 Open Access
Open Access
REVIEW
The Presence of Human Papillomavirus and Epstein-Barr Virus Infection in Gastric Cancer: A Systematic Study
1
Department of Microbiology, Faculty of Basic Sciences, Kazerun Branch, Islamic Azad University, Kazerun, Iran
2
Department of Virology, Faculty of Medicine, Tabriz University of Medical Sciences, Tabriz, Iran
3
Department of Immunology, Faculty of Medicine, Tabriz University of Medical Sciences, Tabriz, Iran
4
Department of Microbiology, Faculty of Basic Sciences, Ahar Branch, Islamic Azad University, Ahar, Iran
* Corresponding Author: Afsoon Shariat. Email:
Oncologie 2022, 24(3), 413-426. https://doi.org/10.32604/oncologie.2022.024161
Received 22 May 2022; Accepted 12 July 2022; Issue published 19 September 2022
Abstract
Background and Aim: Gastric cancer (GC) is one of the most common infection-related malignancies worldwide. Human Papillomavirus (HPV) and Epstein-Barr virus (EBV) are among the most important viruses affecting many people worldwide. The potential role of these viruses in gastric tissue may explain the possibility of GC, as seen in Helicobacter pylori (H. pylori). This study aimed to systematically investigate the presence of HPV and EBV in GC. Methods: According to the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines, this study is a systematic review based on reported cases. The keywords HPV, EBV and GC, were searched in PubMed, Web of Science, Scopus, EMBASE and Google scholar databases from 2012 to 2022. Articles were selected and evaluated by five researchers independently. The odds ratio of HPV and EBV viruses in GC was estimated. Data analysis was performed by SPSS (Version 20) software. Results: Sixty studies with 14949 patients were included in the study after obtaining the inclusion criteria. The mean prevalence of HPV and EBV viruses in GC was 10.58% and 8.58%, respectively. The highest prevalence of HPV and EBV were 37.74% and 44.44% in Turkey and Iraq, respectively. The highest odds of HPV and EBV in GC were observed in Asia (17.54%) and Africa (19.02%), respectively. Conclusion: The findings indicate the presence of HPV and EBV in GC in the study areas. However, the present study’s results are insufficient for a more accurate conclusion. Therefore, further studies are necessary for the conclusion in this regard.Keywords
Cancer is the leading cause of death in most developing and developed countries. Gastric cancer (GC) is the second leading cause of death and the most common malignant tumor globally [1]. GC is one of the multifactorial diseases whose main reason is the presence of infectious, genetic and environmental factors in individuals [2]. The prevalence of GC varies from the resulting mortality rate because the second phenomenon is more due to the late diagnosis of GC in the advanced phase of the disease [3]. Viruses cause many cancers, with about 15%–20% of human cancers associated with viruses [4]. Human Papillomavirus (HPV) is a DNA virus found in all human populations, and sometimes the infection it causes can lead to cancer [5,6]. The virus is divided into high-risk and low-risk types in terms of its potential to cause cancer [7]. HPV16 and HPV18 are among the most common subtypes involved in carcinogenesis [8]. Various studies have indicated the oncogenic properties of HPV, suggesting a role for the virus in the pathogenesis of cancer [9]. Integration of HPV DNA in the host genome is the main cause of carcinogenesis that causes the regulation of cellular oncogenes (mainly E6 and E7). The virus disrupts HPV E2 expression and causes overexpression of E6 and E7 oncoproteins [10]. Epstein-Barr virus (EBV) is a DNA virus in the herpes virus family. EBV is the first human virus responsible for approximately 1.8% of all human cancers, including Hodgkin’s lymphoma, Burkitt’s lymphoma, NK/T cell lymphoma, and Nasopharyngeal [11]. The first association between EBV and gastric epithelial lymphatic carcinoma was identified in 1990, indicating the first association between GC and EBV [12]. Subsequent studies have also shown an association between gastric adenocarcinoma and EBV, which may indicate an important role for EBV in pathogenesis [13]. Thus, these findings suggest that 10% of GCs worldwide are associated with EBV [13–15]. Considering that EBV can continuously infect a cell with monoclonal proliferation, EBV infection is effective in the early stages of GC [16–18]. Although various studies have investigated the mechanism of occurrence and development of GC and positive EBV, they found no association between EBV titer and GC risk [19]. Studies show that latent EBV infection and the expression of latent EBV genes as a co-factor increase carcinogenicity, which causes abnormalities in the host genome (such as aberrant DNA methylation), disruption of cell pathways, and immune cell function [20]. Nowadays, extensive research has reported the prevalence of HPV and EBV in GC. Therefore, the present study aimed to gain new insight into the prevalence of HPV and EBV in GC for a better understanding of carcinogenesis.
The present study was conducted by the indicators of preferred reporting items for systematic reviews and meta-analyses (PRISMA) [21].
This study examined PubMed, Web of Science, Scopus, EMBASE and Google scholar databases to find studies on HPV, EBV and GC. Databases were searched from 2012 to 2022 with the keywords HPV, EBV and GC.
All findings from appropriate databases regarding subject matter, quality, and method were entered into the X9 version of EndNote (Thomson Reuters) software, and duplicate inputs were removed. Two researchers (AJS and BJ) performed screening of the obtained data independently. The full text of the remaining articles was then reviewed, and any disagreements were resolved through discussion by the third and fourth researchers (ASH and HBB).
2.3 Inclusion and Exclusion Criteria
The following eligible studies were included: (1) All research studies that report the prevalence of HPV and EBV infection in patients with GC; (2) Articles with free access; (3) Articles published in ten years in English in reputable journals. Studies with the following characteristics were excluded from the study: (1) Studies at intervals other than ten years, (2) Studies published in languages other than English, (3) Articles in the review process, letters to the editor and case report.
The following data were extracted from eligible studies: country, year, authors’ names, virus type, the total number of samples, positive samples, percentage of positive samples to total samples and source. The data obtained in this section were entered into a checklist and, after evaluation by the fifth researcher (BB), were entered in Table 1.
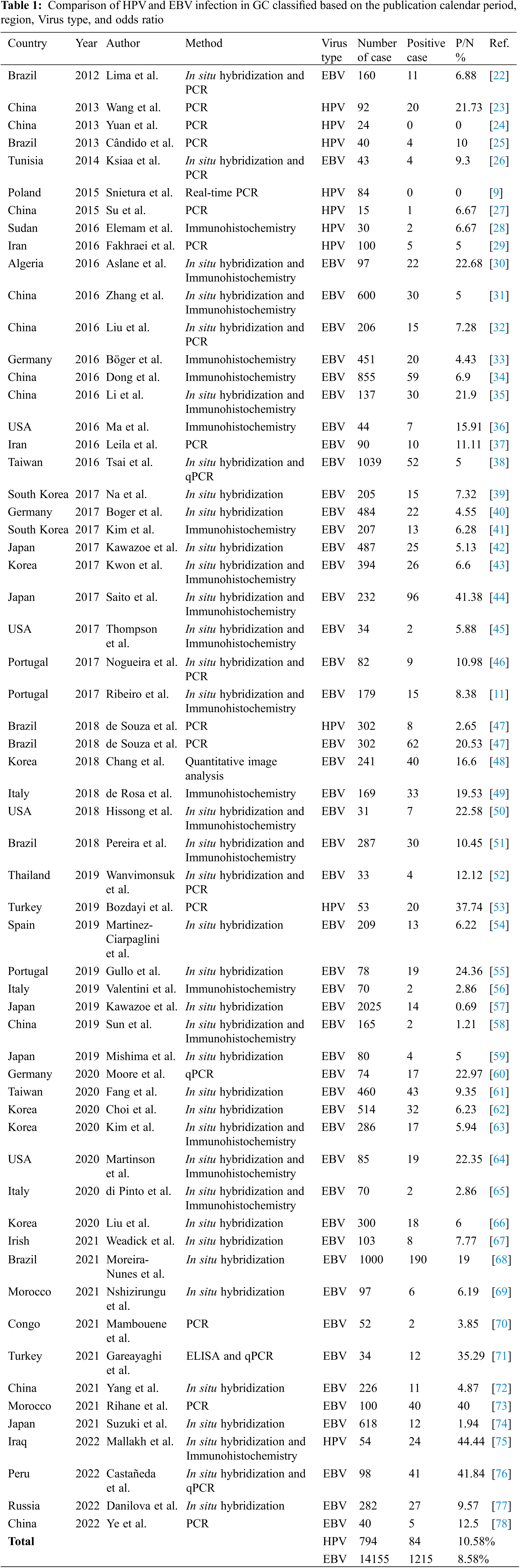
The prevalence of HPV and EBV in the GC and the odds ratio of positive samples (ORs) with a 95% confidence interval (CIs) were used to assess the relationship between HPV and EBV and the occurrence of GC. In this study, SPSS software (version 16) was used for the statistical analysis of data.
A total of 327 articles were searched. After screening, 60 articles were selected (Fig. 1) with the necessary standards to enter the study. A comparison of the prevalence of HPV and EBV viruses in GC is given in Table 1. The prevalence of HPV and EBV in 2249 GC samples was studied in 24 countries, and according to the results, the prevalence varied from 0% to 44.44%. The mean prevalence of HPV and EBV viruses in GC was 10.58% and 8.58%, respectively. Then, after classification based on the research area and calculating the average data of each country, it was found that the highest prevalence of HPV and EBV were 37.74% and 44.44% in Turkey and Iraq, respectively. However, the lowest prevalence of HPV and EBV were 0% and 0.69% in China and Japan, respectively. The total number of HPV and EBV samples in GC was analyzed based on continental segmentation (Table 2 and Fig. 2). By comparing the odds ratios for each continent, it was found that HPV in Asia, Europe, Africa and America was 17.54%, 14.6%, 6.67% and 3.51%, respectively. For the EBV in Africa, America, Europe and Asia, this ratio was 19.02%, 18.08%, 8.71% and 6.07%, respectively.
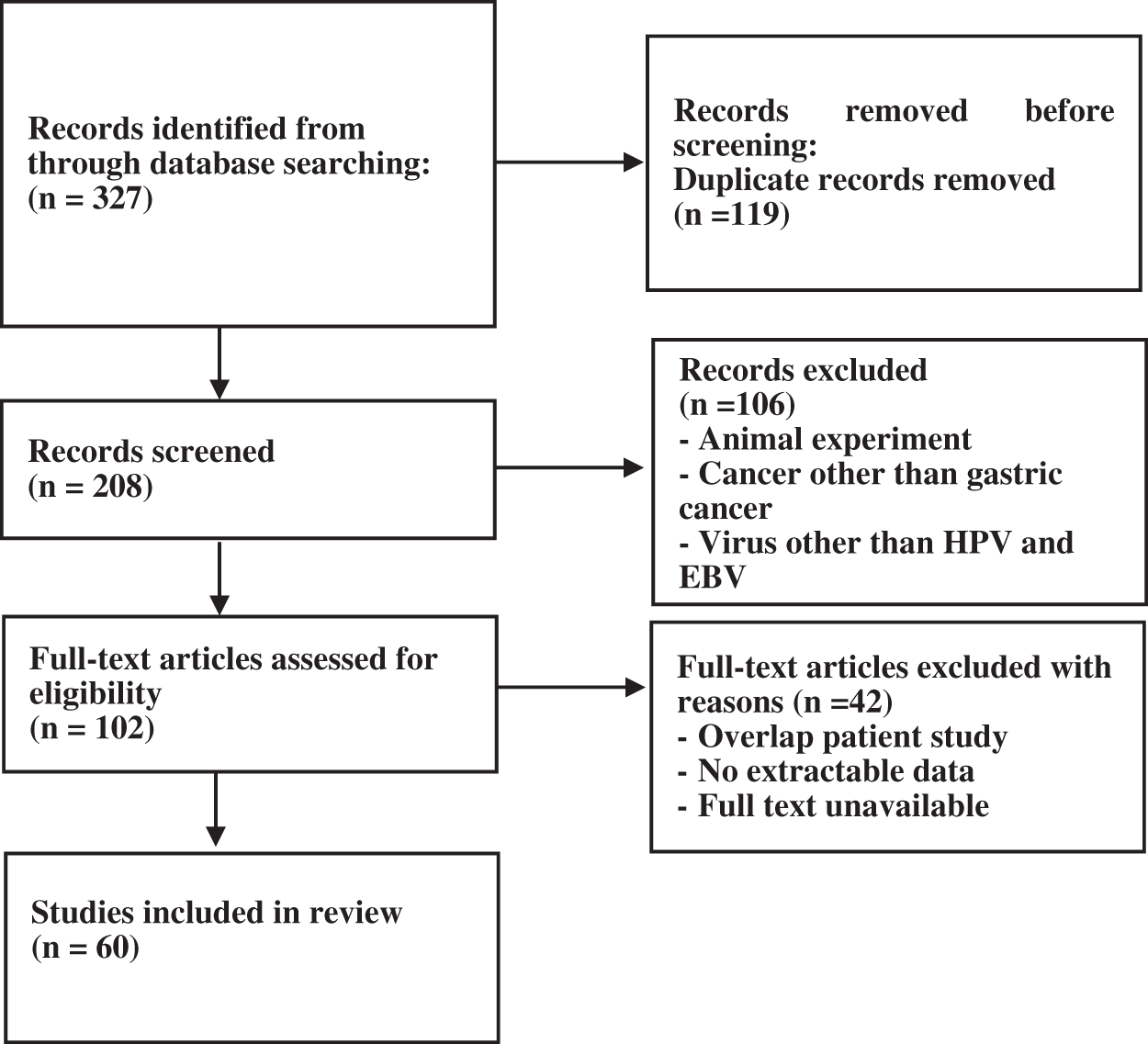
Figure 1: Flow-chart of the literature search and selection
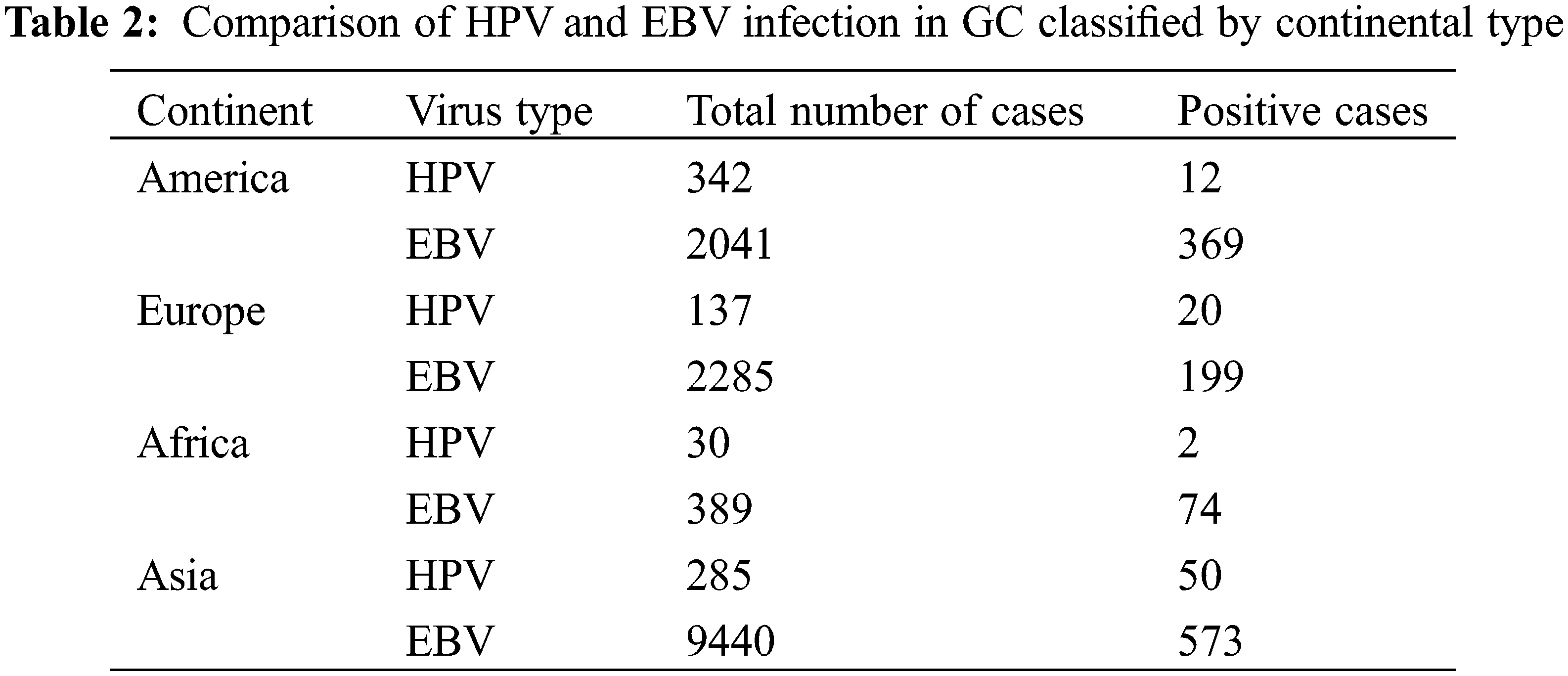
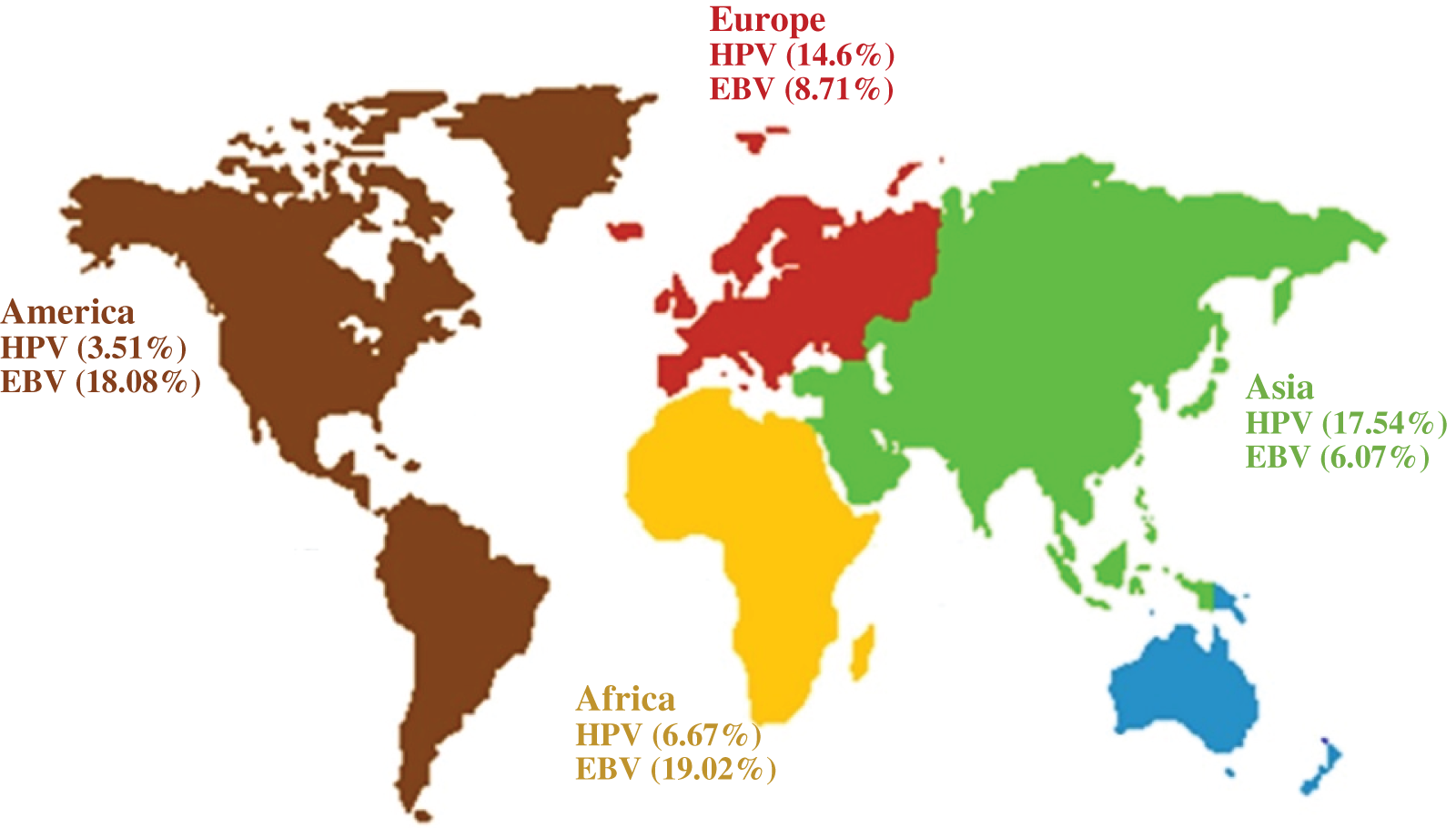
Figure 2: Odds ratios for HPV and EBV positive GC on different continents
GC is one of the most common malignancies associated with infection worldwide. Various studies have proven the carcinogenic mechanisms of Helicobacter pylori (H. pylori) and EBV virus [79–82]. However, comprehensive information on other carcinogenic viruses has not been obtained. Therefore, this systematic study was performed to determine this. The researchers’ findings indicate that HPV is one of the main infectious agents involved in prostate, cervical, anal, and colorectal cancer [83–87]. Most studies suggest that HPV can lead to cancer if it co-occurs with H. pylori, while other studies indicate that the prevalence of HPV is not associated with H. pylori [25,88]. HPV infection enters the esophagus through the mouth and eventually into the stomach, and the integration of HPV DNA into the host genome plays a key role in carcinogenesis [89]. Various studies in HPV-infected cells show that overexpression of E6 and E7 oncoprotein (caused by the integration of HPV in the host genome) in addition to tumorigenesis, can regulate the expression of multiple genes involved in cellular processes (such as proliferation, differentiation, apoptosis, adhesion, angiogenesis, transcription and protein translation), and induce genomic instability in normal human cells [90–96]. Yamato et al. and Jabbar et al. [97,98] have shown that the activity of E6 and E7 is necessary for the persistence of cancer caused by HPV because in the absence of the activity of these oncoproteins, cancer cells to senesce or undergo apoptosis. Our systematic study showed that the prevalence of HPV among GC patients from 24 countries was 10.58%. During a meta-analysis study, Zeng et al. concluded that out of the 1917 patients studied, 28% were HPV positive, which was more than the present study’s findings [89]. In their study, Bae showed that the proportion of HPV-positive cases in Chinese studies was 1.43 times higher than in non-Chinese studies [99]. In 2020, Wang et al. examined the presence of HPV in 901 patients with GC and found that 23.6% of the samples were HPV positive. Therefore, there is a significant relationship between HPV infection and the risk of gastric malignancy [100]. EBV is found in approximately 9% of GC [12,101]. Various studies have revealed the role of EBV infection in the progression of GC, which can be caused by EBV entering B lymphocytes in the oropharyngeal lymphoid tissues and then entering gastric epithelial cells, which can occur through cell-to-cell contact between B lymphocytes and gastric epithelial cells or direct entry into the gastric epithelium. This entry can be facilitated by mucosal damage [102,103]. The prevalence of EBV in the present study was 8.58%. Our findings are consistent with previous meta-analysis studies that reported the presence of EBV in GC from 6.9% to 8.8% [101,104–108]. Pyo et al. [109] estimated the presence of EBV in GC at 0.113%. Tavakoli et al. [110] reported an EBV prevalence of 8.77% in 20361 patients with GC. In addition, for the presence of EBV in GC, genome atlas research showed repeated PIK3CA mutations, severe DNA hypermethylation, amplification of JAK2, CD274, and PDCD1LG2 [111], which can enhance our understanding of the carcinogenic mechanism of EBV. This study contained the following limitations due to the data sources used in the systematic study. Age, sex, and H. pylori infection are important factors in the development of GC. However, the studies used were not mainly classified by age or sex, and there were no reports of an association between H. pylori infection and GC, so information on age, sex, and co-infection was not considered. There was also significant heterogeneity due to differences in sample size and geographical areas.
Overall, the present study’s findings indicated that the prevalence of HPV and EBV infections in GC was 10.58% and 8.58%, respectively. These findings suggest an association between HPV, EBV and GC infections. However, the evidence inferred in the present study is insufficient to conclude that HPV and EBV infection are associated with GC risk. In addition, the prevalence of HPV and EBV was found in 24 countries worldwide. Therefore, extensive studies in other countries are strongly recommended to obtain more reliable results.
Acknowledgement: This article was derived from Ph.D. degree thesis in the Islamic Azad University Kazerun branch. The authors acknowledge the Department of Microbiology, Kazerun Branch, Islamic Azad University, and the Research Deputy.
Authors’ Contributions: All authors contributed to the conception and the main idea of the work. AJS wrote the manuscript. ASH, HBB, BB, and BJ analyzed the data and edited the manuscript. All authors reviewed the results and approved the final version of the manuscript.
Ethics Approval and Informed Consent Statement: Not applicable.
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. Sun, K., Jia, K., Lv, H., Wang, S. Q., Wu, Y. et al. (2020). EBV-positive gastric cancer: Current knowledge and future perspectives. Frontiers in Oncology, 10, 583463. DOI 10.3389/fonc.2020.583463. [Google Scholar] [CrossRef]
2. Zabaleta, J. (2012). Multifactorial etiology of gastric cancer. Cancer Epigenetics, 863, 411–435. DOI 10.1007/978-1-61779-612-8. [Google Scholar] [CrossRef]
3. Lichtenstein, P., Holm, N. V., Verkasalo, P. K., Iliadou, A., Kaprio, J. et al. (2000). Environmental and heritable factors in the causation of cancer analyses of cohorts of twins from Sweden, Denmark, and Finland. New England Journal of Medicine, 343(2), 78–85. DOI 10.1056/NEJM200007133430201. [Google Scholar] [CrossRef]
4. Zur Hausen, H. (1991). Viruses in human cancers. Science, 254(5035), 1167–1173. DOI 10.1126/science.1659743. [Google Scholar] [CrossRef]
5. Pim, D., Thomas, M., Banks, L. (2001). The function of the human papillomavirus oncogenes. In: Perspectives in medical virology, vol. 5, pp. 145–192. USA: Elsevier. [Google Scholar]
6. Walboomers, J. M., Jacobs, M. V., Manos, M. M., Bosch, F. X., Kummer, J. A. et al. (1999). Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. The Journal of Pathology, 189(1), 12–19. DOI 10.1002/(ISSN)1096-9896. [Google Scholar] [CrossRef]
7. Doorbar, J., Quint, W., Banks, L., Bravo, I. G., Stoler, M. et al. (2012). The biology and life-cycle of human papillomaviruses. Vaccine, 30(1), F55–F70. DOI 10.1016/j.vaccine.2012.06.083. [Google Scholar] [CrossRef]
8. Tommasino, M. (2014). The human papillomavirus family and its role in carcinogenesis. Seminars in Cancer Biology, 26, 13–21. DOI 10.1016/j.semcancer.2013.11.002. [Google Scholar] [CrossRef]
9. Snietura, M., Waniczek, D., Piglowski, W., Kopec, A., Nowakowska-Zajdel, E. et al. (2014). Potential role of human papilloma virus in the pathogenesis of gastric cancer. World Journal of Gastroenterology, 20(21), 6632. DOI 10.3748/wjg.v20.i21.6632. [Google Scholar] [CrossRef]
10. Samoff, E., Koumans, E. H., Markowitz, L. E., Sternberg, M., Sawyer, M. K. et al. (2005). Association of Chlamydia trachomatis with persistence of high-risk types of human papillomavirus in a cohort of female adolescents. American Journal of Epidemiology, 162(7), 668–675. DOI 10.1093/aje/kwi262. [Google Scholar] [CrossRef]
11. Ribeiro, J., Oliveira, A., Malta, M., Oliveira, C., Silva, F. et al. (2017). Clinical and pathological characterization of Epstein-Barr virus-associated gastric carcinomas in Portugal. World Journal of Gastroenterology, 23(40), 7292–7302. DOI 10.3748/wjg.v23.i40.7292. [Google Scholar] [CrossRef]
12. Burke, A. P., Yen, T. S., Shekitka, K. M., Sobin, L. H. (1990). Lymphoepithelial carcinoma of the stomach with Epstein-Barr virus demonstrated by polymerase chain reaction. Modern Pathology: An Official Journal of the United States and Canadian Academy of Pathology, Inc, 3(3), 377–380. [Google Scholar]
13. Shibata, D., Weiss, L. M. (1992). Epstein-Barr virus-associated gastric adenocarcinoma. The American Journal of Pathology, 140(4), 769–774. [Google Scholar]
14. Takada, K. (2000). Epstein-Barr virus and gastric carcinoma. Molecular Pathology, 53(5), 255–261. DOI 10.1136/mp.53.5.255. [Google Scholar] [CrossRef]
15. Tokunaga, M., Land, C. E., Uemura, Y., Tokudome, T., Tanaka, S. et al. (1993). Epstein-Barr virus in gastric carcinoma. The American Journal of Pathology, 143(5), 1250–1254. [Google Scholar]
16. Fukayama, M., Hayashi, Y., Iwasaki, Y., Chong, J., Ooba, T. et al. (1994). Epstein-Barr virus-associated gastric carcinoma and Epstein-Barr virus infection of the stomach. Laboratory Investigation, 71(1), 73–81. [Google Scholar]
17. Imai, S., Koizumi, S., Sugiura, M., Tokunaga, M., Uemura, Y. et al. (1994). Gastric carcinoma: Monoclonal epithelial malignant cells expressing Epstein-Barr virus latent infection protein. Proceedings of the National Academy of Sciences, 91(19), 9131–9135. DOI 10.1073/pnas.91.19.9131. [Google Scholar] [CrossRef]
18. Young, L. S., Rickinson, A. B. (2004). Epstein-Barr virus: 40 years on. Nature Reviews Cancer, 4(10), 757–768. DOI 10.1038/nrc1452. [Google Scholar] [CrossRef]
19. Figueiredo, C., Camargo, M. C., Leite, M., Fuentes-Pananá, E. M., Rabkin, C. S. et al. (2017). Pathogenesis of gastric cancer: Genetics and molecular classification. Molecular Pathogenesis and Signal Transduction by Helicobacter Pylori, 400, 277–304. [Google Scholar]
20. Varga, M. G., Cai, H., Waterboer, T., Murphy, G., Shimazu, T. et al. (2018). Epstein-Barr virus antibody titers are not associated with gastric cancer risk in East Asia. Digestive Diseases and Sciences, 63(10), 2765–2772. DOI 10.1007/s10620-018-5154-9. [Google Scholar] [CrossRef]
21. Moher, D., Liberati, A., Tetzlaff, J., Altman, D. G. (2009). Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Annals of Internal Medicine, 151(4), 264–269. DOI 10.7326/0003-4819-151-4-200908180-00135. [Google Scholar] [CrossRef]
22. Lima, M. A. P. D., Ferreira, M. V. P., Barros, M. A. P., Pardini, M. I. D. M. C., Ferrasi, A. C. et al. (2012). Epstein-Barr virus-associated gastric carcinoma in Brazil: Comparison between in situ hybridization and polymerase chain reaction detection. Brazilian Journal of Microbiology, 43(1), 393–404. DOI 10.1590/S1517-83822012000100048. [Google Scholar] [CrossRef]
23. Wang, Z. J., Zhang, Y. Q., Zhang, Y. T. (2013). Analysis of relationship of the infection of human papillomavirus 16 H. pylori cagA gene and ureA gene in gastric carcinogenesis. Journal of Practical Medical Techniques, 20(10), 1061–1064. [Google Scholar]
24. Yuan, X. Y., Wang, M. Y., Wang, X. Y., Chang, A. Y., Li, J. (2013). Non-detection of Epstein-Barr virus and Human Papillomavirus in a region of high gastric cancer risk indicates a lack of a role for these viruses in gastric carcinomas. Genetics and Molecular Biology, 36(2), 183–184. DOI 10.1590/S1415-47572013005000018. [Google Scholar] [CrossRef]
25. Cândido, A. C., de Lima Filho, J. L., Martins, D. B., Mendes, C. M., Vieira, J. R. et al. (2013). Association of human papillomavirus genomic sequences by polymerase chain reaction in gastric carcinomas in Brazil. Analytical and Quantitative Cytopathology and Histopathology, 35, 1–6. [Google Scholar]
26. Ksiaa, F., Ziadi, S., Gacem, R. B., Dhiab, M. B., Trimeche, M. (2014). Correlation between DNA methyltransferases expression and Epstein-Barr virus, JC polyomavirus and Helicobacter pylori infections in gastric carcinomas. Neoplasma, 61(6), 710–717. DOI 10.4149/neo_2014_086. [Google Scholar] [CrossRef]
27. Su, L., He, F. (2015). Analysis of the correlation between human papillomavirus (HPV) and Epstein-Barr virus (EBV) infection and upper gastrointestinal tumor patients. International Journal of Virology, 22, 159–161. [Google Scholar]
28. Elemam, Y., Bakhit, I. (2016). Immunohistochemical based detection of HPV in gastric cancer among sudanese patient. International Journal of Current Research and Academic Review, 4(2), 56–62. DOI 10.20546/ijcrar.2016.402.006. [Google Scholar] [CrossRef]
29. Fakhraei, F., Haghshenas, M. R., Hosseini, V., Rafiei, A., Naghshvar, F. et al. (2016). Detection of human papillomavirus DNA in gastric carcinoma specimens in a high-risk region of Iran. Biomedical Reports, 5(3), 371–375. DOI 10.3892/br.2016.728. [Google Scholar] [CrossRef]
30. Aslane, M., Al Haj, A., Henneb, A., Khenchouche, A., Houali, K. (2016). Characteristics of gastric carcinoma associated with Epstein-Barr virus in Algeria. The Pharma Letter, 8(17), 169–178. [Google Scholar]
31. Zhang, Z. X., Guo, D. L., Jin, M., Zhang, W., Huang, L. H. et al. (2016). Invasion-related signal pathways in Epstein-Barr virus (EBV)-associated gastric carcinoma. International Journal of Clinical and Experimental Medicine, 9(2), 3544–3550. [Google Scholar]
32. Liu, Y., Yang, W., Pan, Y., Ji, J., Lu, Z. et al. (2016). Genome-wide analysis of Epstein-Barr virus (EBV) isolated from EBV-associated gastric carcinoma (EBVaGC). Oncotarget, 7(4), 4903–4914. DOI 10.18632/oncotarget.6751. [Google Scholar] [CrossRef]
33. Böger, C., Behrens, H. M., Mathiak, M., Krüger, S., Kalthoff, H. et al. (2016). PD-L1 is an independent prognostic predictor in gastric cancer of Western patients. Oncotarget, 7(17), 24269–24283. DOI 10.18632/oncotarget.8169. [Google Scholar] [CrossRef]
34. Dong, M., Wang, H. Y., Zhao, X. X., Chen, J. N., Zhang, Y. et al. (2016). Expression and prognostic roles of PIK3CA, JAK2, PD-L1, and PD-L2 in Epstein-Barr virus-associated gastric carcinoma. Human Pathology, 53, 25–34. DOI 10.1016/j.humpath.2016.02.007. [Google Scholar] [CrossRef]
35. Li, Z., Lai, Y., Sun, L., Zhang, X., Liu, R. et al. (2016). PD-L1 expression is associated with massive lymphocyte infiltration and histology in gastric cancer. Human Pathology, 55, 182–189. DOI 10.1016/j.humpath.2016.05.012. [Google Scholar] [CrossRef]
36. Ma, C., Patel, K., Singhi, A. D., Ren, B., Zhu, B. et al. (2016). Programmed death-ligand 1 expression is common in gastric cancer associated with Epstein-Barr virus or microsatellite instability. The American Journal of Surgical Pathology, 40(11), 1496–1506. DOI 10.1097/PAS.0000000000000698. [Google Scholar] [CrossRef]
37. Leila, Z., Arabzadeh, S. A., Afshar, R. M., Afshar, A. A., Mollaei, H. R. (2016). Detection of Epstein-Barr virus and cytomegalovirus in gastric cancers in Kerman, Iran. Asian Pacific Journal of Cancer Prevention, 17(5), 2423–2428. [Google Scholar]
38. Tsai, C. Y., Liu, Y. Y., Liu, K. H., Hsu, J. T., Chen, T. C. et al. (2017). Comprehensive profiling of virus microRNAs of Epstein-Barr virus-associated gastric carcinoma: Highlighting the interactions of ebv-Bart9 and host tumor cells. Journal of Gastroenterology and Hepatology, 32(1), 82–91. DOI 10.1111/jgh.13432. [Google Scholar] [CrossRef]
39. Na, S. J., Park, H. L., Lee, S. Y., Song, K. Y., Kim, S. H. (2017). Correlation between infection status of Epstein-Barr virus and 18F-Fluorodeoxyglucose uptake in patients with advanced gastric cancer. In Vivo, 31(4), 749–753. DOI 10.21873/invivo.11126. [Google Scholar] [CrossRef]
40. Böger, C., Krüger, S., Behrens, H. M., Bock, S., Haag, J. et al. (2017). Epstein-Barr virus-associated gastric cancer reveals intratumoral heterogeneity of PIK3CA mutations. Annals of Oncology, 28(5), 1005–1014. DOI 10.1093/annonc/mdx047. [Google Scholar] [CrossRef]
41. Kim, J. Y., Bae, B. N., Kang, G., Kim, H. J., Park, K. (2017). Cytokine expression associated with Helicobacter pylori and Epstein-Barr virus infection in gastric carcinogenesis. APMIS, 125(9), 808–815. DOI 10.1111/apm.12725. [Google Scholar] [CrossRef]
42. Kawazoe, A., Kuwata, T., Kuboki, Y., Shitara, K., Nagatsuma, A. K. et al. (2017). Clinicopathological features of programmed death ligand 1 expression with tumor-infiltrating lymphocyte, mismatch repair, and Epstein-Barr virus status in a large cohort of gastric cancer patients. Gastric Cancer, 20(3), 407–415. DOI 10.1007/s10120-016-0631-3. [Google Scholar] [CrossRef]
43. Kwon, M. J., Kim, K. C., Nam, E. S., Cho, S. J., Park, H. R. et al. (2017). Programmed death ligand-1 and MET co-expression is a poor prognostic factor in gastric cancers after resection. Oncotarget, 8(47), 82399–82414. DOI 10.18632/oncotarget.19390. [Google Scholar] [CrossRef]
44. Saito, R., Abe, H., Kunita, A., Yamashita, H., Seto, Y. et al. (2017). Overexpression and gene amplification of PD-L1 in cancer cells and PD-L1+ immune cells in Epstein-Barr virus-associated gastric cancer: The prognostic implications. Modern Pathology, 30(3), 427–439. DOI 10.1038/modpathol.2016.202. [Google Scholar] [CrossRef]
45. Thompson, E. D., Zahurak, M., Murphy, A., Cornish, T., Cuka, N. et al. (2017). Patterns of PD-L1 expression and CD8 T cell infiltration in gastric adenocarcinomas and associated immune stroma. Gut, 66(5), 794–801. DOI 10.1136/gutjnl-2015-310839. [Google Scholar] [CrossRef]
46. Nogueira, C., Mota, M., Gradiz, R., Cipriano, M. A., Caramelo, F. et al. (2017). Prevalence and characteristics of Epstein-Barr virus-associated gastric carcinomas in Portugal. Infectious Agents and Cancer, 12(1), 1–8. DOI 10.1186/s13027-017-0151-8. [Google Scholar] [CrossRef]
47. de Souza, C. R. T., Almeida, M. C. A., Khayat, A. S., da Silva, E. L., Soares, P. C. et al. (2018). Association between Helicobacter pylori, Epstein-Barr virus, human papillomavirus and gastric adenocarcinomas. World Journal of Gastroenterology, 24(43), 4928–4938. DOI 10.3748/wjg.v24.i43.4928. [Google Scholar] [CrossRef]
48. Chang, Y. H., Heo, Y. J., Cho, J., Song, S. Y., Lee, J. et al. (2018). Computational measurement of tumor immune microenvironment in gastric adenocarcinomas. Scientific Reports, 8(1), 1–8. DOI 10.1038/s41598-018-32299-0. [Google Scholar] [CrossRef]
49. de Rosa, S., Sahnane, N., Tibiletti, M. G., Magnoli, F., Vanoli, A. et al. (2018). EBV+ and MSI gastric cancers harbor high PD-L1/PD-1 expression and high CD8+ intratumoral lymphocytes. Cancers, 10(4), 102. DOI 10.3390/cancers10040102. [Google Scholar] [CrossRef]
50. Hissong, E., Ramrattan, G., Zhang, P., Zhou, X. K., Young, G. et al. (2018). Gastric carcinomas with lymphoid stroma. The American Journal of Surgical Pathology, 42(4), 453–462. DOI 10.1097/PAS.0000000000001018. [Google Scholar] [CrossRef]
51. Pereira, M. A., Ramos, M. F., Faraj, S. F., Dias, A. R., Yagi, O. K. et al. (2018). Clinicopathological and prognostic features of Epstein-Barr virus infection, microsatellite instability, and PD-L1 expression in gastric cancer. Journal of Surgical Oncology, 117(5), 829–839. DOI 10.1002/jso.25022. [Google Scholar] [CrossRef]
52. Wanvimonsuk, S., Thitiwanichpiwong, P., Keelawat, S., Mutirangura, A., Kitkumthorn, N. (2019). Distribution of the Epstein-Barr virus in the normal stomach and gastric lesions in Thai population. Journal of Medical Virology, 91(3), 444–449. DOI 10.1002/jmv.25318. [Google Scholar] [CrossRef]
53. Bozdayi, G., Dinc, B., Avcikucuk, H., Turhan, N., Altay-Kocak, A. et al. (2019). Is human papillomavirus and Helicobacter pylori related in gastric lesions? Clinical Laboratory, 65(10), 1807–1812. DOI 10.7754/Clin.Lab.2019.181244. [Google Scholar] [CrossRef]
54. Martinez-Ciarpaglini, C., Fleitas-Kanonnikoff, T., Gambardella, V., Llorca, M., Mongort, C. et al. (2019). Assessing molecular subtypes of gastric cancer: Microsatellite unstable and Epstein-Barr virus subtypes. Methods for detection and clinical and pathological implications. ESMO Open, 4(3), e000470. DOI 10.1136/esmoopen-2018-000470. [Google Scholar] [CrossRef]
55. Gullo, I., Oliveira, P., Athelogou, M., Gonçalves, G., Pinto, M. L. et al. (2019). New insights into the inflamed tumor immune microenvironment of gastric cancer with lymphoid stroma: From morphology and digital analysis to gene expression. Gastric Cancer, 22(1), 77–90. DOI 10.1007/s10120-018-0836-8. [Google Scholar] [CrossRef]
56. Valentini, A. M., di Pinto, F., Coletta, S., Guerra, V., Armentano, R. et al. (2019). Tumor microenvironment immune types in gastric cancer are associated with mismatch repair however, not HER2 status. Oncology Letters, 18(2), 1775–1785. DOI 10.3892/ol.2019.10513. [Google Scholar] [CrossRef]
57. Kawazoe, A., Shitara, K., Kuboki, Y., Bando, H., Kojima, T. et al. (2019). Clinicopathological features of 22C3 PD-L1 expression with mismatch repair, Epstein-Barr virus status, and cancer genome alterations in metastatic gastric cancer. Gastric Cancer, 22(1), 69–76. DOI 10.1007/s10120-018-0843-9. [Google Scholar] [CrossRef]
58. Sun, Y., Yu, W., Guan, W., Cai, L., Qiao, M. et al. (2019). Integrated assessment of PD-L1 expression and molecular classification facilitates therapy selection and prognosis prediction in gastric cancer. Cancer Management and Research, 11, 6397–6410. DOI 10.2147/CMAR. [Google Scholar] [CrossRef]
59. Mishima, S., Kawazoe, A., Nakamura, Y., Sasaki, A., Kotani, D. et al. (2019). Clinicopathological and molecular features of responders to nivolumab for patients with advanced gastric cancer. Journal for Immunotherapy of Cancer, 7(1), 1–8. DOI 10.1186/s40425-019-0514-3. [Google Scholar] [CrossRef]
60. Moore, A., Hikri, E., Goshen-Lago, T., Barkan, T., Morgenstern, S. et al. (2020). Young-onset gastric cancer and Epstein-Barr Virus (EBV)–A major player in the pathogenesis? BMC Cancer, 20(1), 1–7. DOI 10.1186/s12885-020-6517-0. [Google Scholar] [CrossRef]
61. Fang, W. L., Chen, M. H., Huang, K. H., Lin, C. H., Chao, Y. et al. (2020). The clinicopathological features and genetic alterations in Epstein-Barr virus-associated gastric cancer patients after curative surgery. Cancers, 12(6), 1517. DOI 10.3390/cancers12061517. [Google Scholar] [CrossRef]
62. Choi, E., Chang, M. S., Byeon, S. J., Jin, H., Jung, K. C. et al. (2020). Prognostic perspectives of PD-L1 combined with tumor-infiltrating lymphocytes, Epstein-Barr virus, and microsatellite instability in gastric carcinomas. Diagnostic Pathology, 15(1), 1–15. DOI 10.1186/s13000-020-00979-z. [Google Scholar] [CrossRef]
63. Kim, D. H., Bae, G. E., Suh, K. S., Ryuman, D., Song, K. S. et al. (2020). Clinical significance of tumor and immune cell PD-L1 expression in gastric adenocarcinoma. In Vivo, 34(6), 3171–3180. DOI 10.21873/invivo.12152. [Google Scholar] [CrossRef]
64. Martinson, H. A., Mallari, D., Richter, C., Wu, T. T., Tiesinga, J. et al. (2020). Molecular classification of gastric cancer among alaska native people. Cancers, 12(1), 198. DOI 10.3390/cancers12010198. [Google Scholar] [CrossRef]
65. di Pinto, F., Armentano, R., Arborea, G., Schena, N., Donghia, R. et al. (2020). Are immunohistochemical markers useful in phenotypic gastric cancer classification? Oncology, 98(8), 566–574. DOI 10.1159/000506077. [Google Scholar] [CrossRef]
66. Liu, X., Choi, M. G., Kim, K., Kim, K. M., Kim, S. T. et al. (2020). High PD-L1 expression in gastric cancer (GC) patients and correlation with molecular features. Pathology-Research and Practice, 216(4), 152881. DOI 10.1016/j.prp.2020.152881. [Google Scholar] [CrossRef]
67. Weadick, C., McLaughlin, R. A., Ramsey, M., Al-Julandani, N., Power, D. G. et al. (2021). The incidence of Epstein-Barr Virus-associated gastric cancer in an Irish population. Journal of Clinical Oncology, 39(3), 236. DOI 10.1200/JCO.2021.39.3_suppl.236. [Google Scholar] [CrossRef]
68. de Fátima Aquino Moreira-Nunes, C., de Souza Almeida Titan Martins, C. N., Feio, D., Lima, I. K., Lamarão, L. M. et al. (2021). PD-L1 expression associated with Epstein-Barr virus status and patients’ survival in a large cohort of gastric cancer patients in northern Brazil. Cancers, 13(13), 3107. DOI 10.3390/cancers13133107. [Google Scholar] [CrossRef]
69. Nshizirungu, J. P., Bennis, S., Mellouki, I., Sekal, M., Benajah, D. A. et al. (2021). Reproduction of the cancer genome atlas (TCGA) and Asian cancer research group (ACRG) gastric cancer molecular classifications and their association with clinicopathological characteristics and overall survival in moroccan patients. Disease Markers, 2021, 9980410. DOI 10.1155/2021/9980410. [Google Scholar] [CrossRef]
70. Mambouene, F., Boumba, A., Mouamba, F., Mbou, V. B., Moudiongui, D. et al. (2021). Prevalence of gastric cancer associated with the Epstein-Barr virus at Brazzaville Chu. International Journal of Health Sciences and Research, 11(9), 137–141. DOI 10.52403/ijhsr. [Google Scholar] [CrossRef]
71. Gareayaghi, N., Akkus, S., Saribas, S., Demiryas, S., Ozbey, D. et al. (2021). Epstein-Barr Virus and Helicobacter pylori co-infection in patients with gastric cancer and duodenale ulcer. New Microbiologica, 44(4), 217–226. [Google Scholar]
72. Yang, N., Wu, Y., Jin, M., Jia, Z., Wang, Y. et al. (2021). Microsatellite instability and Epstein-Barr virus combined with PD-L1 could serve as a potential strategy for predicting the prognosis and efficacy of postoperative chemotherapy in gastric cancer. PeerJ, 9, e11481. DOI 10.7717/peerj.11481. [Google Scholar] [CrossRef]
73. Rihane, F. E., Erguibi, D., Elyamine, O., Abumsimir, B., Ennaji, M. M. et al. (2021). Helicobacter pylori co-infection with Epstein-Barr virus and the risk of developing gastric adenocarcinoma at an early age: Observational study infectious agents and cancer. Annals of Medicine and Surgery, 68(1), 102651. DOI 10.1016/j.amsu.2021.102651. [Google Scholar] [CrossRef]
74. Suzuki, Y., Inoshita, N., Kikuchi, D., Nomura, K., Matsui, A. et al. (2021). Clinicopathological features of Epstein-Barr virus-associated superficial early stage gastric cancer treated with endoscopic submucosal dissection. Digestive and Liver Disease, 21, 764–767. [Google Scholar]
75. Mallakh, M. K., Mahmood, M. M., Ali, S. H. M. (2022). Expression of a cell cycle regulatory protein (P73) correlated with IL-10 levels in HPV-infected gastric cancer patients. Bulletin of National Institute of Health Sciences, 140(1), 1123–1132. [Google Scholar]
76. Castañeda, C., Castillo, M., Bernabe, L., Suarez, N., Fassan, M. et al. (2022). The relationship between tumour infiltrating lymphocytes, Epstein-Barr virus and Helicobacter pylori infection in gastric cancer. Ecancermedicalscience, 16, 1362. [Google Scholar]
77. Danilova, N. V., Mikhailov, I. A., Kalinin, D. V., Oleynikova, N. A., Chayka, A. V. et al. (2022). Study of gastric adenocarcinomas association with the Epstein-Barr virus-encoded small RNAs. Arkhiv Patologii, 84(2), 5–12. DOI 10.17116/patol2022840215. [Google Scholar] [CrossRef]
78. Ye, L. P., Mao, X. L., Zhou, X. B., Wang, Y., Xu, S. W. et al. (2022). Cost-effective low-coverage whole-genome sequencing assay for the risk stratification of gastric cancer. World Journal of Gastrointestinal Oncology, 14(3), 690–702. DOI 10.4251/wjgo.v14.i3.690. [Google Scholar] [CrossRef]
79. Fukayama, M. (2010). Epstein-Barr virus and gastric carcinoma. Pathology International, 60(5), 337–350. DOI 10.1111/j.1440-1827.2010.02533.x. [Google Scholar] [CrossRef]
80. Liang, Q., Yao, X., Tang, S., Zhang, J., Yau, T. O. (2014). Integrative identification of Epstein-Barr virus-associated mutations and epigenetic alterations in gastric cancer. Gastroenterology, 147(6), 1350–1362. DOI 10.1053/j.gastro.2014.08.036. [Google Scholar] [CrossRef]
81. Ohnishi, N., Yuasa, H., Tanaka, S., Sawa, H., Miura, M. (2008). Transgenic expression of Helicobacter pylori CagA induces gastrointestinal and hematopoietic neoplasms in mouse. PNAS, 105(3), 1003–1008. DOI 10.1073/pnas.0711183105. [Google Scholar] [CrossRef]
82. Wroblewski, L. E., PeekJr, R. M., Wilson, K. T. (2010). Helicobacter pylori and gastric cancer: Factors that modulate disease risk. Clinical Microbiology Reviews, 23(4), 713–739. DOI 10.1128/CMR.00011-10. [Google Scholar] [CrossRef]
83. Chen, H., Chen, X. Z., Waterboer, T., Castro, F. A., Brenner, H. (2015). Viral infections and colorectal cancer: A systematic review of epidemiological studies. International Journal of Cancer, 137(1), 12–24. DOI 10.1002/ijc.29180. [Google Scholar] [CrossRef]
84. Harper, D. M., Vierthaler, S. L. (2011). Who should be targeted for vaccination against anal cancer? The Lancet Oncology, 12(9), 828–829. DOI 10.1016/S1470-2045(11)70237-6. [Google Scholar] [CrossRef]
85. Li, N., Bi, X., Zhang, Y., Zhao, P., Zheng, T. et al. (2011). Human papillomavirus infection and sporadic breast carcinoma risk: A meta-analysis. Breast Cancer Research and Treatment, 126(2), 515–520. DOI 10.1007/s10549-010-1128-0. [Google Scholar] [CrossRef]
86. Valentino, K., Poronsky, C. B. (2016). Human papillomavirus infection and vaccination. Journal of Pediatric Nursing, 31(2), e155–e166. DOI 10.1016/j.pedn.2015.10.005. [Google Scholar] [CrossRef]
87. Yang, L., Xie, S., Feng, X., Chen, Y., Zheng, T. et al. (2015). Worldwide prevalence of human papillomavirus and relative risk of prostate cancer: A meta-analysis. Scientific Reports, 5(1), 1–10. DOI 10.1038/srep14667. [Google Scholar] [CrossRef]
88. Ma, T. Y., Liu, W. K., Chu, Y. L., Jiang, X. Y., An, Y. et al. (2007). Detection of human papillomavirus type 16 DNA in formalin-fixed, paraffin-embedded tissue specimens of gastric carcinoma. European Journal of Gastroenterology & Hepatology, 19(12), 1090–1096. DOI 10.1097/MEG.0b013e3282eeb4dc. [Google Scholar] [CrossRef]
89. Zeng, Z. M., Luo, F. F., Zou, L. X., He, R. Q., Pan, D. H. et al. (2016). Human papillomavirus as a potential risk factor for gastric cancer: A meta-analysis of 1,917 cases. OncoTargets and Therapy, 9, 7105. DOI 10.2147/OTT. [Google Scholar] [CrossRef]
90. Mirabello, L., Yeager, M., Yu, K., Clifford, G. M., Xiao, Y. et al. (2017). HPV16 E7 genetic conservation is critical to carcinogenesis. Cell, 170(6), 1164–1174. DOI 10.1016/j.cell.2017.08.001. [Google Scholar] [CrossRef]
91. El Abidine, A. Z., Tomaić, V., Rhouma, R. B. H., Massimi, P., Guizani, I. et al. (2017). A naturally occurring variant of HPV-16 E7 exerts increased transforming activity through acquisition of an additional phospho-acceptor site. Virology, 500(12), 218–225. DOI 10.1016/j.virol.2016.10.023. [Google Scholar] [CrossRef]
92. Lipari, F., McGibbon, G. A., Wardrop, E., Cordingley, M. G. (2001). Purification and biophysical characterization of a minimal functional domain and of an N-terminal Zn2+-binding fragment from the human papillomavirus type 16 E6 protein. Biochemistry, 40(5), 1196–1204. DOI 10.1021/bi001837+. [Google Scholar] [CrossRef]
93. Yim, E. K., Park, J. S. (2005). The role of HPV E6 and E7 oncoproteins in HPV-associated cervical carcinogenesis. Cancer Research and Treatment, 37(6), 319–324. DOI 10.4143/crt.2005.37.6.319. [Google Scholar] [CrossRef]
94. Sen, P., Ganguly, P., Ganguly, N. (2018). Modulation of DNA methylation by human papillomavirus E6 and E7 oncoproteins in cervical cancer. Oncology Letters, 15(1), 11–22. [Google Scholar]
95. Duensing, S., Münger, K. (2004). Mechanisms of genomic instability in human cancer: Insights from studies with human papillomavirus oncoproteins. International Journal of Cancer, 109(2), 157–162. DOI 10.1002/ijc.11691. [Google Scholar] [CrossRef]
96. Castillo, A. (2013). Human papillomavirus and carcinogenesis in the upper aero-digestive tract. Carcinogenesis, 45, 45–62. DOI 10.5772/2982. [Google Scholar] [CrossRef]
97. Yamato, K., Yamada, T., Kizaki, M., Ui-Tei, K., Natori, Y. et al. (2008). New highly potent and specific E6 and E7 siRNAs for treatment of HPV16 positive cervical cancer. Cancer Gene Therapy, 15(3), 140–153. DOI 10.1038/sj.cgt.7701118. [Google Scholar] [CrossRef]
98. Jabbar, S. F., Abrams, L., Glick, A., Lambert, P. F. (2009). Persistence of high-grade cervical dysplasia and cervical cancer requires the continuous expression of the human papillomavirus type 16 E7 oncogene. Cancer Research, 69(10), 4407–4414. DOI 10.1158/0008-5472.CAN-09-0023. [Google Scholar] [CrossRef]
99. Bae, J. M. (2021). Human papillomavirus infection and gastric cancer risk: A meta-epidemiological review. World Journal of Virology, 10(5), 209–216. DOI 10.5501/wjv.v10.i5.209. [Google Scholar] [CrossRef]
100. Wang, H., Chen, X. L., Liu, K., Bai, D., Zhang, W. H. et al. (2020). Associations between gastric cancer risk and virus infection other than Epstein-Barr virus: A systematic review and meta-analysis based on epidemiological studies. Clinical and Translational Gastroenterology, 11(7), e00201. DOI 10.14309/ctg.0000000000000201. [Google Scholar] [CrossRef]
101. Murphy, G., Pfeiffer, R., Camargo, M. C., Rabkin, C. S. (2009). Meta-analysis shows that prevalence of Epstein-Barr virus-positive gastric cancer differs based on sex and anatomic location. Gastroenterology, 137(3), 824–833. DOI 10.1053/j.gastro.2009.05.001. [Google Scholar] [CrossRef]
102. Herrera-Goepfert, R., Akiba, S., Koriyama, C., Ding, S., Reyes, E. et al. (2005). Epstein-Barr virus-associated gastric carcinoma: Evidence of age-dependence among a Mexican population. World Journal of Gastroenterology, 11(39), 6096–6103. DOI 10.3748/wjg.v11.i39.6096. [Google Scholar] [CrossRef]
103. Yue, W., Zhu, M., Zuo, L., Xin, S., Zhang, J. et al. (2019). Early pattern of Epstein-Barr virus infection in gastric epithelial cells by “cell-in-cell”. Virologica Sinica, 34(3), 253–261. DOI 10.1007/s12250-019-00097-1. [Google Scholar] [CrossRef]
104. Camargo, M. C., Murphy, G., Koriyama, C., Pfeiffer, R. M., Kim, W. H. et al. (2011). Determinants of Epstein-Barr virus-positive gastric cancer: An international pooled analysis. British Journal of Cancer, 105(1), 38–43. DOI 10.1038/bjc.2011.215. [Google Scholar] [CrossRef]
105. Camargo, M. C., Koriyama, C., Matsuo, K., Kim, W. H., Herrera-Goepfert, R. et al. (2014). Case-case comparison of smoking and alcohol risk associations with Epstein-Barr virus-positive gastric cancer. International Journal of Cancer, 134(4), 948–953. DOI 10.1002/ijc.28402. [Google Scholar] [CrossRef]
106. Lee, J. H., Kim, S. H., Han, S. H., An, J. S., Lee, E. S. et al. (2009). Clinicopathological and molecular characteristics of Epstein-Barr virus-associated gastric carcinoma: A meta-analysis. Journal of Gastroenterology and Hepatology, 24(3), 354–365. DOI 10.1111/j.1440-1746.2009.05775.x. [Google Scholar] [CrossRef]
107. Li, S., Du, H., Wang, Z., Zhou, L., Zhao, X. et al. (2010). Meta-analysis of the relationship between Epstein-Barr virus infection and clinicopathological features of patients with gastric carcinoma. Science China Life Sciences, 53(4), 524–530. DOI 10.1007/s11427-010-0082-8. [Google Scholar] [CrossRef]
108. Sousa, H., Pinto-Correia, A. L., Medeiros, R., Dinis-Ribeiro, M. (2008). Epstein-Barr virus is associated with gastric carcinoma: The question is what is the significance? World Journal of Gastroenterology, 14(27), 4347–4351. DOI 10.3748/wjg.14.4347. [Google Scholar] [CrossRef]
109. Pyo, J. S., Kim, N. Y., Kang, D. W. (2020). Clinicopathological significance of EBV-infected gastric carcinomas: A meta-analysis. Medicina, 56(7), 345. DOI 10.3390/medicina56070345. [Google Scholar] [CrossRef]
110. Tavakoli, A., Monavari, S. H., Solaymani Mohammadi, F., Kiani, S. J., Armat, S. et al. (2020). Association between Epstein-Barr virus infection and gastric cancer: A systematic review and meta-analysis. BMC Cancer, 20(1), 1–14. DOI 10.1186/s12885-020-07013-x. [Google Scholar] [CrossRef]
111. Cancer Genome Atlas Research Network (2014). Comprehensive molecular characterization of gastric adenocarcinoma. Nature, 513(7517), 202–209. DOI 10.1038/nature13480. [Google Scholar] [CrossRef]
Cite This Article
 Copyright © 2022 The Author(s). Published by Tech Science Press.
Copyright © 2022 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools