
 | Sound & Vibration |  |
DOI: 10.32604/oncologie.2022.019236
ARTICLE
Tumor-Associated Macrophages Facilitate the Proliferation and Migration of Cervical Cancer Cells
1Central Laboratory, University of Chinese Academy of Sciences-Shenzhen Hospital, Shenzhen, 518016, China
2Institute of Virology, School of Basic Medical Sciences, State Key Laboratory of Virology, Wuhan University, Wuhan, 430071, China
*Corresponding Author: Xinxing Wu. Email: wuxinxing9755@163.com
Received: 10 September 2021; Accepted: 16 March 2022
Abstract: Tumor-associated macrophages (TAMs) are important components in tumor microenvironment. This study intended to explore the influence of TAMs on cervical cancer cells proliferation and migration. The expression levels of TAMs markers, CD68 and CD163, in tissues were examined by immunohistochemistry and increased with the progression of cervical lesions (p < 0.05). TAMs with M2-like phenotype (PMA(Polymethacrylate) induced THP-1 cells) were noticed to promote the proliferation of cervical cancer cells and improve the migration ability of tumor cells. These enhancements were attributed to secreting soluble components and the physical contact between macrophages and tumor cells. The tumor formation and tumor growth were significantly faster in the mixed group of induced THP-1 cells and SiHa than in the SiHa cells alone group (p < 0.05). The results indicated that the interaction of macrophages and cervical cancer cells, including the secretion of soluble components and physical contact, were responsible for shaping immunosuppression microenvironments and in promoting tumor cell proliferation and migration.
Keywords: Tumor-associated macrophage; cervical cancer; proliferation; migration
An immunosuppressive microenvironment in various cancers, including cervical cancer, suppresses pro-inflammatory Th1 and cytotoxic lymphocyte responses due to low immunogenicity and promotes the production of cytokines such as transforming growth factor (TGF-β), interleukin (IL-10), indoleamine 2,3-dioxygenase (IDO) and prostaglandin E2 (PGE2). Approximately 50% cervical cancer patients have manifested a weak proliferative response of T cells. This was associated with a functional switch of tumor-infiltrated cells such as T cells, dendritic cells and macrophages from tumor-suppressing to tumor-promoting in the immune suppressive tumor microenvironment [1–3].
Tumor-infiltrated macrophages, also known as tumor-associated macrophages (TAMs) are mainly display both M1-like and M2-like phenotypes. M1 macrophages are activated by interferon (IFN-γ) and lipopolysaccharides (LPS) through Toll-like receptors (TLRs), and granulocyte-monocyte colony-stimulating factor (GM-CSF). These factors retain the properties such as high expression of major histocompatibility complex class II (MHC-II) and co-stimulatory molecules such as CD86/CD80, high production of IL-12, IL-23, tumor necrosis factor (TNF-α), increased generation of reactive oxygen species (ROS) and reactive nitrogen species (RNS). The primary functions of M1 macrophages are to eliminate intracellular pathogens, destroy tumor cells and promote the Th1 immune response. Meanwhile, M2 macrophages are induced by various interleukins such as IL-4, IL-13, IL-10, IL-33, and IL-21 and are characterized by the expressions of CD163, CD68 and CD206. M2 macrophages are involved in parasite clearance, tissue remodeling, wound healing and immunoregulation. Unlike M1, M2 macrophages promote the Th2 immune response, tumor promotion through the expression of vascular endothelial growth factor (VEGF), transforming growth factor (TGF)-β, indoleamine 2,3-dioxygenase (IDO) and programmed death ligand 1 (PD-L1) expression. TAMs are highly plastic in nature possessing features of both M1-like and M2-like phenotypes. In some tumors, the presence of M2 macrophages is an indicator of a poor prognosis [4–6].
The microenvironment of malignant cell promotes the secretion of numerous cytokines to induce the migration of mononuclear cells into the tumor tissue and therefore, allowing the polarization of TAMs [7–9]. The polarized TAMs stimulate tumor cell proliferation and survival, suggesting the key role in cervical cancer formation and development [10,11]. In this study, we mainly focused on the effect of TAMs on proliferation, migration and metastasis of cervical cancer cells.
Cervical cancer cell lines SiHa, HeLa and C33A cells were preserved by our laboratory, and THP-1 cells were purchased from Shanghai Cell Bank of Chinese Academy of Sciences. All cells were cultured in RPMI 1640 or Dulbecco’s Modified Eagle Medium (DMEM) containing 10% fetal bovine serum (FBS) at 37°C in a 5% CO2 incubator. Cervical tissue paraffin specimens were obtained from Departments of Pathology, Zhongnan Hospital of Wuhan University. There were 29 cervical cancer, 24 LSIL and 23 HSIL. All experimental specimens were re-examined by two pathologists independently.
Tissues were 4% formalin-fixed, paraffin-embedded, 4 μm serial sections. Staining method was in strict followed as mentioned in ready-to-use non-biotin immunohistochemical EliVisionTM plus detection kit (Fuzhou Maxim Biotech, China). In brief, tissue slices were washed by ddH2O (pH 7.4) for 5 min thrice after dewaxed and hydrated. Then slices were boiled in citrate buffer (pH 6.0, 10 mmol/ml) for 1–2 min in high pressure boiler in order to repair antigen, followed by risned in ddH2O for 5 min twice and in PBST for 5 min twice. Then tissues were incubated with monoclonal primary antibody at 37°C for 1 h, and rinsed in PBST for 5 min thrice. 50 μl polymer intensifier was added onto each slice and incubated at room temperature for 30 min, then rinsed in PBST for 5 min thrice. 50 μl enzyme-mark polymer against murine was added and hatched at room temperature for 30 min followed by rinsing in PBST for 5 min thrice. 100 μl fresh DAB was dropped onto each slides and incubated for 5 min, then coloration was finished with the ddH2O. Then hematoxylin stained followed by dehydration, clearing and mounting with neutral gums.
2.3 THP-1 and Cervical Cancer Cells Co-Cultured
TAM in vitro model: TAMs are M2-like macrophage. The Phorbol-12-myristate-13-acetate (PMA)-treated THP-1 macrophages had an M2 functional profile and considered as a cell model for functional study of TAM. THP-1 cells were induced with 100 ng/ml PMA to polarizing the THP1 cells to TAMs. After 48 h, the supernatant was discarded and the cells were washed three times with PBS. The cells (PMA-induced THP-1 (PMA-THP-1)) were continued to cultured in RPMI-1640 complete medium and used in the following experiments. The macrophages used in this study were the PMA -treated THP-1 as if no special version.
Contact co-culture: Cervical cancer cells were seeded in a 6-well plate or culture dish in which PMA-THP-1 cells had been induced to grow and mix (the ratio of cervical cancer cells: PMA-THP-1 cells were maintained as 5:1).
Non-contact co-culture: After induction of THP-1 cells in the trans-well upper chamber, Cells in the lower chamber were cervical cancer cells under non-contact co-culture condition to avoid direct contacts among cells.
THP-1 cells and PMA induced THP-1 cells were trypsinized and centrifuged at 4°C at 300 g for 5 min, washed with PBS twice and centrifuged at 4°C at 300 g for 5 min. Then 1 × 106 cells were suspended in 500 µl PBS and mixed with FTIC conjugated CD68 or CD163 antibody at final concentration of 1 µl/ml, and incubated 60 min in the dark at 37°C. Then detected by flow cytometry.
2.5 Tumor Cells Proliferation Experiment
Cells at logarithmic growth phase were harvested and centrifuged at 1000 r/min for 5 min. The supernatant was discarded and cell pellets were re-suspended in PBS. Carboxyfluorescein diacetate succinimidyl ester (CFSE) was added to suspend cells at final concentration of 5 μmol/L and incubated for 5 min at 37°C. Cells were centrifuged for 5 min at 1000 r/min before adding to RPMI-1640 complete medium and incubated for 5 min at 37°C followed by centrifugation thrice, suspenion and cell counting. The labeled cells were fixed in the dark at 4°C as seed cells. Cells co-cultured for 48 h under the contact co-culture or non-contact co-culture conditions, respectively. The unlabeled cells were harvested as a blank control, washed for once with PBS, and then were re-suspended in 500 μl PBS and detected with FACS. Cell proliferation was analyzed using Flowjo Software to calculate the division index. The division index was the average number of cell divisions that a cell in the original population had undergone.
Cervical cancer cells were cultured both separately and under non-contact co-culture in 6-well plates for 24 h. After attaining 90% confluency, cells were mechanically injured to create an incision-like gap with a sterile micropipette tip. With a cell-free gap prepared, optical microscopy was used to observe cells migrating into the wound area. The photographs were taken at 0 and 24 h. The wound healing rate was evaluated by measuring wound closure rat which was the ration of the occupied area in the gap to the area of the initial gap calculated the change in wound area.
2.6.2 Trans-Well Migration Assay
Migration assays were performed in 24-well trans-well chambers (BD Biosciences, Bedford, Massachusetts) containing polycarbonate filters with 8 μm pores (BD Biosciences). Firstly, the 1 × 105 cells (cultured alone or co-cultured with PMA-THP1) that were suspended in serum-free DMEM were added to the upper compartment of the chamber, and medium containing 10% fetal bovine serum was added to the lower compartment of the chamber. At presupposition point, non-migrated cells remaining on the top of the upper chamber were removed with cotton swabs, whereas the cells migrated through the membrane and attached to the lower surface of the membrane were fixed with paraformaldehyde and stained with 1% crystal violet. Photos were captured with a fluorescence microscope. And the number of migrated cells was quantified using the ImageJ software.
Gelatin zymography was prepared essentially as described previously [12]. MMP-9 assay was size-separated via electrophoresis through polyacrylamide gels impregnated with gelatin (ThermoFisher Scientific). Briefly, Cervical cancer cell culture supernatant was collected after 24 h single culture, contact co-culture or non-contact co-culture. Supernatants mixed with sample loading buffer and 55°C water bath for 3–5 min. Electrophoresis was proceeded at 165 V for 1 h. Then gels were eluted in 2.5% Triton X-100 twice for 15 min each time, and incubated in incubation buffer at 37°C for 16–20 h. Gels were then stained with Coomassie Blue and destained overnight to reveal cleared, non-stained regions that resulted from in-gel gelatin hydrolysis by MMP-9.
2.8 Cytometric Beads Array for Cytokines Levels Detections
Levels of IL-8, IL-1β, IL-6, IL-10, IL-12p70 and TNF-ɑ in the supernatant of cultured cells including SiHa cells, PMA-induced THP-1 cells, SiHa cells and THP-1 co-cultures for 24 h were determined using Cytometric Beads Array (CBA) (BD Pharmingen) according to the manufacturer’s instructions. Briefly, culture supernatant was collected after 24 h single culture, contact co-culture or non-contact co-culture. Dilute Standards by serial dilutions using the Assay Diluent, and Assay Diluent was used to be control. 10 µl/test mixed Human Inflammation Capture Bead suspension (vortex before aliquoting) was prepared. 50 µl of mixed beads were transfered to each assay tube. 50 µl Standard Dilutions or test samples were add to the appropriate sample tubes. And then PE Detection Reagent (50 µl/test) was added. After 3 h incubation at RT, samples were washed with 1 ml Wash Buffer and centrifuged at 200 g for 5 min. Finally, 300 µl Wash Buffer was added to each assay tubes and analyzed. The data was acquired on FACS Canto-II (BD Bioscience, USA) and analyzed using FCAP Array software (BD Bioscience).
Female severe combined immuno-deficient (SCID) mice with 15 to 20 g of weight were provided by the Medical Experimental Animal Center of Guangdong Province (Hunan Slack Jingda Company, China). Animal experiments were conducted under the protocol approved by the Institutional Animal Care and Use Committee of Wuhan University. 18 mice were randomly divided into three groups, namely SiHa cells, mixed inoculation group (contact co-cultured SiHa cells and PMA induced THP-1 cells) and PMA-THP-1 group, with 6 mice in each group. Six weeks old female nude mice were inoculated subcutaneously at the right hindlimb with 1.0 × 107 cells/0.2 ml PBS. Tumor size was measured using a caliper, and the weight of each mouse was measured with a scale every 3 days until the end of the experiment. The volume of tumor was calculated using the following formula:
where length represents the largest tumor diameter and width represents the perpendicular tumor diameter.
All mice were sacrificed at day 30 after cell inoculation. The mean tumor volume was used to generate a tumor growth curve. Tumor specimens were then prepared as formalin-fixed, paraffin-embedded sections for hematoxylin-eosin (H&E) staining. CD68 and Ki67 were detected using immunohistochemical method.
All data were expressed as means ± SD and were representative of at least two repeats. The statistical significance of group differences was by Student’s t-test. p < 0.05 was considered statistically significant (StatXact4 software, Cytel Corporation, Cambridge, Massachusetts, USA).
3.1 Expression of Macrophage Markers in Cervical Tissue
Macrophage markers CD68 and CD163 were detected in cervical tissue samples by immunohistochemistry. As shown in Fig. 1, CD68 and CD163 were predominantly expressed in cytoplasm. Interstitium and cancer nest depicted majority of CD68 and CD163 positive cells. The expression of CD68 and CD163 in LSIL (CIN1), HSIL (CIN2 and CIN3) and cervical cancer tissues was significantly increased (p < 0.05) (Table 1). These macrophages exhibited migration to pithelial tissues with the progression of cervical lesions (Fig. 1).
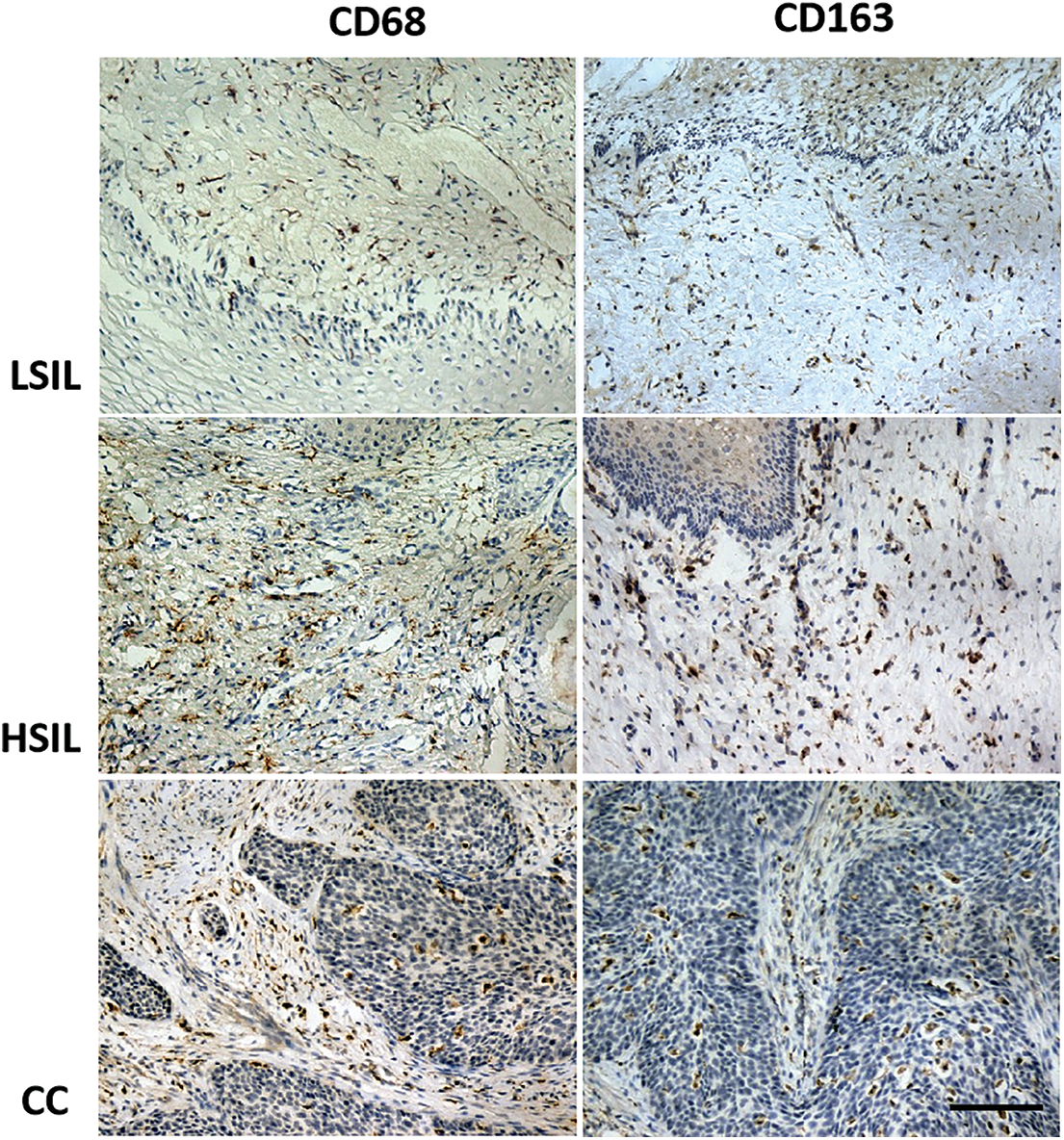
Figure 1: The expression of CD68 and CD163 in LSIL, HSIL and CC tissue using immunohistochemistry analysis (100×). The expressions of CD68 and CD163 in LSIL (CIN1), HSIL (CIN2 and CIN3) and cervical cancer tissues were increased (p < 0.05), as well as an increase of macrophages migrated to epithelial tissues with the progression of cervical lesions. Scale bar = 100 μm

3.2 Effect of Induced Differentiation Macrophages on the Proliferation of Cervical Cancer Cells
Morphological observations showed that THP-1 cells grew in suspension with a round shape (Fig. 2A). After induction by 100 ng/ml PMA for 48 h, THP-1 cells changed to adherent growth, and formed irregular in shape with multiple pseudopods, presenting macrophage morphology (Fig. 2B). The expressions of recognized macrophage markers, CD68 and CD163, were analyzed by flow cytometry. Flow cytometry showed that, in the untreated THP-1 cells, the cell surface expression was 33.17 ± 2.81% for CD68 and 24.93 ± 3.59% for CD163. And, the cell surface of the PMA-THP-1 was 56.40 ± 3.12% for CD68 and 42.90 ± 3.64% for CD163. The CD68 and CD163 cell surface expressions of PMA-THP-1 were significantly higher than those of THP-1 (p < 0.05) (Figs. 2C and 2D), which suggested the successful induction of TAMs.
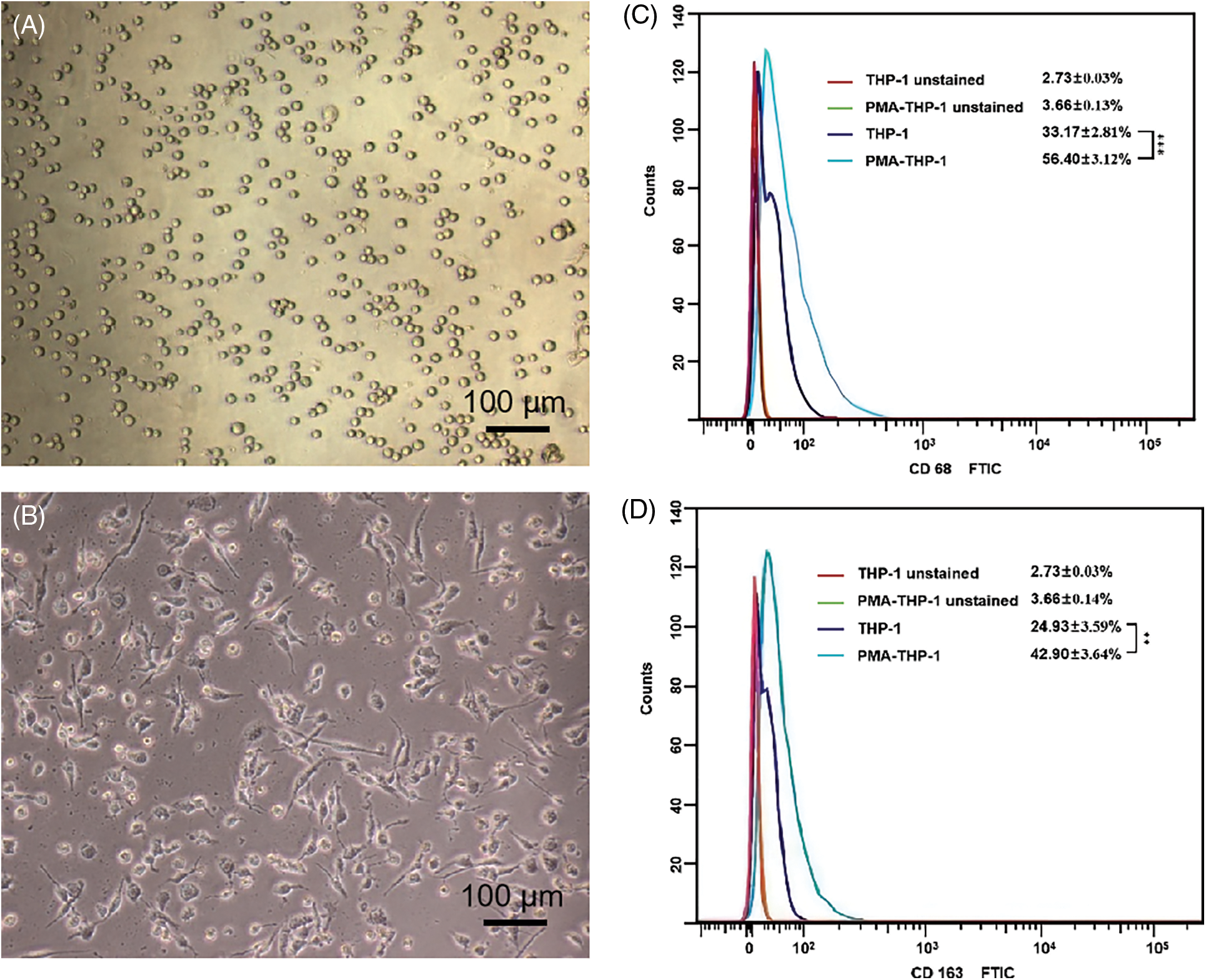
Figure 2: Morphological observation and surface marker determination of TAMs polarized from THP-1 cells induced by PAM in vitro. (A) THP-1 cell morphology; (B) Morphology of THP-1 cell treated with 100 ng/ml PMA for 48 h. (C) The expression of CD68 on the surface of THP-1 cells before and after treated with PMA. (D) The expression of CD163 on the surface of THP-1 cells before and after treated with PMA
The proliferation of 3 categories of contact co-culture and non-contact co-culture cervical cancer cells was detected using carboxyfluorescein diacetate succinimidyl ester (CFSE) method. For HeLa cells and SiHa cells, the division index was observed higher in both co-culture and non-contact co-culture condition compared to culture separately (p < 0.05), indicating that contact co-culture facilitated cell proliferation more under co-culture condition (p < 0.05). However, there was no significant difference in division index for C33A, indicating that co-culture had no effect on the proliferation of C33A (Fig. 3).
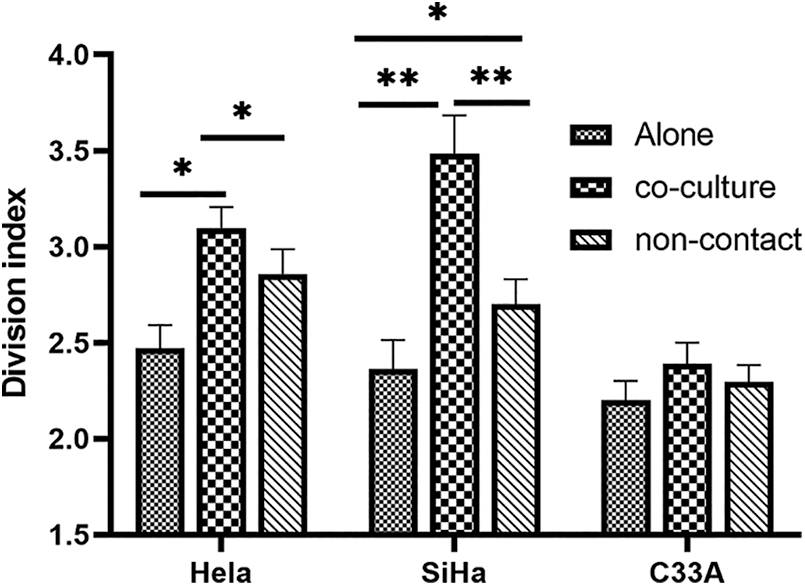
Figure 3: Division index of HeLa, SiHa and C33A cells under contact co-culture and non-contact co-culture conditions was detected using CFSE method. For HeLa cells and SiHa cells, the division index was higher in both co-culture and non-contact co-culture condition than culture alone, whereas there was no significant difference in division index for C33A under either contact co-culture or non-contact co-culture
3.3 Effect of Differentiated Macrophages on the Migration of Cervical Carcinoma
Wound Healing Assay is a simple way to detect cell migration. The co-cultured of SiHa cells and C33A cells promoted enhanced the migration ability. The migration ability of HeLa cells did not change significantly after co-culture (Fig. 4).
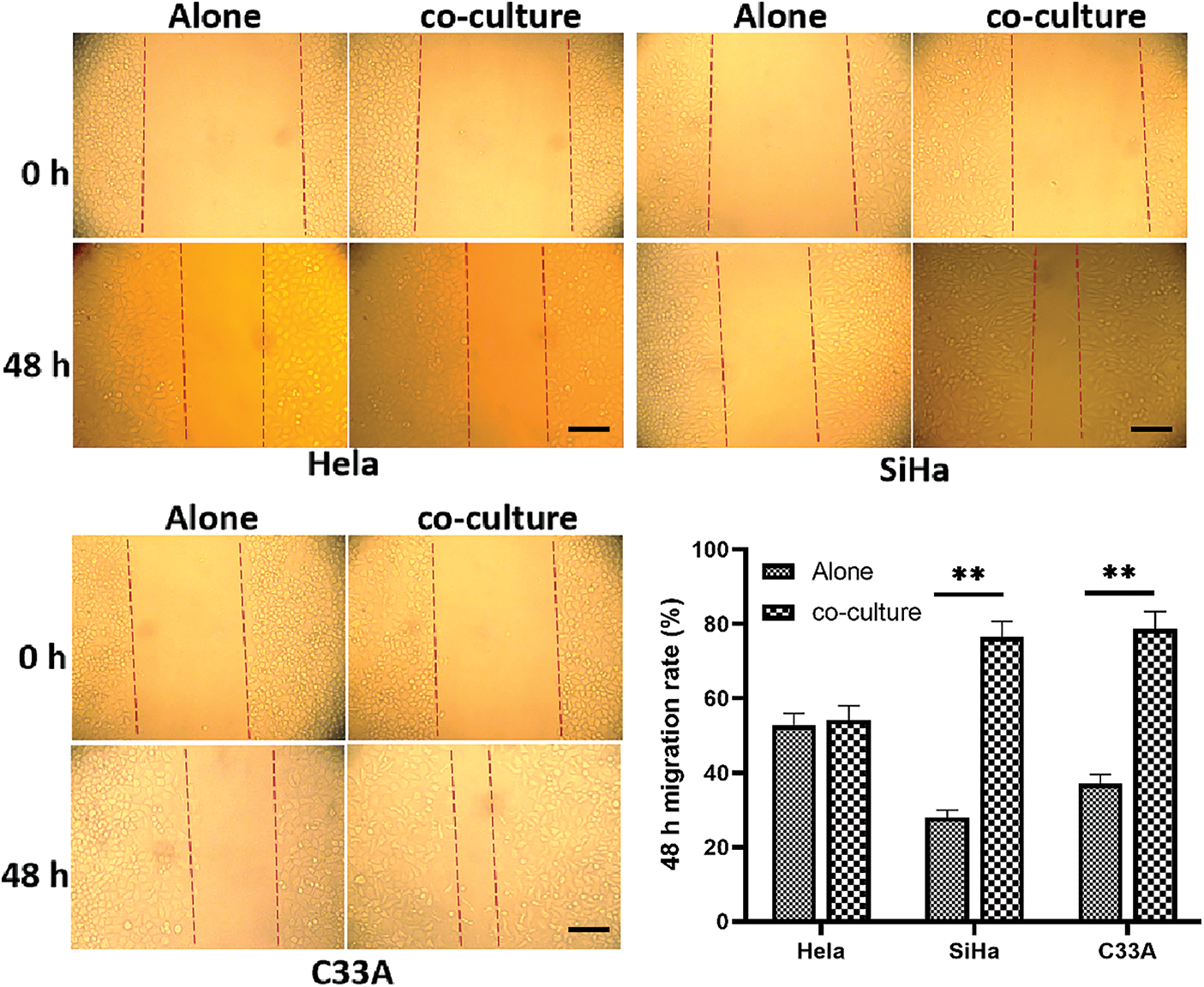
Figure 4: Cell migration under contact co-culture was detected by wound healing assay. The co-cultured SiHa cells and C33A cells migrate faster (p < 0.05). Scale bar = 100 μm
In trans-well migration assay, the migration of C33A and SiHa cells across the membrane increased significantly in indirect contact condition. However, the number of HeLa cells passing through the membrane before and after co-culture showed no difference, indicating that co-culture had no major effect on the migration ability of HeLa cells (Fig. 5).
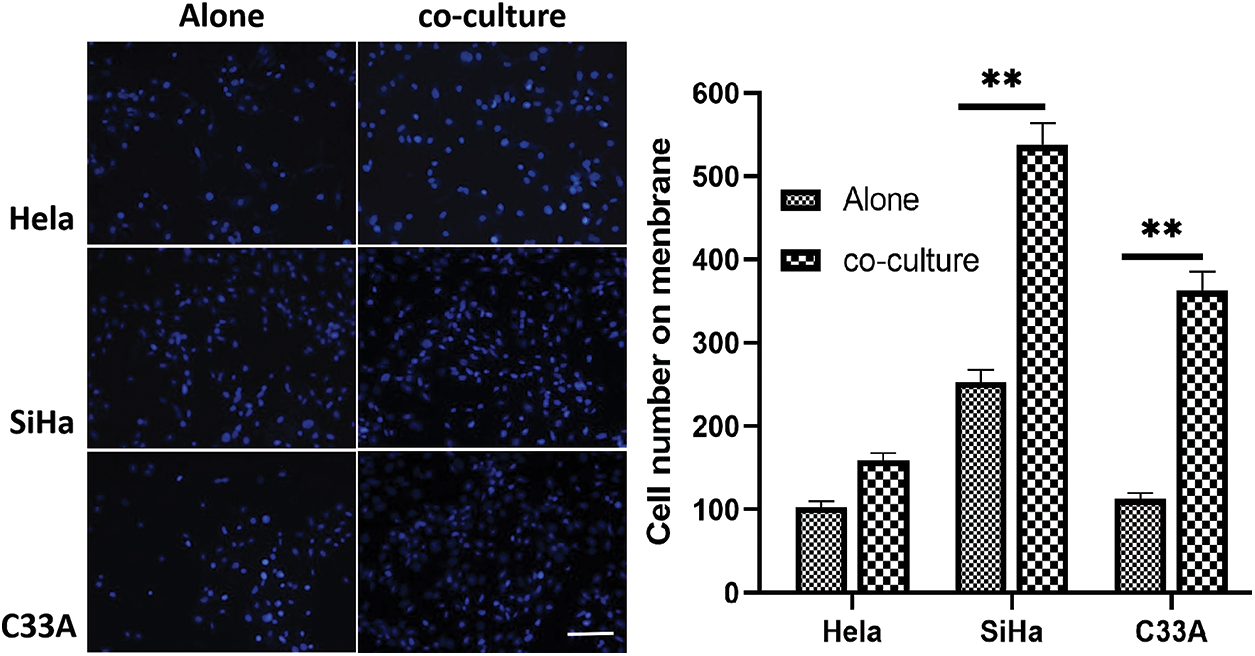
Figure 5: Cell immigration detected by trans-well assay. The non-contact co-cultured SiHa cells and C33A cells migrate faster (p < 0.05). Scale bar = 100 μm
3.4 MMP Activity Detection with Zymography
PMA-THP-1 cells secreted high levels of MMP-9 validated by the translucent band at 92KD. MMP-9 secreted by the three types of cervical cancer cell lines was less, and the bands were vaguely visible. The amount of MMP-9 secreted in the two types of co-culture supernatants was less significantly less than MMP-9 in the supernatant of PMA-THP-1 cells, induced THP-1 are primarily involved in the secretion of MMP-9. The depicted that co-culture was not capable for cells to secrete more MMP-9 (Fig. 6).
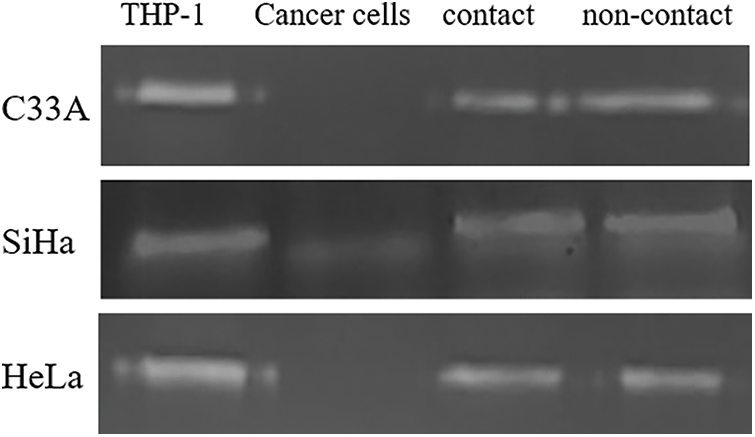
Figure 6: MMP-9 activity was detected by gelatin zymography. MMP-9 was mainly secreted by the induced THP-1 cells, and co-culture did not enable cells to secrete more MMP-9 either in the contact co-culture or non-contact co-culture system
3.5 Cytokine Detection with CBA
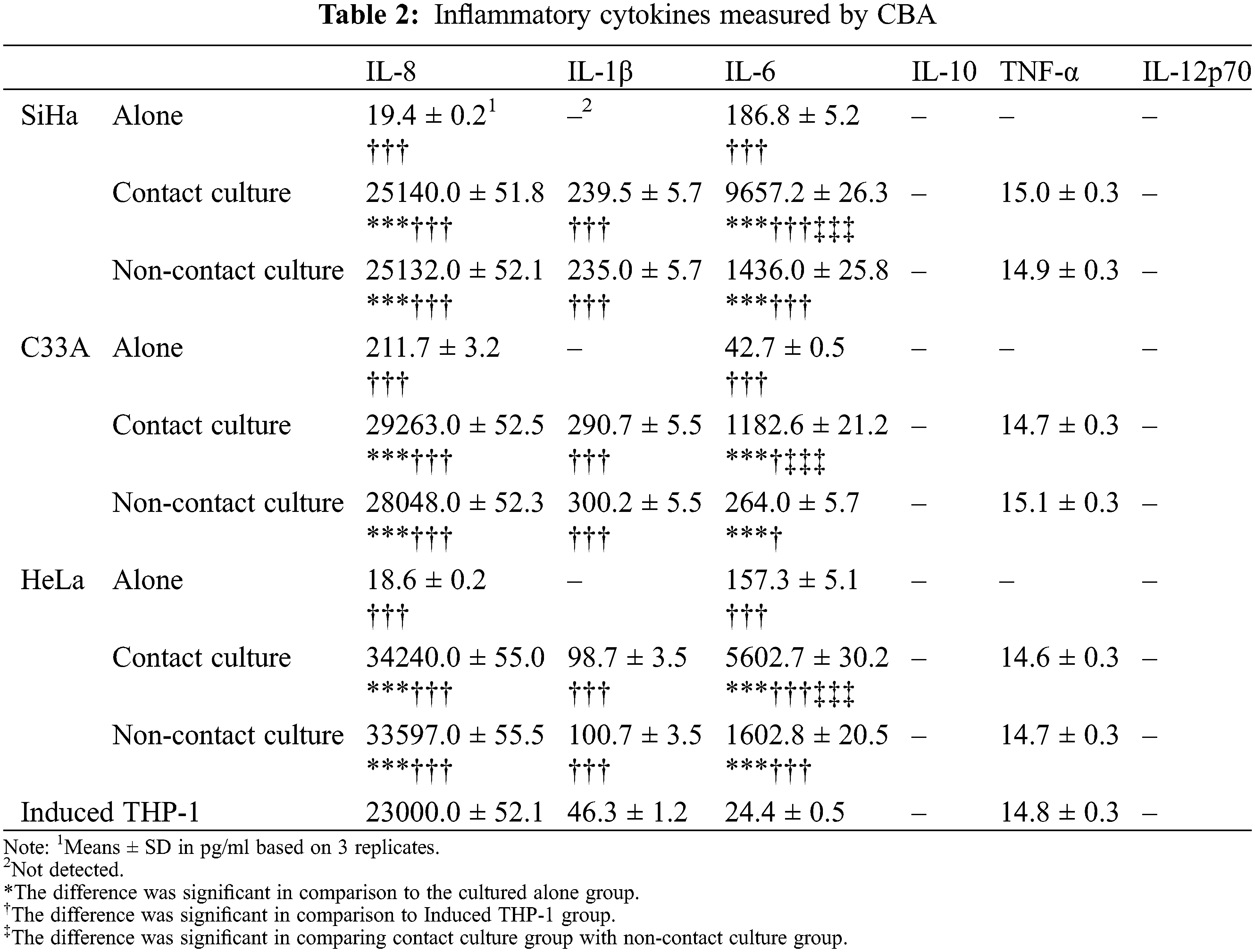
The levels of various cytokines IL-8, IL-1β, IL-6, IL-10, IL-12p70 and TNF-α in each sample were detected simultaneously by CBA. The levels of IL-8, IL-1β and IL-6 in the three co-culture supernatants were higher than culturing separately and THP-1, indicating that the co-culture conditions accelerated the levels of cytokines. The secretion levels of IL-8 and IL-1β were similar to those in the supernatants of the contact culture and non-contact culture, and showed insignificant difference. Contrastingly, the level of IL-6 in the contact culture supernatant was substantially higher than that in the noncontact co-culture. Interestingly, IL-10 and IL-12p70 were not detected in all supernatant samples. Moreover, IL-1β and TNF-α were not detected in cervical cancer cell lines. There was no significant difference in the level of TNF-α in the supernatant of THP-1 cells after co-culture and induction, suggesting that the co-culture had no effect on TNF-α secreting level of the induced THP-1 cells (see Table 2).
3.6 Animal Experiments in Vivo
The palpable tumor in the mixed inoculation groups (THP1 cells and SiHa cells after induction) was observed after 14 days of inoculation, after which the tumor size was measured every 3 days. The results showed that the time of tumor formation and the tumor growth was significantly faster in the mixed culture group than that the SiHa cells alone inoculation group. After dissecting nude mice tumor tissue post 30 days of harvest, the tumor volume was significantly larger in the mixed inoculation group than that the SiHa cells inoculation group (Fig. 7) and the average tumor weight in the mixed culture group was approximately twice as in the SiHa cells alone group (Fig. 7). No tumor formation was found in the PMA-THP1 group at the end of the experiments, supported by 100% survival rate of all nude mice. For H&E staining, the squamous cell carcinoma was found in all tumor specimens (Fig. 8). The fewer macrophage marker CD68 was observed in the SiHa cells alone inoculation group whereas large amount of macrophage infiltration detected in the mixed inoculation group (Fig. 8). In addition, Ki-67 expression in mixed inoculation group was significantly higher than the simple inoculation group, indicating TAMs could enhance tumor cell proliferation (Fig. 8).
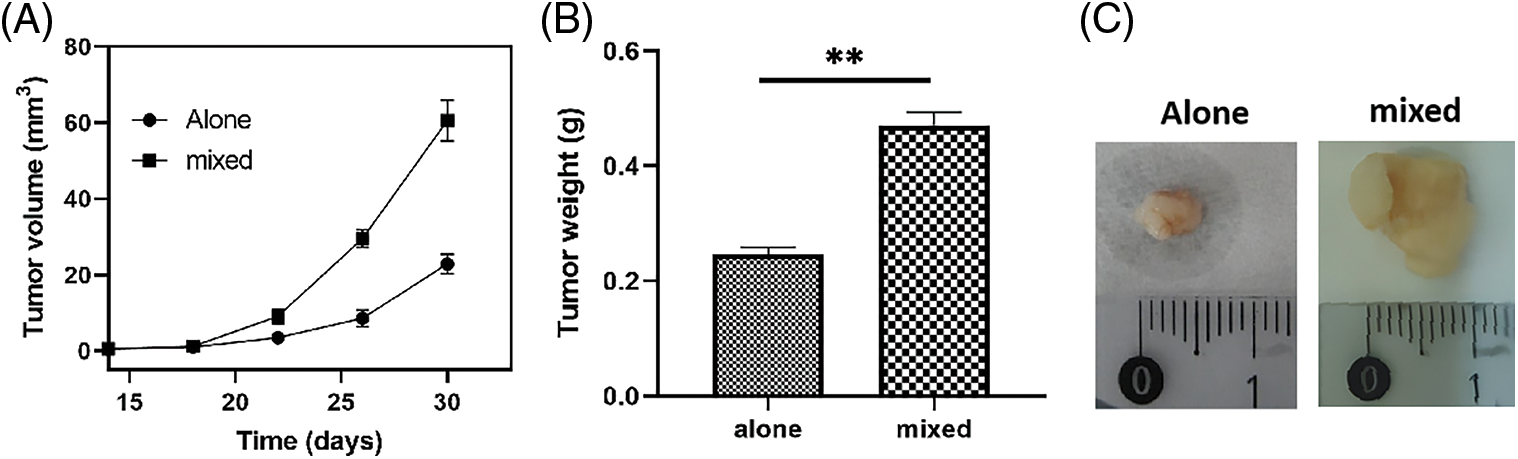
Figure 7: Animal experiments in vivo. The speed of tumor growth was significantly faster and the tumor volume was significantly larger in the mixed culture group than that in the SiHa cells alone inoculation group. (A) The tumor formation curves; (B) The tumor weight at day 30. (C) The pictures of tumors at day 30
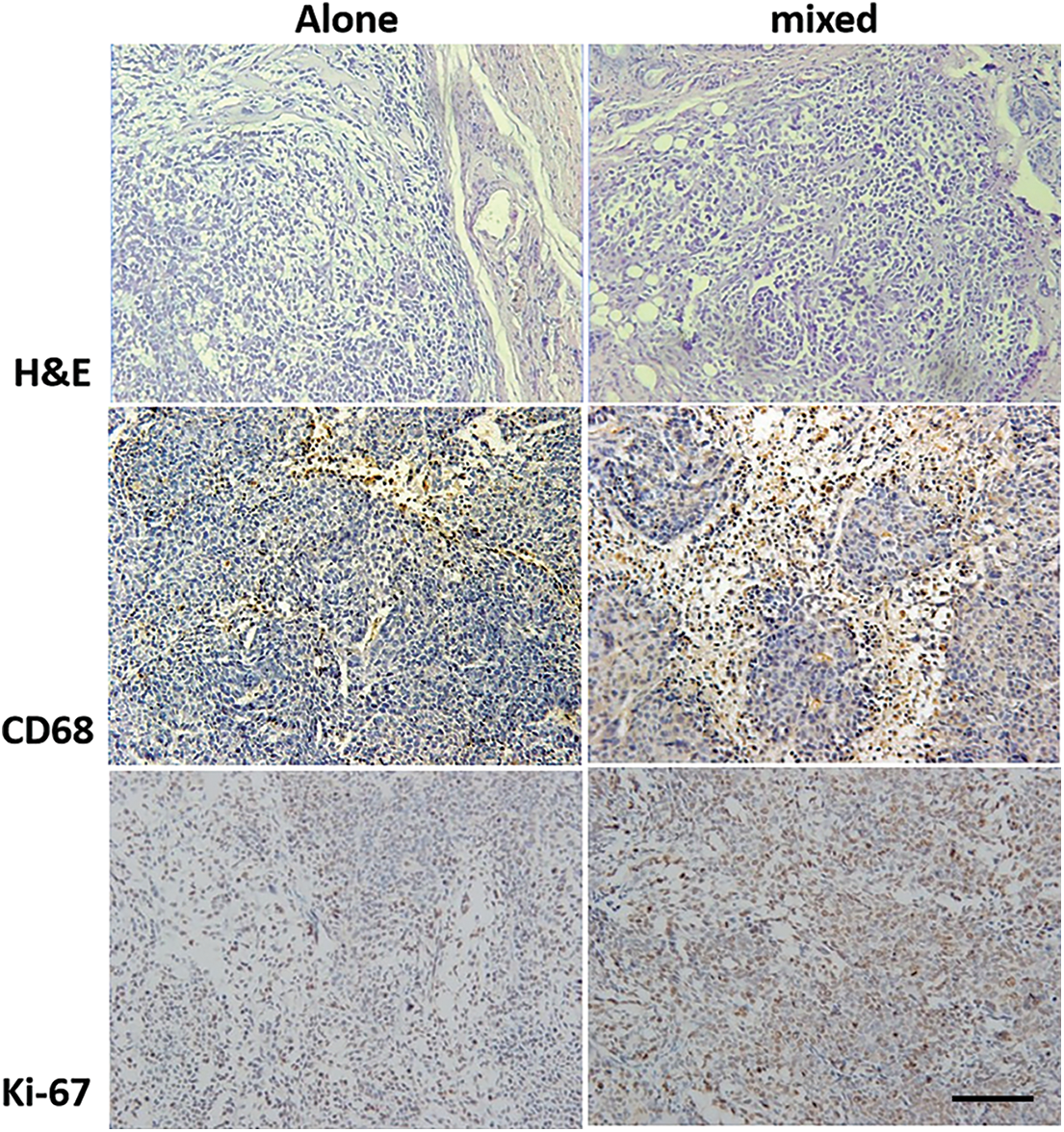
Figure 8: Tumor tissues from mixed SiHa and TPH-1 cells inoculation group and SiHa cells alone inoculation were analyzed by H&E staining, CD68 and Ki-67 immunohistochemistry. For H&E staining, the squamous cell carcinoma was found in all tumor specimens. The detection of macrophage marker revealed that few macrophages were occasionally seen in the SiHa cells alone inoculation group and a large amount of macrophage infiltration in the mixed inoculation group. In addition, Ki-67 expression in mixed inoculation group was significantly higher than the simple inoculation group, indicating TAMs could enhance tumor cell proliferation. Scale bar = 100 μm
In recent years, chronic inflammation has posed a greater concern in accelerating the tumor growth [13–15]. The large body of studies had demonstrated macrophages, a pivotal cell type, involved in anti-inflammation and anti-tumor immune regulation process [16], as they could eliminate tumor cells or remove the tumor by presenting tumor-associated antigens to induce immune response directly [17,18]. Interestingly TAMs are the largest inflammatory cell population in tumor microenviroment (TME), accounting for 30%–50% of inflammatory cells [19]. TAMs could also be considered as an independent poor prognosis marker of various tumors including Hodgkin’s lymphoma, Ewing’s sarcoma, renal clear cell carcinoma and supraglottic carcinoma. However, the role of TAMs in tumorigenesis and progression is contradictory [20]. TAMs are located at adjacent to tumor tissue in colon cancer to prevent tumor progression (TAMs to M1 polarization). Patients manifesting more TAMs infiltration have better prognosis and higher survival rate. The number of intra-tumoral TAMs showed an association with invasion depth, lymph node metastasis and clinical staging, suggesting the aggressive behavior of intra-tumor macrophages [21]. Therefore, the causes of these contradictions need to be further investigated.
In this study, the expression of CD68 and CD163 at several pathological stages were studied, including LSIL(CIN-1), HSIL (CIN2 and CIN3) of cervix tissues and cervical cancer specimens. The findings showed an enhanced expression of CD68 and CD163 in macrophages in mild and severe cervical lesions and cervical cancer tissues. This promoted increased migration of the number of macrophages to epithelial as the cervical lesion progressed. This indicated that the expression of CD163 and the number of macrophages were dominating factors of cervical lesion progression and hence assumed as the one of the most prominent prognoses of CIN progression and cervical cancer.
The polarization of macrophages promotes tumor phenotypic transformation was mainly due to the interaction between the tumor and interstitial cells in tumor microenvironment [20]. Macrophages in the cervical cancer distribute through the interstitial and cancer nest [22]. Interstitial macrophages in tumor microenvironment secrete cytokines allowing to infiltrate into cancer nest and affect carcinoma cells by direct contact between the cells. THP-1 induced by PMA manifest phenotype of M2-like macrophages expressing CD206, CD163 and CD68, and also secrete low levels of TNF-α, IL-6 and IL-1β and high levels of TGF-β. We had employed two co-culture methods to study their interactions. In non-direct contact culture, tumor cells and macrophages interacted via secretion of soluble components. Direct contact co-culture allowed physical interaction between tumor cells and macrophages. The impact of macrophages on the proliferation of three kinds of cervical cancer cells was examined and was observed that co-culture could promote the proliferation of HeLa and SiHa cells, whereas no significant effect was found on C33A cells. Moreover, the direct contact culture ameliorated proliferation than the indirect contact culture. These results suggested that the interaction of tumor cells and macrophages facilitated the proliferation of HeLa and SiHa cells. Additionally, co-culture displayed differential influence of biological functions depending on the type of cervical cancer cells.
Cell migration is an integral phenomenon in various biological processes such as immune, wound healing, inflammation and cancer metastasis. The results depicted enhanced migration ability of SiHa cells and C33A cells following co-culture, whereas HeLa cells showed no obvious change before or after co-culture.
The matrix metalloproteinases (MMPs) are involved in ECM degradation and are associated with tumor invasion and metastasis. The majority of studies showed that MMPs were mainly expressed in interstitial cells and some tumor cells [23–25]. Our finding claimed that the THP-1 cells secreted higher amount of MMP-9 than that of cervical cancer cells. Co-culture conditions, contrastingly, neither promote THP-1 cells nor cervical cancer cells to secrete more MMPs. Furthermore, the results suggested that MMP-9 in co-culture supernatants was mainly secreted by THP-1 cells.
The kinetics of cytokine network is both intricate and play a pivotal role in the tumor microenvironment. The expression profile of various cytokines reflects the immune status in tumor microenvironment. To elucidate the changes of cytokines after the interaction between macrophages and cervical cancer cells, several important inflammatory factors were detected by BCA method. IL-6 is a pro-inflammatory cytokine regulating the immune responses and closely related to tumorigenesis, development, invasion and metastasis [26]. Higher levels of IL-6 in cervical cancer cells promoted angiogenesis and anti-apoptotic capacity enhancement [27]. In this study, the IL-6 level induced by contact co-culture was higher than that in non-contact co-culture. The direct cell contacts led to a significant increase of IL-6. As a result, it was speculated that induced membrane receptor of cells secreted more IL-6. IL-8 is primarily involved in cell migration and invasion. This study demonstrated enhanced IL-8 and IL-1β in both contact and non-contact co-cultured cell supernatant; however, there was no significant difference was observed between the two co-culture conditions. This indicated that IL-8 and IL-1β were induced by the secretion of soluble components of cells.
TNF-α in tumor microenvironment is considered as a marker of many malignancies and normally associated with poor prognosis. TNF-α in cervical secretion from patients with HSIL was higher than that in LSIL patients [28]. In this study, TNF-α was not detected in the three cancer cell supernatants, consistent with previous report [29]. Moreover, co-culture condition manifested lower levels of TNF-α, indicating that the co-culture did not induce THP-1 to produce more TNF-α. As a result, it was found that high levels of IL-10 and low levels of IL-12 were typical characteristics of M2 macrophages. Some studies showed that IL-10 and IL-12 levels in cancer tissues were lower than in CIN [30]. Studied have suggested insignificant difference in the expression levels of IL-10 in CIN and cervical cancer tissues [31]. Quite the opposite, another research shows that IL-10 expression in cervical cancer and HSIL was higher than that in LSIL [32]. In this study, IL-10 and IL-12 were not found in supernatants of the three cervical cancer cells aligning with previous studies [31]. IL-10 and IL-12 were not detected in co-culture supernatant or induced-THP-1 supernatant. High levels of IL-10 and low levels of IL-12 were not found in THP-1 after co-cultured. THP-1 with both M1 and M2 phenotype was induced by tumor cell supernatant. Though THP-1 is closer to M2-like macrophages, they were not able to induce a typical M2 phenotypes. However, incomplete M2-like macrophages could also promote the proliferation and migration of cervical cancer and improve the anti-apoptotic ability.
To further examine the function of co-culture on SiHa cells in-vivo, a tumor model of cervical cancer cells was transplanted in nude mice. The results showed that time duration and size of tumor formation increased in co-culture of SiHa cells and induced-THP1. The expression of Ki67 in mixed inoculation group was higher, indicating an increase of its highly proliferation and degree of malignancy [33,34]. Our study has successfully demonstrated the enhanced macrophage infiltration in mixed inoculation group due to high secretion of chemokines resulting in macrophage recruitment and tumor growth. Congruently, co-inoculation of M2-like macrophages with prostate cancer cells promoted tumor growth effectively [35]. The results above supported the viewpoint that M2-like macrophages in TMEs could stimulate the proliferation of cancer cells.
In conclusion, these results evidently showed that macrophages could promote cervical cancer cell proliferation, migration due to secretion of soluble components and direct contacts among cells. Nevertheless, further investigation is required to dissect the mechanistic role.
Authorship: The authors confirm contribution to the paper as follows: study conception and design: Yi Zheng; data collection: Youyou Wang, Chen Zou; analysis and interpretation of results: Yi Zheng, Bicheng Hu; draft manuscript preparation: Youyou Wang; Review and correction of the manuscripts: Chen Zou, Min Zhao; supervision: Yi Zheng, Xinxing Wu; funding acquisition: Yi Zheng, Xinxing Wu. All authors reviewed the results and approved the final version of the manuscript.
Ethics Approval and Informed Consent Statement: The present study was approved by the Ethics Committees of the Zhongnan Hospital of Wuhan University (Code: LL-KT-2020436). Written informed consent was obtained from all patients.
Funding Statement: This research was funded by Natural Science Foundation of Guangdong Province (No. 2020A1515011353 to Yi Zheng) and The Innovation Program of Shenzhen Guangming District Science and Technology Innovation Bureau (Nos. 2020R01072 and 2021R01120).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Wang, Y., He, M., Zhang, G., Cao, K., Yang, M. et al. (2021). The immune landscape during the tumorigenesis of cervical cancer. Cancer Medicine, 10(7), 2380–2395. DOI 10.1002/cam4.3833. [Google Scholar] [CrossRef]
2. Cao, M., Wang, Y., Wang, D., Duan, Y., Hong, W. et al. (2020). Increased high-risk human papillomavirus viral load is associated with immunosuppressed microenvironment and predicts a worse long-term survival in cervical cancer patients. American Journal of Clinical Pathology, 153(4), 502–512. DOI 10.1093/ajcp/aqz186. [Google Scholar] [CrossRef]
3. Mehraj, U., Qayoom, H., Mir, M. A. (2021). Prognostic significance and targeting tumor-associated macrophages in cancer: New insights and future perspectives. Breast Cancer, 28(3), 539–555. DOI 10.1007/s12282-021-01231-2. [Google Scholar] [CrossRef]
4. Parisi, L., Gini, E., Baci, D., Tremolati, M., Fanuli, M. et al. (2018). Macrophage polarization in chronic inflammatory diseases: Killers or builders? Journal of Immunology Research, 2018(12), 1–25. DOI 10.1155/2018/8917804. [Google Scholar] [CrossRef]
5. Qian, B. Z., Pollard, J. W. (2010). Macrophage diversity enhances tumor progression and metastasis. Cell, 141(1), 39–51. DOI 10.1016/j.cell.2010.03.014. [Google Scholar] [CrossRef]
6. Walsh, J. T., Zheng, J., Smirnov, I., Lorenz, U., Tung, K. et al. (2014). Regulatory T cells in central nervous system injury: A double-edged sword. Journal of Immunology, 193(10), 5013–5022. DOI 10.4049/jimmunol.1302401. [Google Scholar] [CrossRef]
7. Heusinkveld, M., de Vos van Steenwijk, P., Goedemans, R., Ramwadhdoebe, T., Gorter, A. et al. (2011). M2 macrophages induced by prostaglandin E2 and IL-6 from cervical carcinoma are switched to activated M1 macrophages by CD4+ Th1 cells. Journal of Immunology, 187, 1157–1165. DOI 10.4049/jimmunol.1100889. [Google Scholar] [CrossRef]
8. Sánchez-Reyes, K., Bravo-Cuellar, A., Hernández-Flores, G., Lerma-Díaz, J. M., Jave-Suárez, L. F. et al. (2014). Cervical cancer cell supernatants induce a phenotypic switch from U937-derived macrophage-activated M1 state into M2-like suppressor phenotype with change in toll-like receptor profile. BioMed Research International, 2014, 683068. DOI 10.1155/2014/683068. [Google Scholar] [CrossRef]
9. Ding, H., Cai, J., Mao, M., Fang, Y., Huang, Z. et al. (2014). Tumor-associated macrophages induce lymphangiogenesis in cervical cancer via interaction with tumor cells. APMIS, 122(11), 1059–1069. DOI 10.1111/apm.12257. [Google Scholar] [CrossRef]
10. Galdiero, M. R., Marone, G., Mantovani, A. (2018). Cancer inflammation and cytokines. Cold Spring Harbor Perspectives in Biology, 10(8), a028662. DOI 10.1101/cshperspect.a028662. [Google Scholar] [CrossRef]
11. Fu, L. Q., Du, W. L., Cai, M. H., Yao, J. Y., Zhao, Y. Y. et al. (2020). The roles of tumor-associated macrophages in tumor angiogenesis and metastasis. Cellular Immunology, 353, 104119. DOI 10.1016/j.cellimm.2020.104119. [Google Scholar] [CrossRef]
12. Gijbels, K., Galardy, R. E., Steinman, L. (1994). Reversal of experimental autoimmune encephalomyelitis with a hydroxamate inhibitor of matrix metalloproteases. Journal of Clinical Investigation, 94(6), 2177–2182. DOI 10.1172/JCI117578. [Google Scholar] [CrossRef]
13. Balkwill, F., Mantovani, A. (2001). Inflammation and cancer: Back to Virchow? Lancet, 357(9255), 539–545. DOI 10.1016/S0140-6736(00)04046-0. [Google Scholar] [CrossRef]
14. Balkwill, F., Charles, K., Mantovani, A. (2005). Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell, 7(3), 211–217. DOI 10.1016/j.ccr.2005.02.013. [Google Scholar] [CrossRef]
15. Nakamura, K., Smyth, M. J. (2017). Targeting cancer-related inflammation in the era of immunotherapy. Immunology and Cell Biology, 95(4), 325–332. DOI 10.1038/icb.2016.126. [Google Scholar] [CrossRef]
16. Chen, Z., Ni, S., Han, S., Crawford, R., Lu, S. et al. (2017). Nanoporous microstructures mediate osteogenesis by modulating the osteo-immune response of macrophages. Nanoscale, 9(2), 706–718. DOI 10.1039/c6nr06421c. [Google Scholar] [CrossRef]
17. Wang, L. X., Zhang, S. X., Wu, H. J., Rong, X. L., Guo, J. (2019). M2b macrophage polarization and its roles in diseases. Journal of Leukocyte Biology, 106(2), 345–358. DOI 10.1002/JLB.3RU1018-378RR. [Google Scholar] [CrossRef]
18. Gou, L., Yue, G. G., Puno, P. T., Lau, C. B. (2021). A review on the relationship of mast cells and macrophages in breast cancer—Can herbs or natural products facilitate their anti-tumor effects? Pharmacological Research, 164, 105321. DOI 10.1016/j.phrs.2020.105321. [Google Scholar] [CrossRef]
19. Shabo, I., Svanvik, J. (2011). Expression of macrophage antigens by tumor cells. Advances in Experimental Medicine and Biology, 714, 141–150. DOI 10.1007/978-94-007-0782-5_7. [Google Scholar] [CrossRef]
20. Yang, Q., Guo, N., Zhou, Y., Chen, J., Wei, Q. et al. (2020). The role of tumor-associated macrophages (TAMs) in tumor progression and relevant advance in targeted therapy. Acta Pharmaceutica Sinica B, 10(11), 2156–2170. DOI 10.1016/j.apsb.2020.04.004. [Google Scholar] [CrossRef]
21. Michel, M., Kaps, L., Maderer, A., Galle, P. R., Moehler, M. et al. (2021). The role of p53 dysfunction in colorectal cancer and its implication for therapy. Cancers, 13(10), 2296. DOI 10.3390/cancers13102296. [Google Scholar] [CrossRef]
22. Chen, X. J., Han, L. F., Wu, X. G., Wei, W. F., Wu, L. F. et al. (2017). Clinical significance of CD163+ and CD68+ tumor-associated macrophages in high-risk HPV-related cervical cancer. Journal of Cancer, 8(18), 3868–3875. DOI 10.7150/jca.21444. [Google Scholar] [CrossRef]
23. Kessenbrock, K., Plaks, V., Werb, Z. (2010). Matrix metalloproteinases: Regulators of the tumor microenvironment. Cell, 141(1), 52–67. DOI 10.1016/j.cell.2010.03.015. [Google Scholar] [CrossRef]
24. Scheau, C., Badarau, I. A., Costache, R., Caruntu, C., Mihai, G. L. et al. (2019). The role of matrix metalloproteinases in the epithelial-mesenchymal transition of hepatocellular carcinoma. Analytical Cellular Pathology, 2019, 9423907. DOI 10.1155/2019/9423907. [Google Scholar] [CrossRef]
25. Gonzalez-Avila, G., Sommer, B., García-Hernández, A. A., Ramos, C. (2020). Matrix metalloproteinases’ role in tumor microenvironment. Advances in Experimental Medicine and Biology, 1245, 97–131. DOI 10.1007/978-3-030-40146-7_5. [Google Scholar] [CrossRef]
26. Coward, J. I. G., Kulbe, H. (2012). The role of interleukin-6 in gynaecological malignancies. Cytokine & Growth Factor Reviews, 23(6), 333–342. DOI 10.1016/j.cytogfr.2012.08.005. [Google Scholar] [CrossRef]
27. Wei, L. H., Kuo, M. L., Chen, C. A., Chou, C. H., Lai, K. B. et al. (2003). Interleukin-6 promotes cervical tumor growth by VEGF-dependent angiogenesis via a STAT3 pathway. Oncogene, 22(10), 1517–1527. DOI 10.1038/sj.onc.1206226. [Google Scholar] [CrossRef]
28. Peghini, B. C., Abdalla, D. R., Barcelos, A. C. M., Teodoro, G. V. L., Murta, E. F. C. et al. (2012). Local cytokine profiles of patients with cervical intraepithelial and invasive neoplasia. Human Immunology, 73(9), 920–926. DOI 10.1016/j.humimm.2012.06.003. [Google Scholar] [CrossRef]
29. Mehta, A. K., Kadel, S., Townsend, M. G., Oliwa, M., Guerriero, J. L. et al. (2021). Macrophage biology and mechanisms of immune suppression in breast cancer. Frontiers in Immunology, 12, 643771. DOI 10.3389/fimmu.2021.643771. [Google Scholar] [CrossRef]
30. Audirac-Chalifour, A., Torres-Poveda, K., Bahena-Román, M., Téllez-Sosa, J., Martínez-Barnetche, J. et al. (2016). Cervical microbiome and cytokine profile at various stages of cervical cancer: A pilot study. PLoS One, 11(4), e0153274. DOI 10.1371/journal.pone.0153274. [Google Scholar] [CrossRef]
31. Otani, S., Fujii, T., Kukimoto, I., Yamamoto, N., Tsukamoto, T. et al. (2019). Cytokine expression profiles in cervical mucus from patients with cervical cancer and its precursor lesions. Cytokine, 120(1–2), 210–219. DOI 10.1016/j.cyto.2019.05.011. [Google Scholar] [CrossRef]
32. Azar, K. K., Tani, M., Yasuda, H., Sakai, A., Inoue, M. et al. (2004). Increased secretion patterns of interleukin-10 and tumor necrosis factor-alpha in cervical squamous intraepithelial lesions. Human Pathology, 35(11), 1376–1384. DOI 10.1016/j.humpath.2004.08.012. [Google Scholar] [CrossRef]
33. Silva, D. C., Gonçalves, A. K., Cobucci, R. N., Mendonça, R. C., Lima, P. H. et al. (2017). Immunohistochemical expression of p16, Ki-67 and p53 in cervical lesions—A systematic review. Pathology-Research and Practice, 213(7), 723–729. DOI 10.1016/j.prp.2017.03.003. [Google Scholar] [CrossRef]
34. Gutnik, H., Kastelic, P., Oštrbenk Valenčak, A., Poljak, M., Strojan Fležar, M. (2020). Histomorphologic assessment and distribution of high-risk human papillomavirus (HPV) types in cervical high-grade squamous intraepithelial lesions with unusual histomorphologic features. Virchows Archiv, 476(2), 251–260. DOI 10.1007/s00428-019-02694-7. [Google Scholar] [CrossRef]
35. Wang, G., Zhao, D., Spring, D. J., DePinho, R. A. (2018). Genetics and biology of prostate cancer. Genes & Development, 32(17–18), 1105–1140. DOI 10.1101/gad.315739.118. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |