
 | Oncologie |  |
DOI: 10.32604/oncologie.2022.022299
REVIEW
Use of the Naphthoquinone YM155 (Sepantronium Bromide) in the Treatment of Cancer: A Systematic Review and Meta-Synthesis
1Department of Public Health, Graduate School of Medicine, International University of Health and Welfare, Tokyo, 107-8402, Japan
2Department of Pathology, School of Medicine, Mongolian National University of Medical Sciences, Ulan-Bator, 14210, Mongolia
3Division of Immunology, Boston Children’s Hospital, Harvard Medical School, Boston, MA 02115, USA
4Department of Administration, MCS Property, Ulan-Bator, 14200, Mongolia
5Department of Rehabilitation, Kobe International University, Kobe, 658-0032, Japan
6Department of Pathology, School of Medicine, International University of Health and Welfare, Narita, 286-8686, Japan
7New Medical University, Ulan-Bator, 18104, Mongolia
8Institute of Translational Medicine, Semmelweis University, Budapest, 1085, Hungary
9Department of Epidemiology and Social Medicine, Graduate School of Public Health, International University of Health and Welfare, Tokyo, 107-8402, Japan
*Corresponding Author: Yasuhiko Tomita. Email: yasuhiko-tomita@umin.ac.jp
Received: 03 March 2022; Accepted: 03 May 2022
Abstract: Most cancer cells overexpress the anti-apoptotic protein survivin and display redox dysregulation originating from genotypic and phenotypic alterations. These disturbances contribute to the uncontrolled proliferation, invasion, and chemoresistance of cancer cells, yet they also represent a specific vulnerability that could be exploited therapeutically in selected tumors. YM155 (sepantronium bromide) is a naphthoquinone-containing imidazole-based compound that selectively inhibits survivin expression at the transcriptional and post-transcriptional levels. Here, we performed a systematic review and meta-analysis of clinical studies in which YM155 was administered as monotherapy or combination therapy for patients with cancer. We assessed fully or partially reported clinical outcomes and pharmacological parameters, and further performed subgroup analysis based on tumor type and treatment regimen. Our comprehensive analysis, which included patients of many ethnicities, demonstrated that YM155 was effective as a monotherapy or combination therapy. Clinical benefits, including regression and/or stabilization of tumor progression and prolonged survival, were observed within a reasonable time after treatment initiation, and YM155 displayed good synergistic effects with combination drugs. YM155 appears to be effective against a wide range of tumor types and has an acceptable safety profile, with the main toxicities being decreased blood cell counts; fatigue/weakness; renal, hepatic, and/or cardiac issues; and electrolyte disturbance.
Keywords: YM155 (sepantronium bromide); systematic review; safety; anticancer effect; meta-synthesis; Naphthoquinone
Nomenclature
| AE | Adverse events |
| AUC | Area under the curve |
| BIRC5 | Baculoviral inhibitor of apoptosis repeat-containing 5 |
| CBR | Clinical benefit rate |
| CCNS | Cell cycle nonspecific |
| CCS | Cell cycle specific |
| Cl | Clearance |
| Cmax | Maximum plasma concentration |
| Css | Steady-state plasma concentration |
| DLT | Dose-limiting toxicity |
| DOR | Duration of response |
| Exc | Renal excretion |
| HAE | Hematological adverse effect |
| HM | Hematological malignancy |
| IAP | Inhibitor of apoptosis |
| MTD | Maximum tolerated dose |
| NHAE | Non-Hematological adverse effect |
| NHL | Non-Hodgkin lymphoma |
| NQ | Naphthoquinone |
| OR | Overall response |
| OS | Overall survival |
| PFS | Progression-free survival |
| PICOSO | Participants, interventions, outcomes, comparisons, study, other |
| PRISMA | Preferred reporting items for systematic reviews and meta-analyses |
| QNRT | Quantitative non-randomized study |
| QRCT | Quantitative randomized controlled trial |
| ST | Solid tumor |
| T1/2 | Half-life |
| TTR | Time to response |
| Vd | Volume of distribution |
| YM155 | Sepantronium bromide |
Survivin (also known as BIRC5), a member of the inhibitor of apoptosis (IAP) family, is essential for cell division and is highly expressed during embryonic development [1,2]. Although it is rarely expressed in adult tissues, survivin is also upregulated in nearly all human tumors [3,4], where it is associated with a more aggressive phenotype, shorter survival times, and a decreased response to chemotherapy [5–7]. Due to its key role in apoptosis, proliferation, and angiogenesis, survivin has received increasing attention as a potential oncotherapeutic target [8,9]. However, survivin is an intracellular protein, does not have intrinsic enzymatic activity, and has few potential druggable sites, making it a relatively complex and difficult therapeutic target [10].
Despite these problems, various strategies have been employed to develop drugs that interfere with survivin expression at the transcriptional and post-translational levels or impair its function [11–14]. Among the candidate drugs, YM155 (sepantronium bromide) is a selective inhibitor of survivin gene expression that has been comprehensively evaluated in both preclinical and clinical studies with promising results [3].
YM155 is a low molecular weight, Naphthoquinone (NQ)-containing imidazole-based compound that was first described in 2007. YM155 suppresses survivin gene transcription by binding specifically to an SP1-rich region in the survivin core promoter, and its inhibition of cell proliferation is thought to be cell cycle-independent [15,16].
The main goal of cancer chemotherapy is to selectively destroy cancer cells while minimizing toxicity to normal cells [17]. In this regard, YM155 holds promise as a chemotherapeutic agent for several reasons. First, as noted above, survivin is overexpressed in almost all malignancies but is virtually undetectable in most normal adult tissues [18]. Second, YM155-mediated repression of survivin transcription is highly selective, and no activity against other IAP family proteins has been reported to date [15,19]. Third, redox dysregulation originating from metabolic alterations contributes to the control of cancer cell proliferation, survival, invasion, and metastasis, suggesting that these features could provide a specific vulnerability of malignant cells that can be selectively targeted by redox chemotherapeutic agents [20,21]. YM155 has been shown to generate reactive oxygen species in the mitochondria. Its NQ pharmacophore undergoes two-electron reduction within the cell, causing profound depletion of reducing elements and disturbing redox homeostasis in cancer cells [22–26].
Vitamin K was first shown to be an NQ-containing antineoplastic agent in 1967 [27], and was tested in clinical research for the first time in 1987 [28]. Since then, an increasing number of preclinical and clinical studies have explored the therapeutic potential of natural and synthetic NQ compounds. The members of NQs’ large family, all contain a common “quinone” ring, which binds and alters macromolecules through arylation reaction, and the electron-donating group, such as—methoxy (-OCH3) and -methyl (-CH3), which can promote the anti-proliferation effect [29–32]. The common NQs molecular structures are depicted in Fig. 1.
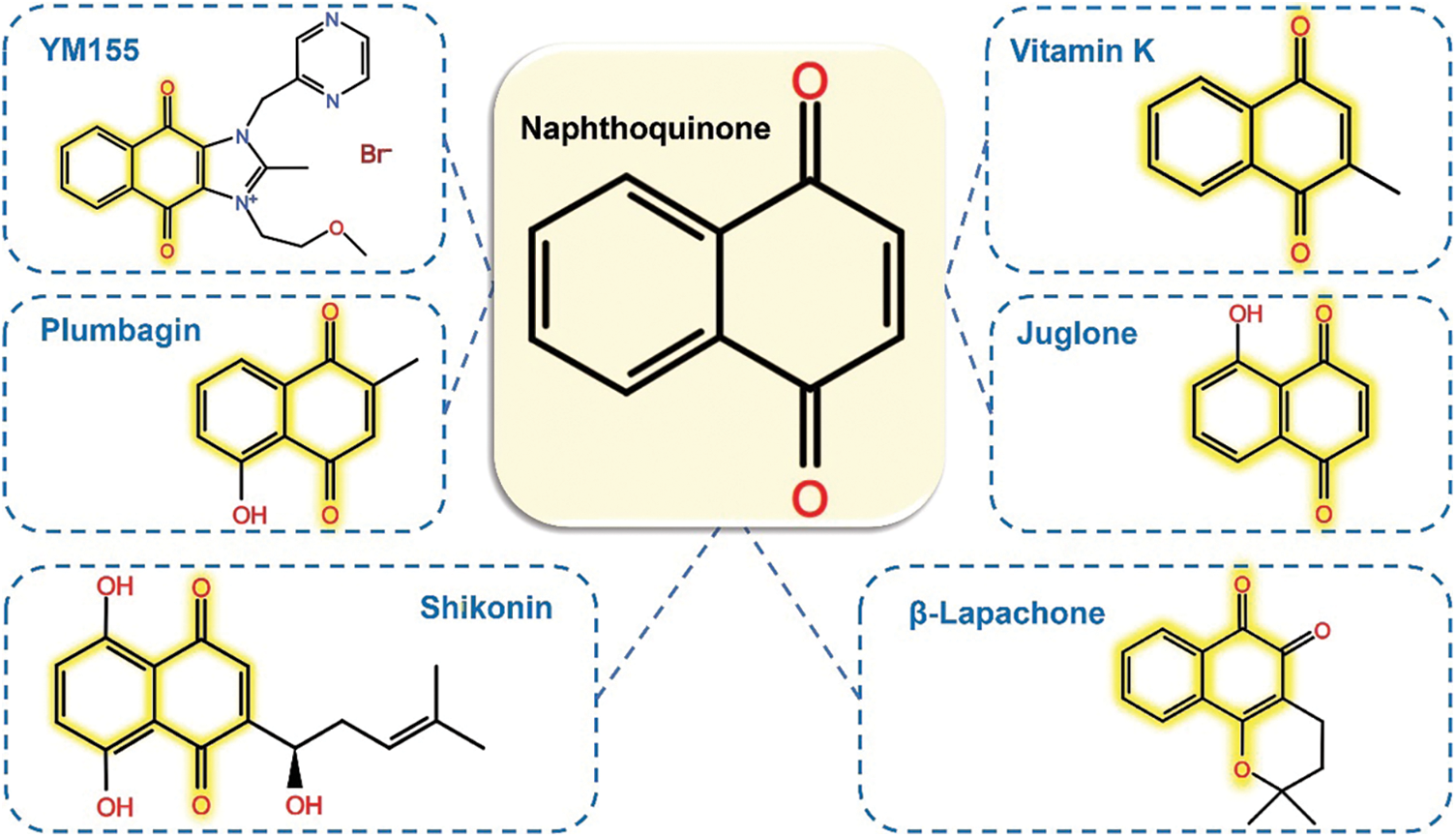
Figure 1: Structural comparison of the most common Naphthoquinone derivatives for anticancer activity
To date, several observational clinical studies have been performed with YM155; however, inconsistent results have been reported with regard to its efficacy in inhibiting cancer progression.
Systematic reviews are the gold standard method of synthesizing and summarizing all relevant primary publications in an unbiased manner, and with high evidence; facilitating decision-making and developing guidelines for patient care [33,34]. Recent advances in systematic review methods have made it possible to combine quantitative and qualitative methods, approaches, and concepts [35–40]. Through this powerful study design, we aimed here to clarify the anticancer effects of YM155 in clinical trials and thereby facilitate clinical decision-making for the treatment of cancer patients.
This systematic review was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [41] and followed an established protocol that has been registered at PROSPERO (record #CRD42019135273, protocol #135273).
2.1 Strategy for Literature Search
To search relevant articles, a concept map was created via MeSH terms and controlled vocabularies based on PICOSO design (Participants, Interventions, Outcomes, Comparisons, Study, Other), then integrated with electronic databases through an advanced filter and update system. The sources were MEDLINE, Cochrane Library, ClinicalTrials.gov, Japan Medical Abstract Society, J-Stage, and Web of Science. Additional manual searches were performed for references from these systematically gathered articles. No restrictions were applied to identify eligible studies, except only articles published in English and Japanese were included. Ethics Committee approval for this study was waived because no human participants or animals were involved.
2.2 Study Selection and Inclusion Criteria
The studies that met the following inclusion criteria were accepted for the qualitative and quantitative analyses: (1) Participants: any patients with cancer, including solid and hematologic malignancies, with no gender, age, or ethnicity restrictions; (2) Intervention: YM155; (3) Controls: quantitative studies included a “non-exposed control group” or “standard of care” as comparators, whereas qualitative studies included “not applicable” as a control; (4) Outcome: studies that assessed the outcomes of clinical and pharmacological parameters; (5) Study design: prospective or retrospective clinical studies focused on the treatment of cancer patients with YM155. Exclusion criteria: (1) Non-cancerous patient, (2) Therapeutic agents other than YM155, (3) Pre-clinical studies or review articles.
2.3 Data Extraction and Management
Data from full texts of potentially eligible studies were extracted independently by two authors as follows. (1) Major characteristics, including the first author, country, year and type of publication, study design and time, comparator, sample size, information about patients, therapeutic strategy, follow-up time with measuring interval, and achievement of the individual study; (2) clinical outcomes, comprising overall response (OR), clinical benefit rate (CBR), overall survival (OS), progression-free survival (PFS), duration of response (DOR), time to response (TTR), and adverse events (AEs); (3) pharmacological parameters, including toxicity (dose-limiting toxicity [DLT] and maximum tolerated dose [MTD]), absorption (maximum plasma concentration [Cmax] and steady-state plasma concentration [Css]), volume of distribution (Vd), elimination (clearance [Cl], renal excretion [Exc], and half-life [T1/2]), and area under the curve (AUC). Microsoft Access was used to collect all information at this stage to improve efficiency and minimize mistakes.
2.4 Quality Assessment and Grading of Evidence
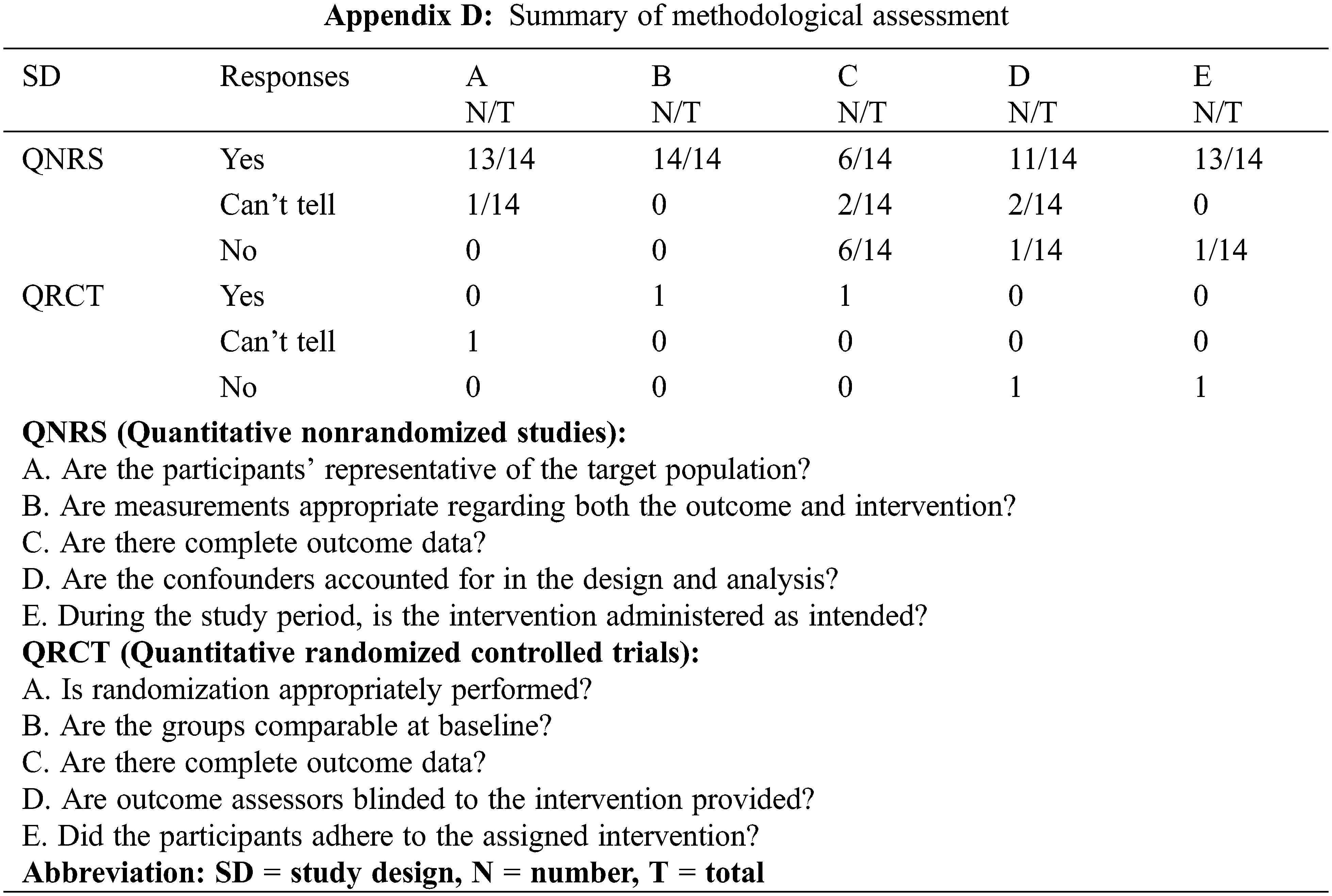
The methodological quality of the included studies was assessed via the Mixed Methods Appraisal Tool (MMAT, version 2018) [42], which permits the appraisal of five categories of studies with a selection algorithm. Each item was classified as “yes”, “no”, or “can’t tell”, which determined the general quality when taken together. Two investigators independently evaluated the data and disagreements were resolved by discussion with a third investigator. Ultimately, a total of 14 studies was included in the analysis (Appendixes A and B) [43–56]. GRADEpro from “Cochrane Consumers and Communication” was employed for assessing the quality of evidence [53].
Using a cutoff date of July 1st, 2020, a total of 83 publications were identified, from which 17 duplicates and 7 considered irrelevant were excluded (Fig. 2). The remaining 59 articles originated from 14 countries and were written in English or Japanese. Detailed information on the included clinical studies is provided in Appendix C. Through the initial screening of titles and abstracts, 15 clinical studies and 44 non-clinical studies were identified, and the non-clinical studies were reserved for a future preclinical systematic analysis.
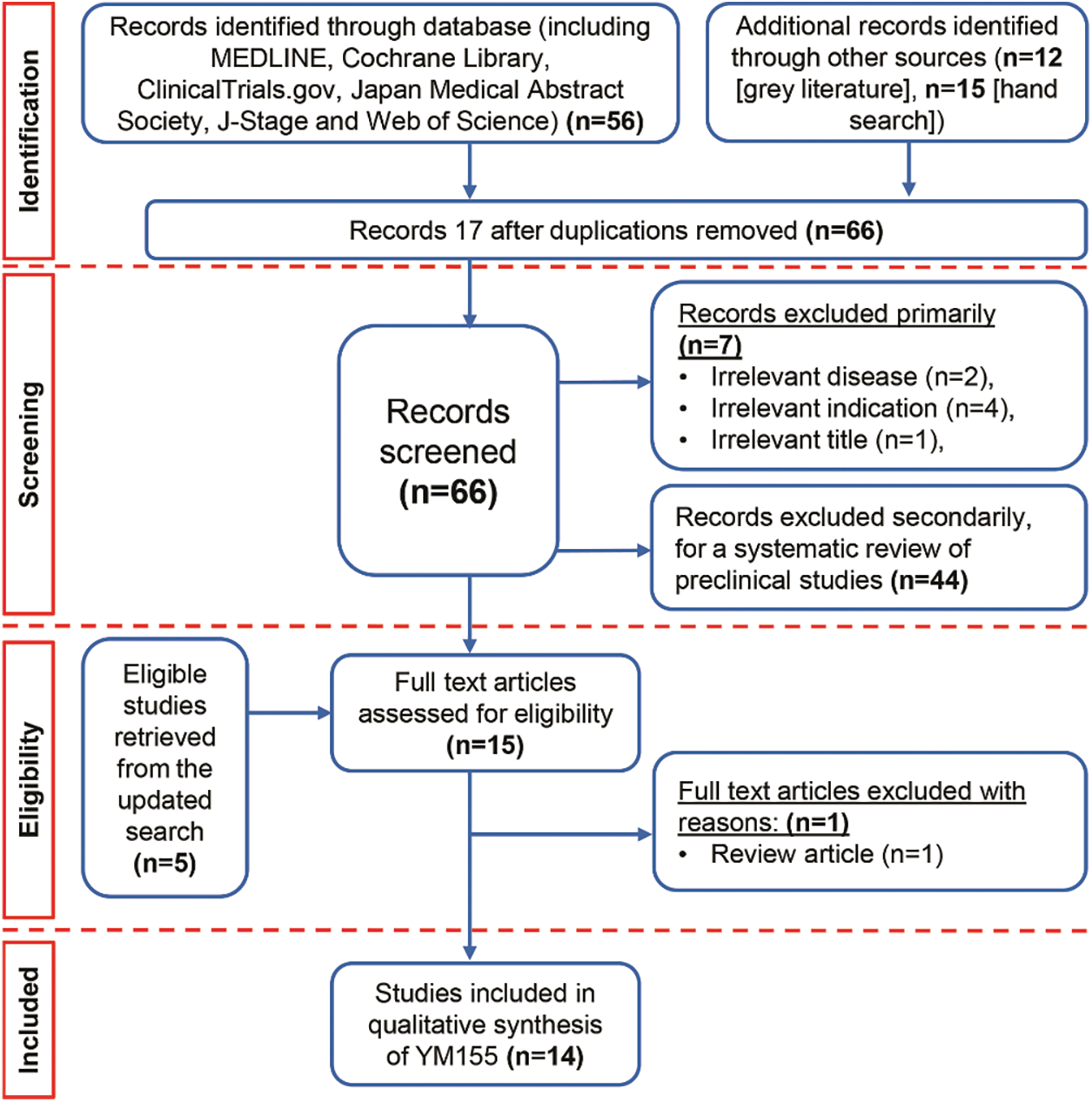
Figure 2: PRISMA chart for YM155
Finally, a total of 14 studies of acceptable quality were included for systematic review, of which 1 was a quantitative randomized controlled trial (QRCT) [47], and 13 were quantitative non-randomized studies (QNRSs) [43–46,48–56]. The quality assessment of all included studies is provided in Appendix D. For the QNRSs, the most common bias was related to completeness of outcomes, with estimations of six (42.8%) high risk and two (14.3%) unclear. This bias may be related to the inclusion of patients with advanced cancer in those studies.
The study characteristics are shown in Table 1. The outcomes were influenced by the trial phase and fell into two main categories: clinical efficacy and pharmacological outcomes. Regarding the clinical outcomes, 13 phase I or II studies included data on AEs (n = 13) [43,44,46–56], OR (n = 11) [46–56], CBR (n = 10) [46–56], OS (n = 8) [46–48,50–53,56], PFS (n = 8) [46–48,50–53,56], TTR (n = 2) [48,53], DOR (n = 8) [47,48,52–56], and synergism (n = 4) [47,50,51,53]. Eleven of the phases I and II studies included pharmacological outcome data on absorption (Cmax [n = 1] [ 55] and Css [n = 11] [43–46,48,50,51,53–56], Vd (n = 4) [43,44,50,55], Cl (n = 8) [43–46,50,54–56], Exc (n = 2) [43,55], T1/2 (n = 6) [43,45,48,50,54,55], AUC (n = 4) [43,45,50,55], and toxicity (DLT [n = 5] [43,50,54–56], and MTD [n = 3] [50,54,55]).
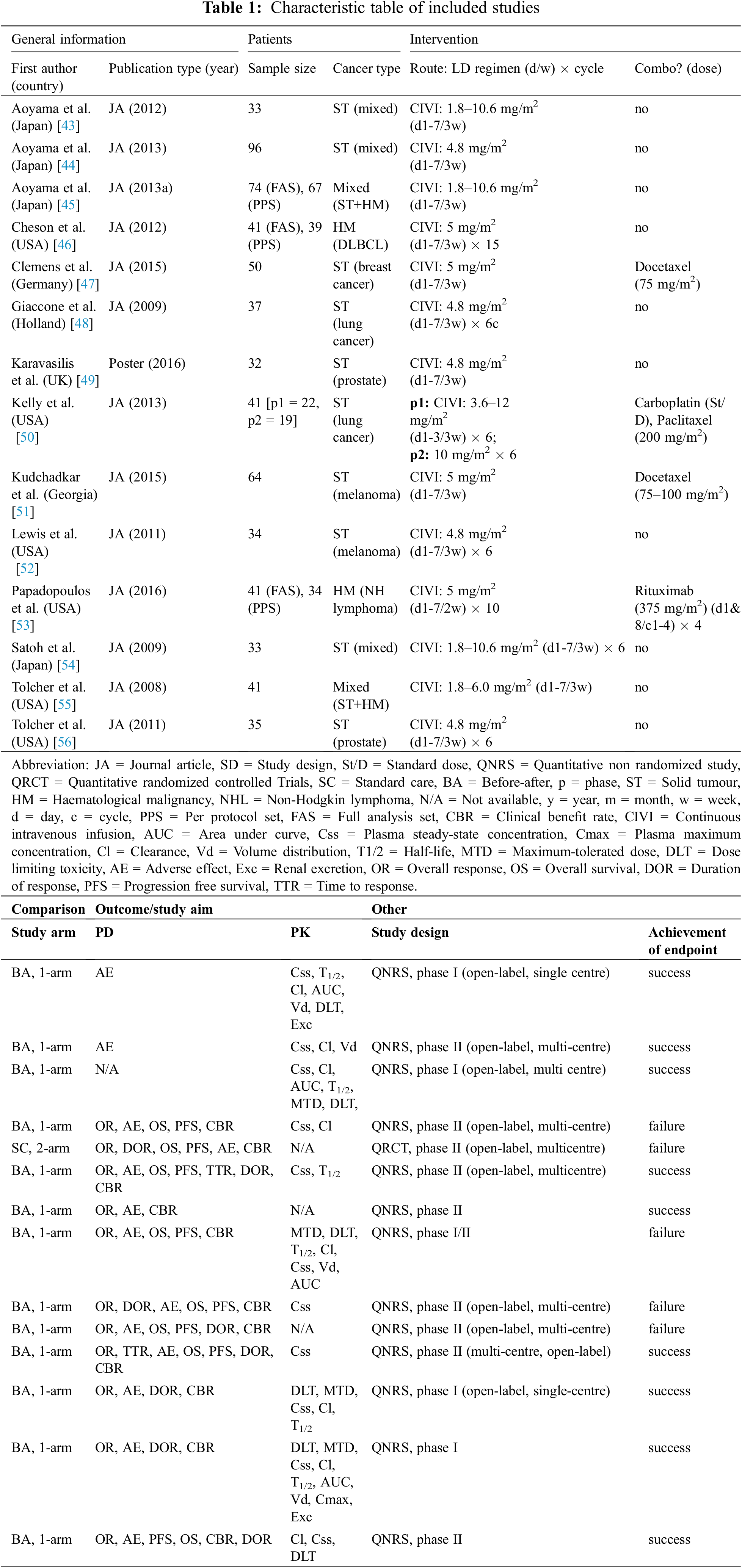
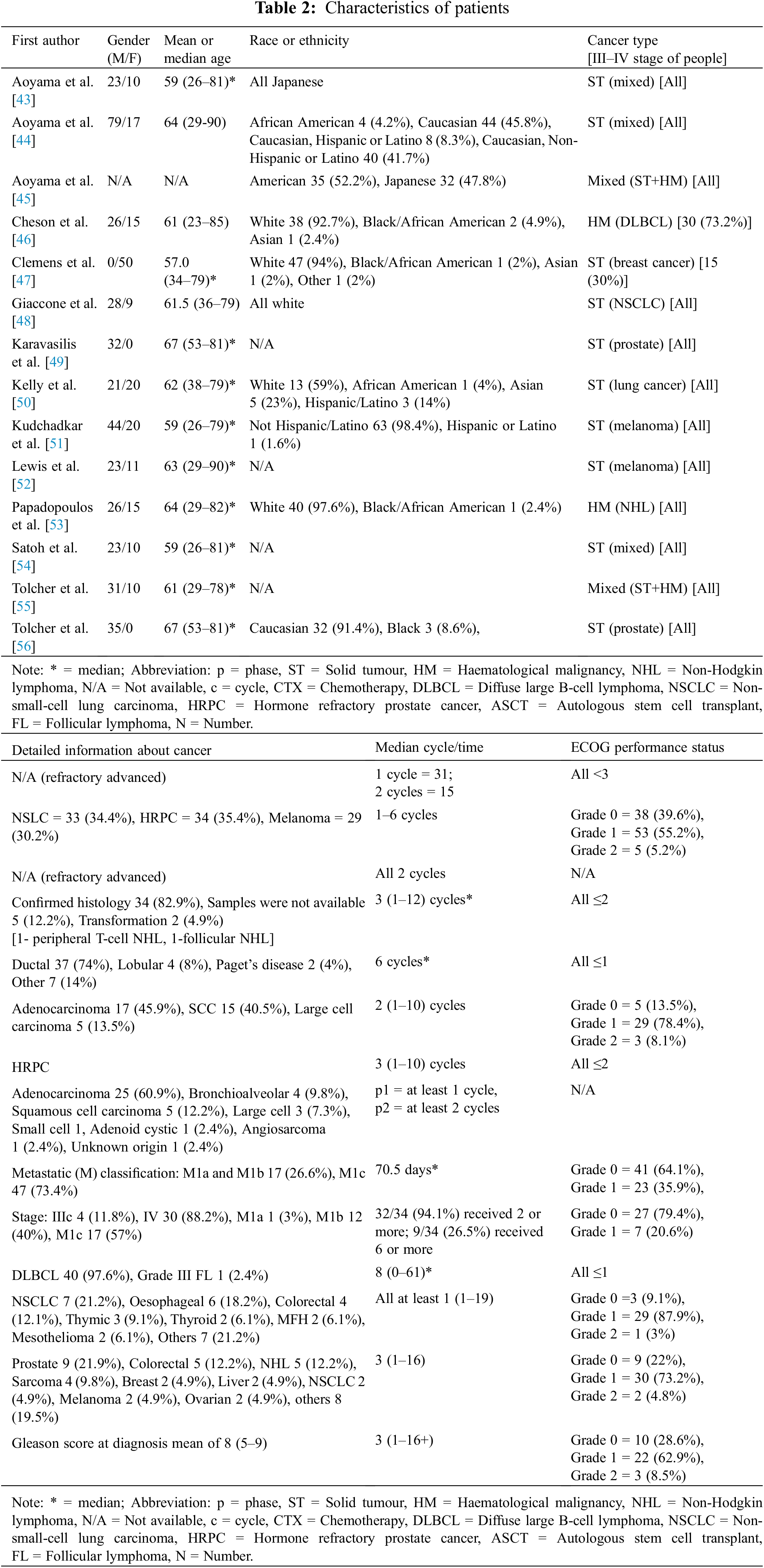
The number of participants in each study ranged from 32 to 96, and the mean and median age both ranged between 59 and 67 years of age. A total of 652 patients were included, of whom 391 (67.7%) were male and 187 (32.3%) were female, with the data being unclear for the remaining 74 patients. The tumor types were heterogeneous, therefore, we formed three subgroups of hematological malignancies (HM), which included 2 studies [46,53]; solid tumors (ST), which included 10 studies [43,44,47–52,54,56]; and mixed HM/ST, which included 2 studies [45,55].
Specifically, the HM group consisted of 87 patients with 4 cancers (79 with diffuse large B-cell lymphoma, 1 with peripheral T-cell non-Hodgkin lymphoma [NHL], 2 with follicular NHL, and 5 with unknown NHL). The ST group consisted of 129 patients with melanoma, 110 with prostate cancer, 52 with breast cancers (37 ductal, 4 lobular, 2 Paget’s disease, 2 unknown breast, and 7 others), and 47 with other STs (9 colorectal, 6 esophageal, 3 thymic, 2 thyroid, 2 malignant fibrous histiocytoma, 2 mesothelioma, 4 sarcoma, 2 liver, 2 ovarian, 15 others), The ST/HM group consisted of 33 patients with unknown mixed STs and 74 with unknown mixed STs and HMs. Detailed information about the patients is given in Table 2.
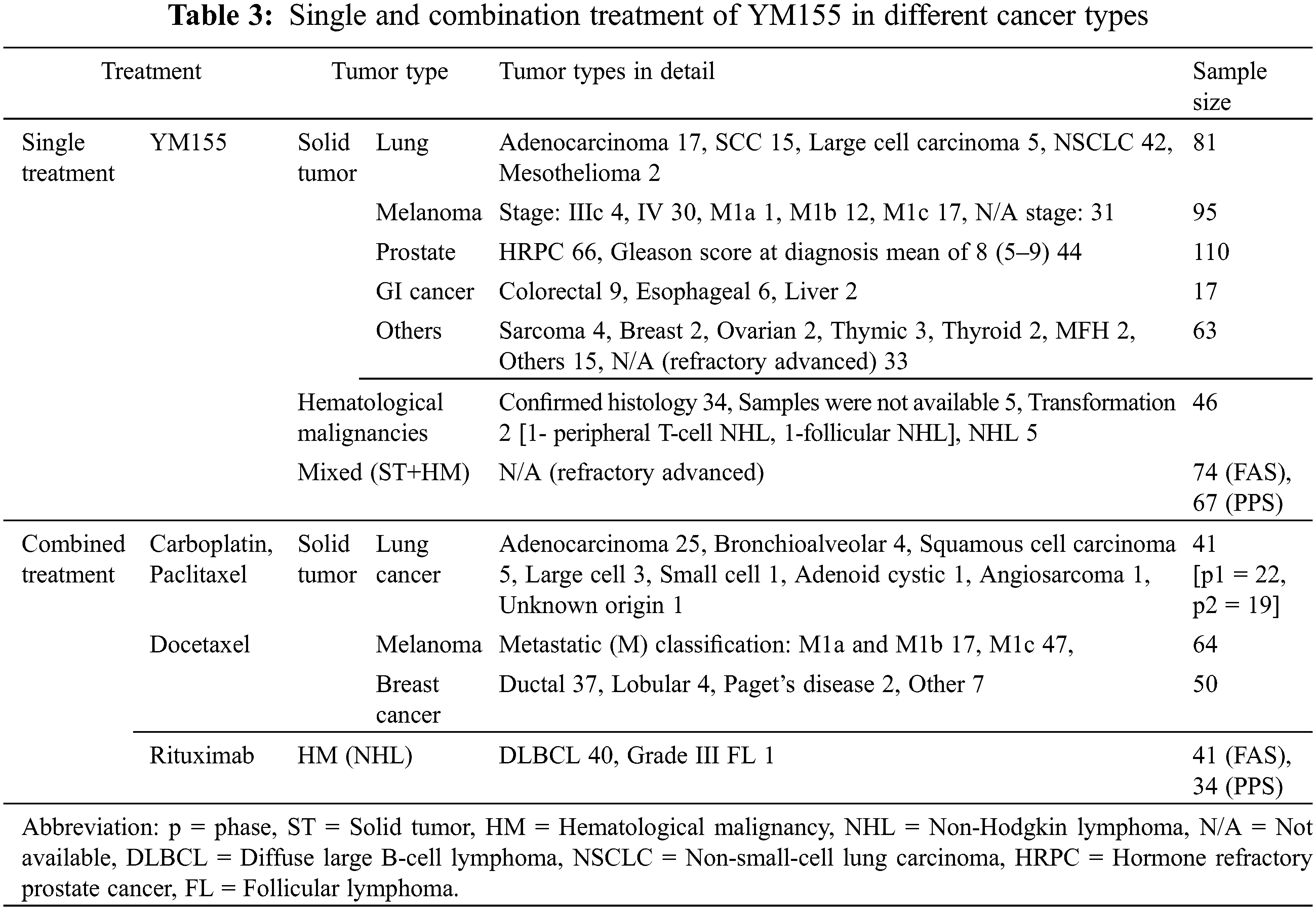
Among the 14 studies, 10 used YM155 as monotherapy [43–46,48,49,52,54–56], and 4 used YM155 in combination with other chemotherapeutic agents [47,50,51,53]. Therefore, we created subgroups based on the therapeutic strategy. In 12 studies, YM155 was administered by continuous intravenous infusion at doses ranging from 1.8 to 12 mg/m2 for 168 h on a 21-day cycle.
The remaining two studies used combined regimens [50,53]: one used an infusion time of 72 h, and the second performed the treatment on a 14-day cycle. The treatment plans are described in Table 1. Depending on the tumor types, the combination therapies consisted of YM155 and either cell cycle-specific and or cell cycle-non-specific agents The cell cycle-specific/mitotic inhibitors (docetaxel, paclitaxel) were administered for melanoma and breast and lung cancers, whereas the cell cycle-non-specific agent was rituximab for patients with B-cell NHL and platinum-based therapy (carboplatin) for patients with lung cancers. Crosstalk between single and combination treatment of YM155 in different cancer types can be seen in Table 3.
All outcomes related to efficacy from the primary studies are listed in Tables 4 and 5. Data on OR were extracted from the QRCT study and 10 QNRS studies [46–56], which in total involved 449 cancer patients treated with YM155 monotherapy or combination therapy. Among the 11 studies, one [56] did not provide CBR outcomes, resulting in data for 408 patients from 10 studies for CBR. The average OR rate (ORR) was 14.04% (range 0%–43.9%, n = 11) and the average CBR was 49.4% (range 6.3%–89.5%, n = 10). For the HM and ST subgroups based on tumor types, the average ORR was 37.1% (7.3%–60.0%, n = 3) and 11.1% (0%–27.3%, n = 10), respectively; and the average CBR was 50.03% (34.2%–65.9%, n = 2) and 49.2% (6.3%–89.5%, n = 9), respectively. After subgrouping based on therapeutic strategy, the YM155 monotherapy and combination therapy subgroups had ORRs of 6.9% (0%–14.6%, n = 7) and 24.04% (10.55%–43.9%, n = 5), respectively, and CBRs of 28.1% (6.35%–43.2%, n = 7) and 74.8% (64.1%–89.5%, n = 5), respectively. The average median TTR was 1.78 months (n = 2, 78 patients), while the average median DOR was greater than 2 months (n = 8, 301 patients) although the precise DOR could not be determined because the original data were expressed in various forms (e.g., range, median). A total of 6 studies involving 261 patients provided data on OS [43,44,46–48,52], and 4 studies involving 176 patients also gave estimates of the 1-year OS rate [44,46–48].
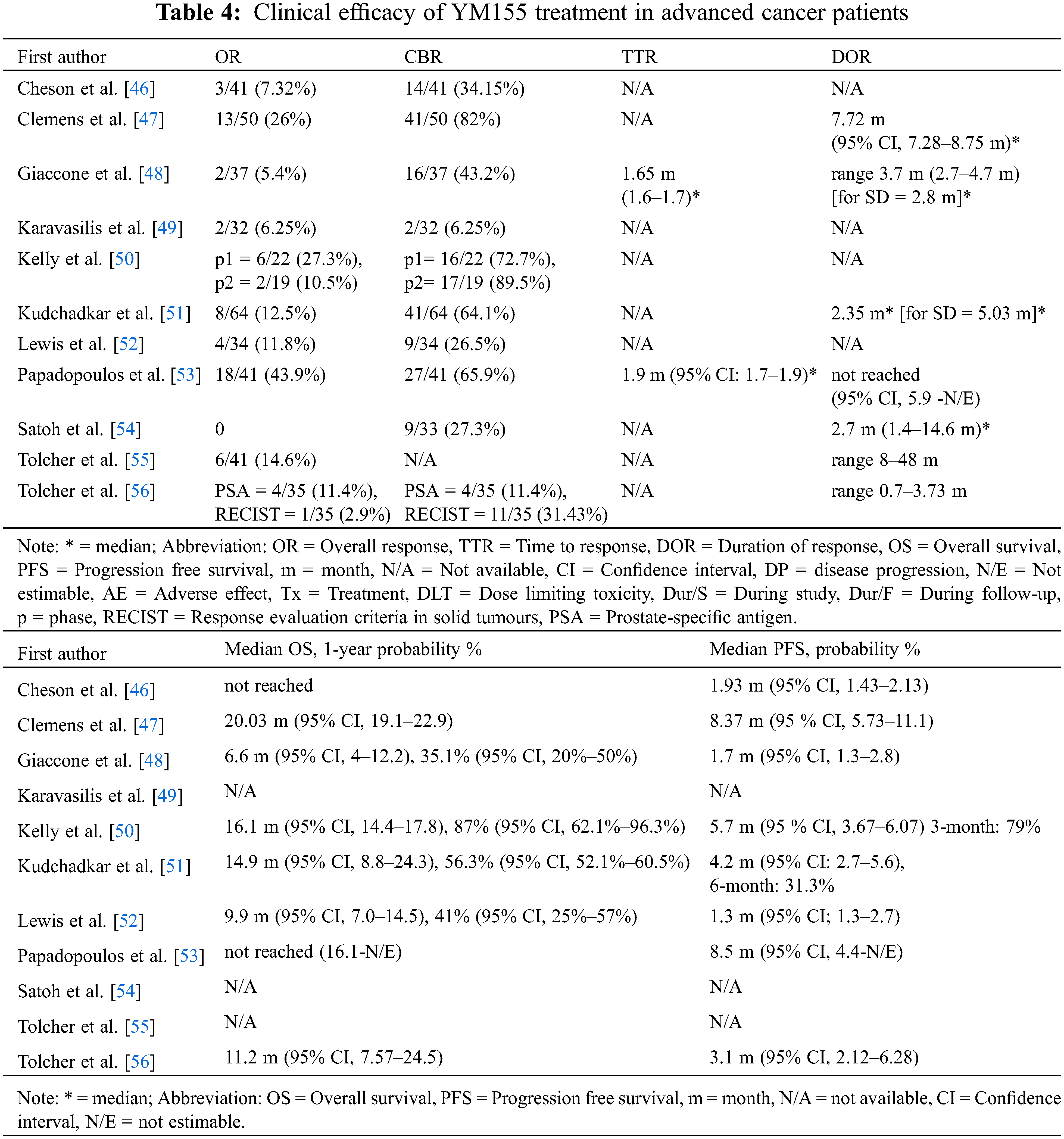
The average median OS was 13.12 months (range 6.6–20.03 months, n = 6) and the average estimated 1-year OS rate was 54.9% (35.1%–87.0%, n = 4). It is noteworthy that all of the studies reporting OS outcomes were of patients with solid tumors. Subgrouping based on the treatment regimens gave an average median OS of 9.23 months (6.6–11.2 months, n = 3) for YM155 monotherapy and 17.01 months (14.90–20.03 months, n = 3) for combination therapy, and the average estimated 1-year OS rates for YM155 monotherapy and combined therapy were 38.1% (35.1%–41.0%, n = 2) and 71.7% (56.3%–87.0%, n = 2), respectively. A total of 8 studies reported on PFS, with the average median PFS being 4.35 months (range 1.3–8.5 months, n = 8). Two studies estimated the 3- and 6-month PFS rates as 79% and 31.3%, respectively [46,47]. Subgrouping according to tumor types, the average median PFS in the HM and ST studies was 5.22 months (1.93–8.5 months, n = 2) and 4.06 months (1.3–8.4 months, n = 6), respectively. For the YM155 regimen subgroups, the average median PFS for YM155 monotherapy and combination therapy was 2.01 months (1.3–3.1 months, n = 4) and 6.69 months (4.2–8.5 months, n = 4), respectively.
3.3.2 Adverse Events Associated with YM155 Treatment
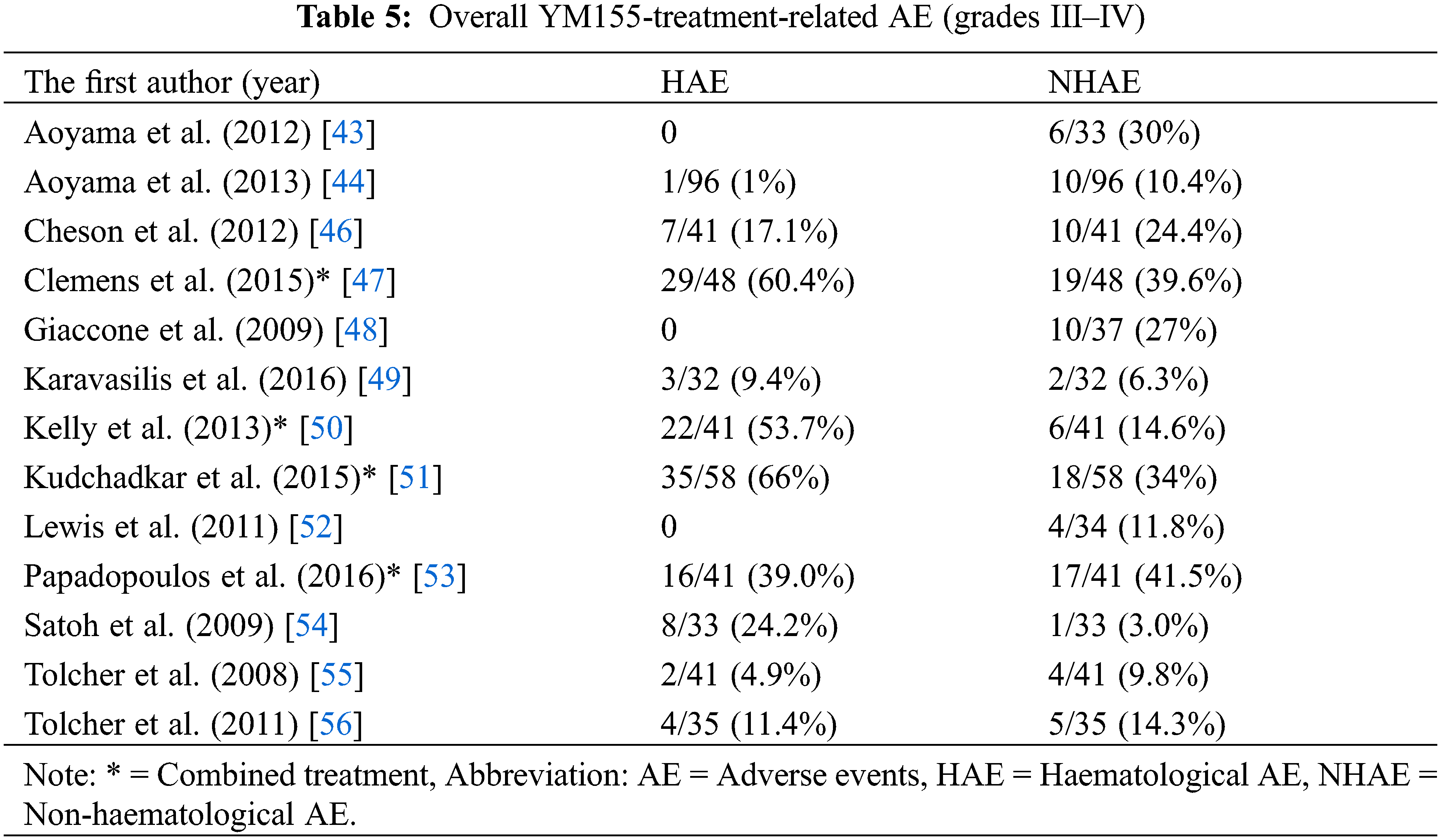
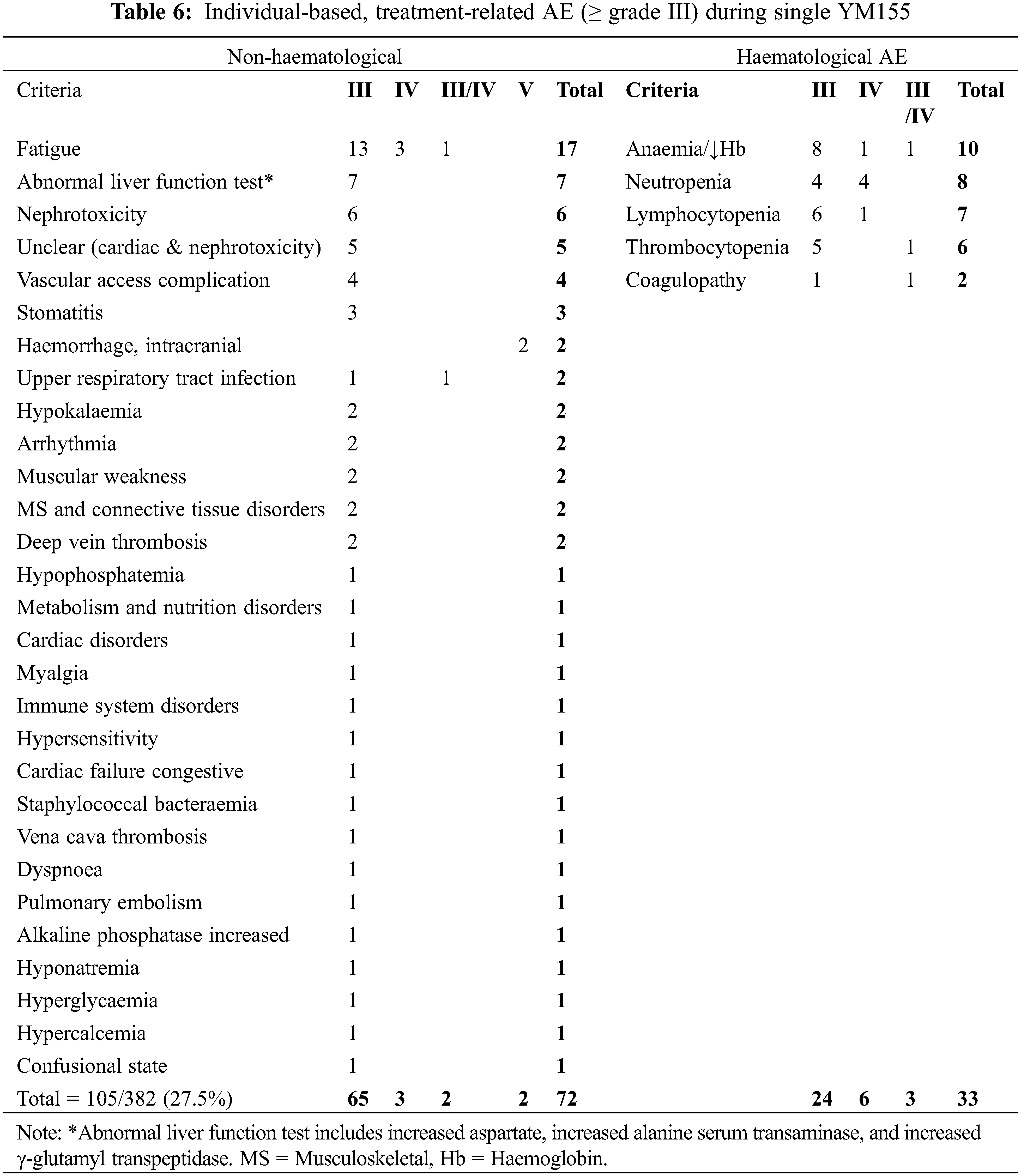
Data on grades III–IV AEs were extracted from 13 studies (12 QNRS and 1 QRCT) involving 578 patients who received YM155 as monotherapy or combination therapy (Tables 5 and 7). For the 13 studies combined, the mean percentage hematological AEs (HAEs) and non-hematological AEs (NHAEs) were 22.1% (range 0%–66.0%) and 20.5% (3.0%–41.5%), respectively. Subgrouping by tumor types, the average HAE and NHAE rates were 28.1% (17.1%–39.0%, n = 2) and 32.95% (24.40%–41.5%, n = 2), respectively, for 82 patients with lymphoma; and 22.6% (0%–66.0%, n = 10) and 19.1% (3%–39.6%, n = 10), respectively, for 455 patients with advanced solid tumors. One study reported the combined rate of AEs for 5 patients with lymphoma and 36 with solid tumors as 4.9% for HAEs and 9.8% for NHAEs. Subgrouping by therapeutic strategy, the average percentage HAEs and NHAEs was 7. 6% (0%–24.2%, n = 9) and 15.2% (35%–30.0%, n = 9), respectively, for 382 patients who received YM155 monotherapy; and 54.8% (39%–66.0%, n = 4) and 32.4% (14.6%–41.5%, n = 4), respectively, for 196 patients who received YM155 as combination therapy. The most common types of AEs resulting from YM155 monotherapy were reported in various ways in the 14 individual studies.
Therefore, we analyzed the AE data reported from 9 studies totaling 382 patients (Tables 6 and 7). A total of 33 types of grade III/IV AEs affecting 105 of the 382 patients (27.5%) were reported. Of the 105 patients, 33 (31.4%) reported HAEs of anemia/decreased hemoglobin (10/33, 30.3%), neutropenia (8/33, 24.2%), lymphocytopenia (7/33, 21.2%), thrombocytopenia (6/33, 18.2%), and coagulopathy (2/33, 6.1%). A total of 29 types of NHAEs were reported, of which the 5 most common (n = 48/72, 66.7%) were fatigue/weakness (19/72, 26.4%), renal and/or cardiac issue (15/72, 20.8%), abnormal liver function test (8/72, 11.1%), and electrolyte disturbance (6/72, 8.3%).

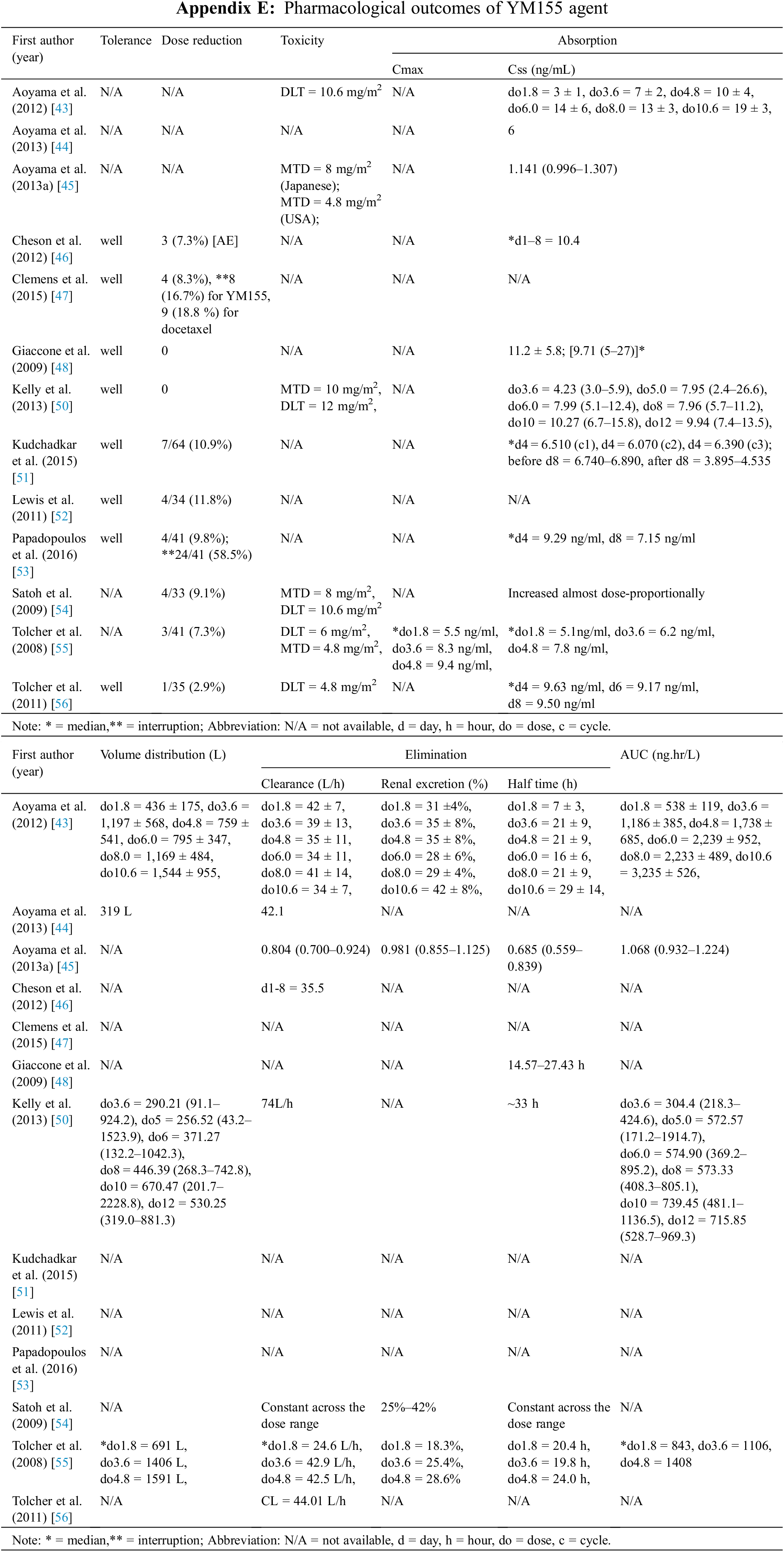
Outcomes related to pharmacological parameters for the individual studies are shown in Appendix E and Table 7. YM155 DLTs ranging from 4.8 to 12 mg/mg2 were reported by 5 studies involving 183 patients [44,50,54–56], and MTDs ranging from 4.8 to 10 mg/mg2 were reported by 4 studies of patients [45,50,54,55]. As a result of toxicity, an average of 6.74% (range 0%–11.8%) of 417 patients in 10 studies required dose reductions [46–48,50–56]. With respect to YM155 pharmacokinetic parameters, Css was shown to increase almost dose-proportionally based on 11 studies of 536 patients [43–46,48,50,51,53–56]. A similar dose-dependence for Vd was reported by 4 studies of 211 patients who received escalating YM155 doses of 1.8–12 mg/m2 [43,44,50,55]. Cl was reported by 8 studies with 394 patients and ranged from 25 to 45 L/h depending on the dosage and infusion day [43–46,50,54–56]. Three phase I escalating dose studies involving 107 patients obtained renal Cl rates of about 20% to 50% [43,54,55]. YM155 T1/2 measurements were reported by 5 studies of 185 patients and the results varied [43,48,50,54,55], with some studies reporting constant T1/2 values across a range of doses and others reporting that T1/2 was dose dependent. AUC was reported to increase with escalating YM155 dose in four studies with a total of 189 patients [43,45,50,55].
Despite rapid advances in the treatment of cancer, it remains a major cause of death and represents a serious public health problem worldwide [58,59]. Thus, there is an urgent need for novel therapeutic strategies based on an understanding of cancer biology. Among the characteristics of cancer cells, two are of particular interest: their universal overexpression of survivin and their poor antioxidant profile [60–63]. YM155, an inhibitor of survivin gene expression and inducer of redox dysregulation, has emerged as a promising chemotherapeutic agent [20,26,64–68].
Here, we performed a systematic literature review and meta-analysis with the goal of assessing pharmacological and clinical outcomes and the safety profile of YM155 as a monotherapy or combination therapy with other chemotherapeutic agents. Our analysis suggests that YM155 provides clinical benefits, such as regression and/or stabilization of tumor progression and prolonged survival, with an acceptable safety profile and good synergistic effects.
Due to the heterogeneity of tumor types and therapeutic regimens in the 14 studies analyzed, we performed subgroup analyses. The average ORR and CBR were higher for patients with HMs vs. STs, and for patients receiving YM155 as a combination therapy vs. monotherapy. Similarly, the median PFS and the estimated 1-year PFS rate were much longer and higher, respectively, in patients with HMs vs STs and in those receiving YM155 combination therapy vs. monotherapy. Although the OS and estimated 1-year OS rates were only assessed in patients with STs, subgroup analysis based on treatment strategy showed that the median OS and 1-year OS rate were almost two-fold longer and higher, respectively, in patients receiving YM155 combination therapy vs monotherapy. TTR and DOR were evaluated in relatively few studies, but it is worth mentioning that the overall median TTR was <2 months, and the DOR was >2 months.
In terms of AEs, both HAEs and NHAEs occurred at acceptable rates and affected an estimated 20% of YM155-treated patients. Subgroup analysis indicated that patients diagnosed with HMs or receiving YM155 combination therapy tended to experience more HAEs and NHAEs than patients with STs or receiving YM155 as a single therapy. To determine specific AEs caused by YM155, we aggregated all observed AEs from patients receiving only YM155. Of the 29 types of grades III–IV AEs reported, fatigue/weakness, renal and/or cardiac issues, abnormal liver function tests, and electrolyte disturbance were the five most common AEs.
The DLT and MTD for YM155 were both between 4.8 and 12 mg/mg2, and related toxicities required dose reduction in 6.74% of the 417 patients in 10 studies analyzed. Other pharmacological parameters were generally affected in a dose-dependent manner. Pharmacological data obtained from a multicenter study concluded that there were no inter-ethnic differences in pharmacokinetics [45]. This is an important observation because ethnicity can potentially affect a drug’s pharmacokinetics, pharmacodynamics, and safety profile, and is thus a key factor in drug development worldwide [69–72]. The patients involved in the studies assessed were from a wide range of races.
The finding YM155 had lower efficacy in patients with STs than HMs could be due to a limitation in the ability of YM155 to penetrate into solid tumors. Currently, the topic of drug delivery for solid tumors is an area of intense preclinical investigation, especially for drug-resistant cancer therapy [73,74]. Two groups have employed YM155-encapsulated liposomes as targeted nanocarriers for YM155 and examined efficacy in xenograft models of cancer. They concluded that nanocarriers extended the half-life of YM155, improved its accumulation in tumor tissues, and induced better antitumor efficacy than did free YM155 [75,76]. In another study, YM155-mediated genotoxicity was found to be dependent on the solute carrier protein SLC35F2 in both in vitro and in vivo experiments, suggesting a new route for targeting DNA damage by exploiting tumor and patient-specific import of YM155 [77].
While numerous therapeutic strategies are under investigation for cancer therapy, the safety and potential side effects of novel drugs are always of great concern. Most of the 14 studies included in the present meta-analysis reported that “YM155 treatment was well tolerated” [46–48,50–52,56]. Likewise, several studies of YM155 combination therapy found that the addition of YM155 allowed the dose of the partner drug to be reduced without sacrificing clinical benefits, and the number of AEs was actually reduced [47,50,51,53]. Our treatment-based subgroup analysis indicated that response rates (OR, CBR, TTR and DOR) and survival (median OS, median PFS and 1-year OS rate) were better in patients receiving combination treatment vs YM155 monotherapy. The next question for YM155 combination therapy is which drug partners would support optimal efficacy. To date, cell cycle-specific (e.g., mitotic inhibitors) and non-specific (rituximab, platinum) inhibitors have been successfully combined with YM155 at the clinical level [47,50,51,53]. We appreciated the idea of combining with mitotic inhibitors. Overexpression of survivin leads to the development of resistance toward different microtubule destabilizers [78,79] and the combination of a microtubule-destabilizing agent, BPR0L075, with survivin siRNA can synergize to restore sensitivity to chemo-resistant cancer cells [80].
Theoretically, the quinone ring of YM155 could synergize with antitumor antibiotics that generate free radicals by converting quinone to semi-quinone, and thus cause profound depletion of cellular reductants [81–86]. YM155 has been shown to cause DNA damage in vitro and in vivo through intercalation between base pairs in a manner contingent on SLC35F2 expression and drug-importing activity [77]. In these contexts, redox-directed cancer therapeutics may be good partners for YM155 combination therapy, since they specifically target redox susceptibilities, such as abnormal metabolism, downregulation of antioxidant enzymes, and impaired mitochondrial function [20,87–91].
It should also be mentioned that many preclinical studies have provided data on the synergism of YM155 with other treatment modalities, including radiotherapy [19,92], and other chemotherapeutic agents such as monoclonal antibodies [93,94], antimetabolites [95,96], DNA-targeted drugs [97,98], and signal transduction inhibitors [99–102]. Thus, the versatile synergistic effects of YM155 suggest that it could be combined with a wide range of agents to form an effective treatment strategy.
Accurate diagnosis and careful monitoring are crucial to the success of cancer management. The main goals of cancer biomarker research are rapid identification of tumor cells and prediction of treatment response, ultimately leading to a favorable therapeutic outcome [103–108]. A large body of evidence has indicated that one such marker can be survivin [109–114]. Indeed, much attention is currently paid to the possibility of using this protein as a diagnostic marker of cancer or a prognostic factor. Therefore, YM155 and other survivin inhibitors are likely to be excellent selective anticancer therapies with their own monitoring biomarker. But it seems relatively different depending on tumor types because surviving’s cancer roles slightly diverge through tissue types. For instance, pro-oncogenic effects in numerous tumors [115–117]; high expression correlates to lower survival in HCC, GIST [118,119], correlates to p53 mutations and metastases, cytoplasmic localization associated with aggressive disease in prostate cancer [120,121], anti-apoptotic function, low survival, chromosomal aberrations in brain tumors [122–126], and resistance to chemotherapy and radiotherapy-induced apoptosis in the lung [127]. In addition to survivin, as digging up monitoring YM155 intervention, observed YM155 therapy responses at pre-clinical levels are chemo and radiosensitization in colorectal cancer, ALL, melanoma, NSCLC, and glioblastoma [128–131]. In neuroblastoma cells, targeted survivin silencing by YM155 causes drug-induced heterogeneity and enables the identification of biomarker candidates for the acquired resistance settings, enhancing chemosensitization [132–135]. At the clinical level, Kelly et al. [50]. performed biomarker analysis in phase II patients, including M30 apoptosense, IL8, and VEGF. However, there was no association between any of these biomarkers, or the changes and response rate. Whereas, decreasing trends of cytokines IL-8, MIP-1b, and granulocyte colony-stimulating factor expression as a prognostic biomarker in non-small cell lung cancer correlated with improved progression-free survival in phase I study [136]. Another prompting result was obtained by Mitsuoka K et al. [137], who investigated 11C-labeled YM155 and assessed tumor cell uptake through positron emission tomography (PET). The authors concluded that high uptake of [11C] YM155 by tumor tissue seems to be a positive predictive marker for a good response to the treatment, suggesting the potential utility of PET/CT imaging with [11C] YM155 for the treatment response [137].
This study has several limitations: First, only one QRCT study satisfied the criteria for meta-analysis. Second, the QNRS studies differed in the outcomes analyzed owing to the variation in trial phases, and they had high heterogeneity based on tumor types and treatment plans. Third, the most common bias was related to completeness of outcome, possibly because of the inclusion of patients with advanced cancer. Fourth, the number of studies associated with some of the subgroups was small, so the results for these analyses may be unreliable. Finally, data were extracted only from articles written in English or Japanese, which might lead to publication bias.
Future work should focus on the delivery of YM155 using nanocarriers, which could improve the efficacy by increasing tumor uptake, especially in solid tumors. Likewise, the application of nanoparticulate systems coupled with biocompatible therapeutics might overcome drug resistance cancer; and improved co-delivery tools with other chemotherapeutic agents can strengthen synergism [138–140]. The selectivity of YM155 may also promote its use in the early stages of cancer when it might have the best opportunity to provide clinical benefit. Additional randomized dual-armed clinical studies with patients at early disease stages might also increase the probability of approaching clinical endpoints, thereby increasing the effect size and accuracy of the results. Ample preclinical evidence suggests that YM155 can enhance sensitization of cancer cells to both radiotherapy and chemotherapy, and future studies should include exploration of optimal partner anticancer agents for combination therapy with YM155 based on tumor types. Moreover, YM155 molecular stabilization should be considered. Currently, it is still unclear whether the tumor growth would be inhibited continuously after stopping infusion [141,142]. YM155 downregulates survivin by blocking Sp1 and ILF3/p54nrb-mediated expression via removing these transcription factors from the nucleoplasm without degradation, raising a suspension that cancer cells might recover after the stopping YM155 intervention or excretion body [143,144].
As a prospective, survivin cancer therapeutics developing rapidly under five categories: 1) survivin-partner protein interaction inhibitors, 2) survivin homodimerization inhibitors, 3) survivin gene transcription inhibitors, 4) survivin mRNA inhibitors, 5) survivin immunotherapy, due to many advantages as compared with other apoptosis-based anticancer agents, including compromises multiple signaling networks required for oncogenesis, a unique target for molecular antagonists, cancer vaccine and gene therapy, survivin antagonists may affect cancer stem cells, does not affect the normal cells or tissues suggesting a favorable toxicity profile [145–148]. Regarding inhibitors, such as Shepherdin and AICAR, which disrupt survivin interactions with its partner proteins, further studies need to clarify the effect of specificity on survivin. Whereas, survivin-directed immunotherapy and survivin-based vaccination have been showing promising efficacy, safety, and associated with antigen-specific immunologic responses, and advancing to several clinical trials.
Concern about inconclusive results from qualitative studies with YM155 prompted us to perform an integrated systematic analysis of relevant clinical trials with the goal of facilitating clinical decision-making in the use of YM155. Our results have several clinical implications. First, they showed significant beneficial clinical outcomes of YM155 treatment, including improved OR, CBR, OS, and PFS. Second, the results suggest that YM155 would be most effective when given in combination with another anticancer agent at an early stage of cancer. Third, the analysis showed that YM155 could be administered to cancer patients of any ethnicity with acceptable efficacy and safety profiles, with the exception of AEs of decreased blood cell counts; fatigue/weakness; renal, hepatic, and/or cardiac issues; and electrolyte disturbance. Finally, YM155 showed efficacy in both solid and hematological malignancies, confirming that the beneficial mechanism of action of YM155 is based on common genotypical and phenotypical features of cancer cells.
Acknowledgement: We thank PhD Anne M. O’Rourke from Edanz Group (https://en-author-services.edanzgroup.com/ac) for editing a draft of this manuscript.
Published Notes: The protocol for the systematic review (#135273; #CRD42019135273) was submitted to PROSPERO (International prospective register of systematic reviews) on October 29, 2019.
Availability of Data and Materials: All data are included in this published article.
Author Contributions: D.G. designed the study and wrote the manuscript. B.E.J., L.G., and M.T. collected and screened the articles. E.D., A.M., and G.G. performed the meta-analysis. Y.T., R.F., I.M., T.S., A.N. edited the manuscript. Y.T. supervised the review. All authors reviewed the results and approved the final version of the manuscript.
Informed Consent Statement and Ethical Approval: No direct studies of humans, animals, or human/animal tissues or cells were performed for this review.
Funding Statement: This work was supported by an internal institutional grant from the International University of Health and Welfare (Project Ref # 10/08/2019).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Wheatley, S. P., Altieri, D. C. (2019). Survivin at a glance. Journal of Cell Science, 132(7), jcs223826. DOI 10.1242/jcs.223826. [Google Scholar] [CrossRef]
2. Uren, A. G., Wong, L., Pakusch, M., Fowler, K. J., Burrows, F. J. et al. (2000). Survivin and the inner centromere protein INCENP show similar cell-cycle localization and gene knockout phenotype. Current Biology, 10(21), 1319–1328. DOI 10.1016/S0960-9822(00)00769-7. [Google Scholar] [CrossRef]
3. Coumar, M. S., Tsai, F. Y., Kanwar, J. R., Sarvagalla, S., Cheung, C. H. A. (2013). Treat cancers by targeting survivin: Just a dream or future reality? Cancer Treatment Reviews, 39(7), 802–811. DOI 10.1016/j.ctrv.2013.02.002. [Google Scholar] [CrossRef]
4. Altieri, D. C. (2008). Survivin, cancer networks and pathway-directed drug discovery. Nature Reviews Cancer, 8(1), 61–70. DOI 10.1038/nrc2293. [Google Scholar] [CrossRef]
5. Mittal, R., Jaiswal, P., Goel, A. (2015). Survivin: A molecular biomarker in cancer. Indian Journal of Medical Research, 141(4), 389. DOI 10.4103/0971-5916.159250. [Google Scholar] [CrossRef]
6. Zhang, Y., Yan, H., Li, R., Guo, Y., Zheng, R. (2019). High expression of survivin predicts poor prognosis in cervical squamous cell carcinoma treated with paclitaxel and carboplatin. Medicine, 98(20), e15607. DOI 10.1097/MD.0000000000015607. [Google Scholar] [CrossRef]
7. Guindalini, R. S. C., Mathias Machado, M. C., Garicochea, B. (2013). Monitoring survivin expression in cancer: Implications for prognosis and therapy. Molecular Diagnosis and Therapy, 17(6), 331–342. DOI 10.1007/s40291-013-0048-1. [Google Scholar] [CrossRef]
8. Ryan, B. M., O’Donovan, N., Duffy, M. J. (2009). Survivin: A new target for anti-cancer therapy. Cancer Treatment Reviews, 35(7), 553–562. DOI 10.1016/j.ctrv.2009.05.003. [Google Scholar] [CrossRef]
9. Tanaka, C., Uzawa, K., Shibahara, T., Yokoe, H., Noma, H. et al. (2003). Expression of an inhibitor of apoptosis, survivin, in oral carcinogenesis. Journal of Dental Research, 82(8), 607–611. DOI 10.1177/154405910308200807. [Google Scholar] [CrossRef]
10. Chantalat, L., Skoufias, D. A., Kleman, J. P., Jung, B., Dideberg, O. et al. (2000). Crystal structure of human survivin reveals a bow tie-shaped dimer with two unusual α-helical extensions. Molecular Cell, 6(1), 183–189. DOI 10.1016/S1097-2765(05)00020-1. [Google Scholar] [CrossRef]
11. Carrasco, R. A., Stamm, N. B., Marcusson, E., Sandusky, G., Iversen, P. et al. (2011). Antisense inhibition of survivin expression as a cancer therapeutic. Molecular Cancer Therapeutics, 10(2), 221–232. DOI 10.1158/1535-7163.MCT-10-0756. [Google Scholar] [CrossRef]
12. Lladser, A., Sanhueza, C., Kiessling, R., Quest, A. F. G. (2011). Is survivin the potential achilles’ heel of cancer? Advances in Cancer Research, 111, 1–37. DOI 10.1016/B978-0-12-385524-4.00001-5. [Google Scholar] [CrossRef]
13. Pennati, M., Folini, M., Zaffaroni, N. (2007). Targeting survivin in cancer therapy: Fulfilled promises and open questions. Carcinogenesis, 28(6), 1133–1139. DOI 10.1093/carcin/bgm047. [Google Scholar] [CrossRef]
14. Fukuda, S., Pelus, L. M. (2006). Survivin, a cancer target with an emerging role in normal adult tissues. Molecular Cancer Therapeutics, 5(5), 1087–1098. DOI 10.1158/1535-7163.MCT-05-0375. [Google Scholar] [CrossRef]
15. Nakahara, T., Takeuchi, M., Kinoyama, I., Minematsu, T., Shirasuna, K. et al. (2007). YM155, a novel small-molecule survivin suppressant, induces regression of established human hormone-refractory prostate tumor xenografts. Cancer Research, 67(17), 8014–8021. DOI 10.1158/0008-5472.CAN-07-1343. [Google Scholar] [CrossRef]
16. Cheng, Q., Ling, X., Wu, J., Nakahara, T., Yamanaka, K. et al. (2008). The novel small chemical molecule YM155 is a bona fide survivin transcription inhibitor and acts on the survivin proximal promoter region: Inhibition of survivin promoter activity by YM155 is cell cycle-independent. Cancer Research, 68(9), 1848. [Google Scholar]
17. Pazdernik, T. L., Kerecsen, L. (2010). Toxicology and drugs of abuse (3rd Ed.). USA: Elsevier Health Sciences, Mosby. [Google Scholar]
18. Shojaei, F., Yazdani-Nafchi, F., Banitalebi-Dehkordi, M., Chehelgerdi, M., Khorramian-Ghahfarokhi, M. (2019). Trace of survivin in cancer. European Journal of Cancer Prevention, 28(4), 365–372. DOI 10.1097/CEJ.0000000000000453. [Google Scholar] [CrossRef]
19. Iwasa, T., Okamoto, I., Suzuki, M., Nakahara, T., Yamanaka, K. et al. (2008). Radiosensitizing effect of YM155, a novel small-molecule survivin suppressant, in non-small cell lung cancer cell lines. Clinical Cancer Research, 14(20), 6496–6504. DOI 10.1158/1078-0432.CCR-08-0468. [Google Scholar] [CrossRef]
20. Wondrak, G. T. (2009). Redox-directed cancer therapeutics: Molecular mechanisms and opportunities. Antioxidants and Redox Signaling, 11(12), 3013–3069. DOI 10.1089/ars.2009.2541. [Google Scholar] [CrossRef]
21. Liew, S. K., Malagobadan, S., Arshad, N. M., Nagoor, N. H. (2020). A review of the structure–activity relationship of natural and synthetic antimetastatic compounds. Biomolecules, 10(1), 138. DOI 10.3390/biom10010138. [Google Scholar] [CrossRef]
22. Ivanova, D., Zhelev, Z., Getsov, P., Nikolova, B., Aoki, I. et al. (2018). Vitamin K: Redox-modulation, prevention of mitochondrial dysfunction and anticancer effect. Redox Biology, 16(Suppl 4), 352–358. DOI 10.1016/j.redox.2018.03.013. [Google Scholar] [CrossRef]
23. Saify, Z. S., Mushtaq, N., Noor, F., Takween, S., Arif, M. (1999). Role of quinone moiety as antitumour agents: A review. Pakistan Journal of Pharmaceutical Sciences, 12(2), 21–31. [Google Scholar]
24. Fisher, G. R., Gutierrez, P. L. (1991). Free radical formation and DNA strand breakage during metabolism of diaziquone by NAD(P)H quinone-acceptor oxidoreductase (DT-diaphorase) and NADPH cytochrome c reductase. Free Radical Biology and Medicine, 11(6), 597–607. DOI 10.1016/0891-5849(91)90141-O. [Google Scholar] [CrossRef]
25. Lu, J. J., Bao, J. L., Wu, G. S., Xu, W. S., Huang, M. Q. et al. (2013). Quinones derived from plant secondary metabolites as anti-cancer agents. Anti-Cancer Agents in Medicinal Chemistry, 13(3), 456–463. DOI 10.2174/187152013804910389. [Google Scholar] [CrossRef]
26. O’Brien, P. J. (1991). Molecular mechanisms of quinone cytotoxicity. Chemico-Biological Interactions, 80(1), 1–41. DOI 10.1016/0009-2797(91)90029-7. [Google Scholar] [CrossRef]
27. von Ardenne, M., Reitnauer, P. G. (1967). Selektive in-vivo-thermosensibilisierung und therapie des Ehrlich-Mäsue-Ascites-Karzinoms durch Vitamin-K3-natrium-bisulfit. Deutsche Gesundheitswesen, 22(40), 1879–1885. [Google Scholar]
28. Nagourney, R., Weisenthal, L., Dill, P., Just, R., Fass, L. et al. (1987). Menadiol in combination with cytotoxic chemotherapies; the feasibility for resistance modification in human cancers: A pilot study.Proceedings-American Society of Clinical Oncology, 6, 35. [Google Scholar]
29. Yuan, J., Liu, Z., Zhang, Z., Yan, D., Zhang, W. (2021). Synthesis and biological evaluation of naphthoquinone phenacylimidazolium derivatives. Bioorganic & Medicinal Chemistry Letters, 41, 127977. DOI 10.1016/j.bmcl.2021.127977. [Google Scholar] [CrossRef]
30. Ourhzif, E. M., Decombat, C., Abrunhosa-Thomas, I., Delort, L., Khouili, M. et al. (2020). Synthesis and biological evaluation of new naphthoquinones derivatives. Current Organic Synthesis, 17(3), 224–229. DOI 10.2174/1570179417666200212111956. [Google Scholar] [CrossRef]
31. Sreelatha, T., Kandhasamy, S., Dinesh, R., Shruthy, S., Shweta, S. et al. (2014). Synthesis and SAR study of novel anticancer and antimicrobial naphthoquinone amide derivatives. Bioorganic & Medicinal Chemistry Letters, 24(15), 3647–3651. DOI 10.1016/j.bmcl.2014.04.080. [Google Scholar] [CrossRef]
32. Fedorov, S. N., Stonik, V. A., Honecker, F., Dyshlovoy, S. A. (2017). Structure-activity relationship studies of new marine anticancer agents and their synthetic analogues. Current Medicinal Chemistry, 24(42), 4779–4799. DOI 10.2174/0929867324666161121115404. [Google Scholar]
33. Julian, P. T. H., James, T., Jacqueline, C., Miranda, C., Tianjing, L. et al. (2019). Cochrane handbook for systematic reviews of interventions (2nd Ed.1–289+321–641. DOI 10.1002/9781119536604. [Google Scholar] [CrossRef]
34. Aromataris, E., Pearson, A. (2014). The systematic review: An overview. American Journal of Nursing, 114(3), 53–58. DOI 10.1097/01.NAJ.0000444496.24228.2c. [Google Scholar] [CrossRef]
35. Munn, Z., Stern, C., Aromataris, E., Lockwood, C., Jordan, Z. (2018). What kind of systematic review should I conduct? A proposed typology and guidance for systematic reviewers in the medical and health sciences. BMC Medical Research Methodology, 18(1), 5. DOI 10.1186/s12874-017-0468-4. [Google Scholar] [CrossRef]
36. Stroup, D. F., Berlin, J. A., Morton, S. C., Olkin, I., Williamson, G. D. et al. (2000). Meta-analysis of observational studies in epidemiology: A proposal for reporting. Journal of the American Medical Association, 283(15), 2008–2012. DOI 10.1001/jama.283.15.2008. [Google Scholar] [CrossRef]
37. Egger, M., Schneider, M. (1998). Meta-analysis spurious precision? Meta-analysis of observational studies. BMJ, 316(7125), 140–144. DOI 10.1136/bmj.316.7125.140. [Google Scholar] [CrossRef]
38. Pearson, A., White, H., Bath-Hextall, F., Salmond, S., Apostolo, J. et al. (2015). A mixed-methods approach to systematic reviews. International Journal of Evidence-Based Healthcare, 13(3), 121–131. DOI 10.1097/XEB.0000000000000052. [Google Scholar] [CrossRef]
39. Barbara, L.,P., Sally, E.,T., Connie, C., Carol, J. (2001). Meta-study of qualitative health research: A practical guide to meta-analysis and meta-synthesis (1st Ed.pp. 1–121. UK: SAGE publisher. [Google Scholar]
40. Siddaway, A. P., Wood, A. M., Hedges, L. V. (2019). How to do a systematic review: A best practice guide for conducting and reporting narrative reviews, meta-analyses, and meta-syntheses. Annual Review of Psychology, 70(1), 747–770. DOI 10.1146/annurev-psych-010418-102803. [Google Scholar] [CrossRef]
41. Page, M. J., Moher, D. (2017). Evaluations of the uptake and impact of the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) Statement and extensions: A scoping review. Systematic Reviews, 6(1), 263. DOI 10.1186/s13643-017-0663-8. [Google Scholar] [CrossRef]
42. Hong, Q. N., Fàbregues, S., Bartlett, G., Boardman, F., Cargo, M. et al. (2018). The Mixed Methods Appraisal Tool (MMAT) version 2018 for information professionals and researchers. Education for Information, 34(4), 285–291. DOI 10.3233/EFI-180221. [Google Scholar] [CrossRef]
43. Aoyama, Y., Nishimura, T., Sawamoto, T., Satoh, T., Katashima, M. et al. (2012). Pharmacokinetics of sepantronium bromide (YM155a small-molecule suppressor of survivin, in Japanese patients with advanced solid tumors: Dose proportionality and influence of renal impairment. Cancer Chemotherapy and Pharmacology, 70(3), 373–380. DOI 10.1007/s00280-012-1913-z. [Google Scholar] [CrossRef]
44. Aoyama, Y., Kaibara, A., Takada, A., Nishimura, T., Katashima, M. et al. (2013). Population pharmacokinetic modeling of Sepantronium bromide (YM155a small molecule survivin suppressant, in patients with non-small cell lung cancer, hormone refractory prostate cancer, or unresectable stage III or IV melanoma. Investigational New Drugs, 31(2), 443–451. DOI 10.1007/s10637-012-9867-x. [Google Scholar] [CrossRef]
45. Aoyama, Y., Katashima, M., Sawamoto, T. (2013). Lack of differences in the pharmacokinetics of sepantronium bromide (YM155) between US and Japanese patients with advanced solid tumors or non-Hodgkin lymphoma. Biopharmaceutics and Drug Disposition, 34(2), 137–140. DOI 10.1002/bdd.1827. [Google Scholar] [CrossRef]
46. Cheson, B. D., Bartlett, N. L., Vose, J. M., Lopez-Hernandez, A., Seiz, A. L. et al. (2012). A phase II study of the survivin suppressant YM155 in patients with refractory diffuse large B-cell lymphoma. Cancer, 118(12), 3128–3134. DOI 10.1002/cncr.26510. [Google Scholar] [CrossRef]
47. Clemens, M. R., Gladkov, O. A., Gartner, E., Vladimirov, V., Crown, J. et al. (2015). Phase II, multicenter, open-label, randomized study of YM155 plus docetaxel as first-line treatment in patients with HER2-negative metastatic breast cancer. Breast Cancer Research and Treatment, 149(1), 171–179. DOI 10.1007/s10549-014-3238-6. [Google Scholar] [CrossRef]
48. Giaccone, G., Zatloukal, P., Roubec, J., Floor, K., Musil, J. et al. (2009). Multicenter phase II trial of YM155, a small-molecule suppressor of survivin, in patients with advanced, refractory, non-small-cell lung cancer. Journal of Clinical Oncology, 27(27), 4481–4486. DOI 10.1200/JCO.2008.21.1862. [Google Scholar] [CrossRef]
49. Karavasilis, V., Mita, A., Hudes, G., Quinn, D., Ferrari, A. et al. (2007). Phase II monotherapy study of YM155, a novel survivin suppressant, administered by 168-hour continuous infusion in previously treated hormone refractory prostate cancer (HRPC). Journal of Clinical Oncology, 25(18_suppl), 5135–5135. DOI 10.1200/jco.2007.25.18_suppl.5135. [Google Scholar] [CrossRef]
50. Kelly, R. J., Thomas, A., Rajan, A., Chun, G., Lopez-Chavez, A. et al. (2013). A phase I/II study of sepantronium bromide (YM155, survivin suppressor) with paclitaxel and carboplatin in patients with advanced non-small-cell lung cancer. Annals of Oncology, 24(10), 2601–2606. DOI 10.1093/annonc/mdt249. [Google Scholar] [CrossRef]
51. Kudchadkar, R., Ernst, S., Chmielowski, B., Redman, B. G., Steinberg, J. et al. (2015). A phase 2, multicenter, open-label study of sepantronium bromide (YM155) plus docetaxel in patients with stage III (unresectable) or stage IV melanoma. Cancer Medicine, 4(5), 643–650. DOI 10.1002/cam4.363. [Google Scholar] [CrossRef]
52. Lewis, K. D., Samlowski, W., Ward, J., Catlett, J., Cranmer, L. et al. (2011). A multi-center phase II evaluation of the small molecule survivin suppressor YM155 in patients with unresectable stage III or IV melanoma. Investigational New Drugs, 29(1), 161–166. DOI 10.1007/s10637-009-9333-6. [Google Scholar] [CrossRef]
53. Papadopoulos, K. P., Lopez-Jimenez, J., Smith, S. E., Steinberg, J., Keating, A. et al. (2016). A multicenter phase II study of sepantronium bromide (YM155) plus rituximab in patients with relapsed aggressive B-cell Non-Hodgkin lymphoma. Leukemia and Lymphoma, 57(8), 1848–1855. DOI 10.3109/10428194.2015.1113275. [Google Scholar] [CrossRef]
54. Satoh, T., Okamoto, I., Miyazaki, M., Morinaga, R., Tsuya, A. et al. (2009). Phase I study of YM155, a novel survivin suppressant, in patients with advanced solid tumors. Clinical Cancer Research, 15(11), 3872–3880. DOI 10.1158/1078-0432.CCR-08-1946. [Google Scholar] [CrossRef]
55. Tolcher, A. W., Mita, A., Lewis, L. D., Garrett, C. R., Till, E. et al. (2008). Phase I and pharmacokinetic study of YM155, a small-molecule inhibitor of survivin. Journal of Clinical Oncology, 26(32), 5198–5203. DOI 10.1200/JCO.2008.17.2064. [Google Scholar] [CrossRef]
56. Tolcher, A. W., Quinn, D. I., Ferrari, A., Ahmann, F., Giaccone, G. et al. (2012). A phase II study of YM155, a novel small-molecule suppressor of survivin, in castration-resistant taxane-pretreated prostate cancer. Annals of Oncology, 23(4), 968–973. DOI 10.1093/annonc/mdr353. [Google Scholar] [CrossRef]
57. Ryan, R. H. S. (2016). How to GRADE the quality of the evidence. Cochrane Consumers and Communication Group. http://cccrg.cochrane.org/author-resources. [Google Scholar]
58. WHO Newsroom (2022). Cancer. https://www.who.int/news-room/fact-sheets/detail/cancer. [Google Scholar]
59. Torre, L. A., Bray, F., Siegel, R. L., Ferlay, J., Lortet-Tieulent, J. et al. (2015). Global cancer statistics, 2012. CA: A Cancer Journal for Clinicians, 65(2), 87–108. [Google Scholar]
60. Garg, H., Suri, P., Gupta, J. C., Talwar, G. P., Dubey, S. (2016). Survivin: A unique target for tumor therapy. Cancer Cell International, 16(1), 49. DOI 10.1186/s12935-016-0326-1. [Google Scholar] [CrossRef]
61. Verrax, J., Taper, H., Buc Calderon, P. (2008). Targeting cancer cells by an oxidant-based therapy. Current Molecular Pharmacology, 1(1), 80–92. DOI 10.2174/1874467210801010080. [Google Scholar] [CrossRef]
62. Chen, X., Duan, N., Zhang, C., Zhang, W. (2016). Survivin and tumorigenesis: Molecular mechanisms and therapeutic strategies. Journal of Cancer, 7(3), 314–323. DOI 10.7150/jca.13332. [Google Scholar] [CrossRef]
63. Cree, I. A. (2011). Cancer biology. Methods in Molecular Biology, 731, 1–11. DOI 10.1007/978-1-61779-080-5. [Google Scholar] [CrossRef]
64. Altieri, D. C. (2013). Targeting survivin in cancer. Cancer Letters, 332(2), 225–228. DOI 10.1016/j.canlet.2012.03.005. [Google Scholar] [CrossRef]
65. Li, F., Ling, X. (2006). Survivin study: An update of what is the next wave? Journal of Cellular Physiology, 208(3), 476–486. DOI 10.1002/(ISSN)1097-4652. [Google Scholar] [CrossRef]
66. Antonio Cheung, C. H., Huang, C. C., Tsai, F. Y., Lee, J. Y. C., Cheng, S. M. et al. (2013). Survivin-biology and potential as a therapeutic target in oncology. OncoTargets and Therapy, 6, 1453–1462. [Google Scholar]
67. Pennati, M., Folini, M., Zaffaroni, N. (2008). Targeting survivin in cancer therapy. Expert Opinion on Therapeutic Targets, 12(4), 463–476. DOI 10.1517/14728222.12.4.463. [Google Scholar] [CrossRef]
68. Li, F. (2013). Discovery of survivin inhibitors and beyond. Fl118 as a proof of concept. International Review of Cell and Molecular Biology, (305), 217–252. DOI 10.1016/B978-0-12-407695-2.00005-6. [Google Scholar] [CrossRef]
69. Kurose, K., Sugiyama, E., Saito, Y. (2012). Population differences in major functional polymorphisms of pharmacokinetics/pharmacodynamics-related genes in Eastern Asians and Europeans: Implications in the clinical trials for novel drug development. Drug Metabolism and Pharmacokinetics, 27(1), 9–54. DOI 10.2133/dmpk.DMPK-11-RV-111. [Google Scholar] [CrossRef]
70. Mizutani, T. (2003). PM frequencies of major CYPs in Asians and Caucasians. Drug Metabolism Reviews, 35(2–3), 99–106. DOI 10.1081/DMR-120023681. [Google Scholar] [CrossRef]
71. Nakashima, K., Narukawa, M., Takeuchi, M. (2011). Approaches to japanese dose evaluation in global drug development: Factors that generate different dosages between Japan and the United States. Clinical Pharmacology and Therapeutics, 90(6), 836–843. DOI 10.1038/clpt.2011.156. [Google Scholar] [CrossRef]
72. Arnold, F. L., Fukunaga, S., Kusama, M., Matsuki, N., Ono, S. (2014). Assessment of factors associated with dose differences between Japan and the United States. Clinical Pharmacology and Therapeutics, 95(5), 542–549. DOI 10.1038/clpt.2013.231. [Google Scholar] [CrossRef]
73. Park, K. (2016). Drug delivery of the future: Chasing the invisible gorilla. Journal of Controlled Release, 240, 2–8. DOI 10.1016/j.jconrel.2015.10.048. [Google Scholar] [CrossRef]
74. Wang, S., Xu, Y., Chan, H. F., Kim, H. W., Wang, Y. et al. (2016). Nanoparticle-mediated inhibition of survivin to overcome drug resistance in cancer therapy. Journal of Controlled Release, 240, 454–464. DOI 10.1016/j.jconrel.2016.04.018. [Google Scholar] [CrossRef]
75. Kawano, H., Shakushiro, K., Nakata, M., Kita, A., Maeda, A. et al. (2014). Antitumor efficacy and biodistribution of liposomal sepantronium bromide (YM155a novel small-molecule survivin suppressant. European Journal of Pharmaceutics and Biopharmaceutics, 88(1), 283–289. DOI 10.1016/j.ejpb.2014.06.015. [Google Scholar] [CrossRef]
76. Shakushiro, K., Kawano, H., Nakata, M., Kita, A., Maeda, A. et al. (2015). Formulation design and evaluation of liposomal Sepantronium bromide (YM155a small-molecule survivin suppressant, based on pharmacokinetic modeling and simulation. Pharmaceutical Research, 32(1), 238–247. DOI 10.1007/s11095-014-1458-4. [Google Scholar] [CrossRef]
77. Winter, G. E., Radic, B., Mayor-Ruiz, C., Blomen, V. A., Trefzer, C. et al. (2014). The solute carrier SLC35F2 enables YM155-mediated DNA damage toxicity. Nature Chemical Biology, 10(9), 768–773. DOI 10.1038/nchembio.1590. [Google Scholar] [CrossRef]
78. Kanwar, J. R., Kamalapuram, S. K., Kanwar, R. K. (2010). Targeting survivin in cancer: Patent review. Expert Opinion on Therapeutic Patents, 20(12), 1723–1737. DOI 10.1517/13543776.2010.533657. [Google Scholar] [CrossRef]
79. Zhou, J., O’Brate, A., Zelnak, A., Giannakakou, P. (2004). Survivin deregulation in β-tubulin mutant ovarian cancer cells underlies their compromised mitotic response to Taxol. Cancer Research, 64(23), 8708–8714. DOI 10.1158/0008-5472.CAN-04-2538. [Google Scholar] [CrossRef]
80. Cheung, C. H. A., Chen, H. H., Kuo, C. C., Chang, C. Y., Coumar, M. S. et al. (2009). Survivin counteracts the therapeutic effect of microtubule de-stabilizers by stabilizing tubulin polymers. Molecular Cancer, 8(1), 1–15. DOI 10.1186/1476-4598-8-43. [Google Scholar] [CrossRef]
81. Rang, H. P., Dale, M. M., Ritter, J. M., Flower, R. J., Henderson, G. (2015). Rang & Dale’s pharmacology (8th Ed.Churchill Livingstone, UK. [Google Scholar]
82. Fry, F. H., Holme, A. L., Giles, N. M., Giles, G. I., Collins, C. et al. (2005). Multifunctional redox catalysts as selective enhancers of oxidative stress. Organic and Biomolecular Chemistry, 3(14), 2579–2587. DOI 10.1039/b502197a. [Google Scholar] [CrossRef]
83. Magda, D., Miller, R. A. (2006). Motexafin gadolinium: A novel redox active drug for cancer therapy. Seminars in Cancer Biology, pp. 466–476. USA: Elsevier Health Sciences. [Google Scholar]
84. Wu, F. Y. H., Sun, T. P. (1999). Vitamin K3 induces cell cycle arrest and cell death by inhibiting Cdc25 phosphatase. European Journal of Cancer, 35(9), 1388–1393. DOI 10.1016/S0959-8049(99)00156-2. [Google Scholar] [CrossRef]
85. Giles, N. M., Gutowski, N. J., Giles, G. I., Jacob, C., Celis, J. (2003). Redox catalysts as sensitisers towards oxidative stress. FEBS Letters, 535(1–3), 179–182. DOI 10.1016/S0014-5793(02)03890-5. [Google Scholar] [CrossRef]
86. Iskander, K., Jaiswal, A. K. (2015). Retraction: NRH: quinone oxidoreductase 2 and NAD(P)H: quinone oxidoreductase 1 protect tumor suppressor p53 against 20S proteasomal degradation leading to stabilization and activation of p53. Cancer Research, 75(3), 615–615. DOI 10.1158/0008-5472.CAN-14-3458. [Google Scholar] [CrossRef]
87. Fruehauf, J. P., Meyskens, F. L. (2007). Reactive oxygen species: A breath of life or death? Clinical Cancer Research, 13(3), 789–794. DOI 10.1158/1078-0432.CCR-06-2082. [Google Scholar] [CrossRef]
88. Cabello, C. M., Bair, W. B., Wondrak, G. T. (2007). Experimental therapeutics: Targeting the redox Achilles heel of cancer. Current Opinion in Investigational Drugs, 8(12), 1022–1037. [Google Scholar]
89. Fried, L., Arbiser, J. L. (2008). The reactive oxygen-driven tumor: Relevance to melanoma. Pigment Cell and Melanoma Research, 21(2), 117–122. DOI 10.1111/j.1755-148X.2008.00451.x. [Google Scholar] [CrossRef]
90. Gius, D., Spitz, D. R. (2006). Redox signaling in cancer biology. Antioxidants and Redox Signaling, 8(7–8), 1249–1252. DOI 10.1089/ars.2006.8.1249. [Google Scholar] [CrossRef]
91. Giles, G. (2006). The redox regulation of thiol dependent signaling pathways in cancer. Current Pharmaceutical Design, 12(34), 4427–4443. DOI 10.2174/138161206779010549. [Google Scholar] [CrossRef]
92. Hu, S., Fu, S., Xu, X., Chen, L., Xu, J. et al. (2015). The mechanism of radiosensitization by YM155, a novel small molecule inhibitor of survivin expression, is associated with DNA damage repair. Cellular Physiology and Biochemistry, 37(3), 1219–1230. DOI 10.1159/000430245. [Google Scholar] [CrossRef]
93. Chen, J., Pise-Masison, C. A., Shih, J. H., Morris, J. C., Janik, J. E. et al. (2013). Markedly additive antitumor activity with the combination of a selective survivin suppressant YM155 and alemtuzumab in adult T-cell leukemia. Blood, 121(11), 2029–2037. DOI 10.1182/blood-2012-05-427773. [Google Scholar] [CrossRef]
94. Kaneko, N., Mitsuoka, K., Amino, N., Yamanaka, K., Kita, A. et al. (2014). Combination of YM155, a survivin suppressant, with bendamustine and rituximab: A new combination therapy to treat relapsed/refractory diffuse large B-cell lymphoma. Clinical Cancer Research, 20(7), 1814–1822. DOI 10.1158/1078-0432.CCR-13-2707. [Google Scholar] [CrossRef]
95. Zhao, X., Puszyk, W. M., Lu, Z., Ostrov, D. A., George, T. J. et al. (2015). Small molecule inhibitor YM155-mediated activation of death receptor 5 is crucial for chemotherapy-induced apoptosis in pancreatic carcinoma. Molecular Cancer Therapeutics, 14(1), 80–89. DOI 10.1158/1535-7163.MCT-14-0229. [Google Scholar] [CrossRef]
96. Purroy, N., Abrisqueta, P., Carabia, J., Carpio, C., Calpe, E. et al. (2014). Targeting the proliferative and chemoresistant compartment in chronic lymphocytic leukemia by inhibiting survivin protein. Leukemia, 28(10), 1993–2004. DOI 10.1038/leu.2014.96. [Google Scholar] [CrossRef]
97. Yu, Y., Zhao, X., Zhang, Y., Kang, Y., Wang, J. et al. (2015). Antitumor activity of YM155, a selective survivin suppressant, in combination with cisplatin in hepatoblastoma. Oncology Reports, 34(1), 407–414. DOI 10.3892/or.2015.3947. [Google Scholar] [CrossRef]
98. Ong, S. M., Saeki, K., Kok, M. K., Nakagawa, T., Nishimura, R. (2019). YM155 enhances the cytotoxic activity of etoposide against canine osteosarcoma cells. Journal of Veterinary Medical Science, 81(8), 1182–1190. DOI 10.1292/jvms.19-0029. [Google Scholar] [CrossRef]
99. Cheng, C. C., Chou, K. F., Wu, C. W., Su, N. W., Peng, C. L. et al. (2018). EGFR-mediated interleukin enhancer-binding factor 3 contributes to formation and survival of cancer stem-like tumorspheres as a therapeutic target against EGFR-positive non-small cell lung cancer. Lung Cancer, 116(3), 80–89. DOI 10.1016/j.lungcan.2017.12.017. [Google Scholar] [CrossRef]
100. Cho, S. Y., Han, J. Y., Na, D., Kang, W., Lee, A. et al. (2017). A novel combination treatment targeting BCL-XL and MCL1 for KRAS/BRAF-mutated and BCL2L1-amplified colorectal cancers. Molecular Cancer Therapeutics, 16(10), 2178–2190. DOI 10.1158/1535-7163.MCT-16-0735. [Google Scholar] [CrossRef]
101. Radic-Sarikas, B., Halasz, M., Huber, K. V. M., Winter, G. E., Tsafou, K. P. et al. (2017). Lapatinib potentiates cytotoxicity of YM155 in neuroblastoma via inhibition of the ABCB1 efflux transporter. Scientific Reports, 7(1), 3091. DOI 10.1038/s41598-017-03129-6. [Google Scholar] [CrossRef]
102. Kiyohara, Y., Yoshino, K., Kubota, S., Okuyama, H., Endo, H. et al. (2016). Drug screening and grouping by sensitivity with a panel of primary cultured cancer spheroids derived from endometrial cancer. Cancer Science, 107(4), 452–460. DOI 10.1111/cas.12898. [Google Scholar] [CrossRef]
103. Wu, L., Qu, X. (2015). Cancer biomarker detection: Recent achievements and challenges. Chemical Society Reviews, 44(10), 2963–2997. DOI 10.1039/C4CS00370E. [Google Scholar] [CrossRef]
104. Bhatt, A. N., Mathur, R., Farooque, A., Verma, A., Dwarakanath, B. S. (2010). Cancer biomarkers-Current perspectives. Indian Journal of Medical Research, 132(8), 129–149. [Google Scholar]
105. Nowsheen, S., Aziz, K., Panayiotidis, M. I., Georgakilas, A. G. (2012). Molecular markers for cancer prognosis and treatment: Have we struck gold? Cancer Letters, 327(1–2), 142–152. DOI 10.1016/j.canlet.2011.11.022. [Google Scholar] [CrossRef]
106. Sawyers, C. L. (2008). The cancer biomarker problem. Nature, 452(7187), 548–552. DOI 10.1038/nature06913. [Google Scholar] [CrossRef]
107. Martinkova, J., Gadher, S. J., Hajduch, M., Kovarova, H. (2009). Challenges in cancer research and multifaceted approaches for cancer biomarker quest. FEBS Letters, 583(11), 1772–1784. DOI 10.1016/j.febslet.2009.03.042. [Google Scholar] [CrossRef]
108. Ma, Y., Gamagedara, S. (2015). Biomarker analysis for oncology. Biomarkers in Medicine, 9(9), 845–850. DOI 10.2217/bmm.15.60. [Google Scholar] [CrossRef]
109. Waligórska-Stachura, J., Jankowska, A., Waśko, R., Liebert, W., Biczysko, M. et al. (2012). Survivin-prognostic tumor biomarker in human neoplasms–Review. Ginekologia Polska, 83(7), 537–540. [Google Scholar]
110. da Veiga, G. L., da Silva, R. D. M., Pereira, E. C., Azzalis, L. A., da Costa Aguiar Alves, B. et al. (2019). The role of Survivin as a biomarker and potential prognostic factor for breast cancer. Revista da Associacao Medica Brasileira, 65(6), 893–901. DOI 10.1590/1806-9282.65.6.893. [Google Scholar] [CrossRef]
111. Petrarca, C. R., Brunetto, A. T., Duval, V., Brondani, A., Carvalho, G. P. et al. (2011). Survivin as a predictive biomarker of complete pathologic response to neoadjuvant chemotherapy in patients with stage II and stage III breast cancer. Clinical Breast Cancer, 11(2), 129–134. DOI 10.1016/j.clbc.2011.03.002. [Google Scholar] [CrossRef]
112. Kren, L., Brazdil, J., Hermanova, M., Goncharuk, V. N., Kallakury, B. V. S. et al. (2004). Prognostic significance of anti-apoptosis proteins survivin and bcl-2 in non-small cell lung carcinomas: A clinicopathologic study of 102 cases. Applied Immunohistochemistry and Molecular Morphology, 12(1), 44–49. DOI 10.1097/00129039-200403000-00009. [Google Scholar] [CrossRef]
113. Zhang, S., Zhang, C., Song, Y., Zhang, J., Xu, J. (2018). Prognostic role of survivin in patients with glioma. Medicine, 97(17), e0571. DOI 10.1097/MD.0000000000010571. [Google Scholar] [CrossRef]
114. Xiong, C., Liu, H., Chen, Z., Yu, Y., Liang, C. (2016). Prognostic role of survivin in renal cell carcinoma: A system review and meta-analysis. European Journal of Internal Medicine, 33(5), 102–107. DOI 10.1016/j.ejim.2016.06.009. [Google Scholar] [CrossRef]
115. Andersen, M. H., Svane, I. M., Becker, J. C., Straten, P. T. (2007). The universal character of the tumor-associated antigen survivin. Clinical Cancer Research, 13(20), 5991–5994. DOI 10.1158/1078-0432.CCR-07-0686. [Google Scholar] [CrossRef]
116. Kanwar, J. R., Kamalapuram, S. K., Kanwar, R. K. (2011). Targeting survivin in cancer: the cell-signalling perspective. Drug Discovery Today, 16(11–12), 485–494. DOI 10.1016/j.drudis.2011.04.001. [Google Scholar]
117. Luong-Gardiol, N., Siddiqui, I., Pizzitola, I., Jeevan-Raj, B., Charmoy, M. et al. (2019). γ-catenin-dependent signals maintain BCR-ABL1+ B cell acute lymphoblastic leukemia. Cancer Cell, 35(4), 649–663.e10. DOI 10.1016/j.ccell.2019.03.005. [Google Scholar] [CrossRef]
118. Ishikawa, C., Senba, M., Mori, N. (2020). Evaluation of artesunate for the treatment of adult T-cell leukemia/lymphoma. European Journal of Pharmacology, 872, 172953. DOI 10.1016/j.ejphar.2020.172953. [Google Scholar] [CrossRef]
119. Wang, D., Liu, J., Liu, S., Li, W. (2020). Identification of crucial genes associated with immune cell infiltration in hepatocellular carcinoma by weighted gene co-expression network analysis. Frontiers in Genetics, 11, 342. DOI 10.3389/fgene.2020.00342. [Google Scholar] [CrossRef]
120. Fujiya, K., Ohshima, K., Kitagawa, Y., Hatakeyama, K., Nagashima, T. et al. (2020). Aberrant expression of Wnt/β-catenin signaling pathway genes in aggressive malignant gastric gastrointestinal stromal tumors. The Journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology, 46(6), 1080–1087. DOI 10.1016/j.ejso.2020.02.036. [Google Scholar] [CrossRef]
121. Turner, D. P., Findlay, V. J., Moussa, O., Semenchenko, V. I., Watson, P. M. et al. (2011). Mechanisms and functional consequences of PDEF protein expression loss during prostate cancer progression. The Prostate, 71(16), 1723–1735. DOI 10.1002/pros.21389. [Google Scholar] [CrossRef]
122. Hennigs, J. K., Minner, S., Tennstedt, P., Löser, R., Huland, H. et al. (2020). Subcellular compartmentalization of survivin is associated with biological aggressiveness and prognosis in prostate cancer. Scientific Reports, 10(1), 3250. DOI 10.1038/s41598-020-60064-9. [Google Scholar] [CrossRef]
123. Faccion, R. S., Bernardo, P. S., de Lopes, G., Bastos, L. S., Teixeira, C. L. et al. (2018). p53 expression and subcellular survivin localization improve the diagnosis and prognosis of patients with diffuse astrocytic tumors. Cellular Oncology, 41(2), 141–157. DOI 10.1007/s13402-017-0361-5. [Google Scholar] [CrossRef]
124. Conde, M., Michen, S., Wiedemuth, R., Klink, B., Schröck, E. et al. (2017). Chromosomal instability induced by increased BIRC5/Survivin levels affects tumorigenicity of glioma cells. BMC Cancer, 17(1), 889. DOI 10.1186/s12885-017-3932-y. [Google Scholar] [CrossRef]
125. Brun, S. N., Markant, S. L., Esparza, L. A., Garcia, G., Terry, D. et al. (2015). Survivin as a therapeutic target in Sonic hedgehog-driven medulloblastoma. Oncogene, 34(29), 3770–3779. DOI 10.1038/onc.2014.304. [Google Scholar] [CrossRef]
126. Lebelt, A., Rutkowski, R., Och, W., Jaczun, K., Dziemiańczyk-Pakieła, D. et al. (2016). Survivin, caspase-3 and MIB-1 expression in astrocytic tumors of various grades. Advances in Medical Sciences, 61(2), 237–243. DOI 10.1016/j.advms.2016.02.001. [Google Scholar] [CrossRef]
127. Brun, S. N., Markant, S. L., Esparza, L. A., Garcia, G., Terry, D. et al. (2015). Survivin as a therapeutic target in Sonic hedgehog-driven medulloblastoma. Oncogene, 34(29), 3770–3779. DOI 10.1038/onc.2014.304. [Google Scholar] [CrossRef]
128. Iwasa, T., Okamoto, I., Suzuki, M., Nakahara, T., Yamanaka, K. et al. (2008). Radiosensitizing effect of YM155, a novel small-molecule survivin suppressant, in non-small cell lung cancer cell lines. Clinical Cancer Research, 14(20), 6496–6504. DOI 10.1158/1078-0432.CCR-08-0468. [Google Scholar] [CrossRef]
129. Morrison, D. J., Hogan, L. E., Condos, G., Bhatla, T., Germino, N. et al. (2012). Endogenous knockdown of survivin improves chemotherapeutic response in ALL models. Leukemia, 26(2), 271–279. DOI 10.1038/leu.2011.199. [Google Scholar] [CrossRef]
130. Yang, W., Cooke, M., Duckett, C. S., Yang, X., Dorsey, J. F. (2014). Distinctive effects of the cellular inhibitor of apoptosis protein c-IAP2 through stabilization by XIAP in glioblastoma multiforme cells. Cell Cycle, 13(6), 992–1005. DOI 10.4161/cc.27880. [Google Scholar] [CrossRef]
131. Yamanaka, K., Nakahara, T., Yamauchi, T., Kita, A., Takeuchi, M. et al. (2011). Antitumor activity of YM155, a selective small-molecule survivin suppressant, alone and in combination with docetaxel in human malignant melanoma models. Clinical Cancer Research, 17(16), 5423–5431. DOI 10.1158/1078-0432.CCR-10-3410. [Google Scholar] [CrossRef]
132. Liang, H., Zhang, L., Xu, R., Ju, X. L. (2013). Silencing of survivin using YM155 induces apoptosis and chemosensitization in neuroblastomas cells. European Review for Medical and Pharmacological Sciences, 17(21), 2909–2915. [Google Scholar]
133. Lamers, F., Schild, L., Koster, J., Versteeg, R., Caron, H. N. et al. (2012). Targeted BIRC5 silencing using YM155 causes cell death in neuroblastoma cells with low ABCB1 expression. European Journal of Cancer, 48(5), 763–771. DOI 10.1016/j.ejca.2011.10.012. [Google Scholar] [CrossRef]
134. Lamers, F., van der Ploeg, I., Schild, L., Ebus, M. E., Koster, J. et al. (2011). Knockdown of survivin (BIRC5) causes apoptosis in neuroblastoma via mitotic catastrophe. Endocrine-Related Cancer, 18(6), 657–668. DOI 10.1530/ERC-11-0207. [Google Scholar] [CrossRef]
135. Michaelis, M., Wass, M. N., Reddin, I., Voges, Y., Rothweiler, F. et al. (2020). YM155-adapted cancer cell lines reveal drug-induced heterogeneity and enable the identification of biomarker candidates for the acquired resistance setting. Cancers, 12(5), 1080. DOI 10.3390/cancers12051080. [Google Scholar] [CrossRef]
136. Shimizu, T., Nishio, K., Sakai, K., Okamoto, I., Okamoto, K. et al. (2020). Phase I safety and pharmacokinetic study of YM155, a potent selective survivin inhibitor, in combination with erlotinib in patients with EGFR TKI refractory advanced non-small cell lung cancer. Cancer Chemotherapy and Pharmacology, 86(2), 211–219. DOI 10.1007/s00280-020-04112-1. [Google Scholar] [CrossRef]
137. Mitsuoka, K., Kita, A., Murakami, Y., Shirasuna, K., Noda, A. et al. (2018). Predicting response to sepantronium bromide (YM155a survivin suppressant, by PET imaging with [11C]YM155. Nuclear Medicine and Biology, 64-65, 41–46. DOI 10.1016/j.nucmedbio.2018.06.005. [Google Scholar] [CrossRef]
138. Wang, S., Xu, Y., Chan, H. F., Kim, H. W., Wang, Y. et al. (2016). Nanoparticle-mediated inhibition of survivin to overcome drug resistance in cancer therapy. Journal of Controlled Release, 240, 454–464. DOI 10.1016/j.jconrel.2016.04.018. [Google Scholar] [CrossRef]
139. Kanwar, J. R., Kamalapuram, S. K., Kanwar, R. K. (2013). Survivin signaling in clinical oncology: A multifaceted dragon. Medicinal Research Reviews, 33(4), 765–789. DOI 10.1002/med.21264. [Google Scholar] [CrossRef]
140. Arista-Romero, M., Cascante, A., Fornaguera, C., Borrós, S. (2021). Role of survivin in bladder cancer: Issues to be overcome when designing an efficient dual nano-therapy. Pharmaceutics, 13(11), 1959. DOI 10.3390/pharmaceutics13111959. [Google Scholar] [CrossRef]
141. Nakahara, T., Kita, A., Yamanaka, K., Mori, M., Amino, N. et al. (2007). YM155, a novel small-molecule survivin suppressant, induces regression of established human hormone-refractory prostate tumor xenografts. Cancer Research, 67(17), 8014–8021. DOI 10.1158/0008-5472.CAN-07-1343. [Google Scholar] [CrossRef]
142. Li, F., Aljahdali, I., Ling, X. (2019). Cancer therapeutics using survivin BIRC5 as a target: What can we do after over two decades of study? Journal of Experimental & Clinical Cancer Research, 38(1), 368. DOI 10.1186/s13046-019-1362-1. [Google Scholar] [CrossRef]
143. Cheng, Q., Ling, X., Haller, A., Nakahara, T., Yamanaka, K. et al. (2012). Suppression of survivin promoter activity by YM155 involves disruption of Sp1-DNA interaction in the survivin core promoter. International Journal of Biochemistry and Molecular Biology, 3(2), 179–197. [Google Scholar]
144. Yamauchi, T., Nakamura, N., Hiramoto, M., Yuri, M., Yokota, H. et al. (2012). Sepantronium bromide (YM155) induces disruption of the ILF3/p54nrb complex, which is required for survivin expression. Biochemical and Biophysical Research Communications, 425(4), 711–716. DOI 10.1016/j.bbrc.2012.07.103. [Google Scholar] [CrossRef]
145. Shojaei, F., Yazdani-Nafchi, F., Banitalebi-Dehkordi, M., Chehelgerdi, M., Khorramian-Ghahfarokhi, M. (2019). Trace of survivin in cancer. European Journal of Cancer Prevention, 28(4), 365–372. DOI 10.1097/CEJ.0000000000000453. [Google Scholar] [CrossRef]
146. Sawyers, C. (2004). Targeted cancer therapy. Nature, 432(7015), 294–297. DOI 10.1038/nature03095. [Google Scholar] [CrossRef]
147. Reya, T., Morrison, S. J., Clarke, M. F., Weissman, I. L. (2001). Stem cells, cancer, and cancer stem cells. Nature, 414(6859), 105–111. DOI 10.1038/35102167. [Google Scholar] [CrossRef]
148. Jaiswal, P. K., Goel, A., Mittal, R. D. (2015). Survivin: A molecular biomarker in cancer. Indian Journal of Medical Research, 141(4), 389–397. DOI 10.4103/0971-5916.159250. [Google Scholar] [CrossRef]
Appendices
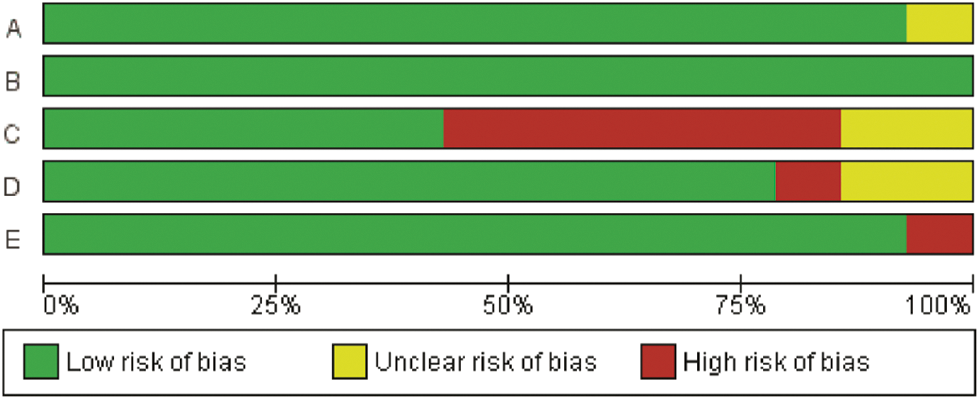
Appendix A: Risk of bias graph YM155
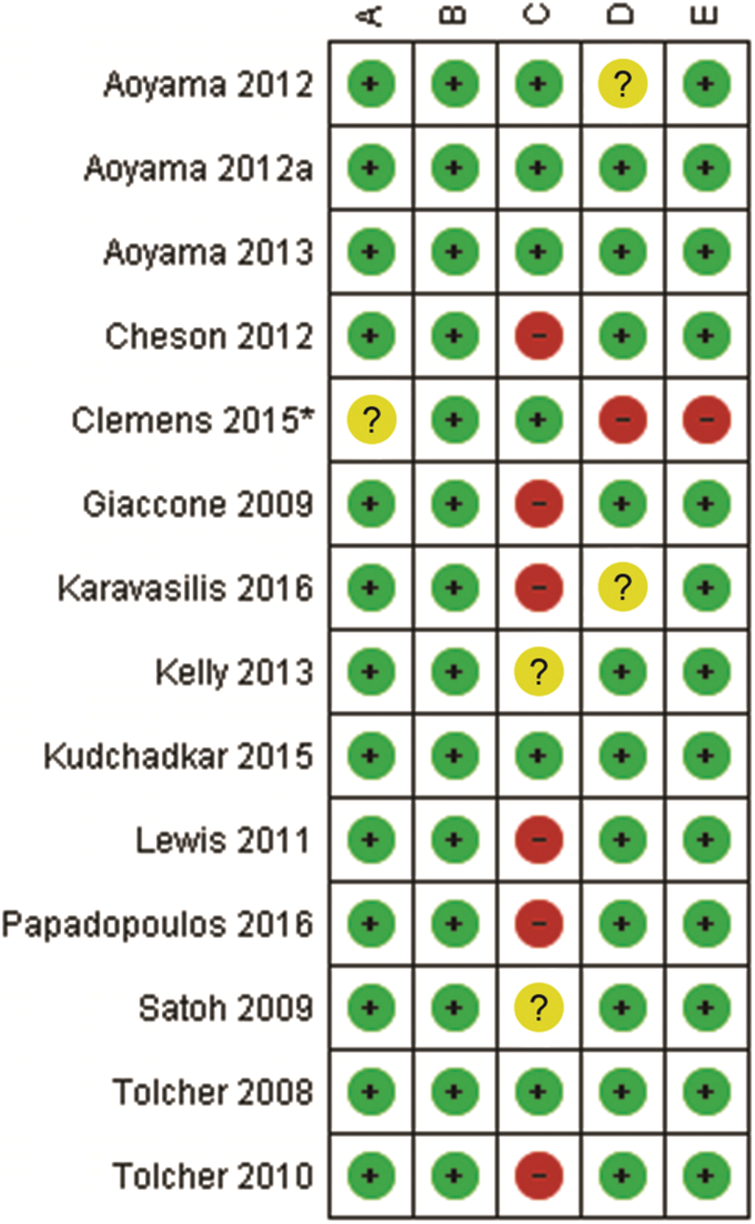
Appendix B: Risk of bias summary YM155
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |