
 | Oncologie |  |
DOI: 10.32604/oncologie.2021.017220
ARTICLE
Clinical Significance of PD-L1 Expression and CD8-Positive Tumor-Infiltrating Lymphocytes in Patients with Cavitary Lung Adenocarcinoma
1Department of Radiography, General Hospital of Central Theater Command, PLA, Wuhan, 430070, China
2Department of Cardio-Thoracic Surgery, General Hospital of Central Theater Command, PLA, Wuhan, 430070, China
3Department of Pathology, General Hospital of Central Theater Command, PLA, Wuhan, 430070, China
*Corresponding Authors: Jiangyong Liu. Email: liuyong4327939@163.com; Wencai Huang. Email: dr_hwang@163.com
#Jiangyong Liu and Mingming Gu contributed equally to this work
Received: 23 April 2021; Accepted: 20 July 2021
Abstract: Cavitary lung cancer is a rare type of lung cancer. Generally, the relationship between cavitary lung adenocarcinoma (LUAD) and specific immune checkpoints remains unknown. In this study, we aimed to detect the expression of programmed cell death ligand-1(PD-L1) and the density of CD8-positive (CD8+) tumor-infiltrating lymphocytes (TILs) to evaluate their clinicopathological significance in the case of patients with cavitary LUAD. This study included 65 patients with cavitary LUAD. Patient specimens were obtained from surgery. The expression of PD-L1 protein and CD8+ TIL status was detected by traditional immunohistochemistry and multiplex quantitative immunofluorescence technology. The correlation of PD-L1 expression and CD8+ TIL status was assessed along with clinicopathological parameters. This included evaluation of overall survival in cavitary LUAD patients based on the follow-up data. High expression of PD-L1 protein was detected in the cancerous tissues of cavitary LUAD patients, whose positive rate was recorded at 44.6% (29/65). PD-L1 expression level was significantly related with the lymph node (P = 0.001), TNM stage (P = 0.024), and CD8+ TIL status (rs = −0.272, P = 0.025). High PD-L1 expression indicated high mortality rate (P < 0.001); however, the CD8+ TIL group showed better survival in cavitary LUAD patients (P = 0.011). This phenotype, with high PD-L1 expression and low CD8+ TILs, can predict poorer overall survival of patients with cavitary LUAD compared with the other phenotypes. Moreover, CD8+ TIL level was an independent good prognosis factor. Initially, we reported that PD-L1 is upregulated in cavitary LUAD patients and that high expression of PD-L1 negatively correlates with CD8+ TIL status. High PD-L1 expression and low CD8+ TILs can predict decreased overall survival of patients with cavitary LUAD.
Keywords: Lung adenocarcinoma; cavity; programmed cell death ligand-1; tumor-infiltrating lymphocytes; multiplex immunohistochemistry
Lung cancer is the most common type of cancer diagnosed in both males and females combined. In addition, lung cancer is the leading cause of cancer death worldwide in 185 countries [1]. Cavitary lung cancer is a solitary and thin-walled tumor that is particularly unique and seldom reported [2]. Cavitary lung cancer occurs in 8% of all lung cancers [3], but other researchers reported an incidence rate of 1.00%–2.07%. Cavitation in a tumor nodule is previously thought to be more prevalent in patients with lung squamous cell carcinoma [4]. Following an increase of lung adenocarcinoma (LUAD), cavitary LUAD has been reported with an incidence of 5.7%–14.9% in patients with LUAD [5]. Cavitary lung cancer is a rare type of lung cancer, which has an irregular cystic wall, wall nodule with septa, or standard uptake value raised on positron emission tomography (PET) [6]. It is not easy for this type of cancer to be accurately diagnosed by clinicians via radiological measures. In addition, compared with noncavitary lung cancer, this cancer has a worse prognosis for affected patients due to a high TNM stage [7–11]. Since computed tomography (CT) can offer reliable messages about the morphology and density of lesions, this is the best method to noninvasively distinguish malignant and nonmalignant cavities [11]. Cavitary lung cancer is significantly associated with elderly, male patients that have tumors with larger maximum diameters, solid nodules, larger pT size, and advanced pTNM stage in multivariable analysis [12]. The biological features of the underlying cavity are still poorly understood, and it remains unclear whether immune checkpoints, for example, programmed cell death ligands 1 (PD-L1), have a different expression pattern.
Immunotherapy is a novel choice in the treatment of a variety of cancers with poor prognosis [13]. The development of immune checkpoint inhibitors has altered the therapy of non-small cell lung cancer (NSCLC) [14]. As one of the typical checkpoint inhibitors, programmed cell death 1 (PD-1) is an inhibitory cell-surface receptor that is expressed on activated T-cells and other immune cells. Anticancer immunotherapy that targets immune checkpoints with antibodies to PD-1 and its ligand PD-L1 is an established treatment modality for NSCLC [15–17]. One of these important mechanisms is the ability of anti-PD-L1 monoclonal antibodies to restrain lymphocyte inhibition by binding to the PD-1 receptor. This prevents PD-1 from binding with its ligands, PD-L1 or PD-L2. In addition, it allows T cells to maintain their tumor cell killing function [18,19]. NSCLC patients with PD-L1-positive had a higher chance of achieving an objective response, when treated with anti-PD-L1 monoclonal antibodies [20,21].
Although these most heartening results, the overall response of lung cancer patients to immune checkpoint treatment is still unsatisfactory [14]. Thus, more and better biomarkers need to be found. The predictable biomarkers of the next area of concern will be detected in the tumor microenvironment. Tumor-infiltrating lymphocytes (TILs) also play a vital role in predicting tumor progression in different kinds of cancers [22]. As the most studied component of the tumor-associated immune response, the expression of cytotoxic CD8-positive (CD8+) T cell could forecast better prognosis of breast or ovarian cancer patients [23,24]. However, the correlation between cavitary lung cancer and immune checkpoints remains unknown. In this study, we take advantage of human specimens to assess PD-L1 expression pattern along with CD8+ TIL density and investigate the prognostic significance of these results in the setting of cavitary LUAD.
2.1 Cavitary LUAD Sample Collection
A total of 65 patients who were diagnosed with cavitary LUAD between September of 2005 and October of 2015 in the General Hospital of Central Theater Command Hospital, People’s Liberation Army of China, were included in the research. These patients had no chemotherapy and/or radiotherapy before surgical resection. All patients underwent a 64-row spiral CT scan with a slice thickness of 1.25 mm, 1.5 mm, or HRCT. Two radiologists (J. Liu and Y. Xue) independently examined and confirmed the imaging features of these samples. In accordance with previous studies, tumor cavitation was defined as an air-filled space with a wall thickness of ≤4 mm along its circumference [5,11,25,26] (Fig. 1).
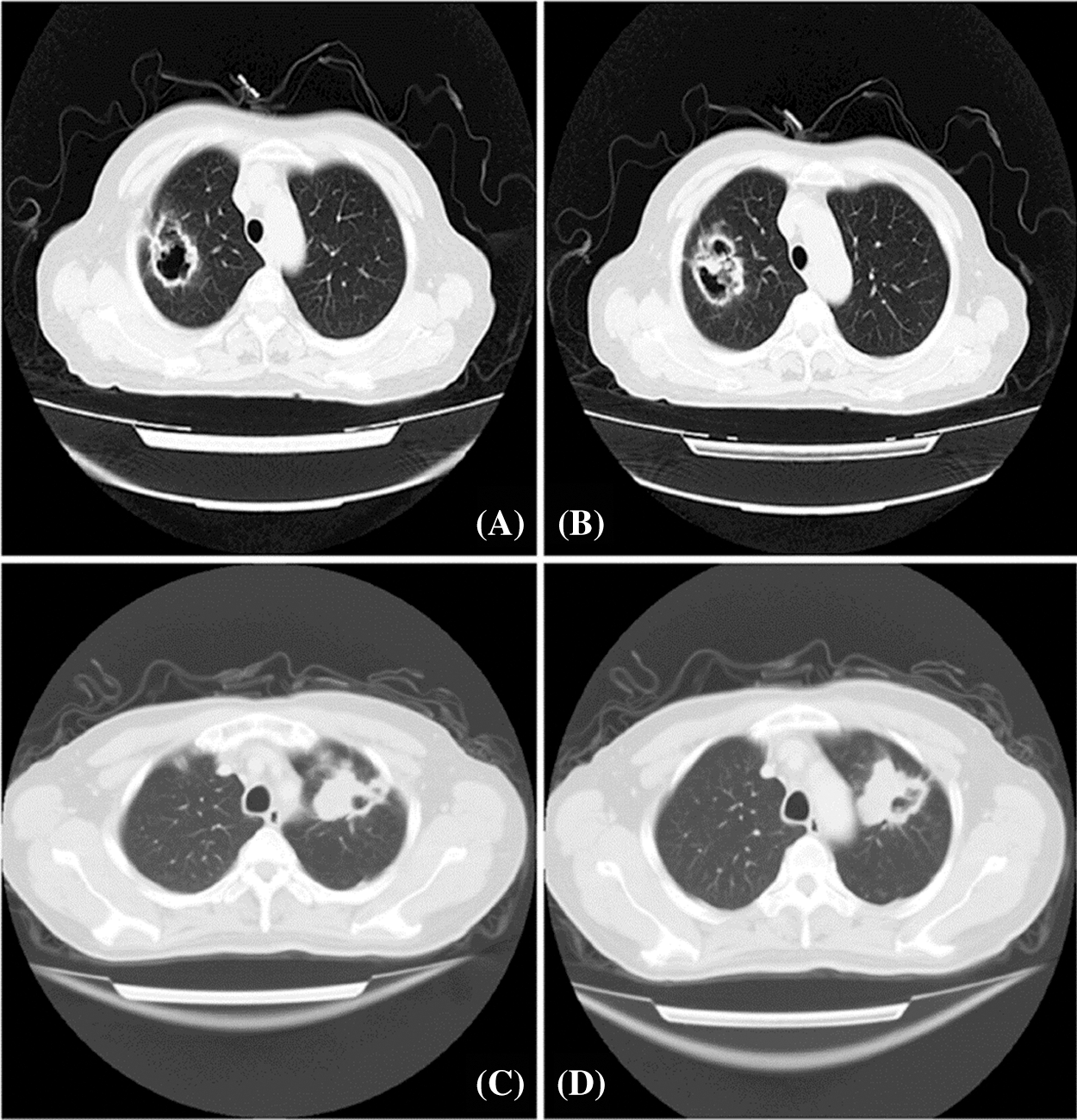
Figure 1: Chest CT scanning presentation of patients with solitary cavitary lung cancer. A and B: The posterior segment of the upper lobe of the right lung showed an irregular and shallowly lobulated mass of 40 × 46 mm2 that was adjacent to pleura traction and depression with irregular cavity. Multiple nodules of varying sizes were observed. C and D: The anterior segment of the upper apex of the left lung showed a 46 × 55 mm2 soft tissue density shadow with eccentric cavity, which was lobulated and pulled by the adjacent pleura
Formalin-fixed, paraffin-embedded (FFPE) tissues of these 65 patients with cavitary LUAD were collected. Two pathologists (Q. Wang and Y. Ren) independently confirmed the histopathologic features of each sample. Clinicopathological data were retrieved from clinical records and histopathology reports. We followed up the patients with cavitary LUAD from the date of surgery or biopsy and ended in October 2018. The median follow-up was 45 months with a range of 1–115 months. We defined overall survival from diagnosis to death or the end of follow-up. We classified cavitary LUAD specimens according to the 8th edition of TNM classification by UICC/AJCC (2017) [27].
2.2 Immunohistochemical Analysis
After samples were deparaffinized and rehydrated, antigen retrieval was applied in citrate (10 mM, pH 6.0) at 95°C for 15 min by microwave (Meidi, China). PD-L1 and CD8 expression in FFPE tumor sections was performed by IHC using a primary rabbit anti-human PD-L1 polyclonal antibody (1:100 dilution, E1L3N, Cell Signaling Technology, USA) and a mouse anti-human CD8 monoclonal antibody (ready-to-use, C8/144B, Dako, Agilent, USA) that were incubated at 4°C overnight. The sections with PD-L1 and CD8 were incubated with horseradish peroxidase (HRP) conjugated to the goat anti-mouse/rabbit second antibody (Dako REAL EnVision Detection System, Agilent, USA) at 37°C for 30 min. After this, the sections were mixed with 3,3’-diaminobenzidine (DAB) (Dako, Agilent, USA) as the chromogen. Lastly, hematoxylin was used for nuclear counterstaining.
2.3 Evaluation of Immunohistochemistry
We observed the intensity of immunostaining using light microscopy (Olympus BX-53 with CCD DP73). Two independent pathologists (Q. Wang and Y. Ren) scored the PD-L1 and CD8 protein results, whose were blinded to the clinicopathological parameters of cavitary LUAD.
The immunohistochemistry characteristics and cutoffs of PD-L1 being considered as positive vary in different research studies. In the current study, the cutoff of PD-L1 protein expression in tumor cells was defined as 5%. PD-L1 ≥ 5% was regarded as high expression. This was consistent with many types of cancers [28–30].
For the evaluation of CD8, the CD8+ TIL number was counted on each slide at ×200 magnification. The mean of these three counts was calculated for each case, and the cutoff value of high or low expression was decided based on the median number of total counts [24,31].
2.4 Multiplex Immunofluorescence Staining
We performed manually multiplex immunofluorescence (mIF) staining on 4-μm FFPE sections of cavitary LUAD with the use of the Opal 4-Color IHC Kit (Akoya Biosciences, USA) [30]. Three markers included PD-L1 (1:200 dilution, E1L3N, Cell Signaling Technology, USA), CK (AE1/AE3) and CD8 (C8/144B), whose were ready-to-use antibodies (1:2 dilution, Agilent/DAKO, California, USA). According to the kit protocol and the reference [30], we completed all the steps. In this study, we selected human tonsil tissues both with and without primary antibody as positive and negative controls, or autofluorescence control, respectively. We scanned the mIF-stained slides by a Vectra 2.3 multispectral microscope system (Akoya Biosciences, USA) from 420 to 720 nm at 20-nm intervals with the same exposure time. Lastly, we analyzed all three markers and gained the significant immune phenotypes using InForm 2.4 software (Akoya Biosciences, USA).
Data were expressed as frequencies for categorical variables and mean ± SD for numeric values. All statistical analyses were completed by SPSS 21.0 (Chicago, IL, USA). We carried out Chi square test or Fisher exact test in order to evaluate the relationship between PD-L1 expression and clinicopathological parameters of cavitary LUAD patients. We used Spearman correlation analysis to explore the correlation between PD-L1 and CD8+ TILs. The survival analysis was assessed using the Kaplan-Meier curve and log-rank test to decide statistical differences. At last, we performed COX proportion hazard regression model to complete univariate and multivariate analysis of survival, and evaluate the independent prognostic values. P-values <0.05 were considered statistically significant.
The cavitation in the lungs was continually evaluated with the use of chest CT scans. Our results found solitary and thin-walled cavities, which localized at pulmonary periphery, and all these cavitary lesions demonstrated suspected signs of malignancy (Fig. 1).
As shown in Tab. 1, among the 65 cavitary LUAD patients, 36 (55.4%) were male and 29 (44.6%) were female, with a mean age of 58 years old (range 48–71). Thirty-one (47.7%) of these patients were alive, and 34 (52.3%) had died by the end of follow-up. The data of the T, N, M, and TNM stages of cavitary LUAD for these patients are also shown in Tab. 1.

3.2 PD-L1 and CD8 Protein Expression
As shown in Figs. 2 and 3, PD-L1 and CD8 proteins were expressed in cavitary LUAD tissues. In cancer tissues, PD-L1 protein was localized on the membrane and the cytoplasm of cancer cells. In some cases, the cytoplasm of immune cells showed negative PD-L1 in the cancer cells (Figs. 2A–2H). Different CD8+ TIL statuses were observed both inside and outside the cancer nest of different cavitary LUAD patients (Figs. 3A–3F). Using mIF, we observed that PD-L1 could be expressed in the cancer cells (PD-L1+CK+) and TILs (PD-L1+CD8+) (Fig. 4).
Of the 65 cases of cavitary LUAD tissues that were studied, 55.4% (n = 36) of cases showed low PD-L1 expression, and 44.6% (n = 29) of cases were found to have high PD-L1 expression. Among the 65 cases of cavitary LUAD patients, 48 (73.9%) cases were low in CD8+ TILs, and 17 (26.1%) were high in CD8+ TILs.
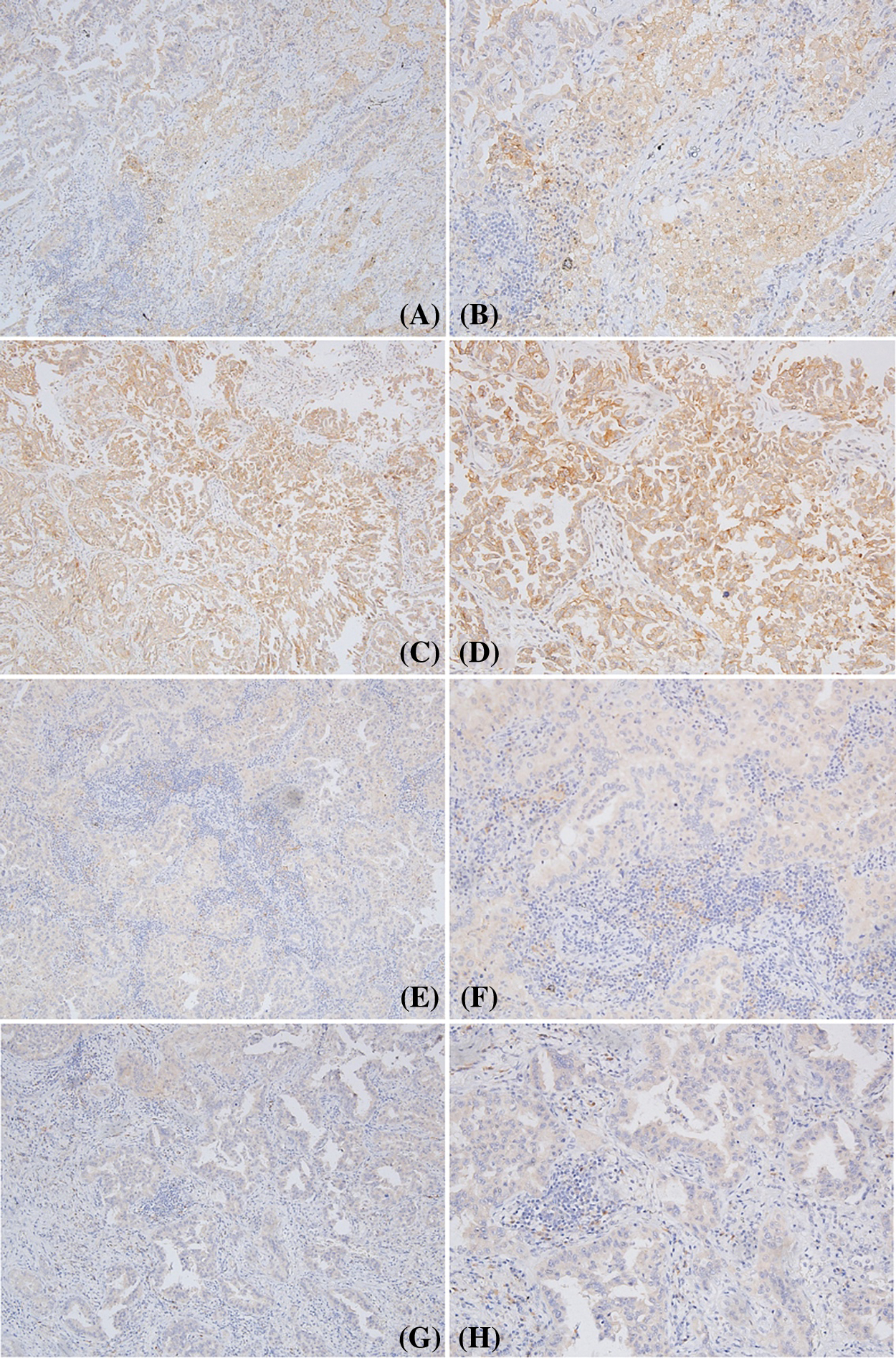
Figure 2: Different pattern of PD-L1 expression in cavitary LUAD. A, B, C, and D: high expression of PD-L1 in the cancer cells; E and F: positive expression of PD-L1 in the immune cells of stroma, negative in the cancer cells. G and H: a few immune cells showed positive PD-L1, but negative in the cancer cells (Positive signal was brown, original magnification A, C, E, G × 100; B, D, F, H × 200)
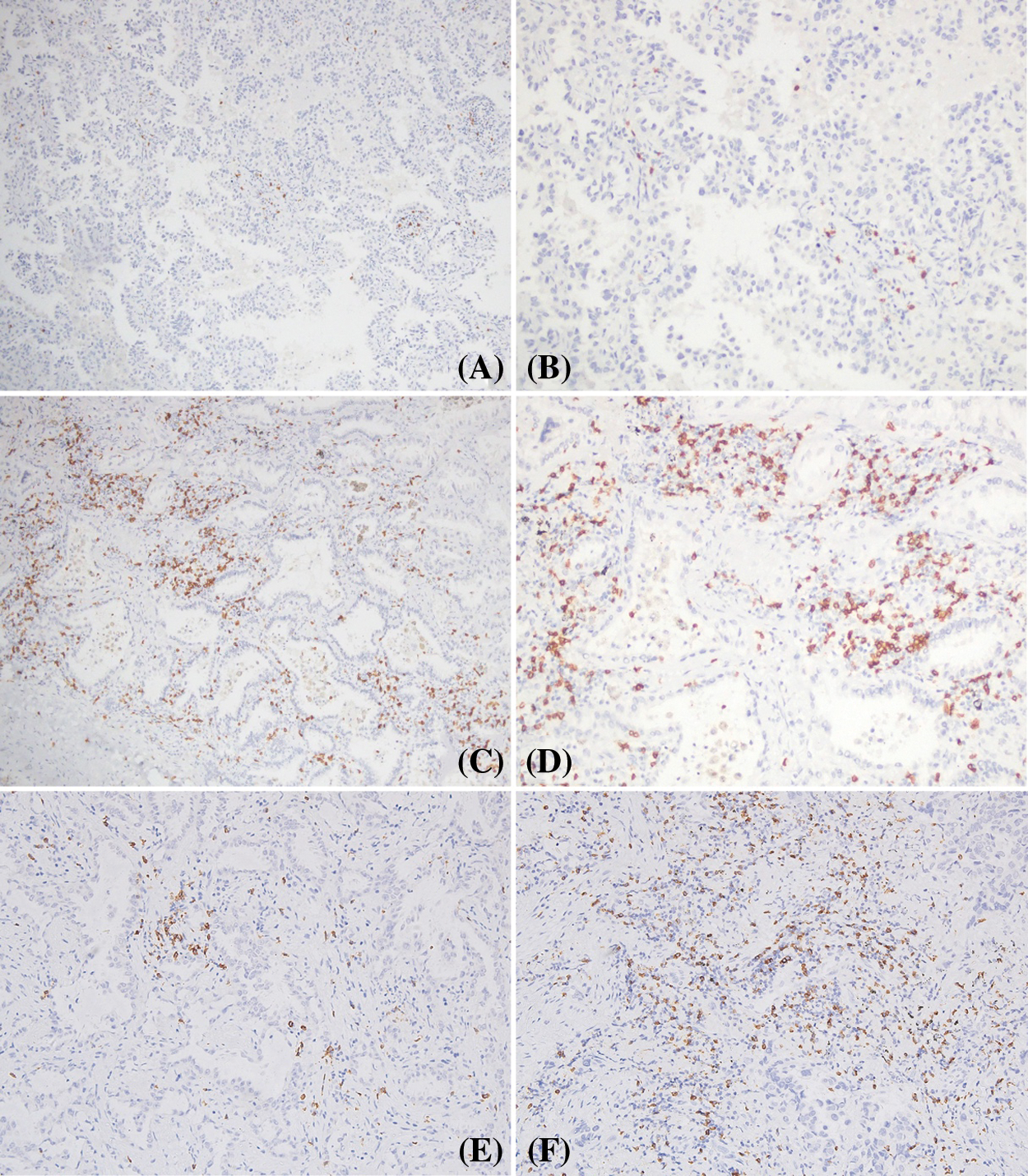
Figure 3: CD8+ TILs in cavitary LUAD. A and B: low expression of CD8+ TILs; C and D: high expression of CD8+ TILs in the stroma and cancer islets. E: low expression of CD8+ TILs in different cases; F: high levels of CD8+ TILs in the stroma of different cases (Positive signal was brown, original magnification A, C × 100; B, D, E, F × 200)
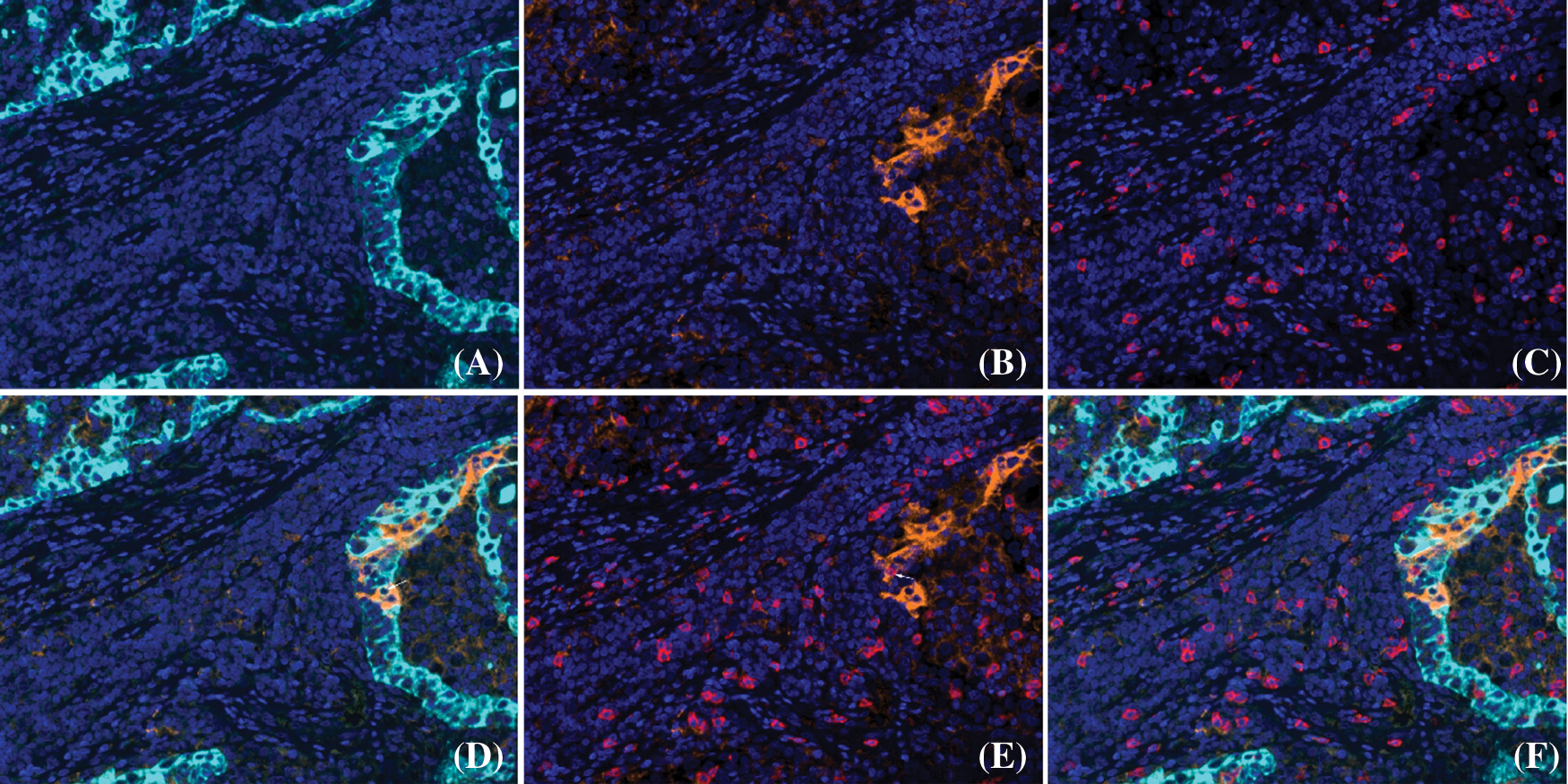
Figure 4: Co-expression of PD-L1 and CD8, CK proteins detected by mIF in cavitary LUAD. A: CK (cyan); B: PD-L1 (orange); C: CD8 (red); D: co-expression of PD-L1 and CK (white arrow) was observed; E: co-expression of PD-L1 and CD8 (white arrow) was observed; F: unmixed composite image for PD-L1, CD8 and CK (original magnification of all images × 400)
3.3 PD-L1 Expression and Clinicopathological Parameters in Cavitary LUAD Patients
The relationship between tumor PD-L1 expression and clinicopathologic variables in cavitary LUAD was analyzed by Chi square test. Tab. 2 showed high expression of PD-L1 protein was significantly associated with lymph node metastasis (N) (P = 0.001) and the TNM stage (P = 0.024). Negative correlation was detected between PD-L1 and CD8+ TIL status (rs = −0.272, P = 0.025; Figs. 5A–5D); but PD-L1 expression was not statistically correlated with age, sex, or tumor size in cavitary LUAD patients.

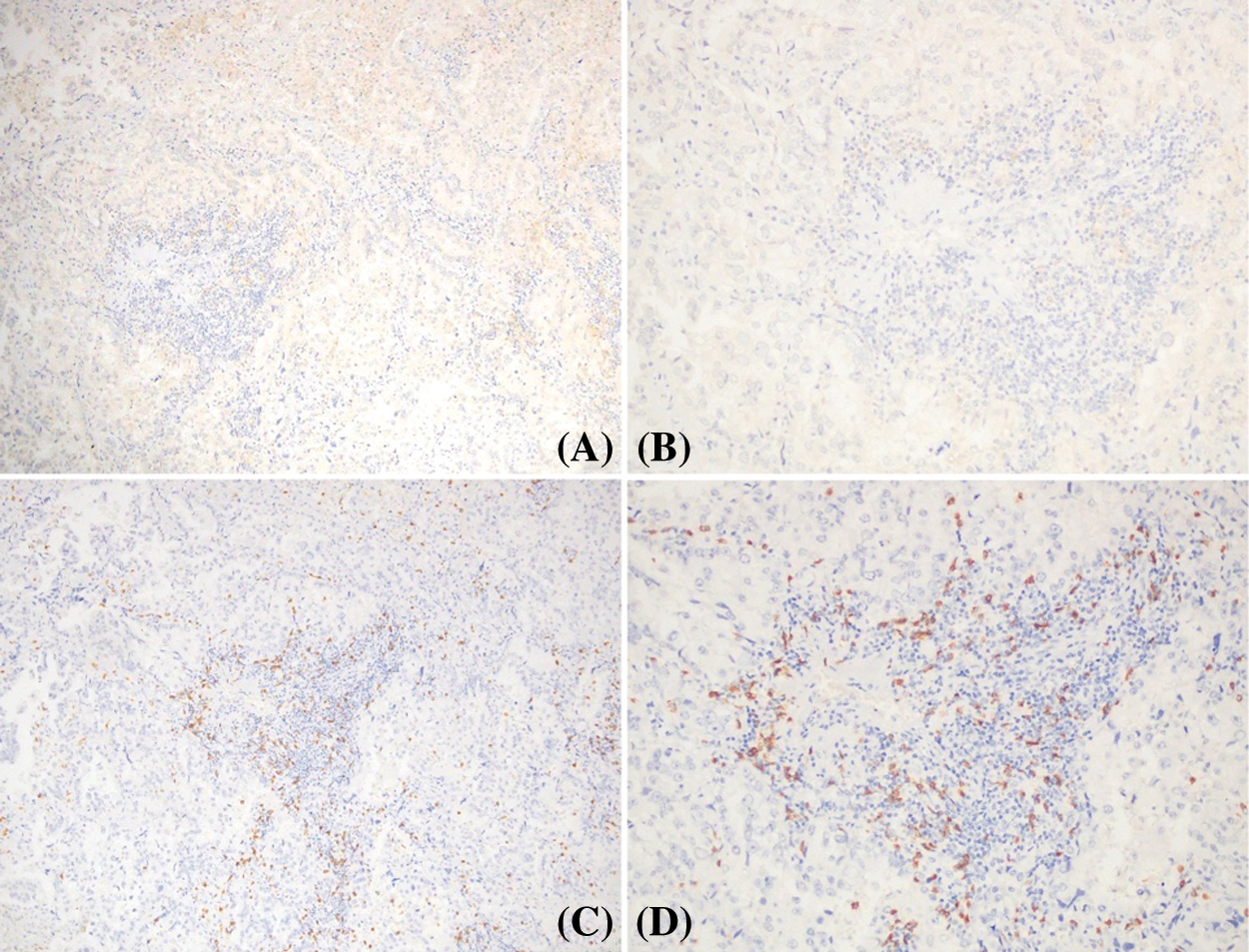
Figure 5: Relation between PD-L1 expression and CD8+ TILs in the same case of cavitary LUAD. A and B: low expression of PD-L1 protein; C and D: high expression of CD8+ TILs in stroma and tumor islets (positive signal was brown, original magnification A, C × 100; B, D × 200)
3.4 Prognostic Value of PD-L1 Expression and CD8+ TILs in Cavitary LUAD Patients
Survival analysis was determined by Kaplan–Meier curve, while log-rank test was used to explore the prognostic value of PD-L1 expression and CD8+ TIL status in cavitary LUAD patients. High expression of PD-L1 was found to predict poor survival and a high mortality rate in cavitary LUAD patients (Fig. 6A, P = 0.004). The CD8+ TIL group had better survival rates of cavitary LUAD patients (Fig. 6B, P = 0.001). In addition, patients with high PD-L1 expression and low CD8+ TILs demonstrated poorer overall survival than those with other phenotypes (Fig. 6C, P = 0.001).
In univariate analysis, both PD-L1 and CD8+ TIL levels were observed to be significantly correlated with the overall survival (OS) of LUAD patients (HR: 2.670, and 95% CI: 1.334–5.345, P = 0.006; HR: 1.995, and 95% CI: 0.991–0.998, P = 0.002, respectively, Tab. 3). In addition, the phenotype of high PD-L1 expression and low CD8+ TILs had higher risk of short OS than the other phenotypes (HR: 2.999, and 95% CI: 1.518–5.923, P = 0.002). Simultaneously, significant correlation was detected between the TNM stage and the OS of cavitary LUAD (Tab. 3). A multivariate COX proportional hazard model on OS was carried out to analyze whether the above univariate analysis as an independent prognostic factor. This multivariate analysis model included the two protein expressions and the clinicopathological parameters of cavitary LUAD. The results confirmed that only CD8+ TILs were an independent prognosis parameter in OS of cavitary LUAD patients (HR: 0.655; and 95% CI: 0.007–0.401, P = 0.004, Tab. 3).

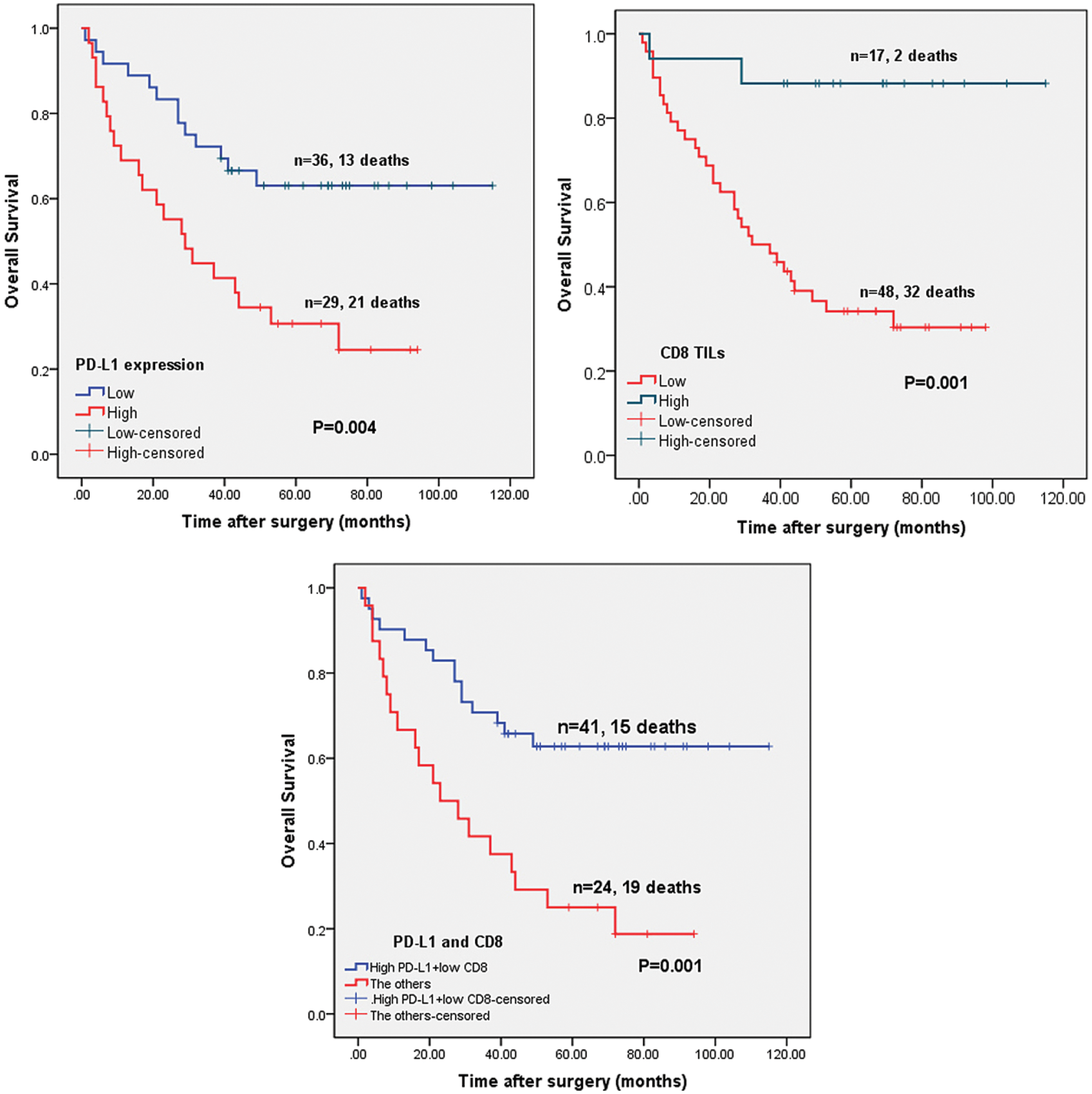
Figure 6: Correlation of PD-L1 and CD8 expression level and OS of patients with cavitary LUAD. A: Patients with low PD-L1 expression demonstrated better OS than those with high PD-L1 expression (P < 0.001). B: Patients with high CD8+ TILs demonstrated better OS than those with low CD8+ TILs (P = 0.010). TILs: tumor-infiltrating lymphocytes. C: Patients with high PD-L1 and low CD8 expression demonstrated poorer OS than those with the other phenotypes (P < 0.001)
This study is among the first to investigate high expression of PD-L1 protein in patients with cavitary LUAD. High expression of PD-L1 protein significantly correlated with lymph node metastasis, TNM stage, and CD8+ TIL status. Interestingly, our study showed that high PD-L1 expression and low CD8+ TILs could predict poorer overall survival of those patients with cavitary LUAD. CD8+ TILs were an independent predictor of LUAD prognosis.
Imaging studies like chest radiography and CT are commonly utilized for the clinical diagnosis of lung cancer. Radiographic features of cavities that indicate malignancy including multiple holes, a nodular ill-defined inner or outer wall, and an eccentric excavation with irregular margins [11]. Patients with cavitary lung cancers often have a poor rate of survival compared with noncavitary NSCLC patients [5,26]. This is because of advanced tumor stage, as well as the vascular, lymphatic, or pleural invasion of cavitary LUAD [5,32]. Based on this finding, cavitary and noncavitary LUAD should be considered separate entities [32]. Currently, there is a lack of cognition concerning the onset and progression of cavitary LUAD. This rare type of cancer is subject to misdiagnosis and missed diagnosis as well [2]. Necrosis may cause a solitary cavity because of primary cancer overgrowth. Tumor growth leads to bronchial obstruction and vascular invasion, which provides an environment of ischemia and hypoxia. This results in tumor necrosis. Furthermore, the autophagy of neoplastic cells can also induce cavitary lesion [7,10].
Immune checkpoint inhibitors targeting the PD-1/PD-L1 axis have previously demonstrated encouraging results in patients with NSCLC. Overexpression of PD-L1 is associated with poor, recurrence-free survival, and OS [33]. In addition, the FDA has approved pembrolizumab as a first-line treatment for advanced PD-L1 positive NSCLC patients [34]. In the current study, we found that high expression of PD-L1 was detected in 44.6% (29/65) cases of cavitary LUAD. This is significantly increased compared with the assessment of patients with noncavitary LUAD, which found that 22.5%–41.2% of cases had high PD-L1 expression [35–38]. Our results demonstrated that tumor necrosis correlates with higher PD-L1 expression in LUAD. Similar results have been reported in previous studies [39]. Recent papers have identified that high expression of PD-L1 has also positive relation with mutations in KRAS, TP53, and MET, which is negatively associated with mutations of EGFR and STK11 in cases of LUAD [17]. In addition, these mutations may be involved with different clones of the PD-L1 antibody and may affect the criteria of a positive rate of PD-L1 protein. In our study, high expression of PD-L1 was correlated with high TNM stage and was able to predict poor prognosis of patients with cavitary LUAD. The above-mentioned results confirmed that high expression of PD-L1 might promote malignant progression, and PD-L1 can be regarded as a cancer immunotherapy target of cavitary LUAD. Because of this, we had to further the specific molecular features of cavitary LUAD to analyze the mechanism of malignant progression and poor prognosis.
Inflammation is a notable feature of cancer that can lead to tumor progression [40]. Cytokines also have an anti-tumor immune effect, and IFN-α, IFN-γ, and TNF-α can all increase the expression of PD-L1 in a variety of cancers [41]. In addition, CD8+ TILs correlate with PD-L1 expression and participate in the inflammation of the anti-tumor immune response [42,43]. Our results also confirmed there is negative correlation between high expression of PD-L1 and CD8+ TIL status in cavitary LUAD. In addition, this immune phenotype of high expression of PD-L1 and low CD8+ TILs could predict poorer overall survival of patients with cavitary LUAD compared with the other phenotypes such as low PD-L1 and high CD8+ TILs. Moreover, we found that CD8+ TILs were an independent marker to predict prognosis of cavitary LUAD. Comprehensively, these results suggested that inflammation or necrosis induced by the dysregulation of tumor growth activates the cytokines secretion and leads to the formation of thin-walled cavity lesions in the development of LUAD. In response to this, cytokines increase the expression and activities of PD-L1, which contributes to tumor cells escaping from immune surveillance and further promotes the malignant progression of cavitary LUAD. CD8+ TILs are involved in the inflammation and immune processes described above. In addition, a negative correlation was observed between PD-L1 and CD8+ TILs in the same cavitary LUAD case. Further studies are required to better understand the mechanism of high expression of PD-L1 and its relationship with inflammation or necrosis in cavitary LUAD cases.
In conclusion, this research demonstrated that PD-L1 expression is upregulated in cavitary LUAD patients and that high expression of PD-L1 negatively correlates with CD8+ TIL status. High PD-L1 expression and low CD8+ TILs can predict poorer overall survival of patients with cavitary LUAD. These results demonstrated that PD-L1 is a pivotal immune checkpoint and can improve our mechanistic understanding of cavitary LUAD.
Funding Statement: This work was supported by a grant from the Natural Science Foundation of Hubei Province Key Project (Grant No. 2020BCB059, W.H.).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Bray, F., Ferlay, J., Soerjomataram, I., Siegel, R. L., Torre, L. A. et al. (2018). Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians, 68(6), 394–424. DOI 10.3322/caac.21492. [Google Scholar] [CrossRef]
2. Qi, Y., Zhang, Q., Huang, Y., Wang, D. (2014). Manifestations and pathological features of solitary thin-walled cavity lung cancer observed by CT and PET/CT imaging. Oncology Letters, 8(1), 285–290. DOI 10.3892/ol.2014.2065. [Google Scholar] [CrossRef]
3. Woodring, J. H., Fried, A. M., Chuang, V. P. (1980). Solitary cavities of the lung: Diagnostic implications of cavity wall thickness. American Journal of Roentgenology, 135(6), 1269–1271. DOI 10.2214/ajr.135.6.1269. [Google Scholar] [CrossRef]
4. Gasinska, A., Kolodziejski, L., Niemiec, J., Dyczek, S. (2005). Clinical significance of biological differences between cavitated and solid form of squamous cell lung cancer. Lung Cancer, 49(2), 171–179. DOI 10.1016/j.lungcan.2005.01.007. [Google Scholar] [CrossRef]
5. Watanabe, Y., Kusumoto, M., Yoshida, A., Shiraishi, K., Suzuki, K. et al. (2016). Cavity wall thickness in solitary cavitary lung adenocarcinomas is a prognostic indicator. Annals of Thoracic Surgery, 102(6), 1863–1871. DOI 10.1016/j.athoracsur.2016.03.121. [Google Scholar] [CrossRef]
6. Opoka, L. M., Szturmowicz, M., Oniszh, K., Korzybski, D., Podgajny, Z. et al. (2019). CT imaging features of thin-walled cavitary squamous cell lung cancer. Advances in Respiratory Medicine, 87(2), 114–117. DOI 10.5603/ARM.2019.0018. [Google Scholar] [CrossRef]
7. Chaudhuri, M. R. (1973). Primary pulmonary cavitating carcinomas. Thorax, 28(3), 354–366. [Google Scholar]
8. Onn, A., Choe, D. H., Herbst, R. S., Correa, A. M., Munden, R. F. et al. (2005). Tumor cavitation in stage I non-small cell lung cancer: epidermal growth factor receptor expression and prediction of poor outcome. Radiology, 237(1), 342–347. DOI 10.1148/radiol.2371041650. [Google Scholar] [CrossRef]
9. Coffey, J. P., Hill, J. C. (2008). 18F-fluoro-2-deoxy-D-glucose standardized uptake value in cavitating non-small-cell lung carcinoma. Nuclear Medicine Communications, 29(12), 1040–1045. DOI 10.1097/MNM.0b013e32831089b2. [Google Scholar] [CrossRef]
10. Farooqi, A. O., Cham, M., Zhang, L., Beasley, M. B., Austin, J. H. et al. (2012). Lung cancer associated with cystic airspaces. American Journal of Roentgenology, 199(4), 781–786. DOI 10.2214/AJR.11.7812. [Google Scholar] [CrossRef]
11. Xue, X. Y., Liu, Y. X., Wang, K. F., Zang, X. F., Sun, J. P. et al. (2015). Computed tomography for the diagnosis of solitary thin-walled cavity lung cancer. Clinical Respiratory Journal, 9(4), 392–398. DOI 10.1111/crj.12172. [Google Scholar] [CrossRef]
12. Liu, Z., Feng, H., Zhang, Z., Sun, H., Liu, D. (2020). Clinicopathological characteristics of solitary cavitary lung cancer: A case-control study. Journal of Thoracic Disease, 12(6), 3148–3156. DOI 10.21037/jtd-20-426. [Google Scholar] [CrossRef]
13. Iwai, Y., Ishida, M., Tanaka, Y., Okazaki, T., Honjo, T. et al. (2002). Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proceedings of the National Academy of Sciences of the United States of America, 99(19), 12293–12297. DOI 10.1073/pnas.192461099. [Google Scholar] [CrossRef]
14. Long, L., Zhao, C., Ozarina, M., Zhao, X., Yang, J. et al. (2019). Targeting immune checkpoints in lung cancer: Current landscape and future prospects. Clinical Drug Investigation, 39(4), 341–353. DOI 10.1007/s40261-018-00746-5. [Google Scholar] [CrossRef]
15. Gettinger, S., Horn, L., Jackman, D., Spigel, D., Antonia, S. et al. (2018). Five-year follow-up of Nivolumab in previously treated advanced non-small-cell lung cancer: Results from the CA209-003 study. Journal of Clinical Oncology, 36(17), 1675–1684. DOI 10.1200/JCO.2017.77.0412. [Google Scholar] [CrossRef]
16. Reck, M., Rodriguez-Abreu, D., Robinson, A. G., Hui, R., Csoszi, T. et al. (2016). Pembrolizumab vs. chemotherapy for PD-L1-positive non-small-cell lung cancer. New England Journal of Medicine, 375(19), 1823–1833. DOI 10.1056/NEJMoa1606774. [Google Scholar] [CrossRef]
17. Schoenfeld, A. J., Rizvi, H., Bandlamudi, C., Sauter, J. L., Travis, W. D. et al. (2020). Clinical and molecular correlates of PD-L1 expression in patients with lung adenocarcinomas. Annals of Oncology, 31(5), 599–608. DOI 10.1016/j.annonc.2020.01.065. [Google Scholar] [CrossRef]
18. Ohaegbulam, K. C., Assal, A., Lazar-Molnar, E., Yao, Y., Zang, X. (2015). Human cancer immunotherapy with antibodies to the PD-1 and PD-L1 pathway. Trends in Molecular Medicine, 21(1), 24–33. DOI 10.1016/j.molmed.2014.10.009. [Google Scholar] [CrossRef]
19. Chen, D. S., Irving, B. A., Hodi, F. S. (2012). Molecular pathways: Next-generation immunotherapy--inhibiting programmed death-ligand 1 and programmed death-1. Clinical Cancer Research, 18(24), 6580–6587. DOI 10.1158/1078-0432.CCR-12-1362. [Google Scholar] [CrossRef]
20. Abdel-Rahman, O. (2016). Correlation between PD-L1 expression and outcome of NSCLC patients treated with anti-PD-1/PD-L1 agents: A meta-analysis. Critical Reviews in Oncology/Hematology, 101(1), 75–85. DOI 10.1016/j.critrevonc.2016.03.007. [Google Scholar] [CrossRef]
21. Aguiar, P. N. Jr., De Mello, R. A., Hall, P., Tadokoro, H., Lima Lopes, G. (2017). PD-L1 expression as a predictive biomarker in advanced non-small-cell lung cancer: Updated survival data. Immunotherapy, 9(6), 499–506. DOI 10.2217/imt-2016-0150. [Google Scholar] [CrossRef]
22. Fu, Q., Chen, N., Ge, C., Li, R., Li, Z. et al. (2019). Prognostic value of tumor-infiltrating lymphocytes in melanoma: A systematic review and meta-analysis. Oncoimmunology, 8(7), 1593806. DOI 10.1080/2162402X.2019.1593806. [Google Scholar] [CrossRef]
23. Li, J., Wang, J., Chen, R., Bai, Y., Lu, X. (2017). The prognostic value of tumor-infiltrating T lymphocytes in ovarian cancer. Oncotarget, 8(9), 15621–15631. DOI 10.18632/oncotarget.14919. [Google Scholar] [CrossRef]
24. Al-Saleh, K., Abd El-Aziz, N., Ali, A., Abozeed, W., Abd El-Warith, A. et al. (2017). Predictive and prognostic significance of CD8(+) tumor-infiltrating lymphocytes in patients with luminal B/HER 2 negative breast cancer treated with neoadjuvant chemotherapy. Oncology Letters, 14(1), 337–344. DOI 10.3892/ol.2017.6144. [Google Scholar] [CrossRef]
25. Xue, X., Wang, P., Xue, Q., Wang, N., Zhang, L. et al. (2012). Comparative study of solitary thin-walled cavity lung cancer with computed tomography and pathological findings. Lung Cancer, 78(1), 45–50. DOI 10.1016/j.lungcan.2012.06.004. [Google Scholar] [CrossRef]
26. Shigefuku, S., Kudo, Y., Yunaiyama, D., Matsubayashi, J., Park, J. et al. (2018). Prognostic factors for surgically resected non-small cell lung cancer with cavity formation. Journal of Thoracic Disease, 10(2), 973–983. DOI 10.21037/jtd.2018.01.61. [Google Scholar] [CrossRef]
27. Detterbeck, F. C., Boffa, D. J., Kim, A. W., Tanoue, L. T. (2017). The eighth edition lung cancer stage classification. Chest, 151(1), 193–203. DOI 10.1016/j.chest.2016.10.010. [Google Scholar] [CrossRef]
28. Herbst, R. S., Soria, J. C., Kowanetz, M., Fine, G. D., Hamid, O. et al. (2014). Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature, 515(7528), 563–567. DOI 10.1038/nature14011. [Google Scholar] [CrossRef]
29. Koganemaru, S., Inoshita, N., Miura, Y., Miyama, Y., Fukui, Y. et al. (2017). Prognostic value of programmed death-ligand 1 expression in patients with stage III colorectal cancer. Cancer Science, 108(5), 853–858. DOI 10.1111/cas.13229. [Google Scholar] [CrossRef]
30. Long, L., Chen, M., Yuan, Y., Ming, A. L., Guo, W. et al. (2020). High expression of PKM2 synergizes with PD-L1 in tumor cells and immune cells to predict worse survival in human lung adenocarcinoma. Journal of Cancer, 11(15), 4442–4452. DOI 10.7150/jca.42610. [Google Scholar] [CrossRef]
31. El-Guindy, D. M., Helal, D. S., Sabry, N. M., Abo El-Nasr, M. (2018). Programmed cell death ligand-1 (PD-L1) expression combined with CD8 tumor infiltrating lymphocytes density in non-small cell lung cancer patients. Journal of the Egyptian National Cancer Institute, 30(4), 125–131. DOI 10.1016/j.jnci.2018.08.003. [Google Scholar] [CrossRef]
32. Watanabe, Y., Kusumoto, M., Yoshida, A., Suzuki, K., Asamura, H. et al. (2015). Surgically resected solitary cavitary lung adenocarcinoma: Association between clinical, pathologic, and radiologic findings and prognosis. Annals of Thoracic Surgery, 99(3), 968–974. DOI 10.1016/j.athoracsur.2014.10.040. [Google Scholar] [CrossRef]
33. Okita, R., Maeda, A., Shimizu, K., Nojima, Y., Saisho, S. et al. (2017). PD-L1 overexpression is partially regulated by EGFR/HER2 signaling and associated with poor prognosis in patients with non-small-cell lung cancer. Cancer Immunology Immunotherapy, 66(7), 865–876. DOI 10.1007/s00262-017-1986-y. [Google Scholar] [CrossRef]
34. Meng, X., Liu, Y., Zhang, J., Teng, F., Xing, L. et al. (2017). PD-1/PD-L1 checkpoint blockades in non-small cell lung cancer: New development and challenges. Cancer Letters, 405(1), 29–37. DOI 10.1016/j.canlet.2017.06.033. [Google Scholar] [CrossRef]
35. Qin, T., Xia, J., Liu, S., Wang, J., Liu, H. et al. (2020). Clinical importance of VEGFC and PD-L1 co-expression in lung adenocarcinoma patients. Thoracic Cancer, 11(5), 1139–1148. DOI 10.1111/1759-7714.13354. [Google Scholar] [CrossRef]
36. Zhang, M. L., Kem, M., Mooradian, M. J., Eliane, J. P., Huynh, T. G. et al. (2019). Differential expression of PD-L1 and IDO1 in association with the immune microenvironment in resected lung adenocarcinomas. Modern Pathology, 32(4), 511–523. DOI 10.1038/s41379-018-0160-1. [Google Scholar] [CrossRef]
37. Song, P., Wu, S., Zhang, L., Zeng, X., Wang, J. (2019). Correlation between PD-L1 expression and clinicopathologic features in 404 patients with lung adenocarcinoma. Interdisciplinary Sciences, Computational Life Sciences, 11(2), 258–265. DOI 10.1007/s12539-019-00329-8. [Google Scholar] [CrossRef]
38. Chen, L., Cao, M. F., Zhang, X., Dang, W. Q., Xiao, J. F. et al. (2019). The landscape of immune microenvironment in lung adenocarcinoma and squamous cell carcinoma based on PD-L1 expression and tumor-infiltrating lymphocytes. Cancer Medicine, 8(17), 7207–7218. DOI 10.1002/cam4.2580. [Google Scholar] [CrossRef]
39. Reiniger, L., Teglasi, V., Pipek, O., Rojko, L., Glasz, T. et al. (2019). Tumor necrosis correlates with PD-L1 and PD-1 expression in lung adenocarcinoma. Acta Oncologica, 58(8), 1087–1094. DOI 10.1080/0284186X.2019.1598575. [Google Scholar] [CrossRef]
40. Dranoff, G. (2004). Cytokines in cancer pathogenesis and cancer therapy. Nature Reviews Cancer, 4(1), 11–22. DOI 10.1038/nrc1252. [Google Scholar] [CrossRef]
41. Zhou, J., Mahoney, K. M., Giobbie-Hurder, A., Zhao, F., Lee, S. et al. (2017). Soluble PD-L1 as a biomarker in malignant melanoma treated with checkpoint blockade. Cancer Immunology Research, 5(6), 480–492. DOI 10.1158/2326-6066.CIR-16-0329. [Google Scholar] [CrossRef]
42. Tumeh, P. C., Harview, C. L., Yearley, J. H., Shintaku, I. P., Taylor, E. J. et al. (2014). PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature, 515(7528), 568–571. DOI 10.1038/nature13954. [Google Scholar] [CrossRef]
43. Nakazawa, N., Yokobori, T., Kaira, K., Turtoi, A., Baatar, S. et al. (2020). High stromal TGFBI in lung cancer and intratumoral CD8-Positive T Cells were associated with poor prognosis and therapeutic resistance to immune checkpoint inhibitors. Annals of Surgical Oncology, 27(3), 933–942. DOI 10.1245/s10434-019-07878-8. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |