 Open Access
Open Access
ARTICLE
Comparison of Time-Varying Pattern of Recurrence in Chinese Breast Cancer Patients with Different Molecular Subtypes: A Single-Center Retrospective Study
1
Department of Breast Surgery, Fudan University Shanghai Cancer Center, Shanghai, 200032, China
2
Human Phenome Institute, Fudan University, Shanghai, 200032, China
3
Department of Oncology, Shanghai Medical College, Fudan University, Shanghai, 200032, China
4
Department of Cancer Prevention, Fudan University Shanghai Cancer Center, Shanghai, 200032, China
* Corresponding Authors: Benlong Yang. Email: ; Jiong Wu. Email:
# These authors contributed equally to this work and share first authorship
Oncologie 2022, 24(3), 451-469. https://doi.org/10.32604/oncologie.2022.025226
Received 29 June 2022; Accepted 12 August 2022; Issue published 19 September 2022
Abstract
Background: To compare the time-varying recurrence patterns of different molecular subtypes of breast cancer in the contemporary era with those in the past era. Patients and Methods: This retrospective study included 14627 consecutive invasive breast cancer patients who underwent surgery from 2008 to 2016 at Fudan University Shanghai Cancer Center. We defined the period from 2013 to 2016 as the contemporary era and that from 2008 to 2012 as the past era. Five subtypes were defined according to the immunohistochemistry results. Emphasis was made on the changing patterns of recurrence for patients with different molecular subtypes changed between the two treatment eras. Kaplan–Meier survival curves were generated, and the hazard function was used to estimate the annual recurrence hazard. Results: By the end of follow-up (median, 68.1 months), 1429 patients (9.77%) experienced recurrence and metastasis. The annual recurrence risks of the entire population and each molecular type in 2013–2016 were reduced compared with those in 2008–2012. Luminal A and triple-negative patients in 2013–2016 showed a significantly lower recurrence hazard curve than those in 2008–2012. The recurrence hazard curve of luminal B (HER2−) and HER2+ subtypes was lower in 2013–2016 than in 2008–2012. The recurrence risk of the luminal B (HER2+) subtype was reduced substantially during 2013–2016, showing a delay of two years. Conclusions: With early detection of disease recurrence and improved treatment strategies, the recurrence patterns of different molecular subtypes of breast cancer have changed. The time-varying recurrence patterns observed during the contemporary era are significant for treatment decision-making and clinical trial planning.Keywords
Even with all the advances in breast cancer treatment, which have decreased the mortality rate of breast cancer patients over the last few years, it remains the most common cancer and the leading cause of cancer death in women worldwide [1]. Disease recurrence and distant metastasis are the major causes of breast cancer-related death. Despite the further development of breast cancer therapeutics, approximately 20% of patients experience relapse or distant metastases at 10 years after breast surgery [2,3]. A better understanding of the risk of breast cancer recurrence will facilitate therapeutic decision-making and help clinicians conduct appropriate follow-up.
Breast cancer is a heterogeneous disease composed of distinct biological subtypes with different risk factors, natural histories, and therapeutic responses and outcomes. Based on the expression of estrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor type 2 (HER2) and Ki67, breast cancer is currently classified into five subtypes [4]: luminal A-like, luminal B-like (HER2 negative), luminal B-like (HER2 positive), HER2 positive (non-luminal) and triple negative (ductal). The luminal A subtype has the best prognosis, whereas the triple-negative subtype is the most aggressive. Although patients continue to experience relapse and metastasis, several studies have shown that patterns of recurrence between different molecular subtypes are different [5–9]. It appeared that luminal A-like patients have a significantly lower risk of recurrence during the first 5 years after treatment compared to other subtypes [10]. Compared to patients with hormone receptor (HR)-positive/HER2-negative tumors, those with triple-negative tumors were found to have a greater risk of brain or lung metastases and had worse breast cancer-specific survival outcomes [11]. In recent years, there have been an increasing number clinical trials on the treatment of different molecular subtypes of breast cancer, and their treatment strategies are constantly changing. Regarding ER/PR-positive breast cancer, extended adjuvant endocrine therapy has significantly improved disease-free survival outcomes and has decreased the relapse of contralateral breast cancer [12]. For breast cancer patients with intermediate or high risk of relapse, especially for ER/PR-negative subtypes, adjuvant chemotherapy is recommended [13,14]. With early detection and advances in precision treatment strategies, the time-varying recurrence risk and patterns of postoperative breast cancer patients have begun to change.
Although many studies abroad have reported differences in survival and recurrence patterns according to breast cancer subtypes, few studies have been conducted in the Chinese population. Furthermore, in both Western and Chinese populations, few studies have compared the contemporary recurrence pattern of breast cancer with that in the past. In this large single-center retrospective study, we aimed to investigate the recurrence risk of early breast cancer patients with different molecular subtypes and explore whether the recurrence pattern has changed during the contemporary treatment era by comparing the latest recurrence patterns of different molecular subtypes (from 2013 to 2016) with those from the past treatment era (from 2008 to 2012).
2.1 Patient Selection and Definition of Molecular Subtypes
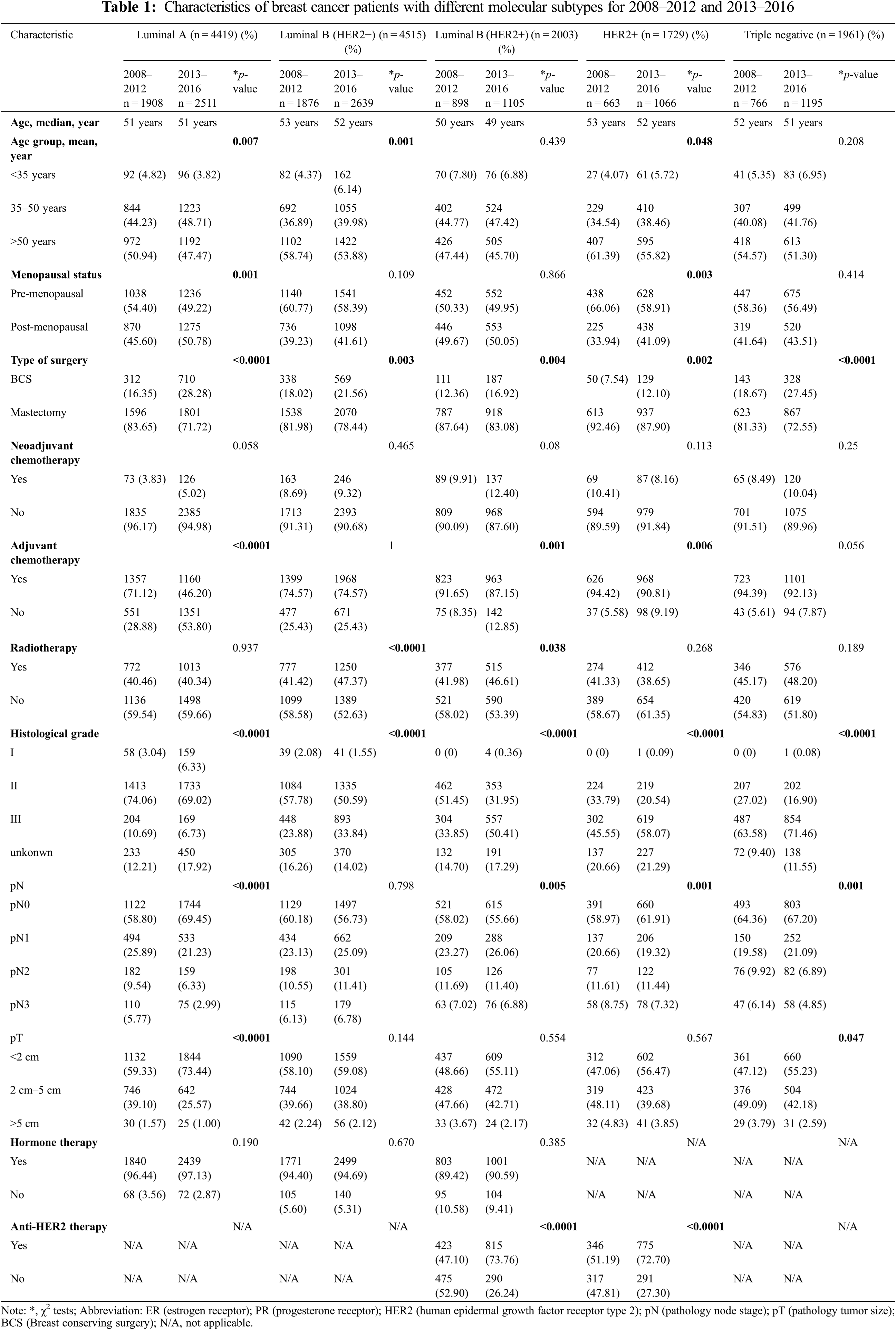
This investigation was a single-center retrospective study conducted at Fudan University Shanghai Cancer Center (FUSCC). From January 2008 to December 2016, a total of 14627 female patients with newly diagnosed I–III unilateral primary invasive breast cancer who underwent surgery were consecutively recruited. We defined the period from 2013 to 2016 as the contemporary era and the period from 2008 to 2012 as the past era. Data on patient and tumor characteristics were collected, including age, menopausal status, type of surgery, neoadjuvant chemotherapy, adjuvant chemotherapy, radiotherapy, histological grade, pathology tumor size (pT), pathology node stage (pN), hormone therapy and anti-HER2 therapy. pT and pN stages were identified according to the eighth edition of the AJCC cancer staging manual [15]. All patients received complete imaging and physical examination and were confirmed to have no distant metastasis before surgery. All primary tumors were treated with mastectomy or lumpectomy, and patients were treated according to the standards used at the time of surgery.
Immunohistochemical (IHC) staining of ER, PR, HER2, Ki67 and FISH (fluorescence in situ hybridization) were carried out in the Pathology Department of Fudan University Shanghai Cancer Center (FUSCC). All slides were examined by two pathologists, and any disagreements were resolved by a third expert. Tumors with HER2 scores of 0 or 1+ were considered negative, and those with scores of 3+ were considered HER2 positive. For borderline HER2 (2+) staining, HER2 status was confirmed by FISH [16]. Based on the 2013 St Gallen consensus [4], we used a cutoff of 20% for the Ki67 index to divide the breast cancer cases into five molecular subtypes: (1) luminal A: ER and PR positive, HER2 negative and Ki67 < 20%; (2) luminal B (HER2−): ER positive, HER2 negative and at least Ki67 ≥ 20% or PR “negative” or “low”; (3) luminal B (HER2+): ER positive, HER2 positive, any PR status and Ki67 ≥ 20%; (4) HER2 positive (HER2+): HER2 overexpression or amplification and the absence of ER and PR expression; and (5) triple negative: HER2 negative and the absence of ER and PR expression.
All patients were told to undergo examinations to ensure complete follow-up data. Routine follow-up data were collected every 3 months during the first 2 years after surgery, every 6 months during the next 2 years and then once a year after 5 years, whether in our hospital or others. The data were updated periodically by retrieving outpatient follow-up medical records or with information collected from follow up visits by telephone. In this study, each patient was followed up once after surgery, and the last follow-up time was March 2021. Recurrence was diagnosed through clinical evaluation including imaging examination or biopsy. Types of recurrence were divided into four types: local relapse (LR), regional relapse (RR), contralateral breast cancer (CBC) and distant metastasis (DM). LR was defined as invasive breast cancer in ipsilateral breast tissue or ipsilateral chest and subcutaneous tissue. RR was defined as recurrence and metastasis in the ipsilateral breast lymph node drainage area, including the ipsilateral axillary lymph nodes, internal mammary lymph nodes, subclavian lymph nodes and supraclavicular lymph nodes. DM was defined as spread to distant organs, including bone, lung, liver, brain and distant lymph node metastasis. Recurrence-free survival (RFS) was defined as the time from surgery to the earliest time of recurrence.
Differences in the clinical characteristics and recurrence types between the different groups were examined by χ2 tests. To compare the RFS outcomes across different molecular subtypes and different years of diagnosis, survival curves were generated by the Kaplan–Meier method with the log-rank test. We used a multivariable Cox regression model to further confirm the factors associated with RFS. The hazard function was used to measure the annual hazard of recurrence, and the Kernel method of smoothing was used to display the time-varying distribution of recurrence in breast cancer [17]. All analyses were performed using IBM SPSS Statistics (Version 26.0), and p < 0.05 was considered statistically significant.
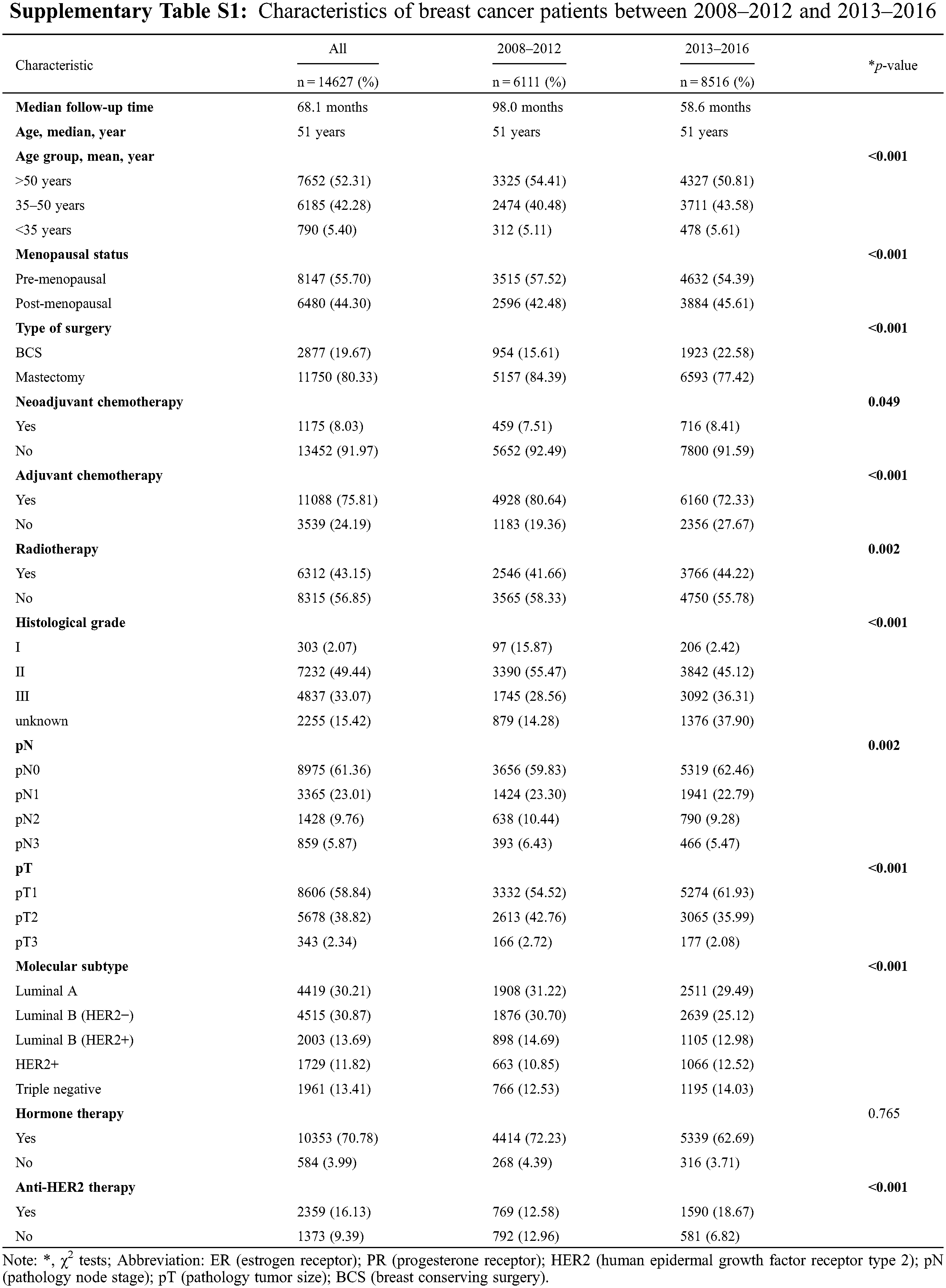
Of the entire population, 6111 patients (41.787%) were diagnosed between 2008 and 2012, while 8516 patients (58.22%) were diagnosed between 2013 and 2016. The characteristics are listed in Supplementary Table S1. The median follow-up time was 68.1 months (98 months in 2008–2012 and 58.6 months in 2013–2016). The majority of patients had luminal A (30.21%, 4419/14627) and luminal B (HER2−) (30.87%, 4515/14627) tumors, followed by luminal B (HER2+) (13.69%, 2003/14627), triple-negative (13.41%, 1961/14627) and HER2+ (11.82%, 1961/14627) tumors. We found that patients were younger and had lower pN and pT stages in 2013–2016 than in 2008–2012 (p < 0.0001). Between 2008 and 2012, 15.61% of patients received BCS, whereas 22.58% of patients received BCS between 2013 and 2016 (p < 0.0001). More patients received radiotherapy in 2013–2016 than in 2008–2012 (p= 0.002). The proportion of patients who received neoadjuvant chemotherapy in 2013–2016 increased from 7.51% to 8.41% (p = 0.049); in contrast, the proportion of who received adjuvant chemotherapy decreased from 80.64% to 72.33% compared with that in 2008–2012 (p < 0.0001).
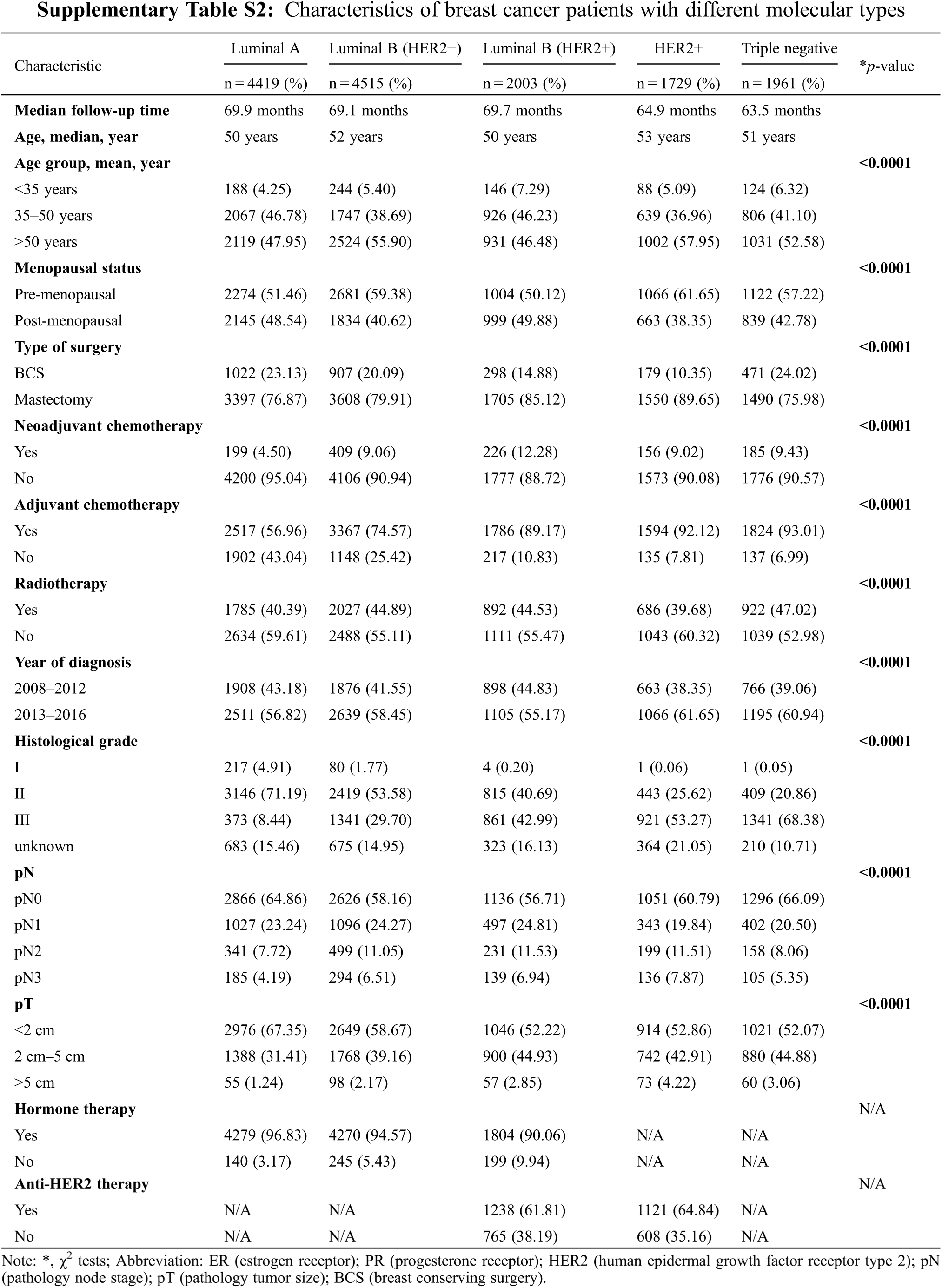
Differences in the baseline characteristics of each molecular subtype are presented in Supplementary Table S2. Luminal A and luminal B (HER−) tumors tended to present with a lower histological grade and smaller tumor size (p < 0.001). More HER2+ and triple-negative patients had received adjuvant chemotherapy compared with other subtypes. The characteristics of patients with different molecular subtypes between 2008–2012 and 2013–2016 are shown in Table 1. During 2013–2016, except for luminal B (HER2−) tumors, each type of tumor presented a lower pN stage than during 2008–2012. Compared with patients in 2008–2012, luminal A tumors had a lower histological grade in 2013–2016; in contrast, other subtypes had a higher histological grade in 2013–2016. More cases of BCS were performed across all subtypes of breast cancer in 2013–2016 than in 2008–2012. In hormone receptor (HR)−positive patients, the proportion of hormone therapy did not change, but the proportion of HER2−positive patients who received anti-HER2 therapy greatly increased in 2013–2016 compared with that in 2008–2012.
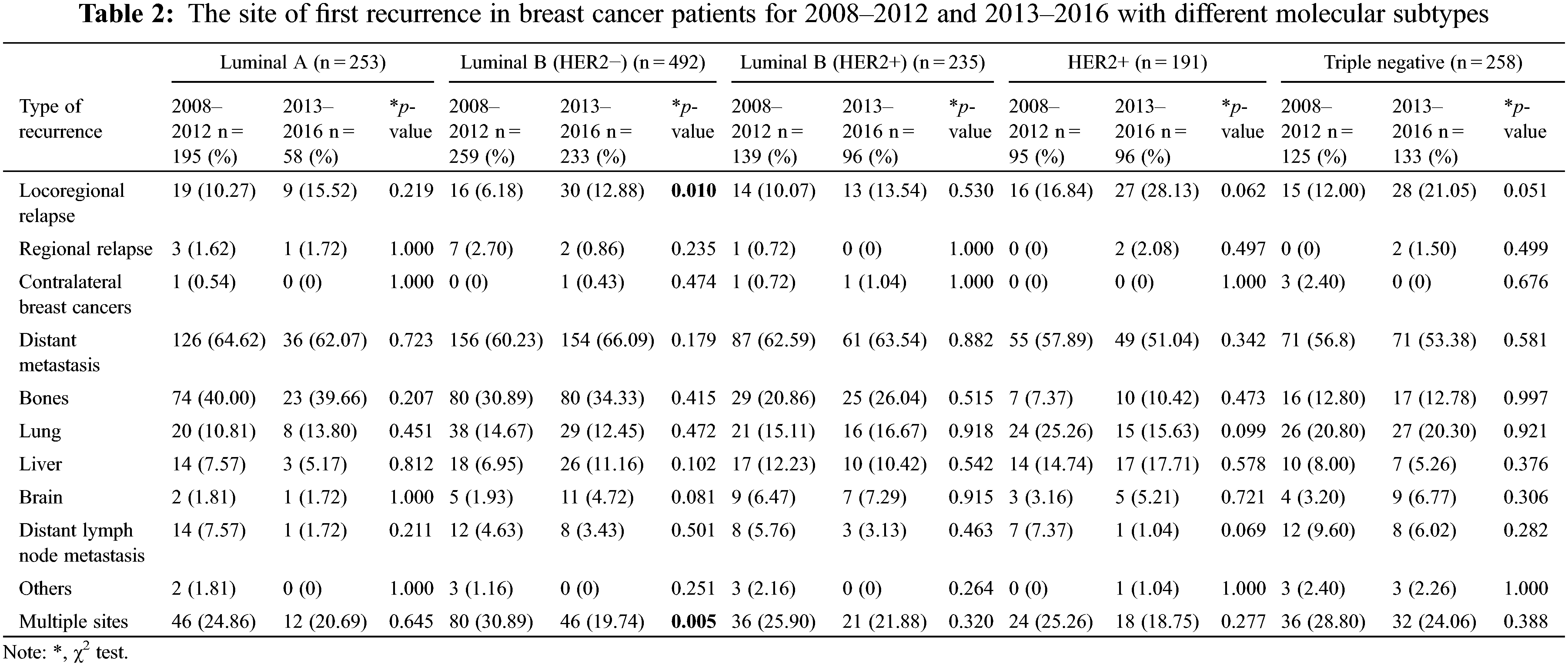
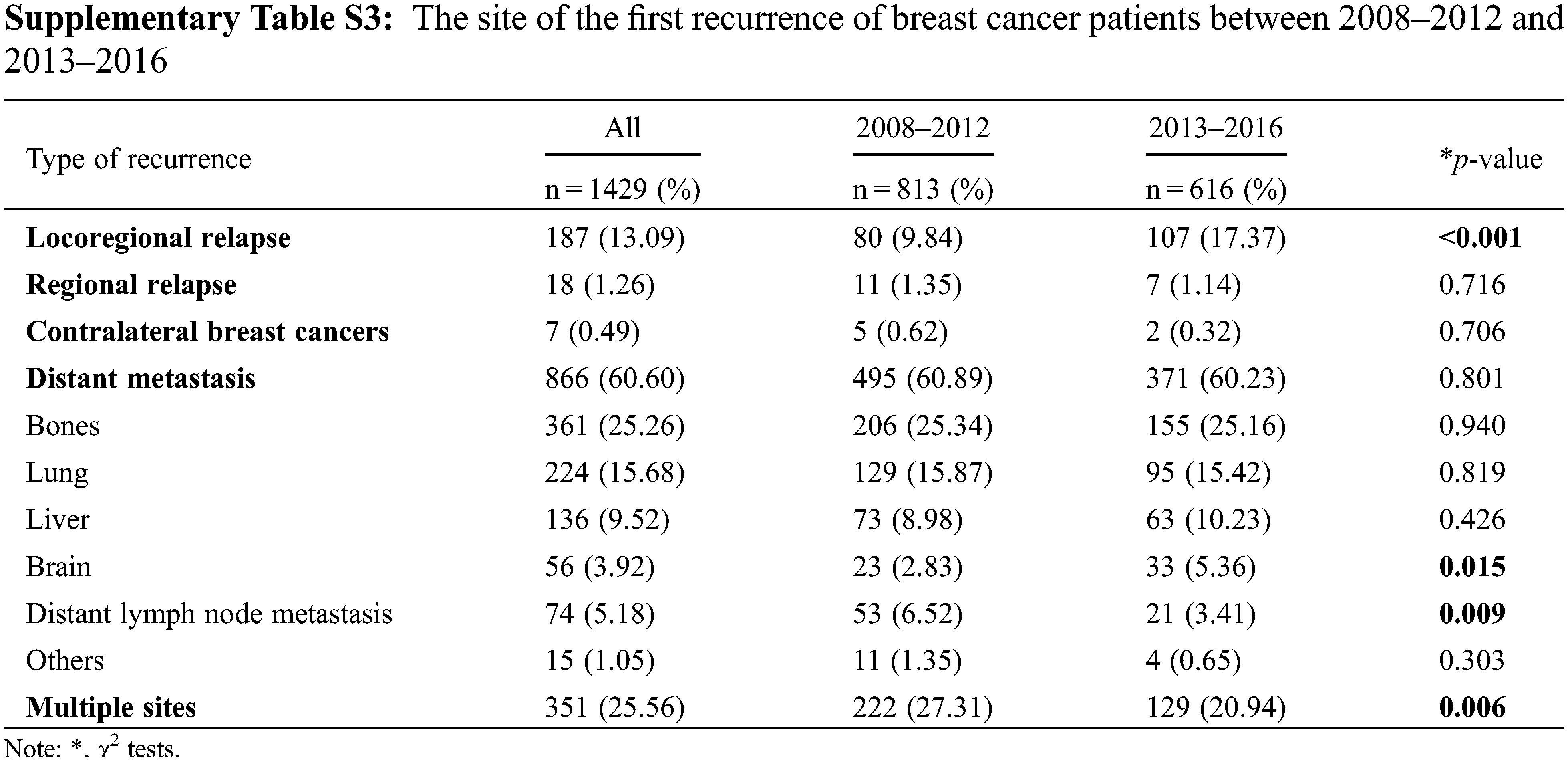
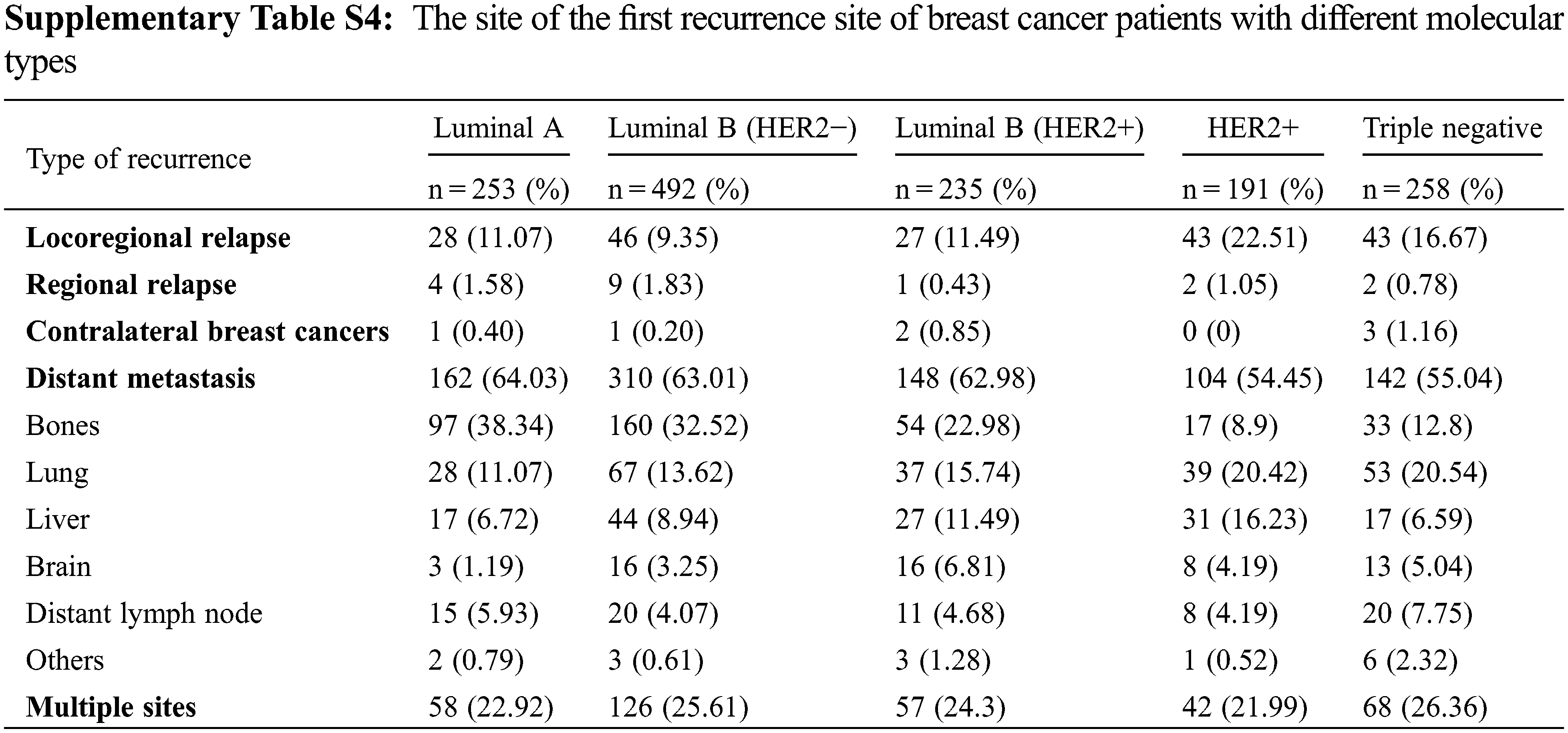
The site of the first recurrence in 2008–2012 and 2013–2016 are presented in Supplementary Table S3. Among all patients, 1429 patients (9.77%) experienced recurrence, of whom 187 patients (13.09%) had LR, 18 patients had RR (1.26%), 7 patients had (0.49%) CBC, and 866 patients (60.60%) had DM. In addition, 351 patients (25.56%) experienced more than one type of recurrence. Compared with the period from 2008–2012, during the period from 2013–2016, the proportion of patients experiencing LR and distant brain metastasis increased, and the proportion of patients experiencing distant lymph node and multiple site metastasis decreased. The site of the first recurrence site of breast cancer patients with different molecular types are presented in Supplementary Table S4. The recurrence rate of triple-negative tumors was the highest (13.16%, 258/1961), followed by that of luminal B (HER2+) tumors (11.73%, 235/2003), HER2+ tumors (11.05%, 191/1729), luminal B (HER2−) tumors (5.91%, 249/4215) and luminal A tumors (5.73%, 253/4419). The proportion of patients with bone metastasis was higher than the proportion of patients with metastases at other sites among those with luminal A (38.34%), luminal B (HER2−) (32.52%) and luminal B (HER2+) (22.98%) tumors. The LR rate and lung metastasis rate were notably high in triple-negative tumors and HER2−positive tumors. The first recurrence sites among different breast cancer subtypes in 2013–2016 did not change relative to 2008–2012, except for luminal B (HER2−) tumors, for which the proportion of patients experiencing LR increased from 6.18% to 12.88% (Table 2).
3.3 Relapse-Free Survival Analysis
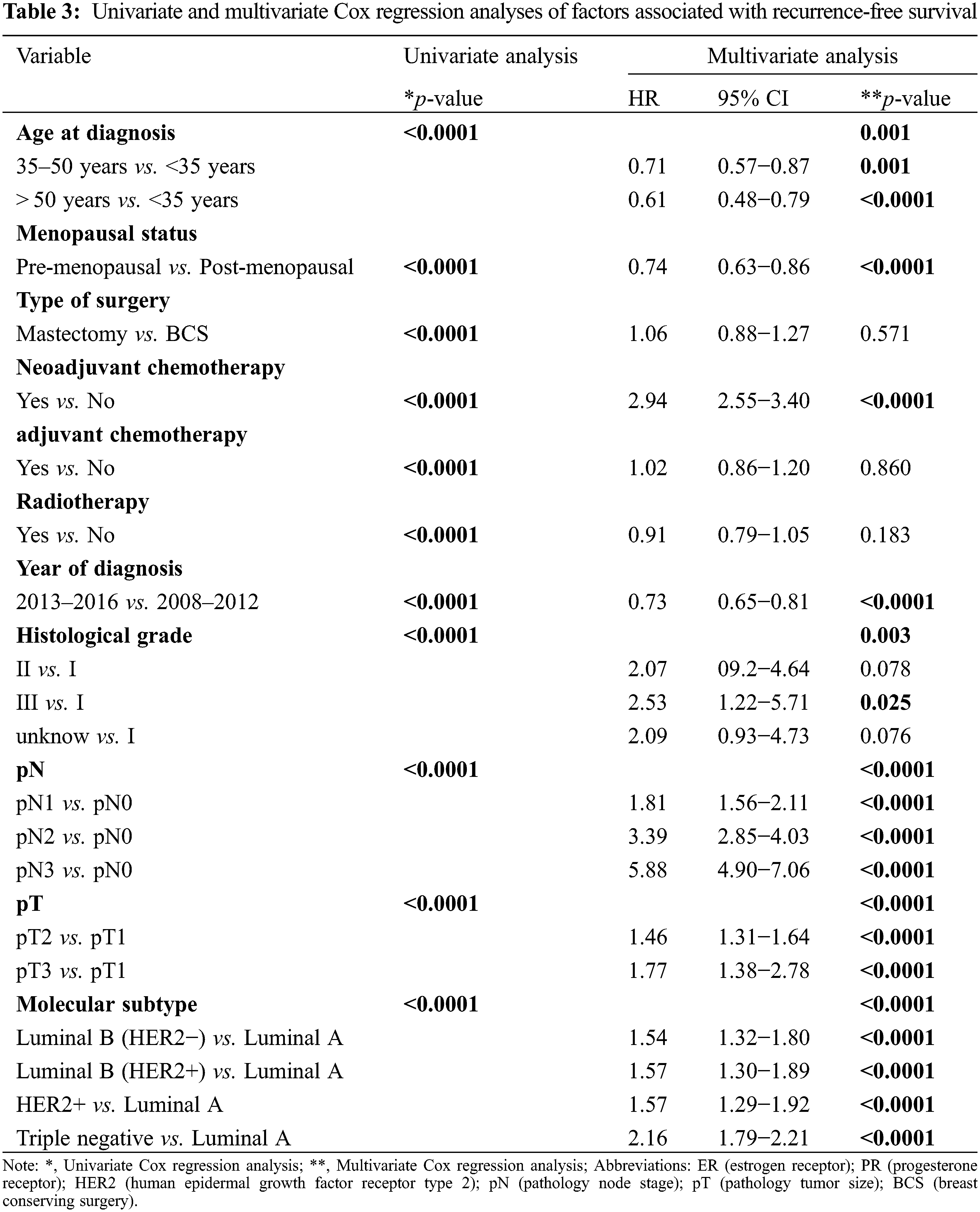
Up to the last follow-up time, 1429 patients (9.77%) experienced disease relapse. Survival analysis showed that age, menopausal status, type of surgery, neoadjuvant chemotherapy, adjuvant chemotherapy, radiotherapy, year of diagnosis, histological grade, pN status, pT status and molecular subtype were related to RFS. A Cox proportional hazards regression model further proved that age, menopausal status, neoadjuvant chemotherapy, year of diagnosis, histological grade, pN status, pT status and molecular subtypes were significant predictors of recurrence-free survival (RFS) (Table 3).
Patients with luminal A tumors showed the best survival after recurrence, and those with triple-negative tumors showed the worst survival after recurrence (Fig. 1A). The patients diagnosed in 2013–2016 showed obvious improvement in recurrence-free survival compared with those diagnosed in 2008–2012 (p < 0.001) (Fig. 1B). In 2013–2018, the recurrence-free survival (RFS) of patients with luminal A (p < 0.001), luminal B (HER2+) (p = 0.005), HER2+ (p = 0.044) and triple-negative tumors (p = 0.014) significantly decreased compared with that in 2008–2012 (Figs. 2A, 2C–2F), except in those with luminal B (HER2−) tumors (Fig. 2B) (p = 0.397).
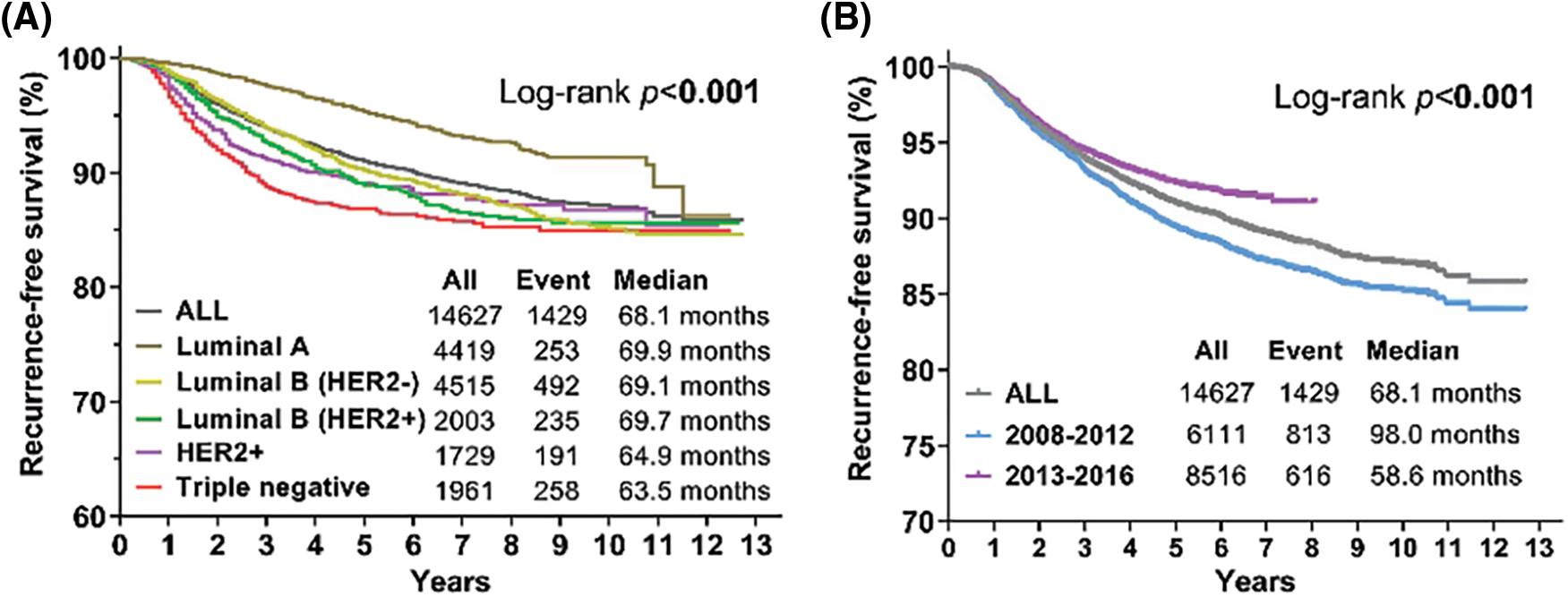
Figure 1: Kaplan-Meier curves for recurrence-free survival (RFS) according to different molecular subtypes and different periods. (A) Kaplan-Meier estimates of RFS of all patients and different molecular types. (B) Kaplan-Meier estimates of RFS between 2008–2012 and 2013–2016. Log-rank p values are shown
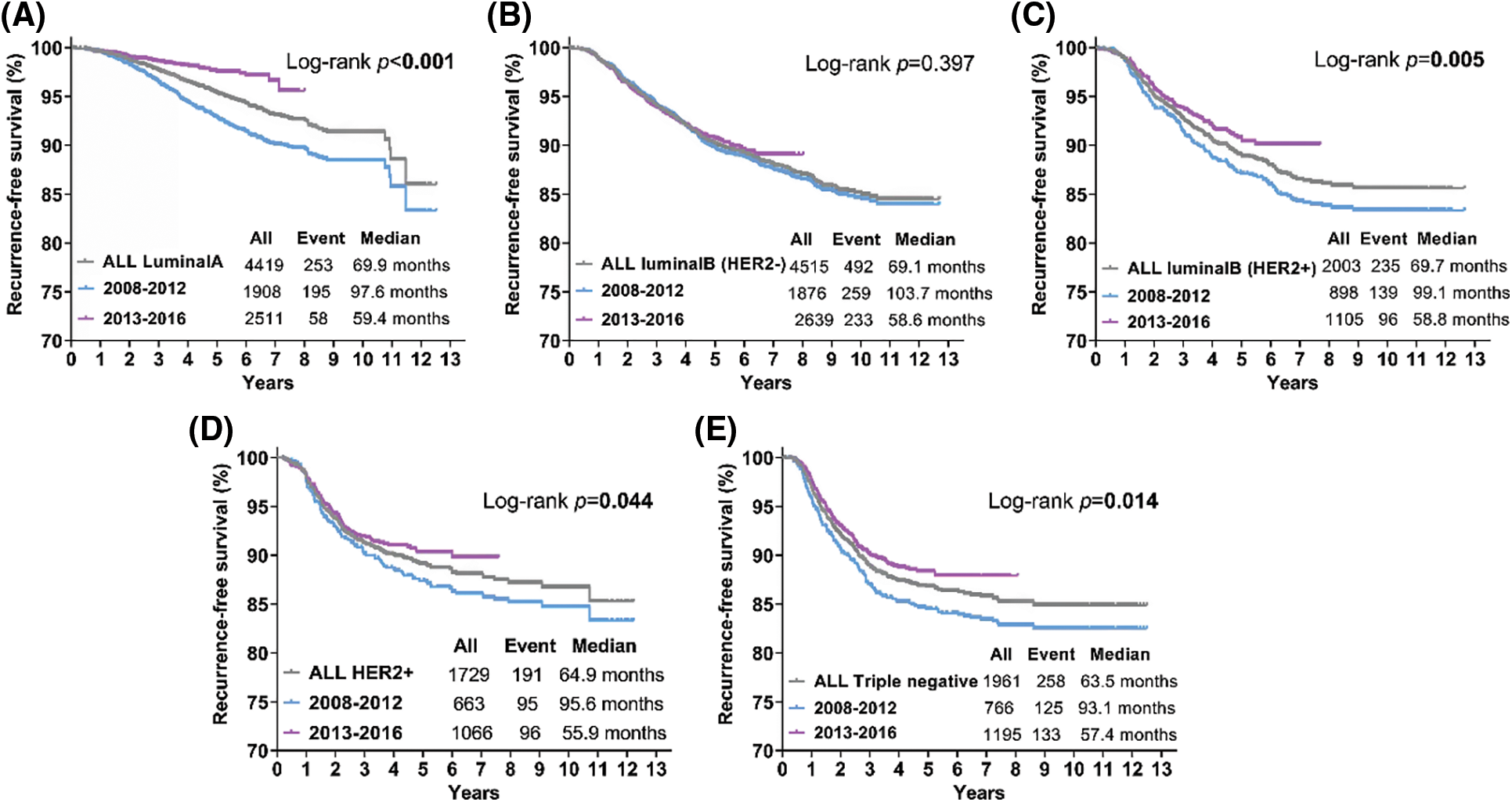
Figure 2: Kaplan-Meier curves for recurrence-free survival (RFS) according to different molecular subtypes for 2008–2012 and 2013–2016. (A) Kaplan-Meier estimates of RFS for luminal A (A), luminal B (HER2−) (B), luminal B (HER2+) (C), HER2+ (D) and triple-negative (E) breast tumors. Log-rank p values are shown
3.4 Recurrence Hazard Analysis
The annual recurrence hazard curve for all populations showed a three-peak pattern, with the first and maximum recurrence peak at the first year after surgery and subsequent recurrence peaks at the sixth and ninth years (Fig. 3A). Similar to the entire population, luminal B (HER2−) tumors showed a three-peaked recurrence pattern, with peaks at the second, sixth and tenth years after surgery. Luminal A tumors displayed a double-peaked recurrence pattern, having the slowest increase in risk compared with other subtypes, reaching its first peak at five years after surgery. Luminal B (HER2+) tumors showed a one-peaked recurrence pattern, with maximum risk at the third year. Among all subtypes, HER2+ and triple-negative tumors demonstrated the earliest and highest risks of recurrence in the first three years after initial treatment, with the first and maximum peak appearing at the first year after surgery (Fig. 3A). For HER2+ tumors, the recurrence risk increased rapidly and remained at a high level from five to ten years after treatment. Triple-negative tumors showed a double-peaked recurrence pattern, with the second peak occurring at the sixth year, with the recurrence then remaining low. According to the year of diagnosis, the annual recurrence risk of the entire population was obviously reduced in the 2013–2016 period compared with the 2008–2012 period, but the double-peaked pattern of recurrence did not change over the seven years of follow-up after surgery (Fig. 3B).
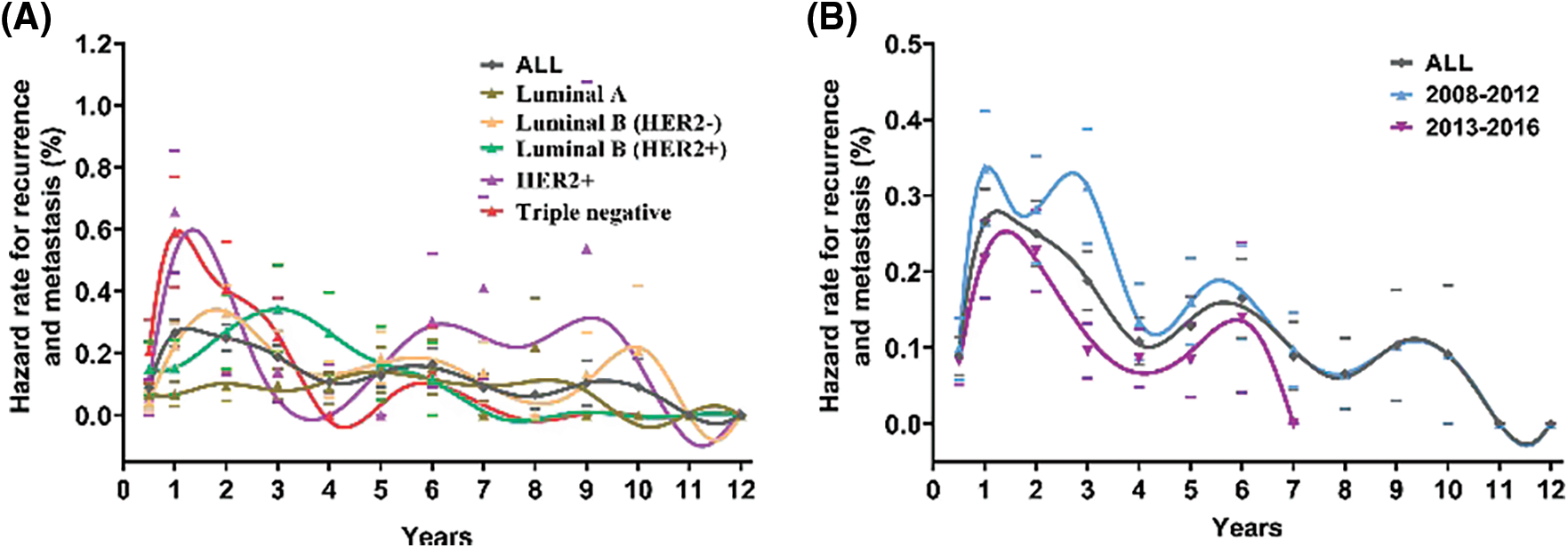
Figure 3: Annal recurrence rate curves for patients. (A) Annal recurrence rate curve for patients with different breast cancer molecular subtypes. (B) Annal recurrence rate curve for all patients for 2008–2012 and 2013–2016. The scale on the y-axis of each figure varies. The hazard rates of recurrence and metastasis were estimated within a 1-year interval. Smoothed curves were obtained by a Kernel-like smoothing procedure. The standard error for single points are also reported
To further investigate whether contemporary treatment changed the recurrence pattern of different molecular subtypes of breast cancer, we compared the annual recurrence hazards between 2008–2013 and 2013–2016. The recurrence hazard analysis revealed that luminal A and triple-negative patients experienced a significantly lower recurrence hazard curve in 2013–2016 than in 2008–2012 (Figs. 4A and 4E). Luminal A tumors in 2008–2012 presented a triple-peaked recurrence pattern, but those in 2013–2016 showed a one-peaked recurrence pattern, with the recurrence risk being reduced to a very low level after the peak at the second year (Fig. 4A). The annual recurrence risk of luminal B (HER2−) and HER2+ tumors was slightly reduced in 2013–2016 compared with 2008–2012 (Figs. 4B and 4D). For luminal B (HER2+) in 2013–2016, its recurrence peak was delayed to the fifth year compared with that in 2008–2012, in which it peaked at the third year after surgery (Fig. 4C).
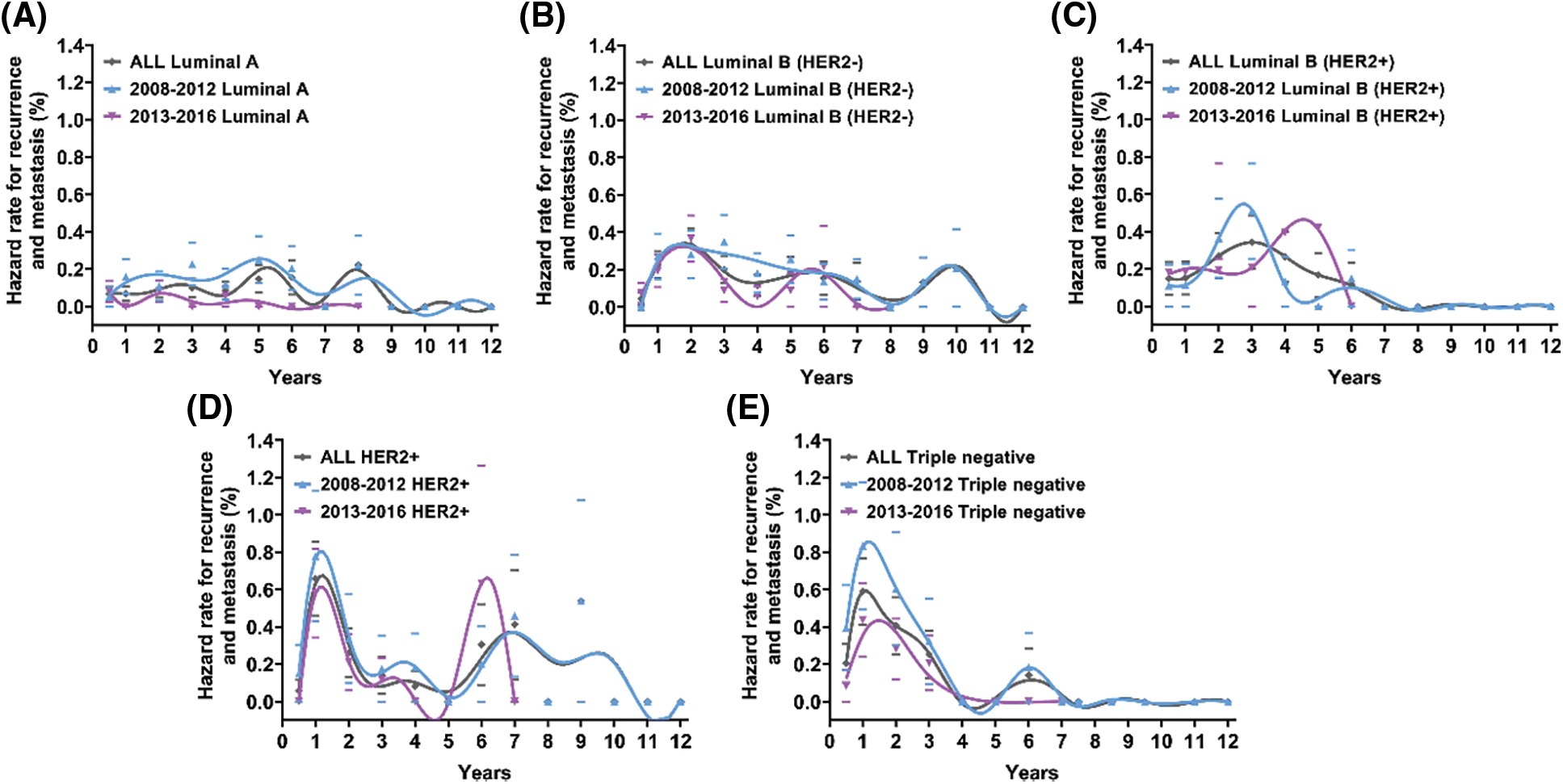
Figure 4: Annal recurrence hazard rate for patients with different molecular subtypes for 2008–2012 and 2013–2016. Hazard rates of recurrence and metastasis were estimated within a 1-year interval. Smoothed curves were obtained by a Kernel-like smoothing procedure. Standard error for single points are also reported
This study is the first and largest retrospective analysis to identify different recurrence patterns of different molecular subtypes of breast cancer and to compare whether the outcomes and recurrence patterns of early breast cancer in the Chinese population had changed during the contemporary treatment era.
The association of different molecular subtypes at diagnosis with survival in breast cancer has been widely analyzed. Consistent with previous studies [10,18], we proved that different breast cancer subtypes and different diagnosis years were related to relapse-free survival. Multivariable analysis illustrated the independent prognostic value of the year of diagnosis and breast cancer subtype. In most recent studies, hazard functions were used to analyze the risk of recurrence for cancer compared with survival curves [6,19]. While survival curves only provide information on the cumulative time distribution of the recurrence rate, hazard functions can describe the recurrence rate at any point in time [20]. In different races or during different periods since diagnosis, the risk of disease recurrence may be different. A 10-year follow-up retrospective study of breast cancer conducted with SEER-Medicare data for the period between 1991 and 1997 showed that the greatest frequency of the first recurrence occurred between 0 and 5 years after the initial diagnosis [21]. In 2009, our center reported a double-peaked recurrence pattern for breast cancer in the Chinese population according to the patients diagnosed in the FUSCC between 1992 and 2003. These results are similar to those obtained with SEER data in the early follow-up years, but the difference is that the recurrence risk suddenly increased between the ninth and tenth years in the Chinese cohort [17]. In our study, we also used hazard function to investigate changes in the risk of breast cancer recurrence over nearly 12 years of follow-up time in patients who underwent surgery at our center from 2008 to 2016. We revealed a triple-peaked recurrence pattern for the entire population, with the risk of recurrence remaining high during the first 3 years after treatment and then gradually decreasing, with two other small recurrence peaks appearing at the sixth and ninth years. Different periods of diagnosis years can represent different treatment eras. We defined the period from 2013 to 2016 as the contemporary era and the period from 2008 to 2012 as the past era. Patients diagnosed during 2013–2018 demonstrated a great reduction of recurrence compared with those diagnosed during 2008–2012. Recurrence hazard curves showed that the double-peaked pattern of recurrence or the peak years did not change over 7 years of follow-up after surgery, but the annual disease recurrence hazard in 2013–2016 decreased meaningfully compared with that in 2008–2012. We also observed that the proportion of patients with pT1 patients increased from 54.52% in 2008–2012 to 61.93% in 2013–2016, while the proportion of pN0 patients increased from 59.83% to 62.46%. During the 2013–2018 period, more patients received BCS, neoadjuvant chemotherapy, radiotherapy and anti-HER2 therapy, while fewer people received adjuvant chemotherapy than in 2008–2012. We considered that the improvements in RFS during 2013–2016 may be attributed to the early detection of breast cancer and the use of more comprehensive and appropriate treatment.
Various studies, including ours, have examined recurrence hazard curves based on the subtypes of breast cancer and concluded that each subtype exhibits a particular pattern of recurrence over time [22]. In our study, we proved that the recurrence risk of luminal A patients was the lowest, and the risk in 2013–2016 showed a decrease to nearly half of that in 2008–2012. The recurrence pattern of luminal A tumors changed to a one-peaked pattern, and the recurrence risk decreased slowly to a very low level after reaching a peak at the second year despite having a triple-peaked recurrence pattern in the past era. The reasons for these changes might be due to some tremendous advances in endocrine therapy for hormone receptor (HR)-positive breast tumors over the past decade. The MA-17 [12] trial proved that extended adjuvant endocrine therapy could improve survival and decrease the relapse of contralateral breast cancer. Evidence has suggested that suppression of ovarian estrogen production could reduce the early recurrence of hormone receptor (HR)-positive breast cancer [23,24]. For young premenopausal women with HR-positive tumors who have sufficient risk of recurrence, the SOFT and TEXT trials [25] showed that treatment with an aromatase inhibitor plus ovarian suppression conferred a great survival benefit compared to tamoxifen plus ovarian therapy. Despite the great survival improvement observed for patients with luminal A tumors, there have been no survival benefits for patients with luminal B (HER2−) tumors. The recurrence pattern of luminal B (HER2−) tumors in the contemporary era remains similar to that in the past era. The Ki67 index was found to be an independent prognostic factor for RFS in luminal-like patients, and the proliferation activity of the Ki67 index is important for distinguishing luminal A from luminal B (HER-) subtypes. Histological grade was also reported to be an independent prognostic factor for all breast cancer patients, especially for luminal-like breast cancer patients [26]. In our population, luminal B (HER2−) patients who were diagnosed in 2013–2016 had a significantly higher histological grade than those diagnosed in 2008–2012, and the proportions of patients who received chemotherapy, radiotherapy or hormone therapy did not change. This may be main reason why patients with luminal B (HER2−) tumors did not receive any survival benefit, indicating that more appropriate treatment should be offered to modern luminal B (HER2−) patients.
It has been proven that standard adjuvant treatment of anti-HER2 therapy in HER2+ positive early breast cancer can substantially reduce the risk of recurrence and improve survival [27,28]. We confirmed this benefit again in the present study. We observed that more than seventy percent of HER2−positive patients received anti-HER2 targeted therapy in 2013–2016, and a notable survival benefit was conferred to both luminal B (HER2+) and HER2+ patients compared with 2008–2012. The recurrence risk of luminal B (HER2+) and HER2+ tumors was significantly attenuated in 2013–2016, even for luminal B (HER2+) tumors, which showed a delayed recurrence peak from the third year to the fifth year after surgery compared with that in 2008–2012. For luminal B (HER2+) tumors, the delayed recurrence peak might be due to not only the progress made in anti-HER2 therapy but also advancements in hormone therapy. With the results of numerous clinical trials on chemotherapy regimens, continuous improvements in outcomes have been achieved for patients with triple-negative breast cancer [29–38]. Fixx [14] demonstrated that adjuvant capecitabine in combination with docetaxel, epirubicin, and cyclophosphamide improved relapse-free survival for triple-negative patients. The CBCSG010 trial [36] illustrated a disease-free survival and overall survival benefit of the addition of capecitabine in early triple-negative tumors. In the present study, triple-negative tumors were the most aggressive breast cancer subtype and exhibited a double-peaked recurrence pattern, showing recurrence peaks in the first and sixth years over 12 years of follow-up. In contrast, triple-negative patients showed a significantly lower recurrence hazard curve than that in 2008–2012, with the double-peaked recurrence pattern changing to a one-peaked pattern.
Above all, we herein describe the latest recurrence patterns of early breast cancer in the Chinese population stratified according to molecular subtype. One of the main strengths of our study is that the clinical features and follow-up data of patients were collected at a single center. The Fudan University Cancer Center is one of the largest cancer centers in China, and the treatment strategies used at this center have been normalized according to the latest clinical trial results, thereby eliminating the bias caused by patient selection or irregular treatment. Another significant strength of the current study is that it is the largest retrospective analysis based on the pattern of recurrence for breast cancer in a Chinese population, and patients were followed over a long period of time. Our study included patients treated up to 2016, and we compared the recurrence patterns of the contemporary era (from 2013 to 2016) with those from the past era (from 2008 to 2012). As a result, our research also covers recent clinical practice and treatment results. The most vital strength of our study is that we not only investigated the recurrence patterns of different molecular subtypes but also compared them between the contemporary era and the past era. We found a significant survival benefit for patients with luminal A, luminal B (HER2+), HER2+ and triple-negative tumors in the contemporary era. However, this benefit was not obvious for patients with luminal B (HER2−) tumors, and we discussed the possible reasons. To our knowledge, few studies [39–41] have compared the differences in recurrence patterns according to different breast cancer subtypes, and our study is the first and largest study of the Chinese population. On the other hand, this study has a few limitations, which should be mentioned. First, the results we observed were only for invasive breast cancer and might not be applicable to other pathological subtypes, such as ductal carcinoma in situ and lobular carcinoma in situ. Second, the follow-up time of the population was shorter during the 2013–2016 period than during the 2008–2012 period, so the calculated recurrence hazards for later years after surgery might be inaccurate. A longer follow-up interval is needed to confirm the recurrence risk beyond 10 years after surgery. Finally, we did not analyze the detailed treatment differences based on each breast cancer subtype. Due to the inherent limitations of retrospective studies, treatment data on ovarian suppression or the type of hormone therapy administered were not included in our database, especially for luminal-like patients. Nevertheless, the improvement in the recurrence hazard during the 2013–2016 period likely reflects the efficacy of modern evidence-based treatment.
We proved that significant improvements in relapse-free survival have been achieved in recent years and that the recurrence patterns of early breast cancer patients with different subtypes have also changed with advancements in breast cancer therapy. Acknowledging the specific disease recurrence patterns of each breast cancer subtype during the contemporary period is of upmost importance to adequately inform patients of their prognosis, as well as to guide physicians in making appropriate treatment decisions and planning future clinical trials.
Acknowledgement: We would like to thank the staff members of the Committee of Breast Cancer Society and the Chinese Society of Breast Surgeons working group for participant recruitment and data collection.
Authorship: The authors confirm contribution to the paper as follows: study conception and design: XJ Z and BL Y; data collection: MM, YT S and JJ H; analysis and interpretation of results: XJ Z and BL Y; draft manuscript preparation: XJ Z, JJ C and JW. All authors reviewed the results and approved the final version of the manuscript.
Ethics Approval and Informed Consent Statement: The study was approved by The Fudan University Shanghai Cancer Center Ethics Committee (Approval No. 1905202-2). Informed consent was not required since this was a retrospective study and all data with personal identifiers were kept confidential.
Availability of Data and Materials: The datasets used or analysed during the current study are available from the corresponding author on reasonable request.
Funding Statement: This work was supported by National Key R&D Program of China (2017YFC1311004).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. IARC (2020). Latest global cancer data: Cancer burden rises to 19.3 million new cases and 10.0 million cancer deaths in 2020. https://www.iarc.who.int/wp-content/uploads/2020/12/pr292_E.pdf. [Google Scholar]
2. Wirtz, H. S., Boudreau, D. M., Gralow, J. R., Barlow, W. E., Gray, S. et al. (2014). Factors associated with long-term adherence to annual surveillance mammography among breast cancer survivors. Breast Cancer Research and Treatment, 143(3), 541–550. DOI 10.1007/s10549-013-2816-3. [Google Scholar] [CrossRef]
3. Early Breast Cancer Trialists’ Collaborative Group, Darby, S., McGale, P., Correa, C., Taylor, C. et al. (2011). Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: Meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet, 378(9804), 1707–1716. DOI 10.1016/S0140-6736(11)61629-2. [Google Scholar] [CrossRef]
4. Goldhirsch, A., Winer, E. P., Coates, A. S., Gelber, R. D., Piccart-Gebhart, M. et al. (2013). Personalizing the treatment of women with early breast cancer: Highlights of the St Gallen International expert consensus on the primary therapy of early breast cancer 2013. Annals of Oncology, 24(9), 2206–2223. DOI 10.1093/annonc/mdt303. [Google Scholar] [CrossRef]
5. Buonomo, O. C., Caredda, E., Portarena, I., Vanni, G., Orlandi, A. et al. (2017). New insights into the metastatic behavior after breast cancer surgery, according to well-established clinicopathological variables and molecular subtypes. PLoS One, 12(9), e0184680. DOI 10.1371/journal.pone.0184680. [Google Scholar] [CrossRef]
6. van Maaren, M. C., de Munck, L., Strobbe, L. J. A., Sonke, G. S., Westenend, P. J. et al. (2019). Ten-year recurrence rates for breast cancer subtypes in the Netherlands: A large population-based study. International Journal of Cancer, 144(2), 263–272. DOI 10.1002/ijc.31914. [Google Scholar] [CrossRef]
7. Shim, H. J., Kim, S. H., Kang, B. J., Choi, B. G., Kim, H. S. et al. (2014). Breast cancer recurrence according to molecular subtype. Asian Pacific Journal of Cancer Prevention, 15(14), 5539–5544. DOI 10.7314/APJCP.2014.15.14.5539. [Google Scholar] [CrossRef]
8. Lee, Y., Kang, E., Lee, A. S., Baek, H., Kim, E. K. et al. (2015). Outcomes and recurrence patterns according to breast cancer subtypes in Korean women. Breast Cancer Research and Treatment, 151(1), 183–190. DOI 10.1007/s10549-015-3390-7. [Google Scholar] [CrossRef]
9. Park, Y. H., Lee, S. J., Jung, H. A., Kim, S. M., Kim, M. J. et al. (2015). Prevalence and clinical outcomes of young breast cancer (YBC) patients according to intrinsic breast cancer subtypes: Single institutional experience in Korea. Breast, 24(3), 213–217. DOI 10.1016/j.breast.2015.01.012. [Google Scholar] [CrossRef]
10. Fernandez, A. G., Chabrera, C., Garcia Font, M., Fraile, M., Gonzalez, S. et al. (2013). Differential survival and recurrence patterns of patients operated for breast cancer according to the new immunohistochemical classification: Analytical survey from 1997 to 2012. Tumour Biology, 34(4), 2349–2355. DOI 10.1007/s13277-013-0782-3. [Google Scholar] [CrossRef]
11. Lin, N. U., Vanderplas, A., Hughes, M. E., Theriault, R. L., Edge, S. B. et al. (2012). Clinicopathologic features, patterns of recurrence, and survival among women with triple-negative breast cancer in the National Comprehensive Cancer Network. Cancer, 118(22), 5463–5472. DOI 10.1002/cncr.27581. [Google Scholar] [CrossRef]
12. Goss, P. E., Ingle, J. N., Pritchard, K. I., Robert, N. J., Muss, H. et al. (2016). Extending aromatase-inhibitor adjuvant therapy to 10 years. New England Journal of Medicine, 375(3), 209–219. DOI 10.1056/NEJMoa1604700. [Google Scholar] [CrossRef]
13. Vaz-Luis, I., Burstein, H. J. (2016). Optimizing adjuvant chemotherapy and surgery for early- and late-stage breast cancer. JAMA Oncology, 2(11), 1399–1400. DOI 10.1001/jamaoncol.2016.3631. [Google Scholar] [CrossRef]
14. Joensuu, H., Kellokumpu-Lehtinen, P. L., Huovinen, R., Jukkola-Vuorinen, A., Tanner, M. et al. (2017). Adjuvant capecitabine in combination with docetaxel, epirubicin, and cyclophosphamide for early breast cancer: The randomized clinical FinXX trial. JAMA Oncology, 3(6), 793–800. DOI 10.1001/jamaoncol.2016.6120. [Google Scholar] [CrossRef]
15. Giuliano, A. E., Edge, S. B., Hortobagyi, G. N. (2018). Eighth edition of the AJCC cancer staging manual: Breast cancer. Annals of Surgical Oncology, 25(7), 1783–1785. DOI 10.1245/s10434-018-6486-6. [Google Scholar] [CrossRef]
16. Wolff, A. C., Hammond, M. E. H., Allison, K. H., Harvey, B. E., Mangu, P. B. et al. (2018). Human epidermal growth factor receptor 2 testing in breast cancer: American society of clinical oncology/College of American pathologists clinical practice guideline focused update. Archives of Pathology & Laboratory Medicine, 142(11), 1364–1382. DOI 10.5858/arpa.2018-0902-SA. [Google Scholar] [CrossRef]
17. Yin, W., Di, G., Zhou, L., Lu, J., Liu, G. et al. (2009). Time-varying pattern of recurrence risk for Chinese breast cancer patients. Breast Cancer Research and Treatment, 114(3), 527–535. DOI 10.1007/s10549-008-0022-5. [Google Scholar] [CrossRef]
18. Wang, Y., Yin, Q., Yu, Q., Zhang, J., Liu, Z. et al. (2011). A retrospective study of breast cancer subtypes: The risk of relapse and the relations with treatments. Breast Cancer Research and Treatment, 130(2), 489–498. DOI 10.1007/s10549-011-1709-6. [Google Scholar] [CrossRef]
19. Siraj, A. K., Parvathareddy, S. K., Qadri, Z., Siddiqui, K., Al-Sobhi, S. S. et al. (2020). Annual hazard rate of recurrence in middle eastern papillary thyroid cancer over a long-term follow-up. Cancers, 12(12), 3624. DOI 10.3390/cancers12123624. [Google Scholar] [CrossRef]
20. Saphner, T., Tormey, D. C., Gray, R. (1996). Annual hazard rates of recurrence for breast cancer after primary therapy. Journal of Clinical Oncology, 14(10), 2738–2746. DOI 10.1200/JCO.1996.14.10.2738. [Google Scholar] [CrossRef]
21. Cheng, L., Swartz, M. D., Zhao, H., Kapadia, A. S., Lai, D. et al. (2012). Hazard of recurrence among women after primary breast cancer treatment--A 10-year follow-up using data from SEER-medicare. Cancer Epidemiology, Biomarkers & Prevention, 21(5), 800–809. DOI 10.1158/1055-9965.EPI-11-1089. [Google Scholar] [CrossRef]
22. Ribelles, N., Perez-Villa, L., Jerez, J. M., Pajares, B., Vicioso, L. et al. (2013). Pattern of recurrence of early breast cancer is different according to intrinsic subtype and proliferation index. Breast Cancer Research and Treatment, 15(5), R98. DOI 10.1186/bcr3559. [Google Scholar] [CrossRef]
23. Bui, K. T., Willson, M. L., Goel, S., Beith, J., Goodwin, A. (2020). Ovarian suppression for adjuvant treatment of hormone receptor-positive early breast cancer. Cochrane Database of Systematic Reviews, 3, CD013538. DOI 10.1002/14651858.CD013538. [Google Scholar] [CrossRef]
24. Francis, P. A., Regan, M. M., Fleming, G. F., Lang, I., Ciruelos, E. et al. (2015). Adjuvant ovarian suppression in premenopausal breast cancer. New England Journal of Medicine, 372(5), 436–446. DOI 10.1056/NEJMoa1412379. [Google Scholar] [CrossRef]
25. Pagani, O., Francis, P. A., Fleming, G. F., Walley, B. A., Viale, G. et al. (2020). Absolute improvements in freedom from distant recurrence to tailor adjuvant endocrine therapies for premenopausal women: Results from TEXT and SOFT. Journal of Clinical Oncology, 38(12), 1293–1303. DOI 10.1200/JCO.18.01967. [Google Scholar] [CrossRef]
26. Liang, Q., Ma, D., Gao, R. F., Yu, K. D. (2020). Effect of Ki-67 expression levels and histological grade on breast cancer early relapse in patients with different immunohistochemical-based subtypes. Scientific Reports, 10(1), 7648. DOI 10.1038/s41598-020-64523-1. [Google Scholar] [CrossRef]
27. Slamon, D. J., Clark, G. M., Wong, S. G., Levin, W. J., Ullrich, A. et al. (1987). Human breast cancer: Correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science, 235(4785), 177–182. DOI 10.1126/science.3798106. [Google Scholar] [CrossRef]
28. Viani, G. A., Afonso, S. L., Stefano, E. J., de Fendi, L. I., Soares, F. V. (2007). Adjuvant trastuzumab in the treatment of her-2-positive early breast cancer: A meta-analysis of published randomized trials. BMC Cancer, 7, 153. DOI 10.1186/1471-2407-7-153. [Google Scholar] [CrossRef]
29. Henderson, I. C., Berry, D. A., Demetri, G. D., Cirrincione, C. T., Goldstein, L. J. et al. (2003). Improved outcomes from adding sequential paclitaxel but not from escalating doxorubicin dose in an adjuvant chemotherapy regimen for patients with node-positive primary breast cancer. Journal of Clinical Oncology, 21(6), 976–983. DOI 10.1200/JCO.2003.02.063. [Google Scholar] [CrossRef]
30. Jones, S. E., Savin, M. A., Holmes, F. A., O’Shaughnessy, J. A., Blum, J. L. et al. (2006). Phase III trial comparing doxorubicin plus cyclophosphamide with docetaxel plus cyclophosphamide as adjuvant therapy for operable breast cancer. Journal of Clinical Oncology, 24(34), 5381–5387. DOI 10.1200/JCO.2006.06.5391. [Google Scholar] [CrossRef]
31. Gluz, O., Nitz, U. A., Harbeck, N., Ting, E., Kates, R. et al. (2008). Triple-negative high-risk breast cancer derives particular benefit from dose intensification of adjuvant chemotherapy: Results of WSG AM-01 trial. Annals of Oncology, 19(5), 861–870. DOI 10.1093/annonc/mdm551. [Google Scholar] [CrossRef]
32. Jones, S., Holmes, F. A., O’Shaughnessy, J., Blum, J. L., Vukelja, S. J. et al. (2009). Docetaxel with cyclophosphamide is associated with an overall survival benefit compared with doxorubicin and cyclophosphamide: 7-year follow-up of US oncology research trial 9735. Journal of Clinical Oncology, 27(8), 1177–1183. DOI 10.1200/JCO.2008.18.4028. [Google Scholar] [CrossRef]
33. Hu, X. C., Zhang, J., Xu, B. H., Cai, L., Ragaz, J. et al. (2015). Cisplatin plus gemcitabine versus paclitaxel plus gemcitabine as first-line therapy for metastatic triple-negative breast cancer (CBCSG006A randomised, open-label, multicentre, phase 3 trial. Lancet Oncology, 16(4), 436–446. DOI 10.1016/S1470-2045(15)70064-1. [Google Scholar] [CrossRef]
34. Zhang, X., Zhou, Y., Mao, F., Lin, Y., Guan, J. et al. (2015). Efficacy and safety of pirarubicin plus capecitabine versus pirarubicin plus cyclophosphamide in Chinese node-negative breast cancer patients: A 4-year open-label, randomized, controlled study. Medical Oncology, 32(10), 240. DOI 10.1007/s12032-015-0686-8. [Google Scholar] [CrossRef]
35. Loibl, S., Weber, K. E., Timms, K. M., Elkin, E. P., Hahnen, E. et al. (2018). Survival analysis of carboplatin added to an anthracycline/taxane-based neoadjuvant chemotherapy and HRD score as predictor of response-final results from GeparSixto. Annals of Oncology, 29(12), 2341–2347. DOI 10.1093/annonc/mdy460. [Google Scholar] [CrossRef]
36. Li, J., Yu, K., Pang, D., Wang, C., Jiang, J. et al. (2020). Adjuvant capecitabine with docetaxel and cyclophosphamide plus epirubicin for triple-negative breast cancer (CBCSG010An open-label, randomized, multicenter, phase III trial. Journal of Clinical Oncology, 38(16), 1774–1784. DOI 10.1200/JCO.19.02474. [Google Scholar] [CrossRef]
37. Zhu, J., Jiao, D., Yan, M., Chen, X., Wang, C. et al. (2021). Establishment and verification of a predictive model for node pathological complete response after neoadjuvant chemotherapy for initial node positive early breast cancer. Frontiers in Oncology, 11, 675070. DOI 10.3389/fonc.2021.675070. [Google Scholar] [CrossRef]
38. Ponde, N. F., Zardavas, D., Piccart, M. (2019). Progress in adjuvant systemic therapy for breast cancer. Nature Reviews Clinical Oncology, 16(1), 27–44. DOI 10.1038/s41571-018-0089-9. [Google Scholar] [CrossRef]
39. Cossetti, R. J., Tyldesley, S. K., Speers, C. H., Zheng, Y., Gelmon, K. A. (2015). Comparison of breast cancer recurrence and outcome patterns between patients treated from 1986 to 1992 and from 2004 to 2008. Journal of Clinical Oncology, 33(1), 65–73. DOI 10.1200/JCO.2014.57.2461. [Google Scholar] [CrossRef]
40. Nishimura, R., Osako, T., Nishiyama, Y., Tashima, R., Nakano, M. et al. (2013). Evaluation of factors related to late recurrence--later than 10 years after the initial treatment--in primary breast cancer. Oncology, 85(2), 100–110. DOI 10.1159/000353099. [Google Scholar] [CrossRef]
41. Lee, E. S., Han, W., Kim, M. K., Kim, J., Yoo, T. K. et al. (2016). Factors associated with late recurrence after completion of 5-year adjuvant tamoxifen in estrogen receptor positive breast cancer. BMC Cancer, 16, 430. DOI 10.1186/s12885-016-2423-x. [Google Scholar] [CrossRef]
Cite This Article
 Copyright © 2022 The Author(s). Published by Tech Science Press.
Copyright © 2022 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools