 Open Access
Open Access
ARTICLE
Study on the Value of Ultrasound Elastography Combined with Plasma miRNA Expression in the Early Detection of Breast Cancer
1
Department of Ultrasound, Nanjing First Hospital, Nanjing Medical University, Nanjing, 210006, China
2
Department of Ultrasound, Nanjing Jiangning District Maternal and Child Health and Family Planning Service Center, Nanjing,
211100, China
3
Department of Healthy Examination, Nanjing jiangning Hospital, Nanjing, 211100, China
* Corresponding Author: Pingyang Zhang. Email:
# These authors contribute equally to this work
(This article belongs to the Special Issue: Biomarkers for Breast Cancer Diagnosis and Treatment Selection: from Basic Research to Practice)
Oncologie 2022, 24(4), 717-727. https://doi.org/10.32604/oncologie.2022.026998
Received 08 October 2022; Accepted 21 November 2022; Issue published 31 December 2022
Abstract
Background: Breast cancer (BC) is the most common malignant tumor in women, and its morbidity and mortality are increasing each year, due to the lack of specific clinical symptoms in the early stage of BC, and the lack of diagnostic methods for early breast cancer. Therefore, identifying an effective diagnostic method for early BC has become urgent. Materials and Methods: Breast lesions with a histological diagnosis that were examined by ultrasonic elastography (UE) in our department from June 2020 to December 2021 were reviewed. qRT-PCR was performed to measure the expression levels of miR-144-5p and miR-26b-5p in the plasma of patients with BC. The receiver operating characteristics (ROC) curve and area under the curve (AUC) were used to investigate the potential diagnostic value of miR- 144-5p, miR-26b-5p and the elastographic score in BC. Results: The ultrasonic elastography score(UES) was found to be significantly upregulated in BC compared with that in benign breast lesions, and the AUC, sensitivity and specificity were 0.809, 0.717 and 0.806 for distinguishing BC from benign breast lesions, respectively. miR-144-5p and miR-26b- 5p were found to be upregulated in the plasma of BC patients, and miR-144-5p+miR-26b-5p had 0.781 sensitivity and 0.780 specificity for the diagnosis of BC. Furthermore, we found that the diagnostic performance of miR-144-5p and miR-26b-5p combined with UES for BC had 0.913 sensitivity and 0.890 specificity. Conclusions: The combination of plasma miR-144-5p, miR-26b-5p and UES has a very high clinical application value for the early detection of BC.Keywords
Breast cancer (BC) is one of the most common malignancies and is the leading cause of cancer-associated mortality in women worldwide [1,2]. BC is classified into five subtypes according to its histology and molecular characteristics (i.e., luminal-A and B, normal-like(normal), triple negative and Her-2 positive), which promote the assessment of its prognosis and the adoption of effective treatment strategies [3].
Although remarkable progress in treating BC has been achieved in recent decades, the rate of 5-year overall survival (OS) rate has not improved significantly. Therefore, identifying an effective diagnostic method for early BC has become urgent.
The concept of ultrasonic elastography (UE) was first proposed by Ophir et al. in 1991 [4]. It is an ultrasonic technology used to evaluate the hardness and elasticity of biological tissues. Recently, an increasing number of studies have reported that UE is highly sensitive and specific for differentiating benign and malignant tumors and it is a potential effective method for diagnosing BC [5,6].
MicroRNAs (miRNAs), a class of functional noncoding RNAs of approximately 18–25 nucleotides (nt), have been reported to be involved in the initiation and progression of several types of cancers [7–9]. In recent years, miRNAs have emerged as effective biomarkers for the early detection of some malignancies. For example, Huang et al. [10] reported that plasma miRNA-92a and miR-29a are dysregulated in colorectal cancer and could act as effective biomarkers for the early diagnosis of colorectal cancer. Zhao et al. [11] used a miRNA screening analysis of plasma samples from patients with BC, and miRNA-19b, miR-16 and miR-106a were identified as diagnostic biomarkers of BC.
In this study, we comprehensively evaluated the value of UE and plasma miRNAs in patients with BC, aiming to identify a new and effective method for the early detection of BC.
The subjects of this study were 82 patients with breast lesions who were hospitalized in Nanjing First Hospital from June 2020 to December 2021. The mean age of these patients was 46 ± 8.8 years (range from 21 to 68 years). Patients with a breast mass of less than 3 cm in diameter were recruited for this study. All masses of these patients were diagnosed as BI-RADS 4 by conventional ultrasound through the US Breast Imaging Reporting and Data System (BI-RADS) criteria [12]. None of the patients underwent puncture biopsy, breast-related surgery, radiotherapy or chemotherapy before receiving an ultrasound examination, and all had pathological results available from breast biopsy or surgically removed tissue. Blood samples were collected from these patients and 30 healthy controls who had no history of any type of tumor. Written informed consent was obtained from all of the participants in this study.
Samsung WS80A ultrasonic equipment (Samsung Medison, Seoul, Korea) was used for ultrasonic elastic imaging in this study, and the probe frequency was 9~13.5. The patients were lying in the supine or lateral position during the exam.
The ultrasound examination was completed by two experienced ultrasound experts. Before ultrasonic elastography, the breast of the subject was scanned comprehensively with conventional ultrasound, the target lesion was observed in multiple sections, and its location, shape, size and internal echo characteristics were recorded. After routine scanning was completed, the elastic imaging program was applied, including the lesion and surrounding tissues in the elastic imaging sampling frame. The sampling frame area was made larger than the lesion area by adjusting the ultrasonic section and the size of the sampling frame. In this study, the elasticity of the breast masses was scored with a 5-point method. Most or the whole lesion was green, 1 point; the periphery of the lesion was green and the center was blue, 2 points; the red and green colors had similar proportions, 3 points; the whole lesion was blue or accompanied by a small amount of green, 4 points; the lesion and surrounding tissue were blue, or accompanied by a small amount of green, 5 points. Among them, breast lesions with 1–3 points were categorized as benign, while breast lesions with 4–5 points were categorized as malignant.
2.3 Blood Collection and RNA Extraction
Blood samples (5 ml) from 82 breast lesions and 30 healthy controls were obtained by using ethylene diamine tetraacetonitrile acid (EDTA)-treated blood collection tubes. The blood samples were centrifuged at 1500 × g for 5 min at 4°C, and the supernatant was then transferred into RNA-free EP tubes and stored at −80°C for further analysis. Total miRNA was extracted by using a miRcute Serum/Plasma miRNA Isolation Kit (TIANGEN, Beijing, China) according to the manufacturer’s instructions.
Total plasma RNA was employed to prepare miRNA first-strand cDNA using a miRcute plus miRNA first-strand cDNA kit following the manufacturer’s instructions. A miRcute plus miRNA qPCR kit was used for qPCR analysis. Cel-miR-39 was used as an external control for normalization, and the results were analyzed using the 2−ΔΔCt method.
All statistical analyses were conducted by using SPSS 25.0, and the data are presented as the mean ± SD. An independent samples Mann–Whitney U test and t test were performed accordingly. The receiver operating characteristics (ROC) and area under the curve (AUC) were used to assess the potential role of miR-144-5p, miR-26b-5p and the elastographic score as diagnostic markers, and p < 0.05 was considered statistically significant.
3.1 Pathological Results of the Breast Lesions
A total of 82 patients with breast lesions were included in this study, all of whom were women. According to the pathological results, Table 1 shows that among the 82 breast lesions, 36 were benign masses, consisting of 22 fibromas, 10 intraductal papillomas, and 4 cysts. There were 46 cases of malignant tumors, including 22 cases of noninvasive carcinoma, 13 cases of early invasive carcinoma, and 11 cases of invasive carcinoma.
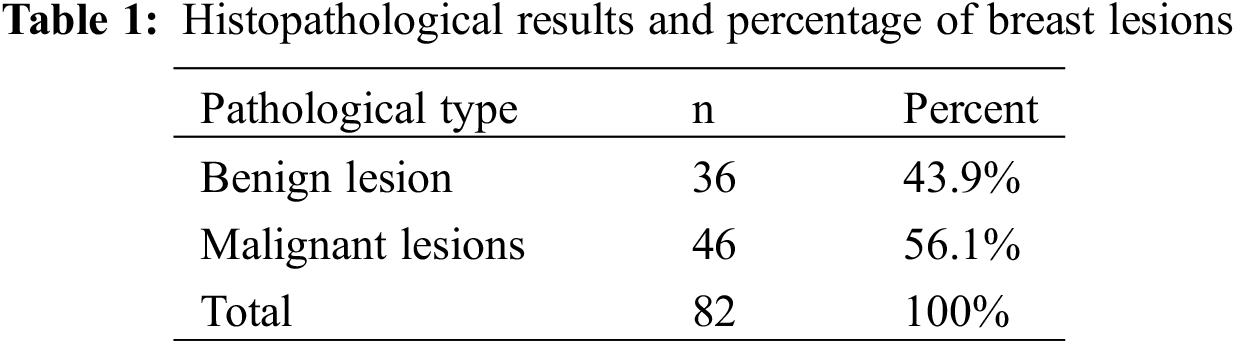
3.2 Ultrasonic Elastography Score
The elasticity score of the 82 breast lesions is shown in Fig. 1: 10 patients had an elastographic score of 2, 32 patients had an elastographic score of 3, 36 patients had an elastographic score of 4, and 4 patients had an elastographic score of 5.
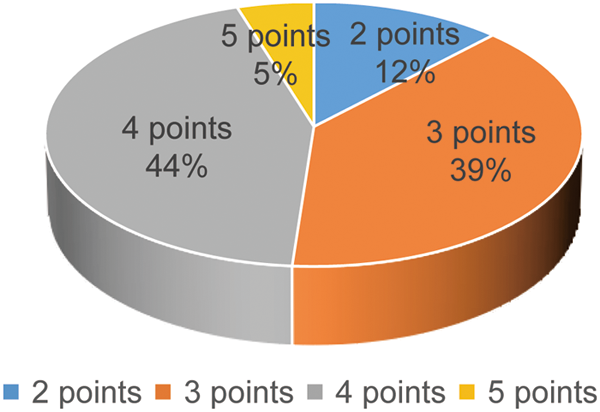
Figure 1: Elasticity score distribution of breast lesions
3.3 The Value of Ultrasonic Elastography Score in the Diagnosis of BC
Our study showed that the average value of the ultrasonic elastography score (UES) of benign breast lesions was 2.91 ± 0.69, and the average value of the UES of malignant breast lesions was 3.80 ± 0.58. The difference between the two groups was statistically significant (Table 2). Representative images of ultrasonic elastography of benign and malignant breast lesions are shown in Fig. 2. To further explore the value of UES in the diagnosis of BC, ROC curve analysis was employed and showed that the AUC was 0.809 (95% CI: 0.714–0.904) (p < 0.001), with a sensitivity of 0.717 and specificity of 0.806 (Fig. 3), indicating that UES has significant clinical significance in the differentiation of benign and malignant breast lesions.
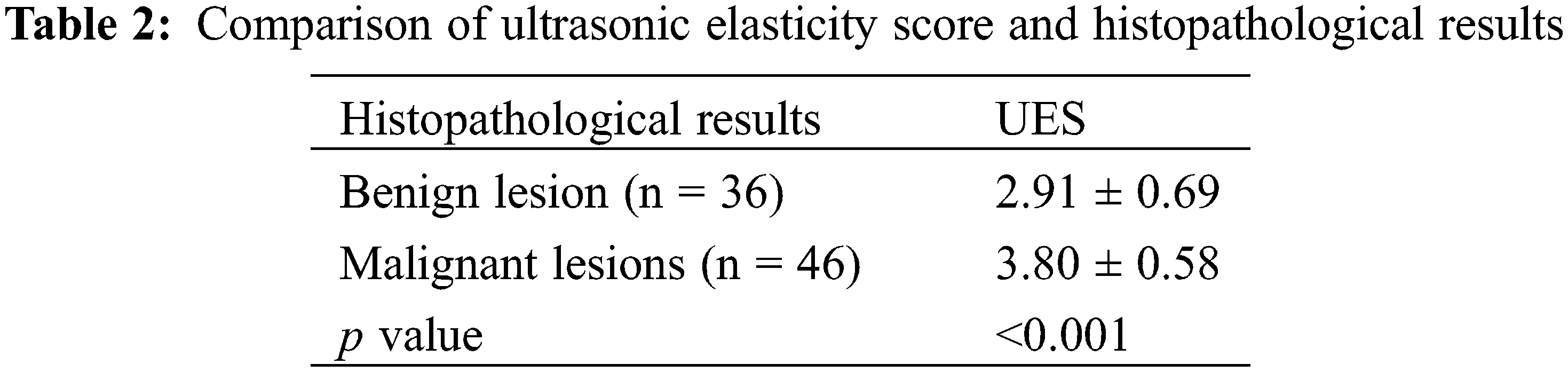
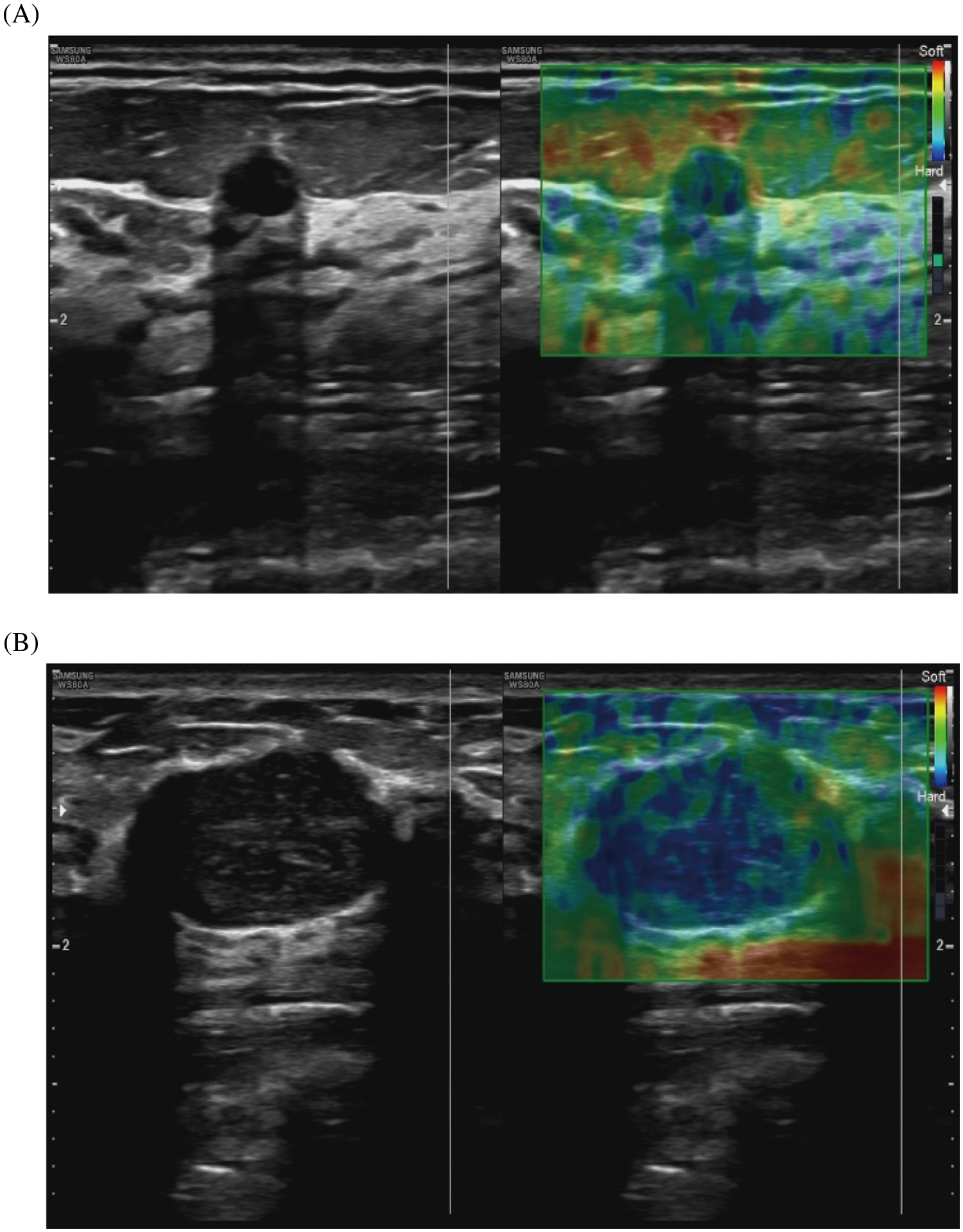
Figure 2: Representative images of ultrasonic elastography of benign and malignant breast lesions. (A) This breast lesion scores 2 points with the 5-point method: (left) the image of conventional ultrasound, (right) the image of ultrasonic elastography. (B) This breast lesion scores 4 points with the 5-point method: (left) the image of conventional ultrasound, (right) the image of ultrasonic elastography
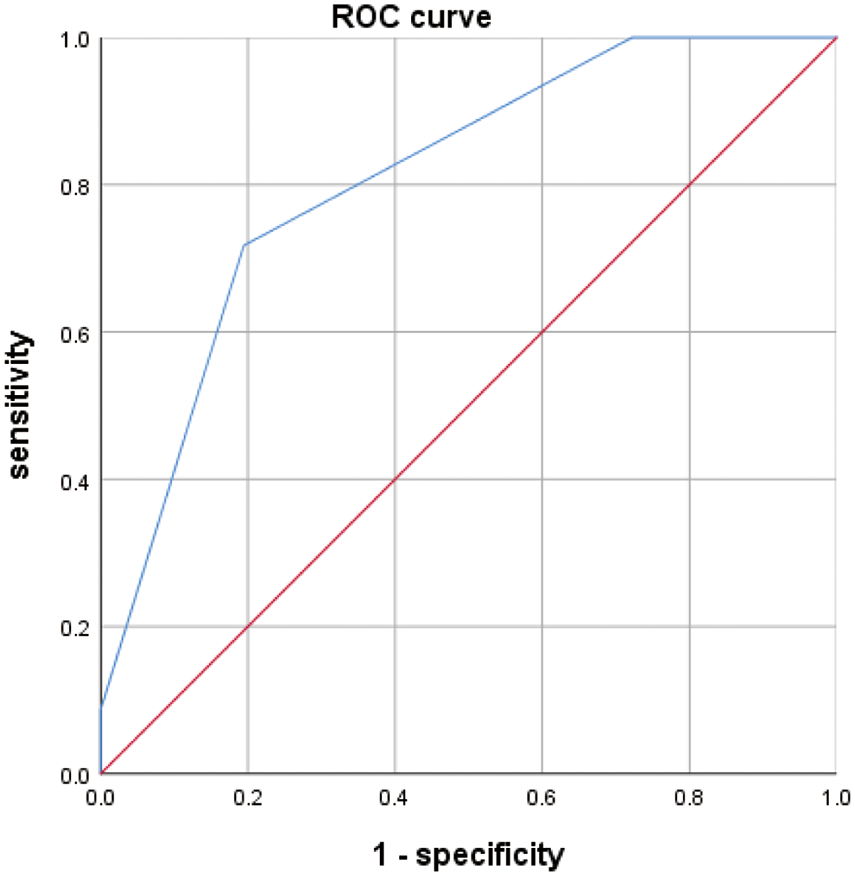
Figure 3: ROC curve analysis results for UES
3.4 Expression Profiling of miRNAs in Whole Blood Samples of BC and Healthy Controls
A miRNA microarray of blood samples from 6 BC patients and 6 healthy controls was downloaded from the GEO database (GSE83270) and identified 171 differentially expressed miRNAs in BC patients by using a fold change (FC) ≥2 and p < 0.05 as the threshold. The heatmap shown in Fig. 4 consists of 8 upregulated miRNAs and 2 downregulated miRNAs with the most obvious differences. The expression levels of these 10 abnormally expressed miRNAs (let-7, g-5p, miR-20b-5p, miR-26b-5p, miR-32-5p, miR-144-5p, miR-190a-5p, miR-204-5p, miR-211-5p, miR-301a-3p, and miR-454-3p) in the peripheral blood of BC and healthy controls are shown in Fig. 5, among which miR-144-5p and miR-26b-5p, with the most obvious difference, were selected for further study.
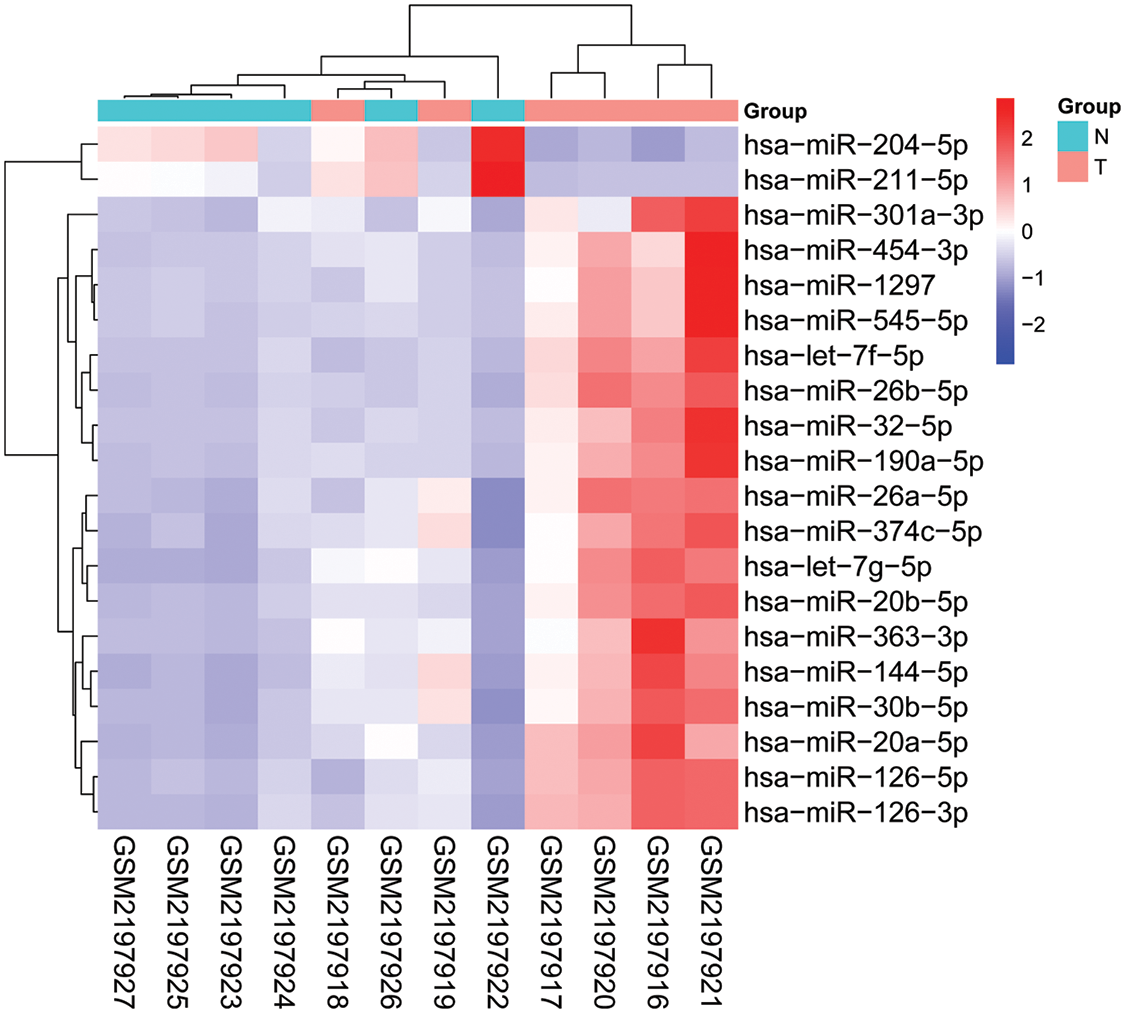
Figure 4: Heatmap of 10 differentially expressed blood miRNAs in BC patients (n = 6) and healthy controls (n = 6) from GSE83270
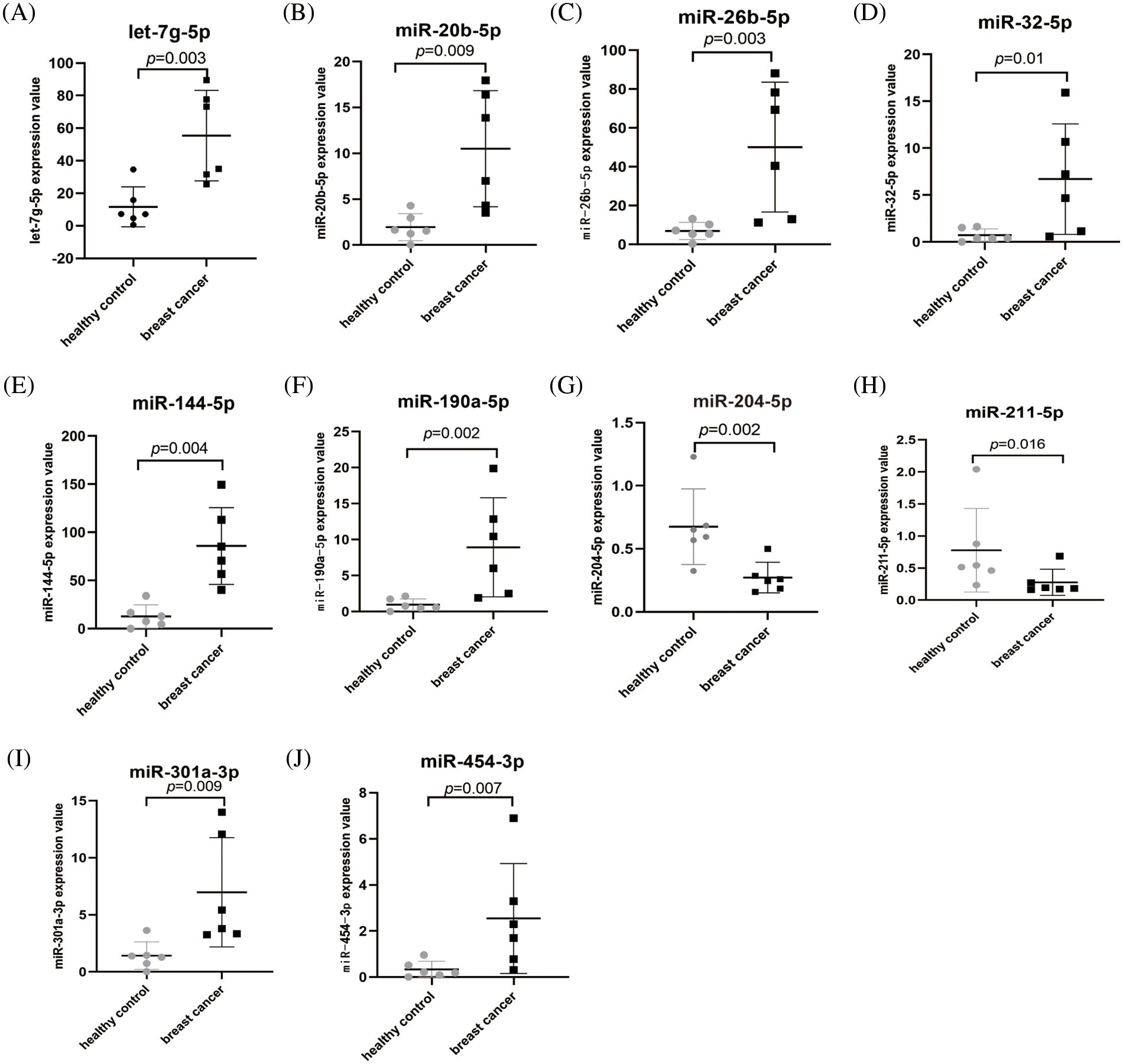
Figure 5: The expression levels of the 10 miRNAs in BC patients (n = 6) and healthy controls (n = 6) from GSE83270. (A) let-7g-5p, (B) miR-20b-5p, (C) miR-26b-5p, (D) miR-32-5p, (E) miR-144-5p, (F) miR-190a-5p, (G) miR-204-5p, (H) miR-211-5p, (I) miR-301a-3p, (J) miR-454-3p
3.5 Expression Levels of miR-144-5p and miR-26b-5p in the Plasma of Participants
To further explore the clinical value of miR-144-5p and miR-26b-5p in the diagnosis of BC, we detected the expression levels of miR-144-5p and miR-26b-5p in the plasma of 82 patients with breast lesions and 30 healthy controls. The results showed that the expression levels of miR-144-5p in the plasma of the BC group, breast benign lesion group and healthy control group were 2.98 ± 1.80, 1.44 ± 0.75, and 1.20 ± 0.59, respectively, and the expression levels of miR-26b-5p in the plasma of the BC group, benign breast lesion group and healthy control group were 2.52 ± 1.59, 1.28 ± 0.63, and 1.12 ± 0.39, respectively. The plasma miR-144-5p and miR-26b-5p in the BC group were significantly upregulated compared with those in the benign breast lesion groups, while there were no significant differences between the benign breast lesion group and the healthy control group (Figs. 6A and 6B). The expression levels of miR-144-5p and miR-26b-5p in the breast cancer group were significantly higher than those in the non-BC group (benign breast lesion group and healthy control group) (Figs. 6C and 6D).
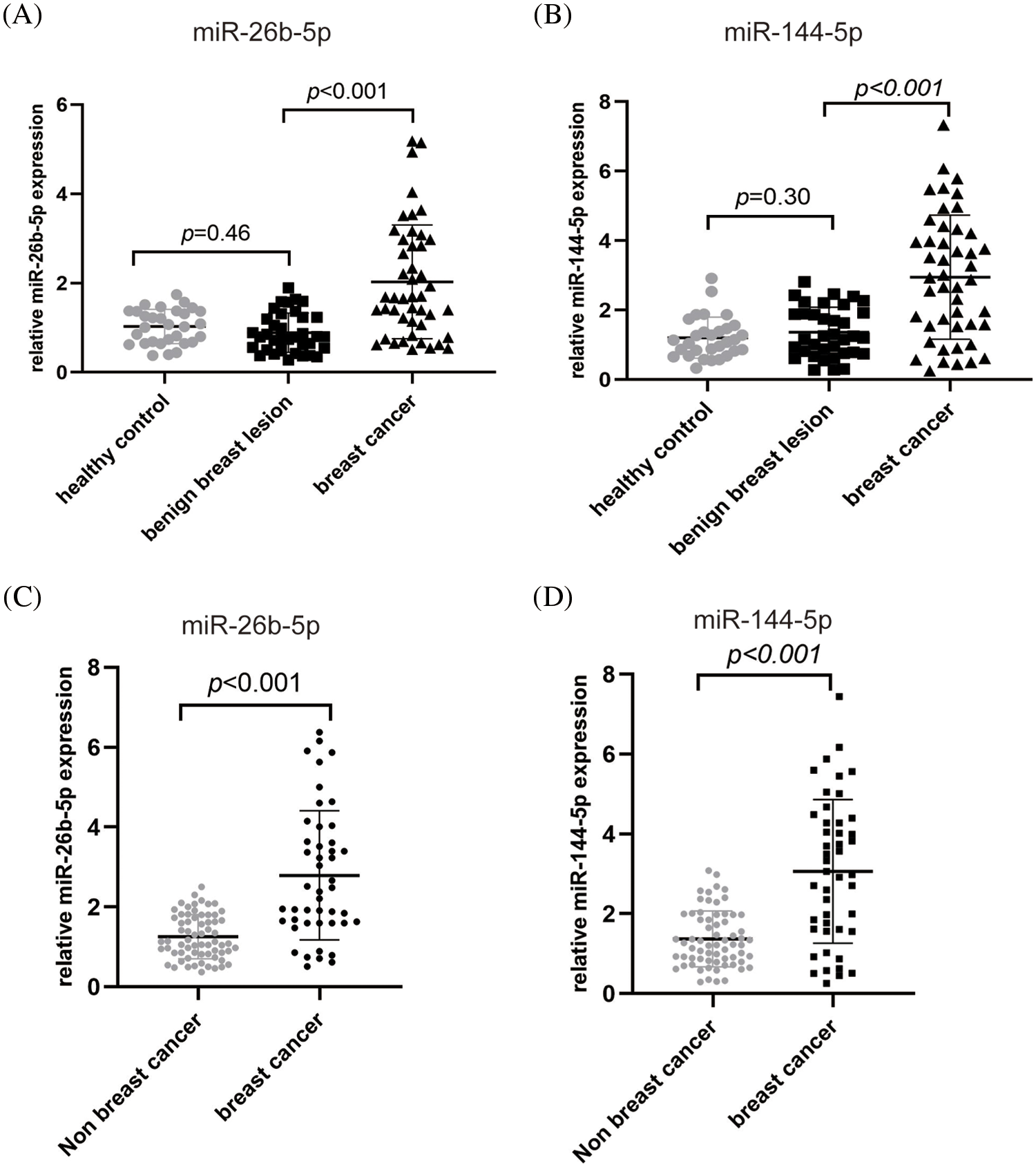
Figure 6: Plasma miR-26b-5p and miR-144-5p expression levels in the breast cancer group (n = 46), benign breast lesion group (n = 36), healthy control group (n = 30) and nonbreast cancer group (n = 66). (A, B) Plasma miR-26b-5p (A) and miR-144-5p (B) expression levels in the healthy control group, benign breast lesion group and breast cancer group. (C, D) Plasma miR-26b-5p (C) and miR-144-5p (D) expression levels in the nonbreast cancer group and breast cancer group
3.6 The Role of Plasma miR-144-5p and miR-26b-5p in the Diagnosis of BC
To further investigate the diagnostic value of plasma miR-144-5p and miR-26b-5p in BC, the ROC curve method was used and showed that the AUC of miR-144-5p in diagnosing BC was 0.763 (95% CI: 0.642–0.851) (p < 0.001). When the cutoff value was 1.462, the Youden index was the highest, the sensitivity was 0.804, and the specificity was 0.590. We also found that the AUC of miR-26b-5p for the diagnosis of BC was 0.747 (95% CI: 0.658–0.868) (p < 0.001). When the cutoff value was 1.305, the Youden index was the highest, and its sensitivity was 0.761 and the specificity was 0.640. It was also found that the AUC of miR-144-5p combined with miR-26b-5p (miR-144-5p+miR-26b-5p) for the diagnosis of BC was 0.832 (95% CI: 0.744–0.919) (p < 0.001), and its sensitivity and specificity for the diagnosis of BC were 0.781 and 0.780, respectively (Fig. 7), indicating that the value of plasma miR-144-5p combined with miR-26b-5p for the diagnosis of BC was greater than either alone.
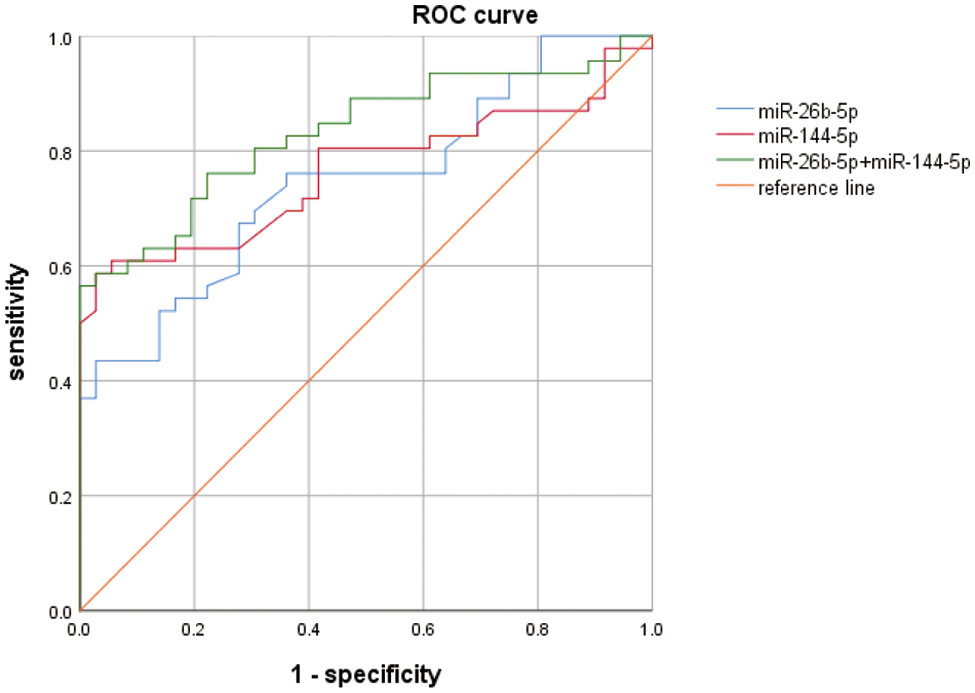
Figure 7: ROC curve analysis results for miRNA-26b-5p, miR-144-5p and their combination
3.7 The Value of miR-144-5p, miR-26b-5p Combined with UES in the Diagnosis of BC
To further explore the value of plasma miRNAs and UES in the diagnosis of BC, ROC curve analysis was performed and found that the AUC of miR-144-5p and miR-26b-5p combined with UES (miR-144-5p+miR-26b-5p+UES) for the diagnosis of BC was 0.934 (95% CI: 0.879–0.988) (p < 0.001), with a sensitivity of 0.913 and a specificity of 0.890 (Fig. 8), suggesting that miR-144-5p+miR-26b-5p+UES is an effective indicator for the early detection of BC.
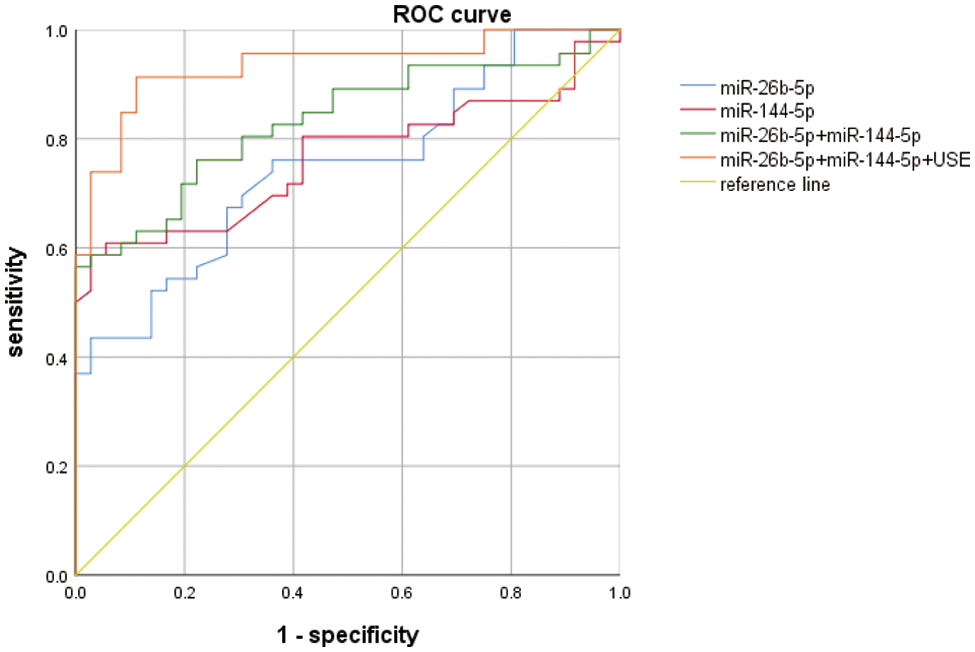
Figure 8: ROC curve analysis results for UES, miRNA-26b-5p, miR-144-5p and their combination
Imaging is currently the main method used for diagnosing breast lesions, among which ultrasound and mammography are the most commonly used for detecting BC. However, the sensitivity and specificity of mammography for the early detection of BC are unsatisfactory. Conventional ultrasound has emerged as a critical examination method for the clinical diagnosis of breast lesions, with high sensitivity when using the Breast Reporting and Data System (BI-RADS); however, due to the lack of specificity of conventional ultrasound, it performs poorly in differentiating benign and malignant breast lesions [13,14].
In recent years, some UE imaging methods have been used in clinical applications, such as touch elasticity imaging, shear wave elastography and strain elastography [15], and have exhibited good efficacy in differentiating benign and malignant breast lesions [16–18]. In our study, a total of 82 patients with breast lesions were included, among which 42 patients had elastographic scores of less than 4, and 40 patients had elastographic scores of more than 4. To investigate the clinical value of UE in BC, ROC curve analysis was employed and showed that the AUC was 0.809 (95% CI: 0.714–0.904) (p < 0.001), with a sensitivity of 0.717 and a specificity of 0.806, indicating that UES has significant potential for the early detection of BC.
Some miRNAs can be secreted into blood, cerebrospinal fluid, urine and saliva and have been reported to be potential diagnostic biomarkers of cancers [19,20]. Urabe et al. [21] measured the expression levels of circulating miRNAs in patients with urothelial carcinoma(UC), and identified miR-1343 and miR-6087 as effective biomarkers for UC. Itani et al. [22] detected 12 miRNAs in plasma samples from patients with breast lesions and developed a signature consisting of 4 miRNAs (miR-145/miR-425-5p/miR-139-5p/miR-130a), which is expected to be a useful marker for the early detection of BC.
In this study, we found that miR-144-5p and miR-26b-5p expression levels in the plasma of patients with BC were significantly higher than those of patients with benign breast diseases, while there was no significant difference in the expression levels of miR-144-5p and miR-26b-5p between the benign breast lesion group and the healthy control group. Moreover, by using ROC curve analysis, we found that the AUC for miR-144-5p to diagnose AUC was 0.763, with sensitivity 0.804 and specificity 0.590, and the AUC for miR-26b-5p to diagnose BC was 0.747, with sensitivity 0.761 and specificity 0.640. It was also found that the AUC of miR-144-5p combined with miR-26b-5p (miR-144-5p+miR-26b-5p) for the diagnosis of BC was 0.832, and its sensitivity and specificity for the diagnosis of BC were 0.781 and 0.780, respectively, suggesting that plasma miR-144-5p combined with miR-26b-5p could be used as potential biomarkers for the early detection of BC.
Furthermore, to investigate the role of plasma miRNAs and UES in the diagnosis of BC, ROC curve analysis was performed and found that the AUC of miR-144-5p and miR-26b-5p combined with UES (miR-144-5p+miR-26b-5p+UES) in the diagnosis of BC was 0.934, with a sensitivity of 0.913 and a specificity of 0.890, revealing that miR-144-5p+miR-26b-5p+UES might be an effective indicator for the early detection of BC.
In summary, miR-144-5p and miR-26b-5p combined with UES have a very high clinical application value for the early detection of BC, with particularly high sensitivity and specificity for distinguishing benign and malignant breast lesions. Our study provides an effective method for the early detection of BC.
Authorship: Study conception and design: Pingyang Zhang and Qian Zhang; data collection: Yuanyuan Cao, Mingming Fang and Qin Yang; analysis and interpretation of results: Yuanyuan Cao and Mingming Fang; draft manuscript preparation: Qian Zhang. All authors in this paper reviewed and approved the final version of the manuscript.
Ethics Approval and Informed Consent Statement: This study was approved by the Ethics Committee of Nanjing First Hospital (No. 2019-KY023). Written informed consent were obtained from all participants in this study.
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. Bray, F., Ferlay, J., Soerjomataram, I., Siegel, R. L., Torre, L. A. et al. (2018). Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians, 68(6), 394–424. DOI 10.3322/caac.21492. [Google Scholar] [CrossRef]
2. Feng, R. M., Zong, Y. N., Cao, S. M., Xu, R. H. (2019). Current cancer situation in China: Good or bad news from the 2018 global cancer statistics? Cancer Communications, 39(1), 1–12. DOI 10.1186/s40880-019-0368-6. [Google Scholar] [CrossRef]
3. Li, Y., Zhan, Z., Yin, X., Fu, S., Deng, X. (2021). Targeted therapeutic strategies for triple-negative breast cancer. Frontiers in Oncology, 11, 731535. DOI 10.3389/fonc.2021.731535. [Google Scholar] [CrossRef]
4. Ophir, J., Cespedes, I., Ponnekanti, H., Yazdi, Y., Li, X. (1991). Elastography: A quantitative method for imaging the elasticity of biological tissues. Ultrasonic Imaging, 13(2), 111–134. DOI 10.1177/016173469101300201. [Google Scholar] [CrossRef]
5. Han, X., Li, J., Zeng, F., Liu, H., He, Y. (2022). Differential diagnosis of basal cell carcinoma by high‐resolution ultrasound elastography. Skin Research and Technology, 28(2), 350–354. DOI 10.1111/srt.13139. [Google Scholar] [CrossRef]
6. Li, H., Cheng, C., Wang, Y., Qin, H., Wang, J. et al. (2021). Combined diagnosis of ultrasonic elastography and BI-RADS classification increases diagnostic value in female patients with breast neoplasms. American Journal of Translational Research, 13(10), 11758–11763. [Google Scholar]
7. Shen, C., Liu, W., Zhang, S., Pu, L., Deng, B. et al. (2020). Downregulation of miR-541 induced by heat stress contributes to malignant transformation of human bronchial epithelial cells via HSP27. Environmental Research, 184, 108954. DOI 10.1016/j.envres.2019.108954. [Google Scholar] [CrossRef]
8. Sole, C., Lawrie, C. H. (2019). MicroRNAs and metastasis. Cancers, 12(1), 96. [Google Scholar]
9. Ma, S., Wei, H., Wang, C., Han, J., Chen, X. et al. (2021). MiR-26b-5p inhibits cell proliferation and EMT by targeting MYCBP in triple-negative breast cancer. Cellular & Molecular Biology Letters, 26(1), 52. DOI 10.1186/s11658-021-00288-3. [Google Scholar] [CrossRef]
10. Huang, Z., Huang, D., Ni, S., Peng, Z., Sheng, W. et al. (2010). Plasma microRNAs are promising novel biomarkers for early detection of colorectal cancer. International Journal of Cancer, 127(1), 118–126. DOI 10.1002/ijc.25007. [Google Scholar] [CrossRef]
11. Zhao, Q., Shen, L., Lu, J., Xie, H., Li, D. et al. (2022). A circulating miR-19b-based model in diagnosis of human breast cancer. Frontiers in Molecular Biosciences, 9, 980841. DOI 10.3389/fmolb.2022.980841. [Google Scholar] [CrossRef]
12. Timmers, J. M., van Doorne-Nagtegaal, H. J., Zonderland, H. M., van Tinteren, H., Visser, O. et al. (2012). The breast imaging reporting and data system (BI-RADS) in the Dutch breast cancer screening programme: Its role as an assessment and stratification tool. European Radiology, 22(8), 1717–1723. DOI 10.1007/s00330-012-2409-2. [Google Scholar] [CrossRef]
13. Kolb, T. M., Lichy, J., Newhouse, J. H. (2002). Comparison of the performance of screening mammography, physical examination, and breast US and evaluation of factors that influence them: An analysis of 27,825 patient evaluations. Radiology, 225(1), 165–175. DOI 10.1148/radiol.2251011667. [Google Scholar] [CrossRef]
14. Lee, S. H., Chang, J. M., Kim, W. H., Bae, M. S., Seo, M. et al. (2014). Added value of shear-wave elastography for evaluation of breast masses detected with screening US imaging. Radiology, 273(1), 61–69. DOI 10.1148/radiol.14132443. [Google Scholar] [CrossRef]
15. Tang, A., Cloutier, G., Szeverenyi, N. M., Sirlin, C. B. (2015). Ultrasound elastography and MR elastography for assessing liver fibrosis: Part 2, diagnostic performance, confounders, and future directions. American Journal of Roentgenology, 205(1), 33–40. DOI 10.2214/AJR.15.14553. [Google Scholar] [CrossRef]
16. Garra, B. S., Cespedes, E. I., Ophir, J., Spratt, S. R., Zuurbier, R. A. et al. (1997). Elastography of breast lesions: Initial clinical results. Radiology, 202(1), 79–86. DOI 10.1148/radiology.202.1.8988195. [Google Scholar] [CrossRef]
17. Burnside, E. S., Hall, T. J., Sommer, A. M., Hesley, G. K., Sisney, G. A. et al. (2007). Differentiating benign from malignant solid breast masses with US strain imaging. Radiology, 245(2), 401–410. DOI 10.1148/radiol.2452061805. [Google Scholar] [CrossRef]
18. Gong, X., Wang, Y., Xu, P. (2013). Application of real-time ultrasound elastography for differential diagnosis of breast tumors. Journal of Ultrasound in Medicine, 32(12), 2171–2176. DOI 10.7863/ultra.32.12.2171. [Google Scholar] [CrossRef]
19. Mitchell, P. S., Parkin, R. K., Kroh, E. M., Fritz, B. R., Wyman, S. K. et al. (2008). Circulating microRNAs as stable blood-based markers for cancer detection. PNAS, 105(30), 10513–10518. DOI 10.1073/pnas.0804549105. [Google Scholar] [CrossRef]
20. Kosaka, N., Iguchi, H., Ochiya, T. (2010). Circulating microRNA in body fluid: A new potential biomarker for cancer diagnosis and prognosis. Cancer Science, 101(10), 2087–2092. DOI 10.1111/j.1349-7006.2010.01650.x. [Google Scholar] [CrossRef]
21. Urabe, F., Matsuzaki, J., Takeshita, F., Kishida, T., Ochiya, T. et al. (2022). Independent verification of circulating miRNA as diagnostic biomarkers for urothelial carcinoma. Cancer Science, 113(10), 3510–3517. DOI 10.1111/cas.15496. [Google Scholar] [CrossRef]
22. Itani, M. M., Nassar, F. J., Tfayli, A. H., Talhouk, R. S., Chamandi, G. K. et al. (2021). A signature of four circulating microRNAs as potential biomarkers for diagnosing early-stage breast cancer. International Journal of Molecular Sciences, 22(11), 6121. DOI 10.3390/ijms22116121. [Google Scholar] [CrossRef]
Cite This Article
 Copyright © 2022 The Author(s). Published by Tech Science Press.
Copyright © 2022 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools