
 | Oncologie |  |
DOI: 10.32604/Oncologie.2021.017833
ARTICLE
Anticancer and Antioxidant Activities of Aqueous and Ethanolic Bark Extracts of Acer Tegmentosum Maxim (Aceaceae) on Tumor Cell Lines
1Department of Food Science and Engineering, College of Agricultural, Yanbian University, Yanji, 133000, China
2Department of Bio-Health Convergence, College of Biomedical Sciences, Kangwon National University, Chuncheon, 200-701, South Korea
3Department of Dental Hygiene, College of Biomedical Sciences, Kangwon National University, Chuncheon, 200-701, South Korea
*Corresponding Authors: Tie-yan Jin. Email: jintieyan@ybu.edu.cn; Hye-Young Kim. Email: khy0606@kangwon.ac.kr
#Both the authors are equally contributed
Received: 09 June 2021; Accepted: 09 August 2021
Abstract: The medicinal plant of Acer tegmentosum Maxim is traditionally used in the southern part of Asia to treat oxidative stress-related diseases, including cancer, diabetes mellitus , wounds, infections, etc. Based on this, the current study was designed to investigate the phytochemical analysis, antioxidants and anti-cancer activities of Acer tegmentosum Maxim (AT). The total phenolic content (TPC), total flavonoid content (TFC), free radicals scavenging (DPPH and ABTS), chemical constituents as well as cytotoxicity potential ATWE (Acer tegmentosum Maxim water extracts) and ATEE (Acer tegmentosum Maxim ethanolic extracts) were tested. The cytotoxic efficacy ATWE and ATEE were studied in Human embryonic kidney 293 cells (HEK 293), human lung cancer cell lines (A549), prostate cancer cells (PC3) and breast cancer cells (MDA-MB 231). The results revealed that, TPC ranged between in 199.97 ± 0.09 mg GAR/g extract in ATWE and 103.48 ± 0.82 mg GAR/g extract in ATEE, TFC were 72.10 ± 0.07 mg RE/g extract in ATWE, and 47.28 ± 0.55 mg RE/g extract in ATEE. Aside it showed a promising antioxidant scavenging activity against DPPH and ABTS radicals. The antioxidant capacity of the two extracts increased in a dose-dependent manner. ATEE and ATWE had little difference in the scavenging rate of DPPH free radicals, and its radical scavenging activity were reached about 70% at 1000 μg/mL. ATWE had significant cytotoxicity to all the tested cancer cell lines of A549, PC3, and MDA-MB 231. The anti-cancer activity of ATWE was better than ATEE, and the IC50 value for A549 cells, PC3 cells, and MDA-MB cells were 96.32 ± 5.96, 198.58 ± 10.35 and 365.27 ± 19.72 μg/mL, respectively. The fluorescent staining (AO/EB, PI, Rh123, ROS) studies explored that ATWE could target the cancer cell via nuclear damage, excessive production of ROS and loss of mitochondrial membrane potential, suggesting that activation of endogenous apoptosis pathways. These results proved that AT extracts (especially ATWE) had significant antioxidants and anti-cancer activities, implying a possible pharmacological application of AT.
Keywords: Acer tegmentosum Maxim; antioxidant; cytotoxicity; anticancer
Cancer has a long history to become a worldwide health problem; it is a serious threat to human life. Cancer is the world’s second most important cause of death [1]. Several reports pointed out that the incidence and mortality of cancer have been drastically increased for the last three decades, including lung cancer, prostate cancer, and breast cancer [2,3]. According to the data provided by the International Agency for Research on Cancer (IARC) in Globocan, the incidence rate (11.7%) and mortality rate (18%) of breast cancer in 2020 ranked first, followed by lung cancer (11.4% of mortality and fifth) and prostate cancer has ranked fourth place (7.3%) [4]. In the treatment of cancer, surgery, radiation therapy, immunotherapy, chemotherapy, targeted therapy and hormone therapy was commonly employed to eradicate the dreadful disease of cancer [5]. However, severe side effects (such as healthy cells will be killed in the process) are usually accompanied by severe side effects, and the treatment is expensive. So, the novel research towards cost-effective findings with minimal side effects treatment methods is critical for cancer therapy. In contrast, as a good source of the anti-cancer drug, plant-based phytocompound and its derivatives have a progressive application in cancer treatment because they are simpler, safer, and more environment-friendly. In terms of chemical structure and pharmacological characteristics, natural drug are quite diverse. Usually the plant-based phytocompounds are specifically targeted and destroy the tumour cells without affecting normal cells, and they are considered as suitable candidates for the development of anti-cancer drugs. Several anticancer medicine such us camptothecin, paclitaxel, podophyllin, etc. were isolated from plants [6,7]. In the recent past, there is a great attention towards the anti-cancer drugs from medicinal plants. Based on these aspects, we were chosen for Acer tegmentosum Maxim to explore its antioxidants and anticancer activity.
Acer tegmentosum Maxim (AT) is a deciduous tree of the Aceaceae family, also known as “Beolnamu”, “Sancheongmok” or “Cheonghaecheck”. It is native to China, South Korea, and Russia [8]. It has a fascinating liver protection activity, and it was traditionally used to treat liver diseases (such as hepatitis, liver cancer and cirrhosis) in China and South Korea [9]. The bark part of the plant material has been used in Chinese and Korean traditional medicine to treat against surgical bleeding, abscesses, leukemia, hepatitis, renal necrosis, diabetes mellitus and hepatic cancer. The Korean Ministry of Food and Drug Safety (MFDS) has approved AT extract as a non-toxic food material. The available literature revealed that AT is rich in phenolic glycosides, flavonoids, coumarins, quinone, salidroside, methyl gallate, catachein and other bioactive compounds [10]. The existence of these phytocompounds makes AT have many physiological and pharmacological functions such as scavenging free radicals [11], reducing atopic dermatitis [12], preventing obesity, diabetes [13], inhibiting bone destruction [14]. However, we found a limited study to documented the anti-cancer activity of AT against liver cancer. Still, relatively few other cancers have been reported, which significantly limits the pharmacological scope and the potential for cancer treatment. As a result, we attempted to study the anti-cancer efficacy of this plant against a variety of cancer cell lines, including liver cancer. The present study was designed to reveal the phytochemical profiling, antioxidants and anti-cancer activity of different extracts of Acer tegmentosum Maxim.
2.1 Preparation of Plant Extracts
The plant material of Acer tegmentosum Maxim was collected from Changbai Mountain in Jilin Province, China. The collected plant material was identified and validated by Botanist MH Wang, and the voucher specimen (KNUH-BMC-2020-017) was deposited in the Herbarium of Department of Biomedical convergence, Kangwon National University, South Korea. The bark part of the plant material was carefully collected and permitted to shade dry and then it was coarsely powered. To obtain the aqueous extracts, 100 gm of powdered sample of AT were mixed with 500 mL distilled water at 100°C for 10 min. Then the extracted material was allowed to filtrated using Whatman No. 1 filter paper. A similar method was used to prepare a 70% ethanol extraction solution (25°C, 24 h). The collected extracts (Water and Ethanol) were concentrated using a rotary evaporator and freeze-dried using a lyophilizer and then stored at 4°C.
2.2 Total Phenolic Content (TPC)
The total phenolic content (TPC) of aqueous and ethanolic extracts of Acer tegmentosum Maxim was determined using the Folin-ciocalteu method [15]. 200 µL of different concentration of ATWE and ATEE (1.9–1,000 μg/mL) was mixed with with 0.5 mL of Folin reagent and it was incubated for 2–3 in room temperature, After the incubation period, 2.5 mL of 20% Na2CO3 solution was added in the solution Then the mixture was incubated at 30°C for 30 min. The absorbance of all the sample was measured at 725 nm. Gallic acid was utilized as a positive control to quantify the total phenolic content in ATWE and ATEE.
2.3 Total Flavonoid Content (TFC)
Total flavonoid content (TFC) in the aqueous and ethanolic extracts of Acer tegmentosum Maxim were revealed by the method described by elsewhere [16]. Briefly, 100 µL different concentration of ATWE and ATEE (1.9–1000 µg/mL) were mixed 0.3 mL 5% NaNO2 and 0.6 mL 10% AlCl3 and the reaction mixture was allowed to stand by for 5 min at room temperature. Afterwards, 2 mL of 1 M NaOH was added to the reaction mixture and mixed well, subsequently the solution volume was adjusted to 10 mL using a distilled water. After 5 min incubation, the absorbance was assessed at 510 nm. Rutin was utilized as positive control to quantify the flavonoid content. The TPC and TFC in ATWE and ATEE were calculated according to the regression equation derived from the standard curve and expressed as mg gallic acid equivalent (GAE)/g extract (Y = 0.0062x-0.1233, R2 = 0.99) and mg Rutin equivalent (RE)/g extract (Y = 0.0035x + 0.0671, R2 = 0.99), respectively.
The activity of DPPH in scavenging free radicals was evaluated using a method described elsewhere [17]. Briefly, 1 mL of different concentration of (1.9–1,000 µg/mL) ATEE and ATWE was mixed with 1 mL of 0.1 mmoL/L DPPH in ethanolic solution was kept in a dark room condition for 30 min. DPPH absorbance was assessed at 517 nm (Ai). As a control group, 1 mL of 100% ethanol was used to replace the DPPH mixed solution (Aj). As a blank group, 1 mL of 100% ethanol was used instead of the sample (Ac). The DPPH free radical scavenging rate was calculated according to formula (1).
The radical scavenging potential of ABTS cation was evaluated using Re et al.’s technique [18]. To this, 0.2 mL of 7.4 mM ABTS and 2.45 mM potassium persulfate solution was mixed in a test tube and permitted to stand in dark conditions for 12–16 h. Anhydrous ethanol was used to dilute the mixed solution until the optical density at 734 nm was 0.700 ± 0.02, and to this ABTS working solution was prepared. For ABTS radical scavenging assay, 1 mL of different concentrations (1.9–1,000 µg/mL) of ATEE and ATWE was mixed with 4 mL of ABTS solution, and then incubated for 6 min in dark at room temperature, After the incubation period, the absorbance was measured at 734 nm (A1). 1 mL of absolute ethanol was used instead of the sample as the blank group (A0). ABTS cation radical scavenging rate was calculated according to formula (2).
Based on the preliminary findings, the phytochemical constituents of ATWE were studied by Gas-chromatography-Mass spectroscopy (GC-MS). Agilent 7890A, 5975C model of GC-MS instrument equipped with DB-5MS, 30 M × 0.25 mm, 0.25 μm of Agilent of J&W GC column with the detection of Mass spectroscopy Saturn 2,000 was used for this study. An electron ionization energy methodology was adopted for GC-MS spectral detection, with a high ionization energy of 70 eV (electron Volts), a scan time of 0.2 seconds, and fragments ranging from 40 to 550 m/z. The initial temperature of the column was fixed with 60°C to 230°C, with a 3 °C/min increase rate and a 10-minute hold interval and the temperature was increased to 250°C at a rate of 10°C per minute. 1 µL of the ATWE sample was introduced into the GC/MS column and as a carrier gas, helium (99.99%) was used at a flow rate of 1 mL/min−1. Finally the phytochemical profile of ATWE were identified using the W8N05ST.L mass spectral library.
WST-1 (4-[3-(4-Iodophenyl)-2-(4-nitro-phenyl)-2H-5-tetrazolio]-1,3-benzene sulfonate) based cytotoxicity assay was used to explore the anticancer efficacy of ATWE and ATEE [19]. Human embryonic kidney cell line of (HEK 293), Liver cancer cell line of A549, Trible negative breast cancer cell line of MDA-MB-231 and prostatic cancer cell line of PC3 were used for this study. Non-cancerous cell line of HEK 293 were cultured in DMEM media and the cancerous cell line of A549, MDA-MB 231, and PC3 cells were cultured in RPMI media comprising of 10% fetal bovine serum and 0.5% antibiotic solution. The cells were allowed to reaching 80%–90% of confluency, Then, 100 μL of cells were seeded into 96-well plate, and the plate was kept in a humidified chamber at 37°C, 5% CO2 for 24 h. After the 24 h seeding, the cells were treated with 10 μL of different concentration of ATWE and ATEE and incubated for 24 h. Then, 10 μL of WST-1 solution was added to each well, and the incubation continued for 1 h. After the incubation, the plates were read in a microplate reader at 450 nm. The IC50 concentration of ATWE was used for fluorescence staining analysis of A549 cells. A549 cells were stained with DCFH-DA, AO/EB, PI, and Rho-123, and cell death was observed under a fluorescence microscope [20,21].
3.1 Phytoconstituents Analysis
Polyphenols and flavonoids are widely found in higher plants, and they are essential sources of secondary metabolites. It has diverse physiological and pharmacological functions such as scavenging free radicals, detoxification mechanism, inhibiting microbial growth and preventing chronic diseases, etc. [22,23]. Based on the regression equation obtained from calibration curve, the water extract (ATWE) showed the highest phenolic content (199.97 ± 0.09 mg of GAE) followed by ethanolic extracts of ATEE (103.48 ± 0.82). Similarly, the water extract ATWE) showed the highest flavonoid content (72.10 ± 0.07 mg of RE) followed by ethanolic extracts (47.28 ± 0.55 mg of RE). As a result, this finding indicated that aqueous extracts were the best method for extracting phenolic/flavonoid compounds from A.tegmentosum Maxim (Tab. 1).
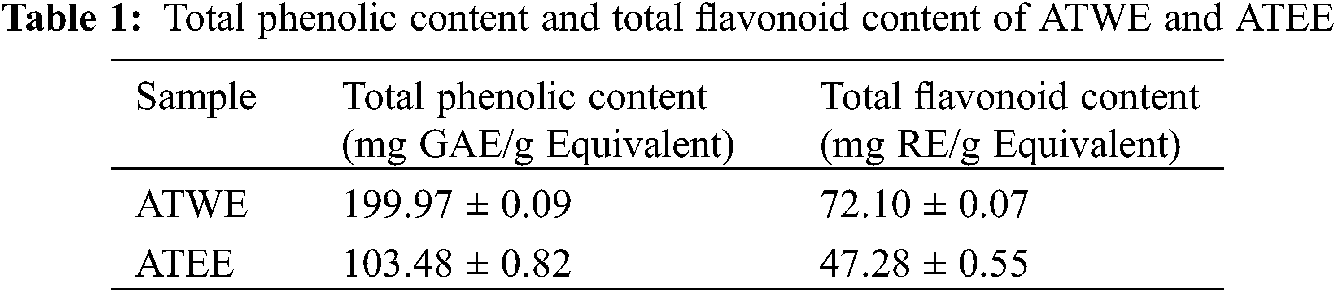
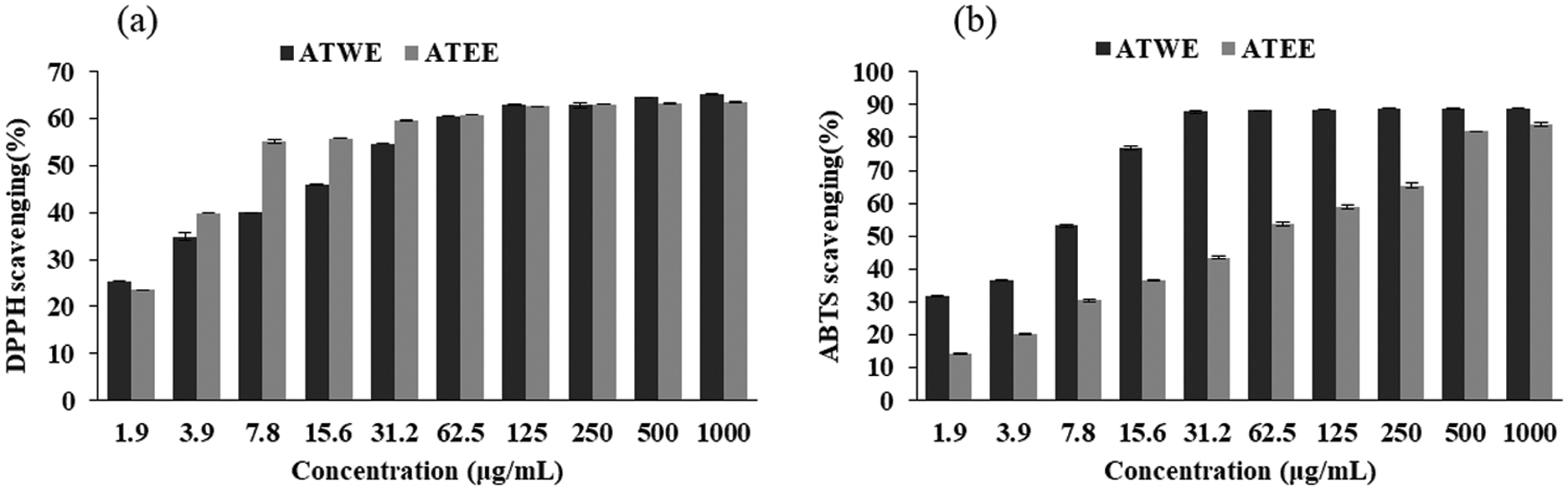
Figure 1: Antioxidant activity of ATWE and ATEE. DPPH radical scavenging rate (a), ABTS radical scavenging rate (b)
The free radical scavenging ability of ATWE and ATEE was measured by DPPH and ABTS assay. DPPH is a synthetic purple coloured free radical has the absorption peak at 517 nm. When it reacts with antioxidants, the DPPH radicals are reduced and the solution turns from dark purple to yellow and it was quantitatively related to the received electrons [24,25]. As shown in Fig. 1a, ATWE and ATEE exhibited a significant DPPH free radical scavenging activity, and the scavenging activities were increased in a dose-dependent manner. The scavenging rate reached about 70% at the highest concentration (1000 μg/mL). At lower concentrations, ATEE was better than ATWE. But when the concentration was higher, the difference between the tested extracts’ DPPH free radical scavenging activity was narrowed. ABTS also synthetic and stable free radicals, but compared with DPPH, a stable free radical, it must be generated by the oxidation of ABTS by potassium persulfate before the measurements [26,27]. As shown in Fig. 1b, ATWE and ATEE showed a significant ABTS radical scavenging activity, and the scavenging rate increased in a dose-dependent manner. The scavenging rate reached 80–90% at 1000 μg/mL, and the IC50 of ATWE is 7.36 μg/mL, the IC50 of ATEE is 48.67 μg/mL.
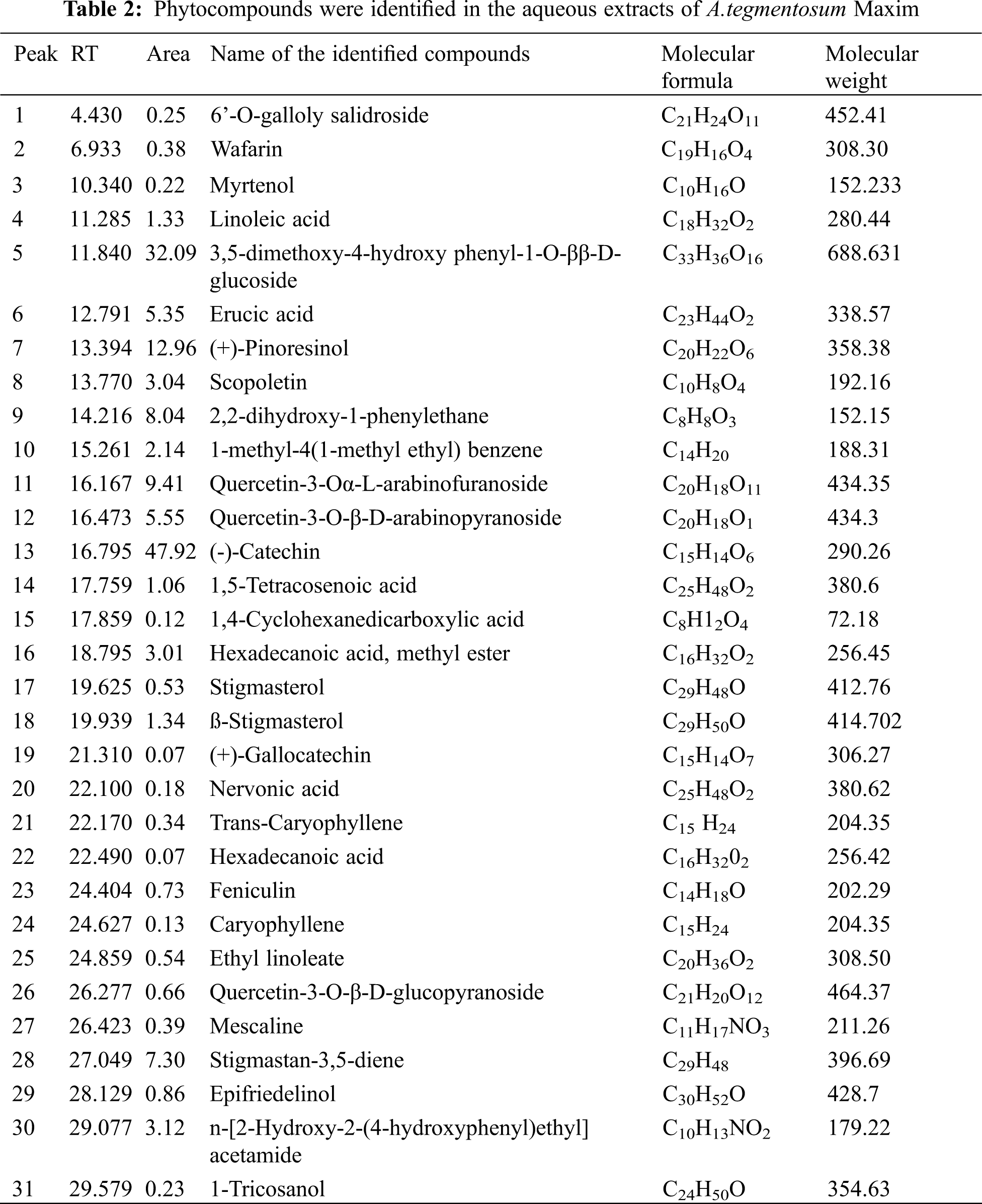
The GC-MS chromatogram of ATWE showed a total of 31 peaks corresponding to bioactive compounds, which were identified by comparing peak, retention period, peak area of known compounds identified by W8N05ST.L mass spectral library. This finding revealed that, different types of phytocompounds were present in methanolic solvent of ATWE (Fig. 2 and Tab. 2). 6’-O-galloly salidroside, Wafarin, Myrtenol, Linoleic acid,3,5-dimethoxy-4-hydroxy phenyl-1-O-ββ-D-glucoside, Erucic acid,(+)-Pinoresinol,Scopoletin,2,2-dihydroxy-1-phenylethane,1-methyl-4(1-methyl ethyl) benzene, Quercetin-3-Oα-L-arabinofuranoside, Quercetin-3-O-β-D-arabinopyranoside,(-)-Catechin,1,5-Tetracosenoic acid,1,4-Cyclohexanedicarboxylic acid, Hexadecanoic acid-methyl ester, Stigmasterol,ß-Stigmasterol,(+)-Gallocatechin, Nervonic acid, Trans-Caryophyllene, Hexadecanoic acid, Feniculin, Caryophyllene, Ethyl linoleate, Quercetin-3-O-β-D-glucopyranoside, Mescaline, Stigmastan-3,5-diene, Epifriedelinol,n-[2-Hydroxy-2-(4-hydroxyphenyl)ethyl]acetamide and 1-Tricosanol were predominantly present in the ATWE.
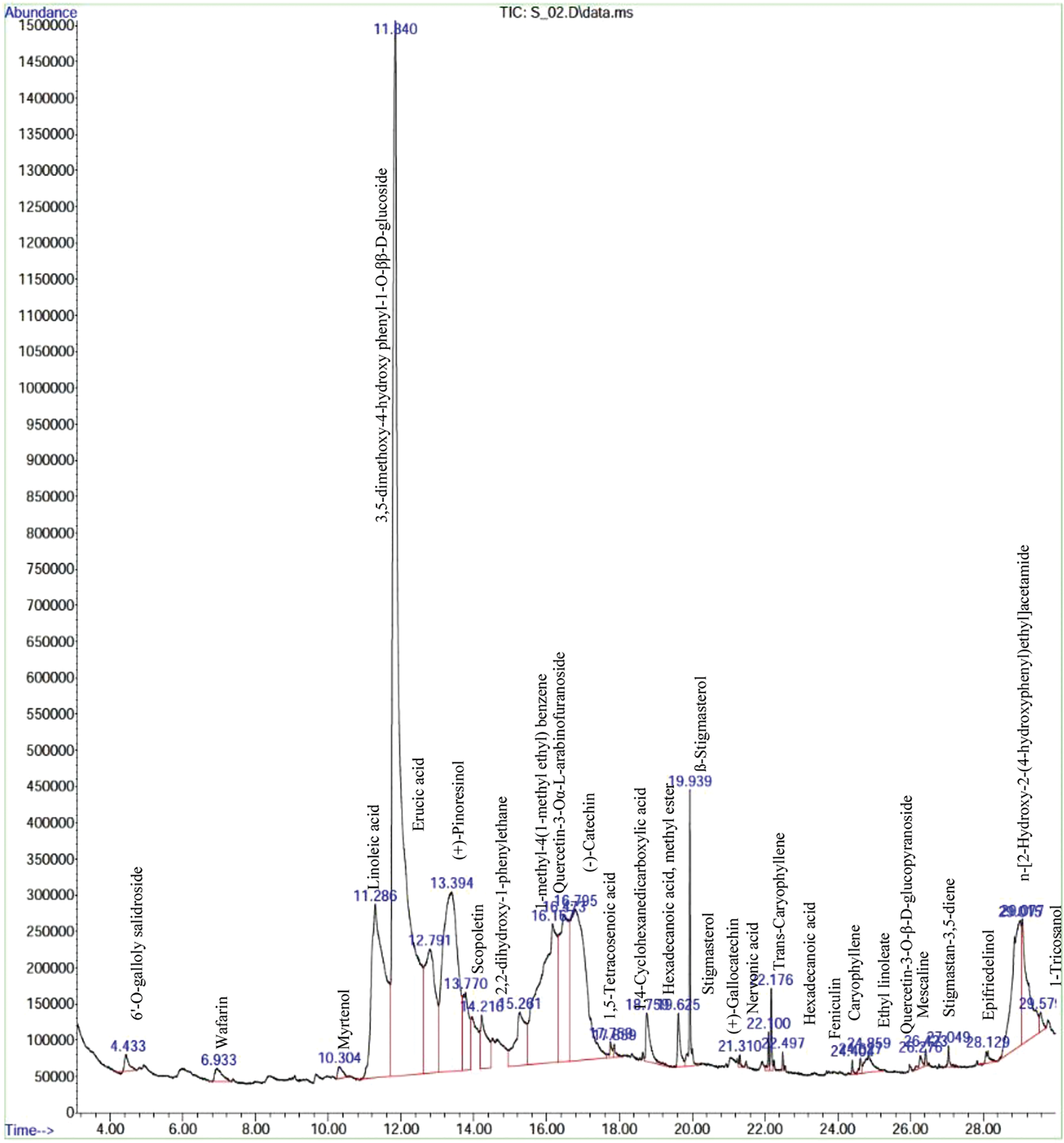
Figure 2: GC-MS Chromatogram of the bioactive compounds presents in aqueous extracts of A.tegmentosum Maxim
In this study, the cytotoxic activity of aqueous and ethanolic extracts of A.tegmentosum was determined by WST based cytotoxicity studies. As shown in Fig. 3a, ATWE and ATEE showed no cytotoxicity to HEK293 cells. The highest concentration of ATWE and ATEE (1000 μg/mL) did not cause a decrease in cell viability. It was indicating the non-toxic nature of the ATWE and ATEE. Comparatively, ATWE was less cytotoxic. Besides, ATWE and ATEE extracts had a significant cytotoxicity towards the cancerous cell line of A549, PC3, and MDA-MB 231 cells. At the higher concentration (100 μg/mL) of the test sample, the cell viability rate has significantly reduced (nearly 70%) for all the cancerous cells. The IC50 concentration was found to be 96.32 ± 5.96, 198.58 ± 10.35, 365.27 ± 19.72 μg/mL (Tab. 3), respectively. These findings revealed that the cytotoxicity of the ATWE and ATEE was increased in a dose-dependent manner (Figs. 3b–3d). Comparatively, ATWE has higher cytotoxicity than ATEE.
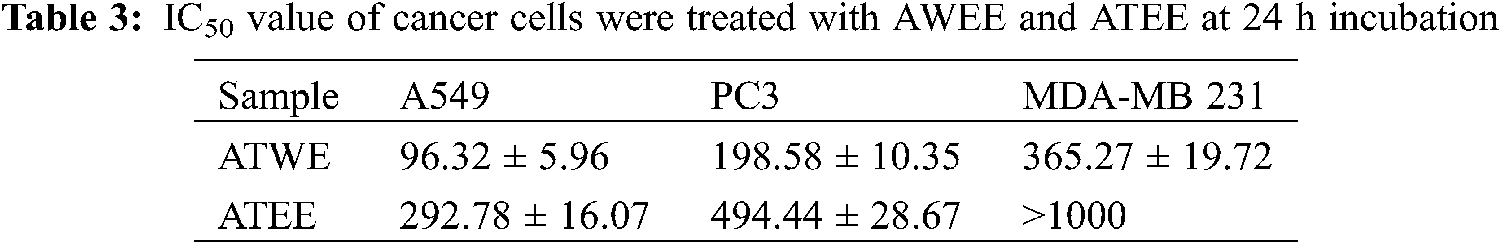
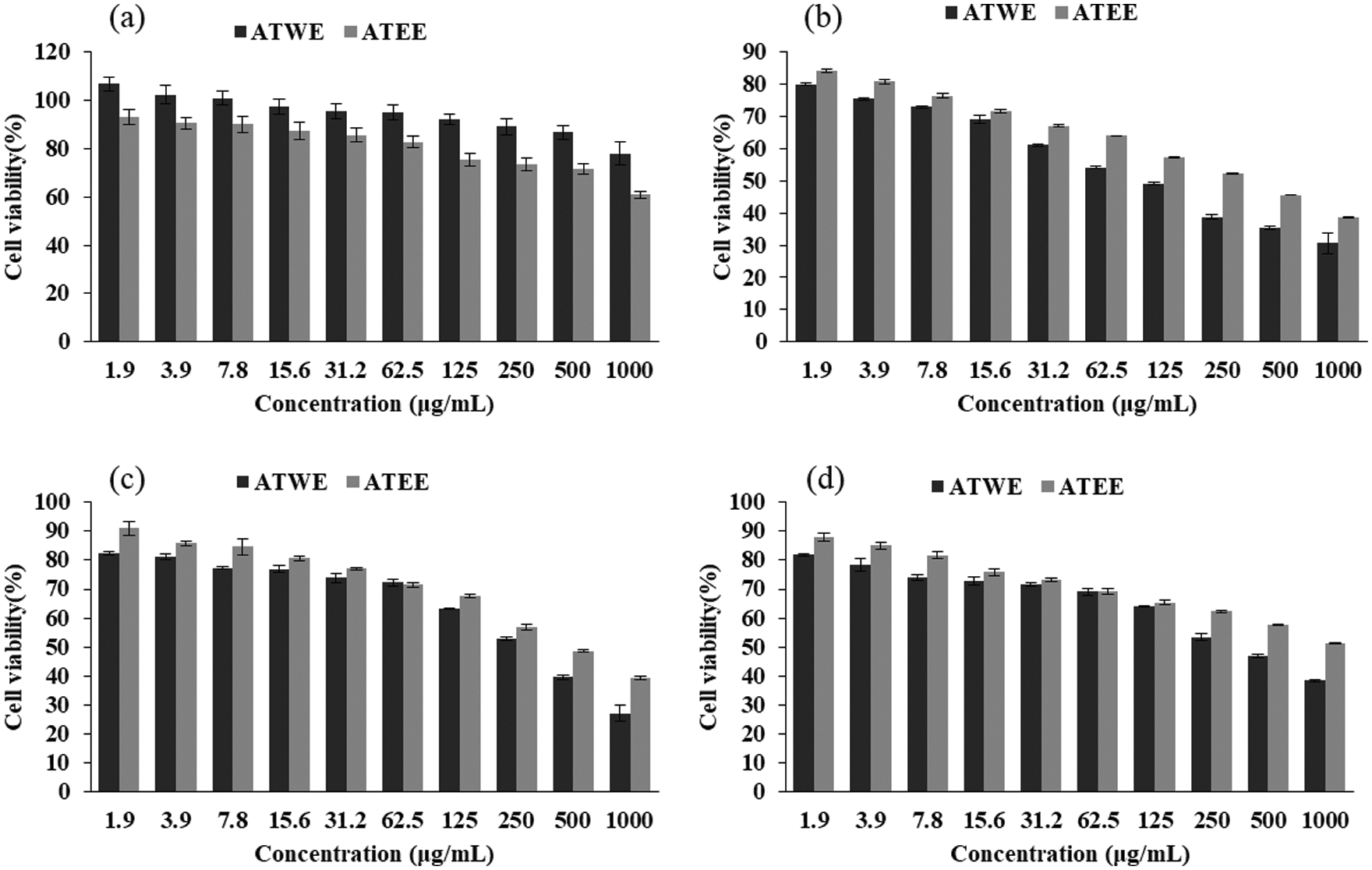
Figure 3: Cytotoxicity of the ATWE and ATEE in HEK293 (a), A549 (b), PC3 (c), MDA-MB cells (d). The values were expressed as Mean ± SD of three independent experiments
As shown in Tab. 4, HEK293 cells were used as a control in the selective analysis, and ATWE showed the best selective index for A549 cells (17.80). According to the selective criteria, ATWE can be considered as a selective extract for A549 cells (selectivity index > 10). On the contrary, the selective index of ATEE for A549 cells and the selective index of the two extracts for the other two cancer cells were less than 10. Extracts with selective indexes lower than 10 but higher than 1 could be considered non-selective [28,29]. Therefore, combining the cytotoxicity results of the two extracts, we chose the extract ATWE and lung cancer cell A549 for further study.
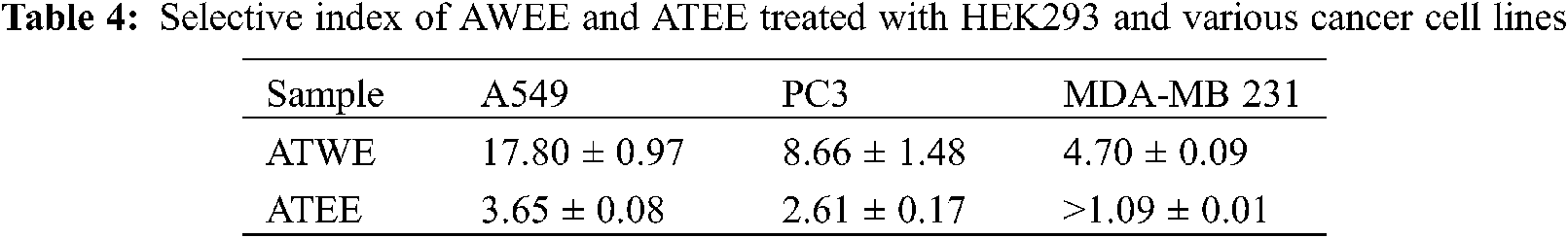
This finding revealed that ATWE significantly restricts lung carcinoma (A549) proliferation, whereas the breast cancer cell lines (MDA-MB 231) require a higher concentration of ATEE. Therefore, the IC50 concentration of ATWE was used for further microscopic studies. Through the bright microscope observation, the untreated A549 cells appeared as normal architecture, and the shape was the same, whereas A549 cells treated with ATWE and positive control staurosporine (STA) showing an increasing dead cell population with shrunken cells, membrane blebbing, ballooning, vacuolization and granulation were apparent in ATWE cells (Fig. 4a). Acridine orange (AO) can penetrate cells with intact cell membranes, embed nuclear DNA, and emit green fluorescence. Only the damaged cell membranes allow Ethidium bromide (EB) to penetrate and embed with nuclear DNA, emitting an orange-red fluorescence [30]. The untreated A549 cells showed uniform green fluorescence, in contrast the cells treated with ATWE showing the early apoptotic cells with green, fluorescent shrinkage and late apoptotic cells with orange-red fluorescence represent the pyknosis or beading (Fig. 4b).
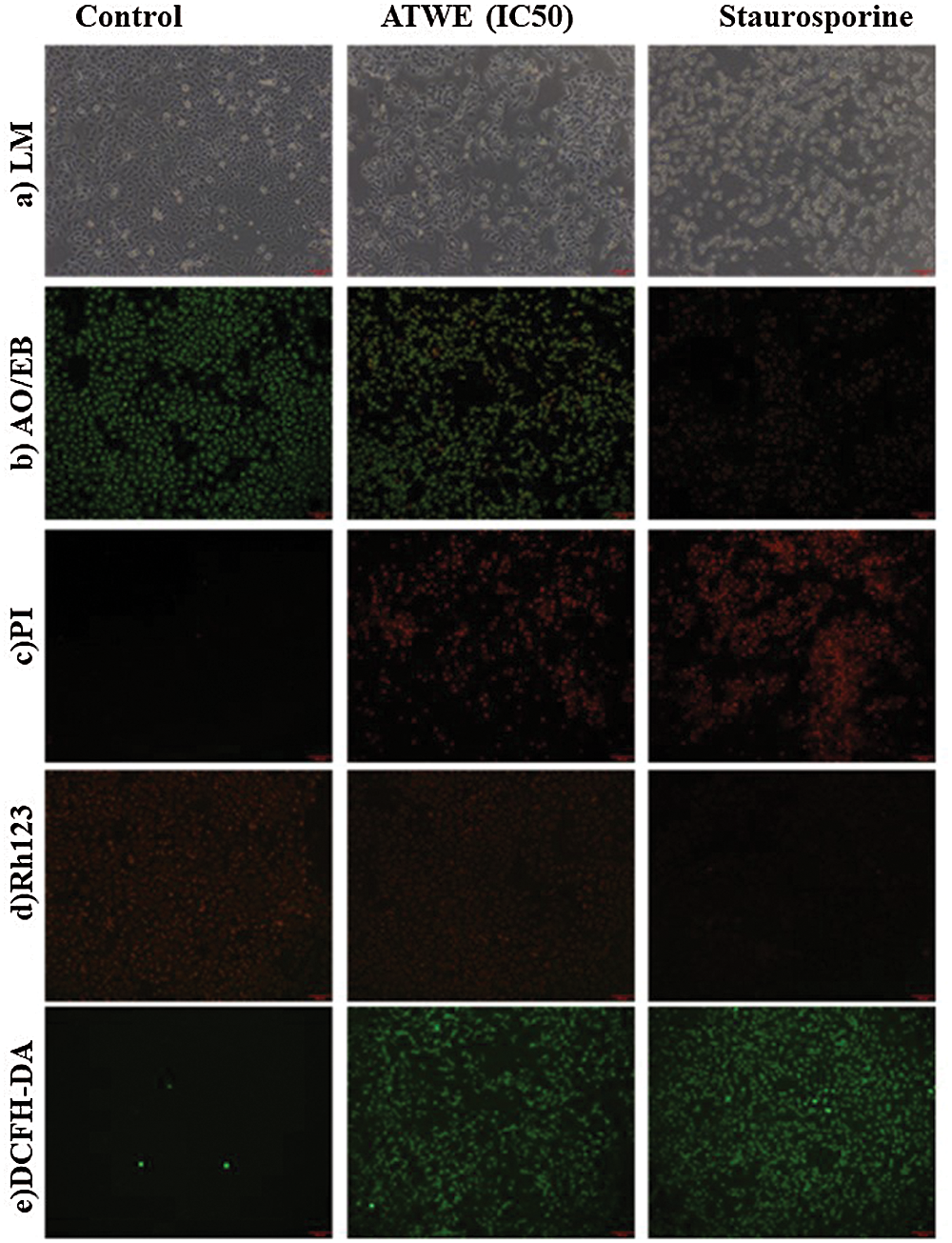
Figure 4: The light microscope photograph (a), AO/EB (b), PI (c), Rh123 (d), DCFH-DA (e) straining photograph of untreated, IC50 of Acer tegmentosum Maxim water extract (ATWE) and 1 μM of Staurosporine (STA) treated cells
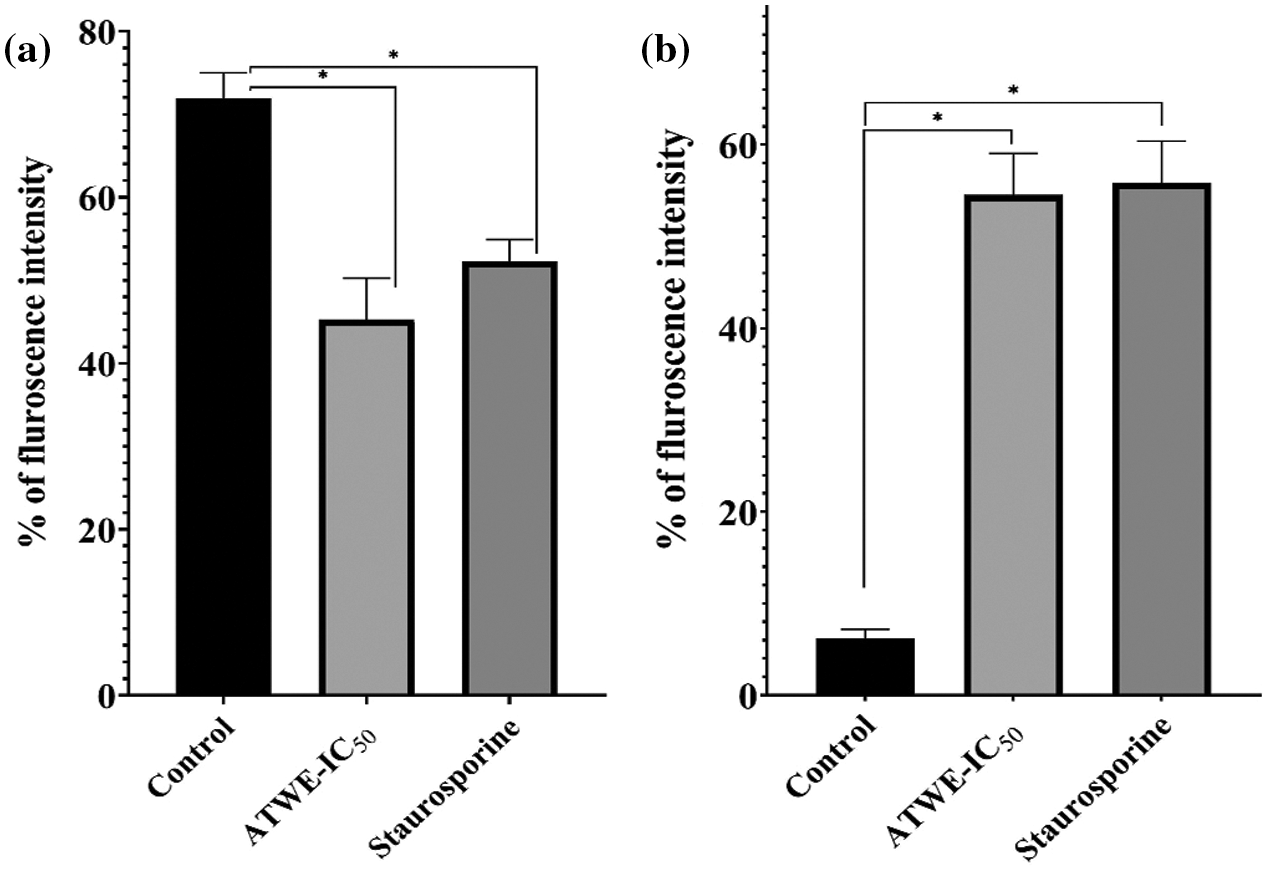
Figure 5: Quantitative fluorescence intensity of Rhodamine-123 stain to measure the ROS generation (a), the loss of mitochondrial membrane potential was measured by DCFH-DA (b). The fluorescence intensity was measured by Image-J analysis. *Values were significantly differing from the untreated control group (p < 0.05)
Propidium iodide is a nuclear staining reagent that is used to detect nuclear damage [31]. As shown in Fig. 4c, no fluorescence response was observed in the control group. On the contrary, ATWE and STA treated cells have appeared as increased red fluorescence intensity. It indicates that ATWE treatment has the ability to damage the A549 cell nucleus. The mitochondrial membrane potential has been identified as one of the hallmarks of apoptosis. Rhodamine 123 stain was commonly used to study the mitochondrial membrane potential. It is a fluorescent dye that can selectively stain the mitochondria of living cells through the cell membrane [32]. Through observation, a large amount of red fluorescence was observed in the untreated control group. After the treatment with ATWE and STA, the decreased fluorescence intensity was observed. After the treatment with ATWE and STA, a decreased fluorescence intensity was observed. It revealed that ATWE treatment might damage the mitochondrial membrane of the A549 cells (Figs. 4d and 5a). DCFH-DA is a non-labelled oxidation-sensitive fluorescent probe, and it is used to measure the intracellular ROS status. Reactive oxygen species (ROS) in cells can oxidize non-fluorescent DCFH to produce DCF with green fluorescent [33]. After treatment with ATWE and STA, the intensity of green fluorescence increased considerably increased in A549 cells (Figs. 4e and 5b). It could be suggesting that the ATWE has the ability to induces ROS mediated cell death.
Medicinal plants have been widely used in traditional complementary and alternative medicine as natural healing remedies with significant antimicrobial, anti-inflammatory, and anti-cancer activity. Based on this scientific background, the antioxidants and anti-cancer activity of A.tegmentosum were clearly documented. The findings of the phytochemical studies confirmed that the TPC and TFC were relatively rich in ATWE and ATEE, which is also an essential reason for A.tegmentosum has many physiological and pharmacological activities [34]. TPC and TFC of ATWE were both higher than that of ATEE. Besides, ATWE and ATEE showed the significant and concentration-dependent free radical and antioxidants activities of DPPH and ABTS. These findings strongly indicate a connection between antioxidant ability and total phenolic and flavonoid content of the ATWE and ATEE. Therefore, the active ingredients and the ratio of active ingredients, the interaction between the ingredients, and the primary structure of the antioxidant ingredients analysis were necessary to validate the pharmacological potential plant extracts [35]. Herein, GC-MS method was used to identify the active ingredients in ATWE. The GC-MS analysis of the aqueous extracts of A. tegmentosum indicated saturated fatty acid, poly unsaturated fatty acid, lignan, phenolic compound, flavonoids, alkaloid, and terpene. As a result, the antioxidant ability of A.tegmentosum investigated in this study might be due to its phenolic compounds, which were found in high concentrations in water and ethanol extracts. Similar antioxidant potentials of some Acer species emerging in various parts of the world have been recently reported.
Based on our preliminary findings, we were mainly focusing the anti-cancer activity of AT against liver cancer. AT showed promising cytotoxicity to lung cancer cells followed by prostate cancer cells and breast cancer cells; on the contrary, non-toxic effects were observed in normal cells HEK293. It revealed the biocompatible nature alongside an excellent and wide-range anti-cancer activity of AT. AT’s aqueous and ethanolic extracts showed considerable cytotoxic action against the A549 cell line (IC50 = 96.32 ± 5.96 µg/mL, 292.78 ± 16.07, respectively). Besides, the breast (MDA-MB 231) and pancreatic cells (PC 3) showed a lesser activity. Of the previous studies with the leaf extracts of A. tegmentosum showed the dose-dependent cytotoxicity in SW480, MCF-7, PC3, AsPC-1, HePG-2, and A549 cells [36]. Our findings were corroborated with observation. ATWE treated A549 cells showed apoptotic associated morphological changes, including nuclear shrinkage, membrane blebbing, apoptotic bodies formation and condensed nuclei formation was observed by light microscopic and AO/EB staining. These morphological changes were indicated that ATWE could damage and destroy the cancer cells. At the same time, under a fluorescence microscope, it could be observed that the increased number of apoptotic cells, nuclear damage, the loss of mitochondrial potential, and the generation of ROS were higher in ATWE treated cells. In AO/EB staining analysis, the apoptotic cell population was significantly increased in ATWE-treated A549 cells, and this finding supports the previously reported observations. It was observed that plant-based phytocompound can trigger-based phytocompound can trigger apoptosis in several types of cancer, including liver cancer [37]. Prior research confirmed that the anti-cancer compound of salidroside from A.tegmentum inhibits apoptotic associated hepatocellular carcinoma cell death. It has been reported that higher ROS levels and loss of mitochondrial membrane potential were attributed to the apoptosis mediated by cancer cell death. At the same time, oxidative stress-mediated ROS production and cell membrane potential loss will also change the expression of cellular antioxidants and apoptosis-related proteins to achieve cytotoxicity and cause apoptosis [38,39]. In the present study, the loss of mitochondrial membrane potential was detected in ATWE treated cells, and it was analyzed using Rhodamine 123 staining. As a result, our findings showed that ATWE treatment of A549 cells causes intracellular ROS and induces apoptosis by lowering the mitochondrial outer membrane potential through activating the endogenous apoptotic pathway. The possible apoptosis inducing mechanism of phytocompounds present in the aqueous extracts of Acer tegmentum Maxim were shown in Scheme 1.
Oxidative stress is caused by when the cell does not have enough capacity to eliminate ROS or balance its existence. The body will produce and accumulate a large number of free radicals under oxidative stress. Excess free radicals will have a destructive effect on cell membrane lipids, proteins, DNA and other biological macromolecules [40]. It has been reported that the ability of anti-cancer agents to induce apoptosis in cancer cells depends on the ability to produce ROS, and a disproportionate increase in ROS can induce apoptosis in cancer cells [41]. Studies have shown that an increase in ROS levels will increase the permeability of mitochondrial membranes, promote the development of mitochondrial permeability transition pores, cause loss of mitochondrial membrane potential, and lead to the initiation of mitochondrial-mediated apoptosis [42,43]. Therefore, the production of ROS in cells is considered an indicator of mitochondrial-mediated cell death pathway activation [44]. In our study, after ATWE treatment, we observed overproduction of ROS and collapse of mitochondrial membrane potential. This result suggests that the mitochondrial apoptosis pathway is involved in the ATWE-induced apoptosis of A549 cells.
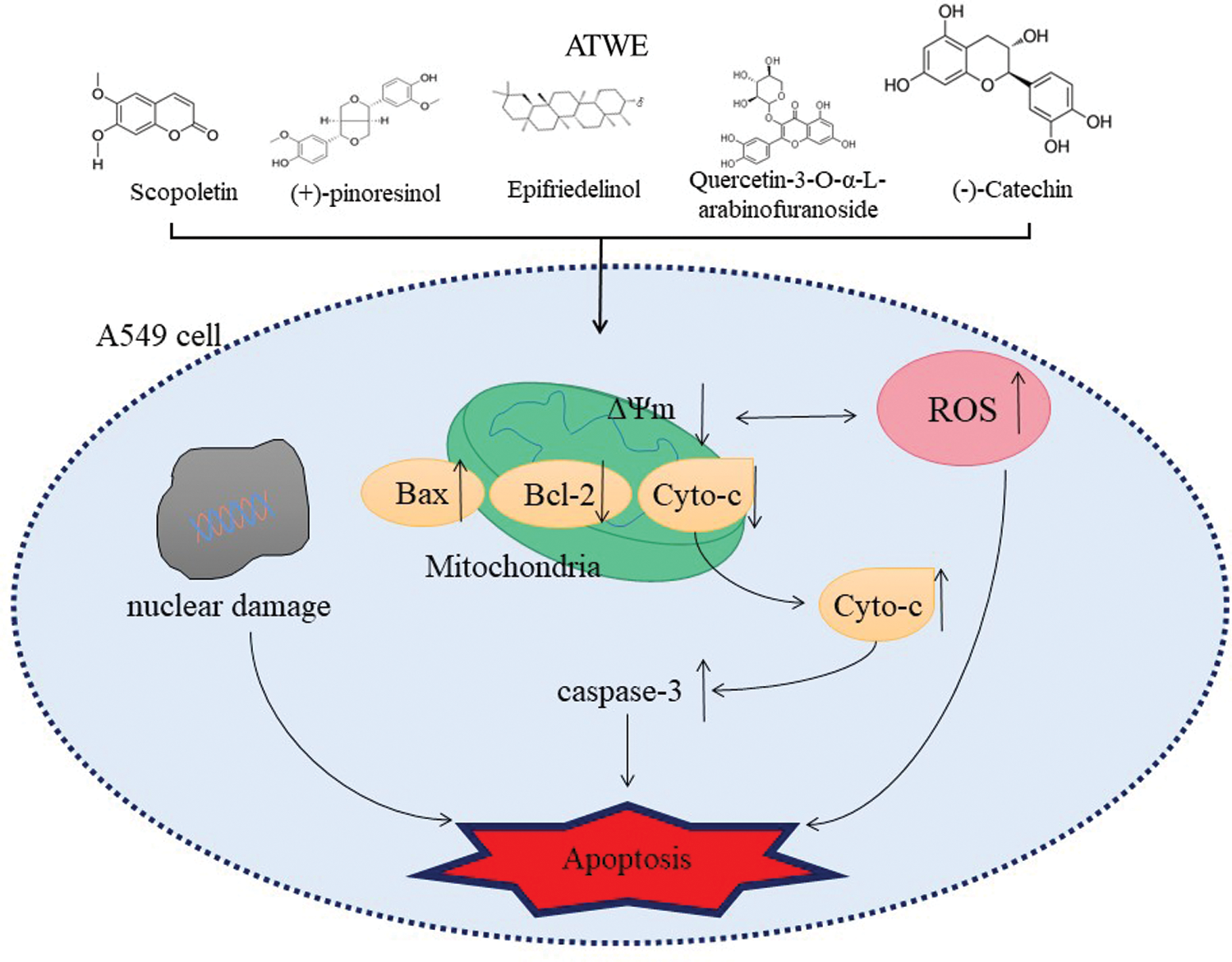
Scheme 1: The possible apoptosis inducing mechanism of phytocompounds present in the aqueous extracts of Acer tegmentum Maxim
Mitochondrial-mediated (endogenous) apoptosis pathway is one of the classic apoptosis pathways, and mitochondrial damage plays a crucial role in apoptosis. The endogenous apoptosis pathway is regulated by the activity of the BCL-2 protein family, including anti-apoptotic proteins and pro-apoptotic proteins [45]. Studies have shown that the down-regulation of anti-apoptotic proteins (Bcl-2 and Bcl-xl) and the up-regulation of pro-apoptotic protein Bax could lead to the production of ROS, the damage of mitochondrial membrane potential and the release of cytochrome c, which in turn activated the downstream caspase cascade and ultimately led to apoptosis. In GCMS analysis, Scopoletin (Phenolic compound), (+)-pinoresinol (Lignan), Epifriedelinol (Triterpene), Quercetin-3-O-α-L-arabinofuranoside(Flavonoid), Hexadecanoic acid(Saturated fatty acid) and other active ingredients were identified. Scopoletin was reported to have a significant inhibitory effect on A549 cells; Scopoletin could significantly increase Bax and Cleaved-caspase3 and significantly reduce the expression of Bcl-2 in A549 cells. The results indicated that Scopoletin’s against non-small cell lung cancer effect was related to the intrinsic apoptosis pathway [46–48]. In addition, (+)-pinoresinol (Pin) is an essential dietary lignan. It has been reported Pin treatment can cause a significant decrease in the mitochondrial membrane potential of HepG2 cells, a significant decrease in Bcl-2, an increase in the ratio of Bax/Bcl-2, and the expression of Bax and caspase 3 [49]. Epifriedelinol has also been observed to increase the caspase enzyme activity and the translocation of cytochrome c in a dose-dependent manner and change the ratio of pro-apoptotic proteins to anti-apoptotic proteins [50]. A study has reported that Quercetin-3-O-α-L-arabinofuranoside (also called Avicularin, AL) dose-dependently inhibited the Cutaneous squamous cell carcinoma (CSCC) cell viability induces cell apoptosis. After AL treatment, the expression level of Bax in SCC13 cells increased, while the expression level of Bcl-2 decreased [51]. Besides, treatment with Hexadecanoic acid isolated from sea pen Virgularia gustaviana could reduce the viability of two cancer cell lines (Hela and MDA-MB 231) in a concentration-dependent manner and induce apoptosis by increasing caspase-3 and significantly decreasing the relative Bcl-2/Bax ratio [52]. These reports were consistent with our research results. In summary, we can reasonably infer that ATWE has anti-cancer-related bioactive components. These components could cause A549 cell apoptosis and inhibit its proliferation through ROS accumulation and activation of the mitochondrial endogenous apoptosis pathway.
Acer tegmentum Maxim plant is enriched with phytoconstituents, and it has remarkable pharmacological activities. In this study, two different extracts (ATWE and ATEE) of were prepared from the bark part of Acer tegmentum. TPC and TFC, antioxidant activity as well as its phytochemical constituents, were analyzed by GC-MS analysis. In addition, the anti-cancer activity was studied in A549, PC3 and MDA-MB 231 cells. ATWE and ATEE were rich in phenols and flavonoids, and the aqueous extracts of Acer tegmentum exhibited significant antioxidant and anti-cancer activities. The results evidenced the development value of functional food and medicine as a natural plant source of Acer tegmentum.
Funding Statement: The authors received no financial support for the research, authorship, and/or publication of this article.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I. et al. (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians, 71, 209–249. DOI 10.3322/caac.21660. [Google Scholar] [CrossRef]
2. Bray, F., Ferlay, J., Soerjomataram, I., Siegel, R. L., Torre, L. A. et al. (2018). Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians, 68, 394–424. DOI 10.3322/caac.21492. [Google Scholar] [CrossRef]
3. Rahib, L., Wehner, M. R., Matrisian, L. M., Nead, K. T. (2021). Estimated projection of US cancer incidence and death to 2040. JAMA Network Open, 4, e214708. DOI 10.1001/jamanetworkopen.2021.4708. [Google Scholar] [CrossRef]
4. Ferlay, J., Colombet, M., Soerjomataram, I., Parkin, D. M., Piñeros, M. et al. (2021). Cancer statistics for the year 2020: An overview. International Journal of Cancer, 149, 778–789. DOI 10.1002/ijc.33588. [Google Scholar] [CrossRef]
5. Arruebo, M., Vilaboa, N., Sáez-Gutierrez, B., Lambea, J., Tres, A. et al. (2011). Assessment of the evolution of cancer treatment therapies. Cancers, 3, 3279–3330. DOI 10.3390/cancers3033279. [Google Scholar] [CrossRef]
6. Arokia, V. A. M., Ramachandran, V., Vinothkumar, R., Vijayalakshmi, S., Sathish, V. et al. (2019). Pharmacological aspects and potential use of phloretin: A systemic review. Mini-Reviews in Medicinal Chemistry, 19, 1060–1067. DOI 10.2174/1389557519666190311154425. [Google Scholar] [CrossRef]
7. Iqbal, J., Abbasi, B. A., Mahmood, T., Kanwal, S., Ali, B. et al. (2017). Plant-derived anti-cancer agents: A green anti-cancer approach. Asian Pacific Journal of Tropical Biomedicine, 7, 1129–1150. DOI 10.1016/j.apjtb.2017.10.016. [Google Scholar] [CrossRef]
8. Park, H. S., Jo, E., Han, J. H., Jung, S. H., Lee, D. H. et al. (2019). Hepatoprotective effects of an Acer tegmentosum Maxim extract through antioxidant activity and the regulation of autophagy. Journal of Ethnopharmacology, 239, 111912. DOI 10.1016/j.jep.2019.111912. [Google Scholar] [CrossRef]
9. Choi, G. E., Hyun, K. Y. (2020). Inhibitory effect of Acer tegmentosum maxim extracts on P. gingivalis LPS-induced periodontitis. Archives of Oral Biology, 109, 104529. DOI 10.1016/j.archoralbio.2019.104529. [Google Scholar] [CrossRef]
10. Piao, Y., Zhang, C., Ni, J., Yin, X., An, R. et al. (2020). Chemical constituents from the stem bark of Acer tegmentosum. Biochemical Systematics and Ecology, 89, 103982. DOI 10.1016/j.bse.2019.103982. [Google Scholar] [CrossRef]
11. Lee, C. E., Jeong, H. H., Cho, J. A., Ly, S. Y. (2017). Antioxidative and anti-inflammatory effects of wild chestnut extract in RAW 264.7 macrophages and inflammation-inducing animal models. Korean Society of Food and Nutrition, 46, 1–9. DOI 10.3746/JKFN.2017.46.1.001. [Google Scholar] [CrossRef]
12. Yang, G., An, D., Lee, M. H., Lee, K., Kim, B. et al. (2016). Effect of Acer tegmentosum bark on atopic dermatitis-like skin lesions in NC/Nga mice. Journal of Ethnopharmacology, 177, 53–60. DOI 10.1016/j.jep.2015.10.033. [Google Scholar] [CrossRef]
13. Cho, H. H., Lee, S. J., Kim, S. H., Jang, S. H., Won, C. et al. (2020). Acer tegmentosum maxim inhibits adipogenesis in 3t3-L1 adipocytes and attenuates lipid accumulation in obese rats fed a high-fat diet. Nutrients, 12, 3753. [Google Scholar]
14. Ha, H., Shim, K. S., Kim, T., An, H., Lee, C. J. et al. (2014). Water extract of Acer tegmentosum reduces bone destruction by inhibiting osteoclast differentiation and function. Molecules (Basel, Switzerland), 19, 3940–3954. DOI 10.3390/molecules19043940. [Google Scholar] [CrossRef]
15. Saravanakumar, K., Sathiyaseelan, A., Mariadoss, A. V. A., Chelliah, R., Hu, X. et al. (2020). Lactobacillus rhamnosus GG and biochemical agents enrich the shelf life of fresh-cut bell pepper (Capsicum annuum L. var. grossum (L.) Sendt). Foods, 9, 1252. [Google Scholar]
16. Saeed, N., Khan, M. R., Shabbir, M. (2012). Antioxidant activity, total phenolic and total flavonoid contents of whole plant extracts Torilis leptophylla L. BMC Complementary and Alternative Medicine, 12, 221. DOI 10.1186/1472-6882-12-221. [Google Scholar] [CrossRef]
17. Arnao, M. B., Cano, A., Acosta, M. (2001). The hydrophilic and lipophilic contribution to total antioxidant activity. Food Chemistry, 73, 239–244. DOI 10.1016/S0308-8146(00)00324-1. [Google Scholar] [CrossRef]
18. Re, R., Pellegrini, N., Proteggente, A., Pannala, A., Yang, M. et al. (1999). Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biology and Medicine, 26, 1231–1237. DOI 10.1016/S0891-5849(98)00315-3. [Google Scholar] [CrossRef]
19. Mariadoss, A. V. A., Saravanakumar, K., Sathiyaseelan, A., Wang, M. H. (2020b). Preparation, characterization and anti-cancer activity of graphene oxide–silver nanocomposite. Journal of Photochemistry and Photobiology B: Biology, 210, 111984. DOI 10.1016/j.jphotobiol.2020.111984. [Google Scholar] [CrossRef]
20. Saravanakumar, K., Sathiyaseelan, A., Mariadoss, A. V. A., Hu, X., Venkatachalam, K. (2021b). Nucleolin targeted delivery of aptamer tagged Trichoderma derived crude protein coated gold nanoparticles for improved cytotoxicity in cancer cells. Process Biochemistry, 102, 325–332. DOI 10.1016/j.procbio.2021.01.022. [Google Scholar] [CrossRef]
21. Mariadoss, A. V. A., Saravanakumar, K., Sathiyaseelan, A., Venkatachalam, K., Wang, M. H. (2020a). Folic acid functionalized starch encapsulated green synthesized copper oxide nanoparticles for targeted drug delivery in breast cancer therapy. International Journal of Biological Macromolecules, 164, 2073–2084. DOI 10.1016/j.ijbiomac.2020.08.036. [Google Scholar] [CrossRef]
22. Mark, R., Lyu, X., Lee, J. J. L., Parra-Saldívar, R., Chen, W. N. (2019). Sustainable production of natural phenolics for functional food applications. Journal of Functional Foods, 57, 233–254. DOI 10.1016/j.jff.2019.04.008. [Google Scholar] [CrossRef]
23. Suresh, K., Manoharan, S., Vijayaanand, M. A., Sugunadevi, G. (2010). Chemopreventive and antioxidant efficacy of (6)-paradol in 7,12-dimethylbenz(a)anthracene induced hamster buccal pouch carcinogenesis. Pharmacological Reports, 62, 1178–1185. DOI 10.1016/S1734-1140(10)70380-7. [Google Scholar] [CrossRef]
24. Moniruzzaman, M., Khalil, M. I., Sulaiman, S. A., Gan, S. H. (2011). Advances in the analytical methods for determining the antioxidant properties of honey: A review. African Journal of Traditional, Complementary, and Alternative Medicines, 9, 36–42. DOI 10.4314/ajtcam.v9i1.5. [Google Scholar] [CrossRef]
25. Sathiyaseelan, A., Saravanakumar, K., Mariadoss, A. V. A., Wang, M. H. (2020). Biocompatible fungal chitosan encapsulated phytogenic silver nanoparticles enhanced antidiabetic, antioxidant and antibacterial activity. International Journal of Biological Macromolecules, 153, 63–71. DOI 10.1016/j.ijbiomac.2020.02.291. [Google Scholar] [CrossRef]
26. Niki, E. (2010). Assessment of antioxidant capacity in vitro and in vivo. Free Radical Biology and Medicine, 49, 503–515. DOI 10.1016/j.freeradbiomed.2010.04.016. [Google Scholar] [CrossRef]
27. Mirzadeh, M., Arianejad, M. R., Khedmat, L. (2020). Antioxidant, antiradical, and antimicrobial activities of polysaccharides obtained by microwave-assisted extraction method: A review. Carbohydrate Polymers, 229, 115421. DOI 10.1016/j.carbpol.2019.115421. [Google Scholar] [CrossRef]
28. PenaMoran, A. O., Villarreal, M. L., Alvarez-Berber, L., Meneses-Acosta, A., Rodriguez-López, V. (2016). Cytotoxicity, post-treatment recovery, and selectivity analysis of naturally occurring podophyllotoxins from Bursera fagaroides var. fagaroides on Breast Cancer Cell Lines. Molecules, 21, 1013. DOI 10.3390/molecules21081013. [Google Scholar] [CrossRef]
29. Calderon-Montaño, J. M., Martinez-Sanchez, S. M., Burgos-Moron, E., Guillen-Mancina, E., Jimenez-Alonso, J. J. et al. (2018). Screening for selective anticancer activity of plants from grazalema natural park. Spain Natural Product Research, 23, 1–5. DOI 10.20944/preprints201802.0091.v1. [Google Scholar] [CrossRef]
30. Mariadoss, A. V. A., Ramachandran, V., Shalini, V., Agilan, B., Franklin, J. H. et al. (2019). Green synthesis, characterization and antibacterial activity of silver nanoparticles by Malus domestica and its cytotoxic effect on (MCF-7) cell line. Microbial Pathogenesis, 135, 103609. DOI 10.1016/j.micpath.2019.103609. [Google Scholar] [CrossRef]
31. Atale, N., Gupta, S., Yadav, U. C. S., Rani, V. (2014). Cell-death assessment by fluorescent and non-fluorescent cytosolic and nuclear staining techniques. Journal of Microscopy, 255, 7–19. DOI 10.1111/jmi.12133. [Google Scholar] [CrossRef]
32. Saravanakumar, K., Mariadoss, A. V. A., Sathiyaseelan, A., Venkatachalam, K., Hu, X. et al. (2021a). pH-sensitive release of fungal metabolites from chitosan nanoparticles for effective cytotoxicity in prostate cancer (PC3) cells. Process Biochemistry, 102, 165–172. DOI 10.1016/j.procbio.2020.12.005. [Google Scholar] [CrossRef]
33. Bao, X. Z., Wang, Q., Ren, X. R., Dai, F., Zhou, B. (2020). A hydrogen peroxide-activated Cu(II) pro-ionophore strategy for modifying naphthazarin as a promising anti-cancer agent with high selectivity for generating ROS in HepG2 cells over in L02 cells. Free Radical Biology and Medicine, 152, 597–608. DOI 10.1016/j.freeradbiomed.2019.12.001. [Google Scholar] [CrossRef]
34. Sofowora, A., Ogunbodede, E., Onayade, A. (2013). The role and place of medicinal plants in the strategies for disease prevention. African Journal of Traditional, Complementary, and Alternative Medicines, 10, 210–229. DOI 10.4314/ajtcam.v10i5.2. [Google Scholar] [CrossRef]
35. Salta, J., Martins, A., Santos, R. G., Neng, N. R., Nogueira, J. M. F. et al. (2010). Phenolic composition and antioxidant activity of Rocha pear and other pear cultivars–A comparative study. Journal of Functional Foods, 2, 153–157. DOI 10.1016/j.jff.2010.02.002. [Google Scholar] [CrossRef]
36. Eo, H. J., Park, G. H., Kim, D. S., Kang, Y., Park, Y. (2020). Antioxidant and anticancer activities of leaves extracts from acer tegmentosum. Korean Journal of Plant Resources, 33, 551–557. DOI 10.7732/kjpr.2020.33.6.551. [Google Scholar] [CrossRef]
37. Feduraev, P., Chupakhina, G., Maslennikov, P., Tacenko, N., Skrypnik, L. (2019). Variation in phenolic compounds content and antioxidant activity of different plant organs from Rumex crispus L. and Rumex obtusifolius L. at different growth stages. Antioxidants, 8, 237. DOI 10.3390/antiox8070237. [Google Scholar] [CrossRef]
38. Nita, M., Grzybowski, A. (2016). The role of the reactive oxygen species and oxidative stress in the pathomechanism of the age-related ocular diseases and other pathologies of the anterior and posterior eye segments in adults. Oxidative Medicine and Cellular Longevity, 2016, 3164734. DOI 10.1155/2016/3164734. [Google Scholar] [CrossRef]
39. Pfeffer, C. M., Singh, A. T. K. (2018). Apoptosis: A target for anticancer therapy. International Journal of Molecular Sciences, 19, 448. DOI 10.3390/ijms19020448. [Google Scholar] [CrossRef]
40. Stefanovic, V., Andersson, S., Vento, M. (2019). Oxidative stress-Related spontaneous preterm delivery challenges in causality determination, prevention and novel strategies in reduction of the sequelae. Free Radical Biology and Medicine, 142, 52–60. DOI 10.1016/j.freeradbiomed.2019.06.008. [Google Scholar] [CrossRef]
41. Zhao, Y., Guo, C., Wang, L., Wang, S., Li, X. et al. (2017). A novel fluorinated thiosemicarbazone derivative-2-(3, 4-difluorobenzylidene) hydrazinecarbothioamide induces apoptosis in human A549 lung cancer cells via ROS-mediated mitochondria-dependent pathway. Biochemical and Biophysical Research Communications, 491, 65–71. DOI 10.1016/j.bbrc.2017.07.042. [Google Scholar] [CrossRef]
42. Ehigie, A. F., Wei, P., Wei, T., Yan, X., Olorunsogo, O. O. et al. (2021). Momordica charantia L. induces non-apoptotic cell death in human MDA-MB-436 breast and A549 lung cancer cells by disrupting energy metabolism and exacerbating reactive oxygen species generation. Journal of Ethnopharmacology, 277, 114036. DOI 10.1016/j.jep.2021.114036. [Google Scholar] [CrossRef]
43. Silvestri, S., Cirilli, I., Marcheggiani, F., Dludla, P., Lupidi, G. et al. (2021). Evaluation of anticancer role of a novel ruthenium (II)-based compound compared with NAMI-A and cisplatin in impairing mitochondrial functionality and promoting oxidative stress in triple negative breast cancer models. Mitochondrion, 56, 25–34. DOI 10.1016/j.mito.2020.11.004. [Google Scholar] [CrossRef]
44. Mondal, A., Bennett, L. L. (2016). Resveratrol enhances the efficacy of sorafenib mediated apoptosis in human breast cancer MCF7 cells through ROS, cell cycle inhibition, caspase 3 and PARP cleavage. Biomedicine & Pharmacotherapy, 84, 1906–1914. DOI 10.1016/j.biopha.2016.10.096. [Google Scholar] [CrossRef]
45. Gao, X., Li, X., Ho, C. T., Lin, X., Zhang, Y. et al. (2020). Cocoa tea (Camellia ptilophylla) induces mitochondria-dependent apoptosis in HCT116 cells via ROS generation and PI3K/Akt signaling pathway. Food Research International, 129, 108854. DOI 10.1016/j.foodres.2019.108854. [Google Scholar] [CrossRef]
46. Qian, C., Wang, J. Q., Song, C. L., Wang, L. L., Ji, L. N. et al. (2013). The induction of mitochondria-mediated apoptosis in cancer cells by ruthenium (II) asymmetric complexes. Metallomics, 5, 844–854. DOI 10.1039/C3MT20270D. [Google Scholar] [CrossRef]
47. Bock, F. J., Tait, S. W. G. (2020). Mitochondria as multifaceted regulators of cell death. Nature Reviews Molecular Cell Biology, 21, 85–100. DOI 10.1038/s41580-019-0173-8. [Google Scholar] [CrossRef]
48. Yuan, C., Wang, M. H., Wang, F., Chen, P. Y., Ke, X. G. et al. (2021). Network pharmacology and molecular docking reveal the mechanism of Scopoletin against non-small cell lung cancer. Life Sciences, 270, 119105. DOI 10.1016/j.lfs.2021.119105. [Google Scholar] [CrossRef]
49. Zhang, Y., Zhao, H., Di, Y., Li, Q., Shao, D. et al. (2018). Antitumor activity of Pinoresinol in vitro: Inducing apoptosis and inhibiting HepG2 invasion. Journal of Functional Foods, 45, 206–214. DOI 10.1016/j.jff.2018.04.009. [Google Scholar] [CrossRef]
50. Yang, J., Fa, J., Li, B. (2017). Apoptosis induction of epifriedelinol on human cervical cancer cell line. African Journal of Traditional, Complementary and Alternative Medicines, 14, 80–86. DOI 10.21010/ajtcam.v14i4.10. [Google Scholar] [CrossRef]
51. Wang, Y., Liu, M., Chen, S., Wu, Q. (2020). Avicularin inhibits cell proliferation and induces cell apoptosis in cutaneous squamous cell carcinoma. Experimental and Therapeutic Medicine, 19, 1065–1071. DOI 10.3892/etm.2019.8303. [Google Scholar] [CrossRef]
52. Sharifi, S., Mostafavi, P. G., Tarasi, R., Moradi, A. M., Givianrad, M. H. et al. (2020). Purified compounds from marine organism sea pen induce apoptosis in human breast cancer cell MDA-MB-231 and cervical cancer cell Hela. European Journal of Pharmacology, 877, 173075. DOI 10.1016/j.ejphar.2020.173075. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |