
 | Sound & Vibration |  |
DOI: 10.32604/oncologie.2022.021705
REVIEW
Ubiquitin Specific Protease 2: Structure, Isoforms, Cellular Function, Related Diseases and Its Inhibitors
1School of Basic Medicine, Weifang Medical University, Weifang, 261053, China
2Central Laboratory, Shanghai Chest Hospital, Shanghai Jiao Tong University, Shanghai, 200030, China
3Hongqiao International Institute of Medicine, Shanghai Tongren Hospital, Faculty of Basic Medicine, Chemical Biology Division of Shanghai Universities E-Institutes, Key Laboratory of Cell Differentiation and Apoptosis of the Chinese Ministry of Education, Shanghai Jiao Tong University School of Medicine, Shanghai, 200025, China
*Corresponding Authors: Hao Luo. Email: luohao@wfmc.edu.cn; Yingli Wu. Email: wuyingli@shsmu.edu.cn
#Hao Luo and Yanjie Ji contributed equally to this work
Received: 30 January 2022; Accepted: 03 March 2022
Abstract: The ubiquitin-proteasome system (UPS) is an important pathway for cellular protein degradation. The components of this pathway, including the proteasome, ubiquitinase, and deubiquitinase, are highly specialized and strictly regulated. The ubiquitin-specific protease 2 (USP2) belongs to the ubiquitin-specific proteases, a subgroup of deubiquitinating enzymes. USP2 plays essential roles in regulating cell survival, cell cycle, circadian rhythm, cell metabolism, inflammatory response, antiviral response, and metastasis by interacting with certain proteins such as Cyclin D1, PER1, CRY1, HDM2/p53, FASN, LDLR, TRAF6, TBK1, and TGFBR1-TGFBR2 complex. Elevation of USP2 has been observed in a variety of cancers, including glioma, testicular cancer, breast cancer, prostate cancer, and some inflammatory diseases. Moreover, USP2 also plays an important role in many non-neoplastic diseases. At present, there is no officially approved USP2 inhibitor in clinic. A few existing inhibitors targeting USP2 have shown certain effects in the treatment of colorectal cancer, but their mechanism of action and binding site information are not clear. Moreover, the efficacy and selection specificity need to be further optimized. The catalytic centers of USP family are relatively conserved, so the design of compounds targeting allosteric site is expected to improve the specificity and inhibitory activity. In this review, we summarize the latest advances of USP2 in its cellular function, related diseases, and small-molecule inhibitors targeting USP2.
Keywords: USP2; structure; isoforms; cellular function; related diseases; inhibitors
The ubiquitin-proteasome system (UPS) executes the majority of protein degradation in eukaryotic cells. Specifically, the UPS-mediated protein degradation comprises two steps: conjugation and degradation. Meanwhile, the UPS also includes a large group of proteases, i.e., deubiquitinases (DUBs), which cleave ubiquitin from the substrate proteins and prevent them from being degraded. By regulating ubiquitination and deubiquitination, the UPS is widely involved in various critical cellular activities, such as cell growth, division, differentiation, and apoptosis [1]. Hence, the UPS is closely associated with various diseases and has become the target of many therapeutic strategies. For example, a series of proteasome inhibitors, such as bortezomib, ixazomib, have been developed and approved for cancer treatment. However, since proteasome is the converging point of lots signaling pathways, inhibiting proteasome usually suffers from drawbacks such as distant toxicity and drug resistance. To circumvent these problems, recent studies have turned to seek new targets from the upstream enzymes in the UPS, especially from DUBs, which possesses vast heterogeneity in both structure and function.
DUBs can be classified into six main superfamilies by structure, the largest one of which is the ubiquitin-specific protease (USP) family that has around 56 members in humans. While the substrates and functions of many USPs still remain unknown, several USPs have been intensely investigated as they play vital roles in disease-related signaling pathways. USP2, a member of the USP family, has three major isoforms and its special structure. Through interacting with and stabilizes a large number of substrates including HDM2/p53, Cyclin D1, PER1, Cry1, FASN, LDLR, TRAF6, TBK1, SKP2 and TGFBR1-TGFBR2 complex, USP2a affects diverse cellular functions such as cell survival, cell cycle, metastasis, metabolism and immune response [2,3]. The dysregulation of USP2 has also been observed in a variety of diseases, including glioma, some inflammatory diseases and testicular cancer. Furthermore, several USP2 inhibitors have been found to exhibit significant anticancer activity [4]. Therefore, USP2 has great potential to become a novel target that brings hope to the treatment of many diseases.
In this review, we aim to summarize recent advances in USP2 research from four aspects, including its structure and isoforms, cellular function, related diseases, and inhibitors. We will first introduce the basic information of USP2, such as its cellular localization and tissue distribution, and then we will show the different function of its isoforms. Subsequently, we will illustrate the essential cellular functions of USP2 with regard to its diverse substrates, followed by an in-depth discussion about how USP2 is associated with different diseases or pathological processes. Finally, we will review the inhibitors of USP2 and their effects in vitro and in vivo. We hope to provide a comprehensive picture of USP2 that inspires more exciting discoveries in both fundamental research and clinical application.
2 Structure and Isoforms of USP2
USP2 has three major isoforms, that share a similar structure composed of an N-terminal domain of variable length, and a 347-amino acid long C-terminal domain that contains the characteristic catalytic triad (Cys, His and Asp/Asn) of the USP family [5]. Specifically, USP2a has the largest N-terminal domain with 258 amino acids, while that of USP2b and USP2c has 49 and 15 amino acids, respectively. Note that these isoforms are produced by the alternative splicing of the USP2 mRNA, see Fig. 1 below. The isoform above has been chosen as the “canonical” sequence named USP2a. The sequence of USP2b differs from the canonical sequence as follows: 1-258: MSQLSSTLKR…RSSSPGRDGM→MRTSYTVTLP…FVGLLLNKAK. The sequence of USP2c isoform differs from the canonical sequence as follows: 1-258: MSQLSSTLKR…RSSSPGRDGM→MLVPGSTRPYSKKRQ. The C terminus, shared by USP2a, USP2b and USP2c, contains the canonical isopeptidase CYS and HIS boxes.
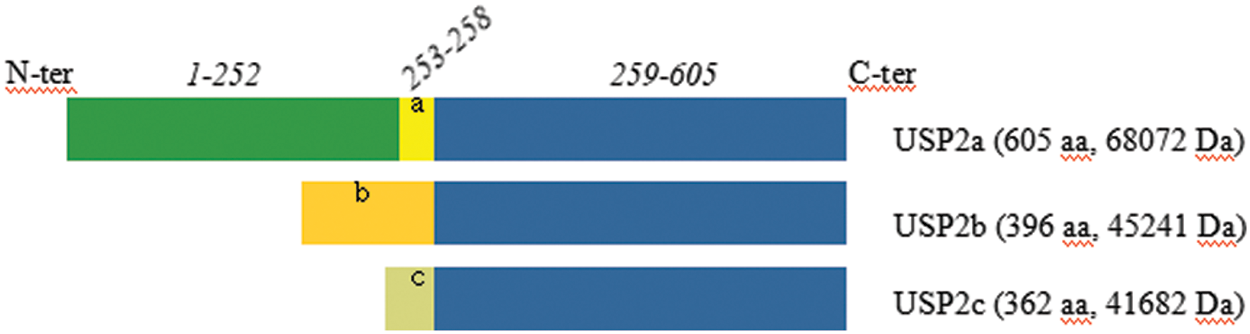
Figure 1: Comparison of amino acid sequences of different variants of human USP2
2.2 The Different Functions of USP2 Isoforms
Although it is not clear yet how the N-terminal domain affects the function of these isoforms, different USP2 isoforms may perform distinct cellular functions [6]. For example, Sendai virus-induced IFN-β activation in HEK293 cells can be inhibited by overexpressing USP2b and USP2c, but not USP2a [7]. Meanwhile, it has been found that specific USP2 isoforms may be selectively expressed in different tissues or organs in mouse and rat models. Further studies suggested that the alternative splicing of USP2 mRNA may be affected by factors including circadian rhythm, nutrition, and androgen. However, different USP2 isoforms may share similar subcellular locations, including cell nucleus, peroxisome, endoplasmic reticulum, and cytoplasm [8].
Despite the difference between the three isoforms, the C-terminal sequence of USP2 is highly conservative and serves as the proteolytic domain. X-ray structure analysis showed that the catalytic domain of USP2 is highly similar to that of USP7, forming a ‘cupped hand’ structure that nicely hosts ubiquitin. In particular, it was demonstrated that the core of ubiquitin interacts with the cupped hand of USP2, which aligns the C-terminus of ubiquitin at the active site cleft of USP2 for hydrolysis. It was also found that the interaction between USP2 and ubiquitin is mediated by an ordered layer of water molecules. Strikingly, SARS-CoV-2 papain-like protease (PLpro) and USP2 share similar structural scaffold and conserved catalytic triplets. The USP folding of these two proteases bears striking similarities to the right-handed thumb-palm-fingers structural scaffold [9]. Overall, USP2 preserves the typical features of the USP family, while its isoforms may differ slightly in structure, cellular function, and tissue distribution.
2.2.1 USP2a and USP2b Play Different Functions in Antiviral Immune Response
pY701-STAT1 was found to be the main form of IFN1-induced STAT1 ubiquitination. pY701-STAT1 is rapidly regulated by the ubiquitin-proteasome system. USP2a plays an important role in antiviral defense by inhibiting the ubiquitination of activated STAT1 in the nucleus and maintaining the antiviral activity of interferon (IFN) [10]. However, USP2b deubiquitinates TBK1 to terminate TBK1 activation and negatively regulate IFN-β signaling, resulting in the decrease of anti Sendai virus reaction in HEK293 cell [11].
2.2.2 USP2a and USP2b Play Different Roles in HCC
Currently, it remains controversial which isoforms of USP2 are expressed in the liver and the isoforms show different function in hepatocellular carcinoma (HCC). Xiong et al. reported that USP2a could stabilized RAB1A by deubiquitinating, which is required for HCC progression [12]. This indicated that targeting USP2a could be a feasible method to treat HCC. However, Christina et al. reported USP2b was significantly down-regulated in HCC and as the notable isoform of the liver [13]. They also found that USP2b exhibited tumorigenic activities through promoting cell proliferation. But on the other hand, USP2b could enhanced bile acid-induced apoptosis and necrosis significantly. Whether USP2b act as tumor promoter or suppressor depend on the cell context or status [13].
By deubiquitinating its substrates, USP2 regulates protein stability, intracellular localization, and protein-protein interactions. As USP2 interacts with a wide range of proteins that involve in various critical processes, resolving the fundamental cellular functions of USP2 is the basis of understanding its pathology and related therapeutics. Here, we introduce how USP2 affects different cellular activities, including cell survival, cell cycle, circadian clock, cell metabolism, metastasis, etc., see Fig. 2 below.
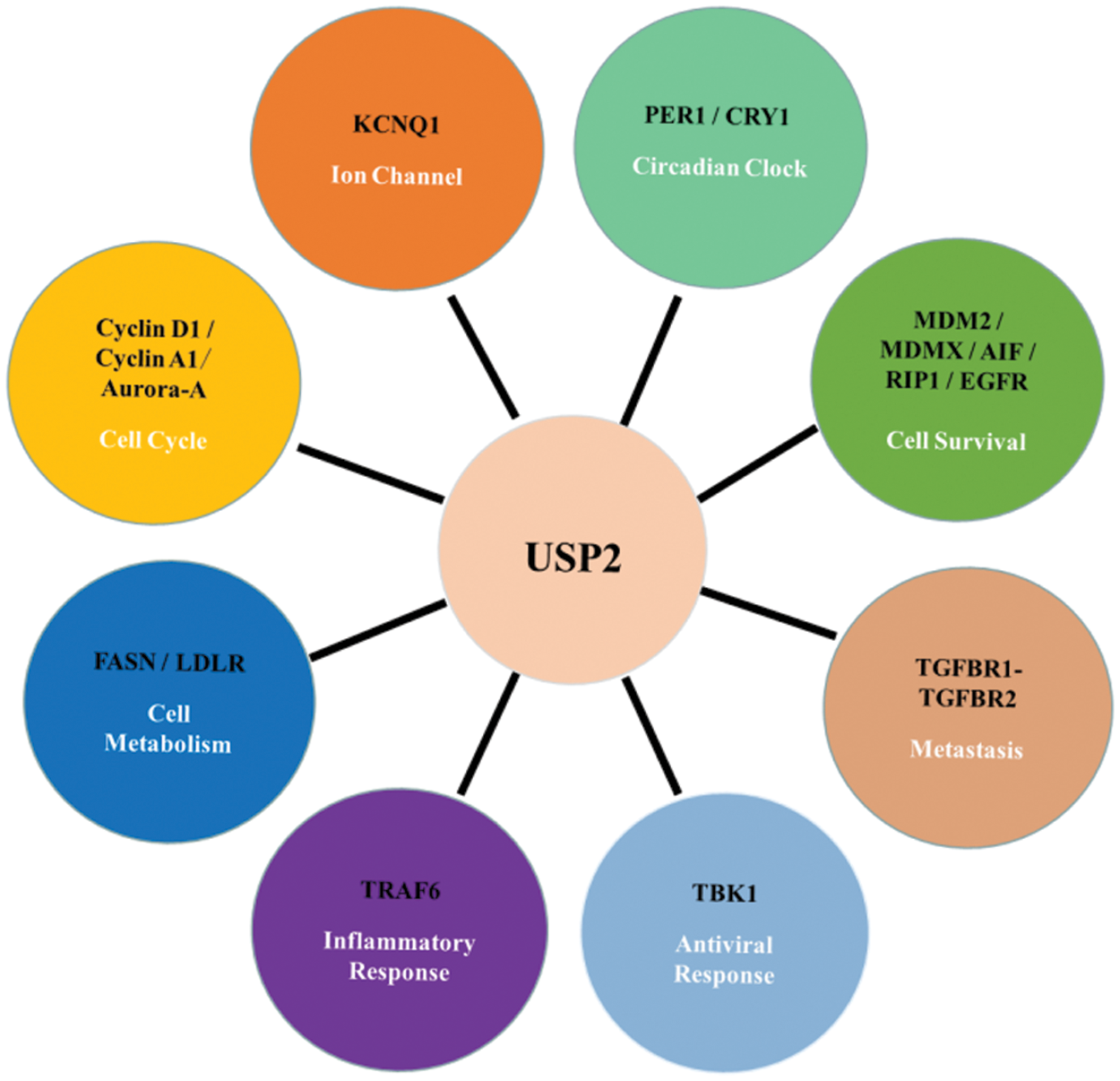
Figure 2: Cellular function of USP2 and its substrates
Ectopically expressed USP2 may either promote or inhibit cell survival through different pathways. For example, USP2 can deubiquitinate and stabilize HDM2, which consequently promotes HDM2-mediated p53 degradation hence inhibits the tumor cells apoptosis. Further studies reveal that USP2-induced p53 degradation downregulates a group of miRNAs and hence increases cellular MYC levels, which enhances prostate cancer cell survival [14]. Additionally, USP2a deubiquitinates the internalized EGFR on early lysosome surface and thus improves the survival of EGFR-dependent cancer cells, such as NSCLC cells. In contrast, USP2a and USP2c have been found to deubiquitinate and stabilize RIP1, the accumulation of which will lead to cell apoptosis [15]. In brief, USP2, or its specific isoforms, may play a double-sided role in cell survival via different mechanisms.
USP2 is involved in cell cycle control by interacting with key regulatory proteins. Specifically, USP2a binds to and stabilizes Cyclin D1, an essential regulatory molecule in G1/S cell cycle progression that is highly expressed in various tumor cells. Correspondingly, the inhibition of USP2a by lithocholic acid hydroxyamide induces G0/G1 arrest and the degradation of Cyclin D1 [16]. Studies have shown that Aurora-A, a threonine/serine kinase that regulates mitotic activities including chromosome separation, mitotic spindle formation, and centrosome maturation, is also a substrate of USP2 [17]. Saroj et al. also found that modulation of USP-2 expression plays a key role in cell cycle regulation by adipokines such as adiponectin and leptin [18]. These functions suggest that targeting USP2 may be a novel anti-cancer strategy via cell cycle manipulation.
USP2 regulates the circadian clock by regulating the internal circadian rhythm and the ability to respond to external signals. USP2 regulates the function of PER1 by controlling the entry and accumulation of its nucleus, thereby regulating the expression of clock-controlled genes. Moreover, USP2 improves the stability of CRY1 protein in serum by de-ubiquitination, so as to inhibit the expression of endogenous PER2 gene and gene promoter activity. In addition, USP2 may regulate the interruption of circadian rhythm by inhibiting CRY1 degradation during inflammation. It depends on USP2 in the process of TNF-α increasing CRY1 protein level and inhibiting rhythmic genes [19]. Scoma et al. [20] have proved that USP2 is associated with the circadian adaptation to environmental day length changes. They identified that USP2 could turnover and regulate the stability of BMAL1, which could alter the CLOCK/BMAL1 related genes [20].
Recent studies and genetic evidence show that USP2a and USP2b are new regulators of lipoprotein clearance. Because the study found that USP2a is a post-transcriptional and one of the androgen regulator isoenzymes, they can control the ubiquitination dependent degradation and subsequent lysosomal degradation of low-density lipoprotein receptor (LDLR) through IDOL, which as an E3-ubiquitin ligase, and finally play a vital role in the survival of tumors such as prostate cancer through stabilizing fatty acid synthase (FAS) [21]. It was also found that USP2 could regulate glucose metabolism by regulating glucocorticoid signaling and inducting of 11β-hydroxysteroid dehydrogenase 1 (HSD1) [22].
USP2 plays roles in controlling ion channel dynamics. The expression of USP2 is important in sodium homeostasis because aldosterone stimulation has been found to increase the expression of USP2 in the distal nephron. Moreover, studies have shown that USP2 also has a protective effect on Nedd4/Nedd4 like down-regulation of ENaC in vitro. KCNQ1 (Kv7.1) together with its KCNE β subunits, plays a pivotal role both in the repolarization of heart tissue and in water and salt transport across epithelial membranes. USP2 may counter Nedd4-2-mediated ubiquitylation by deubiquitylating KCNQ1 as the partial rescue of the membrane localization of the channel [23]. It is also reported that USP2b as the clock output effector acting at the post-translational level at cell membranes, possibly regulates membrane permeability of Ca2+ [24].
It has been demonstrated that TGF-β induces EMT and thereby promotes metastasis. USP2 promotes metastasis by interacting with TGFBR1 and TGFBR2 accompany TGF-β stimulation and can promote the recruitment of SMAD2/3 by removing the K33 linked polyubiquitin chain in lys502 of TGFBR1 [25].
Through activation of various natural receptors downstream to produce IFN-β and IFN regulator 3, one of the essential proteins is TANK-binding kinase 1 (TBK1). Recent studies have shown that USP2b deubiquitinates K63-associated polyubiquitin chains of TBK1 to cease TBK1 activation and controls negative IFN-β signaling and immune responses against viruses [11].
3.8 Cell Inflammatory Response
Recently, USP2 has been shown to inhibit TLR/IL-1β-induced NF-κB, which plays an important role in the immune system as a transcription factor activation, in tumor cells by deubiquitination of TRAF6. USP2 promotes T cell activation and TNF-α-induced cell death or inhibits IL-1β-induced inflammatory responses by deubiquitinating TRAF6, indicating that USP2 might be a tumor suppressor by modulating the tumor micro-environment [26].
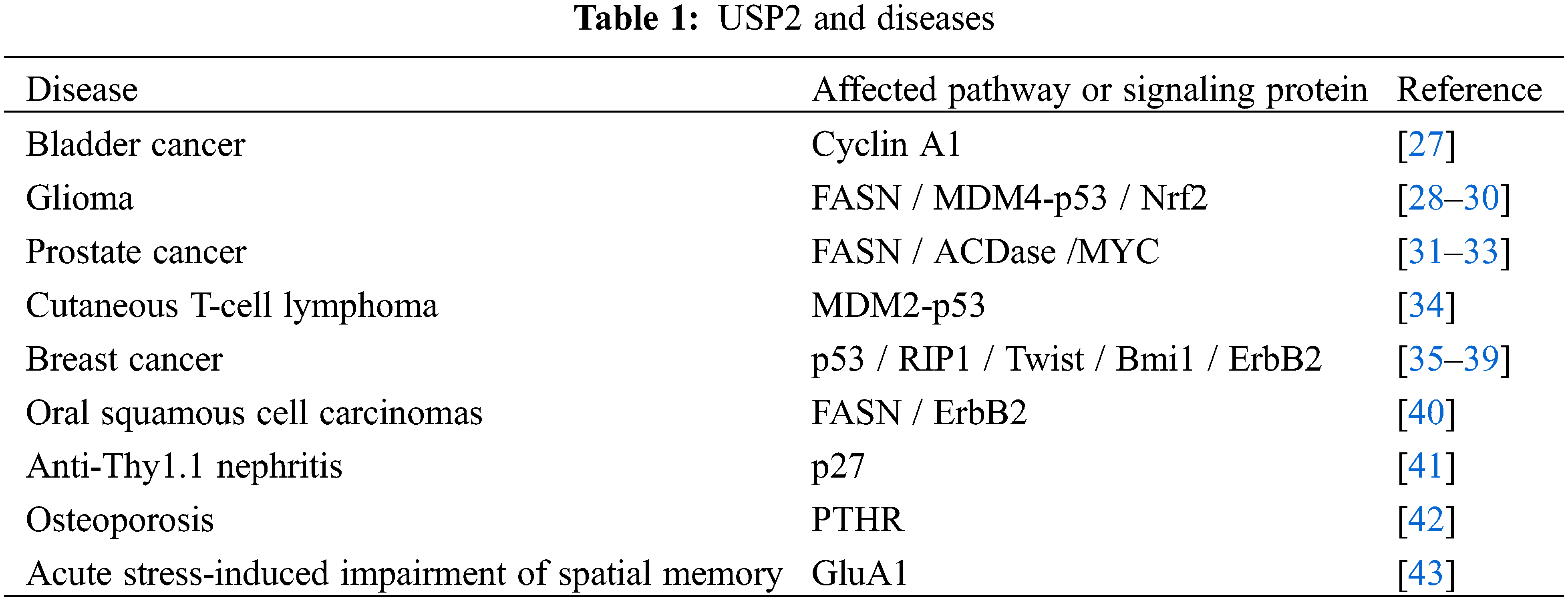
USP2 plays an essential role in regulating cell survival, cell cycle, circadian rhythm, cell metabolism, inflammatory response, antiviral response, and metastasis through interacting with many proteins such as Cyclin A1, MDM2/p53, etc., see Table 1. USP2 plays different roles in different tumors. Besides, it also associated with many functional disorders, such as recurrent miscarriage.
The development of tumor involves the regulation of multi-gene and multi-angle comprehensive. Deubiquitination enzyme as a class of important protein hydrolytic enzymes plays an important role in tumor. USP2 is highly expressed in various cancers such as breast cancer, OSCC, bladder cancer and prostate cancer [27–29]. In hematological tumors, it has been reported that MLL and USP2a form a fusion gene named MLL-USP2a [44]. Moreover, USP2 can promote TGF-β-triggered signals to facilitate tumor metastasis. This implied that USP2 has the potential to be target for the treatment of metastatic cancer. As a deubiquitinating protease, USP2 must function by interacting with other proteins. USP2 can regulate some important tumor-related proteins, such as β-catenin, FASN, MDM2, RAB1A, etc., and plays a part in the development of tumor through influencing tumor-related proteins [45]. USP2 plays different roles in different tumors, which may be related to the different downstream substrates in different tumors or different stages of tumors.
4.1.1 USP2 Acts as a Therapeutic Target for TNBC and ErbB2-Positive Breast Cancer
USP2 is over-expressed in breast invasive ductal carcinoma, and is closely related to proliferation promoting effects [35]. Triple negative breast cancer (TNBC) is an aggressive breast cancer subtype with poor prognosis. USP2 expression can be used as a prognostic biomarker of TNBC, and is significantly associated with the invasion of TNBC cell line and the survival rate of breast cancer patients [36]. In addition, TNBC harbors enriched cancer stem cell (CSC) populations in tumors. USP2 maintains the CSC population by activating self-renewing factor Bmi1 and epithelial-mesenchymal transition through Twist upregulation [37]. The canonical Wnt signaling pathway is activated in multiple types of cancers, including colorectal cancer, breast cancer and hepatocellular carcinoma. Mutation or overexpression of β-catenin often results in tumor formation and metastatic progression [38]. USP2a was found able to deubiquitinate and stabilize β-catenin. Genetic depletion or pharmacological inhibition of USP2 in TNBC cells destabilized β-catenin, suggesting that USP2 can serve as a novel therapeutic target of Wnt/β-catenin signaling in certain cancers such as TNBC. Zhang et al. found that USP2 protects ErbB2 surface levels in ErbB2-positive breast cancer by antagonizing its ubiquitin mediated endocytosis, which could be used to design novel therapeutic strategies for ErbB2-driven malignancies as a combination therapy with HSP90 inhibitors [39]. USP2 could elevate the level of RIP1 to promote apoptosis by deubiquitinating Ub-RIP1 in human breast cancer cells [15]. Over-expression of USP2 in TRAF2 gene knocked-out mouse embryonic fibroblast cell can obviously promote the RIP1 level and cell apoptosis.
4.1.2 USP2a is Associated with Tumor Progression and Poor Prognosis in OSCC
FASN and ErbB2 have been found to be overexpressed in oral squamous cell carcinoma (OSCC). At the same time, USP2a is expressed in OSCC and may play a role in FASN accumulation. Sabrina Daniela da Silva and others studied the correlation between FASN, ErbB2, USP2a gene expression and immunohistochemical status and the clinicopathological characteristics of OSCC patients. It was found that the expression of ErbB2, FASN and USP2a showed a strong positive correlation. In OSCC, FASN, ErbB2 and USP2a mRNA and protein levels are associated with tumor progression and poor prognosis, and there is a molecular link between them [40].
4.1.3 USP2 Acts as an Oncoprotein in Bladder Cancer
USP2 binds to cyclin A1 and prevents ubiquitination of cyclin A1, resulting in inhibition of cyclin A1 degradation. This leads to an accumulation of cyclin A1 and an increase in cell proliferation. Kim et al have found that USP2a is an oncoprotein in bladder cancer [27]. USP2 inhibits ubiquitination and stabilizes cyclin A1, an important cell cycle regulator, raising the possibility of USP2 targeting as a combination of chemotherapy for bladder tumors [27]. In bladder cancer, Liu et al. also discovered that tight junction protein 1 (TJP1) regulates TWIST1 expression by recruiting USP2, thereby regulating CCL2 expression and regulating angiogenesis in bladder cancer [46].
4.1.4 USP2 Acts as an Antioncogene and an Independent Factor for ccRCC Prognosis
USP2 mRNA level is significantly lower in renal cancer, relative to normal, and is interrelated to poor prognosis of renal cell carcinoma. USP2 acts as an antioncogene and an independent factor for clear cell renal cell carcinoma (ccRCC) prognosis [47]. However, the specific mechanism of USP2’s role in ccRCC remains unclear.
USP2 is involved in regulating key transcription factors in the inflammatory response. Increasing evidence shows that USP2 participates in the modulation of inflammatory and immune signaling. He et al. previously reported that USP2a could negatively regulates NF-κB dependent induction of TNF and IL-6 in colorectal carcinoma cells [26]. Conversely, in macrophages Sun et al. reported that USP2 could modifies degradation of TNF-α protein [48]. In addition, Kitamura et al. found that cytokine expression in macrophage-like cells modified by USP2 was systematically monitored so that USP2 inhibited a huge number of cytokines after LPS induction [49].
It was also reported that miR-125b can activate NF-κB through USP2, ultimately, resulting in psoriasis phenotype. Having the high-expression USP2 variant also gives individuals a slightly higher lifetime risk of psoriasis, and knocking down USP2 strongly reduces keratinocyte proliferation [50]. In addition, the function of USP2 is associated with chronic inflammation associated with circadian rhythm, and USP2 plays an important role in the regulation of the stability of crypto chrome CRY1 protein. Circadian clocks and inflammation are important regulators of metabolism, and a more in-depth understanding of the role of USP2 in CRY1 protein regulation could shed light on the interaction between the inflammatory response and the biological clock. Therefore, USP2 is also expected to be a drug target for treating physiological and metabolic disorders.
In addition, the expression of USP2 in human nephritis MSc is related to the pathological type of nephritis. Inflammatory factors and immune complexes can induce USP2 expression in mesangial MSc and up-regulate the expression of P27 protein. Overexpression of USP2 can slow the progression of Thy1.1 nephritis in rats [41].
4.3.1 Increased USP2 Expression is Necessary for Osteoblast Proliferation
Parathyroid hormone (PTH) plays an anabolic role in bone formation and is the main treatment for severe osteoporosis. USP2a and USP2b are predominantly expressed in osteoblasts. In vivo imaging analysis has demonstrated that USP2 is required for PTH1-34 induction of primary osteoblast proliferation in fluorescent ubiquitinated cell cycle indicator (Fucci) transgenic mice. It was found that USP2 may be one of the direct target genes for PTHR signaling in studying the effect of PTH on gene expression in osteoblast [42].
4.3.2 USP2 Participates in Acute Stress-Induced Impairment of Spatial Memory Retrieval
Loss of ubiquitin homeostasis in neurons leads to neurological diseases. Correction of ubiquitinase abnormalities has a high regulatory effect on cognitive function improvement. Recent studies have shown that USP2 is highly expressed in the rat hippocampus, and retigabine alleviated acute stress-induced spatial memory retrieval impairment through adjusting the aberrance of USP2. PGC-1α, E4BP4 and β-catenin are the upstream regulators while mTOR, autophagy and GluA1 are its downstream targets. Stress-induced spatial memory impairment can be enhanced by drugs that reverse the abnormal expression of USP2-related signaling pathways [43].
4.3.3 USP2 is Involved in Maintaining the Integrity of Mitochondrial Membrane
USP2 is thought to be involved in the differentiation of myoblasts into myotubes. The USP2-KO cells produced by Cas9 reduce the accumulation of oxygen consumption and adenosine triphosphate (ATP) content in the cells. In USP2-KO cells, mitochondrial membrane permeability was assessed using calcian AM-cobalt staining. The membrane potential of USP2-KO cells was significantly reduced. ML364, which a USP2 selective inhibitor, also increased the level of mitochondrial ROS and regulated the mitochondrial membrane potential and morphology. These results suggest that USP2 may be involved in maintaining mitochondrial membrane integrity. This process ensures a supply of ATP in myoblasts, possibly leading to proliferation and differentiation [51].
4.3.4 USP2 Plays an Important Role in Spermatogenesis and Sperm Function
USP2 mRNA expression is very high in mouse testis, but the role of USP2 subtypes in spermatogenesis needs further study. Bedard et al. found that although USP2-/-male testicular sperm count and epididymal sperm count were normal, fertility was also a serious drawback, with significantly reduced sperm motility in these animals, only 12% of USP2 +/+ litters [52] . Mayuko et al. demonstrated a distinct role of USP2 that directly affect sperm function in organ-specific macrophages [53]. Testicular macrophage USP2 does not affect the structure of the testis or modulate retinoic acid and testosterone synthesis. However, it could promote hyperactivation, motility, and sustain GM‑CSF expression in testicular interstitial macrophages.
4.3.5 USP2a Is Associates with Recurrent Miscarriage
Wang et al. showed that the placental of recurrent miscarriage patients have lower expression of USP2, which could inhibit human trophoblast-derived cell lines apoptosis and promoted its proliferation [54]. They discovered that TGF-β secreted by M2 macrophages can interact with USP2a in trophoblasts to regulate placental formation. What’s more, USP2a could promote nuclear translocation of β-catenin through activated PI3K/Akt/GSK3β signaling pathway while further activated epithelial-mesenchymal transition (EMT) of trophoblasts.
4.3.6 USP2 Regulate Ubiquitination and Stability of Antithrombin
It was cleared that USP2a overexpression is sufficient to stabilize antithrombin, while it still unclear which role USP2b will play in regulating the expression of antithrombin. It was found that USP2 interacted with antithrombin and regulated the expression of antithrombin, indicating that overexpression of USP2 improve the stability of antithrombin. Cullin 2 E3 ubiquitin ligase and USP2 synergistically regulate antithrombin ubiquitination and degradation. Therefore, targeting USP2 may be a potential strategy for the treatment of hemophilia [55].
4.3.7 USP2 Has Cardiomyocyte-Specific Function
The expression of USP2 in primary cardiac fibroblasts (CFS) induced by angiotensin II in neonatal rats increased. The inhibition of USP2 restrain the synthesis of collagen, the proliferation of CFS, and the progression of cell cycle. USP2 could stabilize β-catenin and cyclin D1 which regulating Ang II-induced cardiac fibroblasts activation [56]. Xing et al. also demonstrated that USP2 overexpression in the heart would inhibit pressure overload induced cardiac remodeling [57]. USP2 is down regulated in the heart under pressure overload. Overexpression of cardiac USP2 inhibits TAC induced cardiac remodeling by inhibiting cardiac hypertrophy, inhibiting fibrosis and inflammatory response, and reducing oxidative stress.
USP2 is a good anti-tumor drug target, and the discovery of its inhibitors is a hotspot of current research, see Table 2. However, the specificity of existing USP2 inhibitors is not ideal, and the vast majority of inhibitors are at the micromole level of IC50. Currently, USP2 inhibitors are not officially approved for clinical use. Current drug screening methods need to be further optimized, and the discovery of USP2 inhibitors is still in its infancy.
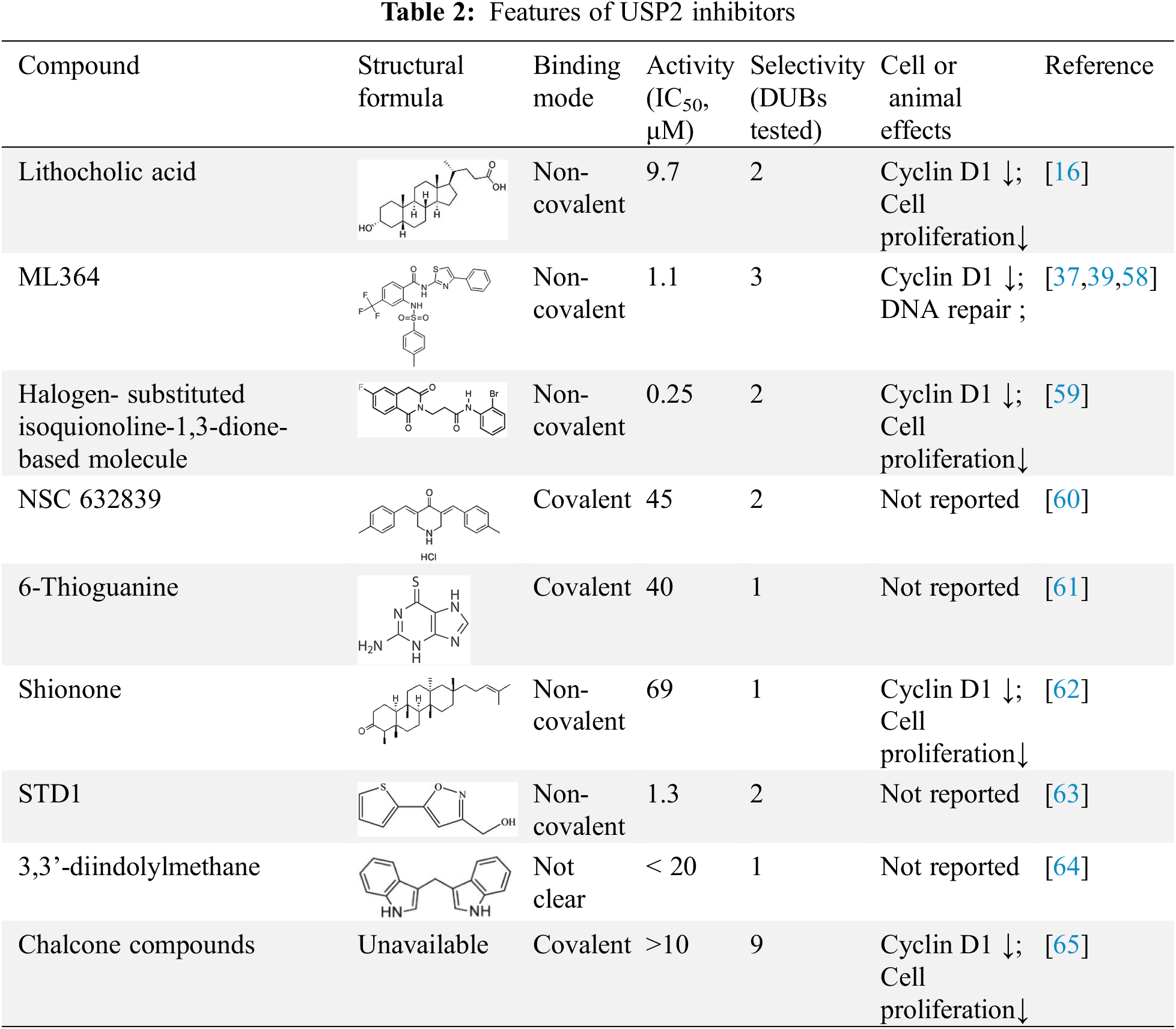
5.1 Isoquinoline-1,3-dione Scaffold-Based Inhibitors
Previously reported USP2 inhibitors, such as isoquinoline-1,3-dione scaffold, the introduction of fluorine atom totally changed the selectivity of inhibitors between USP7 and USP2. It was found that USP2 was inhibited by reactive oxygen species independent non-competitive mechanism with IC50 of 250 nm. When treated HeLa cells with 2 and 10 μM of compound 14, the cyclinD1 levels decreased in a time and concentration dependent, which suggests that compound 14 is able to enter cells and trigger USP2 inhibition in vivo. The research of isoquinoline 1,3 diketone cores and found of halogenated substituents helps for the development of more highly selective deubiquitinase inhibitors [59] .
Inhibition of ubiquitin isopeptase by NSC 632839 can be illustrated by the ability of crude lysates to inhibit z-LRGG-AMC cleavage in the range of middle micro-molars. In order to further characterize the inhibitory effect of NSC 632839 on purified enzyme, the inhibitory effect of NSC 632839 on purified USP2, USP7 and SENP2 was determined, and it was proved that NSC 632839 is not only a DUB inhibitor, but also a inhibitor of deSUMOylase. According to the description, the EC50 values of NSC 632839 inhibits USP2, SENP2 and USP7 are 45 ± 4 μM, 9.8 ± 1.8 μM and 37 ± 1 μM, respectively [60].
In recent studies, 6-thioguanine (6TG), an old drug still in clinical use for the treatment of leukemia and intestinal disease, was identified as an effective USP2 inhibitor. It is reported only 6TG but not 6MP has a significant inhibitory effect of USP2 with an IC50 of 40 ± 2.0 μM [61].
ML364 is a non-covalent USP2 inhibitor reported in 2016, IC50 of 1.1 μM [58]. Among the 6 other proteases tested, USP8 was also inhibited. ML364 induces cyclin D1 degradation resulting in cell-cycle arrest, indicating key role in progression of non-Hodgkin’s lymphoma and colorectal cancer. He et al. used ML364 in combination with doxorubicin and found that ML364 increased the sensitivity of existing TNBC xenografts to doxorubicin [37]. It can effectively inhibit the in vivo growth of ErbB2-positive breast cancer xenograft tumors with the combination of ML364 and HSP90 inhibitors [39].
Domestic researchers found shionone, as a natural compounds, can inhibit USP2 under their screening platform [62]. It was found that shionone can inhibit USP2 substrate Cyclin D1 and inhibit the proliferation of tumor cells. Shionone can provide a lead compound for identification of new USP2 inhibitors.
Indole-3-methanol (I3C) is a derivative of cruciferous vegetables such as cabbage and is known for its many health benefits in chemoprophylaxis and weight loss. It is easily metabolized to more stable 3,3’-diindolylmethane (DIM) under the acidic conditions of the stomach. DIM treatment directly inhibits USP2 deubiquitinase activity, which leads to a decrease in the stability of cyclin Dl and thus increases ubiquitin-mediated proteasome degradation. By inhibiting USP2 to increase the degradation of cyclin D1, USP2 may be responsible for reducing adipose tissue mass and inhibiting HFD-induced obesity in mice [64].
In addition to these, some small molecule inhibitors have also been reported. Lithocholic acid derivatives can inhibit USP2a, but also inhibit the JAMM/MPN+ family in DUBs [16]. A recent study reported small molecules 5-(2-thienyl)-3-isoxazoles (STD1) as the inhibitors of the USP2-ubiquitin protein-protein interaction that bind to USP2 through using NMR-based fragment screening and a biochemical binding assay [63]. Chalcone Compounds (double-digit micro molar potency) could significantly inhibited UCH-L1, UCH-L3, USP2, USP5 and USP8 [65]. The emergence of allosteric modulators provides a new and broader drug development space. The development and design of USP2 allosteric inhibitors will bring a greater prospect for targeted therapy of USP2-related diseases.
In recent years, USP2 related research has made progress in many aspects. USP2 is highly expressed in various tumors and is involved in the regulation of immune and inflammatory signals. This indicates that USP2 may be an important therapeutic target in inflammation and tumorigenesis. From our review, we can also see that different subtype of USP2 exhibit different functions. The specific mechanism of why these differences occur needs to be further explored. Furthermore, we conclude that USP2 plays an important role in many other non-neoplastic diseases such as recurrent abortion and osteoporosis. All of these suggest that USP2, as a deubiquitin protease, may play a role in different diseases by regulating and stabilizing different substrates. To further explore the specific interaction protein of USP2 in different diseases has an important role in the targeted treatment of diseases. We believe that as more research progresses, the role of USP2 in other diseases can also be verified.
In addition, specific USP2 inhibitor in clinical has not been officially approved currently. The existing USP2 small molecule inhibitors lack in vivo activity tests, and the selectivity is not ideal. Because the catalytic domains of USP are highly homologous, it is important to better understand the cross-reactivity between USP2 and other USP members. In previous studies, the eutectic structure of USP2 and Ub has been reported, but no single crystal structure of USP2 has been analyzed so far. The study reveals its crystal structure will provide important theoretical basis and direction for the development of USP2 specific inhibitors. The summary notes that USP2 is an effective target for cancer and other related diseases. These advances are encouraging because they will provide references and protocols for the clinical application of these diseases. However, the search for USP2 inhibitors is still in its infancy, and huge efforts are needed in the next few years.
Author Contributions: Yingli Wu conceived the idea. Hao Luo and Yanjie Ji searched the literature and wrote the manuscript. Xinrong Gao, Xinying Liu and Yunzhao Wu made supplements and corrections to the content of the article. All authors read and approved the final manuscript.
Ethics Approval and Informed Consent Statement: Not applicable.
Funding Statement: This work was supported by grants from the Shandong Provincial Natural Science Foundation (ZR2020QH095) and the National Undergraduate Training Program for Innovation and Entrepreneurship (202110438075).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Nandi, D., Tahiliani, P., Kumar, A., Chandu, D. (2006). The ubiquitin-proteasome system. Journal of Biosciences, 31(1), 137–155. DOI 10.1007/BF02705243. [Google Scholar] [CrossRef]
2. Kitamura, H., Hashimoto, M. (2021). USP2-Related cellular signaling and consequent pathophysiological outcomes. International Journal of Molecular Sciences, 22(3), 1209. DOI 10.3390/ijms22031209. [Google Scholar] [CrossRef]
3. Zhang, F., Zhao, Y., Sun, Y. (2021). USP2 is an SKP2 deubiquitylase that stabilizes both SKP2 and its substrates. Journal of Biological Chemistry, 297(4), 101109. DOI 10.1016/j.jbc.2021.101109. [Google Scholar] [CrossRef]
4. Lin, H. C., Kuan, Y., Chu, H. F., Cheng, S. C., Pan, H. C. et al. (2021). Disulfiram and 6-Thioguanine synergistically inhibit the enzymatic activities of USP2 and USP21. International Journal of Biological Macromolecules, 176(6), 490–497. DOI 10.1016/j.ijbiomac.2021.02.072. [Google Scholar] [CrossRef]
5. Gousseva, N., Baker, R. T. (2003). Gene structure, alternate splicing, tissue distribution, cellular localization, and developmental expression pattern of mouse deubiquitinating enzyme isoforms Usp2-45 and Usp2-69. Gene Expression, 11(3–4), 163–179. DOI 10.3727/000000003108749053. [Google Scholar] [CrossRef]
6. Engel, E., Viargues, P., Mortier, M., Taillebourg, E., Couté, Y. et al. (2014). Identifying USPs regulating immune signals in Drosophila: USP2 deubiquitinates Imd and promotes its degradation by interacting with the proteasome. Cell Communication and Signaling, 12(1), 41. DOI 10.1186/s12964-014-0041-2. [Google Scholar] [CrossRef]
7. Rougier, J. S., Albesa, M., Syam, N., Halet, G., Abriel, H. et al. (2015). Ubiquitin-specific protease USP2-45 acts as a molecular switch to promote α2δ-1-induced downregulation of Cav1.2 channels. Pflügers Archiv-European Journal of Physiology, 467(9), 1919–1929. DOI 10.1007/s00424-014-1636-6. [Google Scholar] [CrossRef]
8. Zhu, H. Q., Gao, F. H. (2017). The molecular mechanisms of regulation on USP2’s alternative splicing and the significance of its products. International Journal of Biological Sciences, 13(12), 1489–1496. DOI 10.7150/ijbs.21637. [Google Scholar] [CrossRef]
9. Freitas, B. T., Durie, I. A., Murray, J., Longo, J. E., Miller, H. C. et al. (2020). Characterization and noncovalent inhibition of the deubiquitinase and deISGylase activity of SARS-CoV-2 papain-like protease. ACS Infectious Diseases, 6(8), 2099–2109. DOI 10.1021/acsinfecdis.0c00168. [Google Scholar] [CrossRef]
10. Ren, Y., Zhao, P., Liu, J., Yuan, Y., Cheng, Q. et al. (2016). Deubiquitinase USP2a sustains interferons antiviral activity by restricting ubiquitination of activated STAT1 in the nucleus. PLoS Pathogens, 12(7), e1005764. DOI 10.1371/journal.ppat.1005764. [Google Scholar] [CrossRef]
11. Zhang, L., Zhao, X., Zhang, M., Zhao, W., Gao, C. (2014). Ubiquitin-specific protease 2b negatively regulates IFN-β production and antiviral activity by targeting TANK-binding kinase 1. Journal of Immunology, 193(5), 2230–2237. DOI 10.4049/jimmunol.1302634. [Google Scholar] [CrossRef]
12. Xiong, B., Huang, J., Liu, Y., Zou, M., Zhao, Z. et al. (2021). Ubiquitin-specific protease 2a promotes hepatocellular carcinoma progression via deubiquitination and stabilization of RAB1A. Cellular Oncology, 44(2), 329–343. DOI 10.1007/s13402-020-00568-8. [Google Scholar] [CrossRef]
13. Nadolny, C., Zhang, X., Chen, Q., Hashmi, S. F., Ali, W. et al. (2021). Dysregulation and activities of ubiquitin specific peptidase 2b in the pathogenesis of hepatocellular carcinoma. American Journal of Cancer Research, 11(10), 4746–4767. [Google Scholar]
14. Benassi, B., Flavin, R., Marchionni, L., Zanata, S., Pan, Y. et al. (2012). MYC is activated by USP2a-mediated modulation of microRNAs in prostate cancer. Cancer Discovery, 2(3), 236–247. DOI 10.1158/2159-8290.CD-11-0219. [Google Scholar] [CrossRef]
15. Mahul-Mellier, A. L., Datler, C., Pazarentzos, E., Lin, B., Chaisaklert, W. et al. (2012). De-ubiquitinating proteases USP2a and USP2c cause apoptosis by stabilising RIP1. Biochimica et Biophysica Acta, 1823(8), 1353–1365. DOI 10.1016/j.bbamcr.2012.05.022. [Google Scholar] [CrossRef]
16. Magiera, K., Tomala, M., Kubica, K., de Cesare, V., Trost, M. et al. (2017). Lithocholic acid hydroxyamide destabilizes cyclin D1 and induces G(0)/G(1) arrest by inhibiting deubiquitinase USP2a. Cell Chemical Biology, 24(4), 458–470.e418. DOI 10.1016/j.chembiol.2017.03.002. [Google Scholar] [CrossRef]
17. Shi, Y., Solomon, L. R., Pereda-Lopez, A., Giranda, V. L., Luo, Y. et al. (2011). Ubiquitin-specific cysteine protease 2a (USP2a) regulates the stability of Aurora-A. Journal of Biological Chemistry, 286(45), 38960–38968. DOI 10.1074/jbc.M111.231498. [Google Scholar] [CrossRef]
18. Nepal, S., Shrestha, A., Park, P. H. (2015). Ubiquitin specific protease 2 acts as a key modulator for the regulation of cell cycle by adiponectin and leptin in cancer cells. Molecular and Cellular Endocrinology, 412(1), 44–55. DOI 10.1016/j.mce.2015.05.029. [Google Scholar] [CrossRef]
19. Tong, X., Buelow, K., Guha, A., Rausch, R., Yin, L. (2012). USP2a protein deubiquitinates and stabilizes the circadian protein CRY1 in response to inflammatory signals. Journal of Biological Chemistry, 287(30), 25280–25291. DOI 10.1074/jbc.M112.340786. [Google Scholar] [CrossRef]
20. Scoma, H. D., Humby, M., Yadav, G., Zhang, Q., Fogerty, J. et al. (2011). The de-ubiquitinylating enzyme, USP2, is associated with the circadian clockwork and regulates its sensitivity to light. PLoS One, 6(9), e25382. DOI 10.1371/journal.pone.0025382. [Google Scholar] [CrossRef]
21. Nelson, J. K., Sorrentino, V., Avagliano Trezza, R., Heride, C., Urbe, S. et al. (2016). The deubiquitylase USP2 regulates the LDLR pathway by counteracting the E3-ubiquitin ligase IDOL. Circulation Research, 118(3), 410–419. DOI 10.1161/CIRCRESAHA.115.307298. [Google Scholar] [CrossRef]
22. Molusky, M. M., Li, S., Ma, D., Yu, L., Lin, J. D. (2012). Ubiquitin-specific protease 2 regulates hepatic gluconeogenesis and diurnal glucose metabolism through 11β-hydroxysteroid dehydrogenase 1. Diabetes, 61(5), 1025–1035. DOI 10.2337/db11-0970. [Google Scholar] [CrossRef]
23. Krzystanek, K., Rasmussen, H. B., Grunnet, M., Staub, O., Olesen, S. P. et al. (2012). Deubiquitylating enzyme USP2 counteracts Nedd4-2-mediated downregulation of KCNQ1 potassium channels. Heart Rhythm, 9(3), 440–448. DOI 10.1016/j.hrthm.2011.10.026. [Google Scholar] [CrossRef]
24. Pouly, D., Chenaux, S., Martin, V., Babis, M., Koch, R. et al. (2016). USP2-45 is a circadian clock output effector regulating calcium absorption at the post-translational level. PLoS One, 11(1), e0145155. DOI 10.1371/journal.pone.0145155. [Google Scholar] [CrossRef]
25. Zhao, Y., Wang, X., Wang, Q., Deng, Y., Li, K. et al. (2018). USP2a supports metastasis by tuning TGF-β signaling. Cell Reports, 22(9), 2442–2454. DOI 10.1016/j.celrep.2018.02.007. [Google Scholar] [CrossRef]
26. He, X., Li, Y., Li, C., Liu, L. J., Zhang, X. D. et al. (2013). USP2a negatively regulates IL-1β- and virus-induced NF-κB activation by deubiquitinating TRAF6. Journal of Molecular Cell Biology, 5(1), 39–47. DOI 10.1093/jmcb/mjs024. [Google Scholar] [CrossRef]
27. Kim, J., Kim, W. J., Liu, Z., Loda, M., Freeman, M. R. (2012). The ubiquitin-specific protease USP2a enhances tumor progression by targeting cyclin A1 in bladder cancer. Cell Cycle, 11(6), 1123–1130. DOI 10.4161/cc.11.6.19550. [Google Scholar] [CrossRef]
28. Tao, B. B., He, H., Shi, X. H., Wang, C. L., Li, W. Q. et al. (2013). Up-regulation of USP2a and FASN in gliomas correlates strongly with glioma grade. Journal of Clinical Neuroscience, 20(5), 717–720. DOI 10.1016/j.jocn.2012.03.050. [Google Scholar] [CrossRef]
29. Boustani, M. R., Khoshnood, R. J., Nikpasand, F., Taleshi, Z., Ahmadi, K. et al. (2016). Overexpression of ubiquitin-specific protease 2a (USP2a) and nuclear factor erythroid 2-related factor 2 (Nrf2) in human gliomas. Journal of the Neurological Sciences, 363(5), 249–252. DOI 10.1016/j.jns.2016.03.003. [Google Scholar] [CrossRef]
30. Wang, C. L., Wang, J. Y., Liu, Z. Y., Ma, X. M., Wang, X. W. et al. (2014). Ubiquitin-specific protease 2a stabilizes MDM4 and facilitates the p53-mediated intrinsic apoptotic pathway in glioblastoma. Carcinogenesis, 35(7), 1500–1509. DOI 10.1093/carcin/bgu015. [Google Scholar] [CrossRef]
31. Priolo, C., Tang, D., Brahamandan, M., Benassi, B., Sicinska, E. et al. (2006). The isopeptidase USP2a protects human prostate cancer from apoptosis. Cancer Research, 66(17), 8625–8632. DOI 10.1158/0008-5472.CAN-06-1374. [Google Scholar] [CrossRef]
32. Mizutani, N., Inoue, M., Omori, Y., Ito, H., Tamiya-Koizumi, K. et al. (2015). Increased acid ceramidase expression depends on upregulation of androgen-dependent deubiquitinases, USP2, in a human prostate cancer cell line, LNCaP. Journal of Biochemistry, 158(4), 309–319. DOI 10.1093/jb/mvv039. [Google Scholar] [CrossRef]
33. Nelson, W. G., de Marzo, A. M., Yegnasubramanian, S. (2012). USP2a activation of MYC in prostate cancer. Cancer Discovery, 2(3), 206–207. DOI 10.1158/2159-8290.CD-12-0027. [Google Scholar] [CrossRef]
34. Wei, T., Biskup, E., Gjerdrum, L. M., Niazi, O., Ødum, N. et al. (2016). Ubiquitin-specific protease 2 decreases p53-dependent apoptosis in cutaneous T-cell lymphoma. Oncotarget, 7(30), 48391–48400. DOI 10.18632/oncotarget.10268. [Google Scholar] [CrossRef]
35. Shrestha, M., Park, P. H. (2016). p53 signaling is involved in leptin-induced growth of hepatic and breast cancer cells. Korean Journal of Physiology & Pharmacology, 20(5), 487–498. DOI 10.4196/kjpp.2016.20.5.487. [Google Scholar] [CrossRef]
36. Qu, Q., Mao, Y., Xiao, G., Fei, X., Wang, J. et al. (2015). USP2 promotes cell migration and invasion in triple negative breast cancer cell lines. Tumour Biology, 36(7), 5415–5423. DOI 10.1007/s13277-015-3207-7. [Google Scholar] [CrossRef]
37. He, J., Lee, H. J., Saha, S., Ruan, D., Guo, H. et al. (2019). Inhibition of USP2 eliminates cancer stem cells and enhances TNBC responsiveness to chemotherapy. Cell Death & Disease, 10(4), 285. DOI 10.1038/s41419-019-1512-6. [Google Scholar] [CrossRef]
38. Zhan, T., Rindtorff, N., Boutros, M. (2017). Wnt signaling in cancer. Oncogene, 36(11), 1461–1473. DOI 10.1038/onc.2016.304. [Google Scholar] [CrossRef]
39. Zhang, J., Liu, S., Li, Q., Shi, Y., Wu, Y. et al. (2020). The deubiquitylase USP2 maintains ErbB2 abundance via counteracting endocytic degradation and represents a therapeutic target in ErbB2-positive breast cancer. Cell Death and Differentiation, 27(9), 2710–2725. DOI 10.1038/s41418-020-0538-8. [Google Scholar] [CrossRef]
40. da Silva, S. D., Cunha, I. W., Nishimoto, I. N., Soares, F. A., Carraro, D. M. et al. (2009). Clinicopathological significance of ubiquitin-specific protease 2a (USP2afatty acid synthase (FASNand ErbB2 expression in oral squamous cell carcinomas. Oral Oncology, 45(10), e134–139. DOI 10.1016/j.oraloncology.2009.02.004. [Google Scholar] [CrossRef]
41. Mao, X., Luo, W., Sun, J., Yang, N., Zhang, L. W. et al. (2016). Usp2-69 overexpression slows down the progression of rat anti-Thy1.1 nephritis. Experimental and Molecular Pathology, 101(2), 249–258. DOI 10.1016/j.yexmp.2016.09.005. [Google Scholar] [CrossRef]
42. Shirakawa, J., Harada, H., Noda, M., Ezura, Y. (2016). PTH-Induced osteoblast proliferation requires upregulation of the ubiquitin-specific peptidase 2 (Usp2) expression. Calcified Tissue International, 98(3), 306–315. DOI 10.1007/s00223-015-0083-5. [Google Scholar] [CrossRef]
43. Li, C., Zhang, J., Xu, H., Chang, M., Lv, C. et al. (2018). Retigabine ameliorates acute stress-induced impairment of spatial memory retrieval through regulating USP2 signaling pathways in hippocampal CA1 area. Neuropharmacology, 135, 151–162. DOI 10.1016/j.neuropharm.2018.02.034. [Google Scholar] [CrossRef]
44. Meyer, C., Lopes, B. A., Caye-Eude, A., Cavé, H., Arfeuille, C. et al. (2019). Human MLL/KMT2A gene exhibits a second breakpoint cluster region for recurrent MLL-USP2 fusions. Leukemia, 33(9), 2306–2340. DOI 10.1038/s41375-019-0451-7. [Google Scholar] [CrossRef]
45. Kim, J., Alavi Naini, F., Sun, Y., Ma, L. (2018). Ubiquitin-specific peptidase 2a (USP2a) deubiquitinates and stabilizes β-catenin. American Journal of Cancer Research, 8(9), 1823–1836. PMID: 30323974. [Google Scholar]
46. Liu, X. Q., Shao, X. R., Liu, Y., Dong, Z. X., Chan, S. H. et al. (2022). Tight junction protein 1 promotes vasculature remodeling via regulating USP2/TWIST1 in bladder cancer. Oncogene, 41(4), 502–514. DOI 10.1038/s41388-021-02112-w. [Google Scholar] [CrossRef]
47. Meng, X., Xiong, Z., Xiao, W., Yuan, C., Wang, C. et al. (2020). Downregulation of ubiquitin-specific protease 2 possesses prognostic and diagnostic value and promotes the clear cell renal cell carcinoma progression. Annals of Translational Medicine, 8(6), 319. DOI 10.21037/atm.2020.02.141. [Google Scholar] [CrossRef]
48. Sun, Y., Qin, Z., Li, Q., Wan, J. J., Cheng, M. H. et al. (2016). MicroRNA-124 negatively regulates LPS-induced TNF-α production in mouse macrophages by decreasing protein stability. Acta Pharmacologica Sinica, 37(7), 889–897. DOI 10.1038/aps.2016.16. [Google Scholar] [CrossRef]
49. Kitamura, H., Ishino, T., Shimamoto, Y., Okabe, J., Miyamoto, T. et al. (2017). Ubiquitin-Specific protease 2 modulates the lipopolysaccharide-elicited expression of proinflammatory cytokines in macrophage-like HL-60 cells. Mediators of Inflammation, 2017(6), 6909415. DOI 10.1155/2017/6909415. [Google Scholar] [CrossRef]
50. Wei, T., Folkersen, L., Biskup, E., Xu, N., Manfe, V. et al. (2017). Ubiquitin-specific peptidase 2 as a potential link between microRNA-125b and psoriasis. British Journal of Dermatology, 176(3), 723–731. DOI 10.1111/bjd.14916. [Google Scholar] [CrossRef]
51. Hashimoto, M., Saito, N., Ohta, H., Yamamoto, K., Tashiro, A. et al. (2019). Inhibition of ubiquitin-specific protease 2 causes accumulation of reactive oxygen species, mitochondria dysfunction, and intracellular ATP decrement in C2C12 myoblasts. Physiological Reports, 7(14), e14193. DOI 10.14814/phy2.14193. [Google Scholar] [CrossRef]
52. Bedard, N., Yang, Y., Gregory, M., Cyr, D. G., Suzuki, J. et al. (2011). Mice lacking the USP2 deubiquitinating enzyme have severe male subfertility associated with defects in fertilization and sperm motility. Biology of Reproduction, 85(3), 594–604. DOI 10.1095/biolreprod.110.088542. [Google Scholar] [CrossRef]
53. Hashimoto, M., Kimura, S., Kanno, C., Yanagawa, Y., Watanabe, T. et al. (2021). Macrophage ubiquitin-specific protease 2 contributes to motility, hyperactivation, capacitation, and in vitro fertilization activity of mouse sperm. Cellular and Molecular Life Sciences, 78(6), 2929–2948. DOI 10.1007/s00018-020-03683-9. [Google Scholar] [CrossRef]
54. Wang, J., Ding, J., Zhang, S., Chen, X., Yan, S. et al. (2021). Decreased USP2a expression inhibits trophoblast invasion and associates with recurrent miscarriage. Frontiers in Immunology, 12, 717370. DOI 10.3389/fimmu.2021.717370. [Google Scholar] [CrossRef]
55. Xu, D., Wu, J., Chen, J., Jiang, L., Chen, J. et al. (2021). Cullin 2-RBX1 E3 ligase and USP2 regulate antithrombin ubiquitination and stability. FASEB Journal, 35(8), e21800. DOI 10.1096/fj.202001146RR. [Google Scholar] [CrossRef]
56. Xu, Q., Liu, M., Zhang, F., Liu, X., Ling, S. et al. (2021). Ubiquitin-specific protease 2 regulates Ang II-induced cardiac fibroblasts activation by up-regulating cyclin D1 and stabilizing β-catenin in vitro. Journal of Cellular and Molecular Medicine, 25(2), 1001–1011. DOI 10.1111/jcmm.16162. [Google Scholar] [CrossRef]
57. Xing, J., Li, P., Hong, J., Wang, M., Liu, Y. et al. (2020). Overexpression of ubiquitin-specific protease 2 (USP2) in the heart suppressed pressure overload-induced cardiac remodeling. Mediators of Inflammation, 2020, 4121750. DOI 10.1155/2020/4121750. [Google Scholar] [CrossRef]
58. Davis, M. I., Pragani, R., Fox, J. T., Shen, M., Parmar, K. et al. (2016). Small molecule inhibition of the ubiquitin-specific protease USP2 accelerates cyclin D1 degradation and leads to cell cycle arrest in colorectal cancer and mantle cell lymphoma models. Journal of Biological Chemistry, 291(47), 24628–24640. DOI 10.1074/jbc.M116.738567. [Google Scholar] [CrossRef]
59. Vamisetti, G. B., Meledin, R., Gopinath, P., Brik, A. (2019). Halogen substituents in the isoquinoline scaffold switches the selectivity of inhibition between USP2 and USP7. Chembiochem, 20(2), 282–286. DOI 10.1002/cbic.201800612. [Google Scholar] [CrossRef]
60. Nicholson, B., Leach, C. A., Goldenberg, S. J., Francis, D. M., Kodrasov, M. P. et al. (2008). Characterization of ubiquitin and ubiquitin-like-protein isopeptidase activities. Protein Science, 17(6), 1035–1043. DOI 10.1110/ps.083450408. [Google Scholar] [CrossRef]
61. Chuang, S. J., Cheng, S. C., Tang, H. C., Sun, C. Y., Chou, C. Y. (2018). 6-Thioguanine is a noncompetitive and slow binding inhibitor of human deubiquitinating protease USP2. Scientific Reports, 8(1), 3102. DOI 10.1038/s41598-018-21476-w. [Google Scholar] [CrossRef]
62. Wang, Z., Xie, W., Zhu, M., Zhou, H. (2017). Development of a highly reliable assay for ubiquitin-specific protease 2 inhibitors. Bioorganic & Medicinal Chemistry Letters, 27(17), 4015–4018. DOI 10.1016/j.bmcl.2017.07.059. [Google Scholar] [CrossRef]
63. Tomala, M. D., Magiera-Mularz, K., Kubica, K., Krzanik, S., Zieba, B. et al. (2018). Identification of small-molecule inhibitors of USP2a. European Journal of Medicinal Chemistry, 150, 261–267. DOI 10.1016/j.ejmech.2018.03.009. [Google Scholar] [CrossRef]
64. Yang, H., Seo, S. G., Shin, S. H., Min, S., Kang, M. J. et al. (2017). 3,3’-Diindolylmethane suppresses high-fat diet-induced obesity through inhibiting adipogenesis of pre-adipocytes by targeting USP2 activity. Molecular Nutrition & Food Research, 61(10), 1700119. DOI 10.1002/mnfr.201700119. [Google Scholar] [CrossRef]
65. Issaenko, O. A., Amerik, A. Y. (2012). Chalcone-based small-molecule inhibitors attenuate malignant phenotype via targeting deubiquitinating enzymes. Cell Cycle, 11(9), 1804–1817. DOI 10.4161/cc.20174. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |