
 | Oncologie |  |
DOI: 10.32604/oncologie.2022.022116
ARTICLE
Chinese Herbal Prescription QYSL Prevents Progression of Lung Cancer by Targeting Tumor Microenvironment
1Oncology Department of Integrated Traditional Chinese and Western Medicine, The First Affiliated Hospital of Anhui Medical University, Hefei, 230022, China
2Scientific Research & Experiment Center, Anhui University of Chinese Medicine, Hefei, 230038, China
3The First Affiliated Hospital of Anhui Medical University, Hefei, 230022, China
4The First Affiliated Hospital of Anhui University of Chinese Medicine, Hefei, 230601, China
5College of Integrated Chinese and Western Medicine, Anhui University of Chinese Medicine, Hefei, 230038, China
*Corresponding Authors: Ping Li. Email: liping64@sina.com; Zegeng Li. Email: li6609@126.com
#These authors contributed equally to this work
Received: 22 February 2022; Accepted: 14 April 2022
Abstract: Objectives: Lung cancer is a common and malignant tumor in adults and ranks first in the incidence and mortality of the top five malignant tumors in China. Our previous studies have shown that QYSL prescription can balance lung cancer mice Th1/Th2 and inhibit tumor cell immune escape. Here, we examined the effects of QYSL on lung cancer associated macrophage and the potential associated mechanism. Methods: C57BL/6 mice were injected with Lewis lung cancer cells and treated with QYSL. FACS, RT-PCR, and western blot were used to examined the effect of QYSL on tumor immune microenvironment. Results: We found QYSL inhibited tumor growth in mice with lung cancer. Further study indicated that QYSL inhibited growth of lung tumor by promoting T cell activation and promoting macrophage polarization to M1 type. We found QYSL could markedly inhibit M2 macrophage related protein expression and promote M1 macrophage related protein expression. Additionally, STAT6 and MTOR expression were reduced in QYSL group. Conclusions: Overall, this study indicated that QYSL potently inhibited the growth of lung cancer by promoting T cell activation and M2 macrophage polarization to M1, which was found to be associated with the STAT6 and MTOR signaling pathway.
Keywords: Lung cancer; TME; traditional Chinese medicine; QYSL
Lung cancer is a common and malignant tumor in adults [1–3]. According to the latest cancer statistics published in 2020 by the International Cancer Institute, lung cancer mortality is the highest [4]. Lung cancer ranks first in the incidence and mortality of the top five malignant tumors in China [5–7]. Currently, the treatment of lung cancer is mainly surgical resection, followed by adjuvant chemotherapy, radiotherapy, targeted therapy and immunotherapy [8–10]. However, in clinical treatment, targeted therapy and immunotherapy still face problems such as drug resistance and adverse reactions [11–14]. Therefore, new therapeutic strategies are still urgently needed.
The occurrence and development of lung cancer is closely related to tumor microenvironment (TME) [15–17]. In the process of anti-tumor immune response, tumor cells can evade the attack of immune cells by inhibiting T cell activation. In recent years, immune checkpoint inhibitors, which have been widely used at home and abroad, have achieved remarkable progress of the treatment of tumors by blocking the inhibitory signal of T cell activation in the tumor microenvironment [18–20]. Therefore, it is particularly important to explore the mechanism affecting the activation of T cells, reactivate the immune response effect of T cells on tumors, and further enhance the efficacy of immunotherapy. Tumor associated macrophages (TAM) in tumor tissue plays an important role in regulating the tumor microenvironment. Different roles played by their subtypes M1 and M2 are related to T cell function, M1 macrophages upregulated the immune response and M2 macrophages suppresses the immune response. Therefore, inducing M2 TAM to M1 polarization is beneficial to regulating the activation of T cells [21–23].
Traditional Chinese medicine has advantages in tumor treatment, which not only inhibit tumor growth, but also reduce drug side effects. Its efficacy has been proven by a large number of clinical practices and experimental studies [24–27]. As a clinical prescription for the treatment of lung cancer, the main ingredients of qiyusanlong (QYSL) prescription are astragalus (Astragalus membranaceus (Fisch.) Bunge), polygonatum odoratum (Polygonatum odoratum (Mill.) Druce), tianlong (Scolopendra), dilong (Pheretima), Solanum nigrum (Solanum nigrum L.), Hedyotis diffusa (Hedyotis diffusa Willd), coix seed (Coicis Semen), zedoary (Euphorbiae helioscopiae), zedoary turmeric (Curcumae rhizoma), and Fritillaria (Fritillariae Cirrhosae Bulbus). QYSL can significantly inhibit tumor growth and improve the quality of life of lung cancer patients. Studies have shown that QYSL can balance lung cancer mice helper T cell 1/2 (Th1/Th2) and inhibit tumor cell immune escape. Further studies found that 21 potential biomarkers were associated with the function of QYSL and the sphingolipid metabolic pathway was significantly associated with Th1-mediated anti-tumor immunity. However, there is no relevant report on whether it affects the tumor associated macrophage. In this study, we want to explore the inhibitory effect of QYSL on tumor growth of lung cancer tumor-bearing mice from the perspective of immune microenvironment, as well as the regulation of M2/M1 macrophage polarization and T cells, which will provide a new theoretical basis of future clinical research of QYSL.
STAT6 Rabbit mAb (#5397), STAT3 Rabbit mAb (#12640), STAT1 Rabbit mAb (#14994), PTEN Rabbit mAb (#9188), MTOR Rabbit mAb (#2983), β-catin Mouse mAb (#3700), CD8α (RPA-T8) FITC-conjugated mAb (#55397), CD4 (RPA-T4) FITC-conjugated mAb (#48705), F4/80 FITC-conjugated mAb (#52267), were purchased from Cell Signaling Technology (Danvers, MA, USA). CD3 APC-conjugated mAb (FAB4841A), CD69 PE-conjugated mAb (FAB2386P), CD206 APC-conjugated mAb (FAB2535A) were purchased from RD (McKinley, MN, USA). CD45 PE-conjugated mAb (ab269346), CD86 APC-conjugated mAb (ab218757) were purchased from Abcam (Cambridge, CB, UK).
5 weeks old female C57BL/6N mice were purchased from Shanghai SLAC Co. (Shanghai, China) and housed in separate stainless steel cages (five mice per cage) atconstant temperature (23°C) with a 12 h light/dark cycle had free access to water and food. It was raised in the animal room of the Key Laboratory of Xinan Medical Education Department of Anhui University of Chinese Medicine. This study has been authorized by the Animal Ethics Committee of Anhui University of Chinese Medicine (approval No. ahucm-mouse-2019013).
The Lewis lung cancer cell line were purchased from the KeBai Biology (Nanjing, China), and cultured in Dulbecco’s modified eagle medium (DMEM) with 10% fetal bovine serum (FBS) and 0.1% penicillin-streptomycin.
The suspension of QYSL prescription was obtained by impregnation, boiling, filtration and water bath concentration, The preparation steps are as follows: Astragalus (30 g), polygonatum odoratum (10 g), Tianlong (6 g), Dilong (6 g), Solanum nigrum (20 g), Hedyotis diffusa (20 g), coix seed (20 g), zedoary (6 g), zedoary turmeric (10 g) and Fritillaria (10 g), which were purchased from the First Affiliated Hospital of Anhui University of Traditional Chinese Medicine. After impregnation, boiling and filtration, QYSL prescription was prepared by concentrating in water bath until the crude drug content was 4.024 g/ml, and stored at 4°C.
In this study, 20 SPF C57BL/6 mice were used to establish the tumor model. 1 × 106 Lewis lung cancer cells were injected into the subcutaneous space on the right flank of C57BL/6 mice. Animals were then randomized into treatment groups for efficacy studies. Daily treatment with the compound was initiated after 2 weeks. SPSS or QYSL was delivered daily by oral gavage. Body weight and tumor growth were measured every day after treatment. tumor volume was calculated:
Total RNA was isolated from tissues using ultrapure RNA Kit (CWBIO, Beijing, China) according to the manufacturer’s instructions. Then, RNA was reverse-transcribed into cDNAs using a Transcriptor First Strand cDNA Synthesis Kit (Roche). The relative expression of specific genes was determined using SYBR Green PCR Mastermix (TaKaRa, Dalian, China) with bio-rad real-time PCR system. The method of 2−ΔΔCt was used to calculate the relative gene expression levels. Related primers were listed below (Table 1):
The effect of QYSL on tumor microenvironment was examined. Cells were harvested and fixed, then stained with antibody. Cell distribution was examined with BD FACS Calibur cytometer. The data was analyzed with the Flow Jo software (Becton Dickinson, NJ, USA).
A total of 4 lung cancer patients were included in this study. All patients provided written informed consents, and the study was approved by the Ethics Committee of the First Affiliated Hospital of Anhui Medical University and complied with the ethical guidelines of the Helsinki Declaration (1975 revision).
The tumor tissues were fully lysed with RIPA buffer, and the supernatants were collected after centrifugation. The protein concentration in the supernatant was determined using the BCA Assay Kit (Beyotime, China). An aliquot of 40 μg total protein from each sample was loaded onto 10% SDS-PAGE gels and transferred to PVDF membranes. PVDF membranes were blocked with 5% BSA for 1 h at room temperature and incubated with primary antibodies. The membranes were visualized using an enhanced chemiluminescence detection system (Tanon, China). The qualification of the protein bands was carried out with Image J software by densitometry.
Student’s t test was used for comparisons among the different groups. All statistical tests were two-tailed, and a p value of less than 0.05 was considered statistically significant.
3.1 QYSL Inhibited Growth of Lung Cancer Tumor in a Subcutaneous Mouse Model
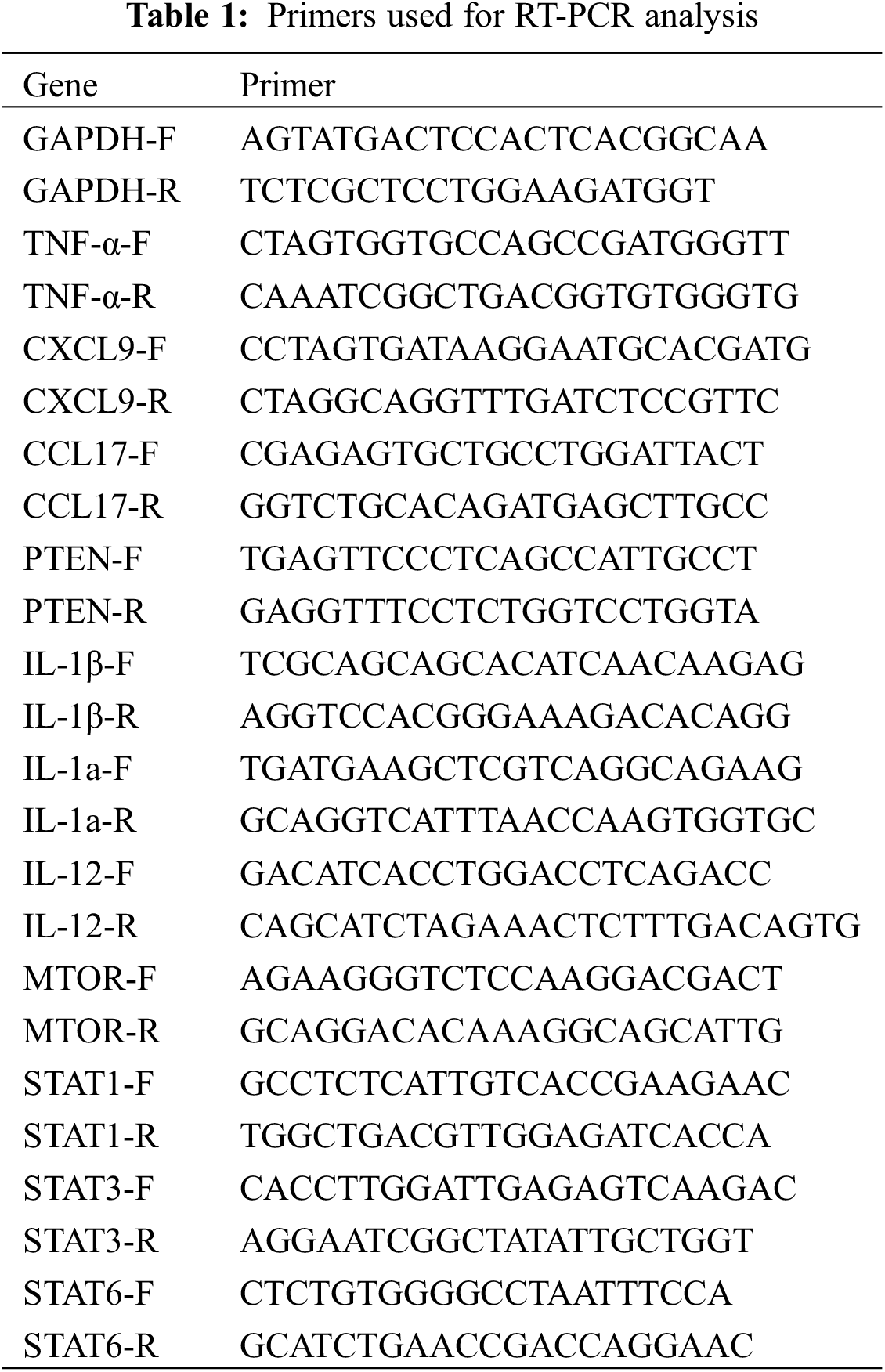
QYSL decoction, as a clinical prescription for the treatment of lung cancer, can significantly improve the quality of life of lung cancer patients. In this study, we mainly explored the mechanism of QYSL from the aspect of tumor immunity. Firstly, subcutaneous mouse model was established by subcutaneous injection of Lewis lung cancer cells to explore the role of QYSL in tumor growth in vivo. The results were shown in Fig. 1A. In vehicle group, the tumor gradually increased in a time-dependent manner. However, treatment with QYSL potently suppressed the tumor growth. Tumor growth inhibition reached 26.2% on day 14 after QYSL administration (Fig. 1B). These results indicate that QYSL potently inhibited the proliferation of lung cancer in vivo. We also observed that QYSL had no effect on the body weight (Fig. 1C). The result indicated that QYSL had high safety. Overall, these results showed that QYSL potently inhibited the growth of lung cancer in vivo.
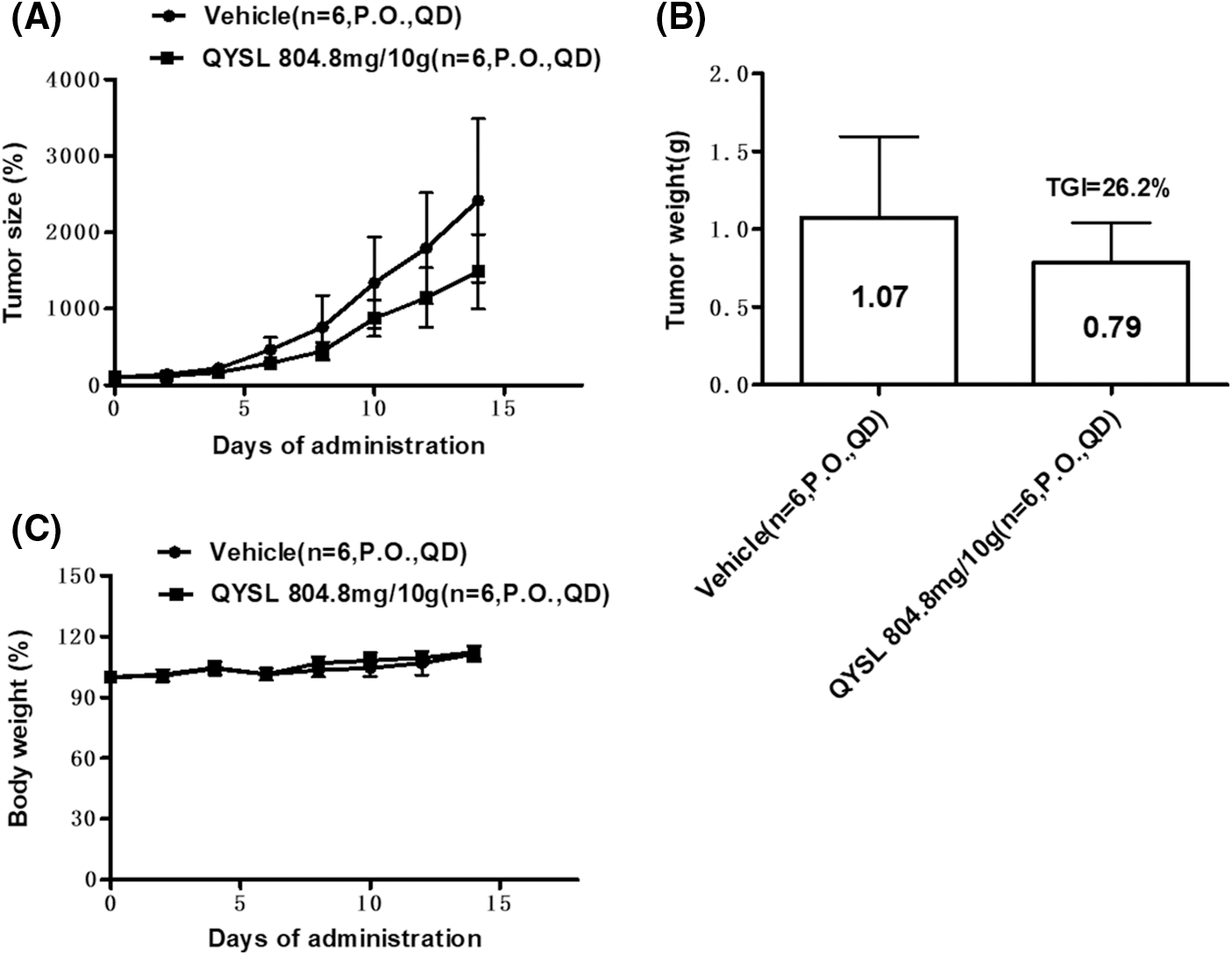
Figure 1: QYSL inhibited growth of lung cancer tumor in a subcutaneous mouse model. (A) In vivo effect of QYSL on Lewis lung tumors. Tumor growth curves of Lewis tumors treated with vehicle control or drugs. (B) Comparison of final tumor weights in each group after a 14 d treatment period. Numbers in columns indicate the mean tumor weight in each group. (C) Body weight measurements of transplanted mice
3.2 QYSL Induces an Anti-Cancer Immune Environment in Lung Cancer
Immune microenvironment is very important to tumor occurrence, development, and drug response, especially activated T cells and macrophages. Current studies have confirmed that M2 macrophages can promote tumor growth, and M1 cells can inhibit tumor growth. Therefore, we want to explore the effects of QYSL on T cells and macrophages. In order to examine the effects of QYSL on T cell and macrophage, flow cytometry was used to examine expression of cluster of differentiation 3 (CD3), CD69, and CD8 for CD8+ T cell, and examine expression of CD86 and CD206 for macrophages. The results shown that total T cells and CD8+ T cells were significantly increased in QYSL group (Figs. 2A and 2B). CD8+ T cells are the main effector cells in the body against tumors and other pathogenic microorganisms. Our results demonstrate that QYSL could exert tumor-killing effects by activating CD8 T cells. We also found that compared with vehicle group, the ratio of M2/M1 was significantly decreased in QYSL group (Fig. 2C). To further confirmed the effect of QYSL on macrophage, we examined the related factors expressed by M1 and M2 macrophages. The result shown that M1 macrophage related factors were increased, such as tumor necrosis factor α (TNF-α), interleukin 1 (IL-1), and IL-12, M2 macrophage related factors were decreased, such as CXC chemokine ligand 9 (CXCL-9) and CC chemokine ligand 17 (CCL-17) (Figs. 2D–2I). M2 macrophages promote tumor growth, while M1 macrophages inhibit tumor growth. These results indicated that QYSL inhibits tumor growth by promoting T cell activation and promoting macrophage polarization to M1 type.
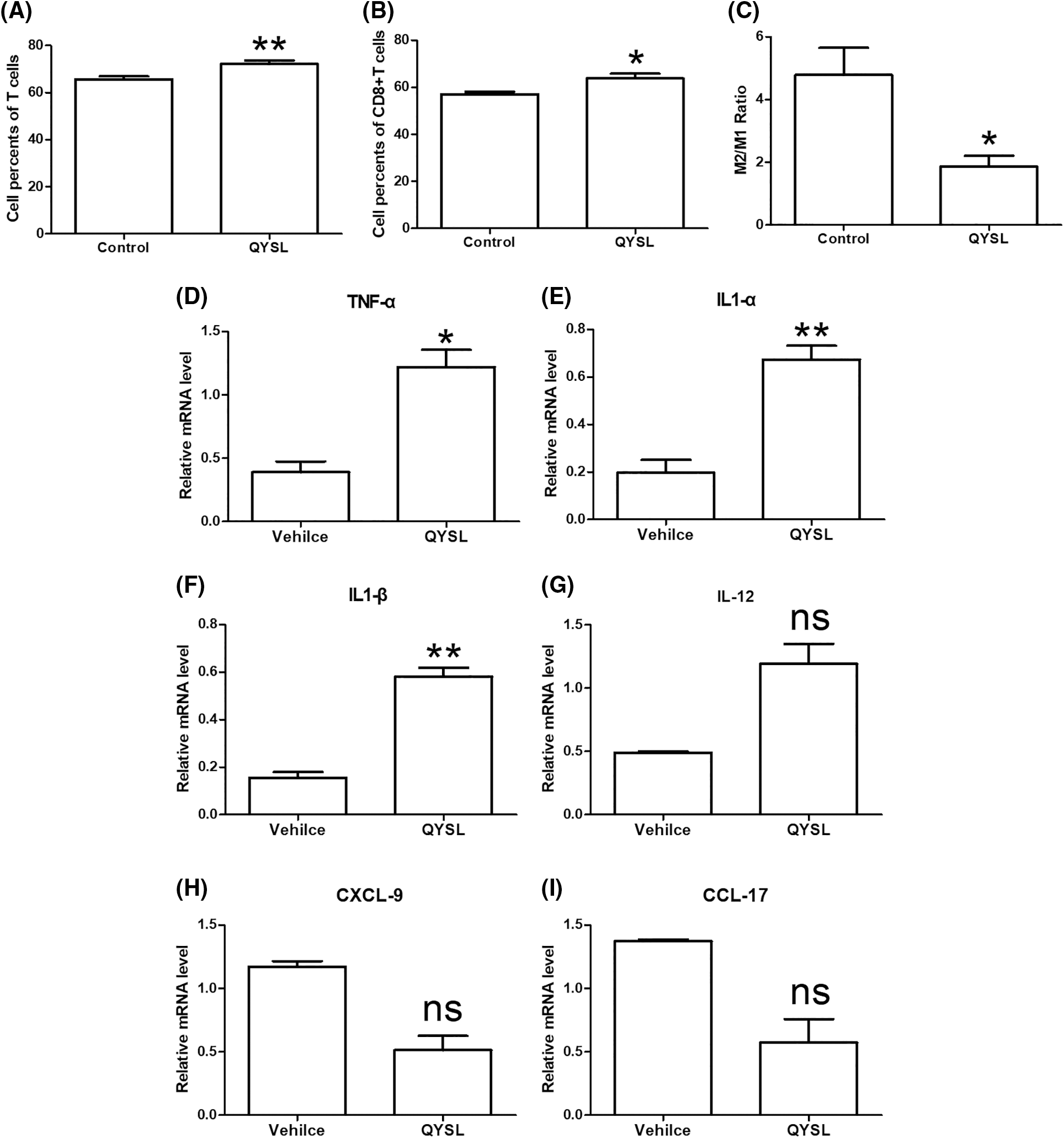
Figure 2: QYSL induces an anti-cancer immune environment in lung cancer. Mice with subcutaneous tumors were dealt with SPSS and QYSL. (A) Total T cell, (B) CD8+ T cell, (C) macrophage, (D–G) M1 macrophage related factors, and (H, I) M2 macrophage related factors in tumor tissue were measured by flow cytometry. Results are mean ± SD of three independent experiments; ns, not statistically significant; (*) p < 0.05, (**) p < 0.01, and (***) p < 0.001
3.3 QYSL Inhibits M2 Macrophage Related Signaling Pathways
JAK/STAT and MTOR are known to be important molecules involved in M2 polarization. To investigate the molecular mechanism underlying QYSL decreasing M2 macrophages, we examined the effect of QYSL on STAT1, STAT3, STAT6, PETN and MTOR. We found that QYSL potently inhibits mRNA level of MTOR and STAT6 (Fig. 3A). we also examined the level of protein with western blot. We also observed that the level of MTOR and STAT6 protein were significantly decreased in QYSL group (Figs 3B and 3C). These results suggested that QYSL promoting macrophage polarization to M1 type possibly through inhibiting MTOR and STAT6 expression.
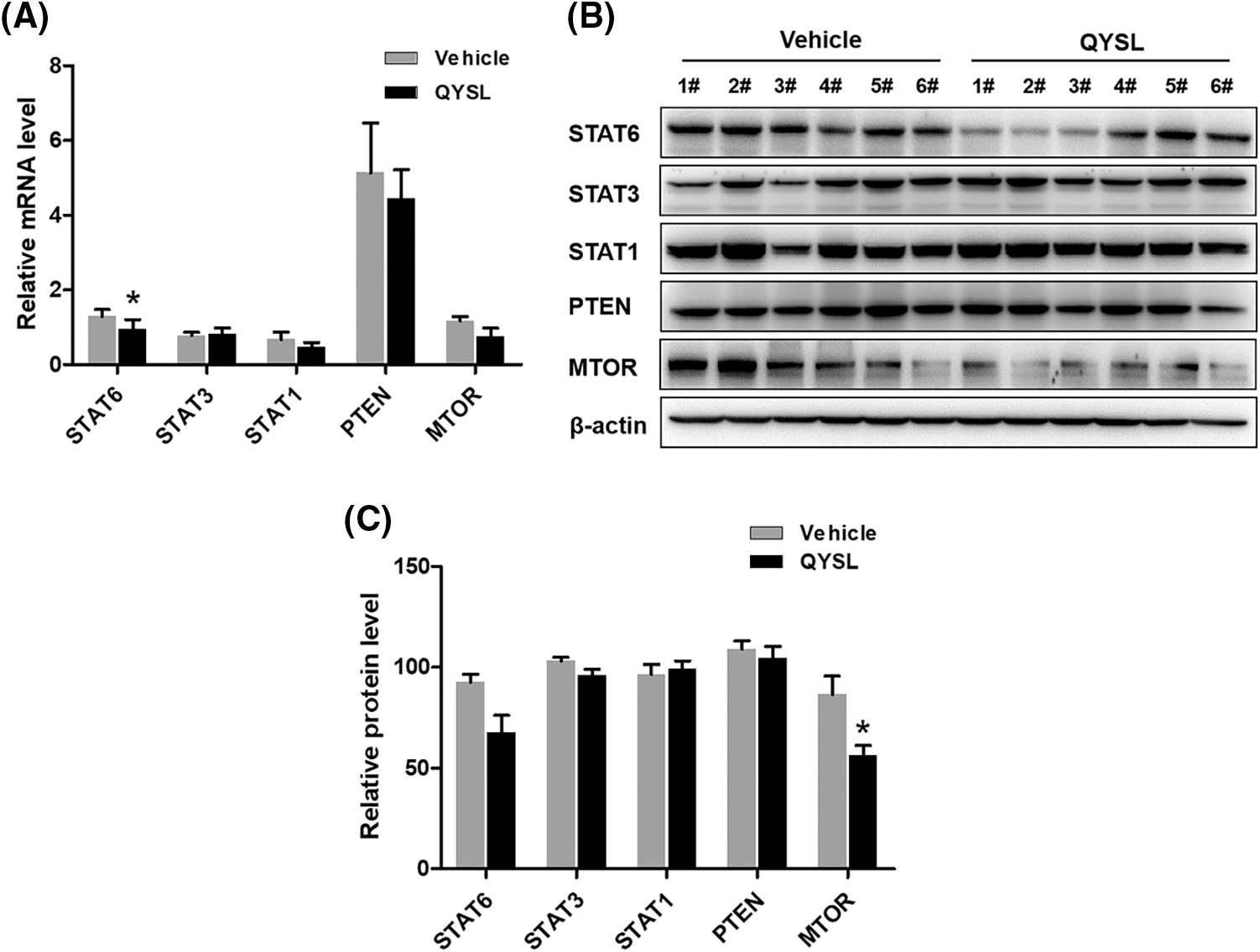
Figure 3: QYSL inhibits M2 macrophage related signaling pathways. Mice with subcutaneous tumors were dealt with SPSS and QYSL. (A) mRNA levels of indicated proteins in tumor tissue were measured by RT-PCR. Results are mean ± SD of three independent experiments. ns, not statistically significant, (*) p < 0.05, (**) p < 0.01, and (***) p < 0.001. (B) The expression of indicated proteins in tumor tissue was measured by western blot. 1#–6# represent tumor samples taken from mice. (C) The level of indicated proteins is quantified by image J
3.4 QYSL Combined with Anti-Tumor Agents Significantly Prevented Tumor Progression in Clinical
QYSL is a commonly used and important formula for the treatment of lung cancer in our department, which has been proved to have good anti-tumor effect. In this study, we share four clinical cases.
A 61 years old female patient had cough and expectoration for more than 2 months. Chest enhanced computed tomography (CT) showed a space occupying lesion in the lower lobe of the left lung, considering lung cancer, with multiple metastasis of mediastinum, bilateral hilar and bilateral supraclavicular lymph nodes. The pathological results showed that the metastatic small cell carcinoma of the right supraclavicular lymph node was likely to originate from the lung. Immunohistochemical results were CK (+), CK7 (−), TTF-1 (+), napsina (−), CK5/6 (−), p63 (−), p40 (−), NSE (+), CD56 (+), CGA (−), syn (+), Cdx-2 (−), CK20 (−), ALK (−), Ki67 (60% +). This patient had received a regimen with Etoposide injection 0.1 g for 9 days, Carboplatin Injection 300 mg for 3 days, combined with Sintilimab 200 mg immunotherapy and QYSL prescription. Finally, this patient was in partial remission (Fig. 4A).
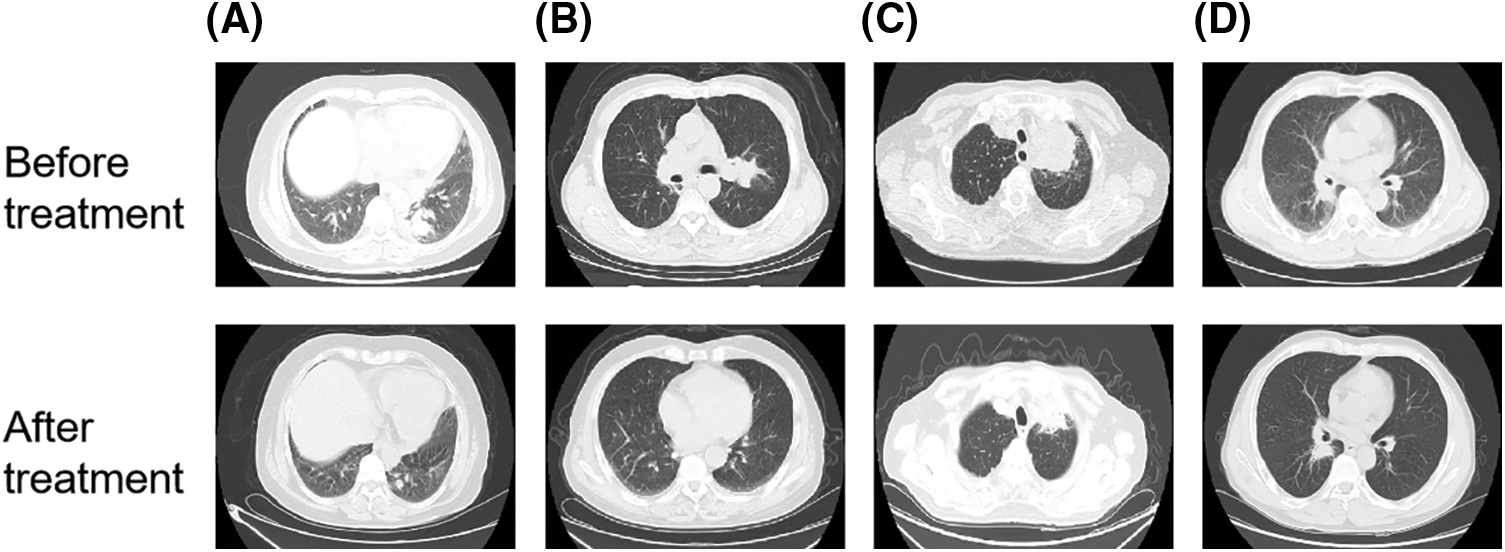
Figure 4: QYSL combined with anti-tumor agents significantly prevented tumor progression in clinical. (A–D) CT image comparison of lung cancer patients before and after combined treatment with QYSL
A 73 years old male patient went to Anhui provincial hospital because no obvious inducement to lose weight. Chest and abdominal CT showed that the right upper lobe mediastinum was occupied with obstructive pneumonia. Considering the possibility of tumor, further examination is recommended. The pathological results of bronchoscopy showed that scattered heterology epithelial cells were found, which was consistent with squamous cell carcinoma. Immunohistochemical results were p40 (+), CK7 (−), TTF-1 (−), Ki67 (60% +). After that, radiotherapy for lung lesions and lymphatic drainage area was started. Gross tumor volume (GTV): 6000cGy/30f, 200cGy/f. The patient received radiotherapy for 22 times and refused to continue radiotherapy because adverse radiation reactions. After discharge, the patient went to our outpatient department for intermittent QYSL prescription treatment. On August 03, 2020, the patient rechecked chest CT. The results showed that the right lung cancer with right pulmonary obstructive pneumonia had unclear boundary with the superior vena cava, and the mass shrank. On August 12, 2020, the patient was admitted to our department. Considering that the patient was lung squamous cell carcinoma and the gene test was negative, the patient had received a regimen with recombinant human endostatin 210 mg 2 ml/h micropump, tegafur bid d1-21, Sintilimab 200 mg, combined with QYSL prescription. Finally, This patient was in partial remission (Fig. 4B).
The 84 years old male patient had chest pain without obvious inducements, which was more obvious on the left. Chest CT showed a space occupying lesion in the posterior segment of the upper lobe tip of the left lung (maximum diameter 5.9 cm), considering lung cancer. On September 02, 2021, the patient was admitted to our department. Lung puncture pathology showed that it was squamous cell carcinoma. After consultation, the thoracic surgery and radiotherapy department would consider that the patient was old, with chronic bronchitis, emphysema and pulmonary bullae, which was not suitable for surgery and radiotherapy. The gene detection showed that there was no mutation in EGFR, ALK, ROS1, and NTRK. Then, this patient had received a regimen with Tislelizumab 100 mg d0, ENDOSTAR 210 mg was continuously micropumped for 7 days Q3W, combined with QYSL prescription. Finally, this patient was in partial remission (Fig. 4C).
A 58 years old male patient coughed and expectorated for more than 1 month. Bronchoscopy showed that new organisms blocked the lumen of the middle bronchus of the right lung. The new biopsies of the middle bronchus of the right lung were sent for pathological examination. The results showed that it was moderately to poorly differentiated squamous cell carcinoma. Immunohistochemical results were CK (+), p63 (+), p40 (+), CK5/6 (+), CK7 (−), TTF-1 (−), napsin-a (−), Ki67 (70% +), ALK (d5f3) (−). This patient had received a regimen with ENDOSTAR continuous micropump, Tislelizumab 200 mg ivgtt, tegafur 40 mg bid po, anti-vascular combined with immune regimen, combined with QYSL prescription. The patient was in stable condition during treatment (Fig. 4D).
Overall, the clinical application results show that the combination of QYSL and other drugs can achieve better clinical therapeutic effect. And because of the strengthening effect of traditional Chinese medicine, the side effects of patients treated with QYSL are significantly reduced.
As the most common malignant tumor, lung cancer has the characteristics of easy metastasis and recurrence [28–32]. Therefore, how to prolong the life of patients and improve the quality of life has become an urgent problem to be solved.
The current treatment of lung cancer mainly includes surgery, radiotherapy, chemotherapy, targeted therapy and immunotherapy. In addition, traditional Chinese medicine also plays an important role in the clinical treatment of lung cancer. Sui et al. [33] found Selaginella could Activate the mitochondrial apoptosis pathway and reduce Ki67 expression to inhibit the growth of NSCLC cells. Xu et al. [34] found Lianjia Shengjiefang could regulate EGFR and p53 signaling pathway to inhibit the proliferation of lung cancer cells. Lee et al. [35] found Calotropis gigantea could stimulate the intrinsic and extrinsic signal transduction pathways of NSCLC cells to induce apoptosis. Reno et al. [36] found Tripterygium wilfordii could inhibit focal adhesion kinase, which can lead to dysregulation of the migration mechanism. Our previous study show that QYSL could Induce autophagy through mTOR signaling pathway, and inhibit Wnt/β-catenin pathway in NSCLC cells and xenografts to inhibit the progress of NSCLC [37,38].
Modern studies have found that in the process of anti-tumor immunity, TME is the main place for the body’s immune system to exert anti-tumor immune response. According to the theory of “cancer immune editing”, infiltrating immune inflammatory cell occupies an important position in TME, and their functions are diverse. By reshaping the tumor microenvironment, tumor cell immune escape can be inhibited [39].
CD8+ T cell can kill antigen expressed cells, which is an important effector cell for anti-infection, acute allograft rejection and killing tumor cells. effector killer T cells exist in normal bodies as inactive resting T cells. Therefore, it can differentiate into effector killer T cell (TC) only after antigen activation and Th cell synergy. The activation of killer T cells also requires dual signaling. Firstly, T cell receptor (TCR) binds to the complex of major histocompatibility complex (MHC) molecules and antigen peptides on the target cell membrane, and delivers the first signal through the CD3 complex molecule. Accesscursory molecules such as CD2, LFA-1, CD8 and CD28 can be combined with corresponding ligand molecules such as LFA-3, ICAM-1, MHC class I molecules and B7 molecules on TC cells. It not only enhanced the adhesion between TC cells and target cells, but also delivered costimulatory signal to TC cells to activate them [40,41]. In this study, we investigated the effect of QYSL on CD8 T cells. The result showed that QYSL significantly promoted the activation of CD8 T cells. It indicated that QYSL can inhibit tumor growth by promoting CD8 T cell-mediated immune response.
In TME, M1 and M2 macrophages play different important roles through a variety of mechanisms. M1 macrophages highly express IL-12 and IL-23, produce killer molecules such as nitric oxide (NO), reactive oxygen species (ROS), a variety of proinflammatory cytokines (IL-1, IL-6, IL-13 and TNF-α), and chemokines (CCL2, CCL3, CXCL-10) [42]. It was found that M1 macrophage polarization can significantly up-regulate the expression of CXCL10 and IL-6, cause acute inflammatory environment, and then reshape immune microenvironment, so as to inhibit the aggregation of inhibitory cells and enhance anti-tumor immune response [43]. We investigated the effect of QYSL on M1 macrophage related factors. The result showed that QYSL significantly promoted the expression of TNF-α, IL-1, and IL-12. These results suggest that QYSL inhibits tumor growth by promoting the polarization of M1 macrophages.
M2 macrophages can promote tumor progression by releasing various cytokines and signal molecules. M2 macrophages can release VEGF, PDGF and other factors to stimulate the formation of abnormal blood vessels and lymphatic vessels. M2 macrophages can release matrix metalloproteinase 2 (MMP2), MMP9 and other molecules to degrade extracellular matrix and promote tumor escape and progression. And, M2 macrophages also secrete IL-6 to promote the growth of tumor stem cells, and leads to the production of chemoresistant cancer cells. On the other hand, M2 macrophages inhibit the anti-tumor immune function of the body through a variety of mechanisms. Arginase-1 (Arg-1), IL-10, CCL17, CCL24, and TGF-β were highly expressed in M2 macrophages, iNOS, IL-6 and TNF-α were low expressed in M2 macrophages. Arg-1 can mediate the depletion of L-arginine, resulting in the loss of CD3ζ chain expression in T cell receptor complex, thus inhibiting the activation of effector T cells [44]. IL-10 and TGF-β secreted by macrophages can further interfere with the function of cytotoxic CD8+ T and CD4+ T cells, so as to inhibit the self-renewal of TME. M2 macrophages can also regulate PI3K γ pathway to inhibit the activation of cytotoxic CD8+ T cells [45]. In addition, M2 macrophages also inhibit the infiltration of CD8+ T cells by reducing the expression of CXCL9 and CXCL10, and can induce dysfunction of tumor infiltrating effector T cells by expressing PD-1 ligand. It is correlated with the prognosis of patients with malignant tumor, such as lung cancer [46].
In this study, we found that the M2 macrophages were increased in tumor and QYSL inhibited tumor growth in the tumor mice model through decreasing activation of M2. We further investigated the mechanism of QYSL regulating macrophage. RT-PCR results showed that QYSL significantly down-regulated the expression of M2 macrophage related genes, such as CXCL-9 and CCL-17. STAT6 and MTOR play important roles in the polarization of M2 macrophages. To further demonstrate whether QYSL inhibited M2-type macrophage polarization by affecting polarization-related signaling pathways, we used RT-PCR and western blot experiments to detect the expressions of STAT1, STAT3, STAT6, PTEN and MTOR proteins. Our results showed that QYSL inhibited the expression of STA6 and MTOR, effectively inhibiting M2 macrophage polarization. Overall, QYSL inhibits tumor growth by inhibiting polarization of M2 macrophages and regulating tumor immune microenvironment.
At present, there are more and more clinical applications of traditional Chinese medicine. The combination of traditional Chinese medicine and anti-tumor drugs can often relieve clinical symptoms, improve the quality of life and the efficacy of chemotherapeutic drugs in clinical practice. It can be seen from the cases provided by us that QYSL combined with anti-tumor drugs has a good therapeutic effect on small cell lung cancer with poor prognosis, especially for poorly differentiated small cell lung cancer. In recent years, for patients with advanced cancer, our department has used QYSL combined with chemotherapy or immunotherapy or radiotherapy to greatly alleviate the side effect of radiotherapy, chemotherapy and immunotherapy, and at the same time enhance the patient’s physique, while ensuring shrinkage of tumor. This lays a solid foundation for radical surgery.
In conclusion, QYSL can inhibit the tumor growth of lung cancer by enhancing the activation of CD8 T cell and polarization of M2 macrophages to M1 macrophages.
Which component in QYSL plays a key role has not been studied.
This study found that QYSL potently inhibited the growth of lung cancer by promoting T cell activation and M2 macrophage polarization to M1, which was found to be associated with the STAT6 and MTOR signaling pathway.
Acknowledgement: We are grateful to Hefei PreceDo Pharmaceuticals Co., Ltd. for their technical support.
Author Contributions: PL and ZGL designed the research. CY and HW developed the methodology. CY, HW, ANJ, JBT, JZ, and MZ performed the experiments and collected the data. CY wrote the manuscript. CY and HW provided the experiment materials. PL and ZGL revised the manuscript.
Ethics Approval and Informed Consent Statement: This study was approved by the Ethics Committee of the First Affiliated Hospital of Anhui medical university with the No. PJ2021-08-08. Written informed consent was obtained from the patients for publication of the related data.
Availability of Data and Materials: The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Funding Statement: This work was supported by the National Natural Science Foundation of China (Grants 82004314, 81874431), the Clinical Research Initiation Plan of the First Affiliated Hospital of Anhui Medical University (Grant LCYJ2021YB017), the Beijing Xisike Clinical Oncology Research Foundation.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Ikonomidis, I., Michalakeas, C. A., Parissis, J., Paraskevaidis, I., Ntai, K. et al. (2012). Inflammatory markers in coronary artery disease. BioFactors, 38(5), 320–328. DOI 10.1002/biof.1024. [Google Scholar] [CrossRef]
2. Liu, B. L., Quan, X. Y., Xu, C. G., Lv, J. C., Li, C. et al. (2019). Lung cancer in young adults aged 35 years or younger: A full-scale analysis and review. Journal of Cancer, 10(15), 3553–3559. DOI 10.7150/jca.27490. [Google Scholar] [CrossRef]
3. Hurria, A., Kris, M. G. (2003). Management of lung cancer in older adults. CA: A Cancer Journal for Clinicians, 53, 325–341. DOI 10.3322/canjclin.53.6.325. [Google Scholar] [CrossRef]
4. Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I. et al. (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians, 71(3), 209–249. DOI 10.3322/caac.21660. [Google Scholar] [CrossRef]
5. Zhou, C. C. (2014). Lung cancer molecular epidemiology in China: Recent trends. Translational Lung Cancer Research, 3(5), 270–279. DOI 10.3978/j.issn.2218-6751.2014.09.01. [Google Scholar] [CrossRef]
6. Gao, S. G., Li, N., Wang, S. H., Zhang, F., Wei, W. Q. et al. (2020). Lung cancer in People’s Republic of China. Journal of Thoracic Oncology, 15(10), 1567–1576. DOI 10.1016/j.jtho.2020.04.028. [Google Scholar] [CrossRef]
7. She, J., Yang, P., Hong, Q. Y., Bai, C. X. (2013). Lung cancer in China: Challenges and interventions. Chest, 143(4), 1117–1126. DOI 10.1378/chest.11-2948. [Google Scholar] [CrossRef]
8. Ni, J., Zhang, L. (2021). Progress in treatment of non-small cell lung cancer harboring HER2 aberrations. OncoTargets and Therapy, 14, 4087–4098. DOI 10.2147/OTT.S312820. [Google Scholar] [CrossRef]
9. Wu, S. Y., Pan, Y., Mao, Y. Y., Chen, Y., He, Y. Y. (2021). Current progress and mechanisms of bone metastasis in lung cancer: A narrative review. Translational Lung Cancer Research, 10(1), 439–451. DOI 10.21037/tlcr. [Google Scholar] [CrossRef]
10. Rubio, X. M., Uribelarrea, E. A., Cortés, L. Q., Moyano, M. S. (2021). Immunotherapy in non-small cell lung cancer: Update and new insights. Journal of Clinical and Translational Research, 7(1), 1–21. DOI 10.18053/jctres.07.202101.001. [Google Scholar] [CrossRef]
11. Shanker, M., Willcutts, D., Roth, J. A., Ramesh, R. (2010). Drug resistance in lung cancer. Lung Cancer (Auckl), 1, 23–36. DOI 10.1097/00001622-199903000-00006. [Google Scholar] [CrossRef]
12. Kim, E. S. (2016). Chemotherapy resistance in lung cancer. Advances in Experimental Medicine and Biology, 893, 189–209. DOI 10.1007/978-3-319-24223-1. [Google Scholar] [CrossRef]
13. Iglesias, V. S., Giuranno, L., Dubois, L. J., Theys, J., Vooijs, M. (2018). Drug resistance in non-small cell lung cancer: A potential for NOTCH targeting? Frontiers in Oncology, 8, 267. DOI 10.3389/fonc.2018.00267. [Google Scholar] [CrossRef]
14. Liu, W. J., Du, Y., Wen, R., Yang, M., Xu, J. (2020). Drug resistance to targeted therapeutic strategies in non-small cell lung cancer. Pharmacology & Therapeutics, 206, 107438. DOI 10.1016/j.pharmthera.2019.107438. [Google Scholar] [CrossRef]
15. Altorki, N. K., Markowitz, G. J., Gao, D. C., Port, J. L., Saxena, A. et al. (2019). The lung microenvironment: An important regulator of tumour growth and metastasis. Nature Reviews Cancer, 19, 9–31. DOI 10.1038/s41568-018-0081-9. [Google Scholar] [CrossRef]
16. Mittal, V., Rayes, T. E., Narula, N., McGraw, T. E., Altorki, N. K. et al. (2016). The microenvironment of lung cancer and therapeutic implications. Advances in Experimental Medicine and Biology, 890, 75–110. DOI 10.1007/978-3-319-24932-2. [Google Scholar] [CrossRef]
17. Díaz, B. C., Botello, D. R., Ostrosky, T. W., Soto, G. R., Sánchez, E. O. et al. (2020). Tumor microenvironment differences between primary tumor and brain metastases. Journal of Translational Medicine, 18(1), 1. DOI 10.1186/s12967-019-02189-8. [Google Scholar] [CrossRef]
18. Jain, P., Jain, C., Velcheti, V. (2018). Role of immune-checkpoint inhibitors in lung cancer. Therapeutic Advances in Respiratory Disease, 12, 1753465817750075. DOI 10.1177/1753465817750075. [Google Scholar] [CrossRef]
19. Gan, J. D., Huang, Y. H., Fang, W. F., Zhang, L. (2021). Research progress in immune checkpoint inhibitors for lung cancer in China. Therapeutic Advances in Medical Oncology, 13, 7–33. DOI 10.1177/17588359211029826. [Google Scholar] [CrossRef]
20. Zhang, Q., Tang, L. S., Zhou, Y. W., He, W. B., Li, W. M. (2021). Immune checkpoint inhibitor-associated pneumonitis in non-small cell lung cancer: Current understanding in characteristics, diagnosis, and management. Frontiers in Immunology, 12, 663986. DOI 10.3389/fimmu.2021.663986. [Google Scholar] [CrossRef]
21. Zhou, J. W., Tang, Z. W., Gao, S. Y., Li, C. Y., Feng, Y. T. et al. (2020). Tumor-associated macrophages: Recent insights and therapies. Frontiers in Oncology, 10, 188. DOI 10.3389/fonc.2020.00188. [Google Scholar] [CrossRef]
22. He, Z. C., Zhang, S. X. (2021). Tumor-associated macrophages and their functional transformation in the hypoxic tumor microenvironment. Frontiers in Immunology, 12, 741305. DOI 10.3389/fimmu.2021.741305. [Google Scholar] [CrossRef]
23. Quatromoni, J. G., Eruslanov, E. (2012). Tumor-associated macrophages: Function, phenotype, and link to prognosis in human lung cancer. American Journal of Translational Research, 4(4), 376–389. DOI 10.1016/s0169-5002(87)80442-7. [Google Scholar] [CrossRef]
24. Xiang, Y. N., Guo, Z. M., Zhu, P. F., Chen, J., Huang, Y. Y. (2019). Traditional Chinese medicine as a cancer treatment: Modern perspectives of ancient but advanced science. Cancer Medicine, 8(5), 1958–1975. DOI 10.1002/cam4.2108. [Google Scholar] [CrossRef]
25. Liu, J., Wang, S., Zhang, Y., Fan, H. T., Lin, H. S. (2015). Traditional Chinese medicine and cancer: History, present situation, and development. Thoracic Cancer, 6(5), 561–569. DOI 10.1111/1759-7714.12270. [Google Scholar] [CrossRef]
26. Wang, S. M., Long, S. Q., Deng, Z. Y., Wu, W. Y. (2020). Positive role of Chinese herbal medicine in cancer immune regulation. The American Journal of Chinese Medicine, 48(7), 1577–1592. DOI 10.1142/S0192415X20500780. [Google Scholar] [CrossRef]
27. Liao, X., Bu, Y., Jia, Q. G. (2020). Traditional Chinese medicine as supportive care for the management of liver cancer: Past, present, and future. Genes & Diseases, 7(3), 370–379. DOI 10.1016/j.gendis.2019.10.016. [Google Scholar] [CrossRef]
28. Popper, H. H. (2016). Progression and metastasis of lung cancer. Cancer and Metastasis Reviews, 35, 75–91. DOI 10.1007/s10555-016-9618-0. [Google Scholar] [CrossRef]
29. Riihimäki, M., Hemminki, A., Fallah, M., Thomsen, H., Sundquist, K. et al. (2014). Metastatic sites and survival in lung cancer. Lung Cancer, 86(1), 78–84. DOI 10.1016/j.lungcan.2014.07.020. [Google Scholar] [CrossRef]
30. Chuang, C. H., Greenside, P. G., Rogers, Z. N., Brady, J. J., Yang, D. et al. (2017). Molecular definition of a metastatic lung cancer state reveals a targetable CD109-Janus kinase-Stat axis. Nature Medicine, 23(3), 291–300. DOI 10.1038/nm.4285. [Google Scholar] [CrossRef]
31. Consonni, D., Pierobon, M., Gail, M. H., Rubagotti, M., Rotunno, M. et al. (2015). Lung cancer prognosis before and after recurrence in a population-based setting. Journal of the National Cancer Institute, 107(6), djv059. DOI 10.1093/jnci/djv059. [Google Scholar] [CrossRef]
32. Sasaki, H., Suzuki, A., Tatematsu, T., Shitara, M., Hikosaka, Y. et al. (2014). Prognosis of recurrent non-small cell lung cancer following complete resection. Oncology Letters, 7(4), 1300–1304. DOI 10.3892/ol.2014.1861. [Google Scholar] [CrossRef]
33. Sui, Y. X., Li, S. G., Shi, P. Y., Wu, Y. J., Li, Y. X. et al. (2016). Ethyl acetate extract from Selaginella doederleinii Hieron inhibits the growth of human lung cancer cells A549 via caspase-dependent apoptosis pathway. Journal of Ethnopharmacology, 190, 261–271. DOI 10.1016/j.jep.2016.06.029. [Google Scholar] [CrossRef]
34. Xu, L., Li, H. G., Xu, Z. Y., Wang, Z. Q., Liu, L. S. et al. (2012). Multi-center randomized double-blind controlled clinical study of chemotherapy combined with or without traditional Chinese medicine on quality of life of postoperative non-small cell lung cancer patients. BMC Complementary and Alternative Medicine, 12, 112. DOI 10.1186/1472-6882-12-112. [Google Scholar] [CrossRef]
35. Lee, J. Y., Jang, H. J., Chun, H., Pham, T., Bak, Y. et al. (2019). Calotropis gigantea extract induces apoptosis through extrinsic/intrinsic pathways and reactive oxygen species generation in A549 and NCI-H1299 non-small cell lung cancer cells. BMC Complementary and Alternative Medicine, 19(1), 134. DOI 10.1186/s12906-019-2561-1. [Google Scholar] [CrossRef]
36. Reno, T. A., Kim, J. Y., Raz, D. J. (2015). Triptolide inhibits lung cancer cell migration, invasion, and metastasis. The Annals of Thoracic Surgery, 100(5), 1817–1824. DOI 10.1016/j.athoracsur.2015.05.074. [Google Scholar] [CrossRef]
37. Gao, Y. T., Wang, X. H., Yang, Q. J., Wang, X. L., Zhang, X. X. et al. (2021). Qiyusanlong formula induces autophagy in non-small-cell lung cancer cells and xenografts through the mTOR signaling pathway. Evidence-based Complementary and Alternative Medicine, 2021, 5575453. DOI 10.1155/2021/5575453. [Google Scholar] [CrossRef]
38. Tong, J. B., Zhang, X. X., Wang, X. H., Zeng, S. J., Wang, D. Y. et al. (2018). Qiyusanlong decoction suppresses lung cancer in mice via Wnt/β-catenin pathway. Molecular Medicine Reports, 17(4), 5320–5327. DOI 10.3892/mmr.2018.8478. [Google Scholar] [CrossRef]
39. Tavakoli, F., Sartakhti, J. S., Manshaei, M. H., Basanta, D. (2021). Cancer immunoediting: A game theoretical approach. Silico Biology, 14(1–2), 1–12. DOI 10.3233/ISB-200475. [Google Scholar] [CrossRef]
40. Hashimoto, M., Kamphorst, A. O., Im, S. J., Kissick, H. T., Pillai, R. N. et al. (2018). CD8 T cell exhaustion in chronic infection and cancer: Opportunities for interventions. Annual Review of Medicine, 69, 301–318. DOI 10.1146/annurev-med-012017-043208. [Google Scholar] [CrossRef]
41. Leun, A. M., Thommen, D. S., Schumacher, T. N. (2020). CD8+ T cell states in human cancer: Insights from single-cell analysis. Nature Reviews Cancer, 20(4), 218–232. DOI 10.1038/s41568-019-0235-4. [Google Scholar] [CrossRef]
42. Liu, J. Y., Geng, X. F., Hou, J. X., Wu, G. S. (2021). New insights into M1/M2 macrophages: Key modulators in cancer progression. Cancer Cell International, 21, 389. DOI 10.1186/s12935-021-02089-2. [Google Scholar] [CrossRef]
43. Martin, E. M., Mellows, T. W., Clarke, J., Ganesan, A. P., Wood, O. et al. (2020). M1 hot tumor-associated macrophages boost tissue-resident memory T cells infiltration and survival in human lung cancer. Journal for ImmunoTherapy of Cancer, 8(2), e000778. DOI 10.1136/jitc-2020-000778. [Google Scholar] [CrossRef]
44. Rőszer, T. (2015). Understanding the mysterious M2 macrophage through activation markers and effector mechanisms. Mediators of Inflammation, 2015, 816460. DOI 10.1155/2015/816460. [Google Scholar] [CrossRef]
45. Pu, J., Xu, Z. M., Nian, J. H., Fang, Q., Yang, M. et al. (2021). M2 macrophage-derived extracellular vesicles facilitate CD8+ T cell exhaustion in hepatocellular carcinoma via the miR-21-5p/YOD1/YAP/β-catenin pathway. Cell Death Discovery, 7, 182. DOI 10.1038/s41420-021-00556-3. [Google Scholar] [CrossRef]
46. Dhupkar, P., Gordon, N., Stewart, J., Kleinerman, E. S. (2018). Anti-PD-1 therapy redirects macrophages from an M2 to an M1 phenotype inducing regression of OS lung metastases. Cancer Medicine, 7(6), 2654–2664. DOI 10.1002/cam4.1518. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |