 Open Access
Open Access
CASE REPORT
Gastric Cancer with Bone Marrow Invasion and Disseminated Intravascular Coagulation: A Case Report
Medical Oncology Department, Affiliated Jinling Hospital, Medical School of Nanjing University, Nanjing, 210093, China
* Corresponding Authors: Guichun Huang. Email: ; Xiaoyuan Chu. Email:
Oncologie 2022, 24(3), 599-604. https://doi.org/10.32604/oncologie.2022.023310
Received 20 April 2022; Accepted 31 May 2022; Issue published 19 September 2022
Abstract
Gastric cancer is the fifth most commonly diagnosed cancer and the fourth leading cause of cancer death worldwide in 2020. Gastric cancer usually undergoes lymph node metastasis and implantation metastasis, but bone metastasis and bone marrow invasion are rare. However, gastric cancer patients with bone marrow invasion usually have cancer emergency, so special attention should be paid in clinical practice. Herein, we analyzed the clinical characteristics of an asian gastric cancer patient with bone marrow invasion and disseminated intravascular coagulation (DIC) in our hospital and summarized the diagnosis and treatment experience to provide a reference for such diseases.Keywords
Abbreviations
| DIC | disseminated intravascular coagulation |
| HER-2 | human epidermal growth factor receptor-2 |
| EGFR | epidermal growth factor receptor |
| c-MET | cellular-mesenchymal epithelial transition factor |
| AFP | alpha fetoprotein |
| VEGFR2 | vascular endothelial growth factor receptor 2 |
| MLH1 | mutL homolog 1 |
| PD-L1 | programmed cell death-ligand 1 |
| CT | computed tomography |
| ECT | emission computed tomography |
| MRI | magnetic resonance imaging |
| APTT | activated partial thromboplastin time |
| PT | prothrombin time |
| MSS | microsatellite stable |
| pMMR | proficient mismatch repair |
| EBER | Epstein-Barr virus-encoded RNA |
| PET-CT | positron emission tomography-computed tomography |
| FDG | fluorodeoxyglucose |
| CEA | carcinoembryonic antigen |
| FDP | fibrin degradation product |
| ALP | alkaline phosphatase |
| RANKL | receptor activator of NF-κB ligand |
| RANK | receptor activator of NF-κB |
Gastric cancer is the fifth most commonly diagnosed cancer and the fourth leading cause of cancer death worldwide in 2020 [1]. Local recurrence and distant metastases are the most common causes of death in patients with gastric cancer [2]. Gastric cancer usually undergoes lymph node metastasis and implantation metastasis, but bone metastasis and bone marrow invasion are rare [3,4]. However, gastric cancer patients with bone marrow invasion usually have cancer emergency, so special attention should be paid in clinical practice [5]. Herein, we analyzed the clinical characteristics of a gastric cancer patient with bone marrow invasion and disseminated intravascular coagulation (DIC) in our hospital and summarized the diagnosis and treatment experience to provide a reference for such diseases.
The patient is female, 58 years old, married, Asian, has one son and one daughter, and was healthy in the past with no history of hypertension and diabetes; no smoking or drinking history; no family history of a malignant tumor.
The patient was diagnosed with gastric cancer four years ago (November 2016) and underwent a radical distal gastrectomy in Affiliated Drum Tower Hospital, Medical School of Nanjing University. Postoperative pathology indicated that the tumor was 1.2 × 1 × 0.5 cm in size and located in the posterior wall of the gastric corpus. The cancer tissue invaded the submucosa of the gastric wall. The tumor was poorly differentiated adenocarcinoma, partly signet ring cell carcinoma, and the Lauren classification of this tumor is diffuse (Fig. 1A). No cancer thrombus was found in the vessels, no invasion was found in the nerves, and no residual cancer was found in the resection margin. Four in sixteen resected lymph nodes had cancer metastasis. The gastric mucosa around cancer showed mild chronic atrophic gastritis with intestinal metaplasia. Immunohistochemistry staining with streptavidin-peroxidase method indicated cancer cells HER-2 (1+), EGFR (weak+), c-MET (+), AFP (-), VEGFR2 (++), E-cadherin (−), MLH1 (++), PD-L1 (−), and Ki-67 (about 60%+) (Fig. 1B). Helicobacter pylori was negative. The patient received no adjuvant chemoradiotherapy postoperatively. In August 2020, the patient developed right chest pain without obvious incentives, and then the symptoms gradually worsened. Chest computed tomography (CT) showed lesions of both lungs, a small amount of pleural effusion on both sides, low-density foci in the liver, and metal density shadows in the stomach wall. Magnetic resonance imaging (MRI) of the thoracic spine revealed abnormal signals in the thoracic 4, 7, and 12 vertebrae. Emission computed tomography (ECT) bone scan showed multiple bone metastases throughout the body. From October 20, 2020, the patient had an intermittent fever every night, with the highest temperature of 37.8°C, without chills, nausea, and vomiting, and the fever could relieve spontaneously. The patient also had a bleeding tendency (spontaneous bleeding of the gums).
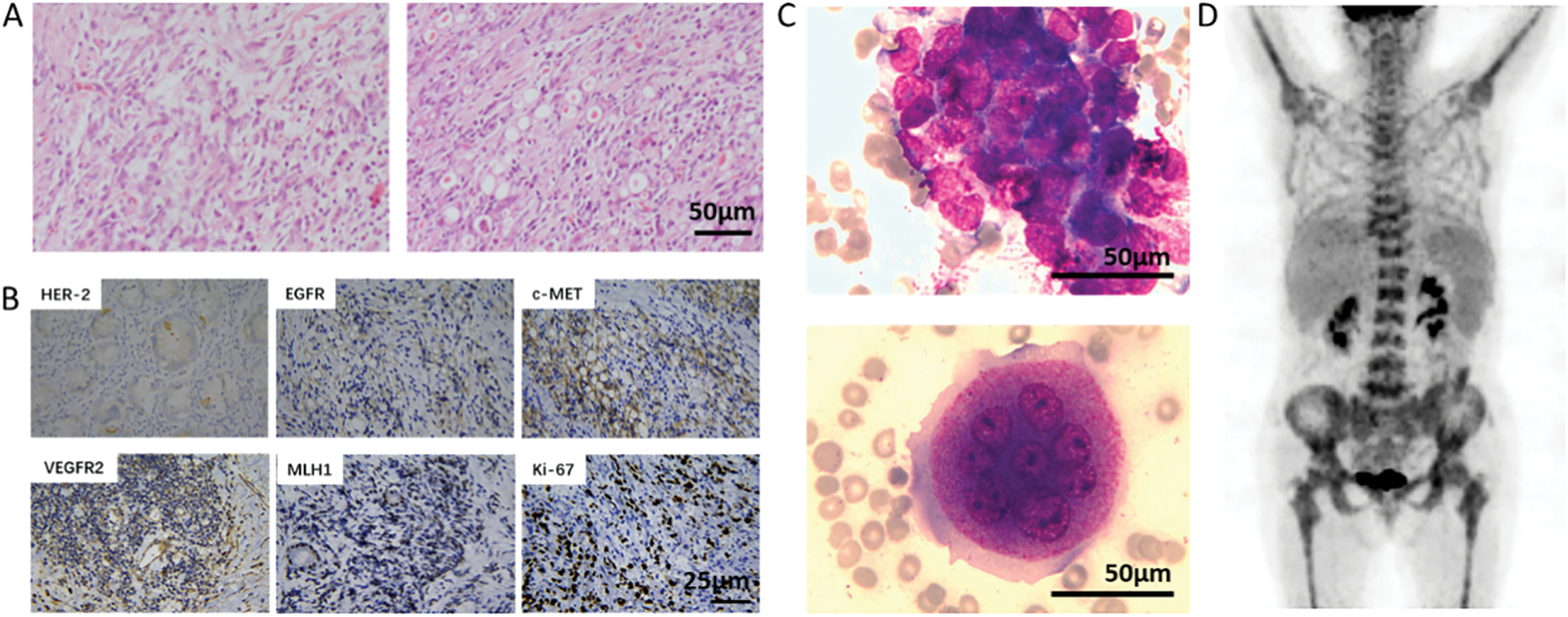
Figure 1: Pathological diagnosis and PET-CT examination of the patient. A, postoperative pathology of the patient (H&E, 200×). Scale bar = 50 μm; B, immunohistochemical staining showed the positive expression of HER-2, EGFR, c-MET, VEGFR2, MLH1, and Ki-67. Scale bar = 25 μm; C, bone marrow smear (Wright’s staining, 400×) of the patient, showing cancer cell mass (left) and multinucleated osteoclasts (right). Scale bar = 50 μm; D, patient’s PET-CT examination showed multiple foci of increased FDG uptake in the whole body, especially in the bone marrow
After admission to our hospital, blood cell count: white blood cell count (2.9 × 109/L), hemoglobin (76 g/L), and platelet count (15 × 109/L). Coagulation function: fibrinogen (1.65 g/L), activated partial thromboplastin time (APTT) (32.2 s, mildly prolonged), prothrombin time (PT) (16.4 s, mildly prolonged), and D-dimer (130.94 mg/L). Liver and kidney function indexes were within the normal range. Molecular pathology of surgical specimens showed: negative for human epidermal growth factor receptor-2 (HER-2) amplification, microsatellite stable (MSS)/proficient mismatch repair (pMMR), Epstein-Barr virus-encoded RNA (EBER) negative, and programmed cell death-ligand 1 (PD-L1) expression negative.
We made the diagnosis before rescue treatment: 1. Gastric cancer with bone metastasis and bone marrow invasion; 2. Grade IV bone marrow suppression; 3. Early-stage disseminated intravascular coagulation (DIC).
Treatment process: After preliminary diagnosis, the patient was given supplementation of platelets and coagulation factors followed by anticoagulation therapy with low molecular weight heparin sodium (reduced dose, 5000 IU, subcutaneous injection every other day). At the same time, the patient received thrombopoietin (15000 IU, once daily), recombinant human interleukin-11 (1.5 mg, once daily), and recombinant human granulocyte-stimulating factor (300 μg, once daily) subcutaneous injection. Zoledronic acid was applied to inhibit osteoclasts. However, the patient’s blood cell count and coagulation function were not significantly improved. Therefore, we prescribed the patient low-dose capecitabine (0.5 g, orally, twice daily) on October 26, 2020. The patient’s D-dimer and fibrinogen degradation products were significantly reduced (Fig. 2A), and then the dose of capecitabine was adjusted (1.0 g, orally, twice daily). On November 14, 2020, blood cell count: white blood cell count (14.6 × 109/L), hemoglobin (65 g/L), and platelet count (45 × 109/L); coagulation function: fibrinogen (1.67 g/L), APTT (32.2 s), PT (15.1 s), and D-dimer (17.79 mg/L). The patient’s condition improved, and we supplemented bone marrow examination and a positron emission tomography-computed tomography (PET-CT) scan. Bone marrow examination showed that the patient’s bone marrow was hyperplasia, especially in granulocytes. The granulocytic lineage and erythroid lineage accounted for 72.0% and 18.0% of bone marrow cells, respectively. And the ratio of granulocyte and erythrocyte was 400:1. Cancer cells can be seen in bone marrow with different sizes, irregular shapes, and densely arranged in piles with unclear boundaries. Meanwhile, multinucleated osteoclasts could be observed (Fig. 1C). PET-CT results showed that: 1, the residual gastric was thickened, but fluorodeoxyglucose (FDG) uptake was not high; 2, uneven bone density in multiple vertebral bodies, slightly increased FDG uptake, and some vertebral bodies became flattened; 3, there was inflammation in both lungs, bilateral pleural thickening, and pleural effusion; 4, there were small lymph nodes in the mediastinum, bilateral axillary and retroperitoneum, but FDG uptake was not high (Fig. 1D). On November 19, 2020, intravenous chemotherapy with cisplatin (30 mg, days 1, 2, 8, and 9) was added to the anti-cancer regimen. The patient had no obvious adverse reactions after chemotherapy, her condition improved significantly, and the persistent fever disappeared. On December 06, 2020, the blood cell count was reexamined: white blood cell count (6.89 × 109/L), hemoglobin (82 g/L), platelet count (114 × 109/L); coagulation function: fibrinogen (5.53 g/L), APTT (27.2 s), PT (14.3 s), D-dimer (4.81 mg/L) (Fig. 2A). The tumor marker carcinoembryonic antigen (CEA) decreased from 315.4 to 42 μg/L. CA724 and CA242 also decreased significantly. Serum alkaline phosphatase (ALP) examined by an automatic biochemistry analyzer (Roche, USA) decreased from 2352 to 1752 U/L (Fig. 2B). After the patient’s condition improved, she received two cycles of the same treatment regimen (capecitabine plus cisplatin) in a local hospital. The patient died of disease progression six months later.
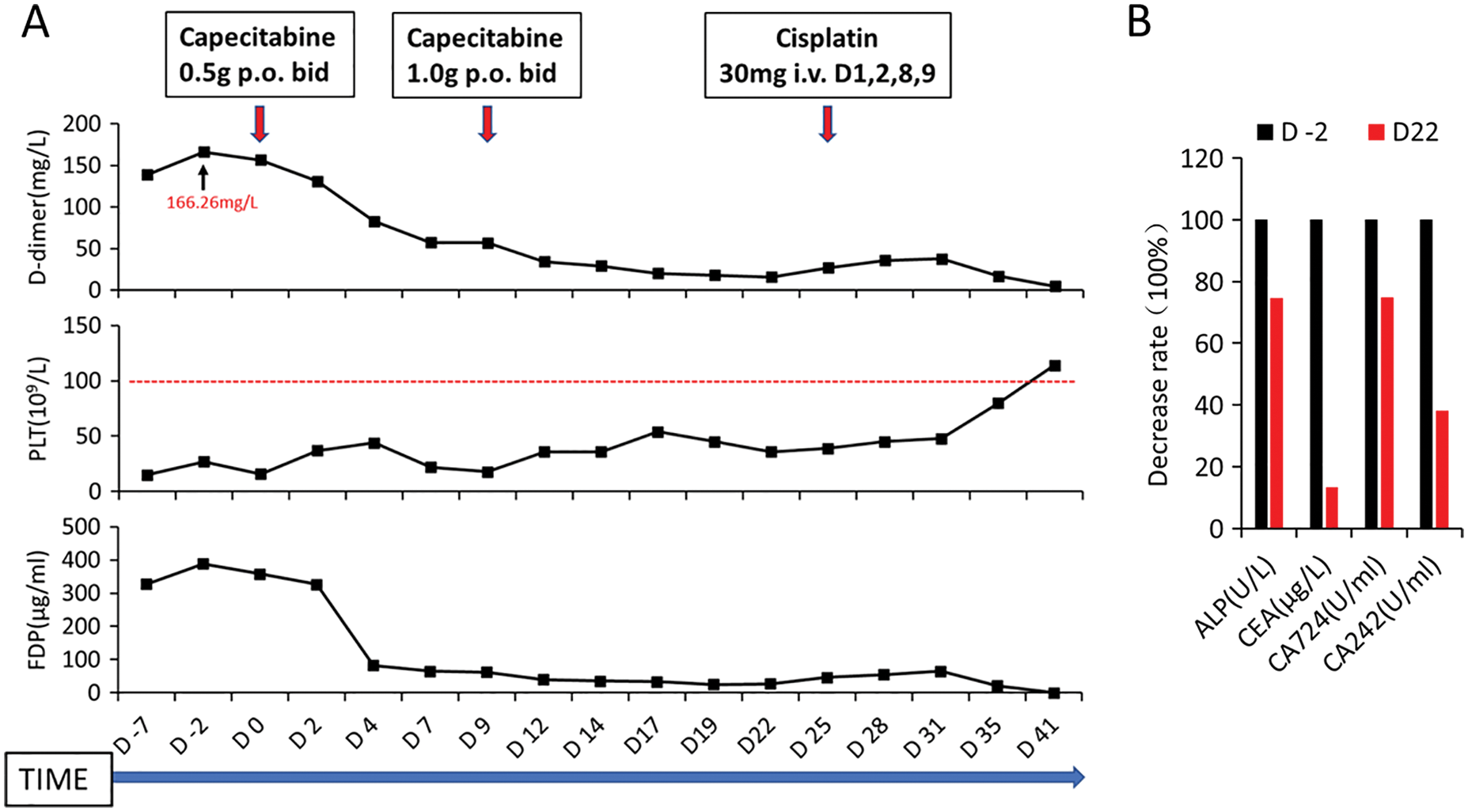
Figure 2: Changes in various blood parameters in the patient before and after Capecitabine and Cisplatin chemotherapy. A, dynamic changes of D-dimer (D-dimer), platelet count (PLT), and fibrin degradation product (FDP) during the patient’s first hospitalization; red arrows indicate the time and dose of chemotherapy drugs. The abscissa axis shows the days of hospitalization of the patient, where D0 refers to the time of the start of chemotherapy; B, the decreased levels of alkaline phosphatase (ALP) and tumor markers (CEA, CA724, and CA242) in the patient before and after Capecitabine chemotherapy
There is no clear distinction between the concepts of bone metastasis, bone marrow metastasis, and bone marrow invasion of gastric cancer, so apparent confusion exists. It significantly affects the analysis of the clinical characteristics of gastric cancer with bone metastasis and bone marrow invasion. Some researchers suggest that gastric cancer bone marrow invasion (or bone marrow metastasis) is one subtype of bone metastasis. However, significant differences remain between gastric cancer with bone marrow invasion and common bone metastasis in clinical manifestations, pathogenesis, treatment response, and prognosis. Although gastric cancer patients with bone marrow invasion and DIC is considered to be highly aggressive, some can obtain a more extended remission period if the disease can be controlled [6]. In this case, the patient has only bone metastasis and bone marrow invasion, while other organ functions are still within the normal range, indicating that this patient has not progressed to multiple organ involvement and is tolerable to specific doses of chemotherapy.
The pathophysiological mechanism of bone marrow invasion in gastric cancer is still unclear. The interaction between tumor cells in a long-term dormant state and the bone marrow microenvironment (especially osteoclasts) might be the key to tumor development [7]. Activation of Receptor Activator of NF-κB Ligand (RANKL) and its receptor RANK signaling pathway in osteoclasts can change tumor cells from the dormant to the proliferating state. At the same time, RANKL released by proliferating tumor cells further activates osteoclasts. The sustained activation of tumor and bone cells releases many inflammatory factors leading to bone marrow suppression and DIC [8,9]. We speculate that the tumor cells of this patient underwent a 4-year dormant period after radical gastrectomy. In addition, apparent tumor cells and osteoclasts were seen in the bone marrow smear, verifying the rationality of the above model. Breaking the vicious feedback loop between tumor cells and the bone marrow microenvironment may be the most important for treating such patients [10,11].
The macroscopic types of gastric cancer with bone marrow invasion are mostly Borrmann type II and Borrmann type III. The histological types are mostly poorly differentiated adenocarcinoma and signet-ring cell carcinoma with a high degree of malignancy [4]. There are no significant characteristics in the molecular pathological classification of these patients, suggesting that the current targeted therapy has limited value in the treatment. In addition, these patients are often accompanied by abnormal coagulation function and bleeding tendency, so the application of anti-vascular targeted therapy drugs should also be cautious [12,13].
Although contraindications to chemotherapy often occur, chemotherapy is still the most effective treatment for bone marrow invasion of gastric cancer so far. Supportive therapy (such as the transfusion of platelet and plasma, anticoagulation with low molecular weight heparin, and inhibition of osteoclasts by bisphosphonate or denosumab) can not improve the condition of these patients. In this case, supportive treatments were given within one week after admission. However, D-dimer, platelets, and fibrin degradation products did not change notably (Fig. 2A). In comparison, symptoms and blood parameters of this patient significantly improved after capecitabine single-agent chemotherapy. Regarding the choice of chemotherapy regimen, fluorouracil, cisplatin, oxaliplatin, and taxane have all been reported [6,14,15]. Still, the authors believe that gastric cancer patients with bone marrow invasion are all accompanied by bone marrow suppression. A fluorouracil single-agent or combined cisplatin regimen should be preferred.
In conclusion, gastric cancer with bone metastasis and bone marrow invasion has a poor clinical prognosis. Early detection anti-tumor therapy can prolong the survival of some patients. Further research on the fundamental mechanisms of pathogenesis can provide a theoretical basis for controlling such diseases.
Acknowledgement: The authors thank the patient who agreed to be included in this study.
Authorship: The authors confirm contribution to the paper as follows: clinical data collection: Lilan Chen, Lu Lu; analysis and interpretation of results: Xiaoyuan Chu and Guichun Huang; draft manuscript preparation: Lilan Chen, Xinlei Gong, and Yichen Xu. All authors reviewed the results and approved the final version of the manuscript.
Ethics Approval and Informed Consent Statement: A written informed consent has been obtained from the patient to publish this paper.
Availability of Data and Materials: There is no additional data regarding to this study and all available data and materials have been shared within the case report.
Funding Statement: The work was supported by National Natural Science of China (No. 81472668) and Jiangsu Provincial Youth Medical Key Talents Project (QNRC2016887).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I. et al. (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians, 71(3), 209–249. DOI 10.3322/caac.21660. [Google Scholar] [CrossRef]
2. Beatty, J. K., Bhargava, A., Buret, A. G. (2014). Post-infectious irritable bowel syndrome: Mechanistic insights into chronic disturbances following enteric infection. World Journal of Gastroenterology, 20(14), 3976–3985. DOI 10.3748/wjg.v20.i14.3976. [Google Scholar] [CrossRef]
3. Qiu, M. Z., Shi, S. M., Chen, Z. H., Yu, H. E., Sheng, H. et al. (2018). Frequency and clinicopathological features of metastasis to liver, lung, bone, and brain from gastric cancer: A SEER-based study. Cancer Medicine, 7(8), 3662–3672. DOI 10.1002/cam4.1661. [Google Scholar] [CrossRef]
4. Iguchi, H. (2015). Recent aspects for disseminated carcinomatosis of the bone marrow associated with gastric cancer: What has been done for the past, and what will be needed in future? World Journal of Gastroenterology, 21(43), 12249–12260. DOI 10.3748/wjg.v21.i43.12249. [Google Scholar] [CrossRef]
5. Kim, Y. J., Kim, S. H., Kim, J. W., Lee, J. O., Kim, J. H. et al. (2014). Gastric cancer with initial bone metastasis: A distinct group of diseases with poor prognosis. European Journal of Cancer, 50(16), 2810–2821. DOI 10.1016/j.ejca.2014.08.003. [Google Scholar] [CrossRef]
6. Zhai, X., Wang, C., Li, S., Cao, T., Du, G. et al. (2022). Bone marrow metastasis from advanced gastric cancer complicated with disseminated intravascular coagulation: A highly aggressive but manageable disease subtype. Cancer Communications, 42(4), 350–354. DOI 10.1002/cac2.12277. [Google Scholar] [CrossRef]
7. Croucher, P. I., McDonald, M. M., Martin, T. J. (2016). Bone metastasis: The importance of the neighbourhood. Nature Reviews. Cancer, 16(6), 373–386. DOI 10.1038/nrc.2016.44. [Google Scholar] [CrossRef]
8. Zhang, X., Song, Y., Song, N., Zhang, L., Wang, Y. et al. (2018). Rankl expression predicts poor prognosis in gastric cancer patients: Results from a retrospective and single-center analysis. Brazilian Journal of Medical Biological Research, 51(3), e6265. DOI 10.1590/1414-431x20176265. [Google Scholar] [CrossRef]
9. Gyori, D. S., Mocsai, A. (2020). Osteoclast signal transduction during bone metastasis formation. Frontiers in Cell and Developmental Biology, 8, 507. DOI 10.3389/fcell.2020.00507. [Google Scholar] [CrossRef]
10. Vijayaraghavalu, S., Gao, Y., Rahman, M. T., Rozic, R., Sharifi, N. et al. (2020). Synergistic combination treatment to break cross talk between cancer cells and bone cells to inhibit progression of bone metastasis. Biomaterials, 227(6), 119558. DOI 10.1016/j.biomaterials.2019.119558. [Google Scholar] [CrossRef]
11. Nakai, Y., Okamoto, K., Terashima, A., Ehata, S., Nishida, J. et al. (2019). Efficacy of an orally active small-molecule inhibitor of RANKL in bone metastasis. Bone Research, 7(1), 1. DOI 10.1038/s41413-018-0036-5. [Google Scholar] [CrossRef]
12. Ekinci, A. S., Bal, O., Ozatli, T., Turker, I., Esbah, O. et al. (2014). Gastric carcinoma with bone marrow metastasis: A case series. Journal of Gastric Cancer, 14(1), 54–57. DOI 10.5230/jgc.2014.14.1.54. [Google Scholar] [CrossRef]
13. Seki, Y., Wakaki, K. (2016). Pathological findings in a case of bone marrow carcinosis due to gastric cancer complicated by disseminated intravascular coagulation and thrombotic microangiopathy. International Journal of Hematology, 104(4), 506–511. DOI 10.1007/s12185-016-2051-x. [Google Scholar] [CrossRef]
14. Smyth, E. C., Nilsson, M., Grabsch, H. I., van Grieken, N. C. T., Lordick, F. (2020). Gastric cancer. The Lancet, 396(10251), 635–648. DOI 10.1016/S0140-6736(20)31288-5. [Google Scholar] [CrossRef]
15. Yu, Y. J., Sun, W. J., Lu, M. D., Wang, F. H., Qi, D. S. et al. (2014). Efficacy of docetaxel combined with oxaliplatin and fluorouracil against stage III/IV gastric cancer. World Journal of Gastroenterology, 20(48), 18413–18419. DOI 10.3748/wjg.v20.i48.18413. [Google Scholar] [CrossRef]
Cite This Article
 Copyright © 2022 The Author(s). Published by Tech Science Press.
Copyright © 2022 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools