
 | Oncologie |  |
DOI: 10.32604/oncologie.2021.019519
CASE REPORT
A Rare Case of Atraumatic Splenic Rupture Due to Metastatic Hepatocellular Carcinoma
1Department of Radiology, Vinmec Healthcare System, Ha Noi, Viet Nam
2Department of Radiology, Pham Ngoc Thach University of Medicine, Ho Chi Minh City, Viet Nam
*Corresponding Author: Nguyen Minh Duc. Email: bsnguyenminhduc@pnt.edu.vn
#These authors contributed equally to this article as co-first authors
ORCID ID: 0000-0001-5411-1492
Received: 27 September 2021; Accepted: 13 October 2021
Abstract: Metastatic spread of hepatocellular carcinoma (HCC) to the spleen is uncommon, only occurring in approximately 1% of cases. Atraumatic splenic rupture due to HCC metastasis is extremely rare and affects patient prognosis, clinical management, and mortality. We report a case of a 65-year-old man with a history of chronic hepatitis B infection who presented with left-sided abdominal pain and fatigue. Clinical examination showed acute anemia with elevated levels of serum alpha-fetoprotein (AFP) and protein induced by vitamin K absence (PIVKA-II). On ultrasound and computed tomography imaging, hemoperitoneum caused by a ruptured splenic tumor was revealed. In addition to multiple hepatic lesions, enlarged abdominal lymph nodes and osteolytic lesions in the thoracic vertebral bodies were detected. The patient underwent total splenectomy and was diagnosed histopathologically with splenic rupture secondary to Grade 2 HCC metastasis. Atraumatic, pathological splenic rupture due to HCC metastasis should be considered in patients with chronic hepatitis B or C infection and increasing serum AFP and PIVKA-II levels, even though splenic metastasis is uncommon.
Keywords: Atraumatic splenic rupture; hepatocellular carcinoma; splenic metastases
Hepatocellular carcinoma (HCC) is the most common type of primary liver cancer that occurs in adults and is associated with a poor prognosis. Extrahepatic metastatic spread confers a worse prognosis. The spleen is an uncommon site of extrahepatic metastatic HCC, accounting for approximately 1% of metastatic cases [1]. The presentation of acute abdominal pain in patients with splenic metastasis can be caused by spontaneous splenic rupture [2]. Although splenic metastasis is a rare cause of the atraumatic splenic rupture, accounting for only 3.8% of cases [3], it should be recognized and diagnosed promptly to reduce mortality. Imaging modalities, including ultrasound and contrast-enhanced computed tomography (CECT), play crucial roles in confirming suspected intra-abdominal bleeding and diagnosing splenic rupture etiologies, in addition to detecting active bleeding events, which require emergency operations [4,5].
A 65-year-old man was admitted to our hospital complaining of fatigue and mild left-side abdominal pain without any history of recent trauma. He had a history of chronic hepatitis B infection for the past 6 years. Physical examination revealed a palpable mass below the left costal margin, which was defined as an enlarged spleen on abdominal ultrasound. Additionally, multiple nodules and masses were detected in the spleen and liver on ultrasonography. The patient was transferred to the oncology department with suspicion of metastatic disease with an unknown primary. Two days after admission, the patient developed severe acute abdominal pain, hypotension (80/50 mmHg), and tachycardia (105 beats per minute). Laboratory tests revealed a low hemoglobin (85 mg/dL), elevated alpha-fetoprotein (AFP; 8757 ng/mL), and elevated protein induced by vitamin K antagonist II (PIVKA-II; 23043.11 AU/mL). Bedside ultrasonography detected free intraperitoneal fluid with mixed internal echogenicity, suggesting hemoperitoneum. Therefore, urgent CECT was performed, which revealed a large quantity of hyperdense, free fluid in the pelvis and surrounding the inferior splenic pole, most consistent with hemoperitoneum (Fig. 1A). Multiple splenic masses were observed, with the largest in the inferior pole measuring 6 x 6 cm. The masses were hypodense on pre-contrast images (Fig. 1A) and demonstrated mild heterogeneous enhancement in the arterial phase (Fig. 1B), without gross change on portal venous (Fig. 1C) or delayed phase images (Fig. 1D). Discontinuity of the splenic capsule consistent with tumor laceration, was observed on post-contrast images without signs of active bleeding (Figs. 1C and 1D). In addition, multiple hepatic masses were observed, which were hypodense on pre-contrast images, and demonstrated marked peripheral hyperenhancement in the arterial phase, and non-peripheral washout in the portal venous and delayed phase images (Figs. 2A–2C). Right anterior portal vein thrombosis (Fig. 2D) was also identified. Multiple enlarged lymph nodes were observed in the hepatic hilum and paraaortic space, in addition to osteolytic lesions in the fourth and eighth thoracic vertebral bodies. The patient was diagnosed with hemoperitoneum due to presumed ruptured splenic metastasis, and an emergency splenectomy was performed. A liter of blood in the abdomen and a lacerated splenic mass were found on surgical exploration. Histopathology revealed a solid tumor with a well-defined margin between the tumor and the splenic parenchyma (Fig. 3A). The tumor cells were arranged in a trabecular pattern, with prominent nucleoli and hyperchromatism (Fig. 3B). Immunohistochemically, the neoplastic cells revealed solid and diffuse positivity for the hepatic marker hepatocyte paraffin 1 (HEPAR-1), indicating an HCC origin (Fig. 3C). The patient was diagnosed with Grade 2 HCC, with splenic and bone metastases, and metastatic abdominal lymphadenopathy. The patient was treated with targeted therapy. Finally, this patient was lost to follow-up.
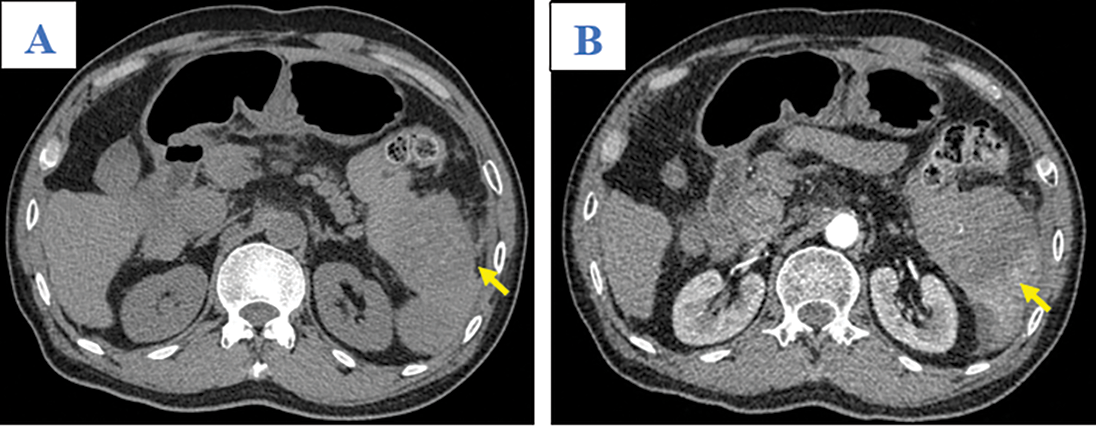
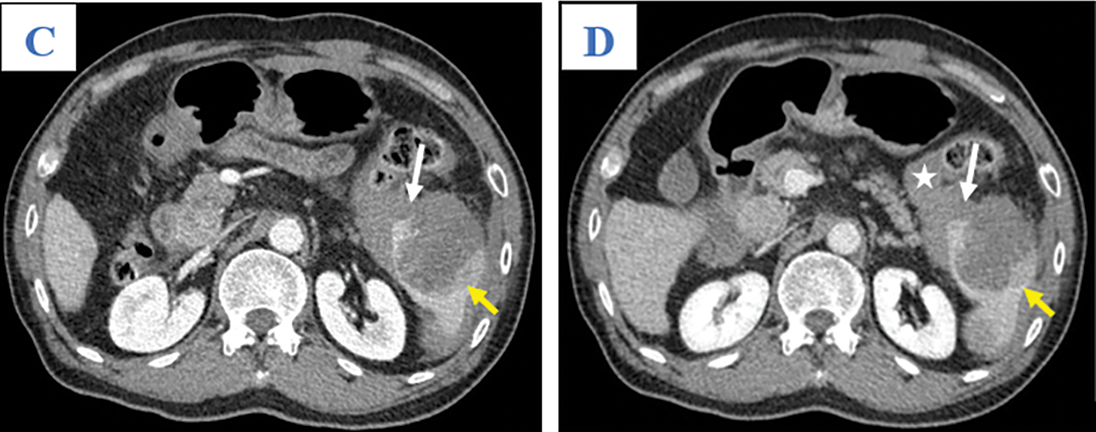
Figure 1: Axial abdominal CT in the pre-contrast (A), Arterial (B), Venous (C), and Late phases (D). This mass presented as mildly hypodense in the pre-contrast phase (A; arrow) and with heterogeneous enhancement, without washout, on the arterial, venous, and late phases (B–D; yellow arrows). A tumor laceration was identified on the splenic capsule (C and D; white arrows) with the presence of hemoperitoneum (asterisk) but no observation of active bleeding
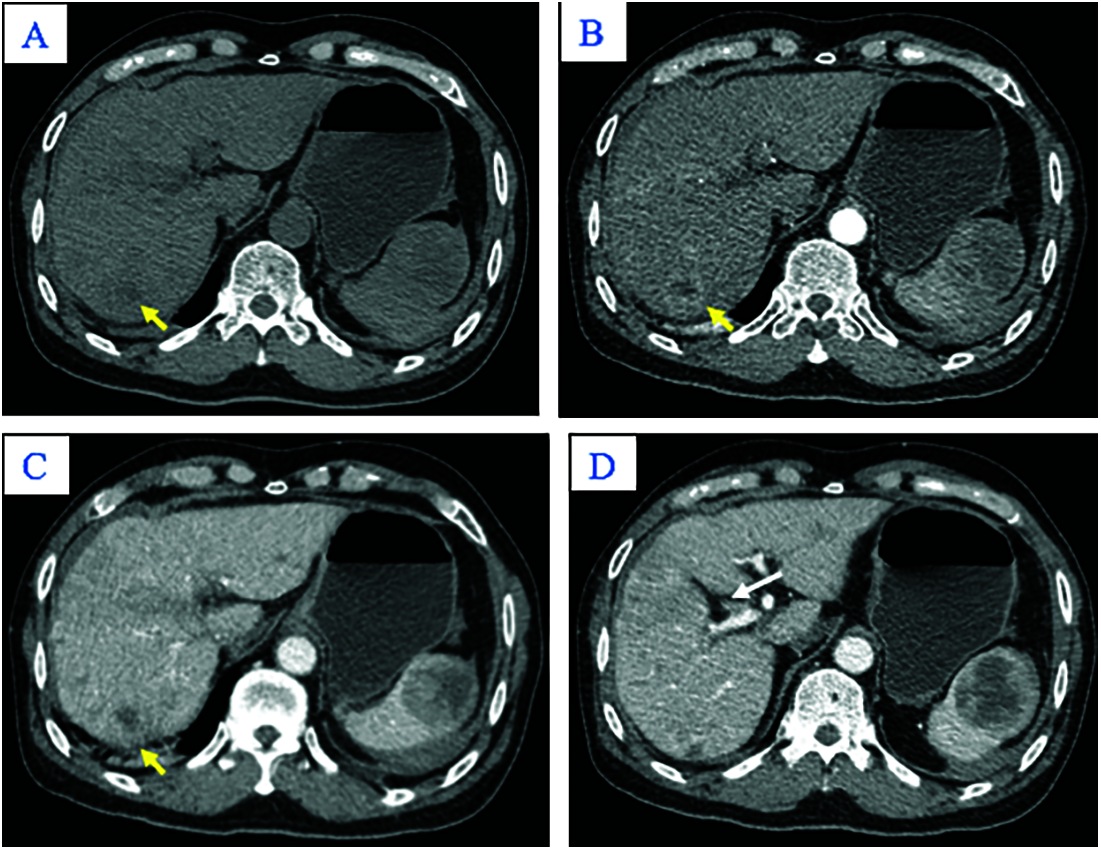
Figure 2: Axial abdominal CT in the pre-contrast (A), Arterial (B), Venous phases (C), and Delayed phase (D). The nodular liver contour in this patient with chronic hepatitis B in segment VII appeared hypodense in the pre-contrast image (A; yellow arrow), peripherally hyper-enhancing in the arterial phase (B; yellow arrow), and without washout in the venous phase (C; yellow arrow). Other hepatic lesions are not shown in these images. Note thrombus in the anterior segmental branch of the right branch of the portal vein (D; white arrow)
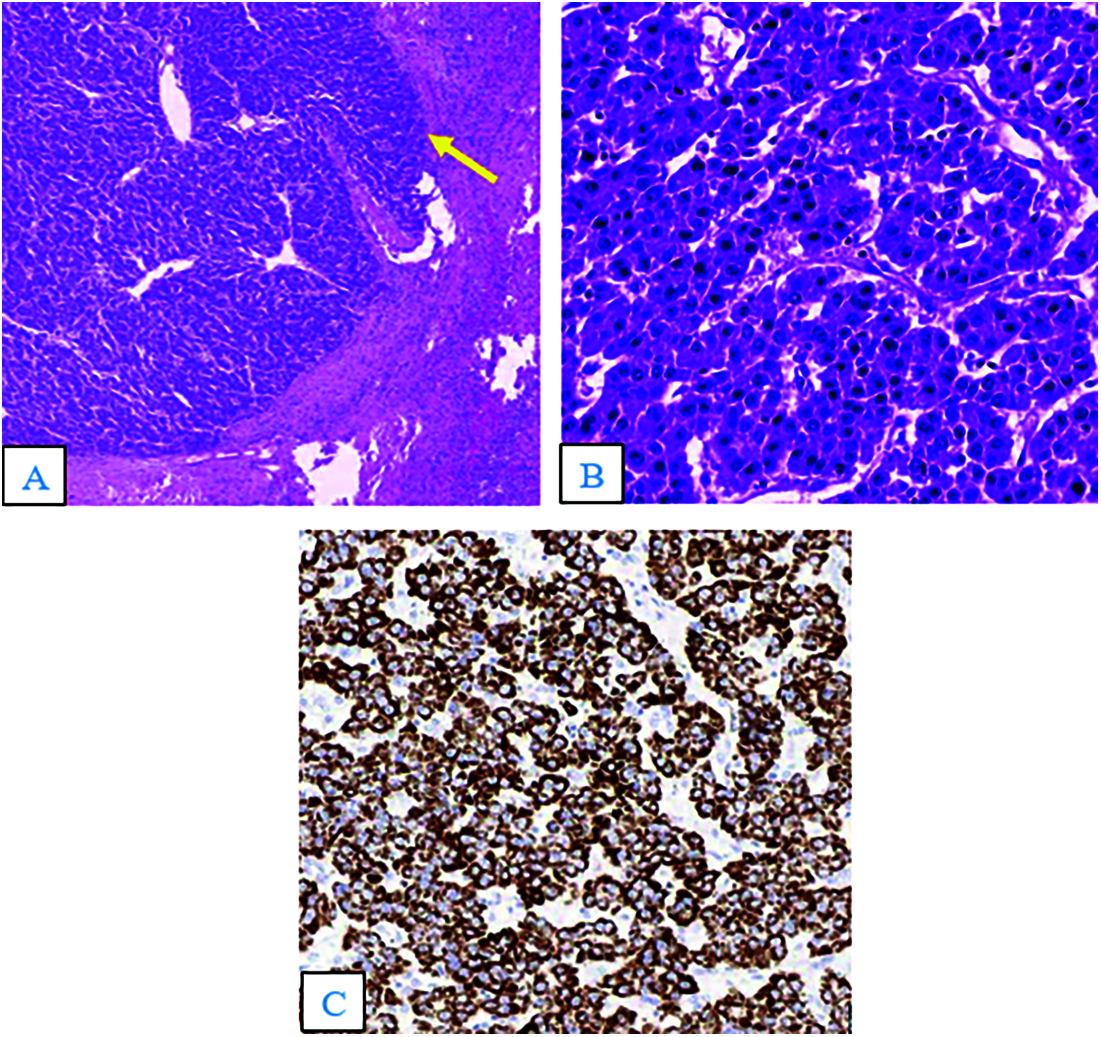
Figure 3: Hematoxylin and eosin staining (A and B). (A) The tumor was solid, with a well-defined margin between the lesion and the splenic parenchyma (arrow; × 40). (B) The tumor cells were arranged in a trabecular pattern, with prominent nucleoli and hyperchromatism (×400). (C) Immunohistochemistry results (×100) showed tumor cells positive for the hepatic marker HEPAR–1
HCC is a common complication of hepatitis B infection or liver cirrhosis from any cause [6]. HCC metastasis occurs in one-third of cases, with common metastatic sites including the lungs (6%), peritoneum (3%), bones (3%), and lymph nodes (2%) [1]. The spleen is an uncommon site for HCC metastasis, accounting for only 1% of metastatic cases [1].
Melanoma, as well as malignancies of the breast, lung, ovary, colon, stomach, and pancreas, are the most frequent cancers to spread to the spleen. The spleen is one of the most well-vascularized organs, yet it is only very infrequently implicated in metastasis. The splenic artery and the coeliac axis form a steep angle, making it troublesome for tumor cells to reach the spleen. Because the splenic parenchyma lacks afferent lymphatic capillaries and the splenic capsule only has a few restricted lymphatics, most metastic tumors are found in the subcapsular area. The splenic sinusoids’ rhythmic contractions may prevent tumor cells from implanting on vascular endothelial cells. The spleen capsule also acts as a physical barrier, preventing tumor cells from entering the spleen. Another protective factor against metastasis is thought to be the spleen’s immunological properties. The spleen, in reality, is a big reticuloendothelial organ with a significant number of lymphocytes and macrophages that generate a variety of chemical compounds that can kill tumor cells [2.3]. Patients presenting with splenic metastasis are often asymptomatic, and metastases are incidentally detected on imaging [2]. On contrast-enhanced CT imaging, splenic metastasis originating from HCC can present as a single or multifocal lesion, strongly enhancing in the arterial phase, and hypodense in the portal phase, similar to the typical appearance of HCC [4,7].
Splenic metastasis is a rare cause of the atraumatic, pathological splenic rupture, accounting for 3.8% of cases [3]. Lymphoma and leukemia are two common diseases that can also trigger spontaneous atraumatic splenic rupture [2,3]. In the English-language literature, only one case, reported by Chin et al. [8], described atraumatic splenic rupture due to HCC metastasis, which was obscured on initial CT images. Risk factors for metastatic splenic rupture include hypervascular features of HCC metastasis, necrotic tumor regions, splenic vascular invasion, splenic capsular invasion by neoplastic cells, a congested or enlarged spleen secondary to portal hypertension, and splenic and portal vein thrombosis [8–10]. Patients with spontaneous splenic metastatic rupture often present with acute left-sided abdominal pain, rigidity, or abdominal distention [11]. In severe cases, patients can develop tachycardia, hypotension, oliguria, or hypovolemic shock [12]. During splenic rupture, pre-contrast phase images can reveal more recent extravascular blood products as low to intermediate attenuation content ranging from 35–60 Hounsfield Units (HU) or later subacute blood products as higher attenuation content ranging from 60–80 HU. A focal hyperdense clot, the sentinel clot, near the spleen provides a clue to splenic injury [4].
Patients with spontaneous splenic ruptures due to metastases should undergo immediate total splenectomy or transcatheter arterial embolization to stop the bleeding [3,8]. There are no definite contraindications to splenectomy surgery. Prior to conducting a splenectomy, however, specific concerns must be taken into account, particularly in patients with splenomegaly or portal vein hypertension. Due to the restricted working area in conducting dissection around surrounding tissues and even removing the specimen from the abdomen, laparoscopic splenectomy becomes challenging when the spleen weighs between 1000 and 2000 grams. When compared to those with a normal-sized spleen, the study has indicated that those with splenomegaly had longer operational times, greater blood loss, and more frequent conversion to open surgery [13]. The spleen can be shrunk via splenic artery embolization so that a laparoscopic splenectomy can be performed [14]. Patients with portal hypertension are at risk of bleeding due to the presence of esophagogastric varices and thrombocytopenia. These patients, according to research, have longer operating hours, more blood loss, and are more prone to convert to open surgery [15]. Splenic artery embolization has recently acquired popularity as a useful adjuvant in the treatment of AAST Grade III or higher splenic injuries. Splenic artery embolization was extremely effective in hemodynamically stable patients with a grade IV to V splenic damage. According to Aiolfi et al. [16], Splenic artery embolization is successful for both hemorrhage management and preserving splenic function, regardless of whether the procedure is proximal or distal.
In our case, urgent CECT facilitated the diagnosis of hemoperitoneum due to splenic lesion rupture. However, the presence of multiple lesions in the liver, spleen, and bone, including abdominal lymphadenopathy, provided a challenge to the diagnosis of the primary tumor. Splenectomy was both therapeutic and diagnostic, leading to the diagnosis of metastatic HCC.
Metastatic HCC that results in atraumatic splenic rupture is a rare phenomenon. Atraumatic splenic rupture can be clinically considered when a patient with known HCC or known chronic liver disease has an acute onset of abdominal pain and signs of hypovolemia. CECT readily provides diagnosis and can expedite ensuing urgent management and reduce patient mortality.
Authors’ Contribution: D.Q.H. and N.M.D. contributed equally to this article, and therefore, they are considered as co-first authors. D.Q.H. and N.M.D. gave a substantial contribution in acquisition, analysis, and data interpretation. N.M.D. prepared, drafted, and revised manuscript critically for important intellectual content. Each author gave the final approval of the version to be published and agreed to be accountable for all aspects of the work, ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All authors have read and agreed to the published version of the manuscript.
Ethical Approval and Informed Consent Statement: Vinmec Healthcare System does not require ethical approval for reporting individual cases or case series. Written informed consent was obtained from the patient(s) for their anonymized information to be published in this article.
Data Availability Statement: No datasets were generated or analyzed during the current study.
Informed Consent Statement: Informed consent was obtained from all subjects involved in the study. All identity of this patient has been protected.
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Abbas, A., Medvedev, S., Shores, N., Bazzano, L., Dehal, A. et al. (2014). Epidemiology of metastatic hepatocellular carcinoma, a nationwide perspective. Digestive Diseases and Sciences, 59(11), 2813–2820. DOI 10.1007/s10620-014-3229-9. [Google Scholar] [CrossRef]
2. Compérat, E., Bardier-Dupas, A., Camparo, P., Capron, F., Charlotte, F. (2007). Splenic metastases: Clinicopathologic presentation, differential diagnosis, and pathogenesis. Archives of Pathology & Laboratory Medicine, 131(6), 965–969. DOI 10.5858/2007-131-965-SMCPDD. [Google Scholar] [CrossRef]
3. Renzulli, P., Hostettler, A., Schoepfer, A. M., Gloor, B., Candinas, D. (2009). Systematic review of atraumatic splenic rupture. The British Journal of Surgery, 96(10), 1114–1121. DOI 10.1002/bjs.6737. [Google Scholar] [CrossRef]
4. Tonolini, M., Ierardi, A. M., Carrafiello, G. (2016). Atraumatic splenic rupture, an underrated cause of acute abdomen. Insights into Imaging, 7(4), 641–646. DOI 10.1007/s13244-016-0500-y. [Google Scholar] [CrossRef]
5. Battula, N., Tsapralis, D., Takhar, A., Coldham, C., Mayer, D. et al. (2012). Aetio-pathogenesis and the management of spontaneous liver bleeding in the west: A 16-year single-centre experience. HPB, 14(6), 382–389. DOI 10.1111/j.1477-2574.2012.00460.x. [Google Scholar] [CrossRef]
6. Ryerson, A. B., Eheman, C. R., Altekruse, S. F., Ward, J. W., Jemal, A. et al. (2016). Annual report to the nation on the status of cancer, 1975–2012, featuring the increasing incidence of liver cancer. Cancer, 122(9), 1312–1337. DOI 10.1002/cncr.29936. [Google Scholar] [CrossRef]
7. Becker, A. K., Tso, D. K., Harris, A. C., Malfair, D., Chang, S. D. (2014). Extrahepatic metastases of hepatocellular carcinoma: A spectrum of imaging findings. Canadian Association of Radiologists Journal, 65(1), 60–66. DOI 10.1016/j.carj.2013.05.004. [Google Scholar] [CrossRef]
8. Chin, K., Luk, Y. S., Khoo, J., Lui, Y. H. (2017). Splenic metastasis of hepatocellular carcinoma with spleen rupture. Hong Kong Journal Radiology, 20, 237–240. DOI 10.12809/hkjr1716436. [Google Scholar] [CrossRef]
9. Smith, R., Massey, D. (2017). Splenic rupture due to metastatic disease: A rare complication. BMJ Case Reports, 2017, bcr2017221336. DOI 10.1136/bcr-2017-221336. [Google Scholar] [CrossRef]
10. Amonkar, S. J., Kumar, E. N. (2009). Spontaneous rupture of the spleen: Three case reports and causative processes for the radiologist to consider. The British Journal of Radiology, 82(978), e111–3. DOI 10.1259/bjr/81440206. [Google Scholar] [CrossRef]
11. Weaver, H., Kumar, V., Spencer, K., Maatouk, M., Malik, S. (2013). Spontaneous splenic rupture: A rare life-threatening condition; diagnosed early and managed successfully. The American Journal of Case Reports, 14, 13–15. DOI 10.12659/AJCR.883739. [Google Scholar] [CrossRef]
12. Gedik, E., Girgin, S., Aldemir, M., Keles, C., Tuncer, M. C. et al. (2008). Non-traumatic splenic rupture: Report of seven cases and review of the literature. World Journal of Gastroenterology, 14(43), 6711. DOI 10.3748/wjg.14.6711. [Google Scholar] [CrossRef]
13. Heniford, B. T., Park, A., Walsh, R. M., Kercher, K. W., Matthews, B. D. et al. (2001). Laparoscopic splenectomy in patients with normal-sized spleens versus splenomegaly: Does size matter? The American Surgeon, 67(9), 854–858. [Google Scholar]
14. Iwase, K., Higaki, J., Yoon, H. E., Mikata, S., Miyazaki, M. et al. (2002). Splenic artery embolization using contour emboli before laparoscopic or laparoscopically assisted splenectomy. Surgical Laparoscopy, Endoscopy & Percutaneous Techniques, 12(5), 331–336. DOI 10.1097/00129689-200210000-00005. [Google Scholar] [CrossRef]
15. Cobb, W. S., Heniford, B. T., Burns, J. M., Carbonell, A. M., Matthews, B. D. et al. (2005). Cirrhosis is not a contraindication to laparoscopic surgery. Surgical Endoscopy, 19(3), 418–423. DOI 10.1007/s00464-004-8722-3. [Google Scholar] [CrossRef]
16. Aiolfi, A., Inaba, K., Strumwasser, A., Matsushima, K., Grabo, D. et al. (2017). Splenic artery embolization versus splenectomy: Analysis for early in-hospital infectious complications and outcomes. The Journal of Trauma and Acute Care Surgery, 83(3), 356–360. DOI 10.1097/TA.0000000000001550. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |