 Open Access
Open Access
REVIEW
Epidemiology of Breast Cancer
Department of VIP, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
* Corresponding Author: Dongkui Xu. Email:
Oncologie 2022, 24(4), 649-663. https://doi.org/10.32604/oncologie.2022.027640
Received 07 November 2022; Accepted 01 December 2022; Issue published 31 December 2022
Abstract
All over the world, the most common malignancy in women is breast cancer. Breast cancer is also a significant factor of death in women. In 2020, approximately 2.3 million cases of breast cancer were newly diagnosed in women globally, and approximately 685,000 people died. Breast cancer incidence varies by region around the world, but it is all increasing. According to the current morbidity and mortality trend of breast cancer, it is estimated that by 2030, the number of incidence and deaths of breast cancer will reach 2.64 million and 1.7 million, respectively. The age-standardized incidence rate was 66.4/100,000 in developed countries and 27.3/100,000 in developing countries. The incidence of breast cancer in the world is increasing rapidly, and the incidence rate has increased by 57.8% in the past 30 years, and the rate of increase is 0.5% per year. The incidence and mortality of breast cancer among Chinese women rank first in the world, and in recent years, the incidence and mortality of breast cancer among Chinese women have also been on the rise. Breast cancer is a disease caused by a variety of factors, such as genetics, estrogen levels, environment, and behavior and lifestyle. The purpose of this review is to explore the global epidemiology, associated risk factors and molecular types of breast cancer to understand breast cancer prevalence, mortality, and to help with its prevention, early detection, treatment and improvement of prognosis.Keywords
In every country on earth, cancer is a major contributor to mortality and a significant impediment to raising life expectancy [1]. The most frequent cancer in women is breast cancer in the majority of countries worldwide, but in a few countries, it may be liver or cervical cancer. The most common cause of mortality from malignant tumors in women is breast cancer in 104 countries around the world, followed by cervical cancer in 44 countries, lung cancer in 29 countries, stomach cancer in several countries, liver cancer and colorectal cancer in a few countries [2]. Globally, there will be about 19.3 million new cases of cancer diagnosed in 2020, and there will be about 10 million cancer-related deaths. As the most common cancer diagnosed, female breast cancer has surpassed lung cancer with an anticipated 2.3 million new cases (11.7%). The following are the most common cancers: stomach cancer (5.6%), colorectal cancer (10.0%), lung cancer (11.4%), and prostate cancer (7.3%). With an estimated 1.8 million deaths (18%), lung cancer remains the leading cause of cancer death, followed by colorectal cancer (9.4%), liver cancer (8.3%), stomach cancer (7.7%), and female breast cancer (6.9%) [3].
The incidence of breast cancer is rising rapidly worldwide, with an increase of 57.8 percent over the past 30 years, and the rate of increase is 0.5% per year. Breast cancer incidence and mortality rates among Chinese women are the highest in the world, and have also been rising in recent years. There are many different factors that can contribute to breast cancer, including environmental factors, genetic factors, behavioral and lifestyle factors. The objective of this review is to investigate the epidemiology, associated risk factors, and molecular subtypes of breast cancer around the world, in order to comprehend its morbidity and offer assistance with prevention, early detection, and early treatment.
From 2000 to 2018, the incidence of breast cancer in women increased sharply all over the world, from 1.05 million in 2000 to 2.09 million in 2018, but the number of deaths decreased. In 2000, global female breast cancer deaths were 370,000, increased to about 520,000 in 2012, and decreased to 310,000 in 2018 [4–8].
Since 2003, breast cancer cases and deaths among women in the United States and Australia have continued to rise, while the incidence has increased slightly, the mortality rate has decreased [9–11]. Compared with China, although the morbidity and mortality of female breast cancer in the United States and Australia are much higher, the incidence of breast cancer in all three countries is on the rise. The mortality rate of female breast cancer in the United States and Australia declined slightly, while breast cancer mortality in China was growing [10–17].
In 2020, breast cancer in women has transcended lung cancer becoming the leading factor of cancer incidence worldwide. There are about 2.3 million new cases, accounting for 11.7% of all cancer cases (Fig. 1) [3]. Breast cancer is the fifth leading cause of cancer death all over the world, with approximately 685,000 deaths each year. In the female population, breast cancer accounts for 1/4 of cancer cases and for 1/6 of cancer deaths. It ranks first in the morbidity and mortality in most countries [3]. There are exceptions especially for cancer deaths, with lung cancer ranking top in Australia/New Zealand, northern Europe, North America and China, and cervical cancer in many countries in sub-Saharan Africa.
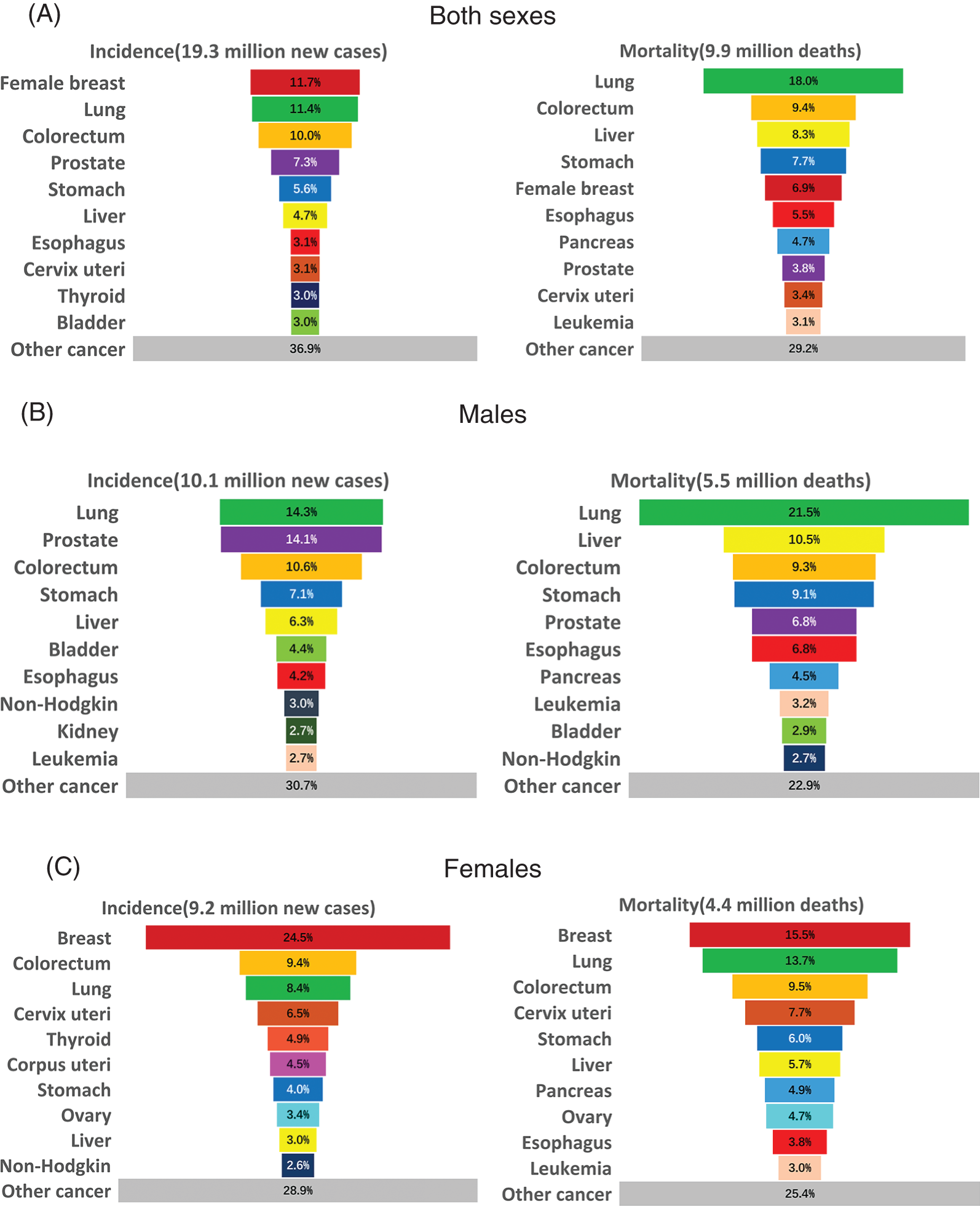
Figure 1: Distribution of cases and deaths for the top 10 most common cancers in 2020 for (A) Both sexes, (B) Men, and (C) Women. For each sex, the area of the pie chart reflects the proportion of the total number of cases or deaths. Source: GLOBOCAN 2020 [3]
The incidence of breast cancer is 88% higher in transitioned nations than in transitioning nations (55.9 and 29.7 per 100,000, respectively). However, compared to women in advanced countries, death rates for women in developing nations are 17% higher (15.0 and 12.8 per 100,000, respectively) (Fig. 2B) [3].
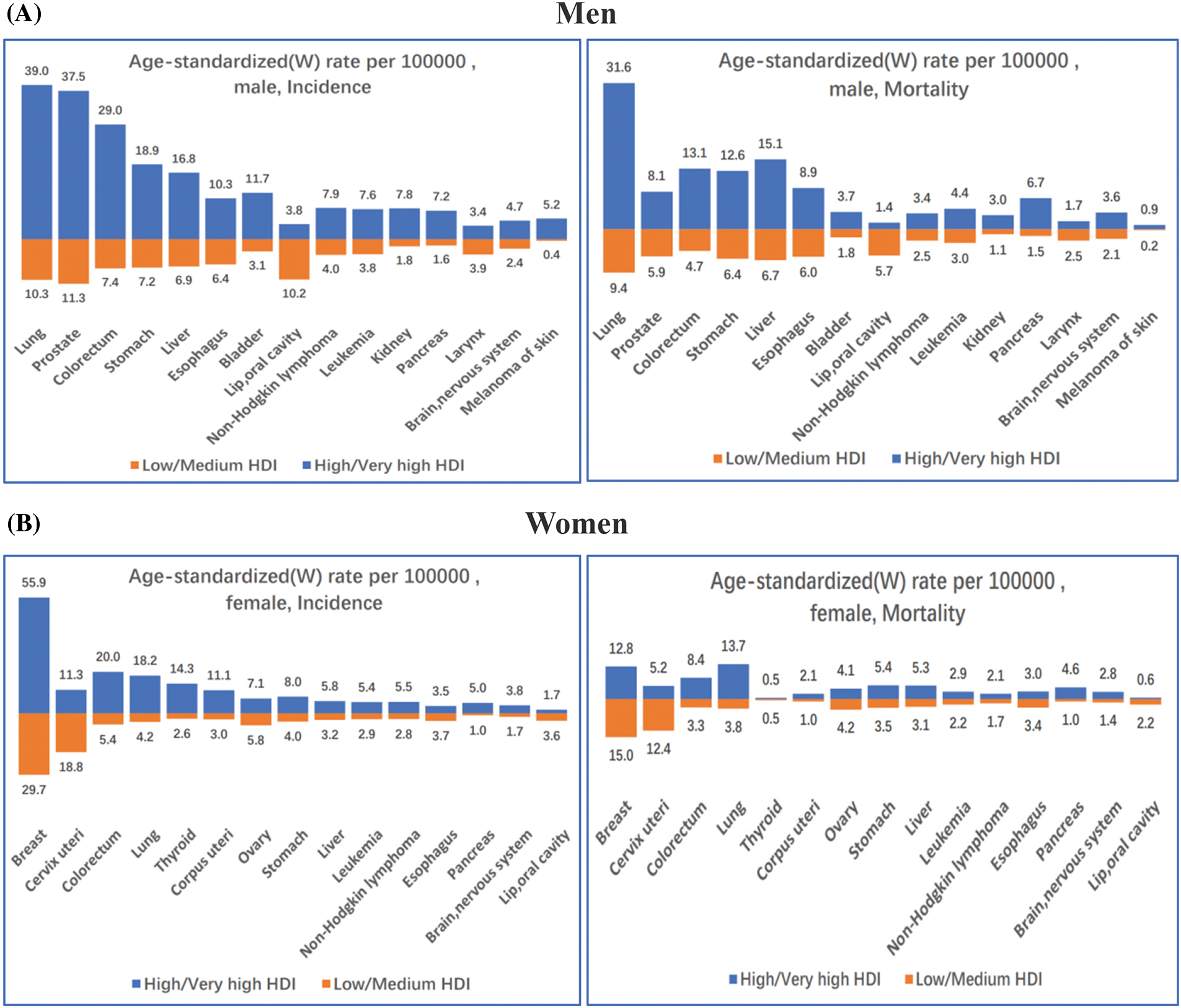
Figure 2: Incidence and mortality age-standardized rates in high/very high human development index (HDI) countries vs. low/medium HDI countries among (A) Men and (B) Women in 2020. The 15 most common cancers in the world (W) are shown in descending order of the overall age-standardized rate for both sexes combined. Source: GLOBOCAN 2020 [3]
Studies have shown that increased breast cancer rates are linked to lifestyle risk factors (drinking, being overweight, being inactive), reproductive and hormonal risk factors (early menarche, later menopause, higher age at first childbearing, fewer children, reduced breastfeeding, menopausal hormone therapy, oral contraceptives), and increased mammography [18].
Breast cancer incidence rates increased rapidly in many countries in North America and Europe during the 1980s and 1990s, possibly reflecting changes in risk factor prevalence associated with increased detection rates from widespread mammography screening. The incidence then started to reduce or stabilize in the early 2000s [19], probably due to a decrease in the use of menopausal hormone therapy and possibly a plateau in screening participation rates [20,21]. Breast cancer incidence is growing rapidly in some transitioning countries and some high-income Asian countries (such as Japan and South Korea, where breast cancer incidence rates are historically low) [22].
The prevalence of breast cancer risk factors has been impacted by the significant changes in lifestyles, sociocultural norms, and environmental factors brought on by the expansion of the world economy and the proportion of women working. Breast cancer incidence rate disparities between industrialized and developing nations are closing as a result of delaying and having fewer children, being overweight, and not exercising.
The fastest growth in breast cancer incidence occurred in sub-Saharan Africa, along with rising mortality rates, which are now ranked highest in the world (Fig. 3) [3], reflecting the association of poor health infrastructure with poor survival outcomes. The 5-year age-standardized relative survival rate for confirmed cases in 12 sub-Saharan African countries was 66% between 2008 and 2015, compared with 85% to 90% in high-income countries between 2010 and 2014 [23].
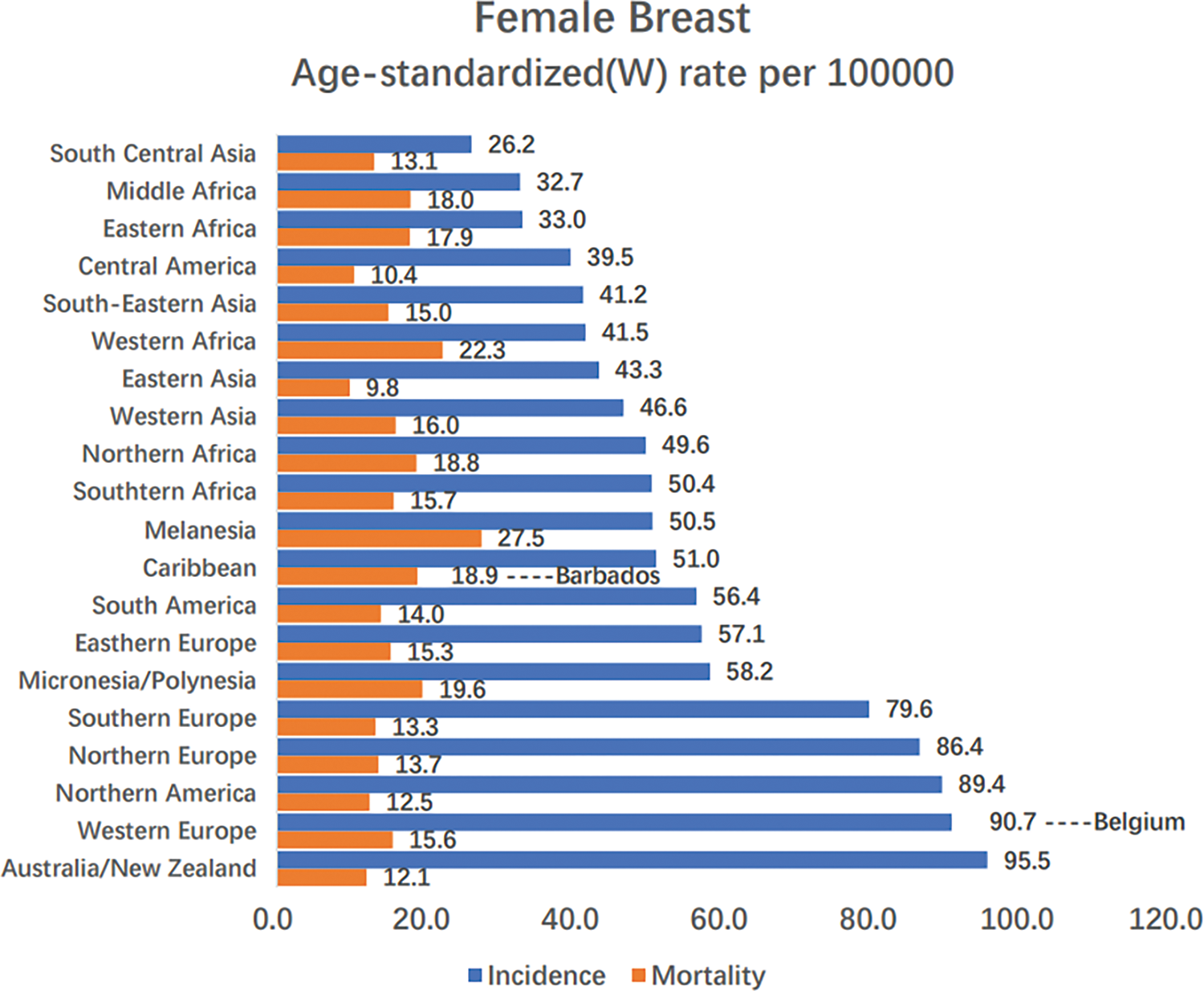
Figure 3: Region-specific incidence and mortality age-standardized rates for female breast cancer in 2020. Rates are shown in descending order of the world (W) age-standardized incidence rate, and the highest national age-standardized rates for incidence and mortality are superimposed. Source: GLOBOCAN 2020 [3]
In sub-Saharan Africa, the low survival rates are largely attributable to advanced disease. Improving breast cancer awareness and breast screening [24,25], and timely and standard treatment are critical to improving survival [26]. With early detection and appropriate treatment, a recent study predicted that 28% to 37% of breast cancer deaths might be avoided [27].
Breast cancer accounts for roughly 30% of female cancers globally, with a mortality-to-incidence ratio of 15% [9]. The global incidence ranges from 27 per 100,000 people in Africa and East Asia to 97 per 100,000 people in North America, reflecting the link between breast cancer incidence and economic development, as well as related social and lifestyle factors [4]. Mortality from breast cancer in women can be better reduced by increasing access to aggressive prevention, early detection, and early treatment [28,29]. About 10 percent of breast cancer cases are linked to family history or a genetic predisposition, which varies by country and ethnicity. The most commonly mutated genes in breast cancer are the BRCA1 and BRCA2 genes, with an average cumulative lifetime risk of 70% approximately [30,31].
3 Risk Factors for Breast Cancer
The majority of occurrences of breast cancer can be linked to hormone therapy factors, pregnancy-related factors, lifestyle factors (such as obesity, inactivity, alcohol use, low-fiber diets, and smoking), as well as other risk factors. Over one-third of breast cancer cases in high-income countries may be avoidable by changing lifestyle [32]. Whether oral hormonal contraceptives increase breast cancer risk has long been debated [33]. On the other hand, it has been more clearly shown that menopausal hormone therapy increases the risk of breast cancer in women [34].
Risk factors for breast cancer include genetic factors, environmental factors, and behavioral and lifestyle factors. The following factors are associated with an increased risk of breast cancer, such as older age, younger menarche, nulliparous or advanced maternal age, dense breasts, family history of breast cancer, history of hormone therapy, obesity, history of smoking or drinking, and consumption of high-calorie foods. However, regular physical activity, breastfeeding, regular work and rest, and a high intake of fruits, vegetables, and dietary fiber can appropriately reduce the risk of breast cancer.
Age is a known important risk factor, and the incidence of breast cancer increases with age [35]. The risk of breast cancer increases by about 50% in postmenopausal women, and the distribution of breast gland density varies among different groups, but most women have a small amount of glandular breast density, and those with extremely dense breasts have the highest risk of breast cancer. The risk of breast cancer is about twice as high as that of the low-gland type and three times that of those with mostly fat breasts, and the high-gland type is second only to the extremely dense type. 40 percent of premenopausal breast cancers and 26 percent of postmenopausal breast cancers could be avoided if all women with extremely dense breasts converted to few glandular breasts [36].
High intakes of red meat, animal fats, and refined carbs are related to an increased risk of breast cancer, while high intakes of fruits, vegetables, whole grains (such as soy milk, soy products, etc.), and dietary fiber are related with a lower risk of the disease [37,38]. The incidence of breast cancer rises as dietary fat intake overall rises [39]. A Western-style diet rich in calories and energy, such as red and/or processed meats, high-fat dairy products, potatoes, and sweets, increases the risk of breast cancer by about 14%. While consuming a lot of fruits, vegetables, fish, whole grains, and low-fat dairy products can cut the risk of breast cancer by about 18% [40]. Recent studies have shown that breast cancer incidence is on the rise as developing countries move toward high-calorie Western diets [39]. Higher natural dietary levels of folic acid, B vitamins, riboflavin, vitamin D, and calcium are associated with a lower risk of breast cancer [41].
Patients who use hormone therapy have a greater risk of breast cancer than patients who have never used it [34]. In African American women, the combination of estrogen and progestin increases the risk of ER+ breast cancer but not ER-breast cancer, whereas estrogen alone has no effect on either risk [42]. Basal insulins are frequently used to treat patients with type 1 diabetes and severe type 2 diabetes. These include the long-acting insulin analogs insulin glargine and Detemir (insulin detemir), as well as neutral protamine zinc (NPH) insulin. In a cohort analysis, using insulin glargine to treat diabetes was linked to a slightly higher incidence of breast cancer in female patients compared to using NPH insulin [43].
Lifestyle factors, such as active or passive smoking, increase the risk of breast cancer, but smoking cessation reduces the risk of death [44]. The risk of breast cancer in drinkers is approximately three times higher than in nondrinkers [38,45]. Regular physical activity is also associated with a lower risk of breast cancer. And people with higher activity levels have a significantly lower risk of breast cancer [37,44].
The risk of breast cancer increases with age at menarche. Women under the age of 12 at menarche have about twice the risk of breast cancer than people over the age of 12 when they have menarche [45]. The risk of breast cancer is increased among nulliparous women or those who have their first childbirth older than 30 years, and the age at first birth older than 30 years is most associated with breast cancer risk, more than six times that of those younger than 30 years of age [36,45]. The risk of breast cancer increases with the age of the first childbirth, and the risk is 3.53 times higher for women whose first childbirth is older than 29 years compared to women whose first childbirth is younger than 20 years [44,46]. Although childbirth reduces the risk of breast cancer, compared with nulliparous women, multiparous women have an 80% increased risk of breast cancer 5 years after delivery and an approximately 25% lower risk of breast cancer 34 years after delivery [47]. Breastfeeding has a preventive effect against breast cancer, however research has shown that it only lowers the incidence of ER-breast cancer, but not ER+ breast cancer [46,48]. It is debatable if parity affects a woman’s risk of developing breast cancer [47].
Familial breast cancer cases account for 15% to 20%, and hereditary cases account for 5% to 10%, of which germline pathogenic variants in BRCA1 and BRCA2 account for more than 30% of hereditary breast cancers. Recent studies have revealed that new genes have become breast cancer susceptibility genes, including rare germline hyper penetrant gene mutations (such as TP53 and PTEN), and the more common moderately penetrant genes mutated in penetrant genes (such as CHEK2, ATM, and PALB2) [49]. In addition, variants in CHEK2, ATM, BARD1, and RAD51D are linked to an increased risk of breast cancer by 1 to 7 times, while variants in MRE11A, RAD50, NBN, BRIP1, RAD51C, MLH1, and NF1 are not linked to an increased risk of breast cancer [50,51]. Germline pathogenic variants in BRCA1 and BRCA2 have been shown to increase lifetime risk of breast cancer.
In comparison to women without a family history, those having a history of breast cancer in first-degree relatives are at higher risk of developing the disease [46]. The 10-year cumulative absolute risk of contralateral breast cancer was 4.3% in those without a family history of breast cancer and 8.1% in those with a family history of breast cancer. If a first-degree relative is diagnosed with breast or bilateral breast cancer before age 40, the risk increases by about three to nine times compared to women without a family history [52]. In addition, the individual risk of breast cancer is proportional to the number of affected relatives and the age at onset of the disease. Among women 65–74 years old, women with fatty breasts had the highest risk associated with first-degree family history, with an HR of 1.67, while women ≥75 years old had the highest risk associated with family history with dense breasts, the HR is 1.55 [53].
Breast conserving surgery (BCS) and adjuvant radiation therapy were performed on 50% of women with early-stage (I or II) breast cancer, while approximately 34% underwent mastectomy, usually without chemotherapy or radiation (Fig. 4) [54]. In contrast, 65% of women with Stage III breast cancer underwent mastectomy, with the majority receiving chemotherapy as well. Black women were less likely than white women to undergo BCS (with or without adjuvant radiation) for Stage I and Stage II breast cancer (60% and 64%, respectively). Black women were more likely to get chemotherapy and/or radiation alone (9% vs. 6%) and less likely to undergo mastectomy (57% vs. 66%) for Stage III cancer. 60% of women who were diagnosed with metastatic breast cancer (Stage IV) received only radiation or chemotherapy.
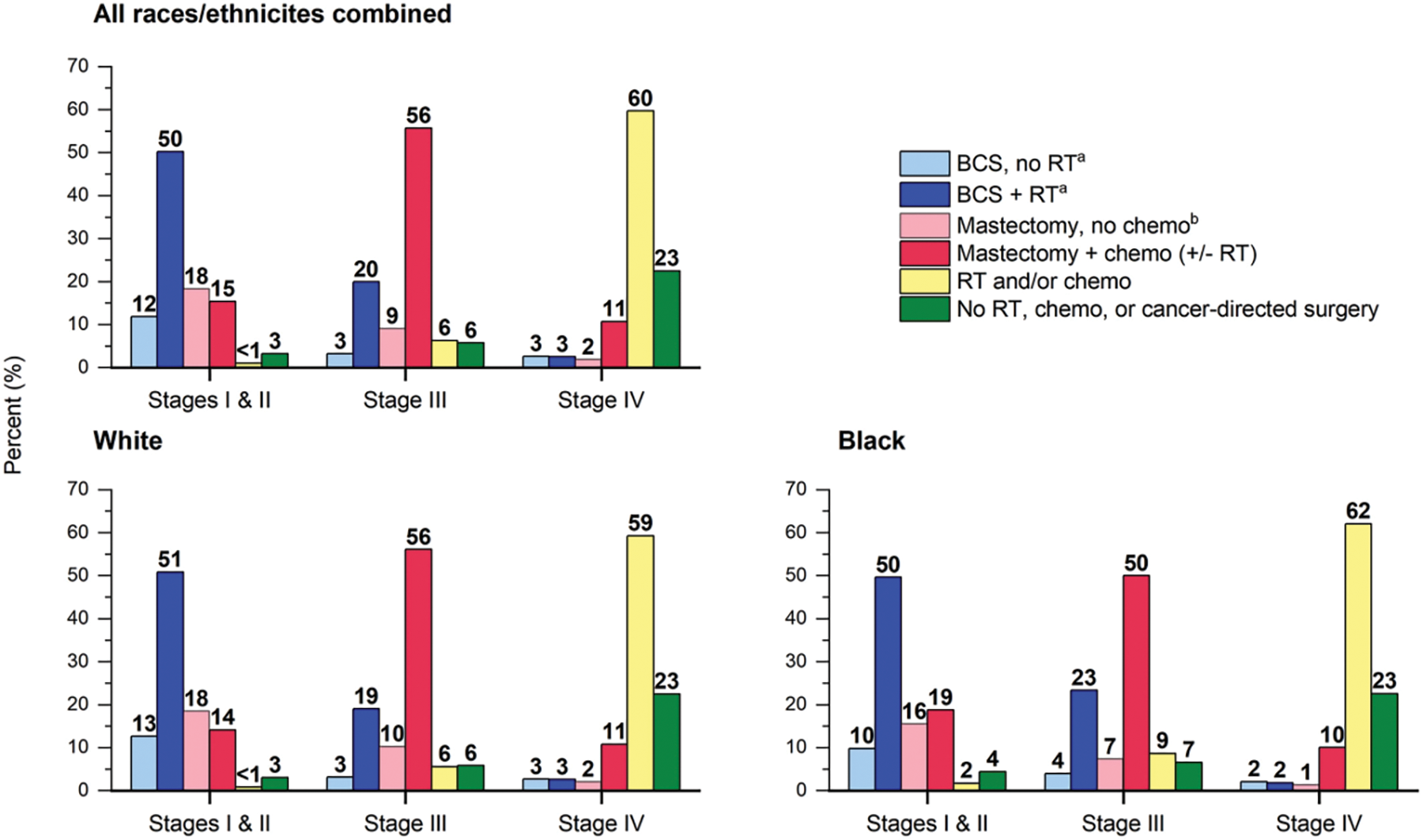
Figure 4: Female breast cancer treatment patterns (%) by Stage, 2018. Categories for white and black race exclude persons of Hispanic ethnicity. a A small number of these patients receive chemotherapy. b A small number of these patients receive radiation therapy (RT). +/− indicates with or without; BCS, breast-conserving surgery; chemo, chemotherapy (includes targeted therapy and immunotherapy) [54]
Long-term survival is the same as mastectomy when local or regional radiation therapy is added following breast conserving surgery [55,56]. However, evidence suggests that in patients with breast cancer aged 70 years and older, with small tumors and estrogen receptor (ER) positive, adjuvant radiation therapy can be omitted without compromising patient survival [57,58]. Some women with breast cancer eligible for breast-conserving surgery choose mastectomy due to reluctance to receive radiation therapy, contraindications to radiation therapy, or fear of recurrence [59–61].
Mastectomy and contralateral preventive mastectomy are more common in younger women (less than 40 years old) and patients with larger and/or more aggressive tumors [62–64]. The proportion of early-stage breast cancer patients who chose mastectomy and were eligible for CPM rose from 2% in 1998 to 30% in 2012. This rise could be attributed to the increased availability of genetic testing as well as increased risk awareness among people [65].
Tumor stage, grade, estrogen receptor (ER) status and progesterone receptor (PR) status, as well as the status of the human epidermal growth factor receptor 2 (HER2), are all facors that affect survival of breast cancer. Triple-negative breast cancers (ER-negative, PR-negative, and HER2 receptor-negative) are treated less effectively than other molecular subtypes and are typically only responsive to cytotoxic chemotherapy. Immunosuppressive combination chemotherapy for patients with early-stage and metastatic breast cancer, however, is becoming a common treatment option for triple-negative breast cancer [66].
A number of additional immunotherapy and targeted therapy treatments are now being researched, but due to the heterogeneous range of molecular profiles present in triple negative tumors, these therapies might only be successful for a portion of patients.
Breast cancer patients had a 75% 5-year relative survival rate in the 1970s, but by 2017 that number had risen to 90% [67,68]. This is mainly due to advances in hormone therapy and improved early diagnosis rates due to increased mammography screening [69]. Patients with stage I breast cancer have a nearly 100% 5-year relative survival rate, whereas patients with stage IV breast cancer only have a 28% 5-year relative survival rate [70].
Breast cancer is a type of malignant tumor that with a relatively good prognosis and a relatively long survival time. Survival rates vary significantly across countries and regions. In most developed western countries, the 5-year relative survival rate of breast cancer is high. For example, from 2005 to 2009, the 5-year relative survival rates of the United States, Australia, Canada, and Germany were 88.6%, 86.2%, 85.8%, and 85.3%, respectively. And the survival rate of breast cancer in these regions showed a gradual upward trend. However, in many Asian countries, due to the combined effects of many factors such as economy, living environment, reproductive pattern, the relative survival rate varies significantly among regions. Breast cancer’s 5-year relative survival rate in South Korea from 2005 to 2009 was 82.7%. In the same period, breast cancer’s 5-year relative survival rate in the Chinese population was 80.9%, while the survival rate in India was only 60.4%, and the survival rate in Jordan was as low as 43.1% [71].
In China, the urban-rural survival gap for breast cancer is still large due to differences in access to breast cancer screening and early diagnosis and treatment for urban and rural women, as well as differences in the treatment levels of breast cancer between urban and rural areas. The survival rate in urban areas is 77.8%, and the survival rate in rural areas is only 55.9% [72]. In recent years, in many economically developed cities, such as Guangzhou, Beijing and other regions, the survival rate of breast cancer has gradually increased [73,74]. And the gap between China and western developed countries is gradually narrowing, while the survival rate of rural and underdeveloped areas is still low.
Establishing primary breast cancer prevention is still difficult. However, weight control, reduced alcohol intake, increased physical activity, and promotion of breastfeeding may reduce the incidence of breast cancer worldwide. Through early detection and efficient treatment, the National Breast Cancer Screening Program seeks to lower the mortality of breast cancer [75–78]. In region with adequate resources, the WHO advises mammograms every two years for women 50 to 69 years old [79]. According to recommendations from the American Cancer Society, women between the ages of 45 and 54 should have annual screening, women between the ages of 40 and 44 should be given the chance to start annual screening, and women above the age of 55 should switch to biennial screening as long as they are healthy. A woman who is in good health and has a life expectancy of more than 10 years, she should continue to be screened [80]. However, the disadvantages of screening mammograms include overdiagnosis and overtreatment [81–83]. Through risk-stratified screening techniques that make use of current and developing risk prediction models, there are chances to increase the cost-effectiveness and benefit-to-hazard ratio of screening [84–87].
There are clinical trials showing that screening mammography can reduce breast cancer mortality by 20% [80]. Routine screening mammograms can detect 2–8 cancers per 1000 x-rays, while using digital tomography, an additional 1.6 cancers per 1000 mammograms could be detected [88]. 4.4 extra tumors were found per 1,000 screening tests using ultrasound, particularly in women with dense breasts, however this test’s positive predictive value was only 3%–8% [89]. MRI screening has a sensitivity of 90%–93% for cancer detection compared to 48%–63% of the combination of mammography and ultrasound screening [90,91]. Simple MRI, also known as contrast-enhanced spectral mammography, may be a new replacement for conventional MRI [92,93]. Bilateral mastectomy can reduce the risk for women with BRCA1 or BRCA2 mutations. Tamoxifen, raloxifene, and aromatase inhibitors have all been demonstrated to lower the chance of developing breast cancer but not the mortality rate [94–97]. In patients with intraepithelial neoplasia, low-dose tamoxifen (5 mg) appears to lower the chance of recurrence [98].
To sum up, the epidemiological analysis of breast cancer indicates that it is a widespread malignant tumor and one of the leading causes of tumor death in women globally. Breast cancer continues to put a strain on society. Breast cancer therapy and prevention are getting more and more complex. Therefore, in the future, the focus of breast cancer prevention and treatment will be on developing scientifically sound breast cancer screening techniques, enhancing breast cancer screening programs, and actively implementing early diagnosis and treatment of breast cancer for women of the appropriate age.
Additionally, the prevention and control of breast cancer should also be strengthened by advocating a healthy lifestyle, controlling weight, quitting smoking and limiting alcohol, regulating the indications for estrogen use, and standardizing treatment. Research on breast cancer in the future will concentrate less on developing new drugs and more on tailoring therapies to each patient’s unique tumor.
Authorship: Conception and design: Dongkui Xu. Analysis and interpretation, drafting and critical revision of the article: Chao Shang. Final approval of the article: Chao Shang.
Ethics Approval and Informed Consent Statement: No ethics approval informed Consent Statement needed.
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. Bray, F., Laversanne, M., Weiderpass, E., Soerjomataram, I. (2021). The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer, 127(16), 3029–3030. DOI 10.1002/cncr.33587. [Google Scholar] [CrossRef]
2. World Health Organization (2020). Global cancer observatory (GCO). Cancer Today. http://gco.iarc.fr/today/home. [Google Scholar]
3. Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I. et al. (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians, 71(3), 209–249. DOI 10.3322/caac.21660. [Google Scholar] [CrossRef]
4. Bray, F., Ferlay, J., Soerjomataram, I., Siegel, R. L., Torre, L. A. et al. (2018). Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians, 68(6), 394–424. DOI 10.3322/caac.21492. [Google Scholar] [CrossRef]
5. Torre, L. A., Bray, F., Siegel, R. L., Ferlay, J., Lortet-Tieulent, J. et al. (2015). Global cancer statistics, 2012. CA: A Cancer Journal for Clinicians, 65(2), 87–108. DOI 10.3322/caac.21262. [Google Scholar] [CrossRef]
6. Jemal, A., Bray, F., Center, M. M., Ferlay, J., Ward, E. et al. (2011). Global cancer statistics. CA: A Cancer Journal for Clinicians, 61(2), 69–90. DOI 10.3322/caac.20107. [Google Scholar] [CrossRef]
7. Parkin, D. M., Bray, F., Ferlay, J., Pisani, P. (2005). Global cancer statistics, 2002. CA: A Cancer Journal for Clinicians, 55(2), 74–108. DOI 10.3322/canjclin.55.2.74. [Google Scholar] [CrossRef]
8. Parkin, D. M. (2021). Global cancer statistics in the year 2000. Lancet Oncology, 2(9), 533–543. DOI 10.1016/S1470-2045(01)00486-7. [Google Scholar] [CrossRef]
9. Siegel, R. L., Miller, K. D., Jemal, A. (2020). Cancer statistics, 2020. CA: A Cancer Journal for Clinicians, 70(1), 7–30. DOI 10.3322/caac.21590. [Google Scholar] [CrossRef]
10. Australian Government, Australian Institute of Health and Welfare (2020). Breast screen Australia monitoring report 2019. https://www.aihw.gov.au/reports/cancer-screening/breastscreen-australia-monitoring-report-2019/data. [Google Scholar]
11. Siegel, R., Naishadham, D., Jemal, A. (2013). Cancer statistics, 2013. CA: A Cancer Journal for Clinicians, 63(1), 11–30. DOI 10.3322/caac.21166. [Google Scholar] [CrossRef]
12. Li, T., Mello-Thoms, C., Brennan, P. C. (2016). Descriptive epidemiology of breast cancer in China: Incidence, mortality, survival and prevalence. Breast Cancer Research and Treatment, 159(3), 395–406. DOI 10.1007/s10549-016-3947-0. [Google Scholar] [CrossRef]
13. Chen, W. Q., Zheng, R. S. (2015). Incidence, mortality and survival analysis of breast cancer in China. Chinese Journal of Clinical Oncology, 42 (13), 668–674. [Google Scholar]
14. Chen, W., Zheng, R., Zeng, H., Zhang, S. W. (2016). The incidence and mortality of major cancers in China, 2012. Chinese Journal of Cancer, 35(1), 73. DOI 10.1186/s40880-016-0137-8. [Google Scholar] [CrossRef]
15. Zuo, T. T., Zheng, R. S., Zeng, H. M., Zhang, S. W., Chen, W. Q. et al. (2017). Female breast cancer incidence and mortality in China, 2013. Thoracic Cancer, 8(3), 214–218. DOI 10.1111/1759-7714.12426. [Google Scholar] [CrossRef]
16. Zheng, R. S., Sun, K. X., Zhang, S. W., Zeng, H. M., Zou, X. N. et al. (2019). Report of cancer epidemiology in China, 2015. Chinese Journal of Oncology, 41(1), 19–28. [Google Scholar]
17. The Centers for Disease Control and Prevention (CDC, The National Cancer Institute (NCI) (2020). United States cancer statistics: Data visualizations. https://gis.cdc.gov/Cancer/USCS/DataViz.html. [Google Scholar]
18. Brinton, L. A., Gaudet, M. M., Gierach, G. L. (2018). Breast cancer. In: Thun, M., Linet, M. S., Cerhan, J. R., Haiman, C. A., Schottenfeld, D. (Eds.Cancer Epidemiology and Prevention, 4th edition, pp. 861–888. Oxford University Press. [Google Scholar]
19. Torre, L. A., Islami, F., Siegel, R. L., Ward, E. M., Jemal, A. (2017). Global cancer in women: Burden and trends. Cancer Epidemiology Biomarkers & Prevention, 26, 444–457. DOI 10.1158/1055-9965.EPI-16-0858. [Google Scholar] [CrossRef]
20. Rossouw, J. E., Anderson, G. L., Prentice, R. L., LaCroix, A. Z., Kooperberg, C. et al. (2002). Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results from the women’s health initiative randomized controlled trial. JAMA, 288, 321–333. DOI 10.1001/jama.288.3.321. [Google Scholar] [CrossRef]
21. Breen, N., Gentleman, J. F., Schiller, J. S. (2011). Update on mammography trends: Comparisons of rates in 2000, 2005, and 2008. Cancer, 117, 2209–2218. DOI 10.1002/cncr.25679. [Google Scholar] [CrossRef]
22. Heer, E., Harper, A., Escandor, N., Sung, H., McCormack, V. et al. (2020). Global burden and trends in premenopausal and postmenopausal breast cancer: A population-based study. The Lancet Global Health, 8(8), e1027–e1037. DOI 10.1016/S2214-109X(20)30215-1. [Google Scholar] [CrossRef]
23. Allemani, C., Matsuda, T., di Carlo, V., Harewood, R., Matz, M. et al. (2018). Global surveillance of trends in cancer survival 2000-14 (CONCORD-3Analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet, 391, 1023–1075. DOI 10.1016/S0140-6736(17)33326-3. [Google Scholar] [CrossRef]
24. Ngan, T. T., Nguyen, N. T. Q., van Minh, H., Donnelly, M., O’Neill, C. (2020). Effectiveness of clinical breast examination as a ‘standalone’ screening modality: An overview of systematic reviews. BMC Cancer, 20, 1070. DOI 10.1186/s12885-020-07521-w. [Google Scholar] [CrossRef]
25. Birnbaum, J. K., Duggan, C., Anderson, B. O., Etzioni, R. (2018). Early detection and treatment strategies for breast cancer in low-income and upper middle-income countries: A modelling study. The Lancet Global Health, 6(8), e885–e893. DOI 10.1016/S2214-109X(18)30257-2. [Google Scholar] [CrossRef]
26. Anderson, B. O., Lipscomb, J., Murillo, R. H., Thomas, D. B. (2015). Chapter 3. Breast cancer. In: Gelband, H., Jha, P., Sankaranarayanan, R., Horton, S. (Eds.Cancer: Disease control priorities, 3rd edition, vol. 3, pp. 45–68. The International Bank for Reconstruction and Development/The World Bank. [Google Scholar]
27. McCormack, V., McKenzie, F., Foerster, M., Zietsman, A., Galukande, M. et al. (2020). Breast cancer survival and survival gap apportionment in sub-saharan Africa (ABC-DOA prospective cohort study. The Lancet Global Health, 8(9), e1203–e1212. DOI 10.1016/S2214-109X(20)30261-8. [Google Scholar] [CrossRef]
28. DeSantis, C. E., Ma, J., Gaudet, M. M. (2019). Breast cancer statistics, 2019. CA: A Cancer Journal for Clinicians, 69, 438–451. DOI 10.3322/caac.21583. [Google Scholar] [CrossRef]
29. Ginsburgh, O., Bray, F., Coleman, M. P., Vanderpuye, V., Eniu, A. et al. (2017). The global burden of women’s cancers: A grand challenge in global health. Lancet, 389(10071), 847–860. DOI 10.1016/S0140-6736(16)31392-7. [Google Scholar] [CrossRef]
30. Kuchenbaecker, K. B., Hopper, J. L., Barnes, D. R., Phillips, K. A., Mooij, T. M. et al. (2017). Risks of breast, ovarian and contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. JAMA, 317(23), 2402–2416. DOI 10.1001/jama.2017.7112. [Google Scholar] [CrossRef]
31. Chen, S., Parmigiani, G. (2007). Meta-analysis of BRCA1 and BRCA2 penetrance. Journal of Clinical Oncology, 25, 1329–1333. DOI 10.1200/JCO.2006.09.1066. [Google Scholar] [CrossRef]
32. Nur, U., El Reda, D., Hashim, D., Weiderpass, E. (2019). A prospective investigation of oral contraceptive use and breast cancer mortality: Findings from the Swedish women’s lifestyle and health cohort. BMC Cancer, 19, 807. DOI 10.1186/s12885-019-5985-6. [Google Scholar] [CrossRef]
33. Mørch, L. S., Skovlund, C. W., Hannaford, P. C., Iversen, L., Fielding, S. et al. (2017). Contemporary hormonal contraception and the risk of breast cancer. The New England Journal of Medicine, 377, 2228–2239. DOI 10.1056/NEJMoa1700732. [Google Scholar] [CrossRef]
34. Collaborative Group on Hormonal Factors in Breast Cancer (2019). Type and timing of menopausal hormone therapy and breast cancer risk: Individual participant meta-analysis of the worldwide epidemiological evidence. Lancet, 394, 1159–1168. DOI 10.1016/S0140-6736(19)31709-X. [Google Scholar] [CrossRef]
35. Giordano, S. H. (2018). Breast cancer in men. The New England Journal of Medicine, 378(24), 2311–2320. DOI 10.1056/NEJMra1707939. [Google Scholar] [CrossRef]
36. Engmann, N. J., Golmakani, M. K., Miglioretti, D. L., Sprague, B. L., Kerlikowske, K. et al. (2017). Population-attributable risk proportion of clinical risk factors for breast cancer. JAMA Oncology, 3(9), 1228–1236. DOI 10.1001/jamaoncol.2016.6326. [Google Scholar] [CrossRef]
37. Tan, M. M., Ho, W. K., Yoon, S. Y., Mariapun, S., Hasan, S. N. et al. (2018). A case-control study of breast cancer risk factors in 7,663 women in Malaysia. PLoS One, 13(9), e0203469. DOI 10.1371/journal.pone.0203469. [Google Scholar] [CrossRef]
38. Heath, A. K., Muller, D. C., van den Brandt, P. A., Papadimitriou, N., Critselis, E. et al. (2020). Nutrient-wide association study of 92 foods and nutrients and breast cancer risk. Breast Cancer Research, 22(1), 5. DOI 10.1186/s13058-019-1244-7. [Google Scholar] [CrossRef]
39. Shetty, P. J., Sreedharan, J. (2019). Breast cancer and dietary fat intake: A correlational study. Nepal Journal of Epidemiology, 9(4), 812–816. DOI 10.3126/nje.v9i4.26961. [Google Scholar] [CrossRef]
40. Xiao, Y. J., Xia, J. J., Li, L. P., Ke, Y. B., Cheng, J. Q. et al. (2019). Associations between dietary patterns and the risk of breast cancer: A systematic review and meta-analysis of observational studies. Breast Cancer Research, 21(1), 16. DOI 10.1186/s13058-019-1096-1. [Google Scholar] [CrossRef]
41. Houghton, S. C., Eliassen, A. H., Zhang, S. M., Selhub, J., Rosner, B. A. et al. (2019). Plasma B-vitamins and one-carbon metabolites and the risk of breast cancer in younger women. Breast Cancer Research and Treatment, 176(1), 191–203. DOI 10.1007/s10549-019-05223-x. [Google Scholar] [CrossRef]
42. Rosenberg, L., Bethea, T. N., Viscidi, E., Hong, C. C., Troester, M. A. et al. (2016). Postmenopausal female hormone use and estrogen receptor-positive and -negative breast cancer in african American women. Journal of the National Cancer Institute, 108(4), djv361. DOI 10.1093/jnci/djv361. [Google Scholar] [CrossRef]
43. Wu, J. W., Azoulay, L., Majdan, A., Boivin, J. F., Pollak, M. et al. (2017). Long-term use of long-acting insulin analogs and breast cancer incidence in women with type 2 diabetes. Journal of Clinical Oncology, 35(32), 3647–3653. DOI 10.1200/JCO.2017.73.4491. [Google Scholar] [CrossRef]
44. Sancho-Garnier, H., Colonna, M. (2019). Épidémiologie des cancers dusein. La Presse Médicale, 48(10), 1076–1084. DOI 10.1016/j.lpm.2019.09.022. [Google Scholar] [CrossRef]
45. Thakur, P., Seam, R. K., Gupta, M. K., Gupta, M., Sharma, M. et al. (2017). Breast cancer risk factor evaluation in a western himalayan state: A case-control study and comparison with the western world. South Asian Journal of Cancer, 6(3), 106–109. DOI 10.4103/sajc.sajc_157_16. [Google Scholar] [CrossRef]
46. Badr, L. K., Bourdeanu, L., Alatrash, M., Bekarian, G. (2018). Breast cancer risk factors: A cross-cultural comparison between the west and the east. The Asian Pacific Journal of Cancer Prevention, 19(8), 2109–2116. [Google Scholar]
47. Nichols, H. B., Schoemaker, M. J., Cai, J., Xu, J. W., Wright, L. B. et al. (2019). Breast cancer risk after recent childbirth: A pooled analysis of 15 prospective studies. Annals of Internal Medicine, 170(1), 22–30. DOI 10.7326/M18-1323. [Google Scholar] [CrossRef]
48. Fortner, R. T., Sisti, J., Chai, B., Collins, L. C., Rosner, B. et al. (2019). Parity, breastfeeding, and breast cancer risk by hormone receptor status and molecular phenotype: Results from the nurses’ health studies. Breast Cancer Research, 21(1), 40. DOI 10.1186/s13058-019-1119-y. [Google Scholar] [CrossRef]
49. Economopoulou, P., Dimitriadis, G., Psyrri, A. (2015). Beyond BRCA: New hereditary breast cancer susceptibility genes. Cancer Treatment Reviews, 41(1), 1–8. DOI 10.1016/j.ctrv.2014.10.008. [Google Scholar] [CrossRef]
50. Park, J. S., Lee, S. T., Nam, E. J., Han, J. W., Lee, J. Y. et al. (2018). Variants of cancer susceptibility genes in Korean BRCA1/2 mutation-negative patients with high risk for hereditary breast cancer. BMC Cancer, 18(1), 83. DOI 10.1186/s12885-017-3940-y. [Google Scholar] [CrossRef]
51. Chen, X., Li, Y., Ouyang, T., Li, J., Wang, T. et al. (2018). Associations between RAD51D germline mutations and breast cancer risk and survival in BRCA1/2-negative breast cancers. Annals of Oncology, 29(10), 2046–2051. DOI 10.1093/annonc/mdy338. [Google Scholar] [CrossRef]
52. Reiner, A. S., Sisti, J., John, E. M., Lynch, C. F., Brooks, J. D. et al. (2018). Breast cancer family history and contralateral breast cancer risk in young women: An update from the women’s environmental cancer and radiation epidemiology study. Journal of Clinical Oncology, 36(15), 1513–1520. DOI 10.1200/JCO.2017.77.3424. [Google Scholar] [CrossRef]
53. Braithwaite, D., Miglioretti, D. L., Zhu, W., Demb, J., Trentham-Dietz, A. et al. (2018). Family history and breast cancer risk among older women in the breast cancer surveillance consortium cohort. JAMA Internal Medicine, 178(4), 494–501. DOI 10.1001/jamainternmed.2017.8642. [Google Scholar] [CrossRef]
54. Miller, K. D., Nogueira, L., Devasia, T., Mariotto, A. B., Yabroff, K. R. et al. (2022). Cancer treatment and survivorship statistics, 2022. CA: A Cancer Journal for Clinicians, 72(5), 409–436. DOI 10.3322/caac.21731. [Google Scholar] [CrossRef]
55. Jatoi, I., Proschan, M. A. (2005). Randomized trials of breast-conserving therapy versus mastectomy for primary breast cancer: A pooled analysis of updated results. American Journal of Clinical Oncology, 28(3), 289–294. DOI 10.1097/01.coc.0000156922.58631.d7. [Google Scholar] [CrossRef]
56. Litiere, S., Werutsky, G., Fentiman, I. S., Rutgers, E., Christiaens, M. R. et al. (2012). Breast conserving therapy versus mastectomy for stage I-II breast cancer: 20 year follow-up of the EORTC 10801 phase 3 randomised trial. Lancet Oncology, 13(4), 412–419. DOI 10.1016/S1470-2045(12)70042-6. [Google Scholar] [CrossRef]
57. Gradishar, W. J., Anderson, B. O., Balassanian, R., Blair, S. L., Burstein, H. J. et al. (2018). Breast cancer, version 4.2017, NCCN clinical practice guidelines in oncology. Journal of the National Comprehensive Cancer Network, 16, 310–320. DOI 10.6004/jnccn.2018.0012. [Google Scholar] [CrossRef]
58. Kunkler, I. H., Williams, L. J., Jack, W. J., Cameron, D. A., Dixon, J. M., on behalf of the PRIME II investigators (2015). Breast-conserving surgery with or without irradiation in women aged 65 years or older with early breast cancer (PRIME IIA randomised controlled trial. Lancet Oncology, 16(3), 266–273. DOI 10.1016/S1470-2045(14)71221-5. [Google Scholar] [CrossRef]
59. Kummerow, K. L., Du, L., Penson, D. F., Shyr, Y., Hooks, M. A. (2015). Nationwide trends in mastectomy for early-stage breast cancer. JAMA Surgery, 150(1), 9–16. DOI 10.1001/jamasurg.2014.2895. [Google Scholar] [CrossRef]
60. Albornoz, C. R., Matros, E., Lee, C. N., Hudis, C. A., Pusic, A. L. et al. (2015). Bilateral mastectomy versus breast-conserving surgery for early-stage breast cancer: The role of breast reconstruction. Plastic and Reconstructive Surgery, 135, 1518–1526. DOI 10.1097/PRS.0000000000001276. [Google Scholar] [CrossRef]
61. Montagna, G., Morrow, M. (2020). Contralateral prophylactic mastectomy in breast cancer: What to discuss with patients. Expert Review of Anticancer Therapy, 20, 159–166. DOI 10.1080/14737140.2020.1732213. [Google Scholar] [CrossRef]
62. Freedman, R. A., Virgo, K. S., Labadie, J., He, Y., Partridge, A. H. et al. (2012). Receipt of locoregional therapy among young women with breast cancer. Breast Cancer Research and Treatment, 135, 893–906. DOI 10.1007/s10549-012-2156-8. [Google Scholar] [CrossRef]
63. Wang, T., Baskin, A. S., Dossett, L. A. (2020). Deimplementation of the choosing wisely recommendations for low-value breast cancer surgery: A systematic review. JAMA Surgery, 155, 759–770. DOI 10.1001/jamasurg.2020.0322. [Google Scholar] [CrossRef]
64. Nash, R., Goodman, M., Lin, C. C., Freedman, R. A., Dominici, L. S. et al. (2017). State variation in the receipt of a contralateral prophylactic mastectomy among women who received a diagnosis of invasive unilateral early-stage breast cancer in the United States, 2004–2012. JAMA Surgery, 152, 648–657. DOI 10.1001/jamasurg.2017.0115. [Google Scholar] [CrossRef]
65. Basu, N. N., Hodson, J., Chatterjee, S., Gandhi, A., Wisely, J. et al. (2021). The angelina jolie effect: Contralateral risk-reducing mastectomy trends in patients at increased risk of breast cancer. Scientific Reports, 11, 2847. DOI 10.1038/s41598-021-82654-x. [Google Scholar] [CrossRef]
66. Schmid, P., Cortes, J., Pusztai, L., McArthur, H., Kümmel, S. et al. (2020). Pembrolizumab for early triple-negative breast cancer. The New England Journal of Medicine, 382(9), 810–821. DOI 10.1056/NEJMoa1910549. [Google Scholar] [CrossRef]
67. Howlader, N., Noone, A. M., Krapcho, M., Miller, D., Brest, A. et al. eds. (2021). SEER cancer statistics review, 1975–2018. National Cancer Institute. [Google Scholar]
68. Surveillance, Epidemiology, and End Results (SEER) Program. SEER*stat database: Incidence—SEER 9 registries research data, November 2020 submission (1973–2018) <Katrina/Rita population adjustment>—Linked to county attributes—Total United States, 1969–2019 counties. National Cancer Institute, Division of Cancer Control and Population Sciences, Surveillance Research Program (2021). [Google Scholar]
69. Berry, D. A., Cronin, K. A., Plevritis, S. K., Fryback, D. G., Clarke, L. et al. (2005). Effect of screening and adjuvant therapy on mortality from breast cancer. The New England Journal of Medicine, 353, 1784–1792. [Google Scholar]
70. Surveillance, Epidemiology, and End Results (SEER) Program. SEER*stat database: Incidence—SEER 18 registries research data + hurricane katrina impacted louisiana cases, November 2020 submission (2000–2018) <Katrina/Rita population adjustment>—Linked to county attributes—Total United States, 1969–2019 counties. National Cancer Institute, Division of Cancer Control and Population Sciences, Surveillance Research Program (2021). [Google Scholar]
71. Allemani, C., Weir, H. K., Carreira, H., Harewood, R., Spika, D. et al. (2015). Global surveillance of cancer survival 1995–2009: Analysis of individual data for 25,676,887 patients from 279 population-based registries in 67 countries (CONCORD-2). Lancet, 385(9972), 977–1010. [Google Scholar]
72. Zeng, H., Zheng, R., Guo, Y., Zhang, S. W., Zou, X. N. et al. (2015). Cancer survival in China, 2003–2005: A population-based study. International Journal of Cancer, 136(8), 1921–1930. [Google Scholar]
73. Wang, Q. J., Zhu, W. X., Xing, X. M. (2006). Analysis of the incidence and survival of female breast cancer in Beijing during the last 20 years. Chinese Journal of Oncology, 28(3), 208–210. [Google Scholar]
74. Ling, L., Liu, Q., Zeng, C. H. (2000). Analysis of malignant neoplasm survival rates in Yuexiu District, Guangzhou City, 1996–1999. Chinese Journal of Cancer, 28(11), 1040–1042. [Google Scholar]
75. Independent UK Panel on Breast Cancer Screening (2012). The benefits and harms of breast cancer screening: An independent review. Lancet, 380, 1778–1786. [Google Scholar]
76. Coldman, A., Phillips, N., Wilson, C., Decker, K., Chiarelli, A. M. et al. (2014). Pan-canadian study of mammography screening and mortality from breast cancer. Journal of the National Cancer Institute, 106(11), dju261. [Google Scholar]
77. Tabar, L., Dean, P. B., Chen, T. H., Yen, M. F., Chen, L. S. et al. (2019). The incidence of fatal breast cancer measures the increased effectiveness of therapy in women participating in mammography screening. Cancer, 125(4), 515–523. [Google Scholar]
78. International Agency for Research on Cancer (IARCWorking Group on the Evaluation of Cancer-Preventive Strategies (2016). Breast cancer screening. In: IARC handbooks of cancer prevention, vol. 15. IARC Press. [Google Scholar]
79. World Health Organization (2014). WHO position paper on mammography screening. Geneva: WHO. [Google Scholar]
80. Oeffinger, K. C., Fontham, E. T., Etzioni, R., Herzig, A., Michaelson, J. S. et al. (2015). Breast cancer screening for women at average risk: 2015 guideline update from the American cancer society. JAMA, 314(15), 1599–1614. DOI 10.1001/jama.2015.12783. [Google Scholar] [CrossRef]
81. Prasad, V., Lenzer, J., Newman, D. H. (2016). Why cancer screening has never been shown to “save lives”—and what we can do about it. BMJ, 352, h6080. [Google Scholar]
82. Puliti, D., Duffy, S. W., Miccinesi, G., de Koning, H., Lynge, E. et al. (2012). Overdiagnosis in mammographic screening for breast cancer in Europe: A literature review. Journal of Medical Screening, 19(suppl 1), 42–56. DOI 10.1258/jms.2012.012082. [Google Scholar] [CrossRef]
83. Michalopoulos, D., Duffy, S. W. (2016). Estimation of overdiagnosis using short-term trends and lead time estimates uncontaminated by overdiagnosed cases: Results from the Norwegian breast screening programme. Journal of Medical Screening, 23, 192–202. DOI 10.1177/0969141315623980. [Google Scholar] [CrossRef]
84. Narod, S. A. (2018). Personalised medicine and population health: Breast and ovarian cancer. Hum Genet, 137, 769–778. DOI 10.1007/s00439-018-1944-6. [Google Scholar] [CrossRef]
85. Pashayan, N., Morris, S., Gilbert, F. J., Pharoah, P. D. P. (2018). Cost-effectiveness and benefit-to-harm ratio of risk-stratified screening for breast cancer: A life-table model. JAMA Oncology, 4, 1504–1510. DOI 10.1001/jamaoncol.2018.1901. [Google Scholar] [CrossRef]
86. Mukama, T., Kharazmi, E., Xing, X., Sundquist, K., Sundquist, J. et al. (2019). Risk-adapted starting age of screening for relatives of patients with breast cancer. JAMA Oncology, 6(1), 68–74. DOI 10.1001/jamaoncol.2019.3876. [Google Scholar] [CrossRef]
87. Pal Choudhury, P., Wilcox, A. N., Brook, M. N., Zhang, Y., Ahearn, T. et al. (2020). Comparative validation of breast cancer risk prediction models and projections for future risk stratification. Journal of the National Cancer Institute, 112(3), 278–285. DOI 10.1093/jnci/djz113. [Google Scholar] [CrossRef]
88. Marinovich, M. L., Hunter, K. E., Macaskill, P., Houssami, N. (2018). Breast cancer screening using tomosynthesis or mammography: A meta-analysis of cancer detection and recall. Journal of the National Cancer Institute, 110: 942–949. DOI 10.1093/jnci/djy121. [Google Scholar] [CrossRef]
89. Melnikow, J., Fenton, J. J., Whitlock, E. P., Miglioretti, D. L., Weyrich, M. S. et al. (2016). Supplemental screening for breast cancer in women with dense breasts: A systematic review for the US preventive services task force. Annals of Internal Medicine, 164, 268–278. DOI 10.7326/M15-1789. [Google Scholar] [CrossRef]
90. Kuhl, C., Weigel, S., Schrading, S., Arand, B., Bieling, H. et al. (2010). Prospective multicenter cohort study to refine management recommendations for women at elevated familial risk of breast cancer: The EVA trial. Journal of Clinical Oncology, 28(9), 1450–1457. DOI 10.1200/JCO.2009.23.0839. [Google Scholar] [CrossRef]
91. Sardanelli, F., Podo, F., Santoro, F., Manoukian, S., Bergonzi, S. et al. (2011). Multicenter surveillance of women at high genetic breast cancer risk using mammography, ultrasonography, and contrast-enhanced magnetic resonance imaging (the high breast cancer risk Italian 1 studyFinal results. Investigative Radiology, 46, 94–105. DOI 10.1097/RLI.0b013e3181f3fcdf. [Google Scholar] [CrossRef]
92. Comstock, C. E., Gatsonis, C., Newstead, G. M., Snyder, B. S., Gareen, I. F. et al. (2020). Comparison of abbreviated breast MRI vs digital breast tomosynthesis for breast cancer detection among women with dense breasts undergoing screening. JAMA, 323(8), 746–756. DOI 10.1001/jama.2020.0572. [Google Scholar] [CrossRef]
93. Sung, J. S., Lebron, L., Keating, D., D’Alessio, D., Comstock, C. E. et al. (2019). Performance of dual-energy contrast-enhanced digital mammography for screening women at increased risk of breast cancer. Radiology, 293, 81–88. DOI 10.1148/radiol.2019182660. [Google Scholar] [CrossRef]
94. Cuzick, J., Sestak, I., Bonanni, B., Costantino, J. P., Cummings, S. et al. (2013). Selective oestrogen receptor modulators in prevention of breast cancer: An updated meta-analysis of individual participant data. Lancet, 381(9880), 1827–1834. DOI 10.1016/S0140-6736(13)60140-3. [Google Scholar] [CrossRef]
95. Fisher, B., Costantino, J. P., Wickerham, D. L., Cecchini, R. S., Cronin, W. M. et al. (2005). Tamoxifen for the prevention of breast cancer: Current status of the national surgical adjuvant breast and bowel project P-1 study. Journal of the National Cancer Institute, 97(22), 1652–1662. DOI 10.1093/jnci/dji372. [Google Scholar] [CrossRef]
96. Vogel, V. G., Costantino, J. P., Wickerham, D. L., Cronin, W. M., Cecchini, R. S. et al. (2010). Update of the national surgical adjuvant breast and bowel project study of tamoxifen and raloxifene (STAR) P-2 trial: Preventing breast cancer. Cancer Prevention Research (Philadelphia), 3(6), 696–706. DOI 10.1158/1940-6207.CAPR-10-0076. [Google Scholar] [CrossRef]
97. Cuzick, J., Sestak, I., Forbes, J. F., Dowsett, M., Cawthorn, S. et al. (2020). Use of anastrozole for breast cancer prevention (IBIS-IILong-term results of a randomised controlled trial. Lancet, 395(10218), 117–122. DOI 10.1016/S0140-6736(19)32955-1. [Google Scholar] [CrossRef]
98. DeCensi, A., Puntoni, M., Guerrieri-Gonzaga, A., Caviglia, S., Avino, F. et al. (2019). Randomized placebo controlled trial of low-dose tamoxifen to prevent local and contralateral recurrence in breast intraepithelial neoplasia. Journal of Clinical Oncology, 37(19), 1629–1637. DOI 10.1200/JCO.18.01779. [Google Scholar] [CrossRef]
Cite This Article
 Copyright © 2022 The Author(s). Published by Tech Science Press.
Copyright © 2022 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools