
 | Oncologie |  |
DOI: 10.32604/oncologie.2022.020864
REVIEW
Technological Innovations in Thyroid Cancer Surgery
Department of Surgical Sciences, University of Cagliari, Policlinico Universitario Duilio Casula, Monserrato (CA), 09042, Italy
*Corresponding Author: Federico Cappellacci. Email: fedcapp94@gmail.com
Received: 16 December 2021; Accepted: 08 February 2022
Abstract: Thyroid cancer is the fifth most common cancer in the USA, with differentiated subtype accounting for more than 95% of neoplasm. Surgery remains the mainstay of treatment, either with lobectomy or total thyroidectomy. In the last decades, many technological innovations have been introduced in this field. The aim of this review is to illustrate the most recent advances regarding the classical surgical approach, particularly regarding hemostatic devices, parathyroid identification with fluorescence systems, intraoperative identification of lymph node metastases, and intraoperative neuromonitoring.
Keywords: Thyroid cancer; thyroid surgery; surgical innovations
Thyroid cancer is the fifth most common cancer in the USA; it is estimated that over 44 000 new cases occurred in men and women during 2021. The incidence of thyroid cancer is still rising worldwide, probably due to the increased use of diagnostic imaging and surveillance [1,2]. Differentiated thyroid cancer (DTC) is the most common subtype, accounting for more than 95% of cases [2,3]. DTC is considered a tumor with a good prognosis, with an overall survival nearly comparable to that of the general population; nevertheless, a certain number of patients experience a poor clinical outcome, with local recurrence, mainly due to nodal metastasis, requiring further medical or surgical treatment, with a considerable worsening of the quality of life [2,4]. Although conservative techniques have been developed for some benign lesions, such as radiofrequency ablation or percutaneous ethanol ablation, surgery remains the mainstay of treatment for thyroid cancer, either with lobectomy or total thyroidectomy; as a result, thyroidectomy represents the most performed operation in endocrine surgery [2,4,5]. Complications are mainly represented by hypoparathyroidism (transient or permanent), recurrent laryngeal nerve (RLN) injury (unilateral or bilateral, transient or permanent), and cervical hematoma [6–15]. Data in the literature shows that re-operative thyroid surgery is a technical challenge with an higher incidence of complications if compared to primary surgery. Scarring, edema, and friability of the tissues together with distortion of the landmarks make re-operative surgery hazardous [16–18]. New technologies have had a positive impact on our ability to diagnose and treat many surgical conditions, including thyroid cancer: in recent decades, many technological innovations, either regarding classical ultrasound diagnosis, i.e., utilizing contrast-enhanced ultrasound (CEUS) in adjunct to standard ultrasound evaluation, or specific molecular tests, have been introduced in this field [5,19–23]. Furthermore, these technological innovations could allow to minimize the need for reoperations, potentially reducing the occurrence of postoperative complications.
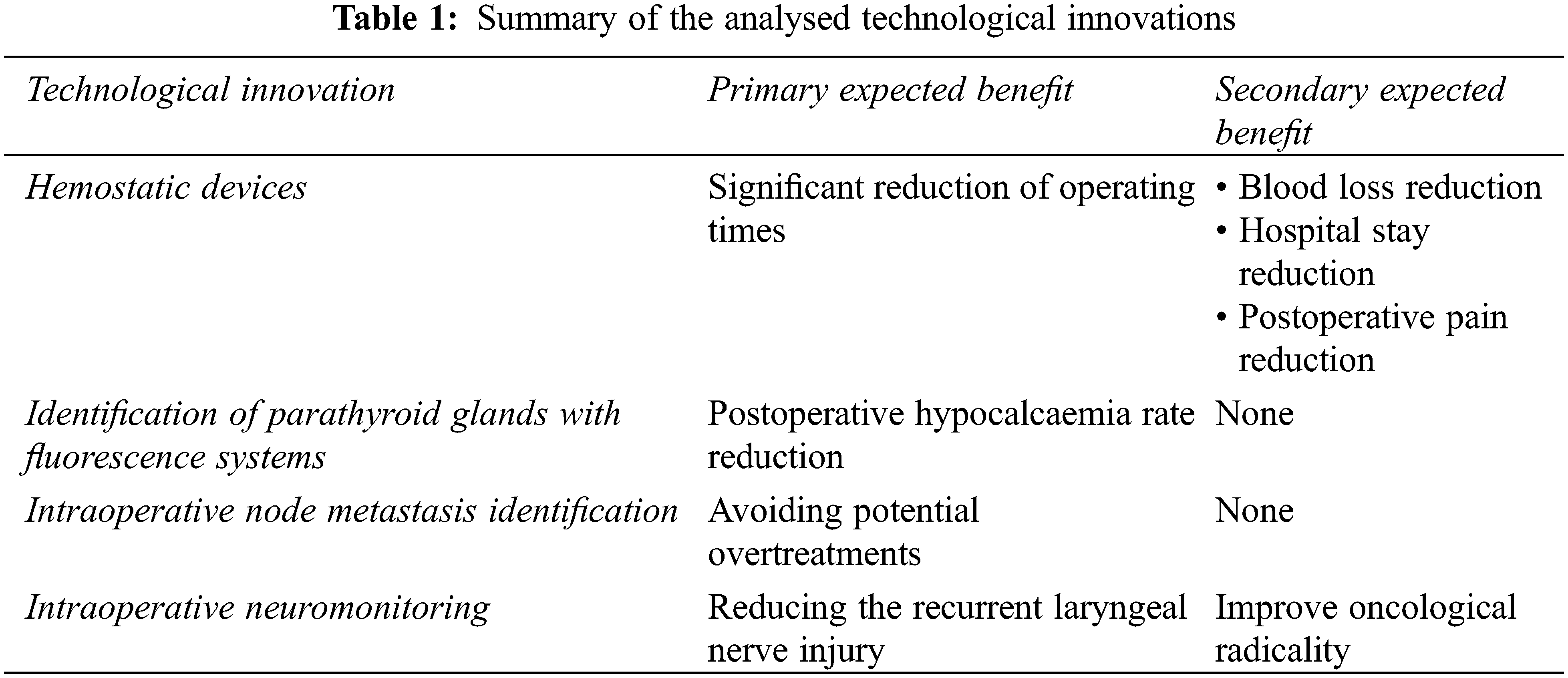
The aim of this review is to illustrate the most recent technological advances, particularly regarding hemostatic devices, parathyroid identification with fluorescence systems, intraoperative identification of lymph node metastases and intraoperative neuromonitoring. A brief summary of the analyzed technologies is present in the Table 1.
The thyroid gland is a highly vascularized organ; therefore, postoperative neck hematoma represents a major concern for surgeons. Achieving accurate hemostasis, preventing bleeding in the operating field, and allowing an adequate view of the anatomic structures is mandatory to avoid the occurrence of complications. Furthermore, postoperative neck bleeding may bring airway compression and respiratory distress, if not promptly treated [11,24].
Hemostasis has historically been considered a crucial point in thyroid surgery. During the 1800s, the mortality rate from thyroidectomy was approximately 40%, mainly due to intraoperative and postoperative hemorrhage. At that time many surgeons warned against performing such procedure: in 1846, Robert Liston stated: “there was a grave risk of death from hemorrhage during thyroid operations and that it was a proceeding by no means to be thought of”; John Dieffenbach, in 1848, described thyroid surgery as “one of the most thankless, most perilous undertakings which, if not altogether prohibited, should at least be restricted”. In 1850, thyroidectomy was condemned by the French Academy of Medicine. In the early 1900s, thyroid surgery was profoundly revolutionized by Emil Theodor Kocher: through a precise surgical technique and accurate hemostasis, he was able to drastically reduce mortality and morbidity [11,24–27].
Nowadays, postoperative hematoma occurs approximately in 0.1% to 1.1% of thyroidectomies, with almost all cases presenting within the first 6 h after the operation, whereas late bleeding after thyroidectomy is anecdotal [9–11].
Since Kocher, numerous technological innovations have been introduced. In particular, the so-called energy-based device (EBD) can be considered a milestone in thyroidectomy. Traditionally, the clamp-and-tie technique, with or without monopolar or bipolar electrocautery, has been used to achieve hemostasis. Over the years, innovative energy-based devices, using different forms of energy, have been introduced. Currently, these instruments are widely used in this kind of surgery [12,22,24,25,27–29]. In addition, so-called hemostatic agents have also been introduced. These agents have been broadly classified into three groups: topical hemostats which cause blood to clot at a bleeding surface, sealants which prevent leakage from tissues including vessels, and adhesives which bond tissues. However, as highlighted in a 2018 meta-analysis performed by Khadra et al. [28–30], their usage in thyroid surgery yields minimal advantages for the management of perioperative bleeding risk, while they effectively reduce the drain output and the hospital stays.
Energy-based devices can be classified, based on the mechanism of hemostatic/vascular sealing provided, into three categories: ultrasonic systems, electrothermal bipolar (radiofrequency) systems, and hybrid systems combining both energy modalities [25,26].
Given this classification, the most commonly used EBDs are Harmonic Focus plus (HF; Ethicon, Johnson and Johnson, Cincinnati, OH, USA), which uses ultrasound energy, LigaSure Small Jaw and the most recent Ligasure Exact dissector (LS and LE; Medtronic, Covidien Products, Minneapolis, MN, USA), which exploits radio frequencies, and the Thunderbeat Open Fine Jaw (TB; Olympus, Japan), which belongs to the hybrid systems category [24,26,31].
Since their introduction, several studies in the literature have examined the differences between the use of EBD and classical clamp and tie techniques and among the devices themselves.
There is now unanimous agreement that EBD significantly reduces operating times compared with the standard clamp and tie technique.
The use of HF is extensively studied in the literature, and all studies agree that it significantly reduces operative times compared to standard clamp and tie techniques [32–36].
A meta-analysis, performed by Ecker et al. [35] in 2010, compared the standard clamp and tie technique vs. the harmonic scalpel (HS), which was the HF precursor. Their results showed a 17 min operative time reduction when HS was compared to clamp and tie, a 23 min reduction when HS was compared to clamp and tie combined with monopolar cautery, and a 35 min reduction when HS was compared to clamp and tie with bipolar cautery. The mean operative time reduction among all the techniques was 22 min. Therefore, this meta-analysis highlights that HS has been associated with lower blood loss, lower postoperative pain, and shorter hospital stays than the standard technique, without an increase in the postoperative complication rate.
Similar results were obtained in several other studies in the literature. Revelli et al. [34], in their 2016 meta-analysis, confronted HS vs. HF vs. standard hemostasis techniques, obtaining a mean reduction in operative time of 25 min, either with HS or HF, with a significant reduction in blood loss, postoperative pain, and hospital stay compared to standard hemostatic techniques.
LS was compared with traditional techniques in a 2017 metanalysis by Luo et al. [37], which included thyroidectomy performed with HS, LS or the standard clamp and tie technique. The authors report a 13 min reduction in operative time when total thyroidectomy was performed utilizing LS compared to standard hemostatic techniques. Interestingly, they found that the use of HS was associated with a lower risk of definitive recurrent laryngeal nerve lesions than standard hemostasis.
A pilot study conducted by Papavramids et al. [31] in 2020 compared LE with other EBD (HS, LS and TB). The authors founded that LE effectively reduce operative time when compared with the others EBDs [31].
While some studies found no difference in complication rates when comparing EBDs vs. conventional clamp and tie techniques [33,35], others highlighted a reduction in postoperative transient hypocalcemia, postoperative cervical hematoma, and definitive recurrent laryngeal nerve palsies compared to standard hemostasis [36,37]. Similar results regarding postoperative hemorrhage were found comparing LS vs. the conventional clamp and tie technique, as highlighted by Grøndal et al. [38], while other authors did not find any difference regarding postoperative cervical hematoma or RLN injury rates when EBD was compared to the standard technique [11,39,40]. It is important to emphasize that some studies in the literature found an increased RLN thermal injuries and palsy in the EBD group, mainly because of the thermal spread around the device and the tissue contraction. Therefore it is important to underline that the EBDs must be used with caution especially in non-expert hands, and in any case at a safe distance from structures considered noble, such as RLN and parathyroid glands, to preserve their functionality [41–43].
Far fewer studies comparing the different devices exist. Shorter operative times in thyroidectomies performed with TB compared to those with HF, as reported in the studies of Back et al. [44] and Papavramidis et al. [31], are probably due to a greater speed of TB in coagulation and cutting of tissues [24,31,44]. Dionigi et al. [45] found no significant difference in the rates of postoperative morbidity comparing HS and LS, while they found differences in clinically less significant endpoints, such as postoperative oral calcium supplementation, which was higher in the HF group.
The reduction in operative times allows optimization of the timing of the operating room, ultimately allowing the treatment of more patients with thyroid cancer during a single surgical session, balancing the higher costs of the devices without an increase in postoperative complication rates.
3 Identification of Parathyroid Glands with Fluorescence Systems
Postoperative hypocalcemia due to postoperative hypoparathyroidism represents the most common complication after thyroid surgery, accounting for more than half of total complications, with rates of approximately 15% to 30% for transient hypocalcemia and 1% to 7% for definitive [4,6–8,46–48]. Therefore, intraoperative identification and preservation of parathyroid glands (PGs) is mandatory during thyroidectomy. Particularly, treatment of thyroid carcinoma may require central neck compartment dissection and, in case of local recurrence, re-do surgeries, both of which increase the risk of postoperative hypoparathyroidism [2,4,8,16,49–51].
Although the current identification method is usually based on naked eye (N-E) individuation, utilizing visual inspection and palpation by the surgeon, with careful preservation of the PGs blood supply, some innovative techniques have gradually established themselves as possible adjuncts to aid the identification of PGs.
Fluorescence imaging is an exponentially developing technology, that is gaining acceptance in many surgical fields, including thyroid surgery [52]. Fluorescence occurs when a material is illuminated by light of shorter wavelengths and, within nanoseconds, emits light with longer wavelengths. In some tissues, such as PGs, this can result in native fluorescence, or “autofluorescence,” which enables the use of a label-free autofluorescence detection technique in tissues [53,54].
This technology allows PGs to spontaneously remit light at a wavelength between 820 and 830 nm (autofluorescence) when exposed to near-infrared light at a wavelength of 785 nm, creating a contrast between thyroid tissues and parathyroid tissues, allowing intraoperative differentiation without the use of a contrast agent (NIR-AF) [53]. One limitation of this technique is that, in some cases, the difference between thyroid and parathyroid tissues can be difficult to distinguish due to the similarity of the autofluorescence intensities of the two tissues [55].
Another possibility is to use a contrast agent, almost always indocyanine green (ICG), which concentrates in the parathyroid tissue and emits fluorescence when the affected area is visualized with dedicated cameras (NIR-ICG). The recommended dose for PG visualization is 0.2–0.5 mg/kg, which may be repeated as required without exceeding the maximal daily dose of 5 mg/kg [56–58]. Both techniques have shown promising results in several studies in the literature [46–48,54,59,60].
The main difference between NIR-AF and NIR-ICG is that the first method allows the identification of PGs without providing information on their vascularization and, therefore, on their functionality. PG autofluorescence is also preserved after gland resection, with the fluorophore known to be resistant to heat, freezing, and formalin fixation [55,61,62].
Using a combination of both methods can correctly identify PGs and provide real-time information regarding their vascularization and functionality after thyroid resection [55,63–65].
A recently published meta-analysis by Kim et al. [54] investigated the diagnostic accuracy of the NIR-AF method to identify the parathyroid glands during thyroidectomy. The authors included 1198 patients from 17 studies. Their results demonstrated that this technique can correctly identify PGs, with a sensitivity, specificity, negative predictive value, and positive predictive value of 0.9693 (0.9491; 0.9816), 0.9248 (0.8885; 0.9499), 0.9517 (0.8981; 0.9778), and 0.9488 (0.9167; 0.9689), respectively. This study proved that NIR-AF PG detection is a useful auxiliary method for identifying PGs during thyroid surgery.
In a multicentric randomized clinical trial, published in 2020, including 241 patients, NIR-AF lowered the temporary postoperative hypocalcemia rate from 22% to 9% and parathyroid inadvertent resection rates from 14% to 3%, suggesting that this technology could limit postoperative hypoparathyroidism [64].
In a 2020 meta-analysis of 13 studies, Barbieri et al. [46] compared the use of NIFI techniques, either NIR-AF, NIR-ICG, or both, in reducing short-, medium-, and long-term hypocalcemia and hypoparathyroidism following total thyroidectomy compared to standard N-E individuation. They found that NIFI techniques significantly reduced short- and medium-term hypocalcemia following total thyroidectomy, with risk differences of 0.10 (0.07; 0.17) and 0.03 (0.01; 0.05), respectively. However, no significant differences were found in long-term hypocalcemia and short-medium- and long-term hypoparathyroidism [46].
Another meta-analysis, published in 2021 by Weng et al. [60], evaluated whether NIR-AF imaging reduces the risk of hypocalcemia after total thyroidectomy. The authors included 6 studies involving 2180 patients. The prevalence of transient hypocalcemia was 8.11% in the NIR-AF group and 25.19% in the N-E group (p < 0.0001), while the prevalence of permanent hypocalcemia was 0% in the NIR-AF group and 2.19% in the N-E group (p = 0.05) [60].
NIR-AF and NIR-ICG necessarily imply longer operative times: Lerchenberger et al. [66], in a study that compared these two methods during thyroid surgery, showed that the additional time needed to perform NIR-AF imaging and NIR-ICG imaging amounts to 5 to 10 min and 8 to 13 min, respectively, when compared to standard N-E identification [66].
To our knowledge, no cost-effectiveness study exists comparing NIFI techniques and N-E identification in thyroid surgery. These techniques could lead to a decrease in postoperative hypoparathyroidism; however, a clear usage protocol has not yet been validated.
4 Intraoperative Node Metastasis Identification
DTC represents the most common subtype of thyroid cancer, mainly due to the high incidence of papillary thyroid cancer (PTC). PTCs are considered slow-growing tumors with an excellent prognosis. However, the incidence of node metastases is high, ranging from 20% to 90% [1–4,51,67–69].
The real impact of node metastases on prognosis is still a matter of debate: reports in the literature demonstrate a reduction in disease-free survival but are divergent in overall survival [49,51,68,70,71].
The American Thyroid Association guidelines suggest prophylactic central lymph node compartment dissection (CLND) in patients with high-risk PTC [4].
Nevertheless, prophylactic CLND is still a controversial topic in the literature, as some authors suggest that it might reduce the incidence of local lymph node recurrence, while others highlight that it has a higher incidence of postoperative complications, especially hypoparathyroidism and RLN palsy [68,70–74].
Molecular biologic techniques have spread widely, both for preoperative diagnosis of many cancers, including DTC, and for intraoperative diagnosis of node metastases, mainly in breast carcinoma [19–20,75].
These assays allow us to quickly isolate, amplify and quantify mRNA encoding proteins selectively present in neoplastic cells, such as cytokeratine-19 (CK-19). One-step nucleic acid amplification (OSNA) is routinely used in the diagnosis of node metastasis in the sentinel lymph node of patients affected by breast cancer [75].
Several studies in the literature have evaluated whether the OSNA technique could be applicable in the intraoperative identification of PTC nodal metastases [75–83]. This could permit to perform a therapeutic CLND, that is based on the objective presence of lymph node metastases, hopefully improving the disease-free survival rate, reducing the locoregional recurrence incidence, and avoiding potential overtreatment of these patients.
There is a consensus among the studies in the literature that the OSNA technique is effective in the identification of lymph node metastases from PTC, with an optimal value of sensitivity, ranging from 87.5% to 89%, specificity, ranging from 86% to 94.4%, and a concordance rate with a standard histological evaluation of more than 90% [75–83].
However, its effectiveness in reducing the recurrence rate has yet to be evaluated, as none of the current studies exploit this aspect. Furthermore, the use of OSNA in thyroid cancer is still “off label”, thus further studies should define a standardized utilization, other than evaluating the cost-effectiveness.
5 Intraoperative Neuromonitoring
RLN injury is the most well-known complication of thyroid cancer surgery. It accounts for approximately 20% of all postoperative complications of thyroid surgery; it can be transient or definitive, unilateral or bilateral, with a total incidence in total thyroidectomy of approximately 4%–6% [84,85].
Unilateral vocal cord paralysis, arising from unilateral RLN injury, can present with symptoms such as dysphonia, aspiration, dysphagia, ineffective cough, and difficulty with maneuvers requiring glottic closure such as lifting. Voice changes may be significant enough to result in a change in vocation, or may be asymptomatic, making it difficult to correctly estimate the incidence of RLN injury [86,87].
Bilateral vocal cord paralysis has been described by Lahey as a “serious surgical calamity”, mainly due to the risk of needing to secure the airway surgically with a tracheostomy; overall, 50% of patients with bilateral vocal cord paralysis require acute airway intervention [88,89].
Visual identification of the RLN is considered the gold standard in the prevention of nerve injury during thyroid surgery [88,90,91]. In recent decades, the introduction of intraoperative neuromonitoring (IONM) has opened new perspectives for the study and preservation of the RLN. This is of relevance in the case of advanced thyroid cancer with local infiltration of the RLN; in fact, nerves invaded with malignancy may maintain electric stimulability. Numerous reports, as well as surgical guidelines, suggest that IONM is necessary for the optimal management of RLN invasion by malignant disease [87,90,92–94].
IONM can be distinguished into intermittent (I-IONM) and continuous (C-IONM).
I-IONM utilizes a stimulation probe to perform nerve stimulation during thyroidectomy; electromyography (EMG) activity is then shown on a screen, which provides useful information regarding nerve functionality [90]. Herein we report the recommendations of the International Neural Monitoring Study Group (INMSG) regarding the basic equipment and the standard procedure for performing IONM [95]:
“The basic equipment include the recording electrodes connected to the endotracheal tube to place in contact with the bilateral vocal fold, the neural stimulating electrodes to stimulate the external branch of superior laryngeal nerve, the Vagus nerve, and the recurrent laryngeal nerve during thyroid surgery. The standard procedure for performing IONM should include the laryngeal examination before and after surgery (L1-L2), stimulating the superior laryngeal nerve before and after upper thyroid pole dissection (S1-S2), and stimulating the vagus nerve and recurrent laryngeal nerve before and after the dissection”.
I-IONM contributes to RLN protection in several ways, allowing early and definite localization of the RLN and confirming the actual identification of the RLN (preventing visual RLN misidentification). However, I-IONM has relevant limitations. With I-IONM, assessment of the functional integrity of the RLN is limited to the short time interval of direct nerve stimulation. Moreover, with I-IONM the integrity of the laryngeal nerve is assessed only at the site of direct nerve stimulation: for proximal neurogenic lesions of the RLN, distal stimulation near the larynx may produce a false negative, “normal”, IONM signal [96,97].
The actual usefulness of I-IONM is still a matter of debate in the literature. Some authors suggest that routine I-IONM does not decrease unilateral RLN injuries, both transient and permanent, compared with visualization alone [90,91,98], while others report a lower incidence of transient vocal cord paralysis following RLN injury when I-IONM is utilized, particularly in high-risk surgery, such as reinterventions and advanced thyroid cancers [99–101]. As reported by Barczyński et al. [102] in 2011, the use of IONM in thyroid cancer surgery decreased the risk of RLN injury by 3.7%, including a 3% drop in the risk of transient damage and a 0.7% drop in the risk of permanent damage. The improvement of these outcomes was accompanied by a significant increase in the radicality of thyroid tissue resection in the group operated on with IONM as assessed by postoperative 131I uptake, and the percentage of patients with iodine uptake below 1% increased when IONM was employed by as much as 45% [90,102].
A 2019 Cochrane library meta-analysis found no difference in transient or permanent RLN injury following thyroidectomy performed either with or without I-IONM [103]. Similar results were obtained in a 2016 meta-analysis performed by Lombardi et al. [98], which found no difference in terms of RLN injury when I-IONM was utilized.
Against that, Yang et al., in their 2017 meta-analysis highlighted a lower incidence of transient RLN lesions when I-IONM was performed, although no difference in terms of permanent injury was found [99].
Some authors suggested that I-IONM could be an educational adjunct. Younger, less experienced surgeons have outcomes equivalent to experienced surgeons if they use IONM during their learning curve; IONM has been shown to lower the incidence of permanent RLN palsy in the hands of low-volume surgeons [104,105].
Moreover, Sari et al. [106] showed that I-IONM decreases the operative time compared to visualization alone by shortening the time needed to identify the RLN [104,106,107].
To overcome the I-IONM limits, continuous IONM technology has been proposed. This technology comprises automatic periodic stimulation of the vagus nerve with an electrode placed on the vagus nerve between the common carotid artery and internal jugular vein and an automatic software-based assessment of changes in EMG amplitude during surgery, providing permanent visual and acoustic feedback to the surgeon of the current RLN conductivity and allowing continuous evaluation of the RLN [96,108–112].
Continuous IONM demonstrated a statistically significant difference in terms of permanent RLN lesions in a retrospective analysis conducted by Schneider et al. [113], in which there were no cases of permanent lesions in the C-IONM group, whereas four cases of permanent injury were noted in the I-IONM group [111,113].
Notably, Terris et al. [114] reported that C-IONM might cause serious patient harm (i.e., hemodynamic instability and reversible vagal neuropraxia) attributable to the monitoring apparatus and the manipulations of the vagus nerve, although it is important to highlight that these are extremely rare complications [114].
While the usefulness of I-IONM in preventing unilateral RLN lesions is still uncertain, the implementation of recent protocols in the case of unilateral loss of signal (LOS) while utilizing IONM, either I-IONM or C-IONM, has made it possible to reduce the risk of bilateral RLN injury to nearly zero, introducing the so-called “two-stage thyroidectomy”, by which we mean the postponement of resection of the contralateral side due to LOS during resection of the first thyroid lobe [86,87,104,115–118].
The INMSG has classified nerve injury into two categories: segmental injury (Type I), which involves a clear-cut RLN segment that is lesioned; and global injury (Type II), where the entire RLN and vagus nerve are nonconductive, indicating an intralaryngeal focus of injury [107,119,120].
The INMSG guideline 2018 on Staging Bilateral Thyroid Surgery with LOS, recommend [86]:
1. That neural monitoring information should be obtained and utilized in the strategy of a planned bilateral procedure by staging the surgery in the setting of ipsilateral LOS. This algorithm should be shared and discussed with the patient during the preoperative informed consent process.
2. The INMSG feels a surgeon should prioritize concern for the obvious significant medical and psychological morbidity of bilateral VCP and possible tracheotomy (even temporary) over perceived surgical convenience, the routine of doing the “planned procedure” or the potential perceived impact on surgical reputation by openly acknowledging the surgical complication of ipsilateral loss of signal. The full benefit of neural monitoring information in this surgical setting is appreciated through both optimization of the patient’s quality of life and surgical cost.
As IONM use in thyroid surgery increased, surgical residency programs have begun including IONM courses in their core curricula. The training evolution for implementation of basic and advanced IONM system (IONM needs, stages and benefits) will improve the surgical residency and surgeon’s practice and maturity of autonomous IONM operations [95].
There is unanimous agreement that when thyroid surgery is performed using IONM it is necessary to give the patient adequate informed consent, which fully explains the various possibilities described in the case of LOS [121].
The implementation of this protocol has made it possible to virtually eliminate the possibility of bilateral RLN injuries and represents the major advantage of IONM.
The past few decades have seen a dramatic improvement in the available technologies for thyroid cancer surgery, aiming for a lower complication incidence and an improvement in patient quality of life. Although the real usefulness of some of these techniques has yet to be proven, such as fluorescence techniques and the intraoperative study of lymph node metastases with the OSNA technique, there is no doubt that the use of EBD and IOMM has brought considerable advantages in the treatment of these malignancies.
Author Contributions: Federico Cappellacci: Design of the study, analysis of data, drafting the manuscript, final approval of the version to be published. Gian Luigi Canu: Acquisition of data, analysis of data, critical revision of the manuscript, final approval of the version to be published. Stefano Piras: Critical revision of the manuscript, final approval of the version to be published. Giacomo Anedda: Interpretation of data, critical revision of the manuscript, final approval of the version to be published. Pietro Giorgio Calò: Interpretation of data, critical revision of the manuscript, final approval of the version to be published. Fabio Medas: Design of the study, analysis and interpretation of data, drafting the manuscript, final approval of the version to be published.
Ethics Approval and Informed Consent Statement: Not applicable.
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Howlader, N., Noone, A. M., Krapcho, M., Miller, D., Brest, A. et al. (2021). SEER Cancer Statistics Review, 1975, 2018, National Cancer Institute. Bethesda, MD. https://seer.cancer.gov/csr/1975_2018/. [Google Scholar]
2. Cabanillas, M. E., McFadden, D. G., Durante, C. (2016). Thyroid cancer. Lancet 3, 388(10061), 2783–2795. DOI 10.1016/S0140-6736(16)30172-6. [Google Scholar] [CrossRef]
3. Ulisse, S., Baldini, E., Lauro, A., Pironi, D., Tripodi, D. et al. (2021). Papillary thyroid cancer prognosis: An evolving field. Cancers. 7, 13(21), 5567. DOI 10.3390/cancers13215567. [Google Scholar] [CrossRef]
4. Haugen, B. R., Alexander, E. K., Bible, K. C., Doherty, G. M., Mandel, S. J. et al. (2016). 2015 American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid, 26(1),1–133. DOI 10.1089/thy.2015.0020. [Google Scholar] [CrossRef]
5. Radzina, M., Cantisani, V., Rauda, M., Nielsen, M. B., Ewertsen, C. et al. (2017). Update on the role of ultrasound guided radiofrequency ablation for thyroid nodule treatment. International Journal of Surgery, 41(1), S82–S93. DOI 10.1016/j.ijsu.2017.02.010. [Google Scholar] [CrossRef]
6. Medas, F., Canu, G. L., Cappellacci, F., Romano, G., Amato, G. et al. (2021). Antibiotic prophylaxis for thyroid and parathyroid surgery: A systematic review and meta-analysis. Otolaryngology–Head and Neck Surgery: Official Journal of American Academy of Otolaryngology-Head and Neck Surgery, 164(3), 482–488. DOI 10.1177/0194599820947700. [Google Scholar] [CrossRef]
7. Canu, G. L., Medas, F., Longheu, A., Boi, F., Docimo, G. et al. (2019). Correlation between iPTH levels on the first postoperative Day after total thyroidectomy and permanent hypoparathyroidism: Our experience. Open Medicine, 14, 437–442. DOI 10.1515/med-2019-0047. [Google Scholar] [CrossRef]
8. Puzziello, A., Rosato, L., Innaro, N., Orlando, G., Avenia, N. et al. (2014). Hypocalcemia following thyroid surgery: Incidence and risk factors. A longitudinal multicenter study comprising 2,631 patients. Endocrine, 47(2), 537–542. DOI 10.1007/s12020-014-0209-y. [Google Scholar] [CrossRef]
9. Calò, P. G., Erdas, E., Medas, F., Pisano, G., Barbarossa, M. et al. (2013). Late bleeding after total thyroidectomy: Report of two cases occurring 13 days after operation. Clinical Medicine Insights. Case Reports, 6, 165–170. DOI 10.4137/CCRep.S13024. [Google Scholar] [CrossRef]
10. Calò, P. G., Pisano, G., Piga, G., Medas, F., Tatti, A. et al. (2010). Postoperative hematomas after thyroid surgery. Incidence and risk factors in our experience. Annali Italiani di Chirurgia, 81(5), 343–347. [Google Scholar]
11. Materazzi, G., Ambrosini, C. E., Fregoli, L., de Napoli, L., Frustaci, G. et al. (2017). Prevention and management of bleeding in thyroid surgery. Gland Surgery, 6(5), 510–515. DOI 10.21037/gs.2017.06.14. [Google Scholar] [CrossRef]
12. Cocchiara, G., Cajozzo, M., Amato, G., Mularo, A., Agrusa, A. et al. (2010). Terminal ligature of inferior thyroid artery branches during total thyroidectomy for multinodular goiter is associated with higher postoperative calcium and PTH levels. Journal of Visceral Surgery, 147(5), e329–e332. DOI 10.1016/j.jviscsurg.2010.08.020. [Google Scholar] [CrossRef]
13. Erdas, E., Medas, F., Podda, F., Furcas, S., Pisano, G. et al. (2015). The use of a biologic topical haemostatic agent (TachoSil(®)) for the prevention of postoperative bleeding in patients on antithrombotic therapy undergoing thyroid surgery: A randomised controlled pilot trial. International Journal of Surgery, 20, 95–100. DOI 10.1016/j.ijsu.2015.06.027. [Google Scholar] [CrossRef]
14. Pisano, G., Canu, G. L., Erdas, E., Medas, F., Calò, P. G. (2019). Tracheostomy after total thyroidectomy: Indications and results in a series of 3214 operations. Minerva Chirurgica, 74(3),277–278. DOI 10.23736/S0026-4733.19.07907-0. [Google Scholar] [CrossRef]
15. Gambardella, C., Polistena, A., Sanguinetti, A., Patrone, R., Napolitano, S. et al. (2017). Unintentional recurrent laryngeal nerve injuries following thyroidectomy: Is it the surgeon who pays the bill? International Journal of Surgery, 41(Suppl 1), S55–S59. DOI 10.1016/j.ijsu.2017.01.112. [Google Scholar] [CrossRef]
16. Medas, F., Tuveri, M., Canu, G. L., Erdas, E., Calò, P. G. (2019). Complications after reoperative thyroid surgery: Retrospective evaluation of 152 consecutive cases. Updates in Surgery, 71(4), 705–710. DOI 10.1007/s13304-019-00647-y. [Google Scholar] [CrossRef]
17. Lefevre, J. H., Tresallet, C., Leenhardt, L., Jublanc, C., Chigot, J. P. et al. (2007). Reoperative surgery for thyroid disease. Langenbeck’s Archives of Surgery, 392(6), 685–691. DOI 10.1007/s00423-007-0201-6. [Google Scholar] [CrossRef]
18. Pironi, D., Panarese, A., Candioli, S., Manigrasso, A., La Gioia, G. et al. (2008). Reoperative thyroid surgery: Personal experience and review of the literature. Il Giornale di Chirurgia, 29(10),407–412. [Google Scholar]
19. Papale, F., Cafiero, G., Grimaldi, A., Marino, G., Rosso, F. et al. (2013). Galectin-3 expression in thyroid fine needle cytology (t-FNAC) uncertain cases: Validation of molecular markers and technology innovation. Journal of Cellular Physiology, 228(5),968–974. DOI 10.1002/jcp.24242. [Google Scholar] [CrossRef]
20. Baldini, E., Tuccilli, C., Pironi, D., Catania, A., Tartaglia, F. et al. (2021). Expression and clinical utility of transcription factors involved in epithelial-mesenchymal transition during thyroid cancer progression. Journal of Clinical Medicine, 10(18), 4076. DOI 10.3390/jcm10184076. [Google Scholar] [CrossRef]
21. Fresilli, D., David, E., Pacini, P., Del Gaudio, G., Dolcetti, V. et al. (2021). Thyroid nodule characterization: How to assess the malignancy risk. Update of the Literature. Diagnostics, 11(8),1374. DOI 10.3390/diagnostics11081374. [Google Scholar] [CrossRef]
22. Sorrenti, S., Dolcetti, V., Fresilli, D., Del Gaudio, G., Pacini, P. et al. (2021). The role of CEUS in the evaluation of thyroid cancer: From diagnosis to local staging. Journal of Clinical Medicine, 10(19), 4559. DOI 10.3390/jcm10194559. [Google Scholar] [CrossRef]
23. Tartaglia, F., Giuliani, A., Tromba, L., Carbotta, S., Karpathiotakis, M. et al. (2016). Fine needle aspiration cytology of 650 thyroid nodules operated for multinodular goiter: A cyto-histological correlation based on the new Italian cytological classification (siapec 2014). Journal of Biological Regulators and Homeostatic Agents, 30(4), 1187–1193. [Google Scholar]
24. Canu, G. L., Medas, F., Podda, F., Tatti, A., Pisano, G. et al. (2020). Thyroidectomy with energy-based devices: Surgical outcomes and complicationscomparison between harmonic focus, LigaSure small Jaw and thunderbeat open fine Jaw. Gland Surgery, 9(3), 721–726. DOI 10.21037/gs.2020.03.31. [Google Scholar] [CrossRef]
25. Bakkar, S., Papavramidis, T. S., Aljarrah, Q., Materazzi, G., Miccoli, P. (2020). Energy-based devices in thyroid surgery-an overview. Gland Surgery, 9(Suppl 1), S14–S17. DOI 10.21037/gs.2019.08.05. [Google Scholar] [CrossRef]
26. Konturek, A., Szpyra, B., Stopa-Barczyńska, M., Barczyński, M. (2020). Energy-based devices for hemostasis in thyroid surgery. Gland Surgery, 9(Suppl 2), S153–S158. DOI 10.21037/gs.2019.10.17. [Google Scholar] [CrossRef]
27. Hannan, S. A. (2006). The magnificent seven: A history of modern thyroid surgery. International Journal of Surgery, 4(3), 187–191. DOI 10.1016/j.ijsu.2006.03.002. [Google Scholar] [CrossRef]
28. Khadra, H., Bakeer, M., Hauch, A., Hu, T., Kandil, E. (2018). Hemostatic agent use in thyroid surgery: A meta-analysis. Gland Surgery, 7(Suppl 1), S34–S41. DOI 10.21037/gs.2018.03.02. [Google Scholar] [CrossRef]
29. Spotnitz, W. D., Burks, S. (2008). Hemostats, sealants, and adhesives: Components of the surgical toolbox. Transfusion, 48(7), 1502–1516. DOI 10.1111/j.1537-2995.2008.01703.x. [Google Scholar] [CrossRef]
30. Spotnitz, W. D., Burks, S. (2010). State-of-the-art review: Hemostats, sealants, and adhesives II: Update as well as how and when to use the components of the surgical toolbox. Clinical and Applied Thrombosis/Hemostasis, 16(5), 497–514. DOI 10.1177/1076029610363589. [Google Scholar] [CrossRef]
31. Papavramidis, T. S., Pliakos, I., Chorti, A., Panidis, S., Kotsovolis, G. et al. (2020). Comparing LigasureTM exact dissector with other energy devices in total thyroidectomy: A pilot study. Gland Surgery, 9(2), 271–277. DOI 10.21037/gs.2020.02.05. [Google Scholar] [CrossRef]
32. Garas, G., Okabayashi, K., Ashrafian, H., Shetty, K., Palazzo, F. et al. (2013). Which hemostatic device in thyroid surgery? A network meta-analysis of surgical technologies. Thyroid, 23(9), 1138–1150. DOI 10.1089/thy.2012.0588. [Google Scholar] [CrossRef]
33. Maeda, H., Kutomi, G., Satomi, F., Shima, H., Mori, M. et al. (2018). Comparison of surgical outcomes and complications between the harmonic FOCUS and conventional surgery for open thyroidectomy. Molecular and Clinical Oncology, 8(4), 553–556. DOI 10.3892/mco.2018.1569. [Google Scholar] [CrossRef]
34. Revelli, L., Damiani, G., Bianchi, C. B., Vanella, S., Ricciardi, W. et al. (2016). Complications in thyroid surgery. harmonic scalpel, harmonic focus versus conventional hemostasis: A meta-analysis. International Journal of Surgery, 28(Suppl 1), S22–S32. DOI 10.1016/j.ijsu.2015.12.050. [Google Scholar] [CrossRef]
35. Ecker, T., Carvalho, A. L., Choe, J. H., Walosek, G., Preuss, K. J. (2010). Hemostasis in thyroid surgery: Harmonic scalpel versus other techniques--A meta-analysis. Otolaryngology--Head and Neck Surgery, 143(1), 17–25. DOI 10.1016/j.otohns.2010.03.018. [Google Scholar] [CrossRef]
36. Calò, P. G., Pisano, G., Medas, F., Tatti, A., Tuveri, M. et al. (2012). The use of the harmonic scalpel in thyroid surgery. Our experience. Annali Italiani di Chirurgia, 83(1), 7–12. [Google Scholar]
37. Luo, Y., Li, X., Dong, J., Sun, W. (2017). A comparison of surgical outcomes and complications between hemostatic devices for thyroid surgery: A network meta-analysis. European Archives of Oto-Rhino-Laryngology, 274(3), 1269–1278. DOI 10.1007/s00405-016-4190-3. [Google Scholar] [CrossRef]
38. Grøndal, A., Høgsbro, M., Pryds, K., Pedersen, H. B., Jacobsen, H. (2021). Intra- and postoperative complications using LigaSure™ small Jaw in patients undergoing thyroidectomy: A register-based study. European Archives of oto-Rhinolaryngology, 278(11), 4491–4500. DOI 10.1007/s00405-021-06685-w. [Google Scholar] [CrossRef]
39. Pacilli, M., Tartaglia, N., Gerundo, A., Pavone, G., Fersini, A. et al. (2020). Energy based vessel sealing devices in thyroid surgery: A systematic review to clarify the relationship with recurrent laryngeal nerve injuries. Medicina, 56(12), 651. DOI 10.3390/medicina56120651. [Google Scholar] [CrossRef]
40. Hua, N., Quimby, A. E., Johnson-Obaseki, S. (2019). Comparing hematoma incidence between hemostatic devices in total thyroidectomy: A systematic review and meta-analysis. Otolaryngology--Head and Neck Surgery, 161(5), 770–778. DOI 10.1177/0194599819865248. [Google Scholar] [CrossRef]
41. Dionigi, G., Wu, C. W., Kim, H. Y., Rausei, S., Boni, L. et al. (2016). Severity of recurrent laryngeal nerve injuries in thyroid surgery. World Journal of Surgery, 40(6), 1373–1381. DOI 10.1007/s00268-016-3415-3. [Google Scholar] [CrossRef]
42. Wang, J. J., Huang, T. Y., Wu, C. W., Lin, Y. C., Tseng, H. Y. et al. (2021). Improving voice outcomes after thyroid surgery-review of safety parameters for using energy-based devices near the recurrent laryngeal nerve. Frontiers in Endocrinology, 12, 793431. DOI 10.3389/fendo.2021.793431. [Google Scholar] [CrossRef]
43. Liu, C. H., Wang, C. C., Wu, C. W., Lin, Y. C., Lu, I. C. et al. (2021). Comparison of surgical complications rates between LigaSure small Jaw and clampand-Tie hemostatic technique in 1,000 neuro-monitored thyroidectomies. Frontiers in Endocrinology, 12, 638608. DOI 10.3389/fendo.2021.638608. [Google Scholar] [CrossRef]
44. Back, K., Hur, N., Kim, M. J., Choe, J. H., Kim, J. H. et al. (2019). A prospective, randomized, controlled comparative study of three energy devices in open thyroid surgery: Thunderbeat. Harmonic, and Ligasure. Journal of Endocrine Surgery, 19(4),106–115. DOI 10.16956/jes.2019.19.4.106. [Google Scholar] [CrossRef]
45. Dionigi, G., Boni, L., Rausei, S., Frattini, F., Ferrari, C. C. et al. (2012). The safety of energy-based devices in open thyroidectomy: A prospective, randomised study comparing the LigaSureTM (LF1212) and the harmonic® FOCUS. Langenbecks Archives of Surgery, 397(5),817–823. DOI 10.1007/s00423-011-0898-0. [Google Scholar] [CrossRef]
46. Barbieri, D., Indelicato, P., Vinciguerra, A., Di Marco, F., Formenti, A. M. et al. (2021). Autofluorescence and indocyanine green in thyroid surgery: A systematic review and meta-analysis. The Laryngoscope, 131(7), 1683–1692. DOI 10.1002/lary.29297. [Google Scholar] [CrossRef]
47. Spartalis, E., Ntokos, G., Georgiou, K., Zografos, G., Tsourouflis, G. et al. (2020). Intraoperative indocyanine green (ICG) angiography for the identification of the parathyroid glands: Current evidence and future perspectives. In Vivo, 34(1), 23–32. DOI 10.21873/invivo.11741. [Google Scholar] [CrossRef]
48. Tjahjono, R., Nguyen, K., Phung, D., Riffat, F., Palme, C. E. (2021). Methods of identification of parathyroid glands in thyroid surgery: A literature review. ANZ Journal of Surgery, 91(9), 1711–1716. DOI 10.1111/ans.17117. [Google Scholar] [CrossRef]
49. Medas, F., Canu, G. L., Cappellacci, F., Anedda, G., Conzo, G. et al. (2020). Prophylactic central lymph node dissection improves disease-free survival in patients with intermediate and high risk differentiated thyroid carcinoma: A retrospective analysis on 399 patients. Cancers, 12(6), 1658. DOI 10.3390/cancers12061658. [Google Scholar] [CrossRef]
50. Canu, G. L., Medas, F., Cappellacci, F., Noordzij, J. P., Marcialis, J. et al. (2021). Risk factors of permanent hypoparathyroidism after total thyroidectomy retrospective analysis of 285 consecutive patients. Annali Italiani di Chirurgia, 92, 339–345. [Google Scholar]
51. Medas, F., Canu, G. L., Boi, F., Lai, M. L., Erdas, E. et al. (2019). Predictive factors of recurrence in patients with differentiated thyroid carcinoma: A retrospective analysis on 579 patients. Cancers, 11(9), 1230. DOI 10.3390/cancers11091230. [Google Scholar] [CrossRef]
52. Falco, J., Dip, F., Quadri, P., de la Fuente, M., Rosenthal, R. (2016). Cutting edge in thyroid surgery: Autofluorescence of parathyroid glands. Journal of the American College of Surgeons, 223(2), 374–380. DOI 10.1016/j.jamcollsurg.2016.04.049. [Google Scholar] [CrossRef]
53. Paras, C., Keller, M., White, L., Phay, J., Mahadevan-Jansen, A. (2011). Near-infrared autofluorescence for the detection of parathyroid glands. Journal of Biomedical Optics, 16(6), 067012. DOI 10.1117/1.3583571. [Google Scholar] [CrossRef]
54. Kim, D. H., Lee, S., Jung, J., Kim, S., Kim, S. W. et al. (2021). Nearinfrared autofluorescence-based parathyroid glands identification in the thyroidectomy or parathyroidectomy: A systematic review and meta-analysis. Langenbeck’s Archives of Surgery. DOI 10.1007/s00423-021-02269-8. [Google Scholar] [CrossRef]
55. Demarchi, M. S., Karenovics, W., Bédat, B., Triponez, F. (2020). Intraoperative autofluorescence and indocyanine green angiography for the detection and preservation of parathyroid glands. Journal of Clinical Medicine, 9(3), 830. DOI 10.3390/jcm9030830. [Google Scholar] [CrossRef]
56. Di Marco, A. N., Palazzo, F. F. (2020). Near-infrared autofluorescence in thyroid and parathyroid surgery. Gland Surgery, 9(Suppl 2), S136–S146. DOI 10.21037/gs.2020.01.04. [Google Scholar] [CrossRef]
57. Vidal Fortuny, J., Belfontali, V., Sadowski, S. M., Karenovics, W., Guigard, W. S. et al. (2016). Parathyroid gland angiography with indocyanine green fluorescence to predict parathyroid function after thyroid surgery. The British Journal of Surgery, 103(5), 537–543. DOI 10.1002/bjs.10101. [Google Scholar] [CrossRef]
58. Jitpratoom, P., Anuwong, A. (2017). The use of ICG enhanced fluorescence for the evaluation of parathyroid gland preservation. Gland Surgery, 6(5), 579–586. DOI 10.21037/gs. [Google Scholar] [CrossRef]
59. Demarchi, M. S., Seeliger, B., Lifante, J. C., Alesina, P. F., Triponez, F. (2021). Fluorescence image-guided surgery for thyroid cancer: Utility for preventing hypoparathyroidism. Cancers, 13(15), 3792. DOI 10.3390/cancers13153792. [Google Scholar] [CrossRef]
60. Weng, Y. J., Jiang, J., Min, L., Ai, Q., Chen, D. B. et al. (2021). Intraoperative near-infrared autofluorescence imaging for hypocalcemia risk reduction after total thyroidectomy: Evidence from a meta-analysis. Head & Neck, 43(8), 2523–2533. DOI 10.1002/hed.26733. [Google Scholar] [CrossRef]
61. McWade, M. A., Paras, C., White, L. M., Phay, J. E., Solórzano, C. C. et al. (2014). Label-free intraoperative parathyroid localization with near-infrared autofluorescence imaging. The Journal of Clinical Endocrinology and Metabolism, 99(12), 4574–4580. DOI 10.1210/jc.2014-2503. [Google Scholar] [CrossRef]
62. de Leeuw, F., Breuskin, I., Abbaci, M., Casiraghi, O., Mirghani, H. et al. (2016). Intraoperative near-infrared imaging for parathyroid gland identification by auto-fluorescence: A feasibility study. World Journal of Surgery, 40(9), 2131–2138. DOI 10.1007/s00268-016-3571-5. [Google Scholar] [CrossRef]
63. Vidal Fortuny, J., Sadowski, S. M., Belfontali, V., Guigard, S., Poncet, A. et al. (2018). Randomized clinical trial of intraoperative parathyroid gland angiography with indocyanine green fluorescence predicting parathyroid function after thyroid surgery. The British Journal of Surgery, 105(4), 350–357. DOI 10.1002/bjs.10783. [Google Scholar] [CrossRef]
64. Benmiloud, F., Godiris-Petit, G., Gras, R., Gillot, J. C., Turrin, N. et al. (2020). Association of autofluorescence-based detection of the parathyroid glands during total thyroidectomy with postoperative hypocalcemia risk: Results of the PARAFLUO multicenter randomized clinical trial. JAMA Surgery, 155(2), 106–112. DOI 10.1001/jamasurg.2019.4613. [Google Scholar] [CrossRef]
65. Sadowski, S. M., Vidal Fortuny, J., Triponez, F. (2017). A reappraisal of vascular anatomy of the parathyroid gland based on fluorescence techniques. Gland Surgery, 6(Suppl 1), S30–S37. DOI 10.21037/gs.2017.07.10. [Google Scholar] [CrossRef]
66. Lerchenberger, M., Al Arabi, N., Gallwas, J., Stepp, H., Hallfeldt, K. et al. (2019). Intraoperative near-infrared autofluorescence and indocyanine green imaging to identify parathyroid glands: A comparison. International Journal of Endocrinology, 2019, 4687951. DOI 10.1155/2019/4687951. [Google Scholar] [CrossRef]
67. Medas, F., Canu, G. L., Cappellacci, F., Boi, F., Lai, M. L. et al. (2020). Predictive factors of lymph node metastasis in patients with papillary microcarcinoma of the thyroid: Retrospective analysis on 293 cases. Frontiers in Endocrinology, 11, 551. DOI 10.3389/fendo.2020.00551. [Google Scholar] [CrossRef]
68. Dobrinja, C., Troian, M., Cipolat Mis, T., Rebez, G., Bernardi, S. et al. (2017). Rationality in prophylactic central neck dissection in clinically node-negative (cN0) papillary thyroid carcinoma: Is there anything more to say? A decade experience in a single-center. International Journal of Surgery, 41(Suppl 1), S40–S47. DOI 10.1016/j.ijsu.2017.01.113. [Google Scholar] [CrossRef]
69. Sorrenti, S., Carbotta, G., di Matteo, F. M., Catania, A., Pironi, D. et al. (2020). Evaluation of clinicopathological and molecular parameters on disease recurrence of papillary thyroid cancer patient: A retrospective observational study. Cancers, 12(12), 3637. DOI 10.3390/cancers12123637. [Google Scholar] [CrossRef]
70. Calò, P. G., Conzo, G., Raffaelli, M., Medas, F., Gambardella, C. et al. (2017). Total thyroidectomy alone versus ipsilateral versus bilateral prophylactic central neck dissection in clinically node-negative differentiated thyroid carcinoma. A retrospective multicenter study. European Journal of Surgical Oncology, 43(1), 126–132. DOI 10.1016/j.ejso.2016.09.017. [Google Scholar] [CrossRef]
71. Conzo, G., Calò, P. G., Sinisi, A. A., de Bellis, A., Pasquali, D. et al. (2014). Impact of prophylactic central compartment neck dissection on locoregional recurrence of differentiated thyroid cancer in clinically node-negative patients: A retrospective study of a large clinical series. Surgery, 155(6), 998–1005. DOI 10.1016/j.surg.2014.02.010. [Google Scholar] [CrossRef]
72. An, X., Yu, D., Li, B. (2019). Meta-analysis of the influence of prophylactic central lymph node dissection on the prognosis of patients with thyroid micropapillary carcinoma. Journal of Clinical Otorhinolaryngology Head & Neck Surgery, 33(2),138–142. DOI 10.13201/j.issn.1001-1781.2019.02.011. [Google Scholar] [CrossRef]
73. Su, H., Li, Y. (2019). Prophylactic central neck dissection and local recurrence in papillary thyroid microcarcinoma: A meta-analysis. Brazilian Journal of Otorhinolaryngology, 85(2), 237–243. DOI 10.1016/j.bjorl.2018.05.004. [Google Scholar] [CrossRef]
74. Chen, L., Wu, Y. H., Lee, C. H., Chen, H. A., Loh, E. W. et al. (2018). Prophylactic central neck dissection for papillary thyroid carcinoma with clinically uninvolved central neck lymph nodes: A systematic review and meta-analysis. World Journal of Surgery, 42(9), 2846–2857. DOI 10.1007/s00268-018-4547-4. [Google Scholar] [CrossRef]
75. Medas, F., Coni, P., Podda, F., Salaris, C., Cappellacci, F. et al. (2019). Evaluation of accuracy of one-step nucleic acid amplification (OSNA) in diagnosis of lymph node metastases of papillary thyroid carcinoma. diagnostic study. Annals of Medicine and Surgery, 46, 17–22. DOI 10.1016/j.amsu.2019.08.006. [Google Scholar] [CrossRef]
76. del Carmen, S., Gatius, S., Franch-Arcas, G., Baena, J. A., Gonzalez, O. et al. (2016). Concordance study between one-step nucleic acid amplification and morphologic techniques to detect lymph node metastasis in papillary carcinoma of the thyroid. Human Pathology, 48, 132–141. DOI 10.1016/j.humpath.2015.09.020. [Google Scholar] [CrossRef]
77. González, O., Iglesias, C., Zafon, C., Castellví, J., García-Burillo, A. et al. (2015). Detection of thyroid papillary carcinoma lymph node metastases using one step nucleic acid amplification (OSNAPreliminary results. Journal of Investigative Surgery, 28(3), 153–159. DOI 10.3109/08941939.2014.990123. [Google Scholar] [CrossRef]
78. Kaczka, K., Fendler, W., Borowiec, M., Młynarski, W., Pomorski, L. (2014). First one-step nucleic acid amplification testing in papillary thyroid cancer lymph nodes-a comparison with histopathology and real-time PCR. Endokrynologia Polska, 65(6),422–430. DOI 10.5603/EP.2014.0059. [Google Scholar] [CrossRef]
79. Iglesias, C., González, O., Temprana-Salvador, J., García-Burillo, A., Caubet, E. et al. (2021). Nodal metastatic load in papillary thyroid carcinoma. morphological and molecular analysis with one-step nucleic acid amplification on more than 550 lymph nodes. Endocrinologia, Diabetes y Nutricion, 68(5), 346–353. DOI 10.1016/j.endinu.2020.04.004. [Google Scholar] [CrossRef]
80. Kaczka, K. A., Pomorski, L. (2017). One-step nucleic acid amplification analysis of sentinel lymph nodes in papillary thyroid cancer patients. Archives of Medical Science, 13(6), 1416–1426. DOI 10.5114/aoms.2017.65466. [Google Scholar] [CrossRef]
81. Iglesias Felip, C, Zafon Llopis, C, Temprana-Salvador, J., García-Burillo, A, Serres Créixams, X. et al. (2019). One-step nucleic acid amplification for intraoperative analysis of sentinel lymph node in papillary thyroid carcinoma. European Journal of Endocrinology, 180(1), 21–29. DOI 10.1530/EJE-18-0624. [Google Scholar] [CrossRef]
82. Zhou, M., Wang, X., Jiang, L., Chen, X., Bao, X. et al. (2018). The diagnostic value of one step nucleic acid amplification (OSNA) in differentiating lymph node metastasis of tumors: A systematic review and meta-analysis. International Journal of Surgery, 56, 49–56. DOI 10.1016/j.ijsu.2018.05.010. [Google Scholar] [CrossRef]
83. Tranoulis, A., Georgiou, D., Yap, J., Attard-Montalto, S., Twigg, J. et al. (2021). The evolving role of one-step nucleic acid amplification (OSNA) for the intraoperative detection of lymph node metastases: A diagnostic accuracy meta-analysis. European Journal of Surgical Oncology, 47(6), 1233–1243. DOI 10.1016/j.ejso.2020.12.001. [Google Scholar] [CrossRef]
84. Rosato, L., Avenia, N., Bernante, P., de Palma, M., Gulino, G. et al. (2004). Complications of thyroid surgery: Analysis of a multicentric study on 14, 934 patients operated on in Italy over 5 years. World Journal of Surgery, 28(3), 271–276. DOI 10.1007/s00268-003-6903-1. [Google Scholar] [CrossRef]
85. Gunn, A., Oyekunle, T., Stang, M., Kazaure, H., Scheri, R. (2020). Recurrent laryngeal nerve injury after thyroid surgery: An analysis of 11, 370 patients. The Journal of Surgical Research, 255, 42–49. DOI 10.1016/j.jss.2020.05.017. [Google Scholar] [CrossRef]
86. Schneider, R., Randolph, G. W., Dionigi, G., Wu, C. W., Barczynski, M. et al. (2018). International neural monitoring study group guideline 2018 part I: Staging bilateral thyroid surgery with monitoring loss of signal. The Laryngoscope, 128(Suppl 3), S1–S17. DOI 10.1002/lary.27359. [Google Scholar] [CrossRef]
87. Wu, C. W., Dionigi, G., Barczynski, M., Chiang, F. Y., Dralle, H. et al. (2018). International neuromonitoring study group guidelines 2018: Part II: Optimal recurrent laryngeal nerve management for invasive thyroid cancer-incorporation of surgical, laryngeal, and neural electrophysiologic data. The Laryngoscope, 128(Suppl 3), S18–S27. DOI 10.1002/lary.27360. [Google Scholar] [CrossRef]
88. Lahey, F. H., Hoover, W. B. (1938). Injuries to the recurrent laryngeal nerve in thyroid operations their management and avoidance. Annals of Surgery, 108(4), 545–562. DOI 10.1097/00000658-193810000-00006. [Google Scholar] [CrossRef]
89. Schneider, R., Sekulla, C., Machens, A., Lorenz, K., Thanh, P. N. et al. (2016). Dynamics of loss and recovery of the nerve monitoring signal during thyroidectomy predict early postoperative vocal fold function. Head & Neck, 38(Suppl 1), E1144–E1151. DOI 10.1002/hed.24175. [Google Scholar] [CrossRef]
90. Calò, P. G., Medas, F., Erdas, E., Pittau, M. R., Demontis, R. et al. (2014). Role of intraoperative neuromonitoring of recurrent laryngeal nerves in the outcomes of surgery for thyroid cancer. International Journal of Surgery, 12(Suppl 1), S213–S217. DOI 10.1016/j.ijsu.2014.05.003. [Google Scholar] [CrossRef]
91. Hayward, N. J., Grodski, S., Yeung, M., Johnson, W. R., Serpell, J. (2013). Recurrent laryngeal nerve injury in thyroid surgery: A review. ANZ Journal of Surgery, 83(1–2), 15–21. DOI 10.1111/j.1445-2197.2012.06247.x. [Google Scholar] [CrossRef]
92. Scharpf, J., Tuttle, M., Wong, R., Ridge, D., Smith, R. et al. (2016). Comprehensive management of recurrent thyroid cancer: An American head and neck society consensus statement: AHNS consensus statement. Head & Neck, 38(12), 1862–1869. DOI 10.1002/hed.24513. [Google Scholar] [CrossRef]
93. Kim, J. W., Roh, J. L., Gong, G., Cho, K. J., Choi, S. H. et al. (2016). Treatment outcomes and risk factors for recurrence after definitive surgery of locally invasive well-differentiated papillary thyroid carcinoma. Thyroid: Official Journal of the American Thyroid Association, 26(2), 262–270. DOI 10.1089/thy.2015.0433. [Google Scholar] [CrossRef]
94. Shindo, M. L., Caruana, S. M., Kandil, E., McCaffrey, J. C., Orloff, L. A. et al. (2014). Management of invasive well-differentiated thyroid cancer: An American head and neck society consensus statement. AHNS consensus statement. Head & Neck, 36(10), 1379–1390. DOI 10.1002/hed.23619. [Google Scholar] [CrossRef]
95. Wu, C. W., Randolph, G. W., Barczyński, M., Schneider, R., Chiang, F. Y. et al. (2021). Training courses in laryngeal nerve monitoring in thyroid and parathyroid surgery-the INMSG consensus statement. Frontiers in Endocrinology, 12, 705346. DOI 10.3389/fendo.2021.705346. [Google Scholar] [CrossRef]
96. Dionigi, G., Donatini, G., Boni, L., Rausei, S., Rovera, F. et al. (2013). Continuous monitoring of the recurrent laryngeal nerve in thyroid surgery: A critical appraisal. International Journal of Surgery, 11(Suppl 1), S44–S46. DOI 10.1016/S1743-9191(13)60014-X. [Google Scholar] [CrossRef]
97. Wu, C. W., Hao, M., Tian, M., Dionigi, G., Tufano, R. P. et al. (2017). Recurrent laryngeal nerve injury with incomplete loss of electromyography signal during monitored thyroidectomy-evaluation and outcome. Langenbeck’s Archives of Surgery, 402(4), 691–699. DOI 10.1007/s00423-016-1381-8. [Google Scholar] [CrossRef]
98. Lombardi, C. P., Carnassale, G., Damiani, G., Acampora, A., Raffaelli, M. et al. (2016). “The final countdown”: Is intraoperative, intermittent neuromonitoring really useful in preventing permanent nerve palsy? Evidence from a meta-analysis. Surgery, 160(6), 1693–1706. DOI 10.1016/j.surg.2016.06.049. [Google Scholar] [CrossRef]
99. Yang, S., Zhou, L., Lu, Z., Ma, B., Ji, Q. et al. (2017). Systematic review with meta-analysis of intraoperative neuromonitoring during thyroidectomy. International Journal of Surgery, 39, 104–113. DOI 10.1016/j.ijsu.2017.01.086. [Google Scholar] [CrossRef]
100. Ling, Y., Zhao, J., Zhao, Y., Li, K., Wang, Y. et al. (2020). Role of intraoperative neuromonitoring of recurrent laryngeal nerve in thyroid and parathyroid surgery. The Journal of International Medical Research, 48(9), 300060520952646. DOI 10.1177/0300060520952646. [Google Scholar] [CrossRef]
101. Duong, W., Grigorian, A., Farzaneh, C., Elfenbein, D., Yamamoto, M. et al. (2021). Nerve monitoring decreases recurrent laryngeal nerve injury risk for neoplasmrelated thyroidectomy. American Journal of Surgery. DOI 10.1016/j.amjsurg.2021.10.013. [Google Scholar] [CrossRef]
102. Barczyński, M., Konturek, A., Stopa, M., Hubalewska-Dydejczyk, A., Richter, P. et al. (2011). Clinical value of intraoperative neuromonitoring of the recurrent laryngeal nerves in improving outcomes of surgery for well-differentiated thyroid cancer. Polski Przeglad Chirurgiczny, 83(4), 196–203. DOI 10.2478/v10035-011-0030-8. [Google Scholar] [CrossRef]
103. Cirocchi, R., Arezzo, A., D’Andrea, V., Abraha, I., Popivanov, G. I. et al. (2019). Intraoperative neuromonitoring versus visual nerve identification for prevention of recurrent laryngeal nerve injury in adults undergoing thyroid surgery. The Cochrane Database of Systematic Reviews, 1(1), CD012483. DOI 10.1002/14651858.CD012483.pub2. [Google Scholar] [CrossRef]
104. Calò, P. G., Medas, F., Conzo, G., Podda, F., Canu, G. L. et al. (2017). Intraoperative neuromonitoring in thyroid surgery: Is the two-staged thyroidectomy justified? International Journal of Surgery, 41(Suppl 1), S13–S20. DOI 10.1016/j.ijsu.2017.02.001. [Google Scholar] [CrossRef]
105. Alesina, P. F., Hinrichs, J., Meier, B., Cho, E. Y., Bolli, M. et al. (2014). Intraoperative neuromonitoring for surgical training in thyroid surgery: Its routine use allows a safe operation instead of lack of experienced mentoring. World Journal of Surgery, 38(3), 592–598. DOI 10.1007/s00268-013-2372-3. [Google Scholar] [CrossRef]
106. Sarı, S., Erbil, Y., Sümer, A., Agcaoglu, O., Bayraktar, A. et al. (2010). Evaluation of recurrent laryngeal nerve monitoring in thyroid surgery. International Journal of Surgery, 8(6), 474–478. DOI 10.1016/j.ijsu.2010.06.009. [Google Scholar] [CrossRef]
107. Deniwar, A., Kandil, E., Randolph, G. (2015). Electrophysiological neural monitoring of the laryngeal nerves in thyroid surgery: Review of the current literature. Gland Surgery, 4(5), 368–375. DOI 10.3978/j.issn.2227-684X.2015.04.04. [Google Scholar] [CrossRef]
108. Schneider, R., Przybyl, J., Hermann, M., Hauss, J., Jonas, S. et al. (2009). A new anchor electrode design for continuous neuromonitoring of the recurrent laryngeal nerve by vagal nerve stimulations. Langenbeck’s Archives of Surgery, 394(5), 903–910. DOI 10.1007/s00423-009-0503-y. [Google Scholar] [CrossRef]
109. Schneider, R., Przybyl, J., Pliquett, U., Hermann, M., Wehner, M. et al. (2010). A new vagal anchor electrode for real-time monitoring of the recurrent laryngeal nerve. American Journal of Surgery, 199(4),507–514. DOI 10.1016/j.amjsurg.2009.04.036. [Google Scholar] [CrossRef]
110. Ulmer, C., Koch, K. P., Seimer, A., Molnar, V., Meyding-Lamadé, U. et al. (2008). Real-time monitoring of the recurrent laryngeal nerve: An observational clinical trial. Surgery, 143(3), 359–365. DOI 10.1016/j.surg.2007.10.007. [Google Scholar] [CrossRef]
111. Stankovic, P., Wittlinger, J., Georgiew, R., Dominas, N., Hoch, S. et al. (2020). Continuous intraoperative neuromonitoring (cIONM) in head and neck surgerya review. HNO, 68(Suppl 2), 86–92. DOI 10.1007/s00106-020-00824-1. [Google Scholar] [CrossRef]
112. Schneider, R., Randolph, G. W., Barczynski, M., Dionigi, G., Wu, C. W. et al. (2016). Continuous intraoperative neural monitoring of the recurrent nerves in thyroid surgery: A quantum leap in technology. Gland Surgery, 5(6), 607–616. DOI 10.21037/gs.2016.11.10. [Google Scholar] [CrossRef]
113. Schneider, R., Sekulla, C., Machens, A., Lorenz, K., Nguyen Thanh, P. et al. (2015). Postoperative vocal fold palsy in patients undergoing thyroid surgery with continuous or intermittent nerve monitoring. The British Journal of Surgery, 102(11), 1380–1387. DOI 10.1002/bjs.9889. [Google Scholar] [CrossRef]
114. Terris, D. J., Chaung, K., Duke, W. S. (2015). Continuous vagal nerve monitoring is dangerous and should not routinely be done during thyroid surgery. World Journal of Surgery, 39(10), 2471–2476. DOI 10.1007/s00268-015-3139-9. [Google Scholar] [CrossRef]
115. Cavicchi, O., Burgio, L., Cioccoloni, E., Piccin, O., Macrì, G. et al. (2018). Intraoperative intermittent neuromonitoring of inferior laryngeal nerve and staged thyroidectomy: Our experience. Endocrine, 62(3), 560–565. DOI 10.1007/s12020-018-1739-5. [Google Scholar] [CrossRef]
116. Calò, P. G., Medas, F., Gordini, L., Podda, F., Erdas, E. et al. (2016). Interpretation of intraoperative recurrent laryngeal nerve monitoring signals: The importance of a correct standardization. International Journal of Surgery, 28(Suppl 1), S54–S58. DOI 10.1016/j.ijsu.2015.12.039. [Google Scholar] [CrossRef]
117. Dralle, H., Sekulla, C., Lorenz, K., Nguyen Thanh, P., Schneider, R. et al. (2012). Loss of the nerve monitoring signal during bilateral thyroid surgery. The British Journal of Surgery, 99(8), 1089–1095. DOI 10.1002/bjs.8831. [Google Scholar] [CrossRef]
118. Sitges-Serra, A., Fontané, J., Dueñas, J. P., Duque, C. S., Lorente, L. et al. (2013). Prospective study on loss of signal on the first side during neuromonitoring of the recurrent laryngeal nerve in total thyroidectomy. The British Journal of Surgery, 100(5), 662–666. DOI 10.1002/bjs.9044. [Google Scholar] [CrossRef]
119. Randolph, G. W., Dralle, H., International Intraoperative Monitoring Study Group, Abdullah, H., Barczynski, M. et al. (2011). Electrophysiologic recurrent laryngeal nerve monitoring during thyroid and parathyroid surgery: International standards guideline statement. The Laryngoscope, 121(Suppl 1), S1–S16. DOI 10.1002/lary.21119. [Google Scholar] [CrossRef]
120. Chiang, F. Y., Lu, I. C., Kuo, W. R., Lee, K. W., Chang, N. C. et al. (2008). The mechanism of recurrent laryngeal nerve injury during thyroid surgery--the application of intraoperative neuromonitoring. Surgery, 143(6), 743–749. DOI 10.1016/j.surg.2008.02.006. [Google Scholar] [CrossRef]
121. Wu, C. W., Huang, T. Y., Randolph, G. W., Barczyński, M., Schneider, R. et al. (2021). Informed consent for intraoperative neural monitoring in thyroid and parathyroid surgery-consensus statement of the international neural monitoring study group. Frontiers in Endocrinology, 12, 795281. DOI 10.3389/fendo.2021.795281. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |