
 | Oncologie |  |
DOI: 10.32604/Oncologie.2021.017267
ARTICLE
Integrated Network Pharmacology and Molecular Docking to Reveal the Mechanism of Tetrandrine in Tumor Chemoresistance
1Department of Traditional Chinese Medicine, The First Affiliated Hospital of Jinan University, Guangzhou, 510630, China
2College of Traditional Chinese Medicine of Jinan University, Guangzhou, 510632, China
3Luogang Street Community Health Service Center, Guangzhou, 510525, China
4Department of Oncology, The First Affiliated Hospital of Jinan University, Guangzhou, 510630, China
*Corresponding Authors: Ling Jin. Email: hljjinling@163.com; Meng Xu. Email: xumengjinan@yahoo.com
#These authors contributed equally to this work
Received: 27 April 2021; Accepted: 28 June 2021
Abstract: Tetrandrine has a variety of anti-tumor effects including against or reversal of tumor chemoresistance, but its mechanism of against tumor chemoresistance is still unclear. Therefore, the analytical method of network pharmacology and molecular docking was used to investigate the mechanism by which tetrandrine acts in tumor chemoresistance. We used public databases (PubChem, SwissADEM, SwissTargetPrediction) to obtain the physicochemical information and targets of tetrandrine, and used gene databases (GeneCards and OMIM) to collected disease targets, respectively. The intersection targets of disease and drug were analyzed by RStudio. We built protein-protein interaction network through the STRING database, and used Cystoscope to screen out hub genes. GO and KEGG pathway enrichment analysis were analyzed by Metascape database and RStudio. “Component-target-pathway” network was erected by Cystoscope. Ultimately, the key targets were chosen to dock with tetrandrine via molecular docking to verify network analysis results. 29 common targets were screened out through intersection. AKT1, PIK3CA, PIK3CB, PIK3CG, JAK2, IGF1R, KDR, SRC and MTOR were the core targets. KEGG pathway enrichment mainly included PI3K-AKT signaling pathway, EGFR tyrosine kinase inhibitor resistance, and Rap1 signaling pathway. Molecular docking indicated that the configuration of protein binding of ligand is stable. In conclusion, the against tumor chemoresistance effect of tetrandrine has the characteristics of multiple targets and multiple pathways, and the prediction of network pharmacology and molecular docking indicated that MTOR, SRC, PIK3CA were the key targets of tetrandrine in tumor chemoresistance, which provides a scientific basis for subsequent research on its anti-tumor chemoresistance mechanism.
Keywords: Network pharmacology; molecular docking; tetrandrine; tumor chemoresistance; mechanism
Tetrandrine, a bisbenzylisoquinoline alkaloidis, is the main active ingredient of Chinese herb medicine called Fang-ji (Stephaniae Tetrandrae Radix), exhibits a variety of anti-tumor activity including against or reversal of tumor chemoresistance. It has been reported that tetrandrine possessed the role as kinase-inhibitor, reversal of drug resistance, inhibition of angiogenesis, inducer of autophagy and caspase pathways in different cancers [1].
Chemotherapy is the main strategy for cancer treatment, but drug resistance is the reason of treatment failure and leads to tumor recurrence. So the exact clarification of the molecular mechanism of tumor chemoresistance and its reversal has always been the key research goal of cancer [2]. It is known that tetrandrine exhibits low toxicity and is safe, showing great potential to enhance chemotherapeutic efficacy [3]. Several recent studies have indicated that tetrandrine can exert anti-MDR activity in Hep-2/v cells [4], act as a TRAIL-sensitizing agent in prostate cancer [5], and tetrandrine could suppress proliferation and induce autophagy in MDA-MB-231 cell by inhibiting the PI3K/AKT/mTOR pathway [6]. Other studies have demonstrated that the combination of tetrandrine and H89 exhibited an enhanced therapeutic effect [7], the combinational therapy using tetrandrine and other anticancer drugs could promote the treatment efficiency of drugs that are substrates of ABCB1 [8]. As an inhibitor of tumor vascular growth, tetrandrine was verified to have antiangiogenic effects in vivo in a liver cancer nude mice xenograft model [9]. Our study found that tetrandrine can reverse cisplatin resistance in non-small cell lung cancer by mediating the PI3K/AKT/mTOR signaling pathway in vivo [10]. However, its mechanism of against tumor chemoresistance is still indefinite.
Network pharmacology, an appropriate approach for modern Traditional Chinese Medicine research, is a brand-new method that uses bioinformatics which contains systems biology, connectivity, network analysis and pleiotropy to predict and discern multiple drug targets and interplay in disease, and network pharmacology will provide more and more meaningful information for drug discovery development [11]. Molecular docking is to predict the main binding mode(s) of a ligand with a protein, and can be utilized to carry out virtual screening on large compounds, rank the result, and put forward structural hypotheses of how the ligand inhibit the target, which is beneficial to test the mechanism of compound [12]. In this research, we aimed to inquire into the mechanism of tetrandrine for anti-tumor chemoresistance by network pharmacology and molecular docking technique.
2.1 Physcochemical Information Collection of Tetrandrine
PubChem database (https://PubChem.ncbi.nlm.nih.gov) provides components’ normal name, PubChem CID, 2D and 3D structure, Canonical SMILES and other information for rectifying the identity of components. SwissADME database (http://swissadme.ch/), a free and simple web tool to assess drug-likeness, pharmacokinetics and medicinal chemistry friendliness of small molecules. we used PubChem database to obtain the 2D structure and Canonical SMILES of tetrandrine, used SwissADME to analyze the other physicochemical information of tetrandrine.
2.2 Target Prediction of Tetrandrine
SwissTargetPrediction (http://www.swisstargetprediction.ch/), a database provides a very intuitive interface to predict small molecule protein targets, and the prediction is established on a combination of 3D and 2D similarity with a library of 370,000 known actives on over 3000 proteins from three different species. Tetrandrine’s 2D structure was imported (limit species to humans) into SwissTargetPrediction to predict the targets.
GeneCards, the human gene database (https://www.genecards.org/), contains exhaustive information about all annotated and predicted human genes. OMIM (https://www.omim.org/) provides information on all known mendelian disorders and over 15,000 genes. Using keywords “tumor/cancer/carcinoma chemoresistance, multidrug resistance” to search for disease related genes by both two databases.
2.4 Candidate Targets Collection
Screening candidate targets related to tetrandrine and disease through RStudio software (version 3.6.3), which offers a wide variety of graphical and statistical techniques. This software is usually the tool of choice for bioinformatics analysis and statistical methods research.
2.5 Component-Disease Target Network
The tetrandrine-disease target network was made by Cytoscape software (version 3.8.2), which is an information data editing and analysis software for designing, constructing, and drawing grids.
2.6 Protein–Protein Interaction Network Construction
The STRING database (https://www.string-db.org/) currently covers 2.4 billion proteins from more than 5 thousand organisms, which is a database of known and predicted protein-protein interactions. We imported common targets into the STRING database and screened human targets with a confidence score >0.4. The TSV format of the Protein-protein interaction network was downloaded. Then screened core targets and subnets by cytoNCA, a plug-in of Cytoscape software, and the degree value, betweenness centrality, closeness centrality was applied for filtering major hub genes. we assessed the tetrandrine PPI network in antitumor chemoresistance.
2.7 Gene Ontology Enrichment and KEGG Pathway Enrichment Analyses
Metascape database (https://metascape.org/) is a powerful gene function annotation analysis tool. We copied and pasted the common genes into the Metascape’s gene list, selected the species as “H. sapiens”. Then in the Enrichment section of the analysis page, we selected KEGG Pathways, GO Biological Processes (BP), GO Cellular Components (CC), and GO Molecular Functions (MF) for enrichment analysis of KEGG Pathways, GO BP, GO CC and GO MF, respectively. Lastly, applying R software to carry out the GO enrichment analysis with the results of top 10 items and KEGG pathway enrichment analysis with the results of top 20 items, drawing into relevant histogram graphs and bubble plots. In the programming language, q value Cutoff = 0.05 was set.
The molecular docking approach was utilized to validate the association of tetrandrine and hub target gene. Importing the 2D structure of tetrandrine to Chemoffice, a chemical drawing tool, and converting it into 3D structure as mol2 format. The three core targets protein crystal structure of MTOR (ID: P42345), SRC (ID: P12931) and PIK3CA (ID: P42336) were downloaded as pdb file from the RCSB PDB database (https://www.rcsb.org/), a protein database, which offers archive-information about the 3D shapes of proteins, nucleic acids, and complex assemblies. We used visual bioinformatics tools AutoDockTools (version 1.5.6) to transform pdb files to pdbqt formats for molecular docking. The conformations of tetrandrine and the key target protein were visualized by PyMol software (version 2.4.1). Running all docking simulations with default settings and presenting with publication. The docking conformation that has docking affinity score –5.0 kcal/mol represents great binding interactions between the compound and its corresponding targets, and the lower the binding energy is, the better the ligand can bind to the protein.
3.1 Physcochemical Information about Tetrandrine
Tetrandrine’s PubChem CID, Canonical SMILES (Tab. 1) and 2D structure (Fig. 1) were obtained by the PubChem database. In accordance with SwissADME, the related parameters of physicochemical properties showed that tetrandrine has a favorable pharmacokinetic profile. The results in Tab. 1 revealed that tetrandrine has high gastrointestinal (GI) absorption due to its better flexibility and polarity and not good oral bioavailability for its poor soluble. The five cytochrome (CYP) subtypes play a key and core role in drug excretion, and tetrandrine inhibited none of them. And the medical chemistry and drug likeness further confirmed the probability of tetrandrine as a drug.
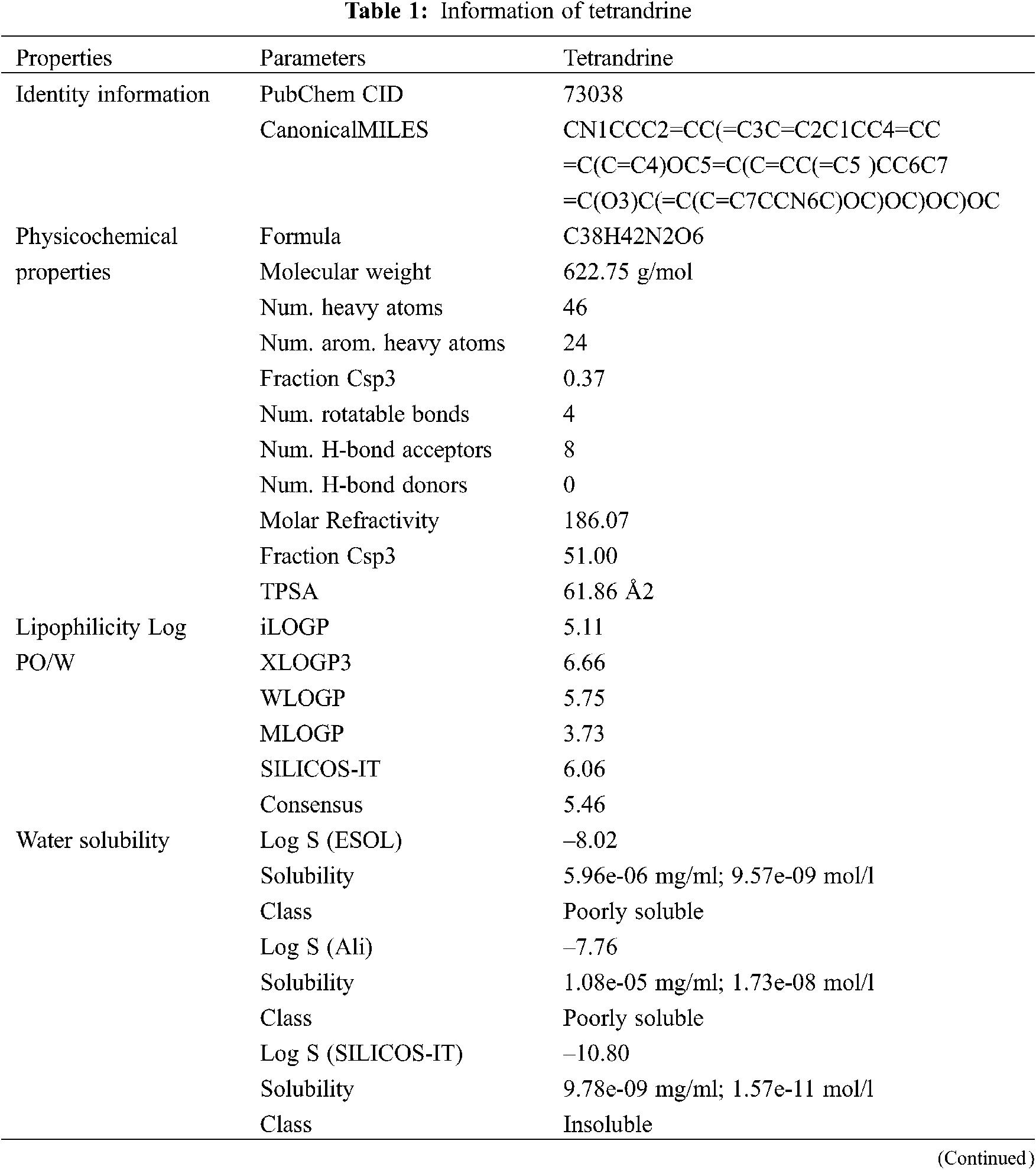
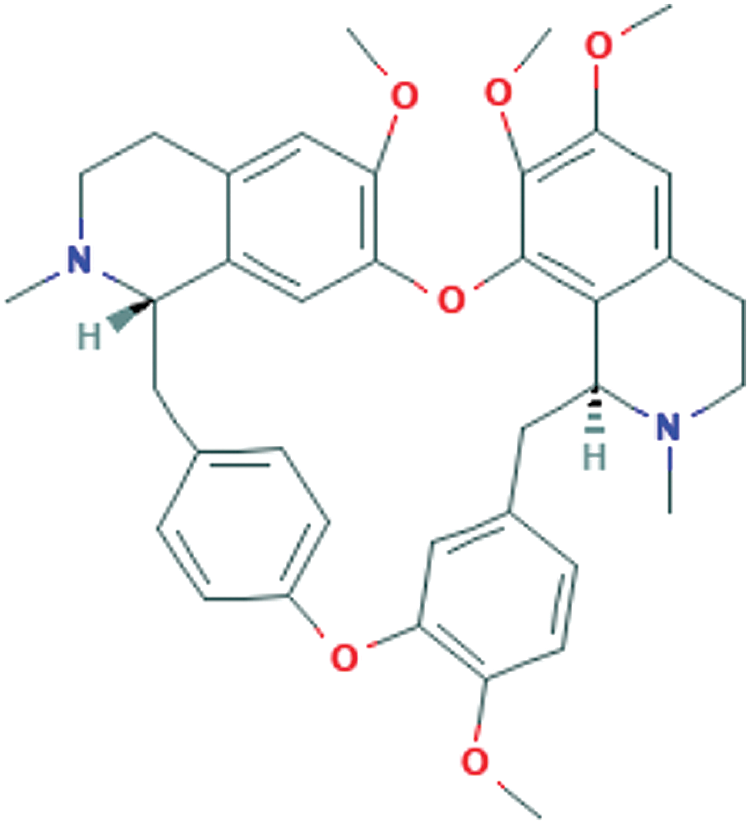
Figure 1: Tetrandrine’s 2D structure
3.2 Screening of Common Targets
A total 98 drug genes were screened by the SwissTargetPrediction database. We finally obtained 753 disease related tumor chemoresistance targets after deleting duplicates from GeneCards and OMIM databases. We used the RStudio to read and obtain 29 common targets of tetrandrine and disease, drawing the Venn diagram (Fig. 2). We used Cytoscape to make a network diagram of common targets for tetrandrine and disease (Fig. 3).
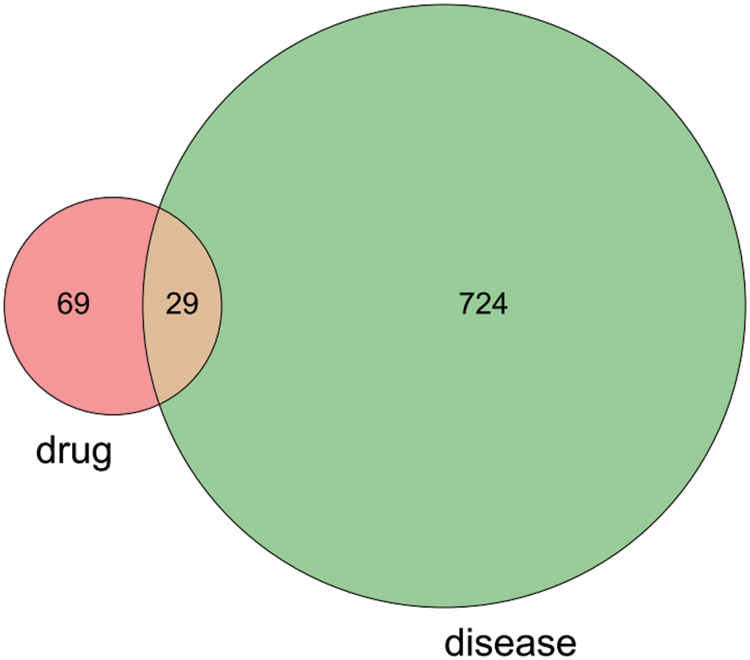
Figure 2: Venn diagram of tetrandrine and disease
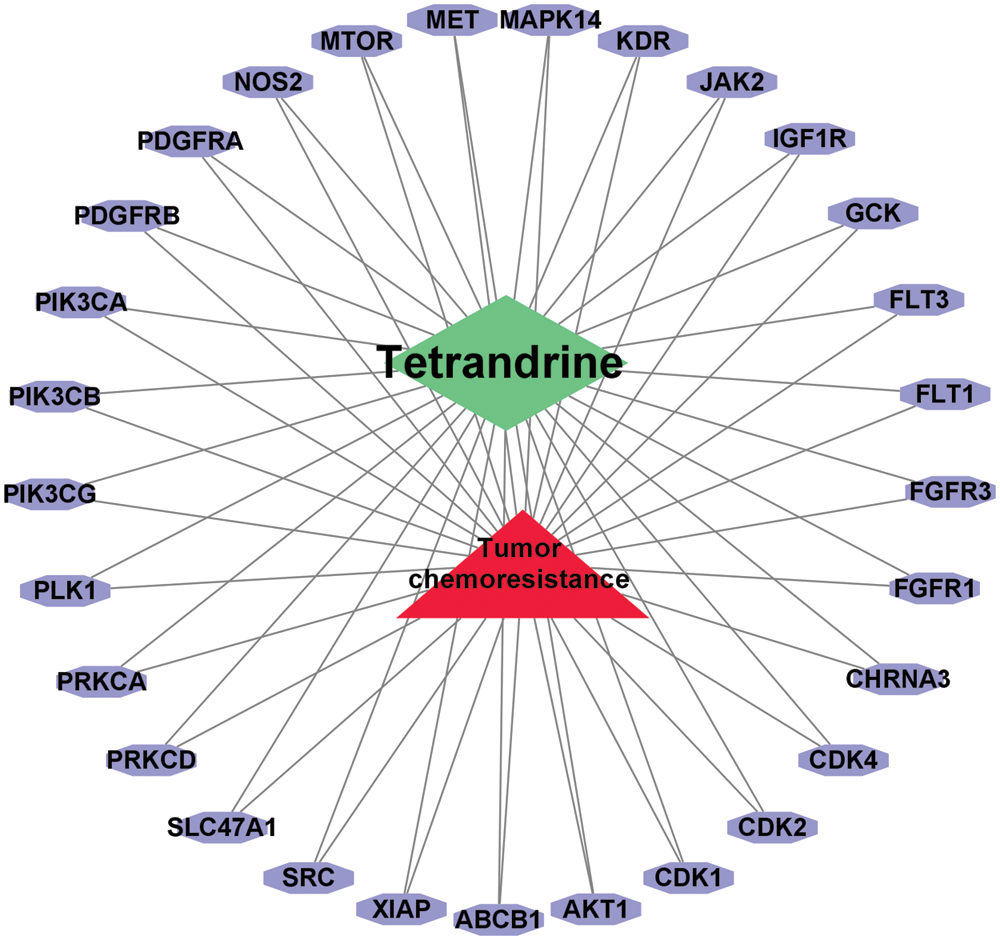
Figure 3: Common targets of tetrandrine and disease
3.3 PPI Network Construction and Analysis
In order to explain the therapeutic mechanism of the drug more comprehensively, we input the common targets to STRING to conduct a PPI network (Fig. 4). Then, according to the exported TSV format, we used RStudio to make a histogram according to the degree (Fig. 5). At last, we screened core targets and subnetwork by Cytoscape according to the degree value, betweenness centrality and closeness centrality (Fig. 6). 9 core genes including AKT1, PIK3CA, PIK3CB, PIK3CG, JAK2, IGF1R, KDR, SRC and MTOR were screened out, among which the most important were MTOR, SRC and PIK3CA, and the darker the color, the more important the gene.
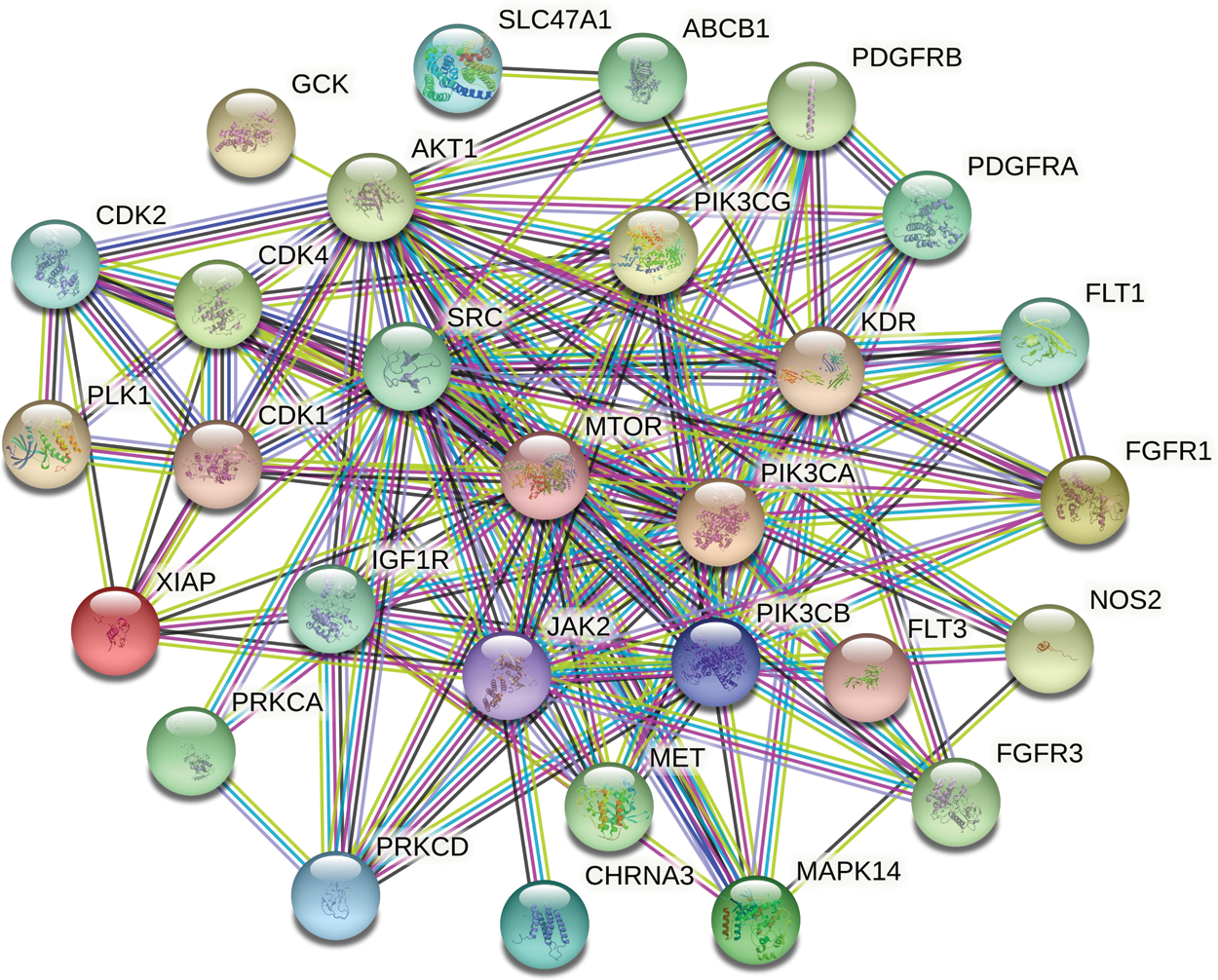
Figure 4: Protein–Protein interaction (PPI) network
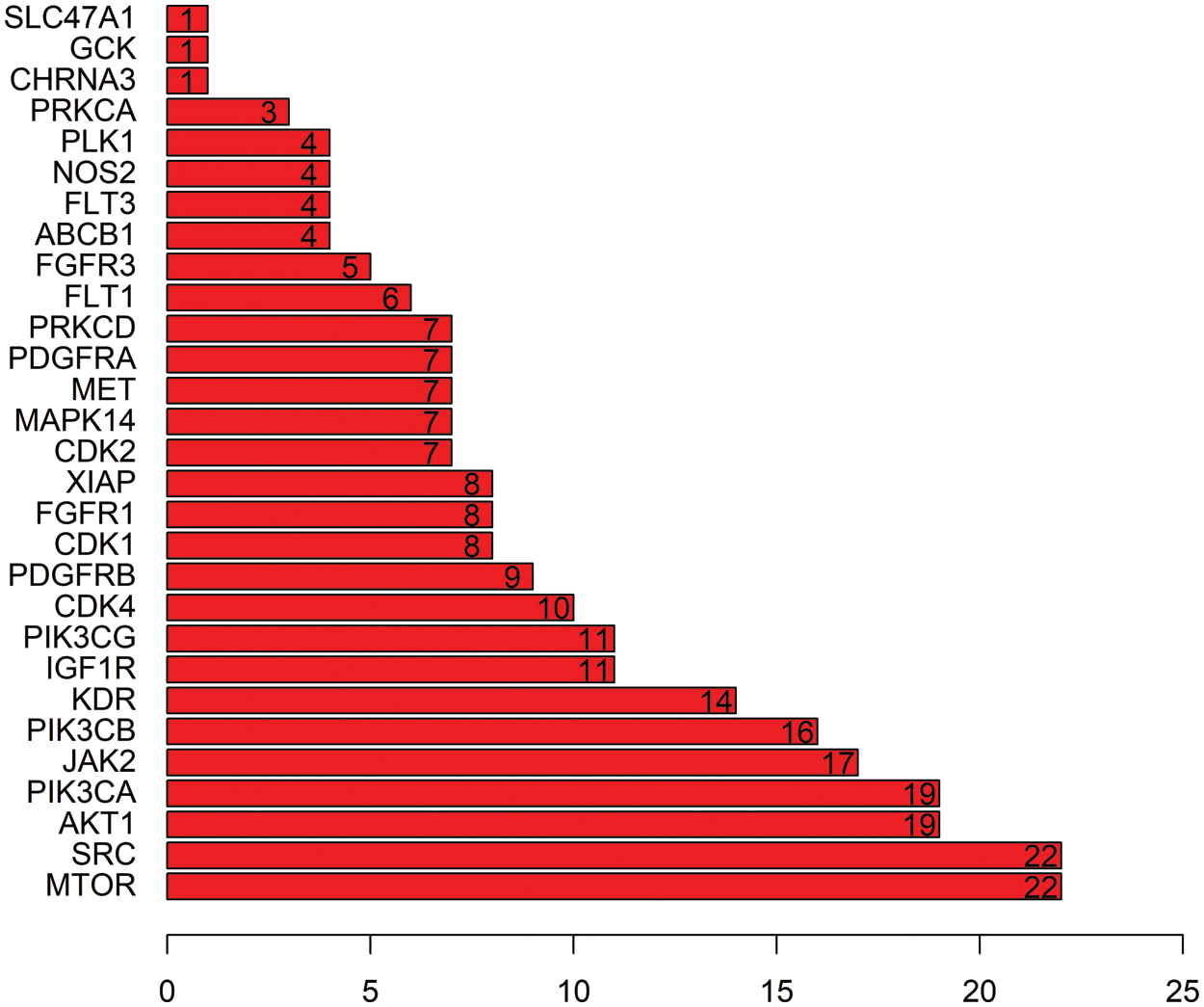
Figure 5: Histogram of common genes
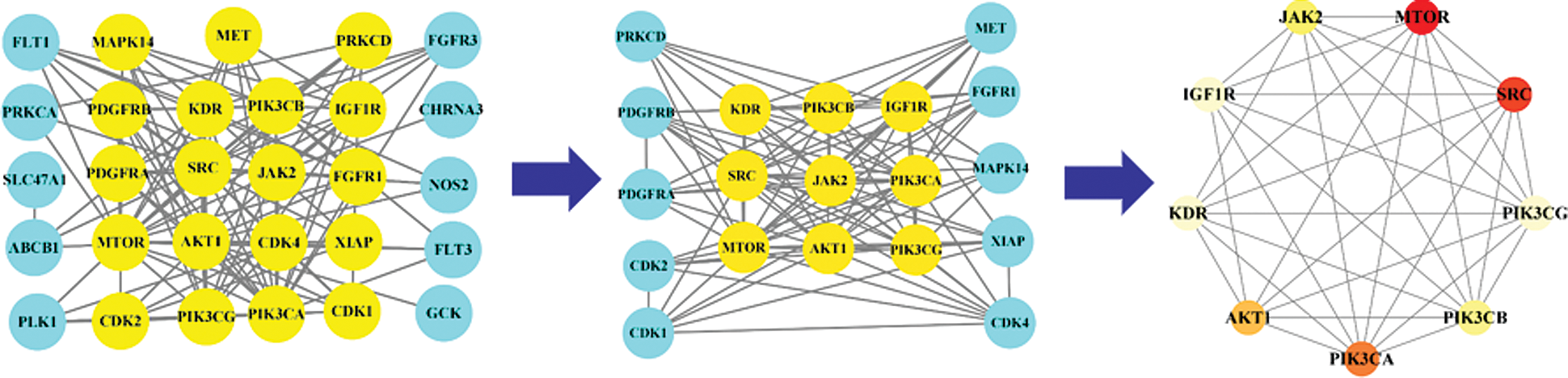
Figure 6: Subnetwork and core genes
3.4 GO Functional and KEGG Pathway Enrichment Analyses
Applying the Metascape database, we did GO functional and KEGG pathway enrichment analysis of intersection targets. For GO functional analyses, we got 776 related items, including 702 biologic processes, 37 cellular component and 37 molecular function. As showed in Fig. 7, the top 3 items of biological processes mainly involving phosphatidylinositol 3-kinase signaling, phosphatidylinositol-mediated signaling, and inositol lipid-mediated signaling. Cellular components analysis revealed transferase complex, transferring phosphorus-containing groups, phosphatidylinositol 3-kinase signaling, protein kinase complex etc. For molecular functions, the targets were enriched in protein tyrosine kinase activity, protein serine/threonine kinase activity, transmembrane receptor protein tyrosine kinase activity etc. The KEGG signal pathway enrichment analysis contained 121 pathways, the top 20 pathways are showed in Fig. 8, mainly including PI3K-AKT signaling pathway, EGFR tyrosine kinase inhibitor resistance, and Rap1 signaling pathway. Lastly, we constructed “component-target-pathway” (C-T-P) network (Fig. 9) by Cytoscape merge tool.
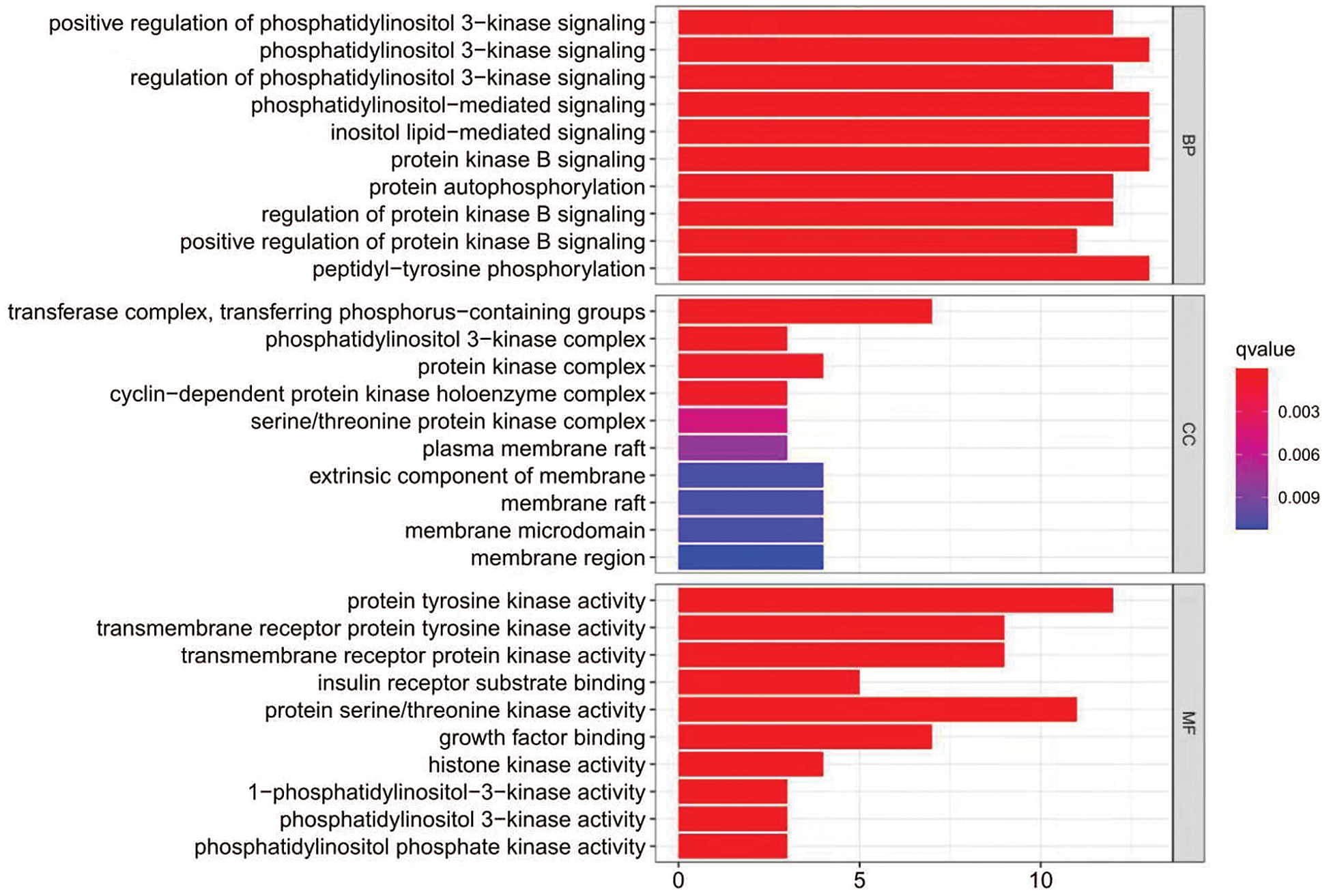
Figure 7: The top 10 pathways for GO enrichment analysis of common targets
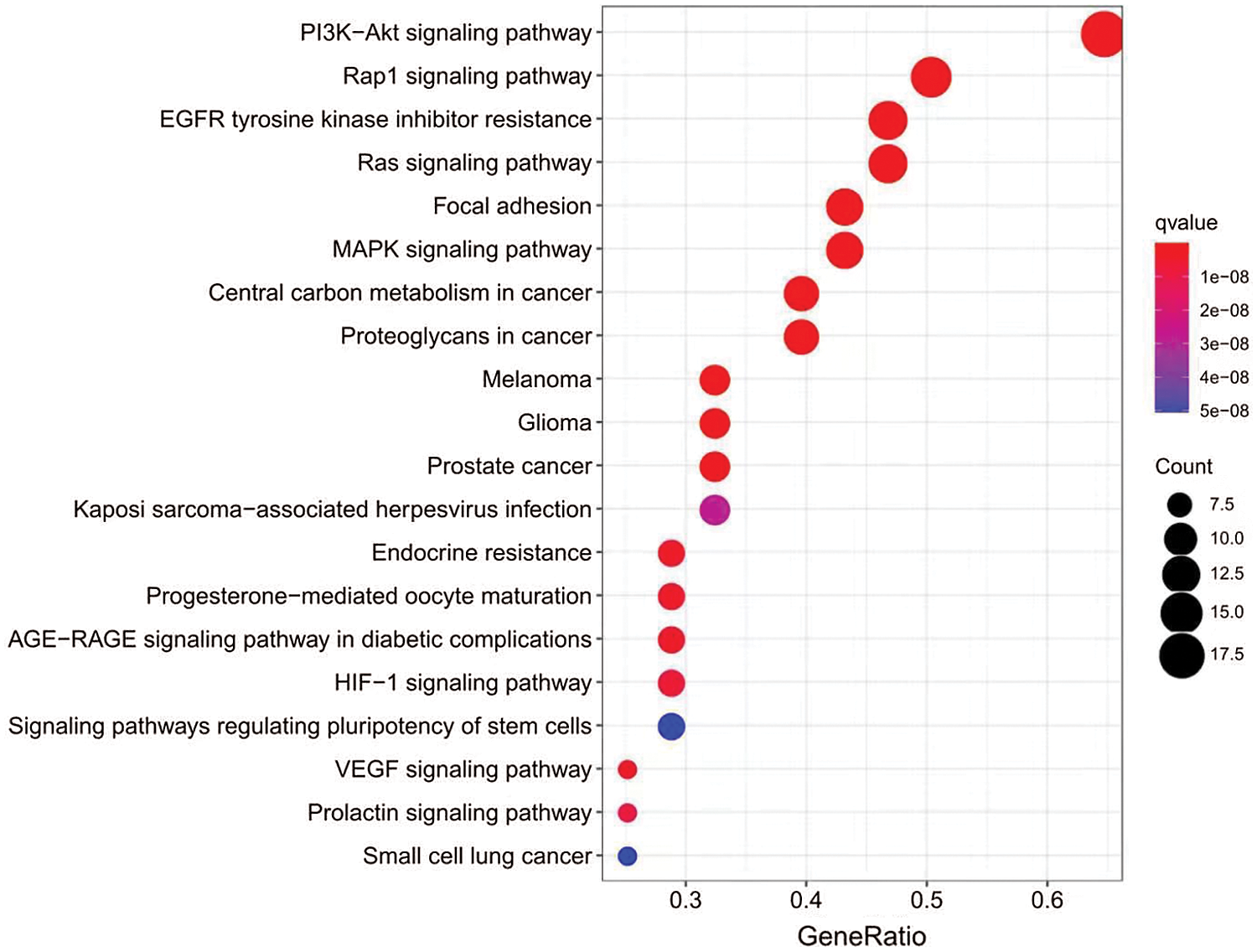
Figure 8: The top 20 pathways for KEGG enrichment analysis of common targets
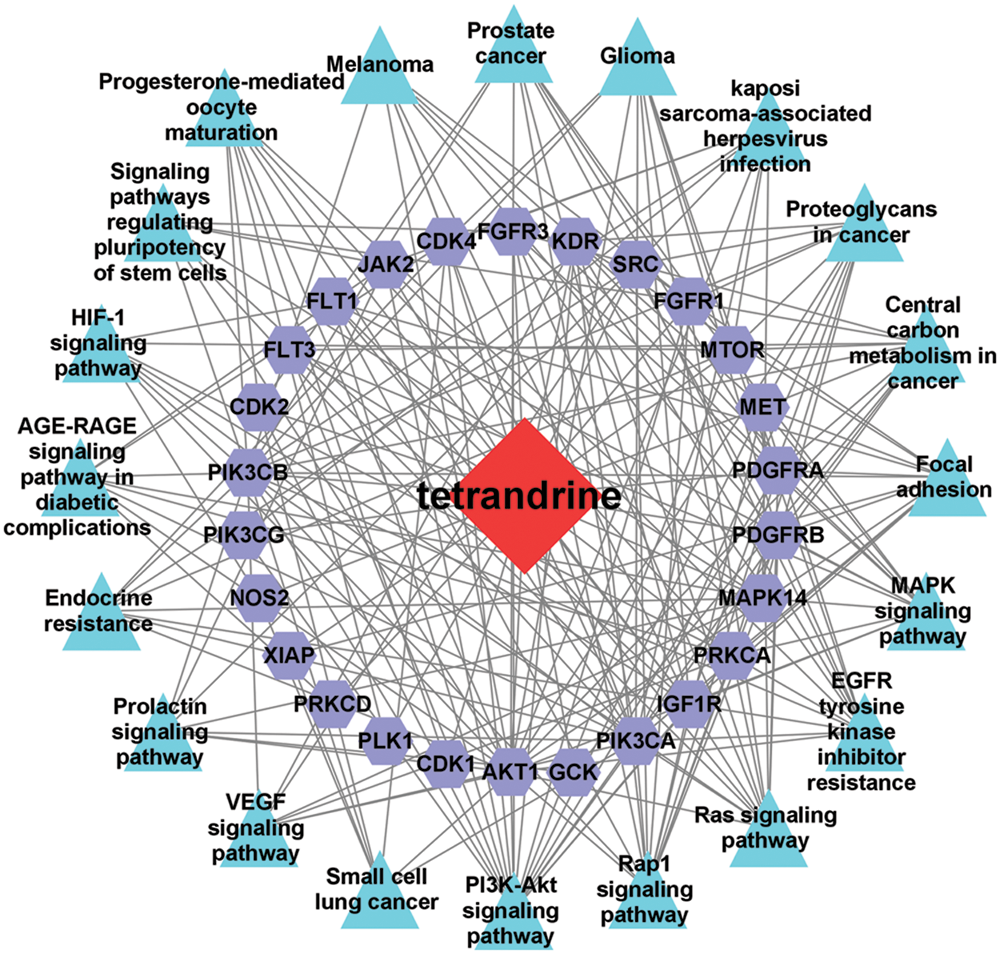
Figure 9: Component-target-pathway (C-T-P) network
Molecular docking simulation was used to test and verify the mechanism of tetrandrine against tumor chemoresistance. we mainly concentrated our docking analysis on the three corn proteins Serine/threonine-protein kinase (MTOR), Proto-oncogene tyrosine-protein kinase (SRC) and Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (PIK3CA). As showed in Fig. 10, the binding energy of MTOR, SRC and PIK3AC with tetrandrine were small, with their affinity croe of –8.6, –9.3, –10.2 kcal/mol, respectively, which pointed out that the conformation of protein binding of tetrandrine are stable.
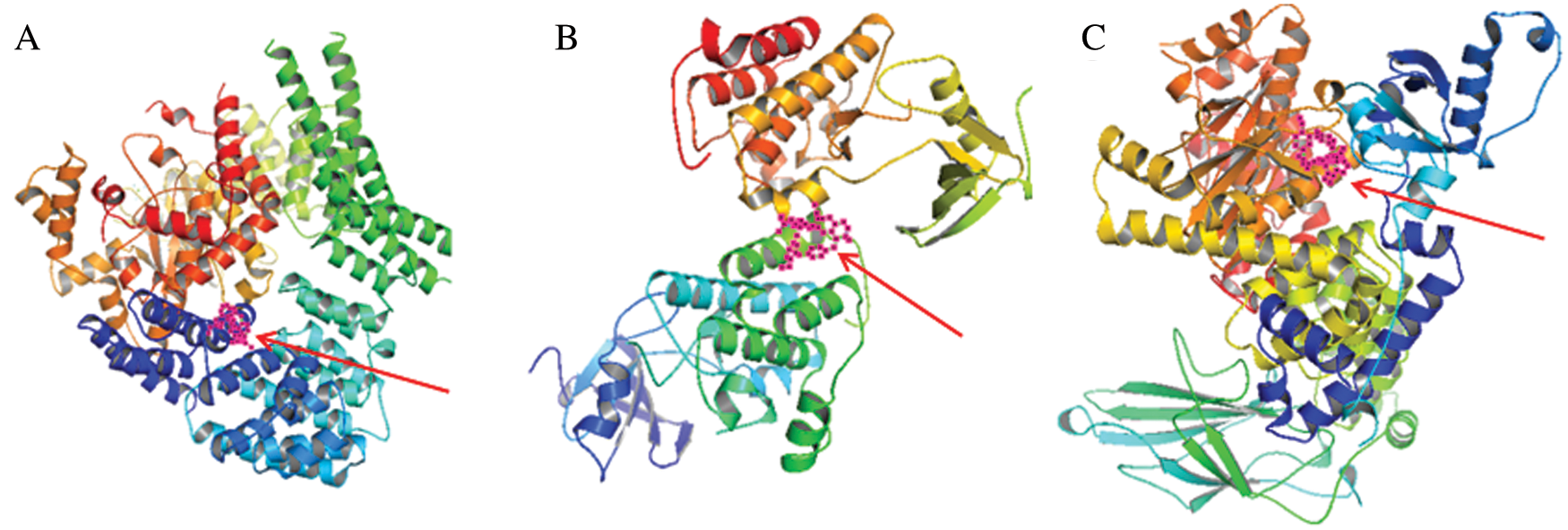
Figure 10: The docking pose of (A) MTOR, (B) SRC, and (C) PIK3AC with tetrandrine (pink with black dots in the middle indicated by red arrow), their affinity croe was –8.6, –9.3, –10.2 kcal/mol, respectively
Tumor chemoresistance is an important factor for tumor treatment failure. Recently, the multi-target and multi-channel treatment of tumor by Traditional Chinese herb has received a lot of attention. Tetrandrine is a natural medicine with a variety of biological activities, and its anti-tumor activity is particularly prominent. A series of studies have shown that tetrandrine has obvious anti-different tumors function in vivo and vitro [13–17]. Tetrandrine can also reverse tumor chemoresistance through different pathways [18,19]. Tetrandrine and similar molecules could inhibit the proliferation of HepG2 cells via inducing apoptosis [20]. Tetrandrine is a radiosensitizer and also a multidrug resistance reversing agent. It helps in reversal of multidrug resistance without changing the pharmacokinetic parameters, which is an additional advantage of tetrandrine compared to other chemotherapy drugs [1]. but its specific mechanism still needs to be revealed. Thence, network pharmacology and molecular docking may be useful to dig the potential mechanism of tetrandrine against tumor chemoresistance.
As the results of physicochemical information about tetrandrine and network pharmacology showed, tetrandrine has a favorable pharmacokinetic profile, and the core genes of tetrandrine in anti-tumor chemoresistance included AKT1, PIK3CA, PIK3CB, PIK3CG, JAK2, IGF1R, KDR, SRC and MTOR, among which the top three were MTOR, SRC and PIK3CA.
Mammalian target of rapamycin (mTOR), a serine/threonine protein kinase in the downstream of the phosphatidylinositol 3-kinases (PI3K) family, indirectly or directly regulates the phosphorylation of more than 800 proteins, which adjusts the maintenance of cellular homeostasis by coordinating transcription, metabolism, translation, and autophagy with availability of amino acids, ATP, growth factors and oxygenis, uncontrolled activation of the mTOR is discovered in cells of the majority tumors [21,22], so this kinase plays a great role in the development and progression of different carcinoma [23,24]. The PIK3 family includes three categories, PIK3CA, PIK3CB and PIK3CG. These three categories are the core genes of tetrandrine against tumor chemoresistance. The catalytic subunit p110 alpha of phosphatidylinositol 3’-kinase (PI3K), called alpha isoform of phosphatidylinositol 3 kinase (PIK3CA), is the most frequently mutated oncogene in human cancers such as colon, breast and endometrial cancers [25]. Both mTOR and PIK3CA are belong to PI3K/AKT/mTOR signaling pathway, which has become a target of tumor chemoresistance or tumor multidrug resistance [26]. Proto-oncogene tyrosine-protein kinase (Src) is the first cancer-causing oncogene, which was identified and discovered by Harold Varmus et al. in 1976. Src kinase also plays a prominent role in promoting tumor invasion, progression, metastasis and chemical resistance [27–29]. Src mediates multiple signal pathways, and it is also the central hub of various signal pathways such as MAPK/ERK, PI3K/AKT, etc.
Currently, several PI3K/Akt/mTOR pathway inhibitors and Src inhibitors have been developed and approved for the treatment of carcinomas. Studies have found that tetrandrine can play an anti-drug resistance effect by regulating the PI3K/Akt/mTOR signaling pathway as an autophagy agonist [30–32]. It was reported that tetrandrine can probably be combined with radiotherapy or other chemotherapeutic agents by reducing MAPK signaling associated proteins to treat glioma [33,34].
The result of KEGG functional enrichment analysis showed that the against tumor chemoresistance mechanism of tetrandrine mainly involve PI3K-AKT signaling pathway, EGFR tyrosine kinase inhibitor resistance, and Rap1 signaling pathway. This result in turn verifies our previous research findings that tetrandrine can reverse cisplatin resistance in non-small cell lung cancer by regulating the PI3K/AKT/ mTOR signaling pathway in vivo [10]. It also reflected the characteristics of tetrandrine’s multi-channel function. Epidermal growth factor receptor (EGFR) belongs to the ErbB family of receptor tyrosine kinases. Activation of EGFR through ligand binding stimulates numerous signals, including MAPKs, PI3K/AKT, and nuclear factor-κB pathways, eventually resulting in playing a great role in the regulation of the cell differentiation, proliferation, migration and survival. Studies have showed that tetrandrine inhibited the phosphorylation of EGFR and its downstream signaling pathways, such as MAPK, PI3K/AKT [35–37]. As is knows, Rap1 is a crucial player in tumor progression such as tumor cell migration, invasion, metastasis and response to chemotherapy [38]. There is no report about tetrandrine acts on the Rap1 signaling pathway.
In this study, the molecular docking technique validated the interactions between tetrandrine and the hub target genes. The interaction between MTOR, SRC, PIK3CA and tetrandrine displayed a strong binding affinity score of –8.6, –9.3, –10.2 kcal/mol, and the molecular docking results revealed that the ligand can bind to the protein well [39]. Through the systematic analysis of the results, it can conclude that tetrandrine has a multi-target and multi-pathway effect in the treatment of tumor chemoresistance. Our study predicted and verified the mechanisms of tetrandrine in anti-tumor chemoresistance. But there still need further experimental confirmations. In summary, tetrandrine has a good application prospect for tumor chemoresistance.
Tetrandrine was proven to have definite anti-tumour activities. However, the bioavailability, safety and pharmacokinetic parameter studies on tetrandrine are very limited in animal models, especially in clinical settings. And more efforts on different pharmacokinetic parameters, potentially involving human subjects, are required before judgment can be passed on the substance as a promising anticancer drug [40].
Integrated the result of network pharmacology and molecular docking, it is indicated that MTOR, SRC, PIK3CA were the key targets of tetrandrine against tumor chemoresistance. And PI3K-AKT signaling pathway, EGFR tyrosine kinase inhibitor resistance and Rap1 signaling pathway were the main mediation pathway for tetrandrine against tumor chemoresistance. In conclusion, our results provide the relevance between tetrandrine and tumor chemoresistance for the first-time using network pharmacology and molecular docking, which serves a new basis for future research on its anti-tumor chemoresistance mechanism.
Funding Statement: This research was financially supported by Project of Administration of Traditional Chinese Medicine of Guangdong Province of China (20182022), Natural Science Foundation of Guangdong Province-the Ph.D. Startup and Vertical Collaboration Project (2018030310186), General Program of the National Natural Science Foundation of China (82074305) and Laboratory Construction of Traditional Chinese Medicine of Guangdong Province of China (89017020).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Bhagya, N., Chandrashekar, K. R. (2018). Tetrandrine and cancer–An overview on the molecular approach. Biomedicine & Pharmacother, 97, 624–632. DOI 10.1016/j.biopha.2017.10.116. [Google Scholar] [CrossRef]
2. Melis, K. Y., Aysun, A. G., Yu, B. (2016). Molecular mechanisms of drug resistance and its reversal in cancer. Critical Reviews in Biotechnology, 36, 716–726. DOI 10.3109/07388551.2015.1015957. [Google Scholar] [CrossRef]
3. Liu, T., Liu, X., Li, W. H. (2016). Tetrandrine, a Chinese plant-derived alkaloid, is a potential candidate for cancer chemotherapy. Oncotarget, 7(26), 40800–40815. DOI 10.18632/oncotarget.8315. [Google Scholar] [CrossRef]
4. Li, Y. C., Li, D. J., Wang, P., Zhu, W., Yin, W. Z. (2020). Tetrandrine partially reverses multidrug resistance of human laryngeal cancer cells. Journal of International Medical Research, 48(8), 1–9. DOI 10.1177/0300060520944706. [Google Scholar] [CrossRef]
5. Shishodia, G., Koul, S., Dong, Q., Koul, H. K. (2018). Tetrandrine (TET) induces death receptors Apo Trail R1 (DR4) and Apo Trail R2 (DR5) and sensitizes prostate cancer cells to trail-induced apoptosis. Molecular Cancer Therapeutics, 17(6), 1217–1228. DOI 10.1158/1535-7163.MCT-17-1157. [Google Scholar] [CrossRef]
6. Guo, Y. B., Pei, X. H. (2019). Tetrandrine-induced autophagy in MDA-MB-231 triple-negative breast cancer cell through the inhibition of PI3K/AKT/mTOR signaling. Evidence-Based Complementary and Alternative Medicine, 2019, 7517431. DOI 10.1155/2019/7517431. [Google Scholar] [CrossRef]
7. Yu, M., Liu, T., Chen, Y. C., Li, Y. F., Li, W. H. (2018). Combination therapy with protein kinase inhibitor H89 and Tetrandrine elicits enhanced synergistic antitumor efficacy. Journal of Experimental & Clinical Cancer Research, 37, 114. DOI 10.1186/s13046-018-0779-2. [Google Scholar] [CrossRef]
8. Liao, D., Zhang, W., Gupta, P., Lei, Z. N., Wang, J. Q. et al. (2019). Tetrandrine interaction with ABCB1 reverses multidrug resistance in cancer cells through competition with anti-cancer drugs followed by downregulation of ABCB1 expression. Molecules, 24, 4383. DOI 10.3390/molecules24234383. [Google Scholar] [CrossRef]
9. Xiao, W. J. Y., Men, Q., Yuan, L., Huang, Z., Liu, T. et al. (2015). Tetrandrine induces G1/S cell cycle arrest through the ROS/Akt pathway in EOMA cells and inhibits angiogenesis in vivo. International Journal of Oncology, 46, 360–368. DOI 10.3892/ijo.2014.2735. [Google Scholar] [CrossRef]
10. Jin, L., Xu, M., Luo, X. H., Zhu, X. F. (2017). Stephania Tetrandra and Ginseng-containing Chinese herbal formulation NSENL reverses cisplatin resistance in lung cancer xenografts. American Journal of Chinese Medicine, 45, 385–401. DOI 10.1142/S0192415X17500240. [Google Scholar] [CrossRef]
11. Zhang, G. B., Li, Q. Y., Chen, Q. L., Su, S. B. (2013). Network pharmacology: A new approach for Chinese herbal medicine research. Journal of Evidence-based Complementary & Alternative Medicine, 2013, 621423. DOI 10.1155/2013/621423. [Google Scholar] [CrossRef]
12. Garrett, M. M., Marguerita, L. W. (2008). Molecular docking. Methods in Molecular Biology, 443, 365–382. DOI 10.1007/978-1-59745-177-2_19. [Google Scholar] [CrossRef]
13. Cheng, Z. X., Wang, K. M., Wei, J., Lu, X., Liu, B. R. (2010). Proteomic analysis of tumor effects by tetrandrine treatment in HepG2 cells. Phytomedicine, 17, 1000–1005. DOI 10.1016/j.phymed.2010.03.018. [Google Scholar] [CrossRef]
14. Chen, Z., Zhao, L., Zhao, F., Yang, G. H., Wang, J. J. (2018). Tetrandrine suppresses lung cancer growth and induces apoptosis, potentially via the VEGF/HIF-1α/ICAM-1 signaling pathway. Oncology Letters, 15(5), 7433–3747. DOI 10.3892/ol.2018.8190. [Google Scholar] [CrossRef]
15. Liu, W. C., Zhang, J., Ying, C., Wang, Q. R., Yan, C. et al. (2012). Tetrandrine combined with gemcitabine and Cisplatin for patients with advanced non-small cell lung cancer improve efficacy. International Journal of Biomedical Science, 8(1), 28–35. [Google Scholar]
16. Wang, C. H., Yang, J. M., Guo, Y. B., Shen, J., Pei, X. H. (2020). Anticancer activity of tetrandrine by inducing apoptosis in human breast cancer cell line MDA-MB-231 in vivo. Journal of Evidence-based Complementary & Alternative Medicine, 2020, 6823520. DOI 10.1155/2020/6823520. [Google Scholar] [CrossRef]
17. Sun, J. Y., Zhang, Y., Zhen, Y. Z., Cui, J., Hu, G. et al. (2019). Tumor activity of tetrandrine citrate in human glioma U87 cells in vitro and in vivo. Oncology Reports, 42(6), 2345–2354. DOI 10.3892/or.2019.7372. [Google Scholar] [CrossRef]
18. Li, X. L., Lu, X. W., Xu, H., Zhu, Z. S., Yin, H. T. et al. (2012). Paclitaxel/tetrandrine coloaded nanoparticles effectively promote the apoptosis of gastric cancer cells based on oxidation therapy. Molecular Biopharmaceutics, 9(2), 222–229. DOI 10.1021/mp2002736. [Google Scholar] [CrossRef]
19. Jiang, L., Hou, R. (2020). Tetrandrine reverses paclitaxel resistance in human ovarian cancer via inducing apoptosis, cell cycle arrest through β-catenin pathway. OncoTargets and Therapy, 13, 3631–3639. DOI 10.2147/OTT.S235533. [Google Scholar] [CrossRef]
20. Lia, D., Liua, H. Z., Liua, Y. F., Zhanga, Q. K., Liu, C. et al. (2017). Design, synthesis and biological activities of tetrandrine and fangchinoline derivatives as antitumer agents. Bioorganic & Medicinal Chemistry Letters, 27(3), 533–536. DOI 10.1016/j.bmcl.2016.12.029. [Google Scholar] [CrossRef]
21. Parkhitko, A. A., Favorova, O. O., Khabibullin, D. I., Anisimov, V. N., Henske, E. P. (2014). Kinase mTOR: Regulation and role in maintenance of cellular homeostasis, tumor development, and aging. Biochemistry, 79, 88–101. DOI 10.1134/S0006297914020023. [Google Scholar] [CrossRef]
22. Timothy, G. C., Dagmar, S. T., Nick, V. H., Elizabeth, D., Mike, S. et al. (2000). Serine/threonine protein kinases and apoptosis. Experimental Cell Research, 256, 34–41. DOI 10.1006/excr.2000.4836. [Google Scholar] [CrossRef]
23. Li, S. Z., Xu, F., Sun, C. Q., Xu, P. (2018). Research advances in the mammalian target of rapamycin signaling pathway and its inhibitors in treatment of hepatocellular carcinoma. Zhonghua Gan Zang Bing Za Zhi, 26(1), 77–80. DOI 10.3760/cma.j.issn.1007-3418.2018.01.018. [Google Scholar] [CrossRef]
24. Feng, M. Y., Xiong, G. B., Cao, Z., Yang, G., Zheng, S. L. et al. (2018). LAT2 regulates glutamine-dependent mTOR activation to promote glycolysis and drug resistance in pancreatic cancer. Journal of Experimental & Clinical Cancer Research, 37(1), 274. DOI 10.1186/s13046-018-0947-4. [Google Scholar] [CrossRef]
25. German, S., Aslam, H. M., Saleem, S., Raees, A., Anum, T. et al. (2013). Carcinogenesis of PIK3CA. Hereditary Cancer in Clinical Practice, 11(1), 5310–5313. DOI 10.1186/1897-4287-11-5. [Google Scholar] [CrossRef]
26. Li, L., Zheng, Y., Zhang, W. H., Hou, L. M., Gao, Y. (2020). Scutellarin circumvents drug resistance, promotes apoptosis, and represses tumor growth by HDAC/miR-34a-mediated down-modulation of Akt/mTOR and NF-κB-orchestrated signaling pathways in multiple myeloma. International Journal of Clinical and Experimental Pathology, 13(2), 212–219. [Google Scholar]
27. Ayse, C., Elif, A., Bulent, O. (2021). SRC signaling in cancer and tumor microenvironment. Advances in Experimental Medicine and Biology, 1270, 57–71. DOI 10.1007/978-3-030-47189-7_4. [Google Scholar] [CrossRef]
28. Guarino, M. (2010). Src signaling in cancer invasion. Journal of Cellular Physiology, 223(1), 14–26. DOI 10.1002/jcp.22011. [Google Scholar] [CrossRef]
29. Lin, Y. P., Wu, J., Tseng, C. W., Chen, H. J., Wang, L. H. (2019). Gjb4 serves as a novel biomarker for lung cancer and promotes metastasis and drug resistance via Src activation. Oncogenes, 8(6), 822–837. DOI 10.1038/s41388-018-0471-1. [Google Scholar] [CrossRef]
30. Wang, H. Q., Liu, T., Wang, Q., Liu, X., Li, W. H. (2015). Tetrandrine is a potent cell autophagy agonist via activated intracellular reactive oxygen species. Cell & Bioscience, 5, 2–9. DOI 10.1186/2045-3701-5-4. [Google Scholar] [CrossRef]
31. Guo, Y. B., Pei, X. H. (2019). Tetrandrine-induced autophagy in MDA-MB-231 triple-negative breast cancer cell through the inhibition of PI3K/AKT/mTOR signaling. Journal of Evidence-based Complementary & Alternative Medicine, 2019(1), 1–11. 7517431. DOI 10.1155/2019/7517431. [Google Scholar] [CrossRef]
32. Wong, W. V. K., Zeng, W., Chen, J., Yao, X. J., Leung, E. L. H. et al. (2017). Tetrandrine, an activator of autophagy, induces autophagic cell death via PKC-α inhibition and mTOR-dependent mechanisms. Frontiers in Pharmacology, 8, 351–364. DOI 10.3389/fphar.2017.00351. [Google Scholar] [CrossRef]
33. Chen, Y., Tseng, S. H. (2010). The potential of tetrandrine against gliomas. Anti-Cancer Agents in Medicinal Chemistry, 10(9), 534–542. DOI 10.2174/187152010793498609. [Google Scholar] [CrossRef]
34. Jiang, Y. W., Cheng, H. Y., Kuo, C. L., Way, T. D., Lien, J. C. et al. (2019). Tetrandrine inhibits human brain glioblastoma multiforme GBM, 8401 cancer cell migration and invasion in vitro. Environmental Toxicology, 34, 364–374. DOI 10.1002/tox.22691. [Google Scholar] [CrossRef]
35. Horng, C. T., Yang, J. S., Chiang, J. H., Lu, C. C., Lee, C. F. (2010). Inhibitory effects of tetrandrine on epidermal growth factor-induced invasion and migration in HT29 human colorectal adenocarcinoma cells. Molecular Medicine Reports, 13(1), 1003–1009. DOI 10.3892/mmr.2015.4635. [Google Scholar] [CrossRef]
36. Bao, G., Li, C., Qi, L., Wang, N., He, B. (2016). Tetrandrine protects against oxygen-glucose-serum deprivation/reoxygenation-induced injury via PI3K/AKT/NF-κB signaling pathway in rat spinal cord astrocytes. Biomedicine & Pharmacotherapy, 84, 925–930. DOI 10.1016/j.biopha.2016.10.007. [Google Scholar] [CrossRef]
37. Xu, W., Wang, X., Tu, Y., Masaki, H., Tanaka, S. et al. (2019). Tetrandrine and cepharanthine induce apoptosis through caspase cascade regulation, cell cycle arrest, MAPK activation and PI3K/Akt/mTOR signal modification in glucocorticoid resistant human leukemia Jurkat T cells. Chemico-Biological Interactions, 310, 108726. DOI 10.1016/j.cbi.2019.108726. [Google Scholar] [CrossRef]
38. Khattar, E., Maung, K. Z. Y., Chew, C. L., Ghosh, A., Huang, M. M. et al. (2019). Rap1 regulates hematopoietic stem cell survival and affects oncogenesis and response to chemotherapy. Nature Communications, 10(1), 5349. DOI 10.1038/s41467-019-13082-9. [Google Scholar] [CrossRef]
39. Saravanakumar, K., Park, S. J., Sathiyaseelan, A., Kim, K. N., Cho, S. H. et al. (2021). Metabolite profiling of methanolic extract of Gardenia jaminoides by LC-MS/MS and GC-MS and its anti-diabetic, and anti-oxidant activities. Pharmaceuticals, 14(2), 102. DOI 10.3390/ph14020102. [Google Scholar] [CrossRef]
40. Luan, F., He, X. R., Zeng, N. (2020). Tetrandrine: A review of its anticancer potentials, clinical settings, pharmacokinetics, and drug delivery systems. Journal of Pharmacy and Pharmacology, 72, 1491–1512. DOI 10.1111/jphp.13339. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |