 Open Access
Open Access
ARTICLE
Nimotuzumab Combined with Neoadjuvant or Induction Chemotherapy for Head and Neck Squamous Cell Carcinoma: A Retrospective Study
Department of Otorhinolaryngology Head and Neck Surgery, The Second Affiliated Hospital of Xi’an Jiaotong University, Xi’an, China
* Corresponding Author: Jin Hou. Email:
Oncologie 2022, 24(4), 707-716. https://doi.org/10.32604/oncologie.2022.027023
Received 10 October 2022; Accepted 06 December 2022; Issue published 31 December 2022
Abstract
Objective: To assess the efficacy and toxicity of nimotuzumab combined with neoadjuvant or induction chemotherapy for head and neck squamous cell carcinoma (HNSCC). Methods: Patients received intravenous nimotuzumab (400 mg, weekly for 1–3 weeks) combined with chemotherapy (5-fluorouracil/paclitaxel/docetaxel + nedaplatin/cisplatin for 1–2 cycles), prior to definitive surgical resection, radiotherapy or other treatments. The primary endpoint was the objective response rate (ORR). The secondary endpoints were tumor downstaging, complete response rate (CRR), partial response rate (PRR), disease control rate (DCR), R0 resection rate, pathological complete response (pCR), larynx preservation rate, overall survival (OS), progression-free survival (PFS), and safety. Results: A total of 71 HNSCC patients with T1-4N0-2M0 were enrolled. After neoadjuvant/induction chemotherapy, the ORR in patients with hypopharyngeal and laryngeal cancer was 100% and 76.1%, respectively. The DCR was 100% in both groups. The T downstaging in patients with hypopharyngeal and laryngeal cancer was 64.0% and 50.0%, the N downstaging was 28.0% and 2.2% (p = 0.001), respectively. At the early stage and locally advanced stage, the T downstaging was 66.7% and 50.0%, the N downstaging was 0% and 16.0% (p = 0.128), respectively. The R0 resection rate and pCR in 39 patients receiving surgery were 94.9% and 20.5%, respectively. The larynx preservation rate was 73.2%. The median PFS was 29.2 months in patients with laryngeal cancer. A mild rash occurred in a single patient and no grade 4 adverse events were encountered. Conclusion: Nimotuzumab combined with neoadjuvant or induction chemotherapy achieved similar short-term efficacy and less adverse events compared with previous studies. The N downstaging rate in patients with hypopharyngeal cancer was significantly higher compared with patients with laryngeal cancer.Keywords
About 745,000 new cases and 365,000 new deaths from head and neck carcinomas were described worldwide in 2020. Among them, 269,000 new deaths were reported from hypopharyngeal cancers and 138,000 deaths were reported from laryngeal cancer [1]. Head and neck carcinomas rank sixth in frequency, with more than 60% of patients presenting at stage III or IV [2]. Hypopharyngeal and laryngeal cancer patients are often diagnosed at an advanced stage, while patients at earlier stages benefit from surgery or radiotherapy alone. Locally advanced patients require total laryngectomy followed by radiotherapy or chemoradiotherapy [3–6], especially for squamous cell cancer [7,8]. Chemotherapy or induction chemotherapy before surgery is an alternative to a total laryngectomy, achieving better larynx preservation rates without affecting the patients’ survival [9,10]. Induction chemotherapy can significantly improve the patient’s survival and laryngeal preservation, with the TPF regimen (docetaxel, carboplatin, and fluorouracil) increasing the rate of laryngeal preservation by more than 70% [11–14].
The epidermal growth factor receptor (EGFR) is a transmembrane glycoprotein of the Erb-B family that regulates various cellular functions, including apoptosis, angiogenesis, cell proliferation, migration, and invasion. EGFR overexpression is widely observed in various cancer cells, including head and neck cancer, breast cancer, and cervical cancer [15–18]. Nimotuzumab is a humanized monoclonal antibody against EGFR, inhibiting the proliferation of tumor cells and promoting apoptosis. Nimotuzumab has been marketed in more than 20 countries for the treatment of advanced head and neck squamous cell cancer (HNSCC), nasopharyngeal carcinoma, glioma, and locally advanced esophageal cancer. Treatment combinations with nimotuzumab are safe and provide a therapeutic option for locally advanced nasopharyngeal carcinoma and HNSCC [19–24].
A few studies investigated nimotuzumab plus induction chemotherapy or concurrent chemoradiotherapy in patients with hypopharyngeal and laryngeal cancer. This study assessed the efficacy and toxicity of nimotuzumab combined with neoadjuvant or induction chemotherapy for HNSCC, especially in patients with hypopharyngeal and laryngeal cancer.
We retrospectively analyzed patients treated with nimotuzumab plus neoadjuvant or induction chemotherapy at the Second Affiliated Hospital of Xi’an Jiaotong University (China) from November 29, 2018 to April 13, 2021. The lesions were histologically confirmed as hypopharyngeal and laryngeal cancer. We collected different types of data, including demographic information, primary tumor location, clinical staging, and the Karnofsky performance status (KPS) score. Efficacy was assessed according to the Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 criteria and while adverse events (AEs) were graded according to Common Terminology Criteria for Adverse Events (CTCAE) 4.0. The study was approved by the Ethics Committee of the Second Affiliated Hospital of Xi’an Jiaotong University (reference: 2021229) and conducted in accordance with the Declaration of Helsinki. Informed consent was obtained from all participants prior to enrollment.
HNSCC patients received intravenous nimotuzumab (400 mg, weekly for 1–3 weeks; Biotech Pharmaceuticals Co., Ltd., Beijing, China) combined with chemotherapy (5-fluorouracil/paclitaxel/docetaxel + nedaplatin/cisplatin for 1–2 cycles) (5-fluorouracil: Shanghai Xudong Haipu Pharmaceutical Co., Ltd., Shanghai, China; Paclitaxel: Nanjing Sike Medicine Industry Co., Ltd., Nanjing, China; Docetaxel: Jiangsu Aosaikang Pharmaceutical Co., Ltd., Nanjing, China; Nedaplatin: Jiangsu Aosaikang Pharmaceutical Co., Ltd., Nanjing, China; Cisplatin: QILU Pharmaceutical Co., Ltd., Jinan, China) prior to definitive surgical resection, radiotherapy or other treatments. The primary endpoint was the tumor objective response rate (ORR). The secondary endpoints consisted of tumor down-staging, complete response rate (CRR), partial response rate (PRR), disease control rate (DCR), R0 resection rate, pathological complete response (pCR), larynx preservation rate, overall survival (OS), progression-free survival (PFS), and safety.
Data were analyzed with SPSS 21.0 (IBM, Chicago, Illinois, USA). Continuous variables are described as mean ± standard deviation (SD). Categorical variables are described using either percentages or frequencies. Quantitative variables were compared by using the t-test while qualtitative variables were compared by using the chi-squared test. Survival curves were plotted using the Kaplan-Meier method with a two-sided log-rank test (significance level of 5%). A p value < 0.05 was considered statistically significant.
A total of 71 HNSCC patients with T1-4N0-2M0 were enrolled. There were 25 patients (35.2%) diagnosed with hypopharyngeal carcinoma and 46 patients (64.8%) diagnosed with laryngeal carcinoma. Twenty-one patients (29.6%) were diagnosed at an early stage (T1NanyM0 + T2N0M0) and 50 patients (70.4%) were diagnosed at a locally advanced stage, according to the American Joint Committee on Cancer (AJCC, 8th edition). Data including age, sex, weight, KPS, concomitant diseases, smoking, and alcohol habits were collected at the baseline (Table 1).

The ORR in patients at the early stage (I + II) and locally advanced stage (III + IV) was 95.2% (20/21) and 80.0% (40/50), respectively. When patients were grouped by primary tumor location, the ORR was 100% (25/25) and 76.1% (35/46) in the hypopharyngeal group and laryngeal group, respectively. The CRR, PRR, and DCR in patients at the early stage were 28.6% (6/21), 66.7% (14/21), and 100% (21/21), respectively. The CRR, PRR, and DCR in patients at the locally advanced stage were 12.0% (6/50), 68.0% (34/50), and 100% (50/50) (all p > 0.05) The CRR, PRR, and DCR in the hypopharyngeal group were 20% (5/25), 80.0% (20/25), and 100% (25/25), respectively. The CRR, PRR, and DCR in the laryngeal group were 15.2% (7/46), 60.9% (28/64), and 100% (25/25), respectively (all p > 0.05) (Table 2).

Following the study treatment, several patients underwent surgery (n = 39), radiotherapy (n = 24), traditional Chinese medicine (n = 2), and chemotherapy (n = 1). The R0 resection rate, pCR of 39 patients receiving sequential surgery were 94.9% (37/39), 20.5% (8/39), respectively. The larynx preservation rate was 73.2% (52/71), including 20 patients with larynx preservation surgery and 32 patients without surgery. Five patients did not perform any sequential therapy: 40% (2/5) reported a complete response, 40% (2/5) reported a partial response (Table 2).
The T downstaging in patients with hypopharyngeal and laryngeal cancer was 64.0% (16/65) and 50.0% (23/46), the N downstaging was 28.0% (7/25) and 2.2% (1/46) (p = 0.001), respectively. In patients at the early stage and locally advanced stage, the T downstaging was 66.7% (14/21) and 50.0% (25/50), the N downstaging was 0% (0/21) and 16.0% (8/50) (p = 0.128), respectively (Table 3).

The median PFS was 29.2 months in the patients with laryngeal cancer (Fig. 1). The OS was not achieved in both groups (Fig. 2). In patients at the early stage, death and disease progression were 4.8% (1/21) and 14.3% (3/21), respectively. In patients at the locally advanced stage, death and disease progression were 12% (6/50) and 28% (14/50), respectively. In patients with hypopharyngeal cancer, death and disease progression were 12% (3/25) and 24% (6/25), respectively. In patients with laryngeal cancer, death and disease progression were 8.7% (4/46) and 23.9% (11/46), respectively (Fig. 3).

Figure 1: Kaplan-Meier estimate of PFS for patients in the hypopharynx and larynx cancer group

Figure 2: Kaplan-Meier estimate of OS for patients in the hypopharynx and larynx cancer group

Figure 3: The OS and PFS events for patients in the early stage and local advanced stage group
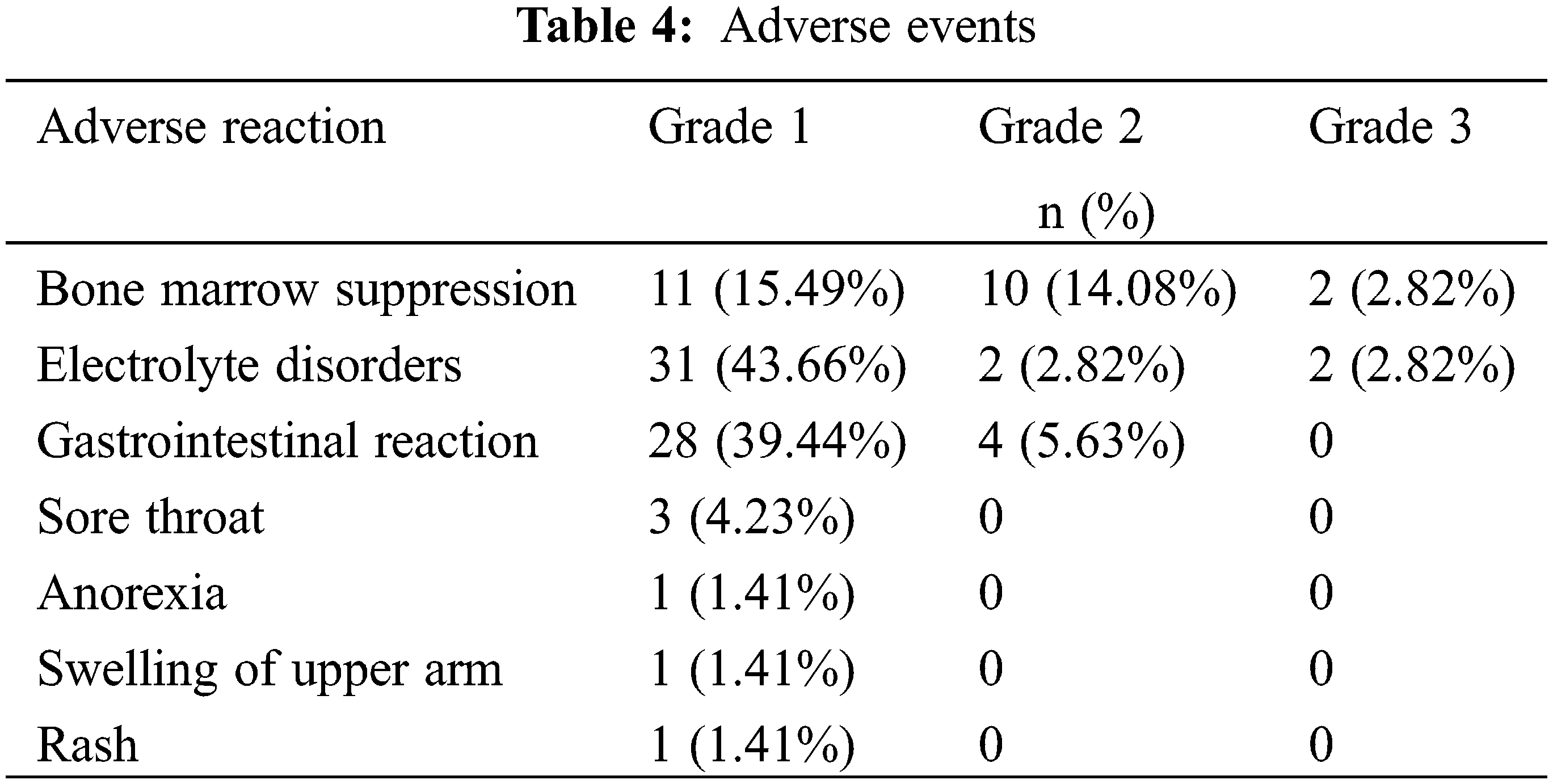
A total of 67 patients reported mild-to-moderate adverse events. The most common adverse events were bone marrow suppression (29.6%), electrolyte disorders (46.5%), and gastrointestinal reaction (45.1%). Grade 3 adverse events were documented, including bone marrow suppression (2.82%) and electrolyte disorders (2.82%). No grade 4 adverse events were encountered (Table 4).
In this study, we administered nimotuzumab combined with chemotherapy in patients with hypopharyngeal or laryngeal cancer. The ORR in hypopharyngeal cancer vs. laryngeal cancer was 100% vs. 76.1%, at the early stage (I + II) group and local advanced stage (III + IV) was 95.2% vs. 80.0%, including 12 cases of complete response and 48 cases of partial response. Overall, 39 patients achieved a T downstaging and 8 patients achieved an N downstaging. The N downstaging was significantly different between hypopharyngeal and laryngeal cancer (28.0% vs. 2.2%, p = 0.001). No grade 4 adverse events occurred during the study. The TPF regimen (docetaxel, cisplatin, and 5-fluorouracil) is the standard induction chemotherapy regimen in locally advanced HNSCC. A phase III study compared the efficacy of TPF vs. PF (cisplatin and 5-fluorouracil) regimens in HNSCC patients, reporting that the ORR was 72% vs. 64% (p = 0.07) and the CRR was 17% vs. 15%. The incidence of grade 3 or 4 hematologic adverse events was high, including neutropenia (83% vs. 56%), anemia (12% vs. 9%) and thrombocytopenia (4% vs. 11%). The incidence of grades 3–4 non-hematologic toxicities was 65% in the TPF group and 62% in the PF group [25]. A retrospective analysis showed that the ORR was significantly increased (83%) in locally advanced or metastatic HNSCC patients receiving a modified TPF (cisplatin, 5-fluorouracil, docetaxel, and leucovorin). Two deaths (4%) occurred during chemotherapy and 10 patients (21%) discontinued the treatment due to adverse events. Febrile neutropenia and grades 3–4 diarrhea occurred during the first cycle of treatment in 2 patients (4%) and 3 patients (6%), respectively [26]. Komatsu et al. [27] retrospectively assessed the efficacy and safety of concurrent chemoradiotherapy with TPF in patients with locally advanced HNSCC, showing that the ORR was 98.6% and 96.4% in patients with primary tumors located in the larynx and hypopharynx, respectively. The most common adverse reaction was neutropenia. Grades 3–4 AEs occurred in 65.0% of patients. Grade 3 dermatitis and mucositis occurring in 60.0% of patients. Compared with previous TPF and PF regimens, the combination of nimotuzumab with induction chemotherapy achieved similar ORR scores without increasing the chemotherapeutic toxicity.
As previously demonstrated, nimotuzumab combined with radiotherapy, chemotherapy, or chemoradiotherapy significantly prolonged the survival of HNSCC patients. Rodríguez et al. [28] assessed the efficacy of nimotuzumab plus radiotherapy in 106 advanced HNSCC patients, showing that nimotuzumab significantly increased CRR (59.5% vs. 34.2%, p = 0.028) and mOS (12.50 months vs. 9.47 months, p = 0.0491). Reddy et al. [22]. found that nimotuzumab with concurrent chemoradiotherapy or radiotherapy was effective in patients with inoperable advanced HNSCC. The ORR was significantly higher in patients treated with nimotuzumab than those treated with chemoradiotherapy or radiotherapy alone (100% vs. 70%, p = 0.020; 76.47% vs. 36.84%, p = 0.023). During the 5-year follow-up, receiving chemoradiotherapy or radiotherapy plus nimotuzumab was associated with a higher OS (49.38 months vs. 16.36 months, p = 0.012) in the patients and reduced the death risk by 48%. Similar findings have been observed in other studies combining nimotuzumab with cisplatin and radiotherapy [24,29].
The studies investigating nimotuzumab combined with induction chemotherapy or chemoradiotherapy in laryngeal and hypopharyngeal cancer are limited. A single-center study in China showed the ORR was significantly increased in patients with unresectable locally advanced hypopharyngeal cancer treated with nimotuzumab plus induction chemotherapy compared with patients treated with chemotherapy alone (91.7% vs. 58.3%, p = 0.029) [30]. The OS at two years was 62.5% vs. 51.8% (p < 0.05), and the PFS at two years was 23 months vs. 18 months, (p < 0.05). Wang et al. assessed the efficacy of nimotuzumab combined with TPF regimen for locally advanced HNSCC [31]. They showed that the PFS and OS rates at two years were 71.2% and 78.3%, respectively. The ORR was 87.1%. Rash and treatment-related deaths were not reported. Zhang et al. [32] found that an EGFR inhibitor combined with non-surgical therapy (induction chemotherapy with the TP regimen, concurrent chemoradiotherapy or concurrent radiotherapy) increased the laryngeal preservation rate in patients with hypopharyngeal carcinoma. The OS and laryngeal preservation rates at three years were 68.9% and 86.7%, respectively. In our study, the ORR in patients with hypopharyngeal and laryngeal cancer was satisfactory.
In conclusion, nimotuzumab plus chemotherapy achieved similar ORRs and downstaging rates compared with previous studies. However, this study is also affected by limitations. First, this is a single-center retrospective study with a relatively small sample size. Second, the long-term survival benefit was only simply analyzed because of short of OS and PFS events. The continued follow-up or an RCT study with a larger sample size focusing on long-term survival is needed.
Nimotuzumab combined with neoadjuvant or induction chemotherapy achieved similar short-term efficacy and less adverse events compared with previous studies. The N downstaging rate in patients with hypopharyngeal cancer was significantly higher compared with patients with laryngeal cancer.
Acknowledgement: The authors would like to thank TopEdit (www.topeditsci.com) for its linguistic assistance during the preparation of this manuscript.
Authorship Contribution Statement: The authors confirm contribution to the paper as follows: study conception and design: Jin Hou, Huihui Zhang. Xiaoyong Ren; data collection: Huihui,Zhang, Jing Yan, Yan Yan, Yangyang Jia, Zhihui Li; analysis and interpretation of results: Jing Yan, Ying Sheng, Zhenghui Wang, Jianmin Liang; draft manuscript preparation: Huihui Zhang, Jin Hou. All authors reviewed the results and approved the final version of the manuscript.
Ethics Approval and Informed Consent Statement: This study was approved by the Ethics Committee of the Second Affiliated Hospital of Xi’an Jiaotong University (reference: 2021229). Informed consent was obtained from all participants prior to enrollment.
Availability of Data and Materials: To gain date access, researchers need to email to corresponding author for obtaining individual participant data that underline the results reported in this article after deidentification.
Funding Statement: Youth Program of Natural Science Foundation of Shaanxi Province, Fund No. 2021-JQ-419.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I. et al. (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians, 71(3), 209–249. DOI 10.3322/caac.21660. [Google Scholar] [CrossRef]
2. Braakhuis, B. J., Brakenhoff, R. H., Leemans, C. R. (2012). Treatment choice for locally advanced head and neck cancers on the basis of risk factors: Biological risk factors. Annals Oncology, 23(Suppl 10), x173–x177. DOI 10.1093/annonc/mds299. [Google Scholar] [CrossRef]
3. Pracy, P., Loughran, S., Good, J., Parmar, S., Goranova, R. (2016). Hypopharyngeal cancer: United Kingdom national multidisciplinary guidelines. The Journal of Laryngology & Otology, 130(S2), S104–S110. DOI 10.1017/S0022215116000529. [Google Scholar] [CrossRef]
4. Jones, T. M., De, M., Foran, B., Harrington, K., Mortimore, S. (2016). Laryngeal cancer: United Kingdom national multidisciplinary guidelines. The Journal of Laryngology & Otology, 130(S2), S75–S82. DOI 10.1017/S0022215116000487. [Google Scholar] [CrossRef]
5. Forastiere, A. A., Ismaila, N., Lewin, J. S., Nathan, C. A., Adelstein, D. J. et al. (2018). Use of larynx-preservation strategies in the treatment of laryngeal cancer: American society of clinical oncology clinical practice guideline update. Journal of Clinical Oncology, 36(11), 1143–1169. DOI 10.1200/JCO.2017.75.7385. [Google Scholar] [CrossRef]
6. Ameri, A., Norouzi, S., Sourati, A., Azghandi, S., Novin, K. et al. (2022). Randomized trial on acute toxicities of weekly vs three-weekly cisplatin-based chemoradiation in head and neck cancer. Cancer Reports, 5(1), e1425. DOI 10.1002/cnr2.1425. [Google Scholar] [CrossRef]
7. Adelstein, D. J., Li, Y., Adams, G. L., Wagner, H. Jr., Kish, J. A. et al. (2003). An intergroup phase III comparison of standard radiation therapy and two schedules of concurrent chemoradiotherapy in patients with unresectable squamous cell head and neck cancer. Journal of Clinical Oncology, 21(1), 92–98. DOI 10.1200/JCO.2003.01.008. [Google Scholar] [CrossRef]
8. Lefebvre, J. L., Chevalier, D., Luboinski, B., Kirkpatrick, A., Collette, L. et al. (1996). Larynx preservation in pyriform sinus cancer: Preliminary results of a European organization for research and treatment of cancer phase III trial. EORTC head and neck cancer cooperative group. Journal of the National Cancer Institute, 88(13), 890–899. DOI 10.1093/jnci/88.13.890. [Google Scholar] [CrossRef]
9. Wolf, G. T., Fisher, S. G., Hong, W. K., Hillman, R., Spaulding, M. (1991). Induction chemotherapy plus radiation compared with surgery plus radiation in patients with advanced laryngeal cancer. New England Journal of Medicine, 324(24), 1685–1690. DOI 10.1056/NEJM199106133242402. [Google Scholar] [CrossRef]
10. Forastiere, A. A., Weber, R. S., Trotti, A. (2015). Organ preservation for advanced larynx cancer: Issues and outcomes. Journal of Clinical Oncology, 33(29), 3262–3268. DOI 10.1200/JCO.2015.61.2978. [Google Scholar] [CrossRef]
11. Pignon, J. P., Bourhis, J., Domenge, C., Designé, L. (2000). Chemotherapy added to locoregional treatment for head and neck squamous-cell carcinoma: Three meta-analyses of updated individual data. MACH-NC collaborative group. Meta-analysis of chemotherapy on head and neck cancer. The Lancet, 355(9208), 949–955. DOI 10.1016/S0140-6736(00)90011-4. [Google Scholar] [CrossRef]
12. Pointreau, Y., Garaud, P., Chapet, S., Sire, C., Tuchais, C. et al. (2009). Randomized trial of induction chemotherapy with cisplatin and 5-fluorouracil with or without docetaxel for larynx preservation. Journal of the National Cancer Institute, 101(7), 498–506. DOI 10.1093/jnci/djp007. [Google Scholar] [CrossRef]
13. Lefebvre, J. L., Pointreau, Y., Rolland, F., Alfonsi, M., Baudoux, A. et al. (2013). Induction chemotherapy followed by either chemoradiotherapy or bioradiotherapy for larynx preservation: The TREMPLIN randomized phase II study. Journal of Clinical Oncology, 31(7), 853–859. DOI 10.1200/JCO.2012.42.3988. [Google Scholar] [CrossRef]
14. Janoray, G., Pointreau, Y., Garaud, P., Chapet, S., Alfonsi, M. et al. (2015). Long-term results of a multicenter randomized phase III trial of induction chemotherapy with cisplatin, 5-fluorouracil, ±docetaxel for larynx preservation. Journal of the National Cancer Institute, 108(4), djv368. [Google Scholar]
15. Byeon, H. K., Ku, M., Yang, J. (2019). Beyond EGFR inhibition: Multilateral combat strategies to stop the progression of head and neck cancer. Experimental & Molecular Medicine, 51(1), 1–14. DOI 10.1038/s12276-018-0202-2. [Google Scholar] [CrossRef]
16. Schlessinger, J. (2002). Ligand-induced, receptor-mediated dimerization and activation of EGF receptor. Cell, 110(6), 669–672. DOI 10.1016/S0092-8674(02)00966-2. [Google Scholar] [CrossRef]
17. Normanno, N., de Luca, A., Bianco, C., Strizzi, L., Mancino, M. et al. (2006). Epidermal growth factor receptor (EGFR) signaling in cancer. Gene, 366(1), 2–16. DOI 10.1016/j.gene.2005.10.018. [Google Scholar] [CrossRef]
18. Rajaram, P., Chandra, P., Ticku, S., Pallavi, B. K., Rudresh, K. B. et al. (2017). Epidermal growth factor receptor: Role in human cancer. Indian Journal of Dental Research, 28(6), 687–694. DOI 10.4103/ijdr.IJDR_534_16. [Google Scholar] [CrossRef]
19. Fei, Z., Xu, T., Li, M., Chen, T., Li, L. et al. (2020). Effectiveness and cost-effectiveness analysis of nimotuzumab for the radiotherapy of locoregionally advanced nasopharyngeal carcinoma. Radiation Oncology, 15(1), 230. DOI 10.1186/s13014-020-01674-5. [Google Scholar] [CrossRef]
20. Liang, R., Yang, L., Zhu, X. (2021). Nimotuzumab, an anti-EGFR monoclonal antibody, in the treatment of nasopharyngeal carcinoma. Cancer Control, 28, 1073274821989301. DOI 10.1177/1073274821989301. [Google Scholar] [CrossRef]
21. You, B., Brade, A., Magalhaes, J., Siu, L., Oza, A. et al. (2011). A dose-escalation phase I trial of nimotuzumab, an antibody against the epidermal growth factor receptor, in patients with advanced solid malignancies. Investigational New Drugs, 29(5), 996–1003. DOI 10.1007/s10637-010-9444-0. [Google Scholar] [CrossRef]
22. Reddy, B. K., Lokesh, V., Vidyasagar, M. S., Shenoy, K., Babu, K. G. et al. (2014). Nimotuzumab provides survival benefit to patients with inoperable advanced squamous cell carcinoma of the head and neck: A randomized, open-label, phase IIb, 5-year study in Indian patients. Oral Oncology, 50(5), 498–505. DOI 10.1016/j.oraloncology.2013.11.008. [Google Scholar] [CrossRef]
23. Ang, M. K., Montoya, J. E., Tharavichitkul, E., Lim, C., Tan, T. et al. (2021). Phase II study of nimotuzumab (TheraCim-hR3) concurrent with cisplatin/radiotherapy in patients with locally advanced head and neck squamous cell carcinoma. Head & Neck, 43(5), 1641–1651. DOI 10.1002/hed.26635. [Google Scholar] [CrossRef]
24. Subramanium, S., Balasundaram, V., Nithya, S., Kiran, P. (2015). Nimotuzumab with induction chemotherapy and chemo-radiation in patients with advanced head and neck cancer. Journal of Cancer Therapy, 6(2), 146–152. DOI 10.4236/jct.2015.62016. [Google Scholar] [CrossRef]
25. Posner, M. R., Hershock, D. M., Blajman, C. R., Mickiewicz, E., Winquist, E. et al. (2007). Cisplatin and fluorouracil alone or with docetaxel in head and neck cancer. New England Journal of Medicine, 357(17), 1705–1715. DOI 10.1056/NEJMoa070956. [Google Scholar] [CrossRef]
26. Fayette, J., Fontaine-Delaruelle, C., Ambrun, A., Daveau, C., Poupart, M. et al. (2016). Neoadjuvant modified TPF (docetaxel, cisplatin, fluorouracil) for patients unfit to standard TPF in locally advanced head and neck squamous cell carcinoma: A study of 48 patients. Oncotarget, 7(24), 37297–37304. DOI 10.18632/oncotarget.8934. [Google Scholar] [CrossRef]
27. Komatsu, M., Shiono, O., Taguchi, T., Sakuma, Y., Nishimura, G. et al. (2014). Concurrent chemoradiotherapy with docetaxel, cisplatin and 5-fluorouracil (TPF) in patients with locally advanced squamous cell carcinoma of the head and neck. Japanese Journal of Clinical Oncology, 44(5), 416–421. DOI 10.1093/jjco/hyu026. [Google Scholar] [CrossRef]
28. Rodríguez, M. O., Rivero, T. C., del Castillo Bahi, R., Muchuli, C. R., Bilbao, M. A. et al. (2010). Nimotuzumab plus radiotherapy for unresectable squamous-cell carcinoma of the head and neck. Cancer Biology & Therapy, 9(5), 343–349. DOI 10.4161/cbt.9.5.10981. [Google Scholar] [CrossRef]
29. Patil, V. M., Noronha, V., Joshi, A., Agarwal, J., Ghosh-Laskar, S. et al. (2019). A randomized phase 3 trial comparing nimotuzumab plus cisplatin chemoradiotherapy versus cisplatin chemoradiotherapy alone in locally advanced head and neck cancer. Cancer, 125(18), 3184–3197. DOI 10.1002/cncr.32179. [Google Scholar] [CrossRef]
30. Tian, X., Xuan, Y., Wu, R., Gao, S. (2020). Nimotuzumab combined with induction chemotherapy and concurrent chemoradiotherapy in unresectable locally advanced hypopharyngeal carcinoma: A single institution experience in China. Cancer Management and Research, 12, 3323–3329. DOI 10.2147/CMAR.S248392. [Google Scholar] [CrossRef]
31. Wang, X., Gu, J., Shao, C., Han, K., Meng, J. (2019). Nimotuzumab plus chemotherapy with docetaxel, cisplatin, 5-fluorouracil for locally advanced head and neck squamous cell carcinoma: A clinical study. Journal of Cancer Research and Therapeutics, 15(2), 312–316. [Google Scholar]
32. Zhang, X., Wang, J., Wu, W., Liu, M., Zhao, F. et al. (2014). Efficacy and safety of combined radiotherapy with EGFR inhibitors and chemotherapy for laryngeal organ preservation in patients with locally advanced hypopharyngeal carcinomas. Current Cancer Drug Targets, 14(6), 589–598. DOI 10.2174/1568009614666140716115349. [Google Scholar] [CrossRef]
Cite This Article
 Copyright © 2022 The Author(s). Published by Tech Science Press.
Copyright © 2022 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools