 Open Access
Open Access
VIEWPOINT
Galectins dysregulation: A way for cancer cells to invade and pervade
1 Molecular Cancer Biology Group, Zoology Department, Faculty of Science, Ain Shams University, Cairo, Egypt
2 Faculty of Basic Science, King Salman International University, South Sinai, Egypt
* Corresponding Author: REHAM HELWA. Email:
Oncology Research 2022, 30(3), 129-135. https://doi.org/10.32604/or.2022.026838
Received 28 September 2022; Accepted 13 December 2022; Issue published 12 January 2023
Abstract
Galectins are sticky molecules that bind to β-galactoside. Their interactions render them essential players in many cellular processes. The imbalance of galectin expression was reported in many diseases. In cancer, galectins interact with the extracellular matrix, evade the immune system, and potentially have broad interactions with blood components. In the last ten years, since 2010, we did focus on galectin research in different cancer types. Our findings showed an interaction between cancer cells and erythrocytes via galectin-4. Moreover, we found that upregulation of galectins was associated with lymph node metastasis in ovarian cancers. Hence, with this, we shortly review some important aspects of galectins and their potential importance in more profound understanding of cancer progression and the field of cancer biomarkers.Keywords
Galectins are Potentially Supporting Cancer Cells to Invade and Metastasize via Interaction with Blood Components
In 2017, an interaction between cancer cells and erythrocytes was reported and interpreted by galectin-4 interaction with the blood group antigen (Fig. 1). Displacement of galectin-4 to attachment points of cancer cells and erythrocytes was noticed. Also, we found in this article, a co-localization of galectin-4 and blood group antigen was seen using double fluorescent immunostaining. Moreover, a morphological deformation of red blood cells was seen to be associated with this interaction [1]. In this model, interacting cells were dividing without the presence of an attachment surface. In addition, developing lamellipodia/filopodia was noticed after interactions [1].
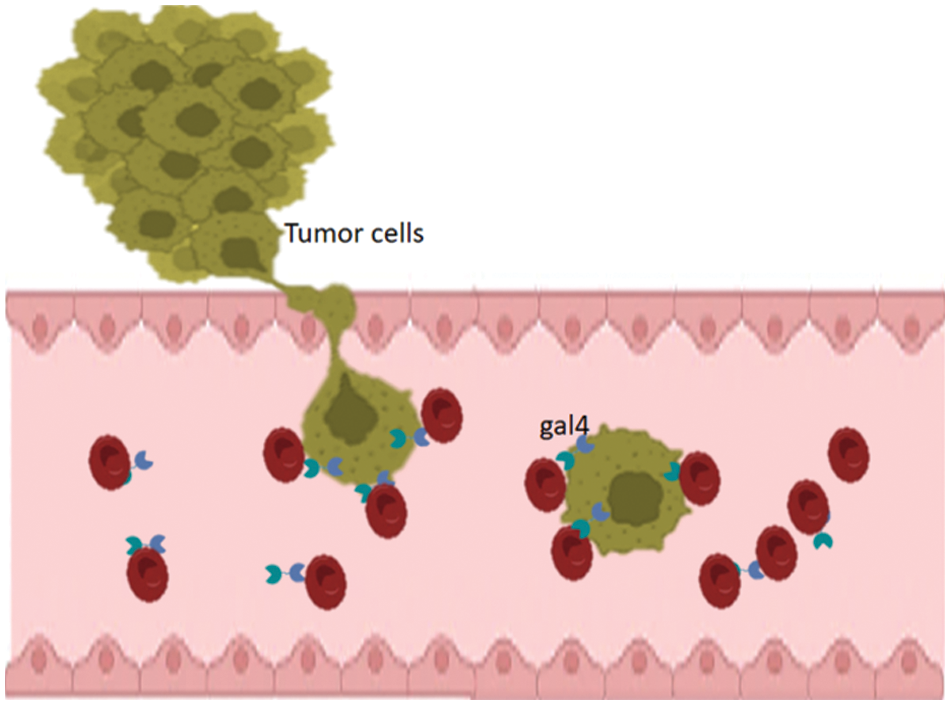
Figure 1: Schematic representation of the results of tumor-blood interaction. Tumor cells that exhibit upregulation of galectin-4 interact with red blood cells (RBCs). Galectins accumulate at the sites of attachment to the erythrocytes.
According to the structure of galectins, all surface/secreted galectins might interact with erythrocytes. Thus, many questions have been raised regarding the dysregulation of galectins in cancer. For instance, is the upregulation of galectins related to invasive cancers or lymph node metastasis? Thus, our group sought mRNA expression in many types of cancers, including AML [2,3], ovarian [4], endometrial, and breast (unpublished). Consistent with our hypothesis, in ovarian cancer, we found that galectin-9 might be a potential marker for lymph node metastasis [4].
Supportive Biological Evidence and Functions Related to Galectins and Cancer
Galectins are protein family that have a high affinity for binding to β-galactoside like N-acetyllactosamine via N-linked or O-linked glycosylation. Galectins are a structurally associated family containing at least one carbohydrate recognition domain (CRD) [5,6]. The CRD of this family is folded into a β-sandwich structure consisting of two stretched antiparallel β-sheets. Galectin’s ligand binds to the groove formed by β-sandwich [7]. Up to now, there are sixteen members of the galectins family. Depending on their structure, galectins are categorized into three different types: prototype, tandem repeat or chimera. Prototypical galectins (LGALS1, 2, 5, 7, 10, 11, 13, 14 and 15) contain one CRD that can dimerize. Tandem galectins (LGALS4, 6, 8, 9, and 12) are at least two CRD linked together by a small peptide domain. Galecin-3 is the only member that contains one CRD linked to the N-terminal non-lectin domain [8,9]. These structural aspects render them a key players in several cellular processes, as shown in Fig. 2.
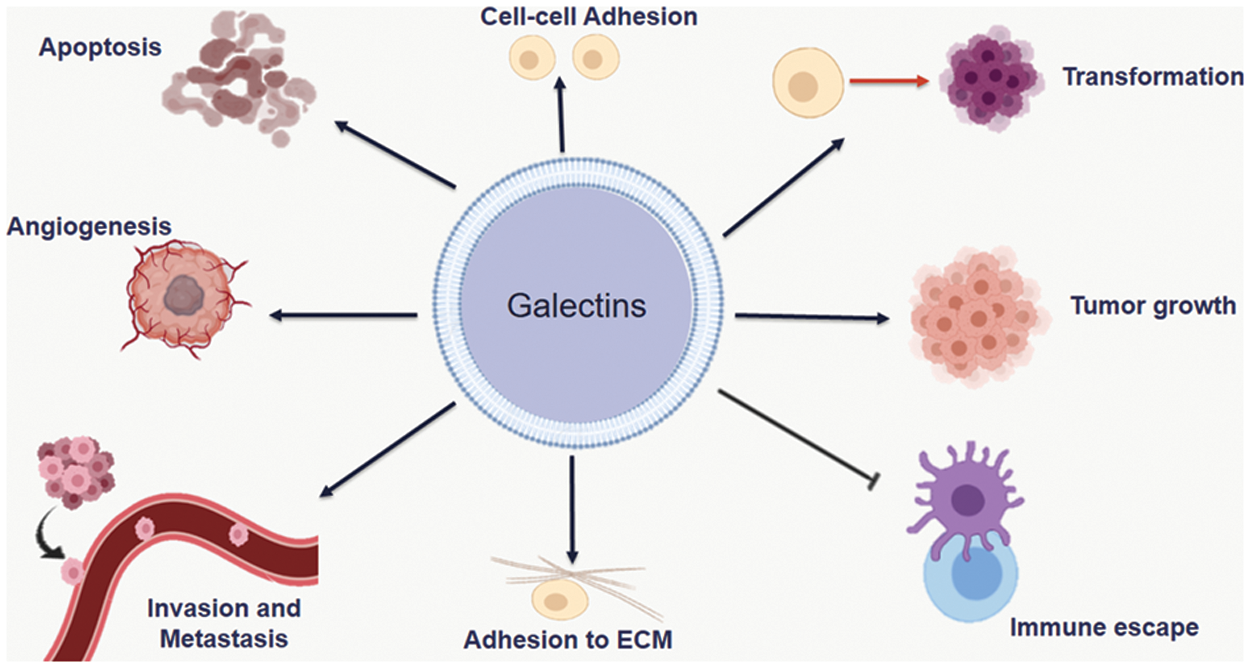
Figure 2: Different roles of Galectins in cancer. Galectins are involved in many events for cancer progression including; transformation, tumor growth, adhesion to extracellular matrux, immune escape, angiogenesis, invasion and metastasis.
Galectins display a wide range of distribution (Table 1). They can be founded in the cytosol; and contribute to protein-protein interactions to mediate intracellular function or in extracellular places in which they depending on carbohydrate binding activities. Galectins family does not have a classical signal for transporting to ER–Golgi pathway. However, some members can be found in the extracellular matrix through non-classical secretion or an understood process [10,11]. Thus, galectins contribute to several functions such as the regulation of signaling pathways, such as cell-cell and cell-matrix interaction, pre-mRNA splicing, apoptosis, cell cycle, metastasis and immune response [12,13].
Galectins Dysregulation in Cancer
High expression of galectin-1 has been reported in several human tumors, such as lung, head and neck [28], colon [29,30], prostate [31], gastric [32], AML [2,3], and ovarian cancers [4]. Moreover, galectin-1 might be involved in tumor angiogenesis because endothelial cells and vascular smooth muscle express it [33]. Galectin-1 binds to neuropilin-1 and promotes VEGFR-2 phosphorylation, which increases cancer cells progression and migration [34–37]. In Colorectal Cancer cells, higher expression of galectin-1 is significantly correlated with metastasis recurrence and can activate the Wnt/β-catenin pathway [38]. It was reported that deletion of galectin-1 in pancreatic ductal adenocarcinoma leads to reduced metastasis levels and a significant increase in the survival, as a result of several mechanisms including improved T cell infiltration, decreased stroma activation, and decreased vascularization [39].
Our group did report overexpression of galectin-2 in monocytic AML. According to FAB classification, in peripheral blood, higher expression of galectin-2 was associated with M4 and M5. mRNA upregulation of galectin-2 was found to be associated with positive CD64, CD11c, and MHC class II [2]. In unpublished results, we could not detect galectin-2 mRNA in ovarian cancer tissues.
Galectin-2 attaches to human monocytes through CD14 and induces the expression of pro-inflammatory IFN-β, TNF-α, IL-6, IL12-p40, and decreased expression of pro-arteriogenic factors VEGF-A, MMP9, MMP2, PDGFB, and HGF [40].
Functions and expression of galectin-3 were repeatdly studied in several types of cancers.
Extracellular galectin-3 has been reported to stimulate the formation of new capillaries and enhance endothelial cell motility [41]. It interacts with N-glycans on αvβ3 integrin and trigger integrin signaling pathways that stimulate VEGF and bFGF angiogenic activity [42]. Overexpression of galectin-3 is associated with enhancing metastasis and poor prognosis in several types of cancers, including colon, thyroid, gastric, liver, pancreatic ductal adenocarcinoma, bladder, and breast cancer [43–46]. It has been revealed that galectin-3 binds to transmembrane mucin (MUC1) and triggers the clustering of MUC1 and exposures to smaller cell surface adhesion molecules, including E-cadherin [47]. Alternatively, the downregulation of LGALS-3 has been detected to be associated with the progression of cancer cells [48,49].
Galectin-4 has been reported in many cancers, and its expression is related to growth and progression. Most research on tumor-associated LGALS-4 levels reported changes at the mRNA level. Galectin-4 was elevated in hepatocellular carcinomas [50] and gastric cancer cells with increased metastatic potential [51]. Similarly, it was found in lung adenocarcinomas and was associated with cancer progression and metastasis [52]. Also, it was found to be elevated in breast cancer [53].
Galectin-7 is a prototype member of the galectins family containing a single CRD that forms a homodimer. Galectin-7 is produced in the cytoplasm, accumulates in the nucleus or cytosol, then secreted to the outer plasma membrane or extracellular matrix [54,55]. Galectin-7 has a reverse effect on tumor growth and progression. It may have an apoptotic effect on certain types of tumors. However, it can promote the development of others. It was reported that a high amount of galectin-7 was found after UVB irradiation of the skin which induces apoptosis and has a significant effect on keratinocyte [56]. Moreover, the ectopic expression of galectin-7 has an apoptotic effect in the human colon carcinoma cell line DLD-1 [57]. In gastric cancer, there was significant statistical correlation between low levels of galectin-7 in tumor tissues compared with corresponding normal tissues [58]. Galectin-7 contributes to apoptosis through interaction with the anti-apoptotic factor Bcl-2 in a carbohydrate-independent manner [59].
Conversely, galectin-7 can enhance invasion activity in human oral squamous cell carcinoma cells, via several mechanisms, including activation of ERK and JNK signaling and the induction of MMP-2 and MMP-9 [60]. Similarly, overexpression of galectin-7 in breast cancer is sufficient to promote metastasis to the bone and lung-related with metastasis of lymph node axillary. Moreover, it can increase the resistance to apoptosis in melanoma cells [61,62].
Galectin-8 is a potential tumor marker of papillary thyroid cancer, since it was detected in the majority of thyroid cancers, is undetectable in normal thyroid [63]. Also, increased concentration of galectin-8 has been reported in lung cancer and correlated to cancer progression and metastasis [64]. Galectin-8 has been reported to bind to CD166 and stimulate angiogenesis [65].
The expression of galectin-8 is significantly associated with relapse-free survival in patients with squamous cancer cells in cervical cancer [66].
Similarly, galectin-8 was found to stimulate the progression and metastasis in prostate cancer. In addition, it has been shown that galectin-8 can bind directly to K-Ras and moderate cell proliferation and migration in lung and pancreatic cancer cells [67].
Galectin-9 was previously described as a protein that is differentially expressed in immune cells and known to be involved in several biological functions, such as regulation of cell adhesion, migration, cell polarity, chemotaxis, proliferation, apoptosis, and differentiation [68,69]. T-cell immunoglobulin and mucin domain-containing molecule 3 (TIM-3) is a transmembrane protein that acts as a receptor for galectin-9 [70]. TIM-3 is expressed over the cell surface of leukemic stem cells (LSCs) in all types of acute myeloid except the subtype M-3, and is not expressed in normal hematopoietic stem cells (HSCs) [71].
The binding of galectin-9/TIM-3 is critical for transformed normal HSCs to LSCs and self-renewal of LSCs, because they induce the phosphorylation of both NF-κB and β-catenin signaling through stimulating phosphorylation of ERK and AKT. Neutralized galectin-9 significantly reduces the self-renewing of LSCs and increases the apoptosis rate of LSCs [72]. In AML cells, TIM-3 and its ligand galectin-9 stimulate the activation of the PI3K/mTOR pathway. Also, it induces the expression of HIF-1α and VEGF [73].
Galectin-9-TIM-3 interaction regulates the immunosuppression by promoting apoptosis of T-cells and inhibits the activity of natural killer NK cells [74]. It also reported that Galectin-9/TIM-3 induced apoptosis of Th1 and improved differentiation of monocyte towards M2 macrophage [75]. Galectin-9 acts in two phasic patterns on the activity of T-cells depending on its concentration and not depending on TIM-3 [76].
Galectin-10 is also known as the Charcot-Leyden crystal (CLCP). This glycan binding protein typically forms bi-pyramidal hexagonal crystals, identified as the Charcot-Leyden crystals [77]. Galectin-10 is found in various granules of eosinophils [78,79]. Galectin-10 is highly expressed in the bone marrow, which play significant role in lymph cell maturation [80]. Galectin-10 was decreased in ovarian cancer cells compared with normal cells, and a higher level of expression is associated with a better OS [81].
Galectin-12 has been described as having a significant role in cell cycle activity. High expression of galectin-12 is associated with apoptosis in adipose tissue [82]. Also, upregulated galectin-12 induces apoptosis through the cell cycle arrest at the G1 phase and inhibits cell proliferation [83]. These results suggest the effect of galectin-12 in pro-apoptotic function and its role in cellular homeostasis. Galectin-12 is also expressed in macrophages. Deletion of galectin-12 reduced the activity of phagocytosis against Escherichia coli and lowered the concentration of nitric oxide. Silencing of galectin-12 induces differentiation of macrophages into the M2 subtype and decreases expression levels of the M1subtype, besides reduced expression levels of numerous M1 pro-inflammatory cytokines [84].
Galectin-13 is a prototype member of the galectins family, which was first isolated from the human placenta [85]. It is expressed in the spleen, kidney, and bladder, as well as in liver cancer, malignant melanoma, and neurogenic tumors [86]. Like other galectins, galectin-13 is expressed in the cytoplasm and can be secreted to the extracellular matrix through a non-classical transport pathway. Galectin-13 can promote apoptosis of activated T-cells and control immune tolerance between maternal and fetal tissues [87]. It was found that galectin-13 can influence neutrophils by reducing the apoptosis rate. Moreover, Galectin-13 increases the expression level of PD-L1, HGF, TNF-α, reactive ROS, and MMP-9 [88].
Galectin-14 is a prototype galectin with significant homology to galectin-13, which is also known as Placental Protein 13 (PPL 13) [89]. Higher expression of Galectin-14 is detected in epithelial ovarian cancer compared to normal tissues and is significantly associated with decreased survival of OC [90].
Galectin-14 was identified in placental tissue [91] and, together with galectins-13 and -14 was found to be elevated in distinguished trophoblast cells to confer immunotolerance at the maternal–fetal interface placenta [92].
There are still many question marks around galectins in cancer. Interactions of galectins with all blood components are still ambiguous. These interactions could be key players in cancer progression and metastasis. Several research projects have to be assigned to confirm the interactions of galectins and explore their pathophysiological role. No doubt that resolving the exact mechanism will unravel many events of cancer progression.
Thus, these molecules are of great interest to be added to the biomarkers panel of several cancers for prognosis or detection. In addition, they could be therapeutic targets to prevent invasion and metastasis.
Acknowledgement: Not applicable.
Funding Statement: The authors received no specific funding for this study.
Author Contribution: Authors are equaly contributed here in collecting data, writing, and designing the figures.
Availability of Data and Materials: Not applicable.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study or research objective of, or hypothesis tested by, the study or observation. Cite only directly pertinent references, and do not include data or conclusions from the work being reported.
References
1. Helwa, R., Heller, A., Knappskog, S., Bauer, A. S. (2017). Tumor cells interact with red blood cells via galectin-4–A short report. Cellular Oncology, 40(4), 401–409. DOI 10.1007/s13402-017-0317-9. [Google Scholar] [CrossRef]
2. Abdelfattah, M. M., Assem, M. M., El Leithy, A. A., Allam, R. M., Hassan, N. M. et al. (2021). Galectins in acute myeloid leukemia: gene expression profiling with clinical emphasis. JBUON, 26(6), 2544–2552. [Google Scholar]
3. El Leithy, A. A., Helwa, R., Assem, M. M., Hassan, N. H. (2015). Expression profiling of cancer-related galectins in acute myeloid leukemia. Tumour Biology, 36(10), 7929–7939. DOI 10.1007/s13277-015-3513-0. [Google Scholar] [CrossRef]
4. Mohamed, R. M., Emam, A., Abdelfattah, M. M., Abdel-Mageed, A. I., Abdelhafeez, M. A. et al. (2022). Assessment of galectins-1, -3, -4, -8, and -9 expression in ovarian carcinoma patients with clinical implications. World Journal of Surgical Oncology, 20(1), 276. DOI 10.1186/s12957-022-02738-4. [Google Scholar] [CrossRef]
5. Barondes, S. H., Castronovo, V., Cooper, D. N., Cummings, R. D., Drickamer, K. et al. (1994). Galectins: A family of animal beta-galactoside-binding lectins. Cell, 76(4), 597–598. DOI 10.1016/0092-8674(94)90498-7. [Google Scholar] [CrossRef]
6. Kamili, N. A., Arthur, C. M., Gerner-Smidt, C., Tafesse, E., Blenda, A. et al. (2016). Key regulators of galectin-glycan interactions. PROTEOMICS, 16(24), 3111–3125. DOI 10.1002/pmic.201600116. [Google Scholar] [CrossRef]
7. Kasai, K., Hirabayashi, J. (1996). Galectins: A family of animal lectins that decipher glycocodes. Journal of Biochemistry, 119(1), 1–8. DOI 10.1093/oxfordjournals.jbchem.a021192. [Google Scholar] [CrossRef]
8. Wdowiak, K., Francuz, T., Gallego-Colon, E., Ruiz-Agamez, N., Kubeczko, M. et al. (2018). Galectin targeted therapy in oncology: Current knowledge and perspectives. International Journal of Molecular Sciences, 19(1), 210. DOI 10.3390/ijms19010210. [Google Scholar] [CrossRef]
9. Rabinovich, G. A., Conejo-García, J. R. (2016). Shaping the immune landscape in cancer by galectin-driven regulatory pathways. Journal of Molecular Biology, 428(16), 3266–3281. DOI 10.1016/j.jmb.2016.03.021. [Google Scholar] [CrossRef]
10. Hughes, R. C. (1999). Secretion of the galectin family of mammalian carbohydrate-binding proteins. Biochimica et Biophysica Acta, 1473(1), 172–185. DOI 10.1016/S0304-4165(99)00177-4. [Google Scholar] [CrossRef]
11. Liu, F. T., Patterson, R. J., Wang, J. L. (2002). Intracellular functions of galectins. Biochimica et Biophysica Acta, 1572(2–3), 263–273. DOI 10.1016/S0304-4165(02)00313-6. [Google Scholar] [CrossRef]
12. Yang, R. Y., Rabinovich, G. A., Liu, F. T. (2008). Galectins: Structure, function and therapeutic potential. Expert Reviews in Molecular Medicine, 10, e17. DOI 10.1017/S1462399408000719. [Google Scholar] [CrossRef]
13. Chou, F. C., Chen, H. Y., Kuo, C. C., Sytwu, H. K. (2018). Role of galectins in tumors and in clinical immunotherapy. International Journal of Molecular Sciences, 19(2), 430. DOI 10.3390/ijms19020430. [Google Scholar] [CrossRef]
14. Cooper, D. N., Barondes, S. H. (1990). Evidence for export of a muscle lectin from cytosol to extracellular matrix and for a novel secretory mechanism. Journal of Cell Biology, 110(5), 1681–1691. DOI 10.1083/jcb.110.5.1681. [Google Scholar] [CrossRef]
15. Dvoránková, B., Lacina, L., Smetana, K., Lensch, M., Manning, J. et al. (2008). Human galectin-2: Nuclear presence in vitro and its modulation by quiescence/stress factors. Histology and Histopathology, 23, 167–178. DOI 10.14670/HH-23.167. [Google Scholar] [CrossRef]
16. Yu, F., Finley Jr, R. L., Raz, A., Kim, H. R. (2002). Galectin-3 translocates to the perinuclear membranes and inhibits cytochrome c release from the mitochondria. A role for synexin in galectin-3 translocation. The Journal of Biological Chemistry, 277(18), 15819–15827. DOI 10.1074/jbc.M200154200. [Google Scholar] [CrossRef]
17. Dumic, J., Dabelic, S., Flögel, M. (2006). Galectin-3: An open-ended story. Biochimica et Biophysica Acta, 1760(4), 616–635. DOI 10.1016/j.bbagen.2005.12.020. [Google Scholar] [CrossRef]
18. Belo, A. I., van der Sar, A. M., Tefsen, B., van Die, I. (2013). Galectin-4 reduces migration and metastasis formation of pancreatic cancer cells. PLoS One, 8(6), e65957. DOI 10.1371/journal.pone.0065957. [Google Scholar] [CrossRef]
19. de Jong, C. G. H. M., Stancic, M., Pinxterhuis, T. H., van Horssen, J., van Dam, A. M. et al. (2018). Galectin-4, a negative regulator of oligodendrocyte differentiation, is persistently present in axons and microglia/macrophages in multiple sclerosis lesions. Journal of Neuropathology & Experimental Neurology, 77(11), 1024–1038. DOI 10.1093/jnen/nly081. [Google Scholar] [CrossRef]
20. Chen, H. L., Chiang, P. C., Lo, C. H., Lo, Y. H., Hsu, D. K. et al. (2016). Galectin-7 regulates keratinocyte proliferation and differentiation through JNK-miR-203-p63 signaling. Journal of Investigative Dermatology, 136(1), 182–191. DOI 10.1038/JID.2015.366. [Google Scholar] [CrossRef]
21. Bibens-Laulan, N., St-Pierre, Y. (2017). Intracellular galectin-7 expression in cancer cells results from an autocrine transcriptional mechanism and endocytosis of extracellular galectin-7. PLoS One, 12(11), e0187194. DOI 10.1371/journal.pone.0187194. [Google Scholar] [CrossRef]
22. Troncoso, M. F., Ferragut, F., Bacigalupo, M. L., Cárdenas Delgado, V. M., Nugnes, L. G. et al. (2014). Galectin-8: A matricellular lectin with key roles in angiogenesis. Glycobiology, 24(10), 907–914. DOI 10.1093/glycob/cwu054. [Google Scholar] [CrossRef]
23. Obino, D., Fetler, L., Soza, A., Malbec, O., Saez, J. J. et al. (2018). Galectin-8 favors the presentation of surface-tethered antigens by stabilizing the B cell immune synapse. Cell Reports, 25(11), 3110–3122.e6. DOI 10.1016/j.celrep.2018.11.052. [Google Scholar] [CrossRef]
24. Zick, Y., Eisenstein, M., Goren, R. A., Hadari, Y. R., Levy, Y. et al. (2002). Role of galectin-8 as a modulator of cell adhesion and cell growth. Glycoconjugate Journal, 19(7–9), 517–526. DOI 10.1023/B:GLYC.0000014081.55445.af. [Google Scholar] [CrossRef]
25. Thijssen, V. L. J. L. (2016). Galectin-9. In: Choi, S. (Ed.Encyclopedia of Signaling Molecules, pp. 1–6. New York, NY: Springer New York. [Google Scholar]
26. Sato, S., Burdett, I., Hughes, R. C. (1993). Secretion of the baby hamster kidney 30-kDa galactose-binding lectin from polarized and nonpolarized cells: A pathway independent of the endoplasmic reticulum-Golgi complex. Experimental Cell Research, 207(1), 8–18. DOI 10.1006/excr.1993.1157. [Google Scholar] [CrossRef]
27. Katzenmaier, E. M., Stark, H. J., Gebert, J., Kopitz, J. (2018). Galectin-12 colocalizes with splicing factor-rich speckles and shuttles between the nucleus and cytoplasm in colon cancer cells. Journal of Molecular Biochemistry, 7, 28–40. [Google Scholar]
28. Lotan, R., Belloni, P. N., Tressler, R. J., Lotan, D., Xu, X. C. et al. (1994). Expression of galectins on microvessel endothelial cells and their involvement in tumour cell adhesion. Glycoconjugate Journal, 11(5), 462–468. DOI 10.1007/BF00731282. [Google Scholar] [CrossRef]
29. Thijssen, V. L., Hulsmans, S., Griffioen, A. W. (2008). The galectin profile of the endothelium: Altered expression and localization in activated and tumor endothelial cells. The American Journal of Pathology, 172(2), 545–553. DOI 10.2353/ajpath.2008.070938. [Google Scholar] [CrossRef]
30. Helwa, R., Ramadan, M., Abdel-Wahab, A. H., Knappskog, S., Bauer, A. S. (2016). Promoter SNPs rs116896264 and rs73933062 form a distinct haplotype and are associated with galectin-4 overexpression in colorectal cancer. Mutagenesis, 31(4), 401–408. DOI 10.1093/mutage/gev086. [Google Scholar] [CrossRef]
31. Clausse, N., van den Brûle, F., Waltregny, D., Garnier, F., Castronovo, V. (1999). Galectin-1 expression in prostate tumor-associated capillary endothelial cells is increased by prostate carcinoma cells and modulates heterotypic cell-cell adhesion. Angiogenesis, 3(4), 317–325. DOI 10.1023/A:1026584523789. [Google Scholar] [CrossRef]
32. You, X., Wang, Y., Wu, J., Liu, Q., Chen, D. et al. (2018). Galectin-1 promotes metastasis in gastric cancer through a sphingosine-1-phosphate receptor 1-dependent mechanism. Cellular Physiology and Biochemistry, 51(1), 11–30. DOI 10.1159/000495157. [Google Scholar] [CrossRef]
33. Moiseeva, E. P., Javed, Q., Spring, E. L., de Bono, D. P. (2000). Galectin 1 is involved in vascular smooth muscle cell proliferation. Cardiovascular Research, 45(2), 493–502. DOI 10.1016/S0008-6363(99)00276-X. [Google Scholar] [CrossRef]
34. Croci, D. O., Juan, P. C., Dalotto-Moreno, T., Santiago, P. M., Ivan, D. M. et al. (2014). Glycosylation-dependent lectin-receptor interactions preserve angiogenesis in anti-VEGF refractory tumors. Cell, 156(4), 744–758. DOI 10.1016/j.cell.2014.01.043. [Google Scholar] [CrossRef]
35. Thijssen, V. L., Barkan, B., Shoji, H., Aries, I. M., Mathieu, V. et al. (2010). Tumor cells secrete galectin-1 to enhance endothelial cell activity. Cancer Research, 70(15), 6216–6224. DOI 10.1158/0008-5472.CAN-09-4150. [Google Scholar] [CrossRef]
36. Hsieh, S. H., Ying, N. W., Wu, M. H., Chiang, W. F., Hsu, C. L. et al. (2008). Galectin-1, a novel ligand of neuropilin-1, activates VEGFR-2 signaling and modulates the migration of vascular endothelial cells. Oncogene, 27(26), 3746–3753. DOI 10.1038/sj.onc.1211029. [Google Scholar] [CrossRef]
37. Thijssen, V. L. J. L., Postel, R., Brandwijk, R. J. M. G. E., Dings, R. P. M., Nesmelova, I. et al. (2006). Galectin-1 is essential in tumor angiogenesis and is a target for antiangiogenesis therapy. PNAS, 103(43), 15975–15980. DOI 10.1073/pnas.0603883103. [Google Scholar] [CrossRef]
38. Peng, K. Y., Jiang, S. S., Lee, Y. W., Tsai, F. Y., Chang, C. C. et al. (2021). Stromal galectin-1 promotes colorectal cancer cancer-initiating cell features and disease dissemination through SOX9 and β-catenin: Development of niche-based biomarkers. Frontiers in Oncology, 11, 1–6. DOI 10.3389/fonc.2021.716055. [Google Scholar] [CrossRef]
39. Orozco, C. A., Martinez-Bosch, N., Guerrero, P. E., Vinaixa, J., Dalotto-Moreno, T. et al. (2018). Targeting galectin-1 inhibits pancreatic cancer progression by modulating tumor-stroma crosstalk. PNAS, 115(16), E3769–E3778. DOI 10.1073/pnas.1722434115. [Google Scholar] [CrossRef]
40. Yıldırım, C., Vogel, D. Y. S., Hollander, M. R., Baggen, J. M., Fontijn, R. D. et al. (2015). Galectin-2 induces a proinflammatory, anti-arteriogenic phenotype in monocytes and macrophages. PLoS One, 10(4), e0124347. DOI 10.1371/journal.pone.0124347. [Google Scholar] [CrossRef]
41. Nangia-Makker, P., Honjo, Y., Sarvis, R., Akahani, S., Hogan, V. et al. (2000). Galectin-3 induces endothelial cell morphogenesis and angiogenesis. The American Journal of Pathology, 156(3), 899–909. DOI 10.1016/S0002-9440(10)64959-0. [Google Scholar] [CrossRef]
42. Markowska, A. I., Liu, F. T., Panjwani, N. (2010). Galectin-3 is an important mediator of VEGF- and bFGF-mediated angiogenic response. Journal of Experimental Medicine, 207(9), 1981–1993. DOI 10.1084/jem.20090121. [Google Scholar] [CrossRef]
43. Song, S., Mazurek, N., Liu, C., Sun, Y., Ding, Q. Q. et al. (2009). Galectin-3 mediates nuclear beta-catenin accumulation and Wnt signaling in human colon cancer cells by regulation of glycogen synthase kinase-3β activity. Cancer Research, 69(4), 1343–1349. DOI 10.1158/0008-5472.CAN-08-4153. [Google Scholar] [CrossRef]
44. Shekhar, M. P., Nangia-Makker, P., Tait, L., Miller, F., Raz, A. (2004). Alterations in galectin-3 expression and distribution correlate with breast cancer progression: Functional analysis of galectin-3 in breast epithelial-endothelial interactions. The American Journal of Pathology, 165(6), 1931–1941. DOI 10.1016/S0002-9440(10)63245-2. [Google Scholar] [CrossRef]
45. Hsu, D. K., Dowling, C. A., Jeng, K. C., Chen, J. T., Yang, R. Y. et al. (1999). Galectin-3 expression is induced in cirrhotic liver and hepatocellular carcinoma. The International Journal of Cancer, 81(4), 519–526. DOI 10.1002/(ISSN)1097-0215. [Google Scholar] [CrossRef]
46. Choi, Y. S., Kim, M. J., Choi, E. A., Kim, S., Lee, E. J. et al. (2022). Antibody-mediated blockade for galectin-3 binding protein in tumor secretome abrogates PDAC metastasis. PNAS, 119(30), e2119048119. DOI 10.1073/pnas.2119048119. [Google Scholar] [CrossRef]
47. Zhao, Q., Barclay, M., Hilkens, J., Guo, X., Barrow, H. et al. (2010). Interaction between circulating galectin-3 and cancer-associated MUC1 enhances tumour cell homotypic aggregation and prevents anoikis. Molecular Cancer, 9(1), 154. DOI 10.1186/1476-4598-9-154. [Google Scholar] [CrossRef]
48. Pacis, R. A., Pilat, M. J., Pienta, K. J., Wojno, K., Raz, A. et al. (2000). Decreased galectin-3 expression in prostate cancer. Prostate, 44(2), 118–123. DOI 10.1002/(ISSN)1097-0045. [Google Scholar] [CrossRef]
49. Ahmed, H., Cappello, F., Rodolico, V., Vasta, G. R. (2009). Evidence of heavy methylation in the galectin 3 promoter in early stages of prostate adenocarcinoma: Development and validation of a methylated marker for early diagnosis of prostate cancer. Translational Oncology, 2(3), 146–156. DOI 10.1593/tlo.09118. [Google Scholar] [CrossRef]
50. Kondoh, N., Wakatsuki, T., Ryo, A., Hada, A., Aihara, T. et al. (1999). Identification and characterization of genes associated with human hepatocellular carcinogenesis. Cancer Research, 59(19), 4990–4996. [Google Scholar]
51. Hippo, Y., Yashiro, M., Ishii, M., Taniguchi, H., Tsutsumi, S. et al. (2001). Differential gene expression profiles of scirrhous gastric cancer cells with high metastatic potential to peritoneum or lymph nodes. Cancer Research, 61(3), 889–895. [Google Scholar]
52. Hayashi, T., Saito, T., Fujimura, T., Hara, K., Takamochi, K. et al. (2013). Galectin-4, a novel predictor for lymph node metastasis in lung adenocarcinoma. PLoS One, 8(12), e81883. DOI 10.1371/journal.pone.0081883. [Google Scholar] [CrossRef]
53. Barrow, H., Guo, X., Wandall, H. H., Pedersen, J. W., Fu, B. et al. (2011). Serum galectin-2, -4, and -8 are greatly increased in colon and breast cancer patients and promote cancer cell adhesion to blood vascular endothelium. Clinical Cancer Research, 17(22), 7035–7046. DOI 10.1158/1078-0432.CCR-11-1462. [Google Scholar] [CrossRef]
54. Varki, A., Cummings, R. D., Esko, J. D., Stanley, P., Hart, G. W. et al. (2015). Essentials of Glycobiology [internet]. [Google Scholar]
55. Bertuzzi, S., Quintana, J. I., Ardá, A., Gimeno, A., Jiménez-Barbero, J. (2020). Targeting galectins with glycomimetics. Frontiers in Chemistry, 8, 593. DOI 10.3389/fchem.2020.00593. [Google Scholar] [CrossRef]
56. Bernerd, F., Sarasin, A., Magnaldo, T. (1999). Galectin-7 overexpression is associated with the apoptotic process in UVB-induced sunburn keratinocytes. PNAS, 96(20), 11329–11334. DOI 10.1073/pnas.96.20.11329. [Google Scholar] [CrossRef]
57. Ueda, S., Kuwabara, I., Liu, F. T. (2004). Suppression of tumor growth by galectin-7 gene transfer. Cancer Research, 64(16), 5672–5676. DOI 10.1158/0008-5472.CAN-04-0985. [Google Scholar] [CrossRef]
58. Kim, S. J., Hwang, J. A., Ro, J. Y., Lee, Y. S., Chun, K. H. (2013). Galectin-7 is epigenetically-regulated tumor suppressor in gastric cancer. Oncotarget, 4(9), 1461–1471. DOI 10.18632/oncotarget.1219. [Google Scholar] [CrossRef]
59. Villeneuve, C., Baricault, L., Canelle, L., Barboule, N., Racca, C. et al. (2011). Mitochondrial proteomic approach reveals galectin-7 as a novel BCL-2 binding protein in human cells. Molecular Biology of the Cell, 22(7), 999–1013. DOI 10.1091/mbc.e10-06-0534. [Google Scholar] [CrossRef]
60. Guo, J. P., Li, X. G. (2017). Galectin‐7 promotes the invasiveness of human oral squamous cell carcinoma cells via activation of ERK and JNK signaling. Oncology Letters, 13(3), 1919–1924. DOI 10.3892/ol.2017.5649. [Google Scholar] [CrossRef]
61. Demers, M., Rose, A. A. N., Grosset, A. A., Biron-Pain, K., Gaboury, L. et al. (2010). Overexpression of galectin-7, a myoepithelial cell marker, enhances spontaneous metastasis of breast cancer cells. The American Journal of Pathology, 176(6), 3023–3031. DOI 10.2353/ajpath.2010.090876. [Google Scholar] [CrossRef]
62. Biron-Pain, K., Grosset, A. A., Poirier, F., Gaboury, L., St-Pierre, Y. (2013). Expression and functions of galectin-7 in human and murine melanomas. PLoS One, 8(5), e63307. DOI 10.1371/journal.pone.0063307. [Google Scholar] [CrossRef]
63. Savin, S., Cvejić, D., Janković, M., Išić, T., Paunović, I. et al. (2009). Evaluation of galectin-8 expression in thyroid tumors. Medical Oncology, 26(3), 314–318. DOI 10.1007/s12032-008-9122-7. [Google Scholar] [CrossRef]
64. Reticker-Flynn, N. E., Malta, D. F., Winslow, M. M., Lamar, J. M., Xu, M. J. et al. (2012). A combinatorial extracellular matrix platform identifies cell-extracellular matrix interactions that correlate with metastasis. Nature Communications, 3(1), 1122. DOI 10.1038/ncomms2128. [Google Scholar] [CrossRef]
65. Troncoso, M. F., Ferragut, F., Bacigalupo, M. L., Cardenas Delgado, V. M., Nugnes, L. G. et al. (2014). Galectin-8: A matricellular lectin with key roles in angiogenesis. Glycobiology, 24(10), 907–914. DOI 10.1093/glycob/cwu054. [Google Scholar] [CrossRef]
66. Beyer, S., Wehrmann, M., Meister, S., Kolben, T. M., Trillsch, F. et al. (2022). Galectin-8 and -9 as prognostic factors for cervical cancer. Archives of Gynecology and Obstetrics, 306(4), 1211–1220. DOI 10.1007/s00404-022-06449-9. [Google Scholar] [CrossRef]
67. Meinohl, C., Barnard, S. J., Fritz-Wolf, K., Unger, M., Porr, A. et al. (2019). Galectin-8 binds to the farnesylated C-terminus of K-Ras4B and Modifies Ras/ERK signaling and migration in pancreatic and lung carcinoma cells. Cancers, 12(1), 30. DOI 10.3390/cancers12010030. [Google Scholar] [CrossRef]
68. Heusschen, R., Griffioen, A. W., Thijssen, V. L. (2013). Galectin-9 in tumor biology: A jack of multiple trades. Biochimica et Biophysica Acta, 1836(1), 177–185. DOI 10.1016/j.bbcan.2013.04.006. [Google Scholar] [CrossRef]
69. Pena, C., Mirandola, L., Figueroa, J. A., Hosiriluck, N., Suvorava, N. et al. (2014). Galectins as therapeutic targets for hematological malignancies: A hopeful sweetness. Annals of Translational Medicine, 2(9), 87. DOI 10.3978/j.issn.2305-5839.2014.09.14. [Google Scholar] [CrossRef]
70. Gonçalves Silva, I., Yasinska, I. M., Sakhnevych, S. S., Fiedler, W., Wellbrock, J. et al. (2017). The tim-3-galectin-9 secretory pathway is involved in the immune escape of human acute myeloid leukemia cells. eBioMedicine, 22, 44–57. DOI 10.1016/j.ebiom.2017.07.018. [Google Scholar] [CrossRef]
71. Kikushige, Y., Shima, T., Takayanagi, S., Urata, S., Miyamoto, T. et al. (2010). TIM-3 is a promising target to selectively kill acute myeloid leukemia stem cells. Cell Stem Cell, 7(6), 708–717. DOI 10.1016/j.stem.2010.11.014. [Google Scholar] [CrossRef]
72. Kikushige, Y., Miyamoto, T., Yuda, J., Jabbarzadeh-Tabrizi, S., Shima, T. et al. (2015). A TIM-3/Gal-9 autocrine stimulatory loop drives self-renewal of human myeloid leukemia stem cells and leukemic progression. Cell Stem Cell, 17(3), 341–352. DOI 10.1016/j.stem.2015.07.011. [Google Scholar] [CrossRef]
73. Prokhorov, A., Gibbs, B. F., Bardelli, M., Rüegg, L., Fasler-Kan, E. et al. (2015). The immune receptor Tim-3 mediates activation of PI3 kinase/mTOR and HIF-1 pathways in human myeloid leukaemia cells. International Journal of Biochemistry and Cell Biology, 59, 11–20. DOI 10.1016/j.biocel.2014.11.017. [Google Scholar] [CrossRef]
74. Zhu, C., Anderson, A. C., Schubart, A., Xiong, H., Imitola, J. et al. (2005). The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nature Immunology, 6(12), 1245–1252. DOI 10.1038/ni1271. [Google Scholar] [CrossRef]
75. Enninga, E. A. L., Nevala, W. K., Holtan, S. G., Leontovich, A. A., Markovic, S. N. (2016). Galectin-9 modulates immunity by promoting Th2/M2 differentiation and impacts survival in patients with metastatic melanoma. Melanoma Research, 26(5), 429–441. DOI 10.1097/CMR.0000000000000281. [Google Scholar] [CrossRef]
76. Su, E. W., Bi, S., Kane, L. P. (2011). Galectin-9 regulates T helper cell function independently of Tim-3. Glycobiology, 21(10), 1258–1265. DOI 10.1093/glycob/cwq214. [Google Scholar] [CrossRef]
77. Ackerman, S. J., Liu, L., Kwatia, M. A., Savage, M. P., Leonidas, D. D. et al. (2002). Charcot-leyden crystal protein (Galectin-10) is not a dual function galectin with lysophospholipase activity but binds a lysophospholipase inhibitor in a novel structural fashion*. Journal of Biological Chemistry, 277(17), 14859–14868. DOI 10.1074/jbc.M200221200. [Google Scholar] [CrossRef]
78. de Re, V., Simula, M. P., Cannizzaro, R., Pavan, A., de Zorzi, M. A. et al. (2009). Galectin-10, eosinophils, and celiac disease. Annals of the New York Academy of Sciences, 1173(1), 357–364. DOI 10.1111/j.1749-6632.2009.04627.x. [Google Scholar] [CrossRef]
79. Dor, P. J., Ackerman, S. J., Gleich, G. J. (1984). Charcot-leyden crystal protein and eosinophil granule major basic protein in sputum of patients with respiratory diseases. American Review of Respiratory Disease, 130(6), 1072–1077. DOI 10.1164/arrd.1984.130.6.1072. [Google Scholar] [CrossRef]
80. Than, N. G., Romero, R., Xu, Y., Erez, O., Xu, Z. et al. (2014). Evolutionary origins of the placental expression of chromosome 19 cluster galectins and their complex dysregulation in preeclampsia. Placenta, 35(11), 855–865. DOI 10.1016/j.placenta.2014.07.015. [Google Scholar] [CrossRef]
81. Jiang, W., Chetry, M., Pan, S., Wang, L., Zhu, X. (2021). Overexpression of galectin10 predicts a better prognosis in human ovarian cancer. Journal of Cancer, 12(9), 2654–2664. DOI 10.7150/jca.54595. [Google Scholar] [CrossRef]
82. Hotta, K., Funahashi, T., Matsukawa, Y., Takahashi, M., Nishizawa, H. et al. (2001). Galectin-12, an adipose-expressed galectin-like molecule possessing apoptosis-inducing activity. Journal of Biological Chemistry, 276(36), 34089–34097. DOI 10.1074/jbc.M105097200. [Google Scholar] [CrossRef]
83. Yang, R. Y., Hsu, D. K., Yu, L., Ni, J., Liu, F. T. (2001). Cell cycle regulation by galectin-12, a new member of the galectin superfamily. Journal of Biological Chemistry, 276(23), 20252–20260. DOI 10.1074/jbc.M010914200. [Google Scholar] [CrossRef]
84. Wan, L., Lin, H. J., Huang, C. C., Chen, Y. C., Hsu, Y. A. et al. (2016). Galectin-12 enhances inflammation by promoting M1 polarization of macrophages and reduces insulin sensitivity in adipocytes. Glycobiology, 26(7), 732–744. DOI 10.1093/glycob/cww013. [Google Scholar] [CrossRef]
85. Bohn, H., Kraus, W., Winckler, W. (1983). Purification and characterization of two new soluble placental tissue proteins (PP13 and PP17). Oncodevelopmental Biology and Medicine, 4(5), 343–350. [Google Scholar]
86. Than, N. G., Sumegi, B., Than, G. N., Berente, Z., Bohn, H. (1999). Isolation and sequence analysis of a cDNA encoding human placental tissue protein 13 (PP13a new lysophospholipase, homologue of human eosinophil charcot-leyden crystal protein. Placenta, 20(8), 703–710. DOI 10.1053/plac.1999.0436. [Google Scholar] [CrossRef]
87. Than, N. G., Romero, R., Goodman, M., Weckle, A., Xing, J. et al. (2009). A primate subfamily of galectins expressed at the maternal-fetal interface that promote immune cell death. PNAS, 106(24), 9731–9736. DOI 10.1073/pnas.0903568106. [Google Scholar] [CrossRef]
88. Vokalova, L., Balogh, A., Toth, E., Van Breda, S. V., Schäfer, G. et al. (2020). Placental protein 13 (Galectin-13) polarizes neutrophils toward an immune regulatory phenotype. Frontiers in Immunology, 11, 297. DOI 10.3389/fimmu.2020.00145. [Google Scholar] [CrossRef]
89. Yang, Q. S., Ying, K., Yuan, H. L., Chen, J. Z., Meng, X. F. et al. (2002). Cloning and expression of a novel human galectin cDNA, predominantly expressed in placenta11 The nucleotide sequence reported in this paper has been deposited in the GenBank dababase under accession number AF267852. Biochimica et Biophysica Acta (BBA)—Gene Structure and Expression, 1574(3), 407–411. DOI 10.1016/S0167-4781(01)00319-0. [Google Scholar] [CrossRef]
90. Oliveira Fernandes de Araujo, L., St-Pierre, Y. (2019). Galectin-14 expression in ovarian cancer. bioRxiv, 717793. DOI 10.1101/717793. [Google Scholar] [CrossRef]
91. Than, N. G., Romero, R., Goodman, M., Weckle, A., Xing, J. et al. (2009). A primate subfamily of galectins expressed at the maternal-fetal interface that promote immune cell death. PNAS, 106(24), 9731–9736. DOI 10.1073/pnas.0903568106. [Google Scholar] [CrossRef]
92. Than, N. G., Romero, R., Xu, Y., Erez, O., Xu, Z. et al. (2014). Evolutionary origins of the placental expression of chromosome 19 cluster galectins and their complex dysregulation in preeclampsia. Placenta, 35(11), 855–865. DOI 10.1016/j.placenta.2014.07.015. [Google Scholar] [CrossRef]
Cite This Article
 Copyright © 2022 The Author(s). Published by Tech Science Press.
Copyright © 2022 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF
 Downloads
Downloads
 Citation Tools
Citation Tools