 Open Access
Open Access
CASE REPORT
Differential Diagnosis between Primary Intracranial Melanoma and Cerebral Cavernoma in Crohn’s Disease: A Case Report and Literature Review
1 Neurosurgery Unit, Department of Biomedicine, Neurosciences & Advanced Diagnostics, School of Medicine, University of Palermo, Palermo, Italy
2 School of Medicine, American University of Integrative Sciences, Cole Bay, Barbados
3 Department of Neurosurgery, Highly Specialized Hospital and of National Importance “Garibaldi”, Catania, Italy
4 Department of Neurosurgery, Trauma Center, Gamma Knife Center, Cannizzaro Hospital, Catania, Italy
* Corresponding Author: Gianluca Scalia. Email:
Oncologie 2022, 24(4), 937-942. https://doi.org/10.32604/oncologie.2022.027155
Received 17 October 2022; Accepted 05 December 2022; Issue published 31 December 2022
Abstract
Primary intracranial melanomas are rare, with a challenging diagnosis based only on clinical and imaging features. The authors described the case of an intracerebral right parieto-temporal melanoma mimicking a cavernoma in a patient affected by Crohn’s disease. A 67-year-old female patient with Crohn’s disease and small bowel stenosis was hospitalized for surgical removal of the terminal ileum and latero-lateral ileo-colic anastomosis. During postoperative week 1, the patient developed psychomotor agitation followed by altered consciousness. An urgent brain CT showed a right intracerebral parieto-temporal hemorrhage with intralesional calcifications. The patient underwent a decompressive craniectomy with hematoma drainage and a complete resection of the lesion. Histologic examination showed characteristics consistent with malignant melanoma. In patients with inflammatory bowel disease (IBD), the differential diagnosis between intracerebral melanoma and cavernoma may be challenging, considering the similar clinical and radiological findings. Thus, a comprehensive multidisciplinary approach is required to exclude the possible coexistence of such intracranial lesions.Keywords
Primary intracranial melanomas are uncommon tumors, accounting for approximately 1% of all reported melanoma cases in the literature. A proper diagnosis can be challenging, even when guided by peculiar clinical presentation and radiological features. In patients with inflammatory bowel diseases (IBD), the occasional detection of melanomas or cerebral vascular malformations has been recently studied to identify potential predisposing factors, such as genetic alterations, drug-induced immunosuppression, or gut microbiota alterations [1]. The authors described the case of a patient with Crohn’s disease affected by an intracerebral right parieto-temporal melanoma with radiological features mimicking a cerebral cavernous malformation.
A 67-year-old female patient suffering from stenosis of the small bowel secondary to Crohn’s disease was urgently hospitalized and treated with surgical removal of the terminal ileum followed by latero-lateral anastomosis between the remaining ileum and the descending colon. During postoperative week 1, the patient developed psychomotor agitation followed by altered consciousness.
2.2 Imaging and Histological Examination
An urgent brain CT-angiography was performed, showing a well-defined right parieto-temporal intracerebral hemorrhage (6 cm × 5 cm) with intralesional calcifications, highly suggestive for a cerebral hemorrhagic cavernous malformation (Fig. 1).
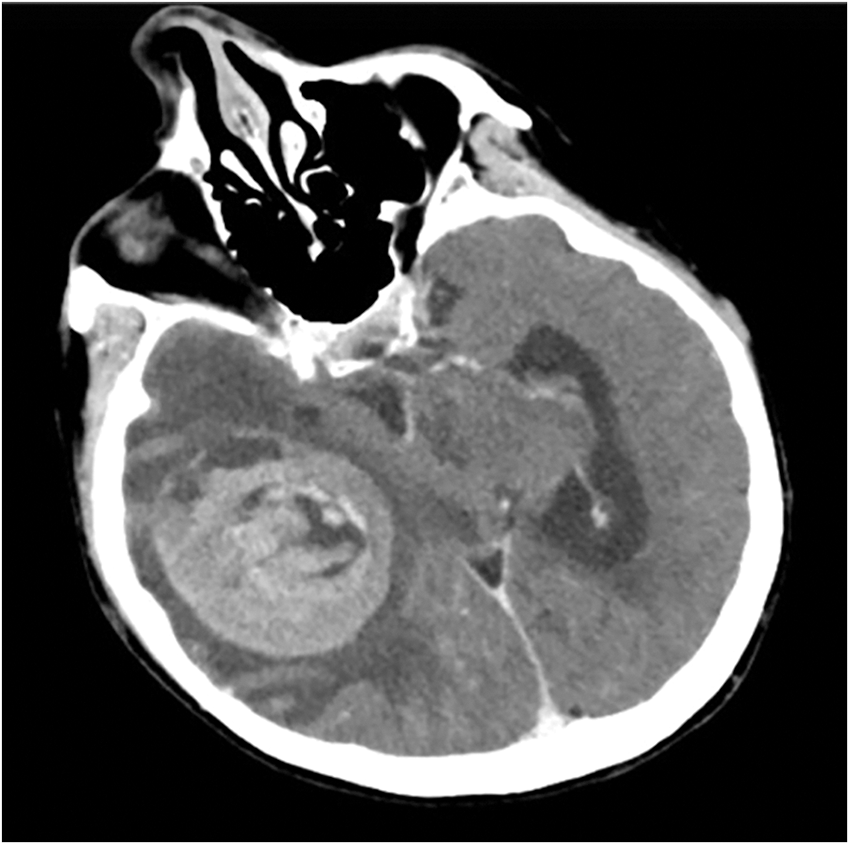
Figure 1: Preoperative axial brain CT-angiogram showing a well-defined right parieto-temporal intracerebral hemorrhage (6 cm × 5 cm) with intralesional calcifications
The patient was admitted to our neurosurgery unit with G.C.S. score of 4/15 and right anisocoria. She underwent a right decompressive craniectomy with hematoma drainage and complete resection of the lesion. Histologic examination showed fragments of highly vascularized neoplastic tissue with epithelioid pattern and characteristic immunophenotype (MART1+, S100+, HMB45+, PANCK-) of melanoma.
2.3 Postoperative Course and Post-Operative Imaging
A whole body 18-FDG PET/CT scan was subsequently performed, revealing no tracer uptake (Figs. 2A, 2B). Postoperative brain CT and MRI scan showed a complete removal of the lesion (Figs. 3A, 3B). The patient progressively improved, most notably after performing hydroxyapatite cranioplasty (Fig. 4). The patient was discharged on postoperative day 20 and transferred to the radiation oncology unit for the adjuvant treatments.
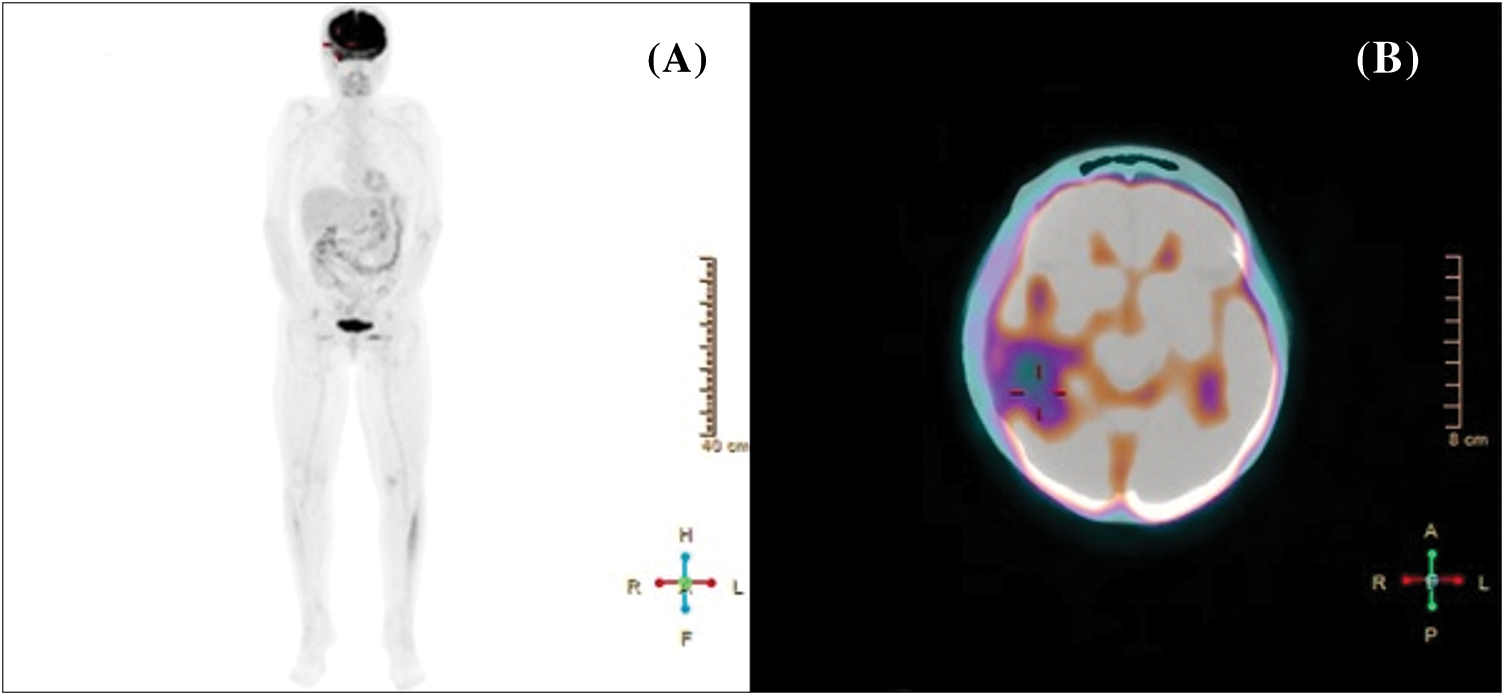
Figure 2: Whole body (A) and cerebral (B) 18-FDG PET/CT scan showing no tracer uptake
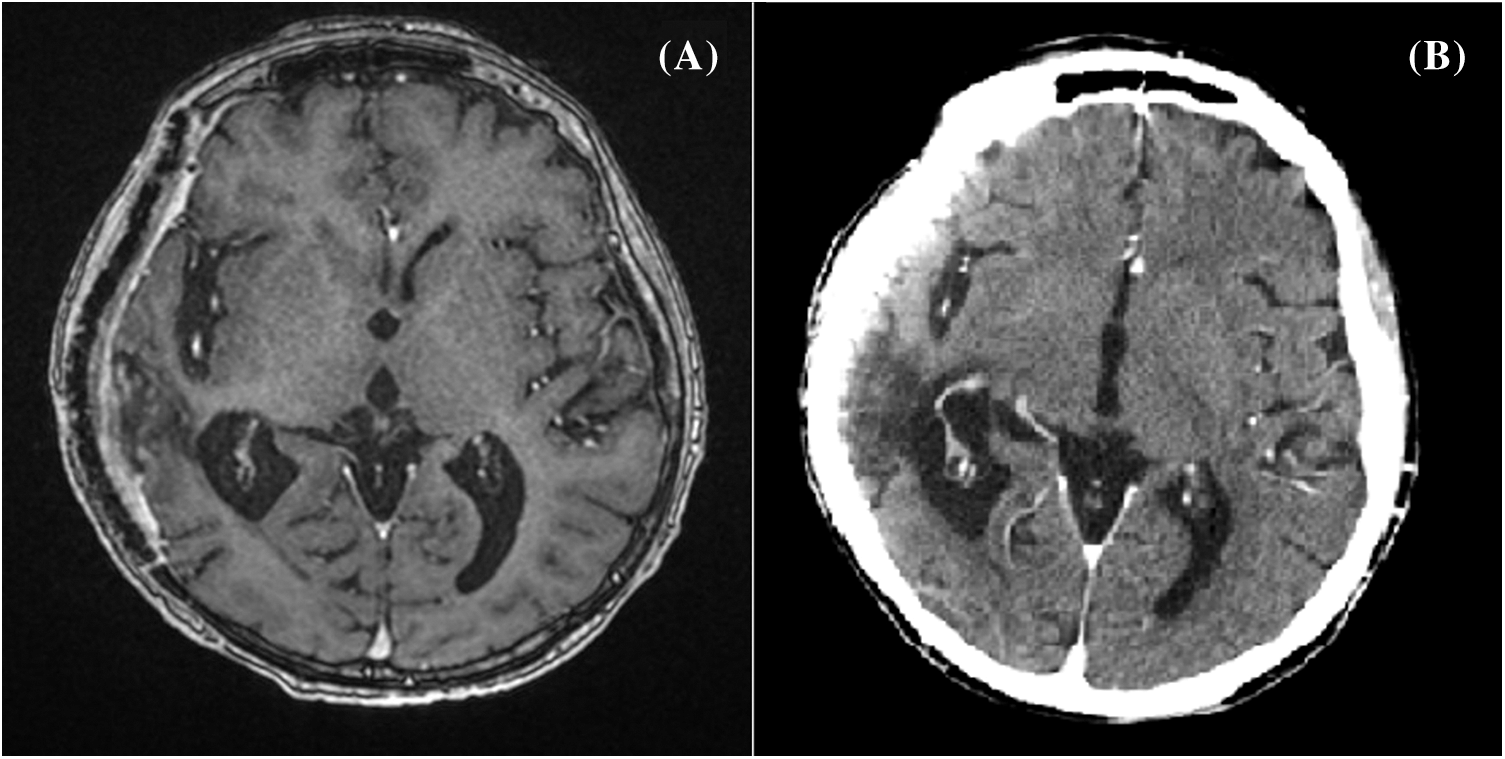
Figure 3: Postoperative brain contrast-enhanced T1-contrast MRI (A) and head CT scan (B) showing a complete removal of the lesion
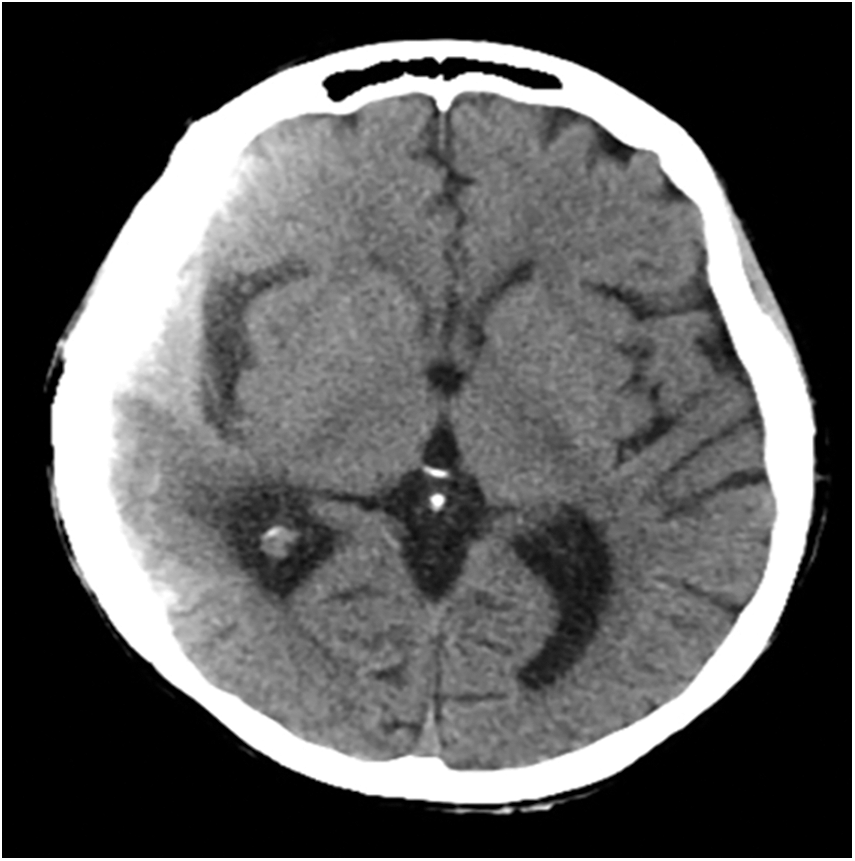
Figure 4: Postoperative brain axial CT scan performed after hydroxyapatite cranioplasty
IBDs represent complex pathologies with high rates of extraintestinal manifestations, including intracranial lesions like cavernous malformations and melanomas. The presence of an intracranial lesion may not always be evident clinically, and symptoms may be insidious or acute. Hence, prompt diagnosis and treatment are crucial to reducing patient’s morbidity and mortality. Although imaging plays an important role in the detection of intracranial lesions, it may be non-specific in differentiating melanomas from cavernomas, as both may appear as space-occupying lesions with hemorrhagic characteristics. Definitive diagnosis requires further histopathological exams with immunohistochemical staining for tumor markers detection.
Crohn’s disease involves transmural inflammation of the whole gastrointestinal tract that causes functional and mechanical impairment of microvascular circulation, leading to a chronic inflammatory state [2]. This condition promotes a hypo-inflammatory state and may facilitate the development of tumors with a predisposition to prothrombotic events [1]. The use of biologic therapies can also induce systemic vasculitis as a side effect, which, when occurring in the cerebral vascular system, may lead to subarachnoid hemorrhages [3]. As demonstrated, a microbiome alteration can influence the appearance of cavernous bleeding malformation [4]. In particular, a stimulation of endothelial TLR-4 and gut microbiome can promote cerebrovascular malformations, justifying microvascular damage and consequent bleeding that can mimic a cavernous malformation [5]. In patients presenting with acute symptoms requiring urgent or emergent intervention, the diagnosis of intracranial melanoma may be missed when solely using imaging.
Considering the current literature, this is the fifth case of a primary malignant melanoma mimicking a cavernoma (Table 1) [6–8].

However, our case is the first to describe these findings in the supratentorial space. Diagnosis based on brain imaging may be further complicated in the presence of recent bleeding, making primary malignant melanomas and cavernomas radiologically indistinguishable, and thus postponing tailored therapy. Metastatic melanoma has a median survival time of 113 days. In the presented case, the patient was treated for Crohn’s disease with adalimumab, and according to recent studies, there is a plausible connection between IBD therapies and development of melanoma, especially in patients treated with anti-TNF alpha biologics [3]. These therapies compromise the immune surveillance, leading to an increased susceptibility to oncogenic retrovirus infections and to oncogene activation, increasing the risk of melanoma onset. There are no studies in literature mentioning an association between adalimumab and the onset of intracranial melanomas.
Nevertheless, Kouklakis et al. [9,10] reported the onset of two primary malignant melanomas following adalimumab therapy in two patients without any risk factors. Moreover, Schaff et al. [11] presented the case of a young male patient with a diagnosis of neurocutaneous melanosis and IBD that has developed leptomeningeal melanoma after adalimumab therapy.
The use of TNF-alpha inhibitors, indeed, promotes the extinguishing of several inflammatory diseases, causing at the same time an increased risk of cancer reactivation. This condition can also promote the ability of immunogenic tumors, such as melanoma, leading to the suppression of immune system and subsequent metastasizing. Patients with IBD, indeed, showed a clear immune dysfunction that can justify an alteration in tumor surveillance, leading to a higher risk of melanoma onset. According to Singh et al. [12], beyond the use of immunomodulator, a 37% increased risk of melanoma is strictly associated to IBD. Finally, the relationship with primary intracranial melanoma is still unknown, requiring a larger patients’ cohort and randomized trials.
Biologic therapy, which induces a systemic modification of microvascular circulation, may result in a chronic inflammatory state, and promote intracranial bleeding that may have the appearance of a cavernous malformation. In these cases, where imaging can mislead a correct diagnosis, histopathology is diagnostic, and guides tailored treatment planning.
The presence of intracerebral melanomas in IBD patients, although rare, is a possible occasional finding in clinical and surgical practice. Differential diagnosis between intracerebral malignant melanoma and cavernoma may be challenging, considering the possible coexistence of these pathologies in the context of IBD. Patients with a long history of IBD may require a more careful and multidisciplinary approach to exclude the possible presence of additional intracranial lesions.
Authors’ Contributions: Conception, diagnosis and design: Gianluca Scalia, Vishal Parmar, Salvatore Marrone, Roberta Costanzo; Manuscript preparation, technical revision, manuscript editing and revision: Gianluca Scalia, Vishal Parmar, Roberta Costanzo, Giovanni Federico Nicoletti, Giuseppe Emmanuele Umana, Domenico Gerardo Iacopino; Literature search: Gianluca Scalia, Roberta Costanzo; Final approval of manuscript: Giuseppe Emmanuele Umana, Gianluca Scalia.
Consent for Publication: Written informed consent was obtained from the patient for publication of this case report and accompanying images.
Ethics Approval and Consent to Participate: Not applicable.
Funding Statement: No funding was received for this research.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. Ghosh, S. S., Wang, J., Yannie, P. J., Ghosh, S. (2020). Intestinal barrier dysfunction, LPS translocation, and disease development. Journal of the Endocrine Society, 4(2), bvz039. DOI 10.1210/jendso/bvz039. [Google Scholar] [CrossRef]
2. Rankin, G. B. (1990). Extraintestinal and systemic manifestations of inflammatory bowel disease. Medical Clinics of North America, 74(1), 39–50. DOI 10.1016/S0025-7125(16)30585-5. [Google Scholar] [CrossRef]
3. Garge, S. S., Vyas, P. D., Modi, P. D., Ghatge, S. (2014). Crohns disease with central nervous system vasculitis causing subarachnoid hemorrhage due to aneurysm and cerebral ischemic stroke. Annals of Indian Acadademy of Neurology, 17(4), 444–447. DOI 10.4103/0972-2327.144035. [Google Scholar] [CrossRef]
4. Polster, S. P., Sharma, A., Tanes, C., Tang, A. T., Mericko, P. et al. (2020). Permissive microbiome characterizes human subjects with a neurovascular disease cavernous angioma. Nature Communications, 11(1), 2659. DOI 10.1038/s41467-020-16436-w. [Google Scholar] [CrossRef]
5. Tang, A. T., Choi, J. P., Kotzin, J. J., Yang, Y., Hong, C. C. et al. (2017). Endothelial TLR4 and the microbiome drive cerebral cavernous malformations. Nature, 545(7654), 305–310. DOI 10.1038/nature22075. [Google Scholar] [CrossRef]
6. Lu, A. Y., Patel, A. R., Kuzmik, G. A., Atsina, K. K., Bronen, R. A. et al. (2014). Brainstem melanomas presenting as a cavernous malformation. Neurochirurgie, 60(4), 184–187. DOI 10.1016/j.neuchi.2014.02.005. [Google Scholar] [CrossRef]
7. Naeem, A., Mubarak, F. (2019). Primary malignant melanoma of brainstem medulla mimicking as cavernoma. International Neuropsychiatric Disease Journal, 12(2), 1–5. DOI 10.9734/indj/2018/v12i230092. [Google Scholar] [CrossRef]
8. Watanabe, M., Nakao, Y., Yamamoto, T., Mori, K., Wada, R. (2008). Intra-axial brainstem malignant melanoma mimicking cavernous angioma. Neurologia Medico-Chirurgica, 48(11), 519–521. DOI 10.2176/nmc.48.519. [Google Scholar] [CrossRef]
9. Kouklakis, G., Efremidou, E. I., Pitiakoudis, M., Liratzopoulos, N., Polychronidis, A. C. (2013). Development of primary malignant melanoma during treatment with a TNF-α antagonist for severe crohn’s disease: A case report and review of the hypothetical association between TNF-α blockers and cancer. Drug Design, Development and Therapy, 7, 195–199. [Google Scholar]
10. Katoulis, A. C., Kanelleas, A., Zambacos, G., Panayiotides, I., Stavrianeas, N. G. (2010). Development of two primary malignant melanomas after treatment with Adalimumab: A case report and review of the possible link between biological therapy with TNF-α antagonists and melanocytic proliferation. Dermatology, 221(1), 9–12. DOI 10.1159/000300136. [Google Scholar] [CrossRef]
11. Schaff, L. R., Marghoob, A., Rosenblum, M. K., Meyer, R., Khakoo, Y. (2019). Malignant transformation of neurocutaneous melanosis (NCM) following immunosuppression. Pediatric Dermatology, 36(4), 497–500. DOI 10.1111/pde.13804. [Google Scholar] [CrossRef]
12. Singh, S., Nagpal, S. J., Murad, M. H., Yadav, S., Kane, S. V. et al. (2013). Inflammatory bowel disease is associated with an increased risk of melanoma: A systematic review and meta-analysis. Clinical Gastroenterology and Hepatology, 12(2), 210–218. DOI 10.1016/j.cgh.2013.04.033. [Google Scholar] [CrossRef]
Cite This Article
 Copyright © 2022 The Author(s). Published by Tech Science Press.
Copyright © 2022 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools