 Open Access
Open Access
ARTICLE
Chitosan capped-NLCs enhanced codelivery of gefitinib and simvastatin into MDR HCC: impact of compositions on cell death, JNK3, and Telomerase
1 Department of Pharmaceutics, College of Pharmacy, King Saud University, Riyadh, 11451, Saudi Arabia
2 Department of Pharmaceutical Chemistry, College of Pharmacy, King Saud University, Riyadh, 11451, Saudi Arabia
3 Department of Pharmacology and Toxicology, College of Pharmacy, King Saud University, Riyadh, 11451, Saudi Arabia
* Corresponding Author: GAMALELDIN I. HARISA. Email:
Oncology Research 2025, 33(2), 477-492. https://doi.org/10.32604/or.2024.053337
Received 29 April 2024; Accepted 08 July 2024; Issue published 16 January 2025
Abstract
Background: Hepatocellular carcinoma (HCC) is a health problem due to multi-drug resistance (MDR). Codelivery of multiple oncotherapy in one cargo as chimeric cancer therapy (CCT) is suggested as a solution for MDR. This study aims to engineer chitosan-coated nanostructure lipid carriers (NLCs) loaded with gefitinib (GF) and simvastatin (SV) as CCT for HCC. Methods: Both GF and SV-loaded nanostructure lipids carriers (GFSVNLC) and chitosan-capped GF and SV-loaded nanostructure lipids carriers (CGFSVNLC) formulations were assembled by top-down techniques. Moreover, particle size (PS), zeta potential (ZP), and polydispersity index (PDI) were measured by Zetasizer. The biosafety of GFSVNLC preparations was investigated by using erythrocytes as a biological model. The cytotoxic, and apoptotic effects of the prepared GFSVNLCs were investigated using HepG2 cell lines as a substitute model for HCC. The effect of GF, SV, and NLC composition on JNK3, HDAC6, and telomerase was studied using molecular docking simulation (MDS). Results: The present results revealed that the obtained GFSVNLC and CGFSVNLC have nanosized and consistent, CS coating shifts anionic ZP of GFSVNLC into CGFSVNLC with cationic ZP. Moreover, both formulations are biocompatible as indicated by their gentle effect on erythrocyte hemolysis. The treatment of HepG2 cells with GFSVNLC, and CGFSVNLC induced marked cell death compared to other groups with a decrease of IC50. Equally, the percentage of the apoptotic HepG2 cells was increased upon treatment of the cells with GFSV, GFSVNLC, and CGFSVNLC compared to the control group. Additionally, GF, SV, stearic acid (SA), and oleic acid (OA) modulate the activity of JNK3, HDAC6, and telomerase. Conclusions: This study suggests CGFSVNLC achieves codelivery, selective targeting, and enhancing the synergistic effect of GF and SV for inducing HepG2 cell death. Mechanistically, CGFSVNLC inhibits key cascades implicated in MDR and HepG2 cell survival. CGFSVNLC is promising for overcoming drug resistance mechanisms and improving therapeutic outcomes against HepG2 cells.Graphic Abstract
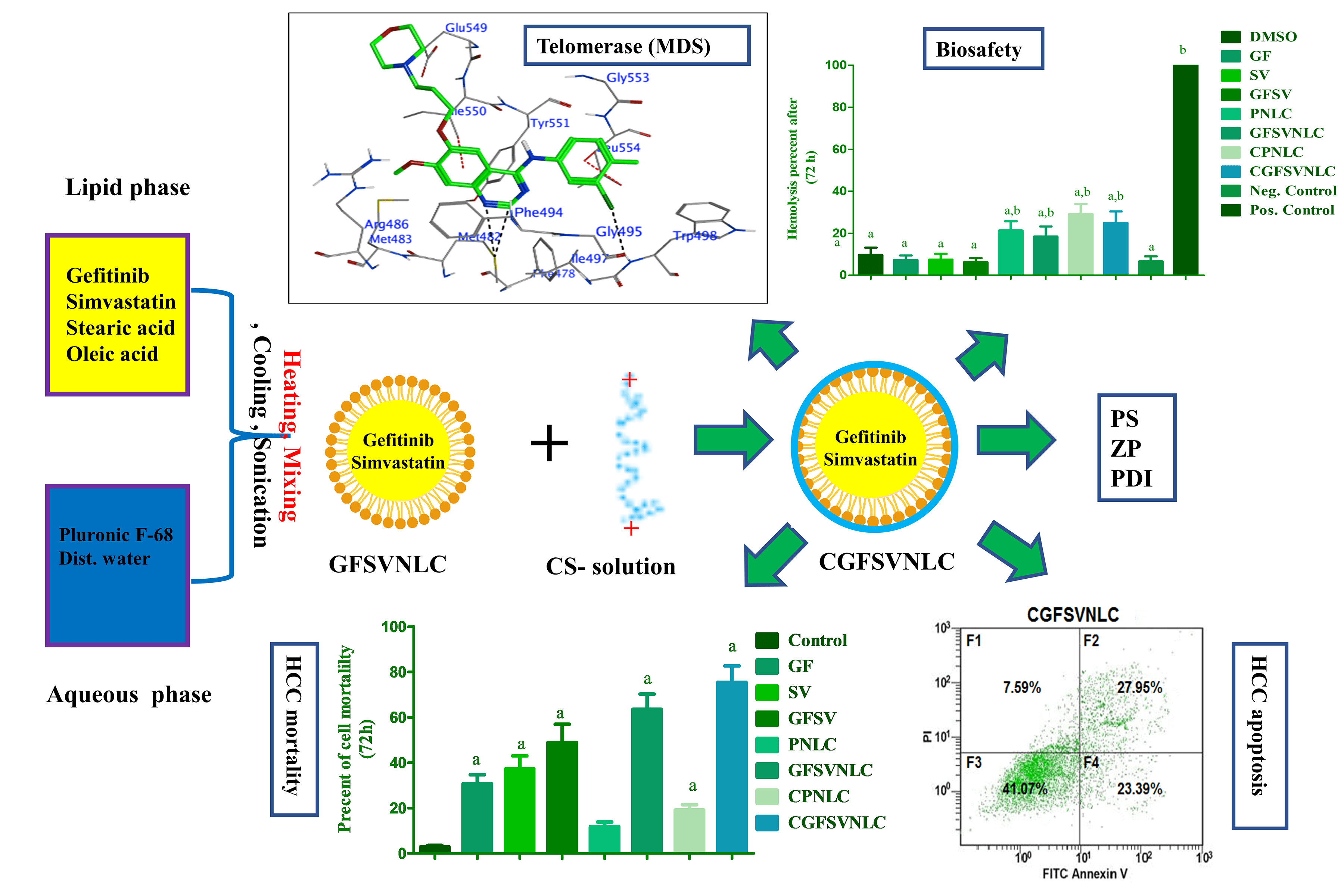
Keywords
Supplementary Material
Supplementary Material FileCite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools