their diagnosis until HNC development, death, or cohort exit. The index date was set as the date one year after the start of AH use (at least 28 cumulative defined daily doses [cDDDs]) or cohort entry. The follow-up period lasted one year from the index date. HNC was the endpoint. AH use was defined as using more than 28 cDDDs. In the study, only H1-antihistamines were considered due to their association with reduced risk of cancer, such as glioma, ovarian cancer, and hepatocellular carcinoma in previous studies [17–19]. We did not include H2-antihistamines in our analysis because they have not been shown to have the same protective effect.
We filtered out participants who met any of the following criteria: (1) received a HNC diagnosis in the first year after T2DM diagnosis, (2) missing information on sex and age in the database or below 18 years of age, (3) received a HNC diagnosis in the first year after index date, (4) had a follow-up duration less than 1 year, (5) diagnosed with any type of cancer within 1 year prior to the cohort entry date to eliminate the impact of HNC metastasis.
An AH prescription was defined as previously reported in the protocol [19,21]. In this study, AHs were used for various conditions including asthma, allergic rhinitis, medication allergies, environmental allergies, or viral infections (such as itchy eyes, runny nose, and pruritus). Information was collected about the type of drug, dose, method of administration, prescription date, and number of pills dispensed, as AH usage was reimbursed by Taiwan’s National Health Insurance (NHI). To account for different AH use patterns over time, we treated AH usage as a time-varying factor in the Cox model. The cumulative dose was calculated by multiplying the number of pills dispensed by the prescribed dose and dividing the result by the number of days’ supply dispensed. The cDDDs were calculated as the sum of the World Health Organization-defined average daily dose per drug. Occasional use was excluded by defining <28 cDDDs as AH non-use and ≥28 cDDDs as AH use in the cohort.
In our analysis, we used a time-varying Cox proportional hazards model after adjusting for potential confounding factors. This model compared the time from the index date to HNC onset between T2DM patients with and without AH use. To minimize the influence of confounders, we used PSM based on patient variables like age, sex, income, urbanization, antidiabetic medication, comorbidities, smoking, alcohol-related diseases, and diabetes severity. The presence of comorbidities was determined using ICD-9-CM or ICD-10-CM codes based on one inpatient visit or two or more outpatient visits. In addition to these factors, we also considered Epstein-Barr virus and human papillomavirus infection, as well as the indications for H1-antihistamine use (e.g., asthma, dermatitis, rhinitis, and urticaria) [22,23].
In this study, we present continuous variables as means ± standard deviations or medians (first and third quartiles) as appropriate. We utilized PSM with a caliper width of 0.2, [24] using the greedy method to match patients at a 1:1 ratio. Matching is a popular method for selecting control subjects with identical important background covariates, aimed at minimizing differences between patient groups.
The primary end point in this study was the diagnosis of HNC, which was verified through the records found in the Registry for Catastrophic Illness Patients [25].
Baseline characteristics of the study population
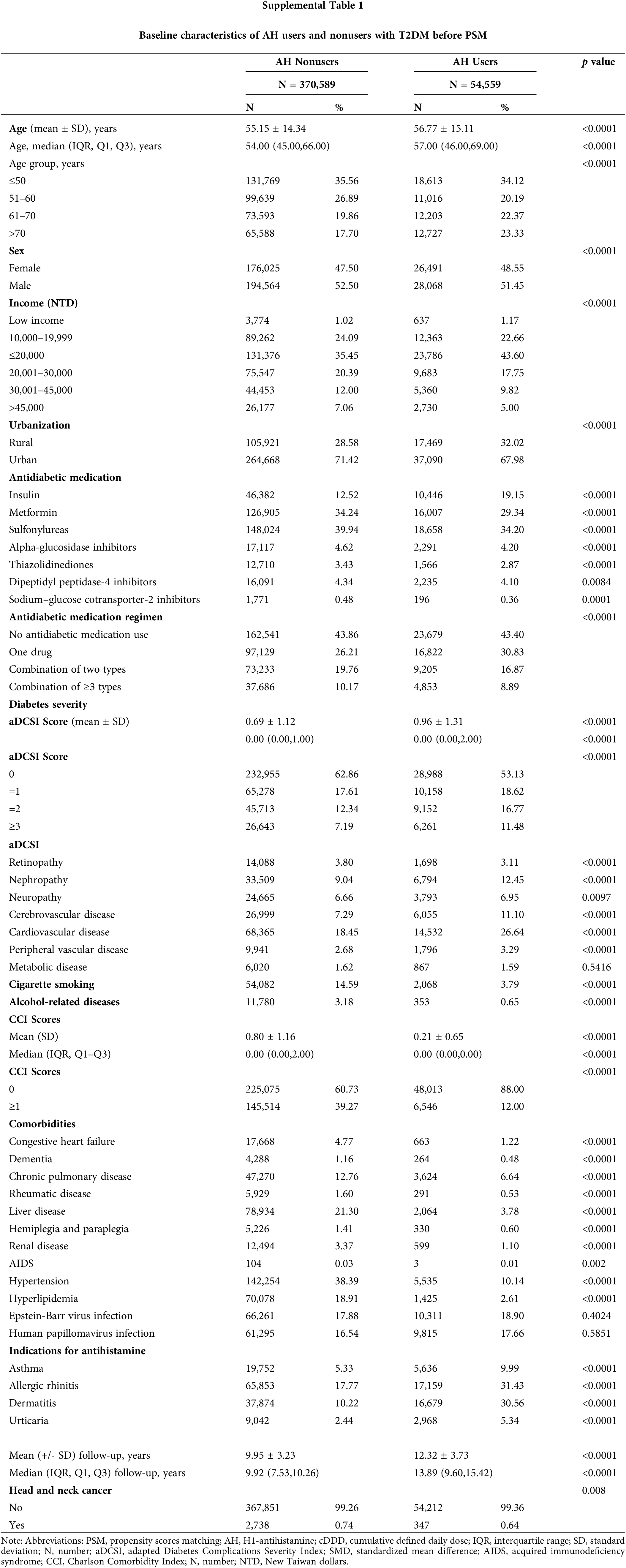
In total, 480,000 individuals diagnosed with T2DM from 2008 to 2018 were included in the study. The final follow-up date was December 31, 2020. Exclusions were made for patients with HNC within a year of T2DM diagnosis (n = 8,341), those without age or sex data or under 18 years old (n = 12,388), those with HNC within a year of the index date (n = 299), those with less than a year of follow-up (n = 22,370), and those with a history of other cancers (n = 11,454). The remaining participants (N = 425,148) were split into two groups: (1) those who did not use AHs (n = 370,589) and (2) those who did (n = 54,559). After 1:1 matching, each group had 54,384 patients. At baseline, relative to the AH nonusers, the AH users were older, tended to be female, had lower income levels, lived more in rural areas, had more insulin use, were less likely to use antidiabetic medication, had more advanced diabetic severity (higher adapted Diabetes Complications Severity Index scores), had lower rates of cigarette smoking and alcohol-related diseases, had more indications for antihistamine, and had lower CCI scores (Suppl. Table 1). Thus, PSM was employed to establish a proper balance between the cases and control groups (Table 1).
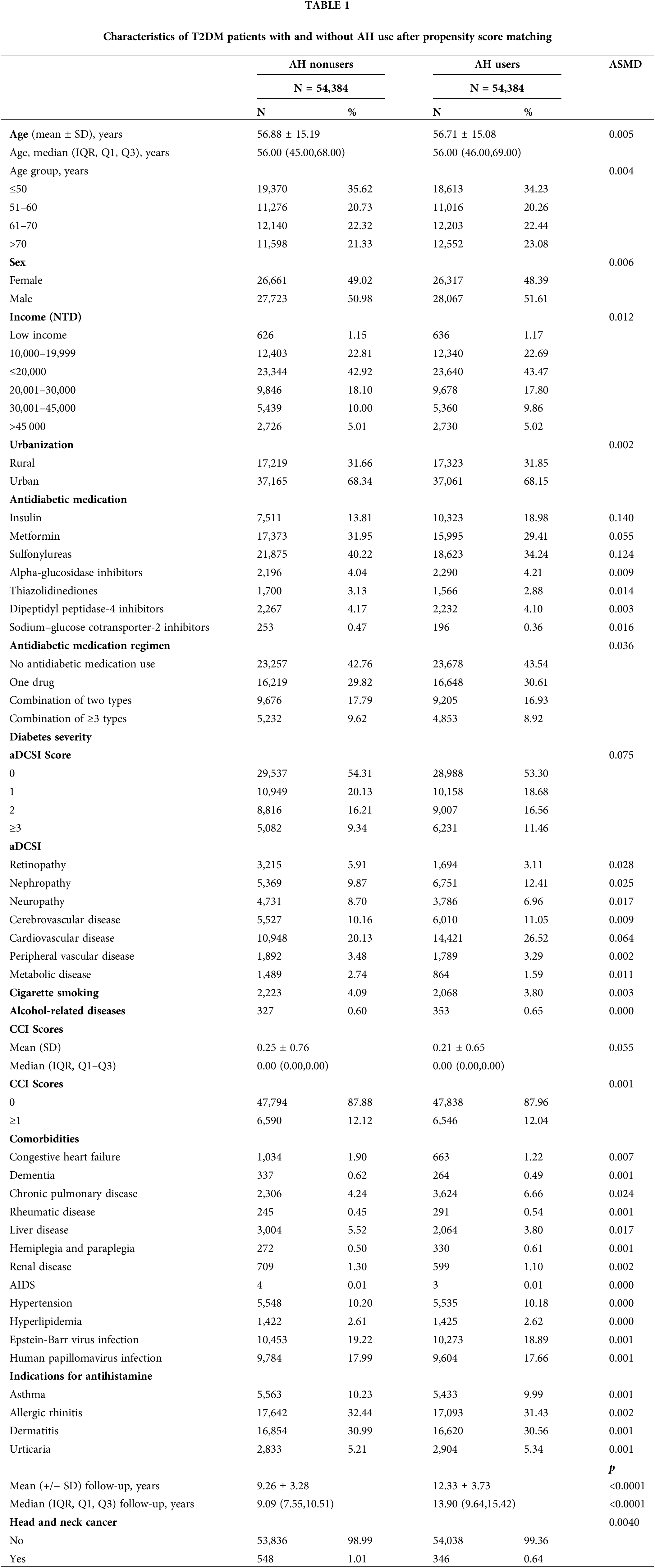
After conducting PSM, the two groups, AH users and non-users, displayed a balanced age distribution. Furthermore, there were no significant differences between the groups in terms of demographic and clinical factors like age, sex, income, urbanization, antidiabetic medication, antidiabetic medication regimen, comorbidities, smoking habits, alcohol-related diseases, and diabetes severity.
Risk factors with HNC incidence
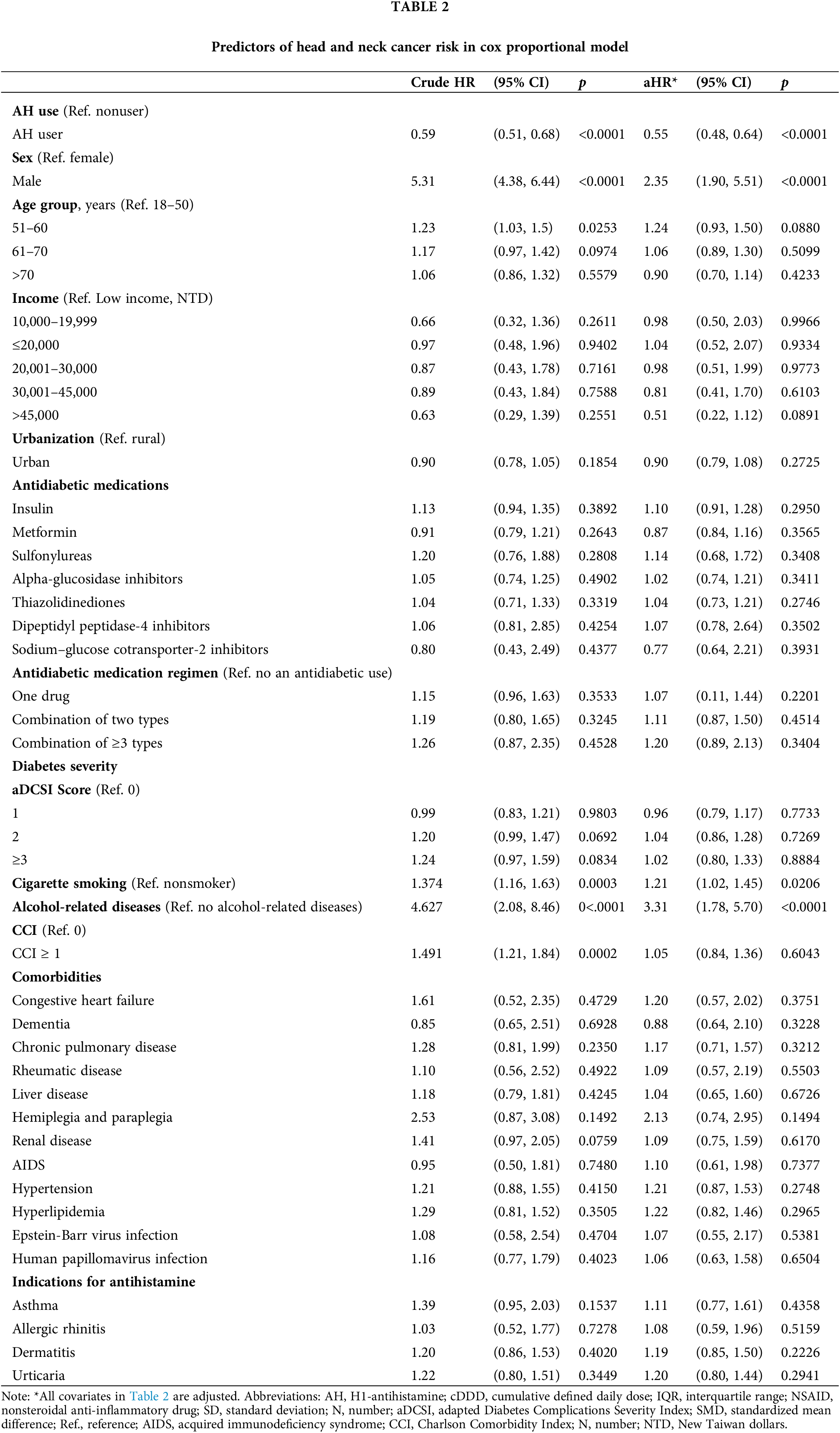
Table 2 shows the relationship between HNC risk and co-occurring medications and health issues. Our study found that HNC risk was higher for men than women (HR: 2.35; 95% CI: 1.90 to 5.51). Additionally, smoking cigarettes (HR: 1.21; 95% CI: 1.02 to 1.45) and having alcoholic liver disease (HR: 3.31; 95% CI: 1.78 to 5.70) were linked to a higher HNC risk.
Risks of HNC in AH users vs. nonusers: IRRs and aHRs
Table 2 displays the relationship between AH use and HNC risk in the population of patients with T2DM. After adjustments for age, sex, income level, urbanization, antidiabetic medication, antidiabetic medication regimen, comorbidities, cigarette smoking, alcohol-related diseases, and diabetes severity, AH exposure was linked to a reduced risk of HNC (aHR: 0.55; 95% CI, 0.48 to 0.64) and a lower IR (5.16 vs. 8.10 per 100,000 person-years) in the T2DM cohort. The IRR (95% CI) was also lower for AH users compared to nonusers (0.63; 0.55 to 0.73) (Table 3).
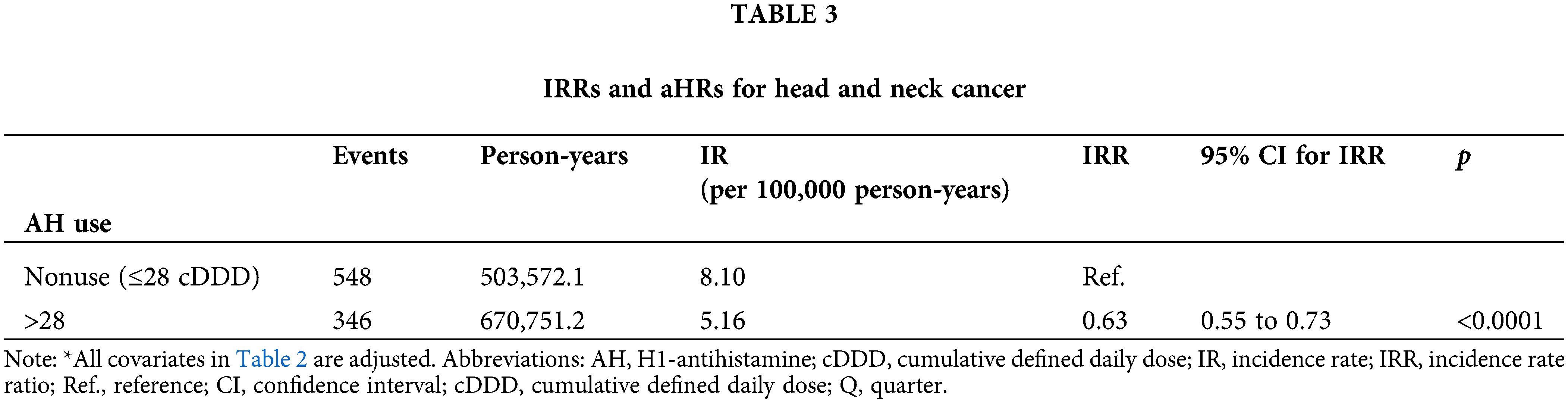
The Kaplan–Meier analysis indicated a reduced HNC risk among AH users compared to nonusers, as shown in Fig. 1 (log-rank test, p < 0.001).
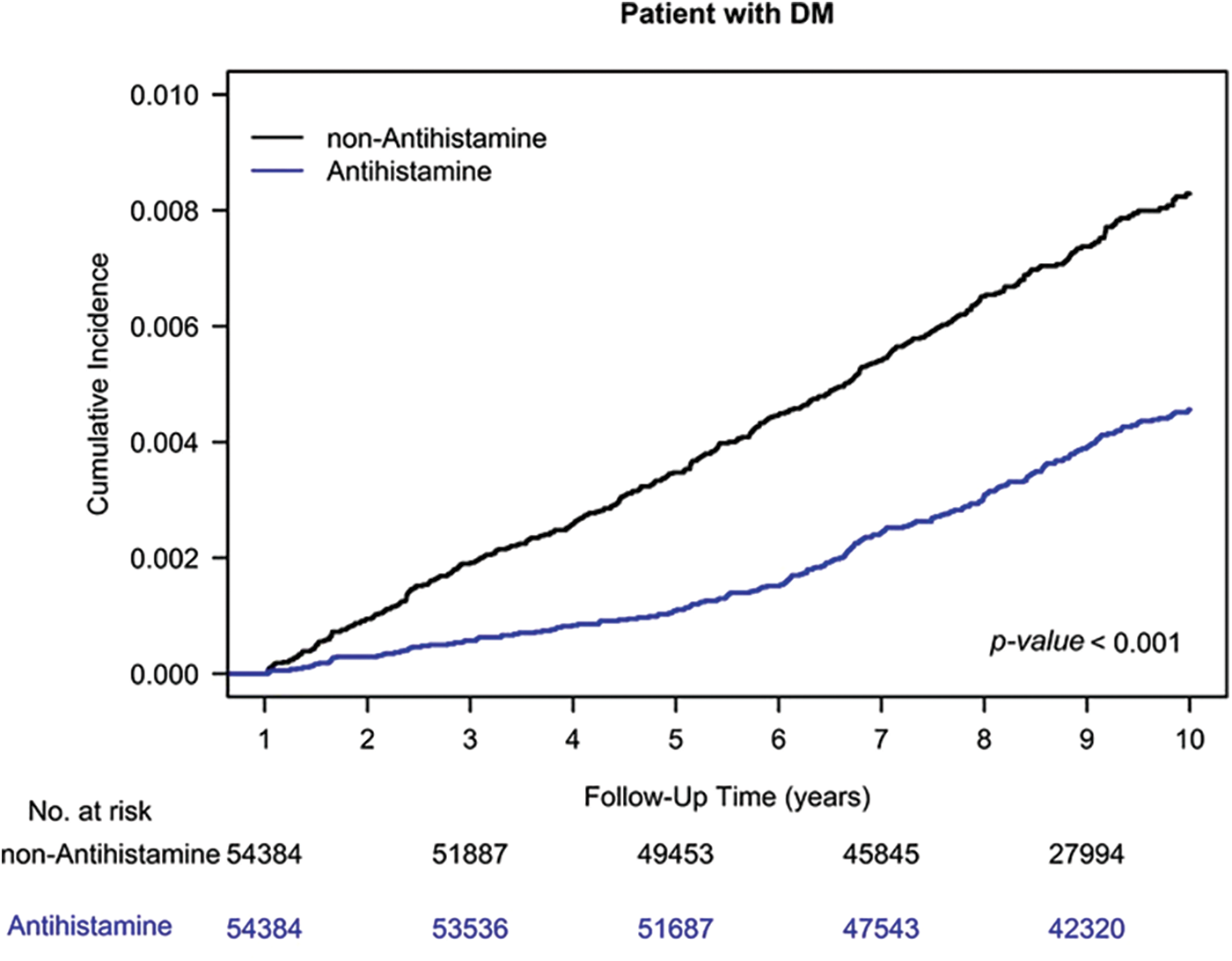
Figure 1: Cumulative incidence of head and neck cancer among AH users and nonusers.
The study indicated that individuals with diabetes faced a 1.47 times greater risk of HNC than those without diabetes did for a first malignant tumor (aHR: 1.48; 95% CI, 1.31 to 1.67) [13]. The rise in T2DM cases could result in a similar increase in HNC risk [13,26,27]. As such, finding drugs that could provide protection against HNC among vulnerable groups is crucial. Chronic inflammation and the purging of insulin-like growth factor-1 (IGF-1) caused by T2DM have been linked to HNC risk [20,26]. A preclinical study indicated that AHs could not only relieve allergies, but also exhibit antitumoral properties [28]. This study is a pioneering examination of the benefits of AHs in reducing HNC risk among patients with T2DM. The results, after taking all influencing factors into account, show that HNC risk was lower for those who used AHs compared to those who did not. The IRR for HNC was also found to be lower for AH users compared to non-users.
Previous studies have established various mechanisms that connect T2DM and HNC risk, such as hyperinsulinemia, hyperglycemia, and chronic inflammation [13,26,29–31]. The growth-promoting properties of insulin, as well as its effect on IGF-I, could fuel carcinogenesis, allowing the survival of both preneoplastic and neoplastic cells with insulin, IGF-I, or hybrid receptors [29]. The contribution of elevated IGF-1 receptor expression to HNC drug resistance and poor survival has been documented in previous studies [31]. Hyperglycemia may directly promote tumor growth by sustaining the increased glucose consumption of cancer cells [13,26,29–31]. Additionally, hyperglycemia could initiate cancer development by acetifying DNA damage and oxidative stress [13,26,29–31]. Hyperglycemia-induced chronic inflammation increases HNC risk as well [26]. Hyperglycemia stimulates inflammation, and inflammatory cells secrete cytokines and chemokines that proliferate malignant cells, inhibit apoptosis, aid angiogenesis, and promote metastasis [32]. Therefore, AH use might modulate chronic inflammation and hyperglycemia in those with T2DM, thereby contributing to the reduction of HNC risk [33,34] this conclusion aligns with previous clinical studies [19,21].
The reason behind the protective role of AHs against HNC associated with diabetes is not clear, and several theories have been proposed. The anti-inflammatory effect of AHs can prevent chronic inflammation, which is a risk factor for HNC [33]. Furthermore, AHs stabilize mast cell membranes and block the release of histamine, which has a proliferative effect on malignant cells [34]. Ellegaard et al. [35] reported that only AHs in the cationic amphiphilic drug (CAD) class can reduce cancer mortality. Thus, the anticancer effect of CADs may be related to their CAD structure rather than their antihistamine effect. Therefore, further research is required for elucidating the mechanisms through which AH use reduces diabetes-related HNC risk, apart from the anti-inflammation effects and stabilization of mast cell [33–35].
Conducting a randomized controlled trial (RCT) to assess the impact of AH on HNC risk in T2DM patients is challenging as AH use cannot be altered through tangible interventions [36]. Designing a RCT to evaluate the impact of AHs on HNC risk in patients with T2DM can be difficult due to the challenge of balancing confounding factors between the case and control groups, as well as the need for long-term follow-up in preventive trials [36]. The current study utilized PSM and retrospective real-world data to balance confounding factors between the case and control groups, eliminating potential bias. PSM is considered the gold standard for estimating the impact of covariates when bias may exist. However, PSM cannot control for unmeasured factors and may result in an explicit selection bias, as individuals who cannot be matched are excluded from the inference.
In our study, male sex, cigarette smoking, and alcoholic liver disease were found to be the risk factors for HNC in patients with T2DM, as shown in Table 2. These risk factors align with those reported in previous studies [2–4,37,38]. This result implies that tobacco and alcohol exposures remain the principal HNC risk factors even in a T2DM cohort. The identification of male sex, cigarette smoking, and alcohol consumption as risk factors might be attributed to residual imbalance, [39,40] although well-designed PSM was performed. In our T2DM cohort, all covariates were matched and adjusted; in our T2DM population, AH usage protects against HNC development independently.
This is the first study, to our knowledge, examining the connection between AH use and HNC risk in a T2DM cohort. The study boasts several strengths, such as a large sample size and validation cohort, extended follow-up period, consistent covariates in case and control groups after PSM, and thorough verification of medication records. However, it has some limitations, including its exclusive focus on an Asian population in Taiwan, and therefore, the findings may not be generalizable to non-Asian populations. Nevertheless, the literature does not suggest that ethnicity plays a role in the effect of AHs, their anti-inflammation effects, or their stabilization of mast cells. Second, PSM may not account for unbalanced characteristics and confounders, and thus, any untracked confounding factors could influence the outcome. Third, H1-antihistamines are available over-the-counter (OTC) in Taiwan and are easily reimbursable through national insurance for patients. Patients don’t have to buy H1-antihistamines over-the-counter, leading to a likelihood of higher usage than what our study recorded, given unreported OTC use. If the OTC availability of H1-antihistamines is higher in the non-AH user group, the protective effects of head and neck cancer for AH users may be underestimated. However, our conclusion would not be overturned, only underestimated. Fourth, patients may not have carefully adhered to their AHs regimen, and The estimated AH intake amount may have been too high. However, in the case of nonadherence to the prescribed AH regimen, the effects of AH use on the reduction of HNC risk would be underestimated; our conclusion is thus robust. Fifth, the diagnosis of coexisting conditions in the Taiwan Cancer Registry Database was determined using ICD-9-CM or ICD-10-CM codes from the Taiwan National Health Insurance Research Database. To ensure the accuracy of these diagnoses, the National Health Insurance Administration reviews patients’ medical records and conducts interviews. The Administration also audits hospitals with unusual charges or practices, imposing strict penalties for any malpractice discovered. Despite these efforts, further research in the form of large-scale RCTs comparing the risk of HNC among users and non-users of AH would still be necessary to provide more precise population information, gather disease occurrence data, and minimize confounding variables. However, conducting an RCT in this context may prove challenging.
Patients with T2DM who use AH have a lower likelihood of HNC, regardless of factors such as age, concurrent health conditions, medications for diabetes, or the severity of their diabetes.
We gathered data on the following patient baseline factors: age (grouped into 10-year increments), sex, income, urbanization, antidiabetic medication type and regimen, coexisting illnesses, smoking, alcohol-related illnesses, diabetes severity, and AH use. The differences between AH users and non-users were analyzed using chi-square and t-tests for categorical and continuous variables respectively, with the Wilcoxon rank-sum test for median values. The baseline was established as the date of cohort entry. To determine the HNC risk for each group, we calculated incidence rates and ratios, and adjusted hazard ratios with 95% confidence intervals were estimated using Cox regression models, controlling for baseline characteristics (except for AH use). The cumulative incidence of HNC was estimated using the Kaplan-Meier method and compared through the log-rank test.
The statistical analyses were conducted using SAS for Windows (version 9.4) by the SAS Institute located in Cary, North Carolina, USA. A two-sided p value less than 0.05 was deemed statistically significant.
Acknowledgement: This work was supported by the Lo-Hsu Medical Foundation, LotungPoh-Ai Hospital.
Funding Statement: Lo-Hsu Medical Foundation, LotungPoh-Ai Hospital, supports Szu-Yuan Wu’s work (Funding Numbers: 10908, 10909, 11001, 11002, 11003, 11006, and 11013).
Author Contributions: The authors contributed as follows: Yi-Nong Chen, Ying-Lin Chen, Wan-Ming Chen, Jenq-Yuh Ko, Mingchih Chen, Ben-Chang Shia, and Szu-Yuan Wu were responsible for the conception and design of the study. Financial support was provided by the Lo-Hsu Medical Foundation and LotungPoh-Ai Hospital and supported Szu-Yuan Wu’s work. Ying-Lin Chen and Szu-Yuan Wu were responsible for the collection and assembly of data. Data analysis and interpretation was carried out by Wan-Ming Chen, Ben-Chang Shia, and Szu-Yuan Wu. Szu-Yuan Wu provided administrative support and Jenq-Yuh Ko and Szu-Yuan Wu were responsible for manuscript writing. All authors approved the final manuscript.
Availability of Data and Materials: The data sets supporting the study conclusions are included in the manuscript. We used data from the National Health Insurance Research Database and Taiwan Cancer Registry database. The authors confirm that, for approved reasons, some access restrictions apply to the data underlying the findings. The data used in this study cannot be made available in the manuscript, the supplemental files, or in a public repository due to the Personal Information Protection Act executed by Taiwan’s government, starting in 2012. Requests for data can be sent as a formal proposal to obtain approval from the ethics review committee of the appropriate governmental department in Taiwan. Specifically, links regarding contact info for which data requests may be sent to are as follows: http://nhird.nhri.org.tw/en/Data_Subsets.html#S3 and http://nhis.nhri.org.tw/point.html.
Ethics Approval: The study protocols were reviewed and approved by the Institutional Review Board of Tzu-Chi Medical Foundation (IRB109-015-B).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I. et al. (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians, 71(3), 209–249. https://doi.org/10.3322/caac.21660 [Google Scholar] [PubMed] [CrossRef]
2. Liu, W. C., Liu, H. E., Kao, Y. W., Qin, L., Lin, K. C. et al. (2021). Definitive intensity-modulated radiotherapy or surgery for early oral cavity squamous cell carcinoma: Propensity-score-matched, nationwide, population-based cohort study. Head and Neck, 43(4), 1142–1152. https://doi.org/10.1002/hed.26575 [Google Scholar] [PubMed] [CrossRef]
3. Lin, K. C., Chen, T. M., Yuan, K. S., Wu, A. T. H., Wu, S. Y. (2020). Assessment of predictive scoring system for 90-day mortality among patients with locally advanced head and neck squamous cell carcinoma who have completed concurrent chemoradiotherapy. JAMA Network Open, 3(3), e1920671. https://doi.org/10.1001/jamanetworkopen.2019.20671 [Google Scholar] [PubMed] [CrossRef]
4. Chang, C. L., Tsai, H. C., Lin, W. C., Chang, J. H., Hsu, H. L. et al. (2017). Dose escalation intensity-modulated radiotherapy-based concurrent chemoradiotherapy is effective for advanced-stage thoracic esophageal squamous cell carcinoma. Radiotherapy and Oncology, 125(1), 73–79. https://doi.org/10.1016/j.radonc.2017.08.025 [Google Scholar] [PubMed] [CrossRef]
5. Health Promotion Administration MoHaW (2020). Taiwan cancer registry annual report. https://www.hpa.gov.tw/Pages/List.aspx?nodeid=269. [Google Scholar]
6. Patterson, R. H., Fischman, V. G., Wasserman, I., Siu, J., Shrime, M. G. et al. (2020). Global burden of head and neck cancer: Economic consequences, health, and the role of surgery. Otolaryngology-Head and Neck Surgery, 162(3), 296–303. https://doi.org/10.1177/0194599819897265 [Google Scholar] [PubMed] [CrossRef]
7. Sun, H., Saeedi, P., Karuranga, S., Pinkepank, M., Ogurtsova, K. et al. (2022). IDF diabetes atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Research and Clinical Practice, 183, 109119. https://doi.org/10.1016/j.diabres.2021.109119 [Google Scholar] [PubMed] [CrossRef]
8. Chatterjee, S., Khunti, K., Davies, M. J. (2017). Type 2 diabetes. Lancet, 389(10085), 2239–2251. [Google Scholar] [PubMed]
9. DeFronzo, R. A., Ferrannini, E. (1991). Insulin resistance. A multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease. Diabetes Care, 14(3), 173–194. https://doi.org/10.2337/diacare.14.3.173 [Google Scholar] [PubMed] [CrossRef]
10. Alberti, K. G., Eckel, R. H., Grundy, S. M., Zimmet, P. Z., Cleeman, J. I. et al. (2009). Harmonizing the metabolic syndrome: A joint interim statement of the international diabetes federation task force on epidemiology and prevention; national heart, lung, and blood institute; American heart association; world heart federation; international atherosclerosis society; and international association for the study of obesity. Circulation, 120(16), 1640–1645. https://doi.org/10.1161/CIRCULATIONAHA.109.192644 [Google Scholar] [PubMed] [CrossRef]
11. Phillips, D. I., Barker, D. J., Hales, C. N., Hirst, S., Osmond, C. (1994). Thinness at birth and insulin resistance in adult life. Diabetologia, 37(2), 150–154. https://doi.org/10.1007/s001250050086 [Google Scholar] [PubMed] [CrossRef]
12. Valdez, R., Athens, M. A., Thompson, G. H., Bradshaw, B. S., Stern, M. P. (1994). Birthweight and adult health outcomes in a biethnic population in the USA. Diabetologia, 37(6), 624–631. https://doi.org/10.1007/BF00403383 [Google Scholar] [PubMed] [CrossRef]
13. Tseng, K. S., Lin, C., Lin, Y. S., Weng, S. F. (2014). Risk of head and neck cancer in patients with diabetes mellitus: A retrospective cohort study in Taiwan. JAMA Otolaryngology-Head & Neck Surgery, 140(8), 746–753. https://doi.org/10.1001/jamaoto.2014.1258 [Google Scholar] [PubMed] [CrossRef]
14. Yan, P., Wang, Y., Yu, X., Liu, Y., Zhang, Z. J. (2021). Type 2 diabetes mellitus and risk of head and neck cancer subtypes: A systematic review and meta-analysis of observational studies. Acta Diabetologica, 58(5), 549–565. https://doi.org/10.1007/s00592-020-01643-0 [Google Scholar] [PubMed] [CrossRef]
15. Lee, O. H., Park, Y. M., Ko, S. H., Lee, K., Kim, Y. et al. (2022). Synergistic association between underweight and type 2 diabetes on the development of laryngeal cancer: A national population-based retrospective cohort study. BMC Cancer, 22(1), 345. https://doi.org/10.1186/s12885-022-09403-9 [Google Scholar] [PubMed] [CrossRef]
16. Choi, S. Y., Cheong, H. K., Lee, M. K., Kang, J. W., Lee, Y. C. et al. (2022). Metabolic diseases and risk of head and neck cancer: A cohort study analyzing nationwide population-based data. Cancers, 14(13), 3277. https://doi.org/10.3390/cancers14133277 [Google Scholar] [PubMed] [CrossRef]
17. Schlehofer, B., Blettner, M., Preston-Martin, S., Niehoff, D., Wahrendorf, J. et al. (1999). Role of medical history in brain tumour development. Results from the international adult brain tumour study. International Journal of Cancer, 82(2), 155–160. https://doi.org/10.1002/(ISSN)1097-0215 [Google Scholar] [CrossRef]
18. Verdoodt, F., Pottegard, A., Dehlendorff, C., Jaattela, M., Hallas, J. et al. (2019). Antihistamine use and risk of ovarian cancer: A population-based case-control study. Maturitas, 120, 47–52. https://doi.org/10.1016/j.maturitas.2018.11.014 [Google Scholar] [PubMed] [CrossRef]
19. Shen, Y. C., Hsu, H. C., Lin, T. M., Chang, Y. S., Hu, L. F. et al. (2022). H1-antihistamines reduce the risk of hepatocellular carcinoma in patients with hepatitis B virus, hepatitis C virus, or dual hepatitis B virus-hepatitis C virus infection. Journal of Clinical Oncology, 40(11), 1206–1219. https://doi.org/10.1200/JCO.21.01802 [Google Scholar] [PubMed] [CrossRef]
20. Wiencke, J. K. (2004). Impact of race/ethnicity on molecular pathways in human cancer. Nature Reviews Cancer, 4(1), 79–84. https://doi.org/10.1038/nrc1257 [Google Scholar] [PubMed] [CrossRef]
21. Wu, S. Y., Chen, W. M., Chen, Y. C., Chiang, M. F., Lee, M. C. et al. (2022). Effects of H1-antihistamines on hepatocellular carcinoma risk in patients with type 2 diabetes mellitus. Diabetes & Metabolism, 49(1), 101393. https://doi.org/10.1016/j.diabet.2022.101393 [Google Scholar] [PubMed] [CrossRef]
22. Chiang, C. H., Chiang, C. H., Peng, C. Y., Hsia, Y. P., See, X. Y. et al. (2022). Efficacy of cationic amphiphilic antihistamines on outcomes of patients treated with immune checkpoint inhibitors. European Journal of Cancer, 174(6382), 1–9. https://doi.org/10.1016/j.ejca.2022.07.006 [Google Scholar] [PubMed] [CrossRef]
23. Chiang, C. H., Chiang, C. H., Chiang, C. H. (2022). Are H1-antihistamines associated with a lower risk of hepatocellular carcinoma? Journal of Clinical Oncology, 40(21), 2389–2390. https://doi.org/10.1200/JCO.22.00179 [Google Scholar] [PubMed] [CrossRef]
24. Austin, P. C. (2011). Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharmaceutical Statistics, 10(2), 150–161. https://doi.org/10.1002/pst.433 [Google Scholar] [PubMed] [CrossRef]
25. Shao, Y. J., Chan, T. S., Tsai, K., Wu, S. Y. (2018). Association between proton pump inhibitors and the risk of hepatocellular carcinoma. Alimentary Pharmacology & Therapeutics, 48(4), 460–468. https://doi.org/10.1111/apt.14835 [Google Scholar] [PubMed] [CrossRef]
26. Wang, X., Wang, H., Zhang, T., Cai, L., Dai, E. et al. (2020). Diabetes and its potential impact on head and neck oncogenesis. Journal of Cancer, 11(3), 583–591. https://doi.org/10.7150/jca.35607 [Google Scholar] [PubMed] [CrossRef]
27. Stott-Miller, M., Chen, C., Chuang, S. C., Lee, Y. C., Boccia, S. et al. (2012). History of diabetes and risk of head and neck cancer: A pooled analysis from the international head and neck cancer epidemiology consortium. Cancer Epidemiology, Biomarkers & Prevention, 21(2), 294–304. https://doi.org/10.1158/1055-9965.EPI-11-0590 [Google Scholar] [PubMed] [CrossRef]
28. Faustino-Rocha, A. I., Ferreira, R., Gama, A., Oliveira, P. A., Ginja, M. (2017). Antihistamines as promising drugs in cancer therapy. Life Sciences, 172, 27–41. https://doi.org/10.1016/j.lfs.2016.12.008 [Google Scholar] [PubMed] [CrossRef]
29. Pollak, M. (2008). Insulin and insulin-like growth factor signalling in neoplasia. Nature Reviews Cancer, 8(12), 915–928. https://doi.org/10.1038/nrc2536 [Google Scholar] [PubMed] [CrossRef]
30. Giovannucci, E., Harlan, D. M., Archer, M. C., Bergenstal, R. M., Gapstur, S. M. et al. (2010). Diabetes and cancer: A consensus report. Diabetes Care, 33(7), 1674–1685. https://doi.org/10.2337/dc10-0666 [Google Scholar] [PubMed] [CrossRef]
31. Denduluri, S. K., Idowu, O., Wang, Z., Liao, Z., Yan, Z. et al. (2015). Insulin-like growth factor (IGF) signaling in tumorigenesis and the development of cancer drug resistance. Genes & Diseases, 2(1), 13–25. https://doi.org/10.1016/j.gendis.2014.10.004 [Google Scholar] [PubMed] [CrossRef]
32. Monkkonen, T., Debnath, J. (2018). Inflammatory signaling cascades and autophagy in cancer. Autophagy, 14(2), 190–198. https://doi.org/10.1080/15548627.2017.1345412 [Google Scholar] [PubMed] [CrossRef]
33. Devillier, P., Roche, N., Faisy, C. (2008). Clinical pharmacokinetics and pharmacodynamics of desloratadine, fexofenadine and levocetirizine: A comparative review. Clinical Pharmacokinetics, 47(4), 217–230. https://doi.org/10.2165/00003088-200847040-00001 [Google Scholar] [PubMed] [CrossRef]
34. Medina, V. A., Rivera, E. S. (2010). Histamine receptors and cancer pharmacology. British Journal of Pharmacology, 161(4), 755–767. https://doi.org/10.1111/j.1476-5381.2010.00961.x [Google Scholar] [PubMed] [CrossRef]
35. Ellegaard, A. M., Dehlendorff, C., Vind, A. C., Anand, A., Cederkvist, L. et al. (2016). Repurposing cationic amphiphilic antihistamines for cancer treatment. EBioMedicine, 9, 130–139. https://doi.org/10.1016/j.ebiom.2016.06.013 [Google Scholar] [PubMed] [CrossRef]
36. Deaton, A., Cartwright, N. (2018). Understanding and misunderstanding randomized controlled trials. Social Science & Medicine, 210(5), 2–21. https://doi.org/10.1016/j.socscimed.2017.12.005 [Google Scholar] [PubMed] [CrossRef]
37. Hashim, D., Genden, E., Posner, M., Hashibe, M., Boffetta, P. (2019). Head and neck cancer prevention: From primary prevention to impact of clinicians on reducing burden. Annals of Oncology, 30(5), 744–756. https://doi.org/10.1093/annonc/mdz084 [Google Scholar] [PubMed] [CrossRef]
38. Auperin, A. (2020). Epidemiology of head and neck cancers: An update. Current Opinion in Oncology, 32(3), 178–186. https://doi.org/10.1097/CCO.0000000000000629 [Google Scholar] [PubMed] [CrossRef]
39. Nguyen, T. L., Collins, G. S., Spence, J., Daures, J. P., Devereaux, P. J. et al. (2017). Double-adjustment in propensity score matching analysis: Choosing a threshold for considering residual imbalance. BMC Medical Research Methodology, 17(1), 78. https://doi.org/10.1186/s12874-017-0338-0 [Google Scholar] [PubMed] [CrossRef]
40. Zhang, Z., Kim, H. J., Lonjon, G., Zhu, Y. (2019). Balance diagnostics after propensity score matching. Annals of Translational Medicine, 7(1), 16. https://doi.org/10.21037/atm.2018.12.10 [Google Scholar] [PubMed] [CrossRef]
Cite This Article
 Copyright © 2023 The Author(s). Published by Tech Science Press.
Copyright © 2023 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools