
 | Oncologie |  |
DOI: 10.32604/oncologie.2022.020890
REVIEW
The Integrated Histopathologic and Molecular Approach to Adult-Type Diffuse Astrocytomas: Status of the Art, Based on the 2021 WHO Classification of Central Nervous System Tumors
1Department of Basic Medical Sciences, Faculty of Medicine, Yarmouk University, Irbid, 21163, Jordan
2Faculty of Medicine, Yarmouk University, Irbid, 21163, Jordan
3Department of Medical, Surgical Sciences and Advanced Technologies “G.F. Ingrassia”, Anatomic Pathology, University of Catania, Catania, 95123, Italy
*Corresponding Author: Giuseppe Broggi. Email: giuseppe.broggi@gmail.com
Received: 17 December 2021; Accepted: 27 January 2022
Abstract: The 2021 World Health Organization (WHO) Classification of Tumors of the Central Nervous System (CNS) improved our understanding of the brain neoplasm biology. In more details, differences between diffuse gliomas that primarily occur in adults and those that primarily occur in children have been identified by the terms “adult-type” and “pediatric-type” diffuse gliomas. More importantly, both diagnostic and grading criteria for adult-type diffuse astrocytomas have been modified, by adopting novel molecular markers: diffuse astrocytomas, IDH-mutant have been grouped into a single entity and graded as CNS WHO grades 2, 3, or 4, with the assignment of Grade 4 in the presence of CDKN2A/B homozygous deletion, regardless of the histology [1]. Additionally, at least one of the following genetic alterations has been considered as sufficient to confer to astrocytomas, IDH wild type, a CNS WHO grade 4: i) TERT promoter mutation, ii) EGFR gene amplification, iii) combined gain of whole chromosome 7 and loss of whole chromosome 10 [+7/−10]. However, histology remains the solid basis to support these new complementary molecular data, and an integrated diagnosis is highly recommended.
Keywords: WHO classification; brain tumors; IDH; diagnosis; adult diffuse astrocytomas
Central Nervous System (CNS) tumor classification has long been based on histological findings supported by ancillary studies like immunohistochemical stains, and ultrastructural changes but recent discoveries have deepened our understanding of the molecular features of CNS tumors. This advancement in molecular characterization of CNS tumors has reframed our understanding of its biology and the development of a new classification system.
The 2021 World Health Organization (WHO) Classification of Tumors of the CNS emphasizes the clinico-pathologic and molecular differences between diffuse gliomas that primarily occur in adults and that occur primarily in children, and accordingly termed as diffuse gliomas “adult-type” and “pediatric-type”, respectively. Additionally, 2021 WHO classification of CNS adopted molecular markers into the revised grading criteria for CNS tumors in general and astrocytoma in particular, consequently, all diffuse astrocytoma, IDH-mutant are considered as a single type and graded as CNS WHO grades 2, 3, or 4, with the assignment of grade 4 in the presence of CDKN2A/B homozygous deletion [1]. Furthermore, the presence of 1 or more of the three genetic alterations including TERT promoter mutation, EGFR gene amplification, and/or combined gain of entire chromosome 7 and loss of entire chromosome 10 [+7/−10]) are sufficient to assign WHO grade 4 for IDH wild type astrocytoma [2], however, the molecular testing to comply with these new WHO criteria is not always available, so an integrated diagnosis combining all available complementary data is still highly recommended [1]. In this report, we will review the integrated histopathologic and molecular approach of adult diffuse astrocytoma, based on 2021 WHO Classification of CNS Tumors, and discuss the new emerging genetic alterations that could be incorporated in the future for further sub-classification, risk stratification and most importantly, targeted therapy that could improve the overall survival of the patients.
2 Diffuse Astrocytoma, IDH-Mutant
The 2016 WHO Classification of Tumors of CNS (updated 4th edition) for the first time emphasizes the diagnostic relevance of IDH1/2 mutations in diffuse gliomas, and provides a clinically meaningful classification for diffuse gliomas, particularly in adults [3]. Although immunohistochemistry is the routine method for the identification of the most frequent IDH mutations (IDH1-R132H), DNA sequencing of IDH1/2 genes is required for identification of the less frequent non-canonical IDH1 and IDH2 mutations.
The 2016 WHO Classification of Tumors of the CNS classifies IDH-mutant gliomas into astrocytoma or oligodendroglioma based on the presence of ATRX and TP53 mutations for former and 1p/19q co-deletion for latter [3]. The 2016 WHO grading system included IDH-mutant, diffuse astrocytoma (WHO grade 2) (Fig. 1), anaplastic astrocytoma (WHO grade 3) (Fig. 2), and glioblastoma (WHO grade 4) [4]. Consequently, testing for IDH mutations in adult diffuse astrocytoma is very important but may be insufficient, since other genetic alterations with prognostic significance maybemissed due to limited molecular testing.
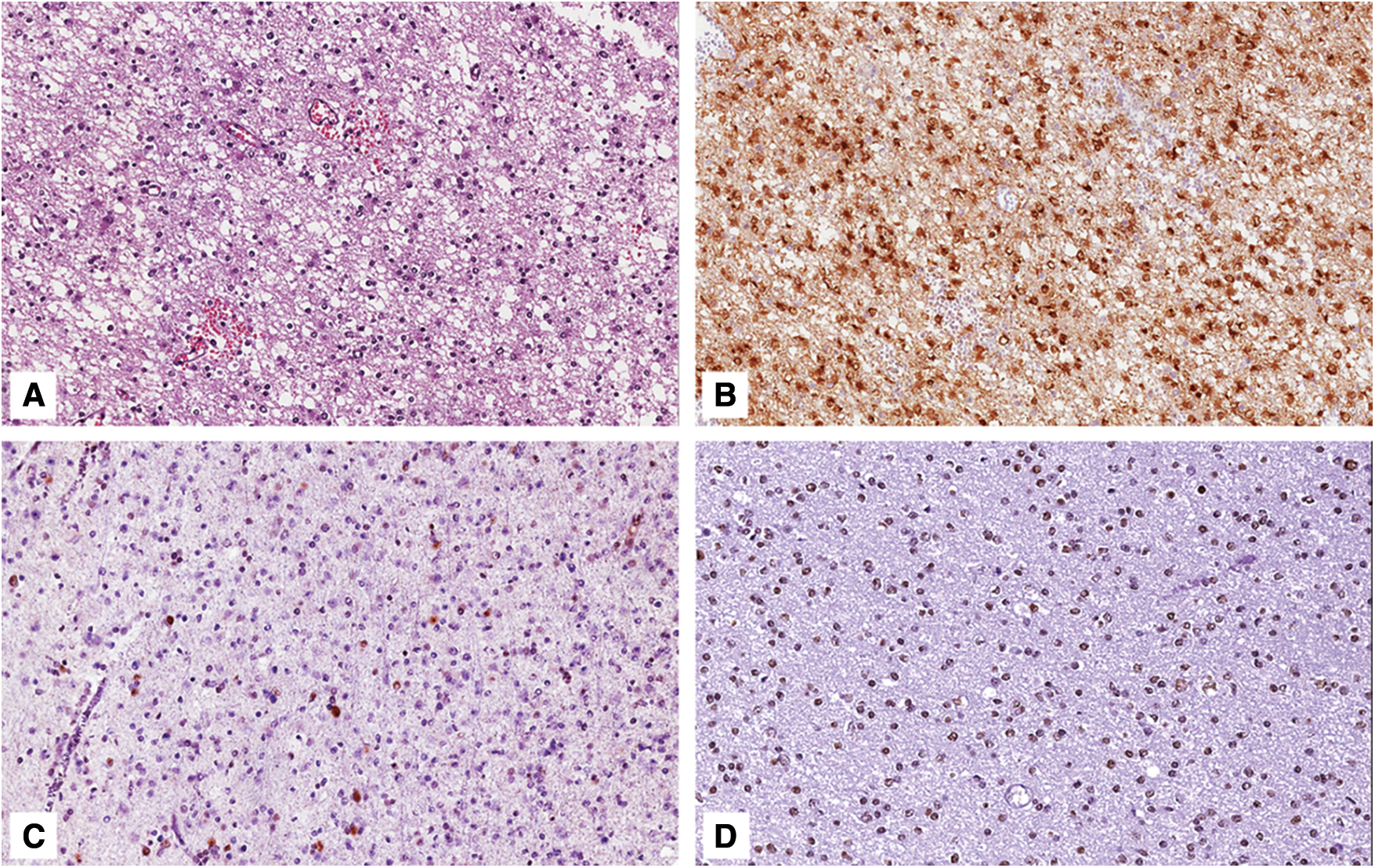
Figure 1: Grade 2 astrocytoma, IDH-mutant. A) Histological examination showing a lowly cellular astrocytic neoplasm composed of mitotically-inactive bland-looking cells with ovoid nuclei (hematoxylin and eosin; original magnification 150x); B) Neoplastic cells are diffusely stained with IDH1 (R132H) (immunoperoxidase; original magnification 150x); C) Tumor exhibits nuclear loss of ATRX; notice the entrapped ATRX-positive glial cells and endothelial cells that serve as internal positive control (immunoperoxidase; original magnification 150x); D) Neoplastic cells are diffusely stained with anti-p53 antibody (immunoperoxidase; original magnification 150x)
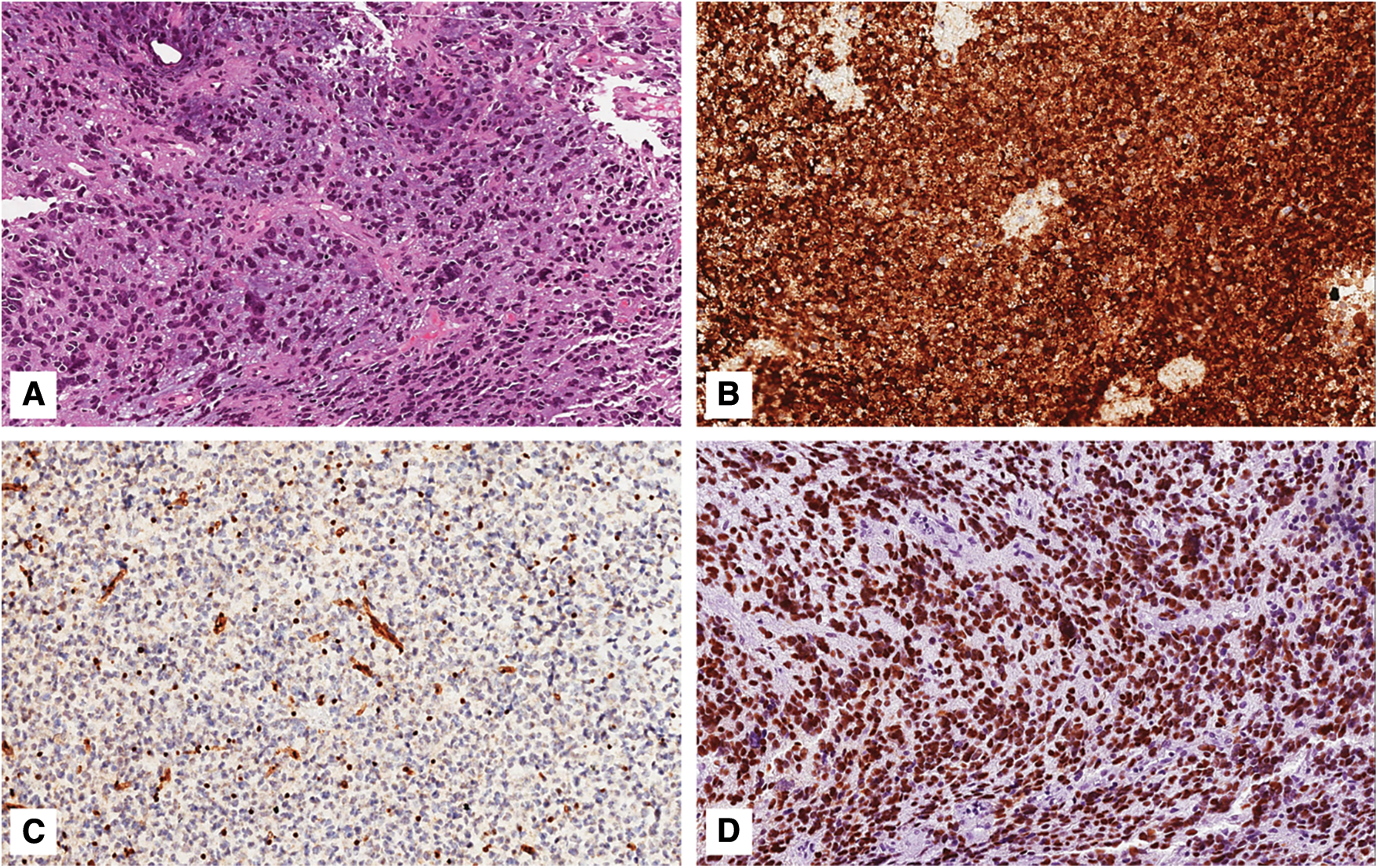
Figure 2: Grade 3 astrocytoma, IDH-mutant. A) Histological examination showing a highly cellular astrocytic tumor composed of mitotically-active cells with nuclear anaplasia; neither necrosis nor microvascular proliferation are seen (hematoxylin and eosin; original magnification 150x); B) Neoplastic cells are diffusely and strongly stained with IDH1 (R132H) (immunoperoxidase; original magnification 150x); C) Tumor exhibits nuclear loss of ATRX; notice the ATRX-positive endothelial cells that serve as internal positive control (immunoperoxidase; original magnification 150x); D) Neoplastic cells are diffusely and strongly stained with anti-p53 antibody (immunoperoxidase; original magnification 150x)
The significant mitotic index, anaplastic nuclear features, and microvascular proliferation or necrosis have classically been the main criteria for performing the histological grading of adult diffuse astrocytomas. In WHO 2016 “significant” proliferative activity distinguishes WHO grade 3 (anaplastic) from WHO grade 2 astrocytoma [3]. In the years preceding the release of 2016 WHO, diffuse astrocytic tumors with ≥2 mitoses/10 HPFs were found to be associated with poorer outcomes and designated as WHO grade 3 [5–7]; moreover, taking into consideration the specimen size, one single mitosis has been also reported as sufficient for a WHO grade 3 diagnosis on a very small biopsy, while greater mitotic activity is necessary for the larger samples [3]. To date, there have been no studies for an alternative mitotic count nor the criteria of the proliferative index (e.g., based on Ki-67) that can reliably stratify risk among histologic grade 2 and 3, IDH-mutant astrocytomas [8].
Recent studies challenge the previously mentioned histopathologic criteria for WHO grading of IDH-mutant gliomas, highlight the importance of associated genetic alterations and emphasize that specific genetic alterations maybe more important than histopathologic features in predicting the prognosis and the outcomes. It has been shown that the risk for patients affected by grades 2 and 3, IDH-mutant astrocytoma cannot be stratified by histological grade alone [9–12] and indicated that the number of mitoses is not accurate for grading IDH-mutant astrocytomas [13]. Additional potential histopathologic and genetic biomarkers, that could act as predictors of more aggressive biological behavior and could be added to the grading system of these tumors, have been investigated [9–10,14–19]. Moreover, the presence of co-occurring, second genetic events that could be class-defining oncogenic drivers in IDH-mutated astrocytoma are important especially since infiltrating gliomas are difficult to treat and identifications of these targetable molecular alterations like pathogenic BRAF, FGFR, and NTRK can help in targeted therapy and improving the overall survival of these patients [20–23], consequently, many molecular genetic alterations were investigated including CDKN2A/B homozygous deletion, alterations of CDK4, RB1, PIK3CA/PIK3R1, PDGFRA, MYCN, and chromosome 14 copy loss; these alterations could reliably stratify risk or identify tumors that would behave most aggressively among patients with IDH-mutant diffuse astrocytomas [24]. Very recently, pathogenic BRAF, FGFR, and NTRK were also investigated [25].
Homozygous deletion of CDKN2A/B and CDK4 amplification was considered as a marker of poor prognosis and associated with decreased global DNA methylation levels in IDH-mutant astrocytomas [10,13–18,26], and the combination of CDK4 amplification with chromosome 14 loss has been linked to poor prognosis and shorter overall survival in these astrocytomas [9,26]. Moreover, subsequent studies have shown that CDKN2A/B homozygous deletion is an independent marker for poor outcomes and shorter survival in all grades (WHO grades 2–4) of IDH-mutant astrocytomas [9,13,14,17,26] and this has been emphasized by a study that found that patients with histologic grade 3 IDH-mutant astrocytomas, and harboring CDKN2A/B homozygous deletions, showed biological behavior more similar to WHO grade 4 glioblastomas [27]. Other investigations have confirmed these findings [13,27,28], but the prognostic significance of RB1 mutation or CDK4 amplification that are functionally equivalent alterations to CDKN2A/B homozygous deletion, remains less well-defined, and are not yet recommended for grading IDH-mutant astrocytomas [24]. Accordingly, The Consortium to Inform Molecular and Practical Approaches to CNS Tumor Taxonomy (cIMPACT-NOW), assigns a grade 4 to astrocytomas, IDH-mutant, with homozygous deletion of CDKN2A/B [29]. As immunohistochemical tests for p16 protein have been demonstrated to have poor correlation with CDKN2A/B status, molecular investigations, such as FISH, quantitative real-time PCR, or next generation sequencing, are the most sensitive and specific tools for detecting CDKN2A/B homozygous deletion [17] cIMPACT-NOW Update 6 also suggested the discontinuation of the term “Glioblastoma, IDH-mutant”, and the conversion to a single name “Astrocytoma, IDH-mutant” with Arabic numeral grades from 2–4; with the use of the previously mentioned histological features [29]. The distinction between histologic grade 2 and grade 3 tumors is based primarily on the detection of brisk mitotic activity, and the presence of necrosis and/or microvascular proliferation remains as the morphologic hallmarks for grade 4 tumors, regardless of CDNK2A/B status. In summary, the proposed grading system according to cIMPACT-NOW Updates 5 and 6 is to designate Astrocytoma, IDH-mutant, WHO grade 2 for tumors that lack significant mitotic activity, anaplastic features, microvascular proliferation, necrosis, and CDKN2A/B homozygous deletion (median overall survival >10 years) [14,15]; WHO grade 3 astrocytoma, IDH-mutant, is for those tumors that exhibit brisk mitotic activity and histologic anaplastic features in the absence of microvascular proliferation, necrosis, and CDKN2A/B homozygous deletion; finally, IDH-mutant astrocytic neoplasms that show at least one of microvascular proliferation, necrosis, and CDKN2A/B homozygous deletion, are designated as WHO grade 4 astrocytomas, IDH-mutant [1,24,29].
Other genetic alterations have also been associated with shorter overall survival, including RB1 homozygous deletion, PIK3CA and PIK3R1 pathogenic mutations, PDGFRA amplification, and MYCN amplification [14–15,19]. Touat et al. also reported shorter survival times in a subset of hypermutated, mismatch repair-deficient gliomas, IDH-mutant [30]; Higher copy number variations (CNV) and somatic mutation levels were also correlated to poorer prognosis in WHO grades 2 and 3 IDH-mutant astrocytic tumors [14–15,31,32], however, there were limitations since the thresholds for high CNV and somatic mutation varied [33]. Additionally, one of the co-occurring, second genetic events that recently studied and could be class-defining oncogenic drivers in IDH-mutated astrocytoma is BRAF, which is known to be altered in different tumor types, including gliomas [34,35]: in more details, KIAA1549-BRAF fusions and BRAF V600E mutations have been identified as common genetic characteristics of pilocytic astrocytomas [36,37] and glial/glioneuronal tumors [38], respectively. Few studies have investigated the coexistence of IDH and BRAF molecular alterations within gliomas; two large studies tested a combined 252 glioma samples for KIAA1549-BRAF fusion, BRAFV600E, and IDH1/2 mutations and concluded that IDH mutations and BRAF alterations were mutually exclusive [28,39]. Similarly, large-scale next generation sequencing studies of pediatrics and adults gliomas showed that IDH and BRAF alterations are mutually exclusive [4,40–42]. However, the co-existence of IDH mutations with BRAF-KIAA1549 fusions or BRAFV600E mutations was reported in about 9% of diffuse gliomas of adult patients [43], but this high incidence is possibly due to a high false positive rate in their molecular methodology. Additionally, the authors of recent study [25] identified IDH1/2 mutations and simultaneous BRAF alterations in 3/1879 glial tumors from their cohort. FGFR alterations also have been recognized as molecular drivers in pediatric and adult low and high-grade gliomas [44–46], with no prior reports of concomitant IDH-mutated gliomas and FGFR alterations, but a recent study by Ahrendsen et al. [25], reported seven gliomas with FGFR alterations with variable histology ranging from WHO grade 2 (diffuse astrocytoma and oligodendroglioma) to WHO grade 4.
NTRK gene rearrangements have also been found as an emerging oncogenic driver in a variety of tumors, including high- and low-grade, in both adults and pediatrics gliomas [47,48]. However, the presence of concomitant IDH mutation in NTRK-rearranged infiltrating gliomas has been described in a few cases with no indications of their significance [49,50]. But, in a recent study by Ahrendsen et al., seven IDH-mutated tumors with co-occurring NTRK fusion were discovered. Interestingly, all the cases that involved second-class defining alterations (BRAF, FGFR, and NTRK) by Ahrendsen et al., were of IDH1 (the majority IDH1 R132H). Additionally, in regard to H3 K27M mutation, many studies demonstrated mutual exclusivity of IDH and H3 K27M mutations in gliomas [25,27,42,50].
3 Diffuse Astrocytomas, IDH-Wild Type
Diffusely infiltrating astrocytomas, IDH-wild type, was considered by 2016 WHO Classification of CNS Tumors as a wide and heterogeneous spectrum of neoplasms, including WHO grade 4 glioblastoma, grade 2 diffuse astrocytoma, and grade 3 anaplastic astrocytoma [51]. The molecular landscape of diffuse astrocytomas, IDH-wild type was classically defined by the absence of IDH1/2 mutations combined with the frequent lack of ATRX and TP53 mutations [51]. In the 2016 edition of WHO Classification, grades 2 and 3 diffuse astrocytomas, IDH wild-type were considered as rare and provisional entities, whose biological behavior was more similar to that of glioblastoma, IDH-wild type than that of their IDH-mutant histologic counterparts [51]. As above-mentioned for IDH-mutant astrocytoma, morphology was the only criterion used to diagnose these entities, as mitoses and nuclear pleomorphism distinguished WHO grade 3 from WHO grade 2, and the presence of necrosis and/or microvascular proliferation defined WHO grade 4 (Fig. 3) [51]. Since then, several studies reported the extreme biological, clinical, and prognostic variability of these tumors, emphasizing the need for further sub-classification. In more details, it has been shown that a subset of adult diffuse astrocytoma, IDH-wildtype, corresponding to histologic grade 2 or 3, had a very aggressive biological behavior, much more similar to that of glioblastoma, despite not exhibiting necrosis and/or microvascular proliferation [4,52–56]. In addition, a variety of glial and glioneuronal tumors, that could also occur in adults, such as pilocytic astrocytoma, pleomorphic xanthoastrocytoma, and other tumors, and lack of IDH1/2 mutations could create differential diagnostic problems. As a result, the need to identify molecular features of adult diffuse adult astrocytoma, that could predict a poor outcome, arose.
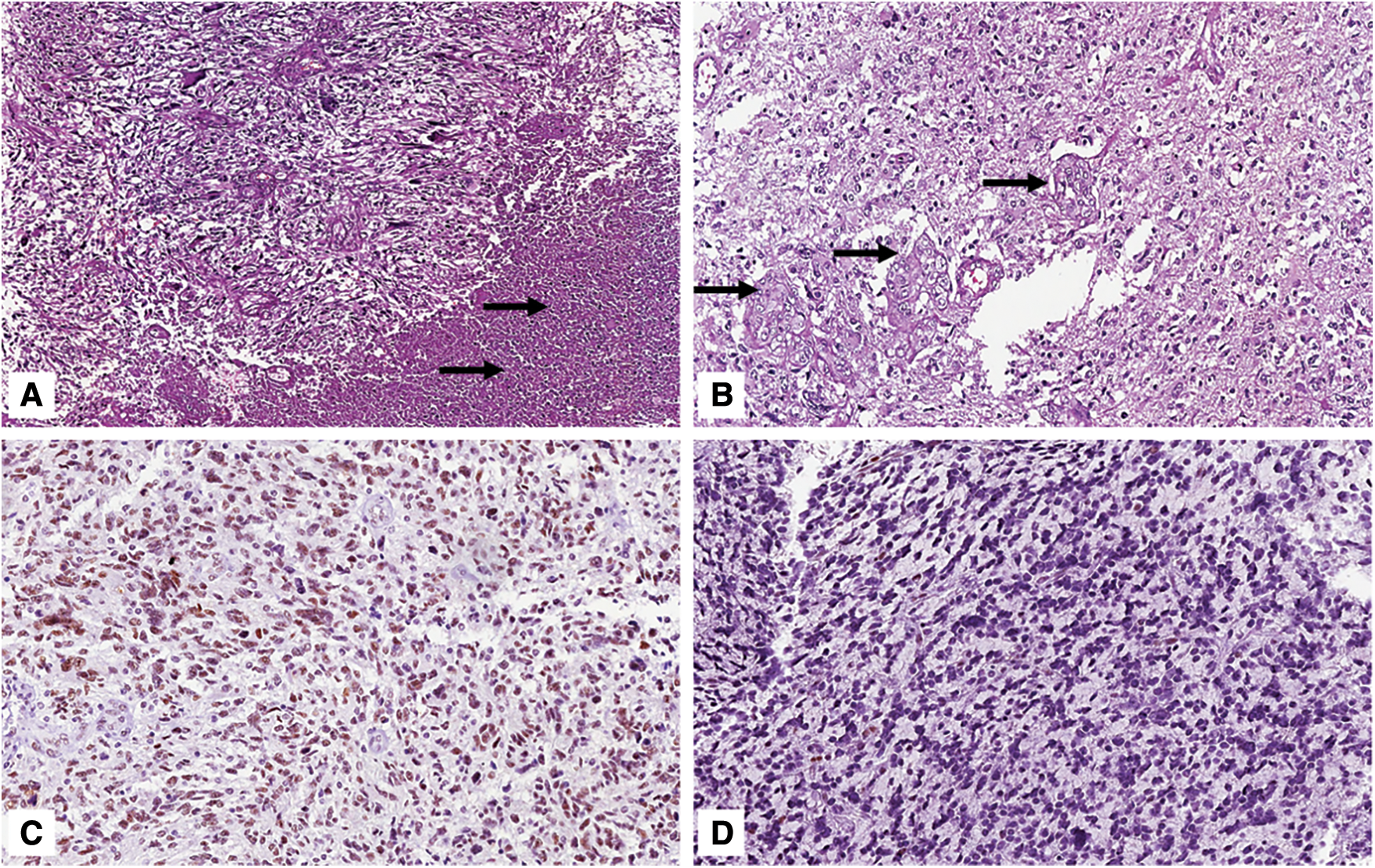
Figure 3: Grade 4 glioblastoma, IDH-wild type. A) Histological examination showing a hypercellular neoplasm composed of variably-shaped malignant cells with extensive foci of necrosis (arrows) (hematoxylin and eosin; original magnification 100x); B) Tumor also exhibits multiple foci of microvascular proliferation (arrows) (hematoxylin and eosin; original magnification 150x); C) Nuclear expression of ATRX is typically retained (immunoperoxidase; original magnification 150x); D) Wild type expression pattern of p53 in tumor cells (immunoperoxidase; original magnification 150x)
In 2018, the cIMPACT-NOW update 3 defined a minimal set of molecular characteristics that were able to reliably predict poor clinical outcomes among adult astrocytic tumors [57]. In this regard it was stated that an IDH-wild type adult diffuse astrocytoma with, at least, one of the following molecular features, would be better designated by the term “diffuse astrocytoma, IDH-wild type with molecular features of glioblastoma WHO grade 4”, regardless of the histological grade [57]: i) EGFR amplification or ii) combined whole chromosome 7 gain and chromosome 10 loss or iii) TERT promoter mutation. It was noteworthy that IDH-wildtype diffuse astrocytoma lacking necrosis and/or microvascular proliferation, but exhibiting, at least, one of the above-mentioned genetic features, corresponding to WHO grade 4 [58,59]; notably, only 6 diffuse astrocytomas, IDH-wildtype, from the TCGA dataset of more than 500 histologic grade 2/3 diffuse gliomas had “molecular features of glioblastoma, WHO grade 4” [57].
In 2020, the cIMPACT-NOW update 6 [29] proposed to simplify the tumor nomenclature, by including the presence of, at least, one of the above-mentioned molecular features (TERT promoter mutation, EGFR amplification, 7 gain/10 loss) among the diagnostic criteria of WHO grade 4 glioblastoma, IDH-wild type. Both EGFR amplification and chromosome 7 gain and chromosome 10 loss signature have high specificity for astrocytomas with poor outcomes [59]. Notably, EGFR amplification, which is defined as high-level EGFR copy number gains should be tested by validated molecular techniques; immunohistochemistry with anti-EGFR antibody should be avoided in diagnostic practice, as it lacks adequate reliability [60]. The prognostic value of TERT promoter mutations deserves a separate discussion, since different studies have yeilded partially contradictory results [4,52,55]. TERT promoter mutations were added to the list of minimal criteria for the diagnosis of “diffuse astrocytoma, IDH-wild type with molecular features of glioblastoma, WHO grade 4” in cIMPACT-NOW Update [57], especially that TERT promoter mutations were also found in almost all oligodendrogliomas and other IDH-wild type gliomas, that lacked both necrosis/microvascular proliferation and/or aggressive biology, such as pleomorphic xanthoastrocytomas, gangliogliomas, ependymomas and high-grade astrocytomas with piloid features [2,61–63]. However, as diffuse astrocytomas, IDH-wild type, frequently harbored TERT promoter mutations along with 7 gain/10 loss or EGFR amplification, the association between these features increased the specificity of TERT promoter mutation alone as a marker of molecular grade 4 astrocytoma [59]. Tesileanu et al. [2] retrospectively compared the overall survival time of 71 diffuse astrocytoma, with low-grade radiologic features, in which 22 of them exhibited only TERT promoter mutation, with those of 197 glioblastomas, IDH-wild type. The authors found that patients with IDH-wild type glioblastomas, and astrocytomas with only TERT promoter mutations, had similar overall survival [2]. A subsequent study by Berzero et al. [64], compared grade 2 to grade 3 diffuse astrocytomas, IDH-wildtype with molecular features of glioblastoma according to cIMPACT-NOW update 3. It has been found that in 62% of cases an isolated TERT promoter mutation was the only molecular alteration of WHO grade 4 glioblastoma, and it was not associated with aggressive biological behavior per se [64]. Based on these findings, Giannini et al. [65] recently questioned if the presence of TERT promoter mutation alone was sufficient to “call” grade 2 astrocytoma, IDH-wild type, as WHO grade 4 glioblastoma, emphasizing that the “old” histological grading still had prognostic utility in adult diffuse astrocytomas, IDH-wild type and that isolated TERT promoter mutation is insufficient to designate grade 2 diffuse astrocytomas, IDH-wild type as WHO grade 4 glioblastomas.
The current 2021 WHO Classification of Tumors of the CNS promoted the integrated molecular and histologic approach to these tumors, by adding TERT promoter mutation, EGFR amplification, 7 gain/10 loss as criteria for grading and, thus, prognostic biomarkers of adult diffuse astrocytomas, IDH-wildtype [1]. Accordingly, grade 2 diffuse astrocytoma, and grade 3 anaplastic astrocytoma, IDH-wild type are no longer included in the current edition of WHO Classification of CNS Tumors, as they have been incorporated into the WHO grade 4 glioblastoma group, which includes adult diffuse astrocytic tumors, IDH-wild type, with the histologic presence of necrosis and/or microvascular proliferation, or one (or more) of the above-mentioned molecular alterations [1].
EGFR amplification and 7 gain/10 loss phenotype are routinely tested by FISH, while DNA sequencing methods are needed to detect TERT promoter mutations.
Homozygous CDKN2A/B deletion, despite being more frequently occurring in astrocytomas, IDH-wild type with EGFR amplification, +7/−10 or TERT promoter mutations [57], is not a prognostic biomarker of WHO grade 4 behavior as what described before for diffuse astrocytoma, IDH-mutant. In addition, IDH-wild type glial tumors that are different from glioblastoma in many aspects including, histology, genetic profile, and clinical outcomes like pleomorphic xanthoastrocytoma and high-grade astrocytoma with piloid features, frequently harbor this deletion [63,66,67].
4 Utility of DNA Methylation Profiling for Brain Tumors Diagnosis and Classification
Genome-wide DNA methylation profiling (DMP) is an analytical technique that in recent years has been increasingly used for the identification and characterization of several CNS neoplasms [1]. Based on the differences in DNA methylation patterns between different tumor entities, DMP is able to reliably assign a neoplasm to one of the already known clusters of CNS tumors and to stratify patients into prognostically distinct subgroups. This technique tends to maintain high reproducibility both on fresh/frozen tissue and formalin-fixed and paraffin-embedded tumor specimens; furthermore, it is also effective when the biological material is represented by small biopsies on which the other molecular methods have limited applicability [1].
Methylome profiling can also detect copy number variations, including combined whole chromosome 7 gain and chromosome 10 loss, 1p/19q codeletion, gene amplifications and deletions. Although DMP is currently used for CNS tumor diagnosis, as an adjunctive tool to the “conventional” methods, such as histopathology, it is probably the most useful technique to classify neoplasms that exhibit unusual morphology and the only technique capable of reliably identifying novel and rare tumor entities [1]. However, when evaluating DMP results, neuropathologists and neuro-oncologists must pay close attention to the calibrated score values, keeping in mind that suggested diagnoses with scores <0.84 or 0.90 should be viewed with caution, while those with scores <0.50 should be probably rejected [1,68].
The 2021 WHO Classification of CNS Tumors included for the first time DMP results among the Definition and Essential and Desirable Diagnostic Criteria for some entities, including high-grade astrocytomas with piloid features, diffuse pediatric-type high-grade gliomas, H3-wild type, and IDH-wild type, diffuse glioneuronal tumors with oligodendroglioma-like features and nuclear clusters, diffuse leptomeningeal glioneuronal tumors and posterior fossa ependymomas [1].
The current review highlights the importance and the clinical significance of an integrated diagnostic approach to brain tumors, based on histology and genetic alterations, which is crucial to stratify prognosis, overall survival, and even grading in both IDH-mutant and IDH-wild type astrocytoma of adults [69–71]. The current 2021 WHO Classification reflects the provisional “state of the art” about the knowledge in the neuro-oncological field and it should be interpreted as a “further stage” in the evolution of the classification of brain tumors [1]. It should provide neuropathologists and other neuro-oncology experts with a practical and applicable guide for standardizing the diagnostic and the therapeutic approach of CNS neoplasms. However, the increasing need for advanced molecular techniques to correctly diagnose brain tumors, makes the global applicability of the 2021 Classification of CNS Tumors matter of debate, especially in low- and middle-income countries [72].
Author Contributions: Conceptualization, H.A. and G.B.; data curation, A.A. and G.B.; writing—original draft preparation, H.A. and G.B.; writing—review and editing, H.A., A.A., R.C. and G.B. All authors have read and agreed to the published version of the manuscript.
Ethics Approval and Informed Consent Statement: Not applicable.
Availability of Data and Materials: All data presented in this study are available from the corresponding author upon reasonable request.
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Louis, D. N., Perry, A., Wesseling, P., Brat, D. J., Cree, I. A. et al. (2021). The 2021 WHO classification of tumors of the central nervous system: A summary. Neuro-Oncology, 23(8), 1231–1251. DOI 10.1093/neuonc/noab106. [Google Scholar] [CrossRef]
2. Tesileanu, C., Dirven, L., Wijnenga, M., Koekkoek, J., Vincent, A. et al. (2020). Survival of diffuse astrocytic glioma, IDH1/2 wildtype, with molecular features of glioblastoma, WHO grade IV: A confirmation of the cIMPACT-NOW criteria. Neuro-Oncology, 22(4), 515–523. DOI 10.1093/neuonc/noz200. [Google Scholar] [CrossRef]
3. Louis, D. N., Ohgaki, H., Wiestler, O. D., Cavenee, W. K. (2016). WHO classification of tumours of the central nervous system (Revised 4th editionLyon, France: World Health Organization. [Google Scholar]
4. Brat, D. J., Verhaak, R. G., Aldape, K. D., Yung, W. K. (2015). Comprehensive, integrative genomic analysis of diffuse lower-grade gliomas. The New England Journal of Medicine, 372(26), 2481–2498. DOI 10.1056/NEJMoa1402121. [Google Scholar] [CrossRef]
5. Coons, S. W., Pearl, D. K. (1998). Mitosis identification in diffuse gliomas: Implications for tumor grading. Cancer, 82(8), 1550–1555. [Google Scholar]
6. Daumas-Duport, C., Scheithauer, B., O’Fallon, J., Kelly, P. (1988). Grading of astrocytomas. A simple and reproducible method. Cancer, 62(10), 2152–2165. DOI 10.1002/1097-0142(19881115)62:10<2152::aid-cncr2820621015>3.0.co;2-t. [Google Scholar] [CrossRef]
7. Giannini, C., Scheithauer, B. W., Burger, P. C., Christensen, M. R., Wollan, P. C. et al. (1999). Cellular proliferation in pilocytic and diffuse astrocytomas. Journal of Neuropathology and Experimental Neurology, 58(1), 46–53. DOI 10.1097/00005072-199901000-00006. [Google Scholar] [CrossRef]
8. Duregon, E., Bertero, L., Pittaro, A., Soffietti, R., Rudà, R. et al. (2016). Ki-67 proliferation index but not mitotic thresholds integrates the molecular prognostic stratification of lower grade gliomas. Oncotarget, 7(16), 21190–21198. DOI 10.18632/oncotarget.8498. [Google Scholar] [CrossRef]
9. Cimino, P. J., Holland, E. C. (2019). Targeted copy number analysis outperforms histologic grading in predicting patient survival for WHO grades II/III IDH-mutant astrocytomas. Neuro-Oncology, 21(6), 819–821. DOI 10.1093/neuonc/noz052. [Google Scholar] [CrossRef]
10. Aoki, K., Nakamura, H., Suzuki, H., Matsuo, K., Kataoka, K. et al. (2018). Prognostic relevance of genetic alterations in diffuse lower-grade gliomas. Neuro-Oncology, 20(1), 66–77. DOI 10.1093/neuonc/nox132. [Google Scholar] [CrossRef]
11. Olar, A., Wani, K. M., Alfaro-Munoz, K. D., Heathcock, L. E., van Thuijl, H. F. et al. (2015). IDH mutation status and role of WHO grade and mitotic index in overall survival in grade II–III diffuse gliomas. Acta Neuropathologica, 129(4), 585–596. DOI 10.1007/s00401-015-1398-z. [Google Scholar] [CrossRef]
12. Reuss, D. E., Mamatjan, Y., Schrimpf, D., Capper, D., Hovestadt, V. et al. (2015). IDH mutant diffuse and anaplastic astrocytomas have similar age at presentation and little difference in survival: A grading problem for WHO. Acta Neuropathologica, 129(6), 867–873. DOI 10.1007/s00401-015-1438-8. [Google Scholar] [CrossRef]
13. Yoda, R. A., Marxen, T., Longo, L., Ene, C., Wirsching, H. G. et al. (2019). Mitotic index thresholds do not predict clinical outcome for IDH-mutant astrocytoma. Journal of Neuropathology and Experimental Neurology, 78(11), 1002–1010. DOI 10.1093/jnen/nlz082. [Google Scholar] [CrossRef]
14. Shirahata, M., Ono, T., Stichel, D., Schrimpf, D., Reuss, D. E. et al. (2018). Novel, improved grading system(s) for IDH-mutant astrocytic gliomas. Acta Neuropathologica, 136(1), 153–166. DOI 10.1007/s00401-018-1849-4. [Google Scholar] [CrossRef]
15. Appay, R., Dehais, C., Maurage, C. A., Alentorn, A., Carpentier, C. et al. (2019). CDKN2A homozygous deletion is a strong adverse prognosis factor in diffuse malignant IDH-mutant gliomas. Neuro-Oncology, 21(12), 1519–1528. DOI 10.1093/neuonc/noz124. [Google Scholar] [CrossRef]
16. Ceccarelli, M., Barthel, F. P., Malta, T. M., Sabedot, T. S., Salama, S. R. et al. (2016). Molecular profiling reveals biologically discrete subsets and pathways of progression in diffuse glioma. Cell, 164(3), 550–563. DOI 10.1016/j.cell.2015.12.028. [Google Scholar] [CrossRef]
17. Reis, G. F., Pekmezci, M., Hansen, H. M., Rice, T., Marshall, R. E. et al. (2015). CDKN2A loss is associated with shortened overall survival in lower-grade (World Health Organization Grades II–III) astrocytomas. Journal of Neuropathology and Experimental Neurology, 74(5), 442–452. DOI 10.1097/NEN.0000000000000188. [Google Scholar] [CrossRef]
18. Weller, M., Weber, R. G., Willscher, E., Riehmer, V., Hentschel, B. et al. (2015). Molecular classification of diffuse cerebral WHO grade II/III gliomas using genome- and transcriptome-wide profiling improves stratification of prognostically distinct patient groups. Acta Neuropathologica, 129(5), 679–693. DOI 10.1007/s00401-015-1409-0. [Google Scholar] [CrossRef]
19. Yang, R. R., Shi, Z. F., Zhang, Z. Y., Chan, A. K., Aibaidula, A. et al. (2020). IDH mutant lower grade (WHO grades II/III) astrocytomas can be stratified for risk by CDKN2A, CDK4 and PDGFRA copy number alterations. Brain Pathology, 30(3), 541–553. DOI 10.1111/bpa.12801. [Google Scholar] [CrossRef]
20. Lang, S. S. (2013). The role of BRAF-targeted therapy in astrocytomas: A review. Neurosurgery, 60(Suppl 1), 110–112. DOI 10.1227/01.neu.0000430768.25844.4d. [Google Scholar] [CrossRef]
21. Bautista, F., Paci, A., Minard-Colin, V., Dufour, C., Grill, J. et al. (2014). Vemurafenib in pediatric patients with BRAFV600E mutated high-grade gliomas. Pediatric Blood & Cancer, 61(6), 1101–1103. DOI 10.1002/pbc.24891. [Google Scholar] [CrossRef]
22. Ardini, E., Menichincheri, M., Banfi, P., Bosotti, R., de Ponti, C. et al. (2016). Entrectinib, a pan-TRK, ROS1, and ALK inhibitor with activity in multiple molecularly defined cancer indications. Molecular Cancer Therapeutics, 15(4), 628–639. DOI 10.1158/1535-7163.MCT-15-0758. [Google Scholar] [CrossRef]
23. Drilon, A., Siena, S., Ou, S. I., Patel, M., Ahn, M. J. et al. (2017). Safety and antitumor activity of the multitargeted pan-TRK, ROS1, and ALK inhibitor entrectinib: Combined results from two phase I trials (ALKA-372-001 and STARTRK-1). Cancer Discovery, 7(4), 400–409. DOI 10.1158/2159-8290.CD-16-1237. [Google Scholar] [CrossRef]
24. Brat, D. J., Aldape, K., Colman, H., Figrarella-Branger, D., Fuller, G. N. et al. (2020). cIMPACT-NOW update 5: Recommended grading criteria and terminologies for IDH-mutant astrocytomas. Acta Neuropathologica, 139(3), 603–608. DOI 10.1007/s00401-020-02127-9. [Google Scholar] [CrossRef]
25. Ahrendsen, J. T., Torre, M., Meredith, D. M., Hornick, J. L., Reardon, D. A. et al. (2021). IDH-mutant gliomas with additional class-defining molecular events. Modern Pathology, 34(7), 1236–1244. DOI 10.1038/s41379-021-00795-w. [Google Scholar] [CrossRef]
26. Cimino, P. J., Zager, M., McFerrin, L., Wirsching, H. G., Bolouri, H. et al. (2017). Multidimensional scaling of diffuse gliomas: Application to the 2016 World Health Organization classification system with prognostically relevant molecular subtype discovery. Acta Neuropathologica Communications, 5(1), 39. DOI 10.1186/s40478-017-0443-7. [Google Scholar] [CrossRef]
27. Sturm, D., Witt, H., Hovestadt, V., Khuong-Quang, D. A., Jones, D. T. et al. (2012). Hotspot mutations in H3F3A and IDH1 define distinct epigenetic and biological subgroups of glioblastoma. Cancer Cell, 22(4), 425–437. DOI 10.1016/j.ccr.2012.08.024. [Google Scholar] [CrossRef]
28. Gierke, M., Sperveslage, J., Schwab, D., Beschorner, R., Ebinger, M. et al. (2016). Analysis of IDH1-R132 mutation, BRAF V600 mutation and KIAA1549-BRAF fusion transcript status in central nervous system tumors supports pediatric tumor classification. Journal of Cancer Research and Clinical Oncology, 142(1), 89–100. DOI 10.1007/s00432-015-2006-2. [Google Scholar] [CrossRef]
29. Louis, D. N., Wesseling, P., Aldape, K., Brat, D. J., Capper, D. et al. (2020). cIMPACT-NOW update 6: New entity and diagnostic principle recommendations of the cIMPACT-Utrecht meeting on future CNS tumor classification and grading. Brain Pathology, 30(4), 844–856. DOI 10.1111/bpa.12832. [Google Scholar] [CrossRef]
30. Touat, M., Li, Y. Y., Boynton, A. N., Spurr, L. F., Iorgulescu, J. B. et al. (2020). Mechanisms and therapeutic implications of hypermutation in gliomas. Nature, 580(7804), 517–523. DOI 10.1038/s41586-020-2209-9. [Google Scholar] [CrossRef]
31. Cohen, A., Sato, M., Aldape, K., Mason, C. C., Alfaro-Munoz, K. et al. (2015). DNA copy number analysis of grade II-III and grade IV gliomas reveals differences in molecular ontogeny including chromothripsis associated with IDH mutation status. Acta Neuropathologica Communications, 3, 34. DOI 10.1186/s40478-015-0213-3. [Google Scholar] [CrossRef]
32. Richardson, T. E., Sathe, A. A., Kanchwala, M., Jia, G., Habib, A. A. et al. (2018). Genetic and epigenetic features of rapidly progressing IDH-mutant astrocytomas. Journal of Neuropathology and Experimental Neurology, 77(7), 542–548. DOI 10.1093/jnen/nly026. [Google Scholar] [CrossRef]
33. Mirchia, K., Snuderl, M., Galbraith, K., Hatanpaa, K. J., Walker, J. M. et al. (2019). Establishing a prognostic threshold for total copy number variation within adult IDH-mutant grade II/III astrocytomas. Acta Neuropathologica Communications, 7(1), 121. DOI 10.1186/s40478-019-0778-3. [Google Scholar] [CrossRef]
34. Chi, A. S., Batchelor, T. T., Yang, D., Dias-Santagata, D., Borger, D. R. et al. (2013). BRAF V600E mutation identifies a subset of low-grade diffusely infiltrating gliomas in adults. Journal of Clinical Oncology, 31(14), e233–e236. DOI 10.1200/JCO.2012.46.0220. [Google Scholar] [CrossRef]
35. Dahiya, S., Emnett, R. J., Haydon, D. H., Leonard, J. R., Phillips, J. J. et al. (2014). BRAF-V600E mutation in pediatric and adult glioblastoma. Neuro-Oncology, 16(2), 318–319. DOI 10.1093/neuonc/not146. [Google Scholar] [CrossRef]
36. Bar, E. E., Lin, A., Tihan, T., Burger, P. C., Eberhart, C. G. (2008). Frequent gains at chromosome 7q34 involving BRAF in pilocytic astrocytoma. Journal of Neuropathology and Experimental Neurology, 67(9), 878–887. DOI 10.1097/NEN.0b013e3181845622. [Google Scholar] [CrossRef]
37. Jacob, K., Albrecht, S., Sollier, C., Faury, D., Sader, E. et al. (2009). Duplication of 7q34 is specific to juvenile pilocytic astrocytomas and a hallmark of cerebellar and optic pathway tumours. British Journal of Cancer, 101(4), 722–733. DOI 10.1038/sj.bjc.6605179. [Google Scholar] [CrossRef]
38. Schindler, G., Capper, D., Meyer, J., Janzarik, W., Omran, H. et al. (2011). Analysis of BRAF V600E mutation in 1,320 nervous system tumors reveals high mutation frequencies in pleomorphic xanthoastrocytoma, ganglioglioma and extra-cerebellar pilocytic astrocytoma. Acta Neuropathologica, 121(3), 397–405. DOI 10.1007/s00401-011-0802-6. [Google Scholar] [CrossRef]
39. Cruz, G. R., Dias Oliveira, I., Moraes, L., Del Giudice Paniago, M., de Seixas Alves, M. T. et al. (2014). Analysis of KIAA1549-BRAF fusion gene expression and IDH1/IDH2 mutations in low grade pediatric astrocytomas. Journal of Neuro-Oncology, 117(2), 235–242. DOI 10.1007/s11060-014-1398-1. [Google Scholar] [CrossRef]
40. Brennan, C. W., Verhaak, R. G., McKenna, A., Campos, B., Noushmehr, H. et al. (2013). The somatic genomic landscape of glioblastoma. Cell, 155(2), 462–477. DOI 10.1016/j.cell.2013.09.034. [Google Scholar] [CrossRef]
41. Zhang, J., Wu, G., Miller, C. P., Tatevossian, R. G., Dalton, J. D. et al. (2013). Whole-genome sequencing identifies genetic alterations in pediatric low-grade gliomas. Nature Genetics, 45(6), 602–612. DOI 10.1038/ng.2611. [Google Scholar] [CrossRef]
42. Frattini, V., Trifonov, V., Chan, J. M., Castano, A., Lia, M. et al. (2013). The integrated landscape of driver genomic alterations in glioblastoma. Nature Genetics, 45(10), 1141–1149. DOI 10.1038/ng.2734. [Google Scholar] [CrossRef]
43. Badiali, M., Gleize, V., Paris, S., Moi, L., Elhouadani, S. et al. (2012). KIAA1549-BRAF fusions and IDH mutations can coexist in diffuse gliomas of adults. Brain Pathology, 22(6), 841–847. DOI 10.1111/j.1750-3639.2012.00603.x. [Google Scholar] [CrossRef]
44. Singh, D., Chan, J. M., Zoppoli, P., Niola, F., Sullivan, R. et al. (2012). Transforming fusions of FGFR and TACC genes in human glioblastoma. Science, 337(6099), 1231–1235. DOI 10.1126/science.1220834. [Google Scholar] [CrossRef]
45. Qaddoumi, I., Orisme, W., Wen, J., Santiago, T., Gupta, K. et al. (2016). Genetic alterations in uncommon low-grade neuroepithelial tumors: BRAF, FGFR1, and MYB mutations occur at high frequency and align with morphology. Acta Neuropathologica, 131(6), 833–845. DOI 10.1007/s00401-016-1539-z. [Google Scholar] [CrossRef]
46. Jones, D. T., Hutter, B., Jäger, N., Korshunov, A., Kool, M. et al. (2013). Recurrent somatic alterations of FGFR1 and NTRK2 in pilocytic astrocytoma. Nature Genetics, 45(8), 927–932. DOI 10.1038/ng.2682. [Google Scholar] [CrossRef]
47. Gambella, A., Senetta, R., Collemi, G., Vallero, S. G., Monticelli, M. et al. (2020). NTRK fusions in central nervous system tumors: A rare, but worthy target. International Journal of Molecular Sciences, 21(3), 753. DOI 10.3390/ijms21030753. [Google Scholar] [CrossRef]
48. Torre, M., Vasudevaraja, V., Serrano, J., DeLorenzo, M., Malinowski, S. et al. (2020). Molecular and clinicopathologic features of gliomas harboring NTRK fusions. Acta Neuropathologica Communications, 8(1), 107. DOI 10.1186/s40478-020-00980-z. [Google Scholar] [CrossRef]
49. Zheng, Z., Liebers, M., Zhelyazkova, B., Cao, Y., Panditi, D. et al. (2014). Anchored multiplex PCR for targeted next-generation sequencing. Nature Medicine, 20(12), 1479–1484. DOI 10.1038/nm.3729. [Google Scholar] [CrossRef]
50. Ferguson, S. D., Zhou, S., Huse, J. T., de Groot, J. F., Xiu, J. et al. (2018). Targetable gene fusions associate with the IDH wild-type astrocytic lineage in adult gliomas. Journal of Neuropathology and Experimental Neurology, 77(6), 437–442. DOI 10.1093/jnen/nly022. [Google Scholar] [CrossRef]
51. Louis, D. N., Perry, A., Reifenberger, G., Deimling, A. V., Figarella-Branger, D. et al. (2016). The 2016 World Health Organization classification of tumors of the central nervous system: A summary. Acta Neuropathologica, 131(6), 803–820. DOI 10.1007/s00401-016-1545-1. [Google Scholar] [CrossRef]
52. Eckel-Passow, J. E., Lachance, D. H., Molinaro, A. M., Walsh, K. M., Decker, P. A. et al. (2015). Glioma groups based on 1p/19q, IDH, and TERT promoter mutations in tumors. The New England Journal of Medicine, 372(26), 2499–2508. DOI 10.1056/NEJMoa1407279. [Google Scholar] [CrossRef]
53. Hartmann, C., Hentschel, B., Wick, W., Capper, D., Felsberg, J. et al. (2010). Patients with IDH1 wild type anaplastic astrocytomas exhibit worse prognosis than IDH1-mutated glioblastomas, and IDH1 mutation status accounts for the unfavorable prognostic effect of higher age: Implications for classification of gliomas. Acta Neuropathologica, 120(6), 707–718. DOI 10.1007/s00401-010-0781-z. [Google Scholar] [CrossRef]
54. Komori, T. (2021). Grading of adult diffuse gliomas according to the 2021 WHO classification of tumors of the central nervous system. Laboratory Investigation, 102(2), 126–133. DOI 10.1038/s41374-021-00667-6. [Google Scholar] [CrossRef]
55. Wijnenga, M., Dubbink, H. J., French, P. J., Synhaeve, N. E., Dinjens, W. et al. (2017). Molecular and clinical heterogeneity of adult diffuse low-grade IDH wild-type gliomas: Assessment of TERT promoter mutation and chromosome 7 and 10 copy number status allows superior prognostic stratification. Acta Neuropathologica, 134(6), 957–959. DOI 10.1007/s00401-017-1781-z. [Google Scholar] [CrossRef]
56. Yan, H., Parsons, D. W., Jin, G., McLendon, R., Rasheed, B. A. et al. (2009). IDH1 and IDH2 mutations in gliomas. The New England Journal of Medicine, 360(8), 765–773. DOI 10.1056/NEJMoa0808710. [Google Scholar] [CrossRef]
57. Brat, D. J., Aldape, K., Colman, H., Holland, E. C., Louis, D. N. et al. (2018). cIMPACT-NOW update 3: Recommended diagnostic criteria for “Diffuse astrocytic glioma, IDH-wildtype, with molecular features of glioblastoma, WHO grade IV”. Acta Neuropathologica, 136(5), 805–810. DOI 10.1007/s00401-018-1913-0. [Google Scholar] [CrossRef]
58. Reuss, D. E., Kratz, A., Sahm, F., Capper, D., Schrimpf, D. et al. (2015). Adult IDH wild type astrocytomas biologically and clinically resolve into other tumor entities. Acta Neuropathologica, 130(3), 407–417. DOI 10.1007/s00401-015-1454-8. [Google Scholar] [CrossRef]
59. Stichel, D., Ebrahimi, A., Reuss, D., Schrimpf, D., Ono, T. et al. (2018). Distribution of EGFR amplification, combined chromosome 7 gain and chromosome 10 loss, and TERT promoter mutation in brain tumors and their potential for the reclassification of IDHwt astrocytoma to glioblastoma. Acta Neuropathologica, 136(5), 793–803. DOI 10.1007/s00401-018-1905-0. [Google Scholar] [CrossRef]
60. Lee, M., Kang, S. Y., Suh, Y. L. (2019). Genetic alterations of epidermal growth factor receptor in glioblastoma: The usefulness of immunohistochemistry. Applied Immunohistochemistry & Molecular Morphology, 27(8), 589–598. DOI 10.1097/PAI.0000000000000669. [Google Scholar] [CrossRef]
61. Koelsche, C., Sahm, F., Capper, D., Reuss, D., Sturm, D. et al. (2013). Distribution of TERT promoter mutations in pediatric and adult tumors of the nervous system. Acta Neuropathologica, 126(6), 907–915. DOI 10.1007/s00401-013-1195-5. [Google Scholar] [CrossRef]
62. Vinagre, J., Almeida, A., Pópulo, H., Batista, R., Lyra, J. et al. (2013). Frequency of TERT promoter mutations in human cancers. Nature Communications, 4, 2185. DOI 10.1038/ncomms3185. [Google Scholar] [CrossRef]
63. Reinhardt, A., Stichel, D., Schrimpf, D., Sahm, F., Korshunov, A. et al. (2018). Anaplastic astrocytoma with piloid features, a novel molecular class of IDH wildtype glioma with recurrent MAPK pathway, CDKN2A/B and ATRX alterations. Acta Neuropathologica, 136(2), 273–291. DOI 10.1007/s00401-018-1837-8. [Google Scholar] [CrossRef]
64. Berzero, G., Di Stefano, A. L., Ronchi, S., Bielle, F., Villa, C. et al. (2021). IDH-wildtype lower-grade diffuse gliomas: The importance of histological grade and molecular assessment for prognostic stratification. Neuro-Oncology, 23(6), 955–966. DOI 10.1093/neuonc/noaa258. [Google Scholar] [CrossRef]
65. Giannini, C., Giangaspero, F. (2021). TERT promoter mutation: Is it enough to call a WHO grade II astrocytoma IDH wild-type glioblastoma?. Neuro-Oncology, 23(6), 865–866. DOI 10.1093/neuonc/noab052. [Google Scholar] [CrossRef]
66. Vaubel, R. A., Caron, A. A., Yamada, S., Decker, P. A., Eckel Passow, J. E. et al. (2018). Recurrent copy number alterations in low-grade and anaplastic pleomorphic xanthoastrocytoma with and without BRAF v600e mutation. Brain Pathology, 28(2), 172–182. DOI 10.1111/bpa.12495. [Google Scholar] [CrossRef]
67. Phillips, J. J., Gong, H., Chen, K., Joseph, N. M., van Ziffle, J. et al. (2019). The genetic landscape of anaplastic pleomorphic xanthoastrocytoma. Brain Pathology, 29(1), 85–96. DOI 10.1111/bpa.12639. [Google Scholar] [CrossRef]
68. Capper, D., Stichel, D., Sahm, F., Jones, D. T. W., Schrimpf, D. et al. (2018). Practical implementation of DNA methylation and copy-number-based CNS tumor diagnostics: The Heidelberg experience. Acta Neuropathologica, 136(2), 181–210. DOI 10.1007/s00401-018-1879-y. [Google Scholar] [CrossRef]
69. Broggi, G., Salvatorelli, L., Barbagallo, D., Certo, F., Altieri, R. et al. (2021). Diagnostic utility of the immunohistochemical expression of serine and arginine rich splicing factor 1 (SRSF1) in the differential diagnosis of adult gliomas. Cancers, 13(9), 2086. DOI 10.3390/cancers13092086. [Google Scholar] [CrossRef]
70. Certo, F., Altieri, R., Maione, M., Schonauer, C., Sortino, G. et al. (2021). FLAIRectomy in supramarginal resection of glioblastoma correlates with clinical outcome and survival analysis: A prospective, single institution, case series. Operative Neurosurgery, 20(2), 151–163. DOI 10.1093/ons/opaa293. [Google Scholar] [CrossRef]
71. Stella, M., Falzone, L., Caponnetto, A., Gattuso, G., Barbagallo, C. et al. (2021). Serum extracellular vesicle-derived circHIPK3 and circSMARCA5 are two novel diagnostic biomarkers for glioblastoma multiforme. Pharmaceuticals (Basel), 14(7), 618. DOI 10.3390/ph14070618. [Google Scholar] [CrossRef]
72. Moudgil-Joshi, J., Kaliaperumal, C. (2021). Letter regarding louis et al: The 2021 WHO classification of tumors of the central nervous system: A summary. Neuro-Oncology, 23(12), 2120–2121. DOI 10.1093/neuonc/noab190. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |