 Open Access
Open Access
ARTICLE
Serum Level of Tumor-Specific Growth Factor in Patients with Cervical Cancer and Its Potential Prognostic Role
1
Clinical Laboratory of Hunan Cancer Hospital and the Affiliated Cancer Hospital of Xiangya School of Medicine, Central South
University, Hunan Key Laboratory of Oncotarget Gene, Changsha, China
2
Hunan University of Chinese Medicine, Changsha, China
* Corresponding Author: Faqin Tang. Email:
# Author has equal contribution
Oncologie 2022, 24(3), 499-512. https://doi.org/10.32604/oncologie.2022.024951
Received 17 June 2022; Accepted 17 August 2022; Issue published 19 September 2022
Abstract
Background: Tumor-specific growth factor (TSGF) is associated with invasion and metastasis of various malignancies and adverse clinical outcomes. TSGF is also highly expressed in cervical cancer (CC), but its clinical value is unclear. Materials and Methods: Serum samples from malignancies, including CC, non-Hodgkin’s lymphoma (NHL), nasopharyngeal carcinoma (NPC), colorectal cancer (CRC), lung cancer (LCA), ovarian cancer (OC), breast cancer (BC), gastric carcinoma (GC), and pancreatic cancer (PC) were collected, and TSGF was detected using a chemiluminescence assay. The patients with CC were followed-up over five years, and their clinicopathological parameters were analyzed. Meanwhile, the pre- and post-treatment TSGF levels of patients with CC were compared. The predictive and prognostic roles of TSGF were evaluated in the patients with CC using Kaplan-Meier survival curves, multivariate COX analyses, and ROC curves. Results: CC, CRC, and OC patients had higher serum TSGF than the healthy population (P < 0.05), and the serum TSGF of patients with CC was higher than that of other cancers (P < 0.05). Clinicopathologic analysis showed that TSGF was linked to CC tumor size, tumor invasion depth, histologic grade, and the International Federation of Gynecology and Obstetrics (FIGO) stage, and TSGF had a high sensitivity of 56%. The post-treatment TSGF in patients with CC was significantly decreased (P < 0.05). Kaplan-Meier survival curves identified high TSGF as a significant poor predictor of metastasis-free, recurrence-free, and overall survival (OS). Multivariate COX analyses showed that TSGF was an independent prognostic factor for OS rate. The ROC curve revealed that serum TSGF levels had a significant ability to predict recurrence, while the cut-off value for TSGF was 66.94 U/mL. Serum TSGF levels notably decreased in post-therapeutic patients and gradually with treatment progress. Conclusions: Serum TSGF may be a novel potential indicator for predicting prognosis and recurrence after surgery and adjuvant therapy in patients with CC.Keywords
Cervical cancer (CC) is one of the most common gynecological malignancies and the fourth most common cancer in women worldwide [1]. In 2018, there were approximately 570,000 cases of CC and 311,000 deaths [2]. Globally, the estimated age-standardized incidence of CC was 13.1/100,000 women and varies widely among countries [3]. However, survival rates are relatively favorable if CC is diagnosed and treated in the early stages [4]. Despite recent advances in cancer treatment, the primary treatment for early-stage CC is either surgery or radiation therapy. For example, bilateral pelvic lymph node (LN) dissection is a necessary component of primary surgical treatment. Currently, the prediction of CC recurrence and poor prognosis is mainly based on LN status, histological grade and depth of invasion [5]. Applying these pathological factors to the prediction of prognosis in CC is not accurate, because the clinical stage is frequently inaccurate, especially in cases of advanced cancer [6,7]. Tumor biomarkers may provide better risk stratification for progression. Therefore, a readily accessible pre-therapeutic test to estimate survival probability and recurrence is required for CC.
Tumor-specific growth factor (TSGF) is a tumor marker that is associated with vascular proliferation of malignant tumors and was discovered by Koukourakis et al. [8]. Its relative molecular weight is low, and it can be significantly increased in the early stages of cancer, and induces dominant expression related to malignant transformation [9]. Since then, many studies have confirmed that serum TSGF levels in patients with cancer are associated with deep myometrial invasion, LN metastasis, recurrence, advanced stage, and reduced survival [10]. However, there were many limitations in these studies, such as the small number of patients, and the inconsistent appropriate reference cutoff values of serum TSGF between these studies, which limited its clinical research. In the present study, large samples of serum TSGF were detected in patients with CC, who were followed-up for a long time, and their relationship to clinicopathological parameters was evaluated. Initially, we evaluated TSGF levels in patients with various types of cancer and compared them with serum levels in healthy people. TSGF levels in patients with CC caught our attention, and we subsequently decided to investigate TSGF levels in patients with CC and their clinical value in predicting prognosis.
This study was conducted in accordance with the National Institutes of Health Guide, approved by the institutional board with patients’ written consent, and evaluated and approved by the Ethics Committee of Hunan Cancer Hospital. This retrospective study included 200 patients with CC, 100 cases of histologically identified uterine myoma (UM), 70 cases of non-Hodgkin’s lymphoma (NHL), 88 patients with nasopharyngeal carcinoma (NPC), 95 patients with colorectal cancer (CRC), 94 patients with lung cancer (LCA), 103 patients with ovarian cancer (OC), 81 patients with breast cancer (BC), 84 patients with gastric carcinoma (GC), 81 patients with pancreatic cancer (PC), UM inclusion criteria were as follows: age > 21 years and diagnosis of UM was confirmed by ultrasound or histologically. The NHL inclusion criteria were histologically confirmed diagnosis of NHL involving any vertebra, available magnetic resonance imaging of the affected site, and a positive wrap-around sign. The NPC inclusion criteria were histological diagnosis of NPC. The CRC inclusion criterion was pathologically confirmation of CRC. LCA inclusion criteria were the patients with biopsy-proven lung cancer. The inclusion criterion was histologically confirmed OC. The BC inclusion criteria included complete medical records with at least one type of clinical imaging modality, mammogram, ultrasound, or MRI documenting the size of the breast lesion, biopsy-proven breast cancer, or in situ lesions. The GC inclusion criterion was histologically proven adenocarcinoma located in the upper third of the stomach (high body, fundus, or cardia). The inclusion criterion was a diagnosis of PC. CC was staged according to the International Federation of Gynecology and Obstetrics (FIGO) guidelines. LN metastases were diagnosed by lymphadenectomy. The tumor size was measured using magnetic resonance imaging, and the greatest dimension was recorded. The patients were followed up until death or until December 2021. The CC inclusion criteria were as follows: (a) age > 21 years; (b) CC confirmed by histology; and (c) patients who underwent received either radical hysterectomy with pelvic lymphadenectomy and/or radiotherapy with or without chemotherapy according to the national standard guidelines during the period 2017–2021. The exclusion criteria were as follows: (a) ≤3 months of follow-up data; (b) malignant disease in other organs, such as hematologic diseases, severe liver disease, renal function failure; and (c) incomplete case data. A total of 100 age-matched patients with UM from the Hunan Cancer Hospital with no evidence of malignant tumors were enrolled. A total of 44 age-matched healthy individuals were included in the control group.
The TSGF assay kit was purchased from Hunan Haiyuan Medical Technology Company (Zhengzhou, China), and the positive threshold was 64 U/mL. Cancer antigen 125 (CA125) assay kits and serum squamous cell carcinoma (SCC) assay kits were purchased from Fujirebio Diagnostics Lincoln National Corp. (Malvern, PA, USA), and the positive thresholds were 35 U/mL and 1.5 ng/mL, respectively. The Epididymis protein 4 (HE4) assay kit (Autobio Company, Changsha, China) was used, with a positive threshold of 140 pmol/L.
2.3 TSGF, CA125, HE4, and SCC Assay
The TSGF assay was performed as described in the manufacturer’s instructions. Briefly, venous blood was drawn from patients with CC, and the serum was separated by centrifugation. 20 μL of serum and 100 μL of Reagent 1 from the TSGF assay kit were added and mixed. The mixture was incubated for 5 min at 37°C and 100 μL of Reagent 2 from the TSGF assay kit was added. After incubation for 90 s at 37°C, the absorbance was measured at wavelength–560–580 nm. The CA125 assay was performed as described in the kit’s instructions. Briefly, serum samples were diluted 10 times, followed by incubation and washing. The combination solution from the kit was added into the diluted serum, followed by incubation and washing. The activation and pre-activation fluids from the kit were added to the mixture and subjected to a chemiluminescence assay. The SCC assay method was consistent with the CA125 assay, as described in the manufacturer’s instructions. The HE4 assay was performed as described in the manufacturer’s instructions. Briefly, 25 μL of serum, 20 μL of magnetic particle suspension, and 50 μL of sample diluent were added and mixed. The mixture was incubated for 15 min at 37°C and washed. The enzyme conjugate (100 μL) was then added to the mixture and incubated for 17 min at 37°C. After washing, substrates A and B from the kit were added and mixed, and the luminescence intensity of the mixture was measured. A standard curve was constructed for each assay, and the antigen activity in each sample was determined by comparison with the standard curve.
Two clinical experts carefully reviewed demographics of 200 patient with CC, clinical characteristics, such as pathologic (including histologic type, differentiation) and staging information, tumor size, deep stromal invasion, LN metastasis status, and treatment variables. In total, demographics and clinical characteristics of 103, 81 and 100 patients with OC, BC, and UM, respectively, were carefully reviewed by two clinical gynecologists. A total of 70, 88, 95, 94, 84, and 81 cases of NHL, NPC, CRC, LCA, GC, and PC patients’ demographics, clinical characteristics, respectively, were carefully reviewed by two clinical oncologist experts. In addition, clinical information was collected from patients at pre- and after treatment. Two hundred patients who met the inclusion criteria were enrolled in this study. A total of 100 patients with UM and 44 healthy individuals served as the controls.
Continuous variables were expressed as mean values ± standard deviation, The Mann-Whitney U test was used for the comparison of two groups, and the Kruskal-Wallis test was used for the comparison of more than three groups. Categorical variables were described as frequencies and percentages, and the chi-square test or Fisher’s exact test was used for categorical variables. Receiver operating characteristic (ROC) curve analysis was used to estimate the value of serum TSGF levels in predicting CC recurrence. Survival analysis was performed using Kaplan–Meier estimation and the multivariate COX analysis. Pearson correlation analysis was used to analyze the association of TSGF with post-therapy time. Statistical significance was set at P < 0.05. All statistical analyses were carried out by SPSS 22.0.
3.1 Serum TSGF Level in CC and Association with CC Development
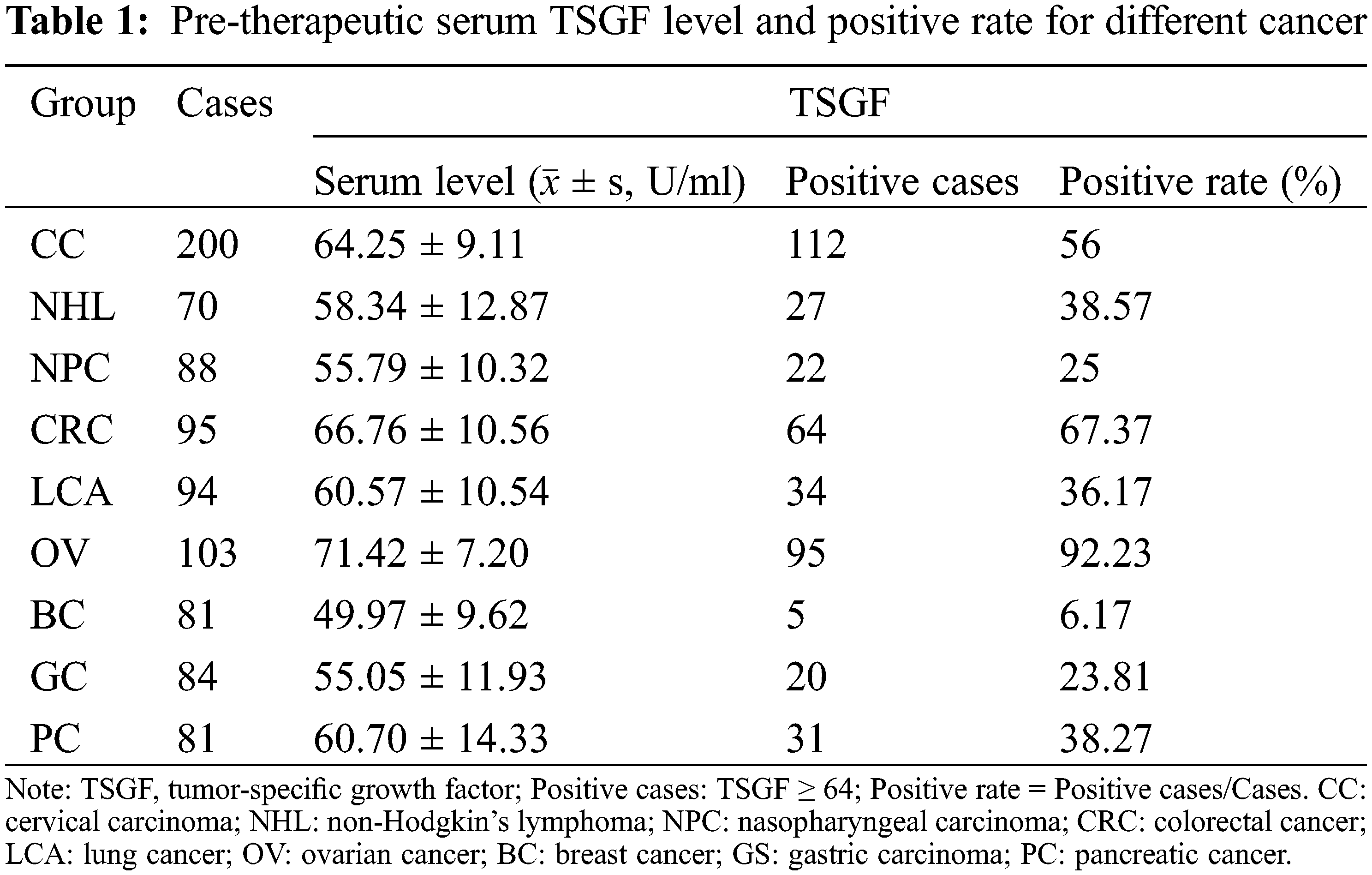
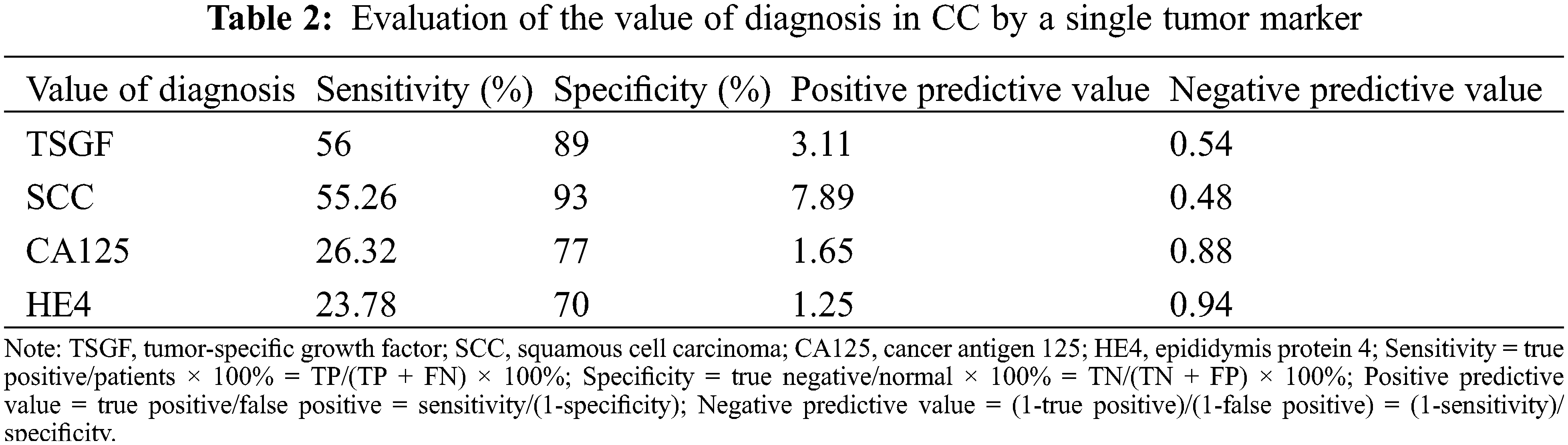
To determine the serum TSGF levels in various cancers, NHL, NPC, CRC, LCA, OC, BC, GC, and PC patients were collected, and their serum TSGF levels were detected. The healthy population were detected. The results showed that TSGF levels of the patients with CC were 64.59 ± 9.54 U/mL, patients with NHL were 58.34 ± 12.87 U/mL TSGF, patients with NPC were 55.79 ± 10.32 U/mL, patients with CRC were 66.76 ± 10.56 U/mL, patients with LCA were 60.57 ± 10.54 U/mL, patients with OC were 71.42 ± 7.20 U/mL, patients with BC were 49.97 ± 9.62 U/mL, patients with GC were 55.05 ± 11.93 U/mL, patients with PC were 60.70 ± 14.33 U/mL (Fig. 1A, Table 1); CC, CRC, and OC patients had higher serum TSGF than the healthy population (57.14 ± 6.09 U/mL, P < 0.05), suggesting that TSGF may be a novel biomarker for those cancers diagnosis and progression evaluation. SCC, CA125, and HE4 are currently considered important biomarkers for CC, and the diagnostic value of TSGF, SCC, CA125, and HE4 for CC was also evaluated. The data showed that, using a single tumor biomarker to diagnose CC, TSGF and SCC had the highest sensitivity (56%) and specificity (93%), respectively. In addition, TSGF specificity was higher than that of SCC and HE4. (Table 2). TSGF has a relatively high sensitivity for diagnosing CC compared with SCC, CA125, and HE4. To further investigate the relationship between serum TSGF and CC development, 100 patients with UM were used for comparison with CC in this study, with a median age of 47 years (range: 27–68 years). The serum TSGF levels of the healthy population, patients with UM and CC were detected and compared. The results showed that serum TSGF of the CC group (64.25 ± 9.11 U/mL) was significantly higher than that of the UM group (40.9 ± 10.77 U/mL) and the healthy population (57.14 ± 6.09 U/mL) (Fig. 1B. P < 0.05).
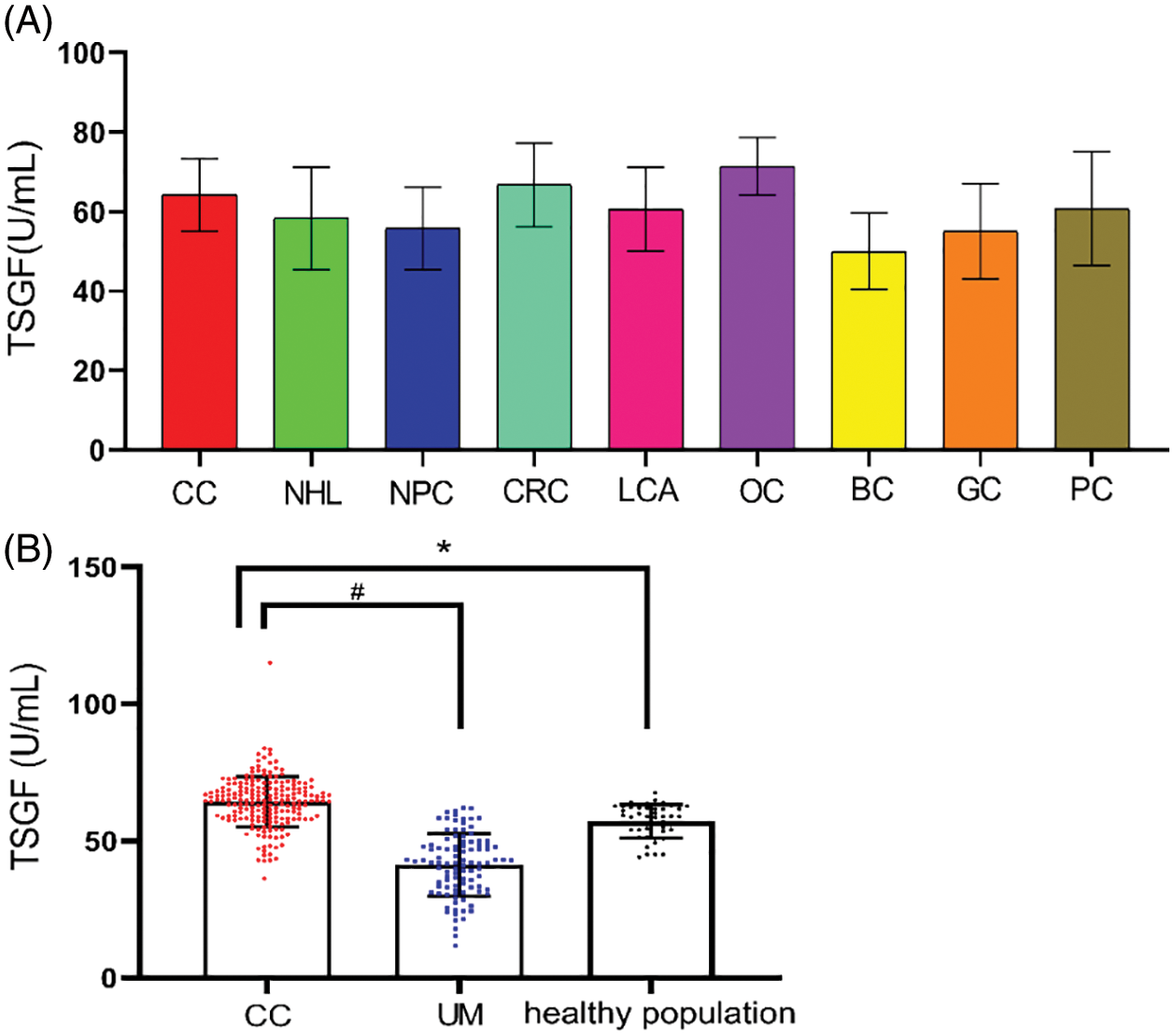
Figure 1: Serum TSGF levels in various cancer (A); TSGF, tumor-specific growth factor; CC, cervical carcinoma; NHL, non-Hodgkin’s lymphoma; NPC, nasopharyngeal carcinoma; CRC, colorectal cancer; LCA, lung cancer; OC, ovarian cancer; BC, breast cancer; GS, gastric carcinoma; PC, pancreatic cancer. Analyses of the sensitivity of serum tumor biomarker for patients with CC; Comparison of serum TSGF levels between patients of cervical cancer and UM, *P < 0.001 compared with healthy population; #P < 0.001 compared with UM (B); UM: uterine myoma
3.2 Serum TSGF Level is Associated with CC Stages
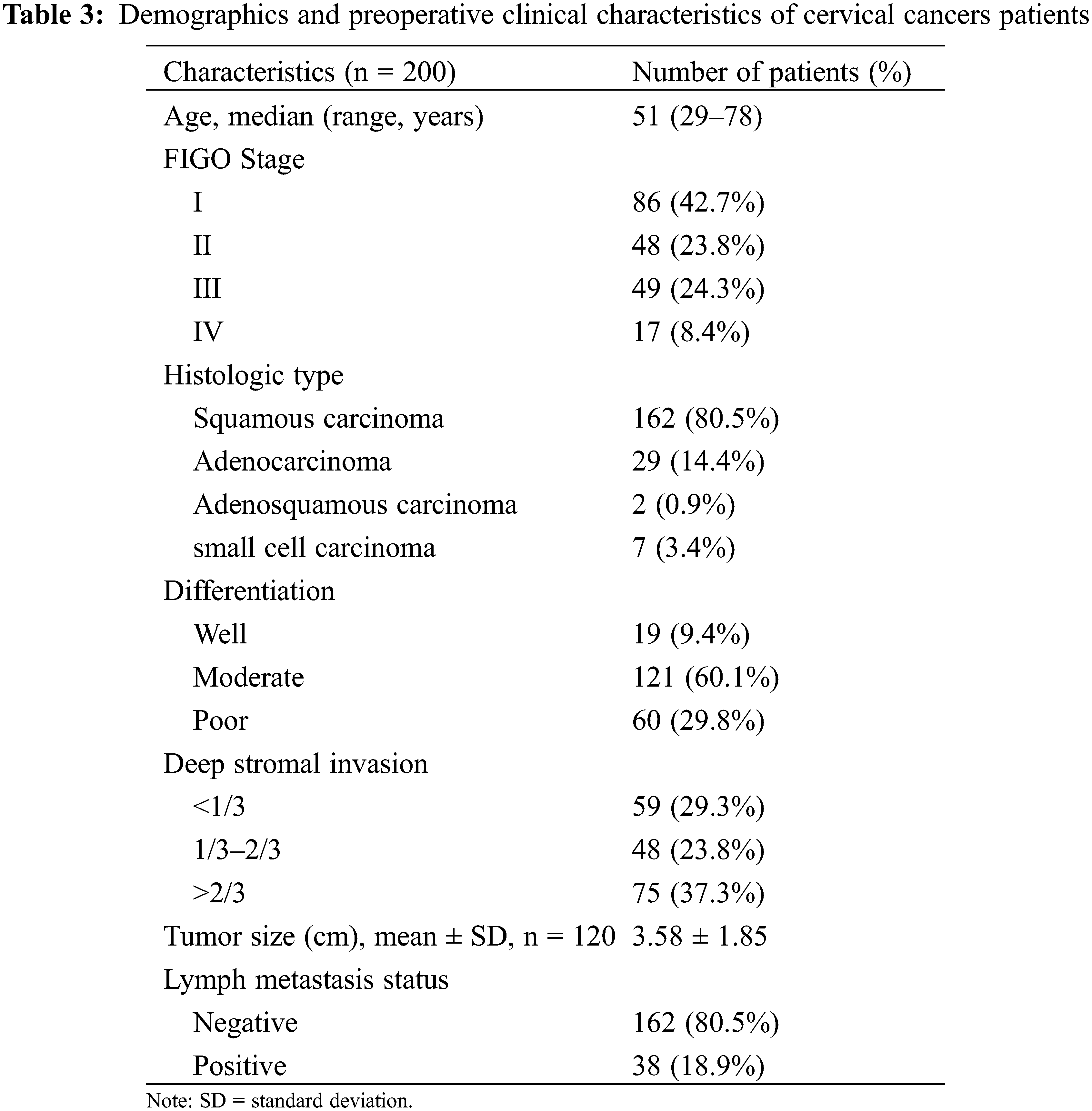
To further analyze the association between TSGF and CC progression, 200 patients with CC at various clinical stages were enrolled in the analysis. Patients with CC were of a median age of 51 years (range: 29–78 years). The demographic and clinicopathological characteristics of the patients with CC are shown in Table 3. The distribution of FIGO stages was as follows: 86 (42.7%), 48 (23.8%), 49 (24.3%), and 17 (8.4%) patients had stages I, II, III, and IV cancer, respectively. In terms of pathological classification, there were 162 (80.5%) cases of squamous cell carcinoma, 29 (14.4%) cases of adenocarcinoma, two (0.9%) cases of adenosquamous carcinoma, and seven (3.4%) cases of small cell carcinoma. LN metastasis was present in 38 (18.9%) patients, and absent in 162 (80.5%) patients, while 60 (29.8%) patients had poor tumor differentiation. The main tumor lesions were 0.5–12.3 cm in diameter, with an average diameter of 3.58 ± 1.85 cm. The association of serum TSGF levels with CC clinical stages was analyzed, showing the relationship between clinicopathological characteristics and pre- therapeutic serum TSGF. No significant differences were observed with respect to age, histology type, or LN metastasis status (P > 0.05) (Table 4). Serum TSGF levels were significantly associated with FIGO stage, differentiation, deep stromal invasion and tumor size (P < 0.05) (Table 4). Serum TSGF levels of patients at stages II, III, and IV showed a significantly higher expression compared with FIGO stage I (P < 0.05) (Table 4). Serum TSGF levels in patients with good and moderate differentiation showed significantly lower expression than those in patients with poor differentiation (P < 0.05) (Table 4). Serum TSGF levels in patients with ≥2/3 deep stromal invasion were significantly higher than those in patients with <1/3 deep stromal invasion. Tumor size ≥ 6 of TSGF levels in patients showed a significantly higher expression than that in patients with tumor size < 4 (P < 0.05) (Table 4).
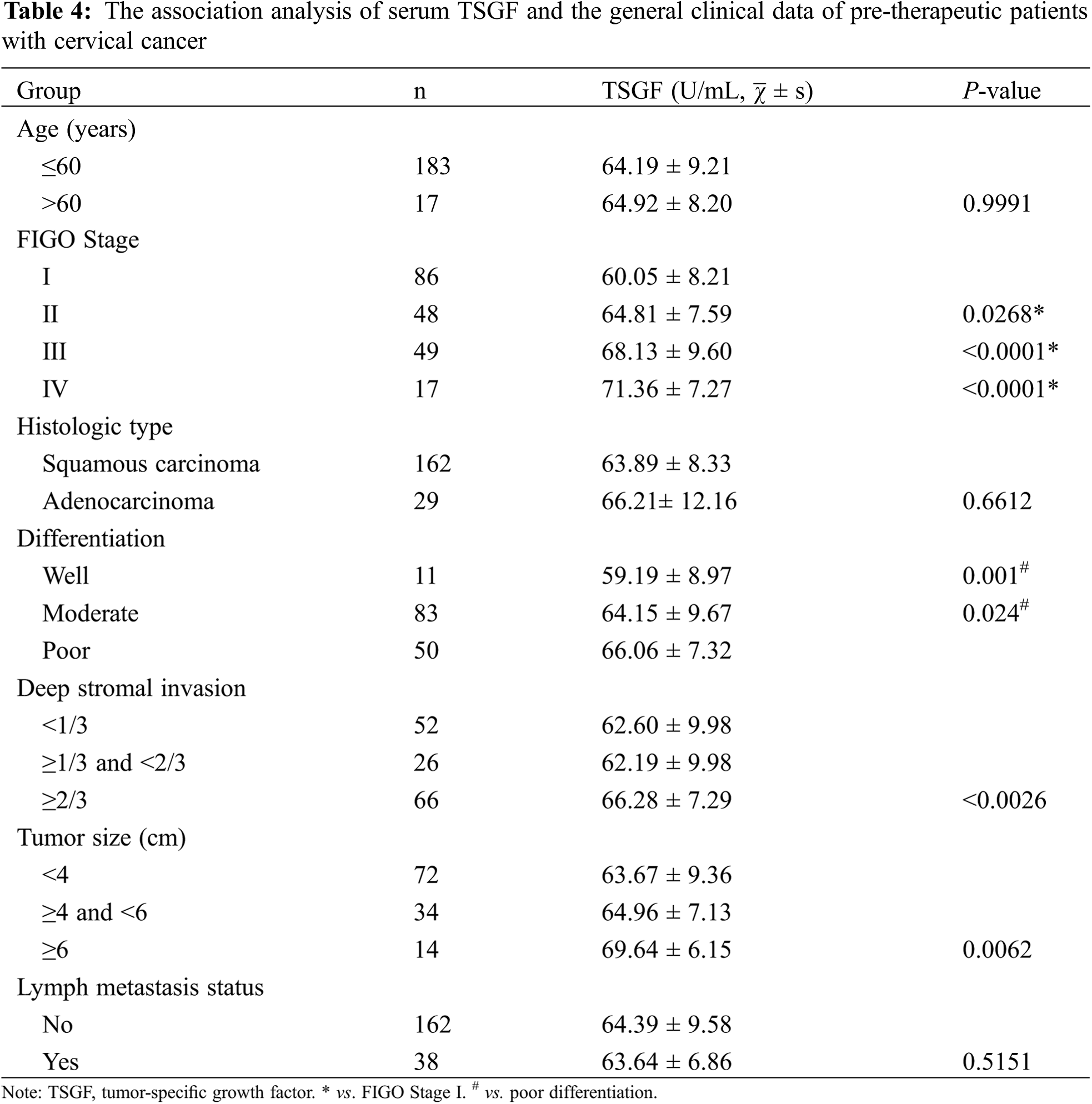
3.3 Serum TSGF Changes in Pre- and Post-Therapeutic CC
To observe the relationship between serum TSGF and CC therapy, TSGF changes of CC patients at pre- and post-therapy was analyzed. Compared with pre-radical operation, the levels of serum TSGF decreased notably in patients’ post-radical operation, and a significant difference was observed (Fig. 2A). Compared with pre-chemotherapy, the levels of serum TSGF decreased notably in patients’ post-chemotherapy, and a significant difference was observed (Fig. 2B). Compared with pre-radiotherapy, the levels of serum TSGF decreased notably in patients’ post- radiotherapy, and a significant difference was observed (Fig. 2C). Furthermore, dynamic changes in the serum TSGF levels were analyzed during the therapeutic process using ANOVA statistical test and Pearson correlation analysis. Fig. 3 shows the dynamic curve of serum TSGF, excluding those who experienced recurrence at different time points post-therapy. Serum TSGF was 60.65 ± 11.88 U/mL, 59.81 ± 10.31 U/mL, 58.41 ± 13 U/mL, 55.35 ± 11.37 U/mL, and 56.95 ± 11.97 U/mL at 1, 3, 6, 12, and 18 months, respectively. TSGF decreased significantly in a time-dependent manner after 1, 3, 6, 12, and 18 months of treatment compared with pre-therapy. In addition, serum TSGF levels of patients with CC after 12 months of treatment decreased significantly compared with that after 1 months of treatment. Pearson correlation analysis was used to analyze the association of TSGF with post-therapy time, the results showed that TSGF decreases were negatively associated with time (R = 0.027. P < 0.001).
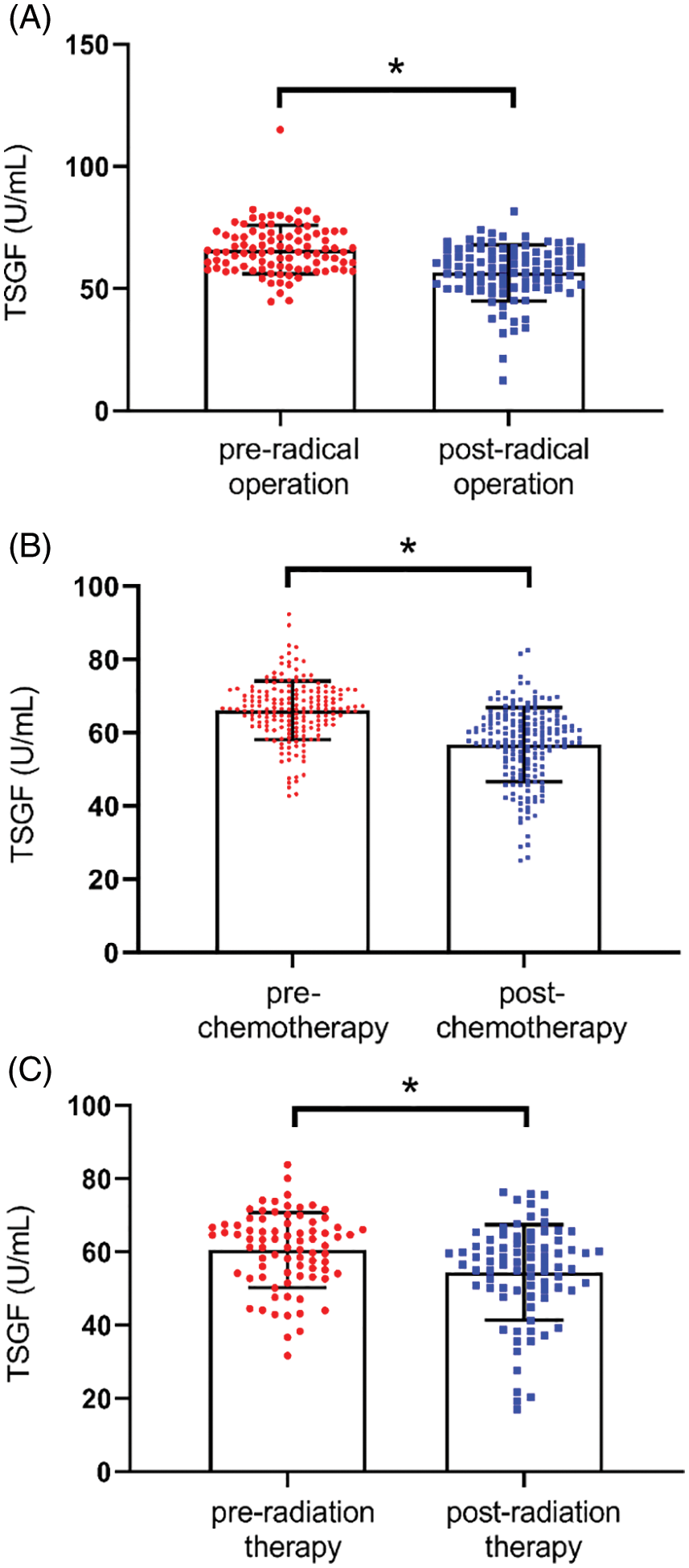
Figure 2: Serum TSGF levels in pre-and post-radical operation patients, *P < 0.001 compared with pre-radical operation patients (A). Serum TSGF levels pre- and post-chemotherapy, *P < 0.001 compared with pre-chemotherapy (B). Serum TSGF levels pre- and post-radiotherapy, *P < 0.001 compared with pre-radiotherapy (C). TSGF, tumor-specific growth factor
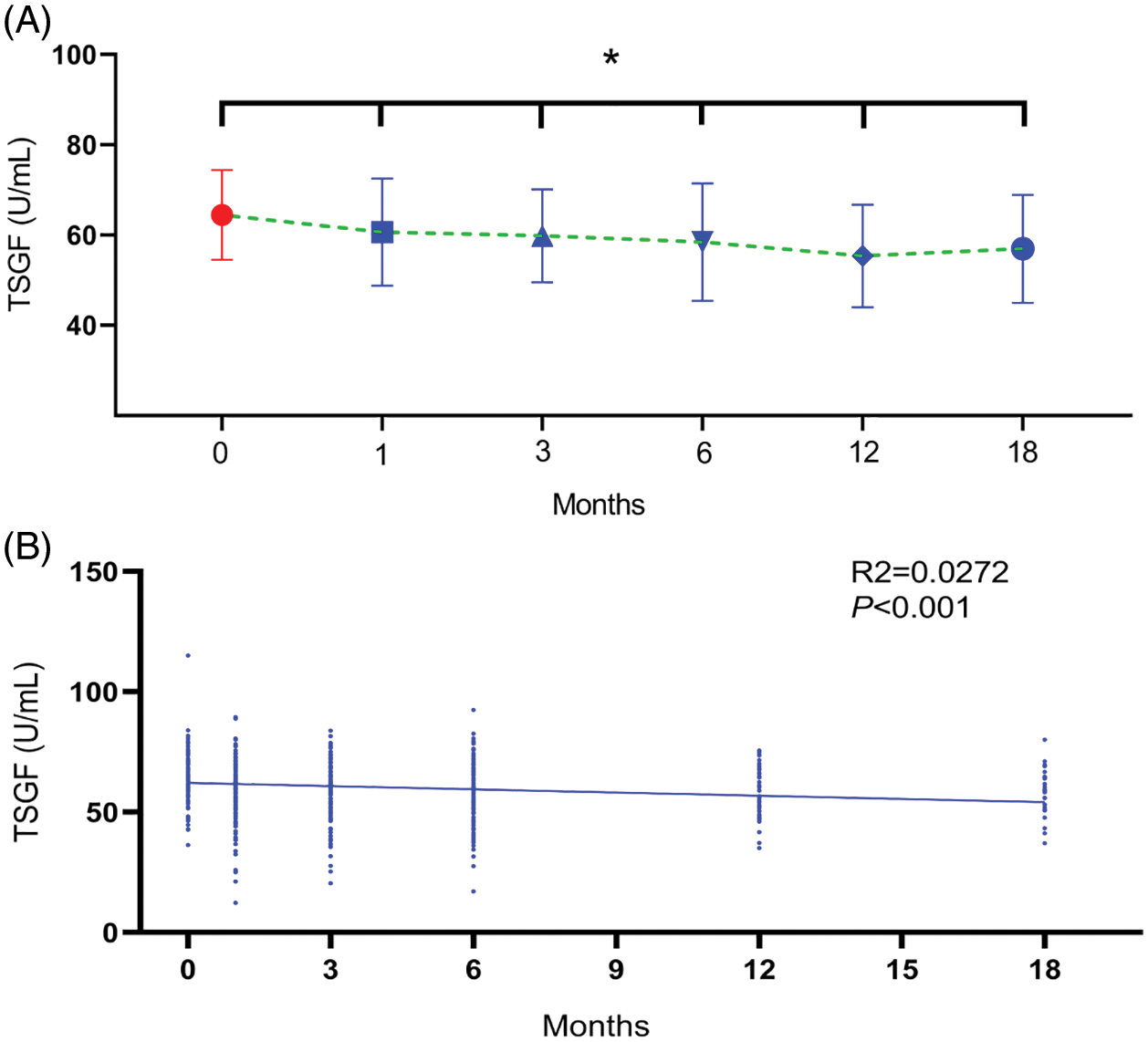
Figure 3: Dynamic curve of serum TSGF at different time points of post-treatment. (A) ANOVA analysis was used to compare serum TSGF at pre-therapy with post-therapy. *P < 0.001. (B) Pearson correlation analysis was used to analyze the association of TSGF with time. TSGF, tumor-specific growth factor
3.4 Serum TSGF Predicts CC Resurgence
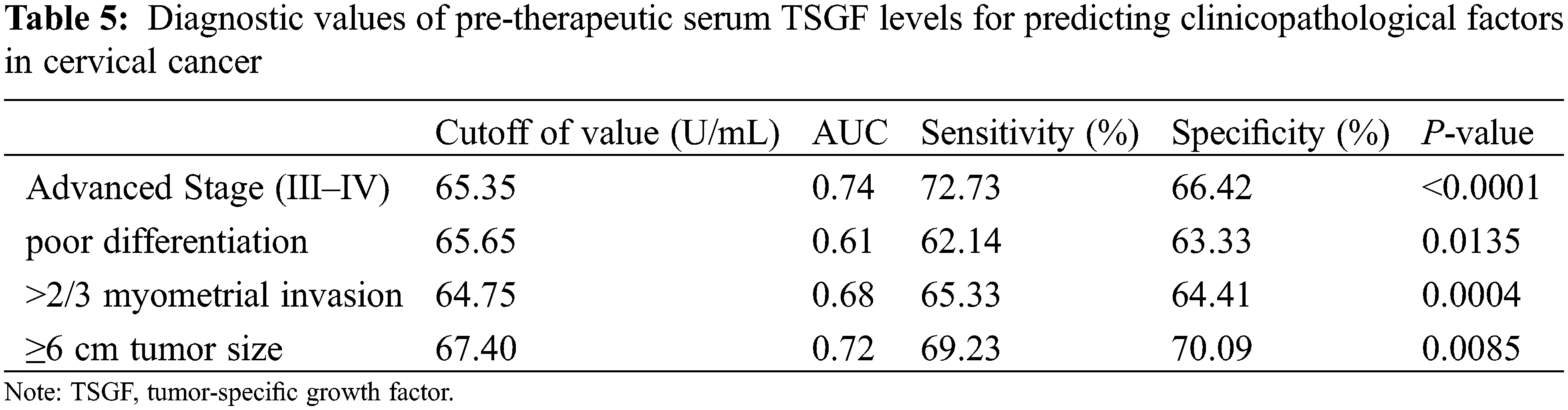
To determine whether TSGF can predict the resurgence of CC, as depicted in Fig. 4, ROC analysis was performed in patients with CC to determine the value of serum TSGF levels. The AUC for TSGF was 0.614 (95% CI: 0.502–0.726). The cutoff value for TSGF was 66.94 U/mL, with 57.78% sensitivity and 63.71% specificity. The most suitable cutoff level of clinicopathologic factors was evaluated using a ROC curve analysis, the TSGF serum levels ranged from 65.35 to 67.40 U/mL with 62.14%–72.73% sensitivity and 63.33%–70.09% specificity (Table 5).
Figure 4: Receiver operating characteristic curve (ROC) analysis. Serum TSGF for predicting all patients with recurrence of cervical cancer. ROC, receiver operating characteristic curve; TSGF, tumor-specific growth factor
3.5 Serum TSGF is Linked with CC Prognosis
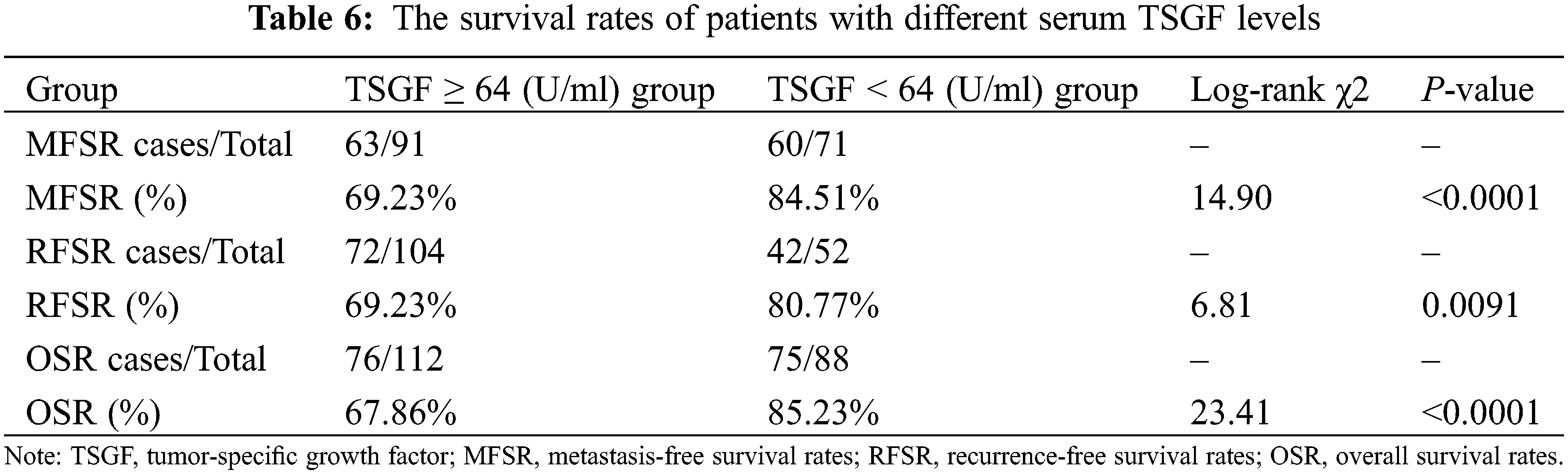
The next step is to analyze the relationship of serum TSGF levels with the survival rate of CC patients with CC. A total of 200 patients with CC were followed-up for over 5 years. Of them, 48 died within 6 years of treatment, and the 6-year survival rate was 76% (152/200). Based on the cutoff value, the patients were divided into TSGF ≥ 64 U/mL and TSGF < 64 U/mL groups. The result of Kaplan Meier curve analysis showed that the metastasis-free survival rates of the TSGF < 64 U/mL and TSGF ≥ 64 U/mL groups were 84.51% and 69.23%, respectively (Fig. 5A, Table 6). The recurrence-free survival rates of the TSGF ≥ 64 U/mL and TSGF < 64 U/mL groups were 69.23%, and 80.77%, respectively (Fig. 5B, Table 6). The overall survival rate of the TSGF ≥ 64 U/mL group was 67.86%, and that of the TSGF < 64 U/mL group was 85.23% (Fig. 5C, Table 6). Multivariate COX analyses in conjunction with the TSGF levels, FIGO stage and differentiation showed that the TSGF levels was an independent prognostic factor for OS in patients with CC (95% CI: 2.104~7.850, P < 0.001) (Table 7).
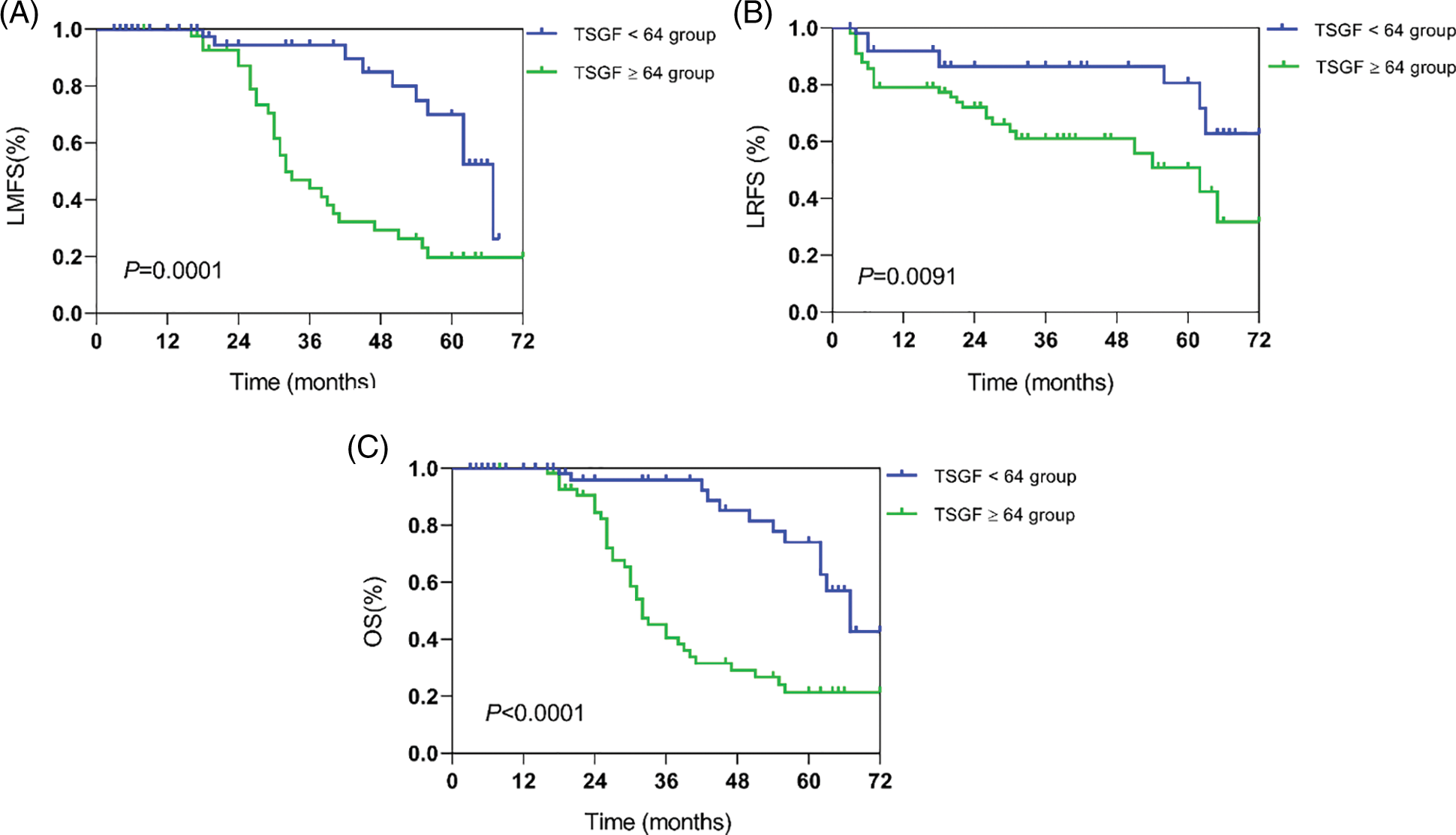
Figure 5: Kaplan-Meier survival curves analysis for overall survival outcomes according to different serum TSGF groups. Lymphatic metastasis-free survival (LMFS) (A), locoregional recurrence-free survival (LRFS) (B), overall survival (OS) (C). TSGF, tumor-specific growth factor
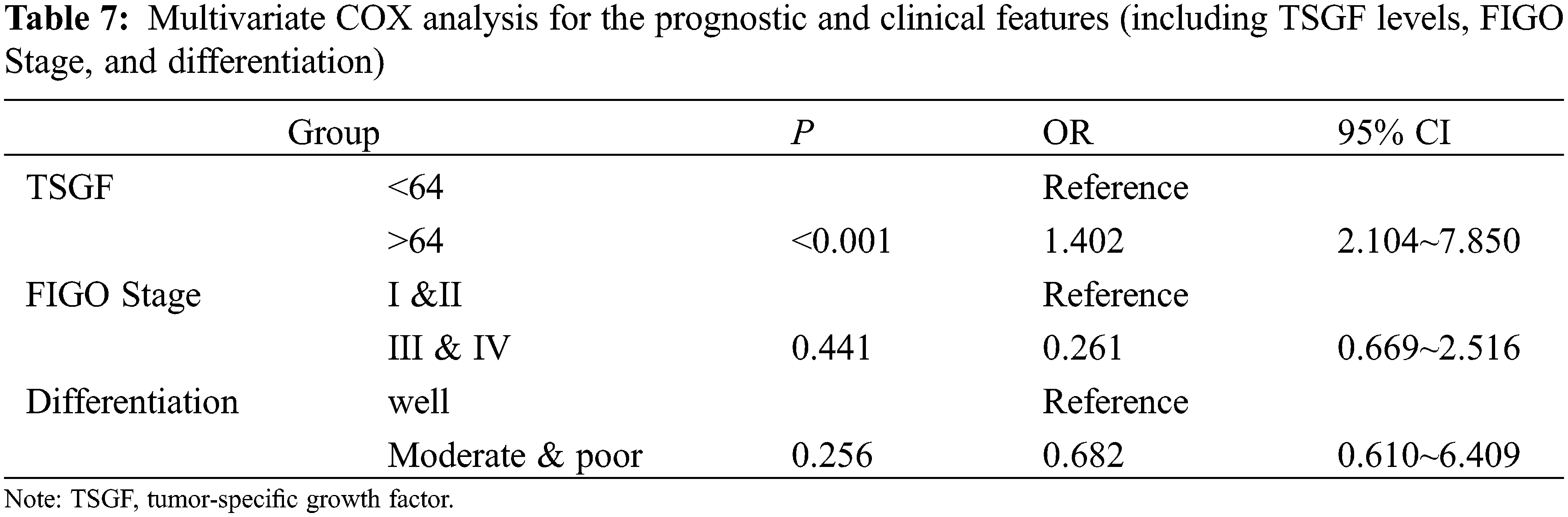
The evaluation of CC therapeutic prognosis requires a practical and effective method; in particular tumor biomarkers, need to be identified. TSGF is associated with invasion, metastasis, recurrence, advanced stage, and survival [11]. In the present study, we focused on patients with massive CC at various stages, with a follow-up period of 4–6 years and analyzed the relationship between serum TSGF and CC. Serum TSGF levels are associated with overall survival rates; patients with high TSGF (TSGF ≥ 64 U/mL) have a low survival rate, and a low TSGF group (TSGF < 64 U/mL) has a high survival rate. In addition, TSGF gradually decreased with prolonged post-treatment, whereas serum TSGF levels significantly increased when CC resurged. Previous reports have shown that serum TSGF levels correlate with poor prognosis in hepatocellular carcinoma [12], colon cancer [13], and PC [14]. Survival analysis showed that high TSGF levels were significantly associated with locoregional progression, distant metastasis, and inferior survival outcomes. Patients with high pre-therapeutic TSGF levels had a poor prognosis, which supports this explanation. We believe that serum TSGF may serve as an important marker for evaluating of CC therapeutic prognosis.
The serum biomarkers above referred to as SCC, CA125, and HE4 have been used as tumor biomarkers for the early diagnosis of various tumors including OC, endometrial cancer and CC, because they have the high levels of sensitivity and specificity. Currently, alterations in CA125 and SCC antigen levels are more likely to predict CC outcomes [15,16]. Moreover, HE4 is a prognostic marker for survival in individuals who have high risk of CC [17]. Recent studies have shown that serum SCC is a better biomarker, with a sensitivity of 80% in patients with CC [18,19]. Therefore, we tested the diagnostic sensitivity of these biomarkers in CC. However, an interesting phenomenon in our study was that the diagnostic sensitivity of TSGF was higher than that of the SCC, CA125 and HE4 tumor biomarkers. In the current study, to improve methods for tumor diagnosis, the cutoff value of tumor biomarkers was used to evaluate pathological status [20]. In our study, the cutoff value of TSGF was used for CC diagnosis and evaluation of prognosis was set at 65.35 U/mL, which was the best cutoff to determine the advanced stage (III–IV) and had high sensitivity and specificity.
In addition, 65.65 U/mL may be used to predict only poor differentiation. Overall, these results suggest that TSGF-level profiles can be used as diagnostic markers for CC. In addition, serum TSGF levels were different in various cancers, TSGF in CC was relatively high compared to NHL, NPC, BC, and GC. Meanwhile, it was higher in CC than in UM. This result was similar to previous studies [21], which reported that serum TSGF levels were significantly higher in patients with endometrial cancer than in those with non-malignant UM and the rate of TSGF-positive cases was also significantly higher.
The strengths of the current study are as follows, First, our study was the largest retrospective study on the value of pre-therapeutic serum TSGF levels in predicting the treatment and the prognosis of patients with CC. Secondly, we excluded the patients who might have other medical comorbidities or undergone preoperative radiotherapy or chemotherapy that contributed to elevated serum TSGF levels. Third, we focused on the post-therapeutic serum TSGF levels in patients with CC. In particular, we created a dynamic curve of serum TSGF according to different treatment times, which provided a better visualization of the variation in serum TSGF levels in patients with CC. However, the present study had some limitations. In this retrospective study, there was a selection bias; some patients were excluded due to a lack of preoperative serum TSGF levels, incomplete tumor size, or loss of follow-up. The follow-up periods of the individual studies are not unified, and may also be a potential source of heterogeneity. Therefore, a large multicenter study is required to confirm these results.
Serum TSGF may be a novel potential indicator for predicting efficacy prognosis and recurrence after surgery and adjuvant therapy in patients with CC. Serum TSGF levels is of great clinical value for evaluating treatment efficacy and can be valuable for monitoring the recurrence and prognosis of patients with CC.
Acknowledgement: We thank the members in Clinical Laboratory of Hunan Cancer Hospital and The Affiliated Cancer Hospital of Xiangya Medical School University for contributions.
Author Contributions: The authors confirm contribution to the paper as follows: data collection and drafted the paper: Wu Y., Deng H.; detected TSGF, SCC, HE4, and CA125: Qu B., Shen H.; conceive and revise the paper: Tang F. All authors reviewed the results and approved the final version of the manuscript.
Data Accessibility: All data generated or analyzed during this study are included in this published article.
Ethical Approval Statement: This study was permitted by the Ethics Committee of Hunan Cancer Hospital and the Affiliated Cancer Hospital of Xiangya School of Medicine, Central South University (No. KYJJ-2018-071).
Funding Statement: This work was supported in part by National Key Research & Development Plan of Ministry of Science and Technology of China [Grant No. 2019YFF0216502 to F Tang], Hunan Provincial Natural Science Foundation of China [Grant No. 2018JJ6131 to F Tang; Grant No. 2019JJ40175 to F Tang], Changsha Science and Technology Project [Grant No. kg1801107 to F Tang].
Conflict of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. Buskwofie, A., David-West, G., Clare, C. A. (2020). A review of cervical cancer: Incidence and disparities. Journal of the National Medical Association, 112(2), 229–232. DOI 10.1016/j.jnma.2020.03.002. [Google Scholar] [CrossRef]
2. Songane, M., Grossmann, V. (2021). The patent buyout price for human papilloma virus (HPV) vaccine and the ratio of R&D costs to the patent value. PLoS One, 16(1), e0244722. DOI 10.1371/journal.pone.0244722. [Google Scholar] [CrossRef]
3. Shi, J. F., Canfell, K., Lew, J. B., Qiao, Y. L. (2012). The burden of cervical cancer in China: Synthesis of the evidence. International Journal of Cancer, 130(3), 641–652. DOI 10.1002/ijc.26042. [Google Scholar] [CrossRef]
4. Pimple, S. A., Mishra, G. A. (2019). Global strategies for cervical cancer prevention and screening. Minerva Ginecologica, 71(4), 313–320. DOI 10.23736/S0026-4784.19.04397-1. [Google Scholar] [CrossRef]
5. Chen, L., Zhang, F., Sheng, X. G., Zhang, S. Q., Chen, Y. T. et al. (2016). Peripheral platelet/lymphocyte ratio predicts lymph node metastasis and acts as a superior prognostic factor for cervical cancer when combined with neutrophil: Lymphocyte. Medicine, 95(32), e4381. DOI 10.1097/MD.0000000000004381. [Google Scholar] [CrossRef]
6. Lee, Y. Y., Choi, C. H., Kim, H. J., Kim, T. J., Lee, J. W. et al. (2012). Pretreatment neutrophil: Lymphocyte ratio as a prognostic factor in cervical carcinoma. Anticancer Research, 32(4), 1555–1561. [Google Scholar]
7. Suh, D. H., Kim, J. W., Aziz, M. F., Devi, U. K., Ngan, H. Y. et al. (2010). Asian society of gynecologic oncology workshop 2010. Journal of Gynecologic Oncology, 21(3), 137–150. DOI 10.3802/jgo.2010.21.3.137. [Google Scholar] [CrossRef]
8. Koukourakis, G. V., Sotiropoulou-Lontou, A. (2011). Targeted therapy with bevacizumab (Avastin) for metastatic colorectal cancer. Clinical & Translational Oncology, 13(10), 710–714. DOI 10.1007/s12094-011-0720-z. [Google Scholar] [CrossRef]
9. Zhou, G., Niu, L., Chiu, D., He, L., Xu, K. (2012). Changes in the expression of serum markers CA242, CA199, CA125, CEA, TNF-α and TSGF after cryosurgery in pancreatic cancer patients. Biotechnology Letters, 34(7), 1235–1241. DOI 10.1007/s10529-012-0908-5. [Google Scholar] [CrossRef]
10. Chen, Y., Gao, S. G., Chen, J. M., Wang, G. P., Wang, Z. F. et al. (2015). Serum CA242, CA199, CA125, CEA, and TSGF are biomarkers for the efficacy and prognosis of cryoablation in pancreatic cancer patients. Cell Biochemistry and Biophysics, 71(3), 1287–1291. DOI 10.1007/s12013-014-0345-2. [Google Scholar] [CrossRef]
11. Song, X., Liang, B., Wang, C., Shi, S. (2020). Clinical value of color Doppler ultrasound combined with serum CA153, CEA and TSGF detection in the diagnosis of breast cancer. Experimental and Therapeutic Medicine, 20(2), 1822–1828. DOI 10.3892/etm.2020.8868. [Google Scholar] [CrossRef]
12. Tang, L., Zhang, X. M. (2020). Serum TSGF and miR-214 levels in patients with hepatocellular carcinoma and their predictive value for the curative effect of transcatheter arterial chemoembolization. Annals of Palliative Medicine, 9(4), 2111–2117. DOI 10.21037/apm-20-1224. [Google Scholar] [CrossRef]
13. Hu, Y., Wang, J. L., Tao, H. T., Wu, B. S., Sun, J. et al. (2013). Expression and significance of TSGF, CEA and AFP in patients before and after radical surgery for colon cancer. Asian Pacific Journal of Cancer Prevention, 14(6), 3877–3880. DOI 10.7314/APJCP.2013.14.6.3877. [Google Scholar] [CrossRef]
14. Jiang, J. T., Wu, C. P., Deng, H. F., Lu, M. Y., Wu, J. et al. (2004). Serum level of TSGF, CA242 and CA19-9 in pancreatic cancer. World Journal of Gastroenterology, 10(11), 1675–1677. DOI 10.3748/wjg.v10.i11.1675. [Google Scholar] [CrossRef]
15. Fu, J., Wang, W., Wang, Y., Liu, C., Wang, P. (2019). The role of squamous cell carcinoma antigen (SCC Ag) in outcome prediction after concurrent chemoradiotherapy and treatment decisions for patients with cervical cancer. Radiation Oncology, 14(1), 146. DOI 10.1186/s13014-019-1355-4. [Google Scholar] [CrossRef]
16. Laengsri, V., Kerdpin, U., Plabplueng, C., Treeratanapiboon, L., Nuchnoi, P. (2018). Cervical cancer markers: Epigenetics and microRNAs. Laboratory Medicine, 49(2), 97–111. DOI 10.1093/labmed/lmx080. [Google Scholar] [CrossRef]
17. Bignotti, E., Zanotti, L., Todeschini, P., Zizioli, V., Romani, C. et al. (2020). Pre-treatment serum HE4 level as a novel independent prognostic biomarker for uterine cervical carcinoma patients. Frontiers in Oncology, 10, 584022. DOI 10.3389/fonc.2020.584022. [Google Scholar] [CrossRef]
18. Radhika, T., Jeddy, N., Nithya, S., Muthumeenakshi, R. M. (2016). Salivary biomarkers in oral squamous cell carcinoma—An insight. Journal of Oral Biology and Craniofacial Research, 6(Suppl 1), S51–S54. DOI 10.1016/j.jobcr.2016.07.003. [Google Scholar] [CrossRef]
19. Grimm, M., Krimmel, M., Hoefert, S., Kraut, W., Calgéer, B. et al. (2016). Monitoring a ‘metabolic shift’ after surgical resection of oral squamous cell carcinomas by serum lactate dehydrogenase. Journal of Oral Pathology & Medicine, 45(5), 346–355. DOI 10.1111/jop.12374. [Google Scholar] [CrossRef]
20. Aliyari Ghasabeh, M., Shaghaghi, M., Pandey, A., Ameli, S., Ambale Venkatesh, B. et al. (2021). Integrating baseline MR imaging biomarkers into BCLC and CLIP improves overall survival prediction of patients with hepatocellular carcinoma (HCC). European Radiology, 31(3), 1630–1641. DOI 10.1007/s00330-020-07251-4. [Google Scholar] [CrossRef]
21. Yu, B., Xu, P. Z., Wang, Q. W., Zhou, H., Zhou, H. X. (2009). Clinical value of tumour specific growth factor (TSGF) and carbohydrate antigen-125 (CA-125) in carcinoma of the endometrium. The Journal of International Medical Research, 37(3), 878–883. DOI 10.1177/147323000903700333. [Google Scholar] [CrossRef]
Cite This Article
 Copyright © 2022 The Author(s). Published by Tech Science Press.
Copyright © 2022 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools