
 | Sound & Vibration |  |
DOI: 10.32604/oncologie.2022.019881
ARTICLE
A Real-World Study on Oral Vinorelbine for the Treatment of Metastatic Breast Cancer
1Department of Medical Oncology, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital & Shenzhen Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Shenzhen, China
2Department of Gynecology and Obstetrics, The University of Hong Kong-Shenzhen Hospital, Shenzhen, China
3College of Medical, Shenzhen University, Shenzhen, China
* Corresponding Author: Caiwen Du. Email: dusumc@aliyun.com
Received: 21 October 2021; Accepted: 18 February 2022
Abstract: Background: Vinorelbine can be used to treat metastatic breast cancer as a single agent or in combination with other chemotherapy agents, although there is little real-world data for its use, particularly the oral form, in China. The current study aimed to explore the efficacy and safety of oral vinorelbine in patients with metastatic breast cancer in real-world clinical practice. Methods: A total of 194 patients with metastatic breast cancer received oral vinorelbine as a treatment between February 2017 and January 2021 at the National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital & Shenzhen Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College. The efficacy, in terms of progression-free survival and overall survival, and safety of oral vinorelbine were evaluated. Results: At a median follow-up period of 17.0 months, 152 patients finally exhibited disease progression, and 55 succumbed to the disease. During the follow-up, 53 patients demonstrated a partial response, and 106 achieved a stable disease, with an objective response rate of 27.3%. Additionally, 118 patients demonstrated a partial response or stable disease for ≥24 weeks, with a clinical benefit rate of 60.8%. The median progression-free survival was 6.2 months (95% confidence interval, 5.0–7.4), and the median overall survival was not evaluated. No treatment-associated mortalities occurred. The most common adverse events included leukopenia (73.2%), neutropenia (72.7%), anemia (65.5%), and diarrhea (46.9%). Conclusions: Oral vinorelbine appears to be efficacious for metastatic breast cancer with acceptable toxicity for real-world use in China.
Keywords: Metastatic breast cancer; oral vinorelbine; real-world study
Breast cancer is the most frequently diagnosed cancer in female individuals worldwide, followed by colorectal, lung, and cervical cancers. It is also the leading cause of cancer death, followed by lung, colorectal, and cervical cancers [1]. In China, breast cancer also ranks first among cancer types diagnosed in female individuals, with 270,000 new cases per year and an increased incidence among those aged between 30 and 59 years and those living in urban areas. Urban areas had twice the incidence rate compared with rural areas [2]. Despite the declining trend in the mortality rate of breast cancer, advanced breast cancer is predominantly an incurable malignancy, with an overall survival (OS) ranging from 2 to 3 years [3,4].
For most patients with metastatic breast cancer (MBC), the disease is incurable, and the primary treatment goal becomes symptomatic palliation and disease control to maintain or improve quality of life and possibly to extend survival [5]. Treatment options for advanced breast cancer include chemotherapy, hormone therapy, and human epidermal growth factor receptor 2 (HER2)-targeted therapy. However, the majority of patients eventually develop drug resistance [6].
Hormone receptor-positive breast cancer is a subtype of breast cancer that expresses estrogen receptors, progesterone receptors, or both. Endocrine therapy is preferred for bone or soft tissue metastasis and minor visceral metastasis [7]. The expression of HER2, which occurs in 20%–30% of breast cancers, is also associated with breast cancer prognosis. HER2-positive cancers are more aggressive and have a poorer prognosis than the HER2-negative disease [8]. Triple-negative breast cancer (TNBC) is a subtype of breast cancer with the absence of estrogen receptor, progesterone receptor, and HER-2 expression. Different studies have reported that TNBC accounts for approximately 10%–20% of all breast cancers [9–11]. TNBC has a poor prognosis and is characterized by high heterogeneity, invasiveness, metastatic potential, likelihood to relapse, and poor prognosis [12,13].
Vinorelbine is a semi-synthetic third-generation vinca-alkaloid with a modified catharanthine ring. At the molecular level, it acts on the dynamic equilibrium of tubulin in the microtubulin apparatus of the cell [14]. Unlike other vinca-alkaloids, vinorelbine binds preferentially to the mitotic spindle, with less effect on the microtubules in the neural structures [15]. It is associated with lower neurotoxicity [16] and has been shown to be effective and well-tolerated in the treatment of MBC [17,18].
Vinorelbine is available for clinical use in intravenous (IV) and oral forms. The oral form is made from gelatin capsules [16] and compared with the IV form, has the advantage of maintaining or improving the quality of life of patients. Furthermore, the oral form has been reported to lower the cost of medical care through avoidance of hospitalization [19,20]. Therefore, oral vinorelbine is a useful alternative to the IV form and warrants further clinical investigation.
The NORBREAST-228 phase II study showed that the clinical benefit rate of oral vinorelbine as a mono-therapy was 56%, and the median progression-free survival (PFS) was 8.2 months [17]. Other clinical trials reported the overall response rate (ORR) of single-agent oral vinorelbine to be 19.7%–29%, and the median PFS was between 5.2 and 5.5 months [21,22]. Meanwhile, the ORR of vinorelbine combined with chemotherapy was reported to be 24%–39.4%, and the PFS was between 4.5 and 7.1 months [23,24]. In addition to the above clinical trials, some real-world studies conducted in European countries, such as France and Spain, showed that the PFS of vinorelbine was between 2.7 and 4.9 months, varying with different treatment regimens [25,26]. Oral vinorelbine was recognized in the Chinese mainland in 2015, with little real-world data in this area. Thus, we aimed to evaluate the efficacy and safety of oral vinorelbine in patients with MBC at the National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital & Shenzhen Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College. The primary endpoint was the efficacy of oral vinorelbine therapy in the treatment of MBC in a real-world setting. This study could be a potential landmark on vinorelbine oral treatment for Chinese patients, since there were no previous real-world studies involved in the treatment of Chinese.
The current study retrospectively reviewed the medical data of 194 Chinese female patients with pretreated MBC who received oral vinorelbine (monotherapy or in combination) at the National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital & Shenzhen Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College (Shenzhen, China) between February 2017 and January 2021. The median age of the patients included in the study was 49 years (range, 26–85 years).
Eligible patients had the following characteristics: i) ≥18 years of age; ii) had at least one metastatic lesion (non-visceral, visceral or any metastasis); iii) had not received any vinorelbine (oral or IV) therapy previously. If patients had a history of other malignancies within the previous 5 years, abnormal laboratory findings, or severe comorbid illnesses, they were excluded from the current study. Patients were also excluded if they were enrolled in clinical trials that impacted their daily clinical condition.
Demographic and baseline clinical information of patients was described using standard descriptive and analytical methods. PFS was defined as the time from the start of oral vinorelbine treatment to the date of documented disease progression or mortality from any cause. OS was defined as the time from the start of treatment to the date of mortality from any cause or to the most recent date patients were confirmed to be alive. ORR was defined as the proportion of patients who achieved a partial response (PR) or a confirmed complete response (CR). The clinical benefit rate (CBR) was defined as the proportion of evaluable subjects with CR, PR, or stable disease (SD) for ≥24 weeks.
All statistical analyses were completed using SPSS software (IBM Corp., Armonk, NY, USA; version 26.0). PFS and OS were estimated using the Kaplan-Meier method. In addition, the Kaplan-Meier method and log-rank test were used to analyze the univariate discrimination of PFS and OS by demographic data, baseline clinical information, and toxicities. Furthermore, the combined effects of these variables on both PFS and OS were examined in multivariate analysis using the Cox proportional hazards regression model. P < 0.05 was considered a statistically significant difference.
A total of 194 patients with MBC received oral vinorelbine (monotherapy or in combination) (NAVELBINE®, PIERRE FABRE MEDICAMENT) at the National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital & Shenzhen Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College between February 2017 and January 2021. The baseline patient characteristics are presented in Table 1. The majority of patients (81.4%) had an Eastern Cooperative Oncology Group (ECOG) performance status of 0–1. For the molecular type of breast cancer, 115 patients (59.3%) had hormone receptor-positive breast cancer, 93 (47.9%) had HER2-positive breast cancer, and 27 (13.9%) were diagnosed with TNBC. Furthermore, 61 patients (31.4%) exhibited histological grades I–II tumors, 70 (36.1%) exhibited grade III tumors, and the remaining 63 (32.5%) exhibited unknown tumor grades. A total of 54 patients (27.8%) had stages I–II disease, 74 (38.1%) had stage III disease, and 42 (21.6%) had stage IV disease. Tumors >2 cm were detected in 130 patients (67.0%).
The most common metastatic site for tumor metastasis was the lymph nodes (n = 168, 86.6%). In the study, 159 patients (82.0%) had regional lymph node metastases, and 156 (80.4%) had distant lymph node metastases. Other metastatic sites included the muscle and soft tissue (69.1%), bone (54.1%), lung (50.0%), liver (45.4%), chest wall (41.2%), brain (29.4%), pleura (23.2%), skin (12.4%), and contralateral breast (7.2%). A total of 115 patients (59.3%) had more than four metastatic sites.
Additionally, 74.2% of patients had previously received anthracycline- or taxane-based neoadjuvant or adjuvant therapy, 31.4% had not been treated previously after metastasis, and 30.4% had been treated with more than three lines of treatment. Furthermore, 69 patients (35.6%) had a disease-free survival (DFS) of >24 months following initial treatment, while 95 (49.0%) had a DFS of ≤24 months.
With a median follow-up of 17.0 months (range, 1.5–46.8 months) for 194 patients, there were 152 patients finally had progressive disease (PD) and 55 deaths. In the first efficacy evaluation after two cycles of treatment, there were 53 patients achieved PR, 106 patients achieved SD, and 35 patients were PD, no patients reached CR. As demonstrated in Fig. 1, the median PFS was 6.2 months [95% confidence interval (CI), 5.0–7.4 months], and the median OS was not evaluated because more than half of the patients were still alive. Among 194 patients, a total of 53 achieved PR with an ORR of 27.3% at the best response. Additionally, 118 patients had a PR or SD for ≥24 weeks, demonstrating a CBR of 60.8%.
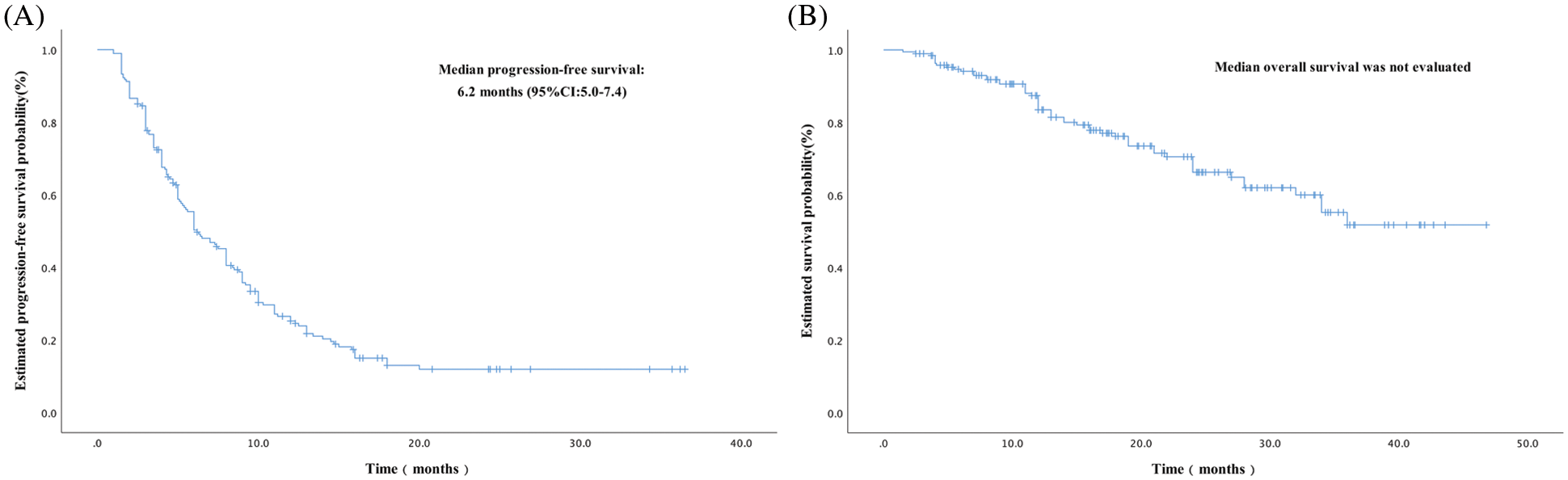
Figure 1: Kaplan-Meier curve of progression-free survival (PFS) and overall survival (OS) of patients with metastatic breast cancer who received oral vinorelbine. (A) Kaplan-Meier curve of PFS, which indicated a median PFS of 6.2 months (95% confidence interval [CI], 5.0–7.4). (B) Kaplan-Meier curve of OS, wherein median OS was not evaluated
Although the PFS was longer for patients who achieved remission (comprising patients with CR or PR; n = 53) compared with those who did not (n = 141), no statistically significant difference was identified (8.0 months [95% CI, 6.9–9.1 months] vs. 6.0 months [95% CI, 4.9–7.1 months]; P = 0.931; Fig. 2A). Furthermore, no statistically significant difference was identified in OS between these patients (both groups were not evaluated for median OS; P = 0.467; Fig. 2B). The PFS and OS were also compared between patients who achieved clinical benefit (referring to patients with CR, PR, or SD for ≥24 weeks; n = 118) and those who did not (n = 76). A significantly longer PFS and OS were identified in patients who gained a clinical benefit compared with those who did not (PFS: 10.0 months [95% CI, 8.6–11.4 months] vs. 3.0 months [95% CI, 2.6–3.4 months], P < 0.001; OS: not evaluated [95% CI, not evaluated] vs. 19.0 months [95% CI, 6.0–32.0 months], P < 0.001, Figs. 2C and 2D).
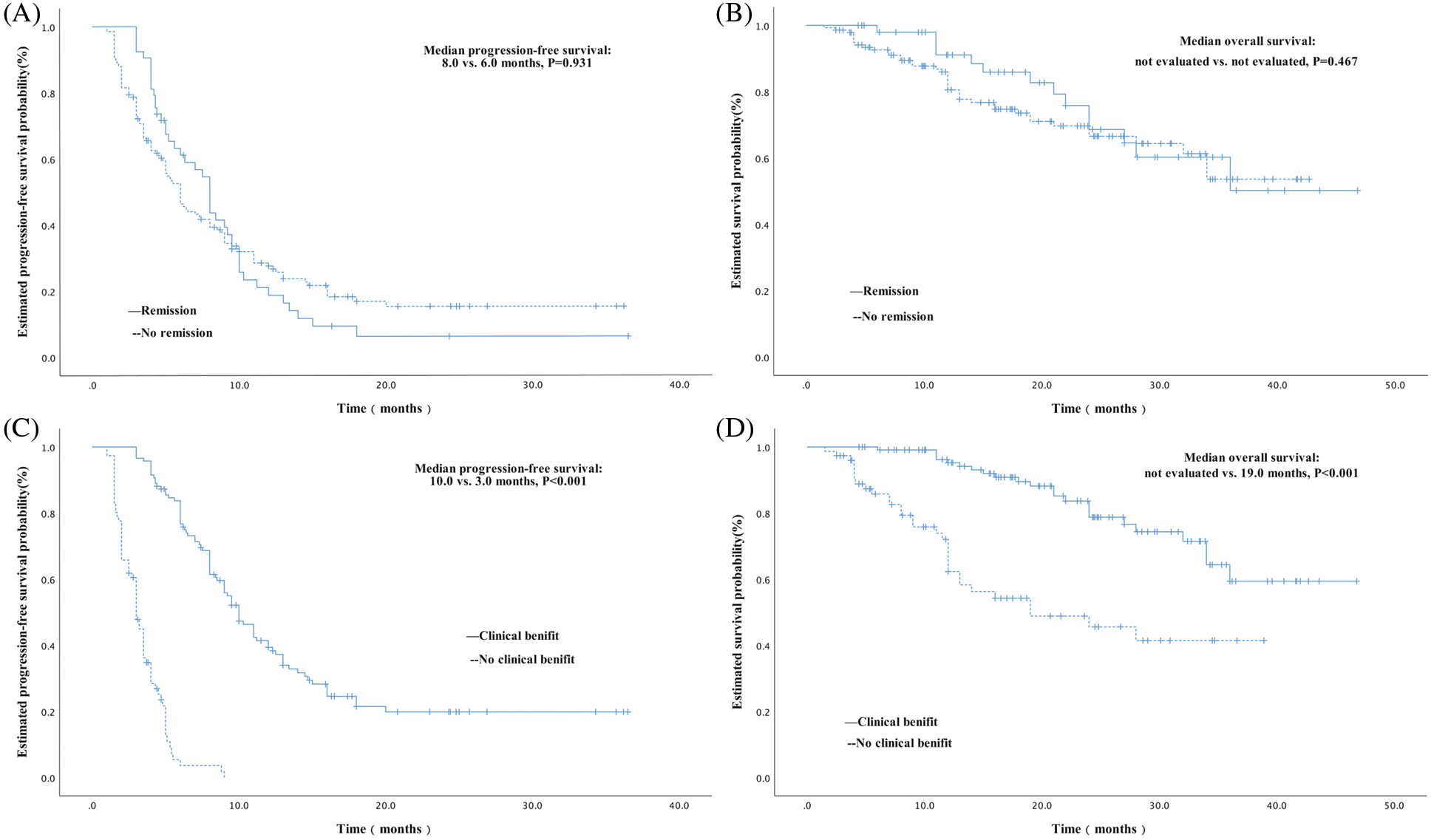
Figure 2: Kaplan-Meier curves of PFS and OS in subgroup analysis. (A) Kaplan-Meier curve of PFS comparing patients who achieved remission following treatment with oral vinorelbine, which had a median PFS of 8.0 months, and those who did not, which had a median PFS of 6.0 months. No significant difference was identified between these two groups (P = 0.931). (B) Kaplan-Meier curve of OS comparing patients who achieved remission following oral vinorelbine and those who did not, wherein a median OS was not evaluated. No significant difference was identified between these two groups (P = 0.467). (C) Kaplan-Meier curve of PFS comparing patients who achieved a clinical benefit following treatment with oral vinorelbine, which had a median PFS of 10.0 months, and those who did not, which had a median PFS of 3.0 months. A statistically significant difference was identified between these two groups (P < 0.001). (D) Kaplan-Meier curve of OS comparing patients who achieved a clinical benefit following oral vinorelbine, wherein a median OS was not evaluated, and those who did not, with a median OS of 19.0 months. A significant difference was identified between these two groups (P < 0.001)
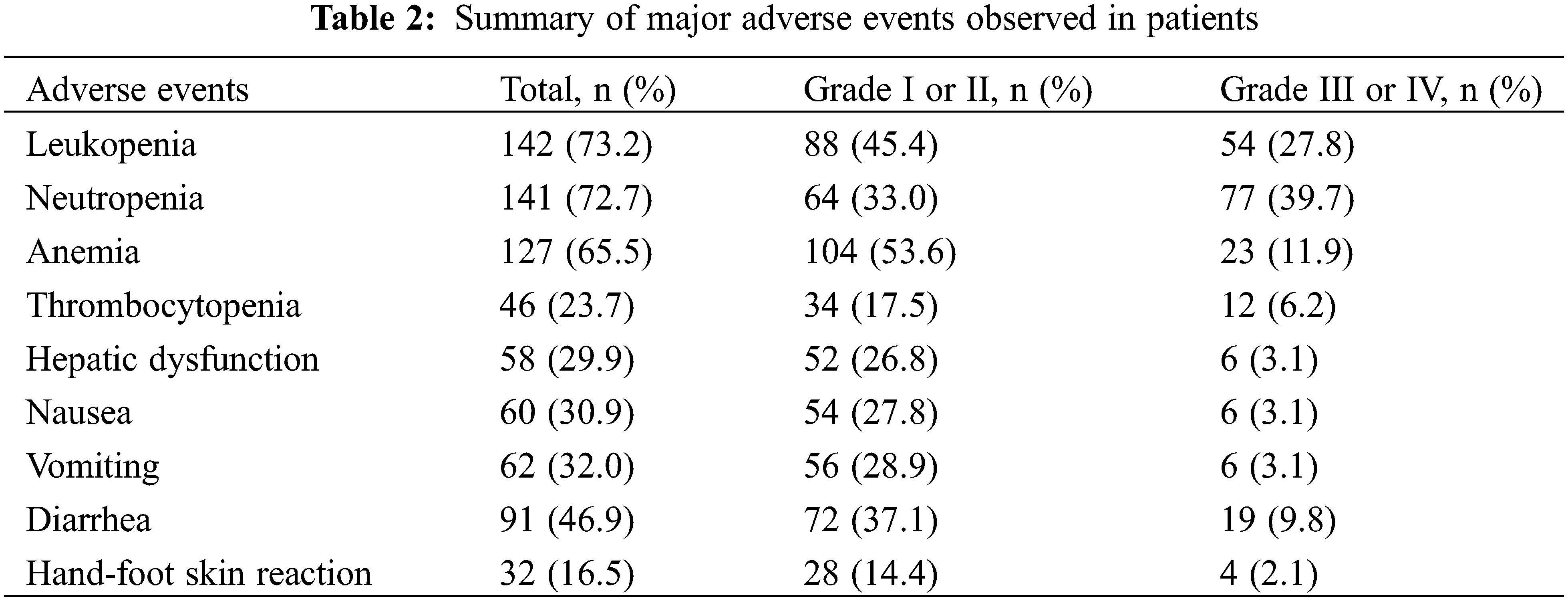
No treatment-associated mortalities occurred. The major adverse events (AEs) for all grades are presented in Table 2. The most common AEs for all grades included leukopenia (73.2%), neutropenia (72.7%), anemia (65.5%), diarrhea (46.9%), vomiting (32.0%), nausea (30.9%), hepatic dysfunction (29.9%), thrombocytopenia (23.7%), and hand-foot skin reaction (16.5%). Neutropenia (39.7%), leukopenia (27.8%), anemia (11.9%), and diarrhea (9.8%) were the most common AEs for grades III or IV. Most toxicities were limited to patients with grades I or II disease and were therefore tolerable and manageable.
3.4 Univariate and Multivariate Analyses
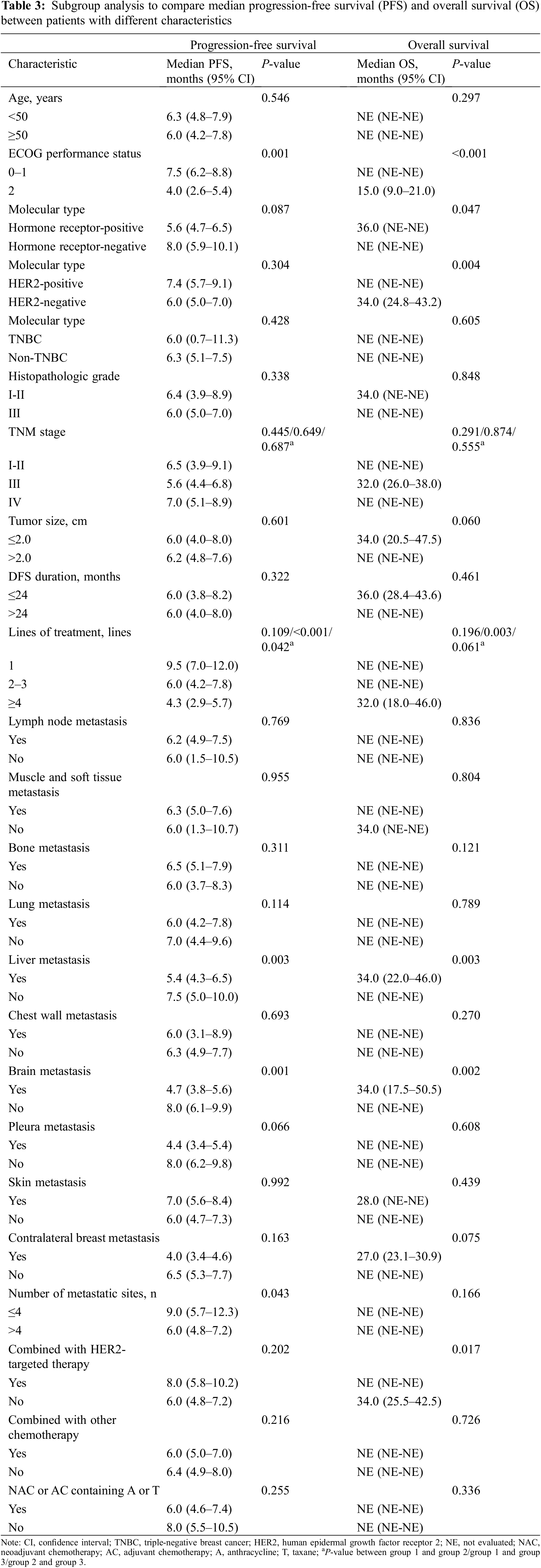
By univariate analysis, the difference in PFS and OS among patients with different demographic data and baseline clinical information was first assessed using Kaplan-Meier analysis and a log-rank test. As presented in Table 3, a significantly longer PFS was identified in patients whose ECOG performance status was 0–1 (P = 0.001), had received combined treatment with 1 line of therapy (P < 0.001) or 2–3 lines of therapy (P = 0.042) compared with ≥4 lines of therapy, had liver metastasis (P = 0.003), had brain metastasis (P = 0.001), or had more than four metastatic sites (P = 0.043). Furthermore, the following factors were identified to be significantly associated with OS: ECOG performance status (P < 0.01), hormone receptor status (P = 0.047), HER-2 status (P = 0.004), lines of treatment (P = 0.003), liver metastasis (P = 0.003), brain metastasis (P = 0.002), and combination HER2-targeted therapy (P = 0.017).
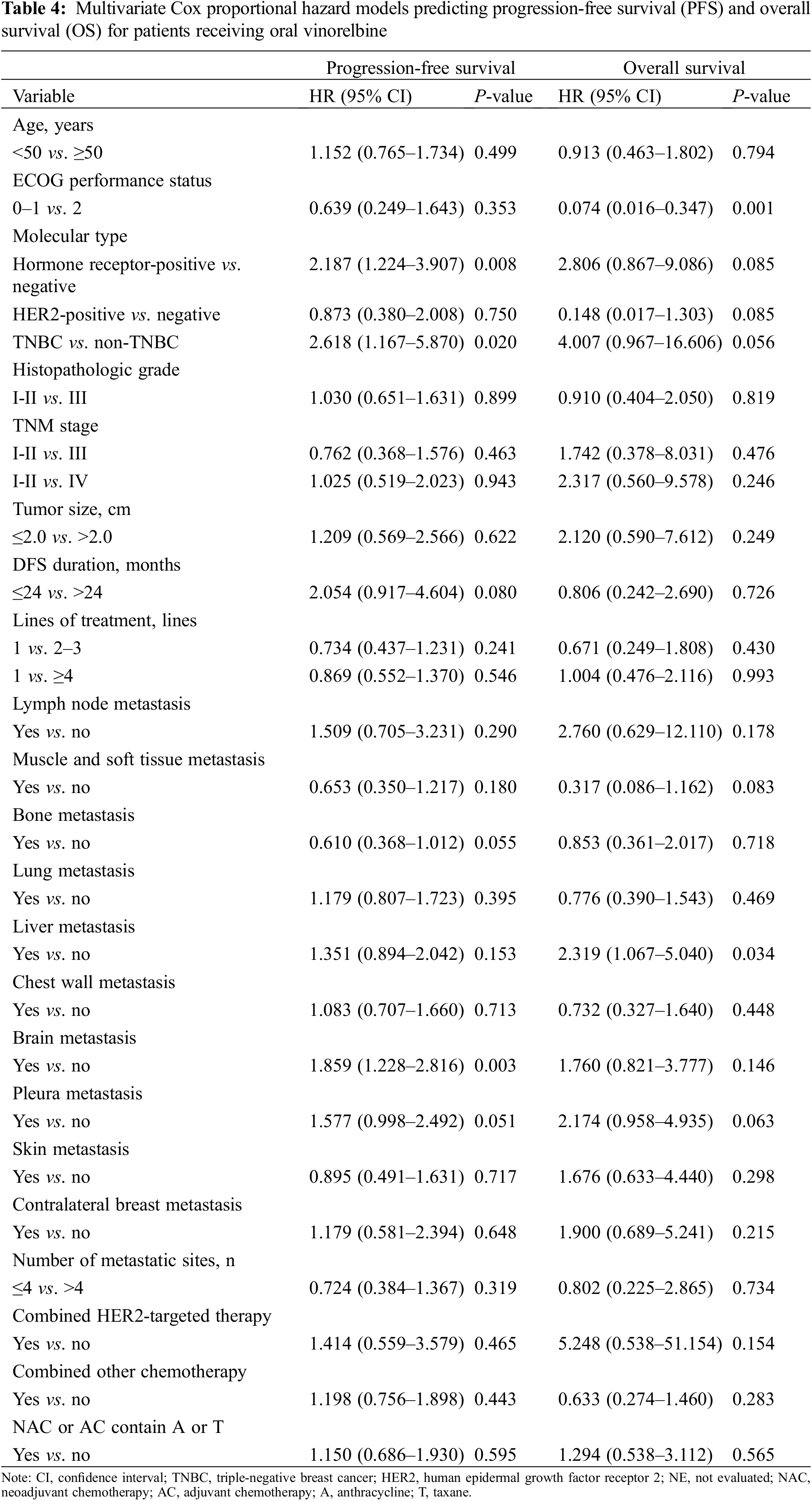
In addition, a multivariate model containing all these variables was established (Table 4). In the multivariate analysis, the hormone receptor status (hazard ratio [HR], 2.187; 95% CI, 1.224–3.907; P = 0.008), TNBC status (HR, 2.618; 95% CI, 1.167–5.870; P = 0.020), and brain metastasis (HR, 1.859; 95% CI, 1.228–2.816; P = 0.003) were associated with a significantly shorter PFS. Additionally, a significantly longer OS was identified in patients whose ECOG performance status was 0–1 (HR, 0.074; 95% CI = 0.016–0.347; P = 0.001), and a significantly shorter OS was found in patients with liver metastasis (HR, 2.319; 95% CI = 1.067–5.040; P = 0.034).
This study included 194 patients with MBC who received treatment with oral vinorelbine between February 2017 and January 2021 at the National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital & Shenzhen Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College. We aimed to evaluate the efficacy and safety of oral vinorelbine. In this study, the median PFS of all 194 patients was 6.2 months (95% CI, 5.0–7.4 months), while the median OS was not evaluated because more than half of the patients were still alive upon assessment. Among the 194 patients eligible for efficacy analysis, the ORR was 27.3% (53/194), and the CBR was 60.8% (118/194). These results indicated that oral vinorelbine had a high efficacy for the treatment of MBC. Blancas I. performed a real-world study on oral vinorelbine in metastatic breast cancer patients [25]. The study results showed that the ORR and CBR were 29.1% and 49.1%, which is consistent with the results of our study.
Some studies have demonstrated the equivalence of oral and IV formulations in pharmacokinetic studies [27]. In clinical practice, the efficacies of the two formulations were also confirmed to be equivalent. A retrospective comparison of two consecutive phase II studies showed that there was no significant difference in TTP between the two formulations. In the IV group, disease control was 61% (37% PR, 24% SD), median TTP was 6.8 months, and median survival was 11.3 months. Meanwhile, in the oral group, disease control was 77% (5.4% CR, 34% PR, 38% SD), median TTP was 7 months, and median survival was 10 months [28]. A phase II clinical study in China confirmed that there was no significant difference in efficacy or toxicity between oral vinorelbine and IV vinorelbine [29]. For patients with MBC, the oral formulation of vinorelbine is advantageous in its ease of ad-ministration and ability to improve quality of life without sacrificing efficacy and safety.
Most patients diagnosed with MBC who received oral vinorelbine received anthracycline- or taxane-containing neoadjuvant or adjuvant therapies (74.2%). Oral vinorelbine was mostly prescribed as a treatment for MBC, either as a single-agent chemotherapy (81.4%; including those in combination with other therapies, such as anti-HER2 therapy, endocrine therapy, and anti-angiogenesis therapy, except chemotherapy) or as combined chemotherapy (18.6%). Single-agent and combined chemotherapy did not demonstrate a statistically significant difference in PFS (6.4 months [95% CI, 4.9–8.0 months] vs. 6.0 months [95% CI, 5.0–7.0 months], P = 0.216). Several studies have shown that the median PFS of single-agent oral vinorelbine for MBC ranged between 5.2 and 5.5 months, while that of vinorelbine combined with chemotherapy was between 4.5 and 7.1 months. However, in previous real-world studies, the PFS was shorter than in clinical studies (2.7–4.9 months). In this study, the median PFS was 6.2 months, which appears to be comparable to that in previous clinical studies and longer than that in other real-world studies. It may be because most patients in our study were treated with combination therapy.
In the overall study population, the median PFS of oral vinorelbine was 6.2 months (95% CI, 5.0–7.4). The median PFS for patients who received oral vinorelbine as first-line chemotherapy was 9.5 months (95% CI, 7.0–12.0), compared with 6.0 months (95% CI, 4.2–7.8) when given two to three lines of chemotherapy and 4.3 months (95% CI, 2.9–5.7) when given four or more lines of chemotherapy. Steger et al. [17] performed an open-label single-arm international phase II study, which enrolled 70 patients with histologically confirmed hormone receptor-positive breast carcinoma and documented bone involvement with or without other non-visceral metastatic disease sites. All patients were treated with first-line chemotherapy. The study results showed that the median PFS was 8.2 months (95% CI, 5.5–9.8). Our study observed a longer PFS, which may be because most patients in our study were treated with combination therapy, while those in the NORBREAST-228 trial were treated with monotherapy. Some studies have reported that oral vinorelbine combined with capecitabine as first-line chemotherapy has a PFS between 8.4 and 8.6 months and an OS between 27.2 and 29.2 months [30,31]. In our study, the median PFS of patients combined chemotherapy was 6.0 months, which was shorter than other two studies’ PFS (oral vinorelbino combined with capecitabine). This is because their studies were first-line treatment, while our study was multiple lines treatment.
No treatment-associated deaths were observed in our study. Most of the AEs were manageable through symptomatic treatment, dose adjustment, or dose interruption. As reported previously, myelosuppression is the most common hematologic toxicity following oral vinorelbine administration [17,25,29]. The main manifestations of myelosuppression observed in our study were leukopenia, neutropenia, anemia, and thrombocytopenia. We found that 142/194 (73.2%) patients exhibited leukopenia during oral vinorelbine treatment, and of these, 54/194 (27.8%) achieved grades 3–4 AEs. In addition, nausea, vomiting, anorexia, fatigue, and diarrhea have been reported to be the most common non-hematologic toxicities during oral vinorelbine treatment [17,29]. Our results showed that the most common non-hematologic toxicities were diarrhea (46.9%), vomiting (32.0%), nausea (30.9%), and hepatic dysfunction (29.9%). The toxicity incidence in our study was similar to that reported previously [17,25,29].
The current study is a real-world observational study. Like most retrospective and real-world cohort studies, our study has some limitations. The retrospective design and single-center nature of this study may inevitably lead to bias. Additionally, the difference in combination regimens may increase the difference in efficacy and AEs.
The results of the current study demonstrated that oral vinorelbine as single agent or combination chemo-therapy might bring clinical benefits for patients with MBC. Considering its high efficacy, manageable toxicity, ease of administration, and ability to improve quality of life, oral vinorelbine is a good alternative therapy for patients with MBC.
The findings of this single center real-world study in China showed that oral vinorelbine appeared to be efficacious for MBC, with acceptable toxicity, and can be used for the treatment of MBC patients.
Authors’ Contribution: Study conception and design: JH, CD; data collection: JH, XB, LC, XL, LS and PH; analysis and interpretation of results: JH, XX and QZ; draft manuscript preparation: JH, CD. All authors reviewed the results and approved the final version of the manuscript.
Ethical Approval and Informed Consent Statement: The present study was approved by the National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital & Shenzhen Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College. Additionally, due to the retrospective design of the current study and patient anonymization, the ethical review board determined that informed consent was not required. All methods were performed in accordance with relevant guidelines and regulations.
Funding Statement: This work was supported by funds from Shenzhen Basic Research Program (2018, JCYJ20180306171227129), the National Natural Science Foundation of China (No. 81671750, 2016) and the National Natural Science Foundation of Guangdong Province (No. 2016A030312008).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Bray, F., Ferlay, J., Soerjomataram, I., Siegel, R. L., Torre, L. A. et al. (2018). Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians, 68(6), 394–424. DOI 10.3322/caac.21492. [Google Scholar] [CrossRef]
2. Chen, W., Zheng, R., Baade, P. D., Zhang, S., Zeng, H. et al. (2016). Cancer statistics in China, 2015. CA: A Cancer Journal for Clinicians, 66(2), 115–132. DOI 10.3322/caac.21338. [Google Scholar] [CrossRef]
3. Mayer, E. L., Burstein, H. J. (2007). Chemotherapy for metastatic breast cancer. Hematology/Oncology Clinics of North America, 21(2), 257–272. DOI 10.1016/j.hoc.2007.03.001. [Google Scholar] [CrossRef]
4. Fedele, P., Ciccarese, M., Surico, G., Cinieri, S. (2018). An update on first line therapies for metastatic breast cancer. Expert Opinion on Pharmacotherapy, 19(3), 243–252. DOI 10.1080/14656566.2018.1425680. [Google Scholar] [CrossRef]
5. Cardoso, F., Fallowfield, L., Costa, A., Castiglione, M., Senkus, E. et al. (2012). Locally recurrent or metastatic breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Annals of Oncology: Official Journal of the European Society for Medical Oncology, 23(suppl 7), vii11–vii19. DOI 10.1093/annonc/mds232. [Google Scholar] [CrossRef]
6. Gradishar, W. J., Anderson, B. O., Balassanian, R., Blair, S. L., Burstein, H. J. et al. (2018). Breast cancer, version 4. 2017, NCCN clinical practice guidelines in oncology. Journal of the National Comprehensive Cancer Network, 16(3), 310–320. DOI 10.6004/jnccn.2018.0012. [Google Scholar] [CrossRef]
7. Ngan, R. K. C. (2018). Management of hormone-receptor positive human epidermal receptor 2 negative advanced or metastatic breast cancers. Annals of Translational Medicine, 6(14), 284. DOI 10.21037/atm.2018.06.11. [Google Scholar] [CrossRef]
8. Heinemann, V., di Gioia, D., Vehling-Kaiser, U., Harich, H. D., Heinrich, B. et al. (2011). A prospective multicenter phase II study of oral and i.v. vinorelbine plus trastuzumab as first-line therapy in HER2-overexpressing metastatic breast cancer. Annals of Oncology, 22(3), 603–608. DOI 10.1093/annonc/mdq409. [Google Scholar] [CrossRef]
9. Vona-Davis, L., Rose, D. P., Hazard, H., Howard-McNatt, M., Adkins, F. et al. (2008). Triple-negative breast cancer and obesity in a rural Appalachian population. Cancer Epidemiology, Biomarkers & Prevention, 17(12), 3319–3324. DOI 10.1158/1055-9965.EPI-08-0544. [Google Scholar] [CrossRef]
10. Carey, L. A., Perou, C. M., Livasy, C. A., Dressler, L. G., Cowan, D. et al. (2006). Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA, 295(21), 2492–2502. DOI 10.1001/jama.295.21.2492. [Google Scholar] [CrossRef]
11. Rakha, E. A., El-Rehim, D. A., Paish, C., Green, A. R., Lee, A. H. S. et al. (2006). Basal phenotype identifies a poor prognostic subgroup of breast cancer of clinical importance. European Journal of Cancer, 42(18), 3149–3156. DOI 10.1016/j.ejca.2006.08.015. [Google Scholar] [CrossRef]
12. Yin, L., Duan, J. J., Bian, X. W., Yu, S. C. (2020). Triple-negative breast cancer molecular subtyping and treatment progress. Breast Cancer Research, 22(1), 61. DOI 10.1186/s13058-020-01296-5. [Google Scholar] [CrossRef]
13. Lehmann, B. D., Bauer, J. A., Chen, X., Sanders, M. E., Chakravarthy, A. B. et al. (2011). Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. Journal of Clinical Investigation, 121(7), 2750–2767. DOI 10.1172/JCI45014. [Google Scholar] [CrossRef]
14. Fellous, A., Ohayon, R., Vacassin, T., Binet, S., Lataste, H. et al. (1989). Biochemical effects of Navelbine on tubulin and associated proteins. Seminars in Oncology, 16(2 Suppl 4), 9–14. [Google Scholar]
15. Binet, S., Chaineau, E., Fellous, A., Lataste, H., Krikorian, A. et al. (1990). Immunofluorescence study of the action of navelbine, vincristine and vinblastine on mitotic and axonal microtubules. International Journal of Cancer, 46(2), 262–266. DOI 10.1002/(ISSN)1097-0215. [Google Scholar] [CrossRef]
16. Galano, G., Caputo, M., Tecce, M. F., Capasso, A. (2011). Efficacy and tolerability of vinorelbine in the cancer therapy. Current Drug Safety, 6(3), 185–193. DOI 10.2174/157488611797579302. [Google Scholar] [CrossRef]
17. Steger, G. G., Dominguez, A., Dobrovolskaya, N., Giotta, F., Tubiana-Mathieu, N. et al. (2018). Single-agent oral vinorelbine as first-line chemotherapy for endocrine-pretreated breast cancer with bone metastases and no visceral involvement: NORBREAST-228 Phase II Study. Clinical Breast Cancer, 18(1), e41–e47. DOI 10.1016/j.clbc.2017.05.012. [Google Scholar] [CrossRef]
18. Bruno, S., Puerto, V. L., Mickiewicz, E., Hegg, R., Texeira, L. et al. (1995). Phase II trial of weekly IV vinorelbine as a single agent in first-line advanced breast cancer chemotherapy. The Latin-American experience. American Journal of Clinical Oncology, 18(5), 392–396. DOI 10.1097/00000421-199510000-00006. [Google Scholar] [CrossRef]
19. Banna, G. L., Collovà, E., Gebbia, V., Lipari, H., Giuffrida, P. et al. (2010). Anticancer oral therapy: Emerging related issues. Cancer Treatment Reviews, 36(8), 595–605. DOI 10.1016/j.ctrv.2010.04.005. [Google Scholar] [CrossRef]
20. Barni, S., Freier, B., Garau, I., Mouysset, J. L., Sediva, M. et al. (2016). Burden of advanced breast cancer for patients and caregivers in Europe: Comparison of two treatment forms of vinorelbine, oral and intravenous. Current Medical Research Opinion, 32(11), 1807–1812. DOI 10.1080/03007995.2016.1211518. [Google Scholar] [CrossRef]
21. Aapro, M., Ruiz-Borrego, M., Hegg, R., Kukielka-Budny, B., Morales, S. et al. (2019). Randomized phase II study evaluating weekly oral vinorelbine versus weekly paclitaxel in estrogen receptor-positive, HER2-negative patients with advanced breast cancer (NorBreast-231 trial). Breast, 45(7), 7–14. DOI 10.1016/j.breast.2019.01.009. [Google Scholar] [CrossRef]
22. Mansour, M., Mourad, C. (2013). Phase II study of single agent oral vinorelbine as first-line treatment in patients with HER-2 negative metastatic breast cancer. Cancer Chemotherapy and Pharmacology, 72(2), 429–435. DOI 10.1007/s00280-013-2216-8. [Google Scholar] [CrossRef]
23. Brems-Eskildsen, A. S., Linnet, S., Danø, H., Luczak, A., Vestlev, P. M. et al. (2020). Metronomic treatment of vinorelbine with oral capecitabine is tolerable in the randomized phase 2 study XeNa including patients with HER2 non-amplified metastatic breast cancer. Acta Oncologica, 60(2), 157–164. DOI 10.1080/0284186X.2020.1851045. [Google Scholar] [CrossRef]
24. Lorusso, V., Spada, M., Giampaglia, M., Misino, A., Calabrese, R. et al. (2006). Oral vinorelbine plus capecitabine (oral vincap) combination in patients with advanced breast cancer (ABC). A phase II study of the GOIM (Gruppo Oncologico dell’Italia Meridionale). Annals of Oncology, 17(suppl 7), vii15–vii17. DOI 10.1093/annonc/mdl942. [Google Scholar] [CrossRef]
25. Blancas, I., Aguirre, E., Morales, S., Gonzalvez, M. L., Servitja, S. et al. (2019). Real-world data on the efficacy and safety of weekly oral vinorelbine in breast cancer patients previously treated with anthracycline or taxane-based regimens. Clinical & Translational Oncology, 21(4), 459–466. DOI 10.1007/s12094-018-1946-9. [Google Scholar] [CrossRef]
26. Heudel, P., Delaloge, S., Parent, D., Madranges, N., Levy, C. et al. (2020). Real-world evaluation of oral vinorelbine in the treatment of metastatic breast cancer: An ESME-MBC study. Anticancer Research, 40(7), 3905–3913. DOI 10.21873/anticanres.14381. [Google Scholar] [CrossRef]
27. Marty, M., Fumoleau, P., Adenis, A., Rousseau, Y., Merrouche, Y. et al. (2001). Oral vinorelbine pharmacokinetics and absolute bioavailability study in patients with solid tumors. Annals of Oncology, 12(11), 1643–1649. DOI 10.1023/A:1013180903805. [Google Scholar] [CrossRef]
28. Lorusso, V., Cinieri, S., Giampaglia, M., Ciccarese, M., Tinelli, A. et al. (2010). Intravenous versus oral vinorelbine plus capecitabine as second-line treatment in advanced breast cancer patients. A retrospective comparison of two consecutive phase II studies. Breast, 19(3), 214–218. DOI 10.1016/j.breast.2010.01.015. [Google Scholar] [CrossRef]
29. Huang, L., Wang, X., Zhou, L., Di, L., Zheng, H. et al. (2020). Oral vinorelbine versus intravenous vinorelbine, in combination with epirubicin as first-line chemotherapy in Chinese patients with metastatic breast cancer. Cancer Chemotherapy and Pharmacology, 85(1), 205–215. DOI 10.1007/s00280-019-04000-3. [Google Scholar] [CrossRef]
30. Tubiana-Mathieu, N., Bougnoux, P., Becquart, D., Chan, A., Conte, P. F. et al. (2009). All-oral combination of oral vinorelbine and capecitabine as first-line chemotherapy in HER2-negative metastatic breast cancer: An international phase II trial. British Journal of Cancer, 101(2), 232–237. DOI 10.1038/sj.bjc.6605156. [Google Scholar] [CrossRef]
31. Tawfik, H., Rostom, Y., Elghazaly, H. (2013). All-oral combination of vinorelbine and capecitabine as first-line treatment in HER2/Neu-negative metastatic breast cancer. Cancer Chemotherapy and Pharmacology, 71(4), 913–919. DOI 10.1007/s00280-013-2082-4. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |