

 | Oncologie |  |
DOI: 10.32604/Oncologie.2021.014772
REVIEW
The Functions of MicroRNAs and Their Potential Applications in the Diagnosis and Treatment of Gastric Cancer
1Food and Drug Department, Luoyang Polytechnic, Luoyang, 471000, China
2Department of Pharmacology and Toxicology, Wright State University, Dayton, 45435, USA
3Department of Pharmacy, The First People’s Hospital of Zhengzhou City, Zhengzhou, 450004, China
*Corresponding Author: Man Sun. Email: sunman0135@163.com
Received: 28 October 2020; Accepted: 02 April 2021
Abstract: Gastric cancer is a highly malignant disease with complex pathogenic mechanisms, and has high incidence and mortality rate. At present, the diagnosis of gastric cancer mainly includes gastroscopy, serum analysis and needle biopsy, and the treatment methods include conventional surgical resection, radiotherapy and chemotherapy. Yet, some limitations were involved in these diagnostic and therapeutic methods, so accurate targeted therapy has received considerable attention. MicroRNAs (miRNAs) are non-coding RNA that can interact with the 3-terminal non-translational region of the target gene mRNA to reduce the expression of the target gene, participate in the regulation of multiple signaling pathways, and play an important role in life activities of the cell. More and more studies have shown that miRNAs can participate in the formation and development of cancer, and many abnormally expressed miRNAs in gastric cancer cells are considered to be potential targets for clinical diagnosis and treatment of gastric cancer. This paper summarizes research progress of miRNAs in gastric cancer, and aims to provide new ideas for the diagnosis and treatment of gastric cancer.
Keywords: Gastric cancer; miRNAs; diagnosis; treatment; drug resistance
As one of the five most common malignant tumors in the world, gastric cancer has a high incidence and fatality rate. According to GLOBOCAN statistics, the number of new cases of gastric cancer reached 783,000 as of year 2018 [1]. Gastric cancer is one of the important causes of cancer-related deaths in some Asian countries, and its incidence in these regions has shown an upward trend year by year [2]. Although the overall medical level of the world continues to improve, and various diagnosis and treatment methods for gastric cancer are constantly improving and perfecting, the incidence and mortality of gastric cancer in various countries still remain high. Studies have shown that Helicobacter pylori infection in the gastrointestinal tract, long-term excessive intake of nitrite, and obesity are all considered to promote the formation and development of gastric cancer to a large extent [3–6]. The pathogenesis of gastric cancer is rather complicated, and the causes are various. A large number of studies have confirmed that the formation and development of gastric cancer are closely related to changes in the activity of multiple signaling pathways in cells, changes in the expression levels of factors such as protein and non-coding RNA, and changes in epigenetic modifications of certain intracellular factors. These changes further induce the deterioration of normal cells, cancer formation with immortal proliferation capacity, high invasiveness and metastasis capacity, and ultimately lead to the loss of organ function. Currently, physical examination, blood chemistry test, gastroscopy, acupuncture biopsy and other diagnostic methods are mainly used to differentiate, locate and stage the patient’s pathological tissue in clinic; the patient’s condition is intervened by conventional means such as surgical resection, radiotherapy and chemotherapy [7–10]. Yet, gastric cancer patients cannot be completely cured, which leads to a generally low survival rate for gastric cancer worldwide. For this reason, the research focus on the treatment of gastric cancer has gradually shifted from traditional methods to targeted therapies concentrating on the discovery and use of precise biomarkers. miRNA is a kind of non-coding RNA in cells, which can participate in a variety of cell life activities. As the research on cancers deepens, miRNAs have been proved to be closely related to the formation and development of cancer. As a result, miRNA targeted therapy is also considered to be a new direction for cancer treatment. This paper summarizes the research status of miRNA in the diagnosis and treatment of gastric cancer, with a aim to propose new ideas for the treatment of gastric cancer.
MicroRNAs (miRNAs) are single-stranded non-coding RNAs with a small molecular weight in cells, usually only about 19-23bp in mammalian cells. Initially, miRNA is usually transcribed by RNA polymerase II or III to form primary miRNA (Pri-miRNA), and the primary RNA is cleaved by the Drosha/DGCR8 complex to form precursor miRNAs (pre-miRNAs) with a hairpin structure [11]; Subsequently, the pre-miRNAs will be transported from the nucleus to the cytoplasm by the Exportin5-Ran-GTP complex, and the Dicer enzyme that binds to the binding protein TRBP on the double-stranded RNA will cleave the pre-miRNA to the length of the mature miRNA [11]; Eventually, double-stranded miRNAs will form RNA-induced silencing complexes (RISCs) with AGO2, in which one single strand of miRNAs will be separated from RISCs and be degraded immediately, while the other single strand will be combined with target mRNAs along with the RISCs complex [11]. By complementing the 3’-UTR of the target mRNA, miRNA inhibits the translation process of the protein, and then participates in the process of cell proliferation, differentiation and apoptosis, and has an important impact on the life activities of cells [12–14].
3 miRNA is Involved in the Formation and Development of Gastric Cancer
Extensive studies have demonstrated that the expression levels of certain miRNAs in gastric cancer cells are quite different from those in normal cells. miRNA can participate in a variety of signal transduction, therefore, the abnormal expression of some miRNAs can activate or inhibit the expression of a variety of oncogenes, participate in the regulation of cell proliferation, invasion, metastasis and apoptosis, and play a role in the formation and development of cancer [15–17]. Ye et al. [18] found in the study that miR-7 is significantly low-expressed in gastric cancer cells. The lack of miR-7 can cause abnormal activation of p65-mediated NF-κB signaling pathway, and miR-7 can target to inhibit the expression of p65 and p65 of phosphoric acid, consequently inhibiting the proliferation and migration of gastric cancer cells by inhibiting the expression of NF-κB and its downstream factors VEGF, MMP-2, MMP-9, ICAM-1 and VCAM-1. Wang et al. [19] found that miR-575 is over-expressed in gastric cancer cell lines such as BGC823, GC9811, SGC-7901, MGC-803 and NCI-N87, and miR-575 can promote the progression of gastric cancer by targeting the PTEN signaling pathway. And knocking down miR-575 can significantly inhibit the proliferation of gastric cancer cells and promote the apoptosis of gastric cancer cells. Xu et al. [20] found in the study that miR-122-5p is significantly lower-expressed in gastric cancer cell lines such as BCG-823, SGC-7901, MGC-803 and HGC-27, while over-expression of miR-122-5p can inhibit the proliferation, migration and invasion of gastric cancer cells by reducing the expression level of DUSP4 in cells. These studies suggest that miRNAs are closely related to the formation and development of gastric cancer.
4 miRNA Serves as a Diagnostic Marker for Gastric Cancer
Endoscopic biopsy and histopathological evaluation are the gold standard of gastric cancer diagnostics, but it is difficult to implement in routine screening on a population basis, particularly for asymptomatic individuals. Serum tumor markers, such as carcinoembryonic antigen (CEA), cancer antigen 19-9 (CA19-9) and cancer antigen 72-4 (CA72-4), have been developed as a clinical method for the diagnosis of gastric cancer. However, the sensitivity and specificity of these markers are not high. In addition, serum tumor markers may useful for monitor patients after surgery since positive conversion of tumor markers normally occurs 2 or 3 months before imaging abnormalities. However, serum markers can not be used to predict early cancer. MiRNAs are abnormally expressed in tumor tissues and body fluids of patients with gastric cancer. Since detection of miRNAs in plasma, serum, urine, and gastric juice can be used for diagnosis, multiple studies evaluated the performance of diagnostic miRNAs in body fluids. Several miRNAs exhibited values in diagnosis, recurrence monitoring and/or clinical staging. The degree of malignancy and clinical stage of the tumor have a strong correlation with the survival and prognosis of patients. Therefore, early diagnosis is of great significance to the survival and prognosis of patients. With the development of technology, many studies have used miRNA microarray technology to detect the expression level of miRNA in pathological tissues of gastric cancer patients, so as to obtain the differential expression map of miRNA in gastric cancer tissues. Hwang et al. [21] detected the changes in the expression of 132 miRNAs in the miRNA microarray analysis in 24 samples of gastric cancer patients (7 cases of early gastric cancer, 3 cases of high-grade dysplastic adenoma, 4 cases of low-grade dysplastic adenoma, and 10 cases of paracancerous tissue), of which 42 miRNAs have significant abnormal expressions in the diseased tissues of patients with early gastric cancer. Among these miRNAs, the expression levels of miR-200C and miR-29a in gastric cancer tissues of patients were significantly lower than those of normal gastric mucosal tissues, while miR-601, mir-107, miR-18a, miR-370, miR-300 and miR-96 increased significantly. And in subsequent further screening using RT-qPCR, 5 representative miRNAs were obtained as markers of early gastric cancer. Liu et al. [22] selected lesion specimens of 15 patients from 40 gastric cancer patients admitted to the Shanghai Cancer Center of Fudan University, and used microRNAs arrays to identify 2006 differentially expressed miRNAs (302 highly expressed miRNAs and 1704 lowly expressed miRNAs). In addition, he further screened out 6 significantly abnormally expressed miRNA (miR-16-2-3p, miR-340-5p, miR-338-3p, miR-142-3p, miR-142-5p and miR-582-5p) through RT-PCR, and finally confirmed that miR-338-3p and miR-142-3p can be used as biomarkers for patients with advanced gastric cancer. These studies show that the expression levels of certain miRNAs have specificity in different stages of gastric cancer tissues, have the potential as gastric cancer markers, and have great application value in the diagnosis of different stages.
Helicobacter pylori infection is also one of the main causes of gastric cancer, and studies have confirmed that the mechanism of Helicobacter pylori inducing gastric cancer is closely related to certain miRNAs. Chang et al. [23] compared the miRNA differential expression profiles of the adjacent and pathological tissues of gastric cancer patients through miRNA microarrays and found that there are about 219 miRNAs in gastric cancer tissues of H. pylori positive and negative patients with significant differential expression. And among them, miR-99b-3p, miR-564 and miR-638 were significantly up-regulated in the cancer tissues of Helicobacter pylori-positive patients, while miR-204-5p, miR-338-5p, miR-375 and miR-548c-3p were significantly up-regulated in the pathological tissues of Helicobacter pylori-negative patients, which suggests that Helicobacter pylori-positive and negative gastric cancer may have different pathogenesis. Lee et al. [24] revealed that there are 156 significantly differentially expressed miRNAs in the positive and negative noncancerous gastric mucosa of Pylori. For the negative normal gastric mucosa, the expression of miR-135b-5p, mir-196-5p and miR-145-5p is significantly dysregulated, while the expression of miR-18a-5p, miR-135b-5p and miR-196a-5p in gastric cancer tissues is significantly dysregulated compared to the positive normal gastric mucosa. Therefore, it is concluded that there are certain differences in the pathogenesis of Helicobacter pylori positive and negative gastric cancer. Rai et al. [25] analyzed cancer and adjacent tissues in 40 cases of gastric cancer patients and found that compared with Helicobacter pylori-negative tissues, tumor-promoting factors miRNA-223 and miR-221 in cancer tissues and adjacent tissues infected with Helicobacter pylori showed significant high expression, and it is speculated that the abnormal expression of miR-223 and miR-221 may be related to the carcinogenicity of Helicobacter pylori. In addition, the abnormal expression profile of miRNA can not only be obtained by detecting gastric cancer tissues, but also by non-invasive methods to detect patients’ peripheral blood, gastric juice and urine, so as to achieve rapid and accurate screening for early gastric cancer [26–28]. Therefore, miRNA has broad application prospects in cancer diagnosis, clinical staging and tumor development prediction.
5 miRNA Serves as a Therapeutic Target for Gastric Cancer
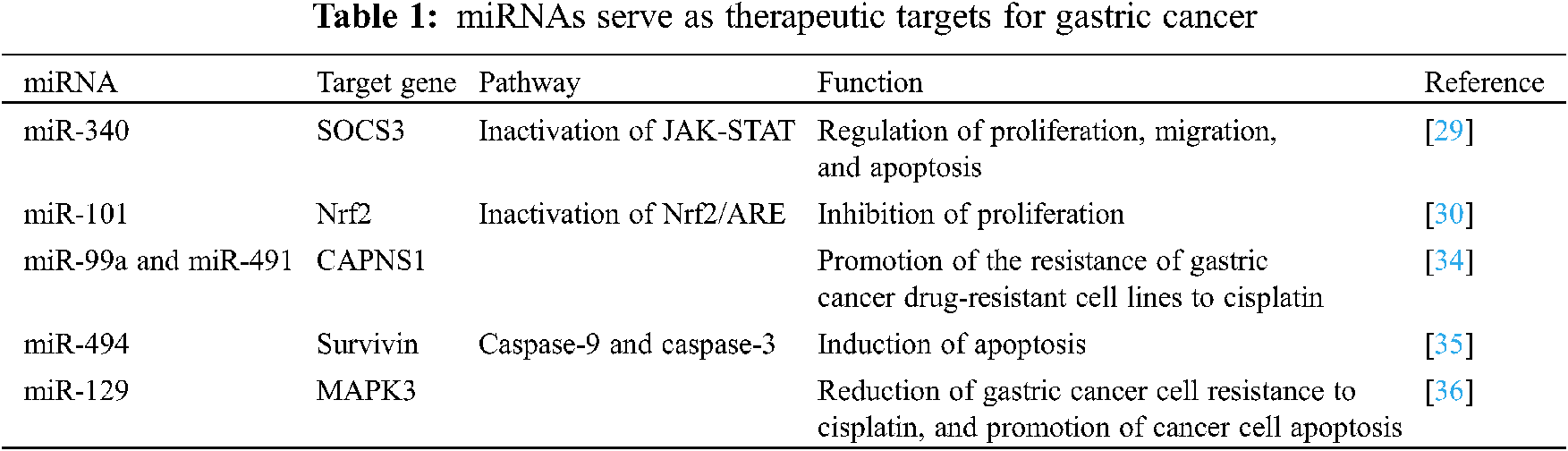
miRNAs play a vital role in cell life activities and can participate in almost all intracellular signal pathway regulation. Therefore, taking miRNAs as targets, by intervening in the expression levels and epigenetic modifications of some key miRNAs, can play a positive role in the treatment of cancer. Currently, studies on miRNA targeting anticancer use miRNA inhibitors or mimics to interfere or promote the expression of target miRNAs in cancer cells, thereby inhibiting the proliferation, migration and invasion of cancer cells by affecting the activity of multiple signaling pathways. Xiao et al. [29] found that miR-340 can bind to the 3’-UTR end of SOCS3 gene mRNA to inhibit its expression in cancer cells, and then participate in the regulation of life activities such as proliferation, migration and apoptosis of gastric cancer cells by inactivating the JAK-STAT signaling pathway. And Dong et al. [30] found that miR-101 can target to inhibit the expression of Nrf2, and up-regulating the expression of miR-101 in gastric cancer cell lines can inhibit the activity of the Nrf2/ARE signaling pathway, thereby effectively inhibiting the proliferation of gastric cancer cells, and promoting its apoptosis. These studies show that the intervention of some key miRNAs can effectively kill cancer cells, so as to achieve the purpose of cancer treatment.
Radiotherapy, chemotherapy and surgical resection are currently the main methods of treatment of tumors, and drug resistance is one of the main reasons affecting the treatment [31–33]. A large number of studies have shown that the resistance of cancer cells to chemotherapy drugs is related to certain miRNAs, and changing the expression level of these miRNAs can significantly improve the killing effect of chemotherapy drugs on cancer cells. miRNA can interfere with the autophagy and other signal pathways of cancer cells by regulating the expression levels of its downstream genes, and weaken the resistance of tumors to drugs. Therefore, miRNA targeted therapy may provide a new strategy for overcoming the drug resistance of cancer cells. Zhang et al. [34] found that miR-99a and miR-491 can promote the resistance of gastric cancer drug-resistant cell lines to cisplatin by targeted inhibiting the expression of CAPNS1. Xu et al. [35] revealed that miR-494 is low expressed in gastric cancer tissues, and up-regulation of miR-494 can increase the sensitivity of gastric cancer cells to TRAIL by targeted inhibiting the expression of survivin, and promote TRAIL to induce decrease of cancer cell mitochondrial membranes potential, release of cytochrome C and AIF, interaction of apaf-1 and caspase-9, and activation of caspase-9 and caspase-3, and finally induce apoptosis of gastric cancer cells. Cao et al. [36] found that miR-129 is low-expressed in gastric cancer cells, and up-regulation of miR-129 can inhibit MAPK3 expression to reduce gastric cancer cell resistance to cisplatin, and promote cancer cell apoptosis. These studies suggest that certain miRNAs can be used as potential targets to reduce the sensitivity of gastric cancer cells to chemotherapy drugs, thereby improving the therapeutic effect of clinical interventions (Tab. 1).
As an important factor in cell life activities, miRNA is closely related to the formation and development of gastric cancer. Regulating the expression and epigenetic modification of some miRNAs in cancer cells has a positive effect on the diagnosis and treatment of gastric cancer. Although the regulatory role of multiple miRNAs in gastric cancer has been widely reported, the understanding of the regulatory mechanism of miRNAs involved in the formation and development of gastric cancer still remains limited due to the complexity of the cancer itself and the diversity of miRNA types. Moreover, although many current studies have confirmed that by interfering with the expression of certain miRNAs in cancer cells and their epigenetic modification, they can effectively kill cancer cells and obtain satisfactory anti-cancer effects, the application value of miRNAs as biomarkers and therapeutic targets for clinical diagnosis and its safety still need further research. As research continues to deepen coupled with the experience gained from the past, miRNA targeted therapy may be widely used in the diagnosis and treatment of gastric cancer in the future.
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Bray, F., Ferlay, J., Soerjomataram, I., Siegel, R. L., Torre, L. A. et al. (2018). Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians, 68(6), 394–424. DOI 10.3322/caac.21492. [Google Scholar] [CrossRef]
2. Kai, K., Satake, M., Tokunaga, O. (2018). Gastric adenocarcinoma of fundic gland type with signet-ring cell carcinoma component: A case report and review of the literature. World Journal of Gastroenterology, 24(26), 2915–2920. DOI 10.3748/wjg.v24.i26.2915. [Google Scholar] [CrossRef]
3. Xu, H. M., Wang, X. (2020). Current status and prospects of clinical research on diagnosis and treatment of gastric cancer in China. Chinese Journal of Gastrointestinal Surgery, 23(2), 109–114. DOI 10.3760/cma.j.issn.1671-0274.2020.02.003. [Google Scholar] [CrossRef]
4. de Souza, C. R. T., Almeida, M. C. A., Khayat, A. S., da Silva, E. L., Soares, P. C. et al. (2018). Association between Helicobacter pylori, Epstein-Barr virus, human papillomavirus and gastric adenocarcinomas. World Journal of Gastroenterology, 24(43), 4928–4938. DOI 10.3748/wjg.v24.i43.4928. [Google Scholar] [CrossRef]
5. Hernández-Ramírez, R. U., Galván-Portillo, M. V., Ward, M. H., Agudo, A., González, C. A. et al. (2009). Dietary intake of polyphenols, nitrate and nitrite and gastric cancer risk in Mexico City. International Journal of Cancer, 125(6), 1424–1430. DOI 10.1002/ijc.24454. [Google Scholar] [CrossRef]
6. Olefson, S., Moss, S. F. (2015). Obesity and related risk factors in gastric cardia adenocarcinoma. Gastric Cancer, 18, 23–32. DOI 10.1007/s10120-014-0425-4. [Google Scholar] [CrossRef]
7. Wang, F. H., Shen, L., Li, J., Zhou, Z. W., Liang, H. et al. (2019). The Chinese Society of Clinical Oncology (CSCOClinical guidelines for the diagnosis and treatment of gastric cancer. Cancer Communications, 39(11–31. DOI 10.1186/s40880-019-0349-9. [Google Scholar] [CrossRef]
8. Zhou, D., Yuan, X., Wang, X., Ren, J., Yang, R. et al. (2015). A case of gastric adenocarcinoma metastasis to the esophagus possibly caused by gastroscopy or gastric reflux. International Journal of Clinical and Experimental Pathology, 8(11), 15386–15390. [Google Scholar]
9. Candido, M. V., Syrjä, P., Kilpinen, S., Spillmann, T. (2018). Canine breeds associated with gastric carcinoma, metaplasia and dysplasia diagnosed by histopathology of endoscopic biopsy samples. Acta Veterinaria Scandinavica, 60(11–9. DOI 10.1186/s13028-018-0392-6. [Google Scholar] [CrossRef]
10. Shen, J., Liu, J., Li, C., Wen, T., Yang, J. (2018). The prognostic significance of serum HBeAg on the recurrence and long-term survival after hepatectomy for hepatocellular carcinoma: A propensity score matching analysis. Journal of Viral Hepatitis, 25(9), 1057–1065. DOI 10.1111/jvh.12911. [Google Scholar] [CrossRef]
11. Link, A., Kupcinskas, J. (2018). MicroRNAs as non-invasive diagnostic biomarkers for gastric cancer: Current insights and future perspectives. World Journal of Gastroenterology, 24(30), 3313–3329. DOI 10.3748/wjg.v24.i30.3313. [Google Scholar] [CrossRef]
12. Chen, J., Wu, L., Sun, Y., Luo, C., Chen, X. et al. (2020). Diagnostic value and clinical significance of circulating miR‐650 and CA211 in detecting of gastric carcinoma. Oncology Letters, 20(5). DOI 10.3892/ol.2020.12117. [Google Scholar] [CrossRef]
13. Li, Z. W., Zhang, T. Y., Yue, G. J., Tian, X., Wu, J. Z. et al. (2020). Small nucleolar RNA host gene 22 (SNHG22) promotes the progression of esophageal squamous cell carcinoma by miR-429/SESN3 axis. Annals of Translational Medicine, 8(16), 1007. DOI 10.21037/atm-20-5332. [Google Scholar] [CrossRef]
14. Liu, Y., Zhang, X., Zhang, Y., Hu, Z., Yang, D. et al. (2020). Corrigendum to “Identification of miRNomes in human stomach and gastric carcinoma reveals miR-133b/a-3p as therapeutic target for gastric cancer” [Canc. Lett. 369 (2015) 58-66]. Cancer Letters, 494, 3–4. DOI 10.1016/j.canlet.2020.07.016. [Google Scholar] [CrossRef]
15. Zhou, K., Song, B., Wei, M., Fang, J., Xu, Y. (2020). MiR-145-5p suppresses the proliferation, migration and invasion of gastric cancer epithelial cells via the ANGPT2/NOD_LIKE_RECEPTOR axis. Cancer Cell International, 20(11–11. DOI 10.1186/s12935-020-01483-6. [Google Scholar] [CrossRef]
16. Zheng, J., Zhang, Y. W., Li, T. K., Bao, Y., Zhang, S. X. (2020). Effect of miR-204-5p on the proliferation, migration, and invasion on tongue squamous cell carcinoma SCC25 cells by targeting bromodomain-containing protein 4. West China Journal of Stomatology, 38(2), 185–192. DOI 10.7518/hxkq.2020.02.013. [Google Scholar] [CrossRef]
17. Yan, F., Ma, Y., Liu, L., Li, L., Deng, J. et al. (2020). Long noncoding RNA HOXD-AS1 promotes the proliferation, migration, and invasion of colorectal cancer via the miR-526b-3p/CCND1 axis. Journal of Surgical Research, 255, 525–535. DOI 10.1016/j.jss.2020.05.078. [Google Scholar] [CrossRef]
18. Ye, T., Yang, M., Huang, D., Wang, X., Xue, B. et al. (2019). MicroRNA-7 as a potential therapeutic target for aberrant NF-κB-driven distant metastasis of gastric cancer. Journal of Experimental & Clinical Cancer Research, 38(11–18. DOI 10.1186/s13046-019-1074-6. [Google Scholar] [CrossRef]
19. Wang, Y., Xu, F., Zhang, P., Wang, P., Wei, Y. et al. (2019). MicroRNA-575 regulates development of gastric cancer by targeting PTEN. Biomedicine & Pharmacotherapy, 113, 108716. DOI 10.1016/j.biopha.2019.108716. [Google Scholar] [CrossRef]
20. Xu, X., Gao, F., Wang, J., Tao, L., Ye, J. et al. (2018). MiR-122-5p inhibits cell migration and invasion in gastric cancer by down-regulating DUSP4. Cancer Biology & Therapy, 19(5), 427–435. DOI 10.1080/15384047.2018.1423925. [Google Scholar] [CrossRef]
21. Hwang, J., Min, B. H., Jiang, J., Kang, S. Y., Bae, H. et al. (2018). MicroRNA expression profiles in gastric carcinogenesis. Scientific Reports, 8(11–8. DOI 10.1038/s41598-018-32782-8. [Google Scholar] [CrossRef]
22. Liu, X., Cai, H., Sheng, W., Huang, H., Long, Z. et al. (2018). microRNAs expression profile related with response to preoperative radiochemotherapy in patients with locally advanced gastric cancer. BMC Cancer, 18(11–8. DOI 10.1186/s12885-018-4967-4. [Google Scholar] [CrossRef]
23. Chang, H., Kim, N., Park, J. H., Nam, R. H., Choi, Y. J. et al. (2015). Different microRNA expression levels in gastric cancer depending on Helicobacter pylori infection. Gut and Liver, 9(2), 188–196. DOI 10.5009/gnl13371. [Google Scholar] [CrossRef]
24. Lee, J. W., Kim, N., Park, J. H., Kim, H. J., Chang, H. et al. (2017). Differential MicroRNA expression between gastric cancer tissue and non-cancerous gastric mucosa according to Helicobacter pylori status. Journal of Cancer Prevention, 22(1), 33–39. DOI 10.15430/JCP.2017.22.1.33. [Google Scholar] [CrossRef]
25. Rai, R. P., Prasad, K., Khatoon, J., Mohindra, S., Ghoshal, U. et al. (2016). Elevated expression of miR-223 and miR-21 in Helicobacter pylori induced gastric cancer patients. International Journal of Infectious Diseases, 45(S1), 147–148. DOI 10.1016/j.ijid.2016.02.356. [Google Scholar] [CrossRef]
26. You, X., Wang, Y., Wu, J., Liu, Q., Chen, D. et al. (2019). Aberrant cytokeratin 20 mRNA expression in peripheral blood and lymph nodes indicates micrometastasis and poor prognosis in patients with gastric carcinoma. Technology in Cancer Research & Treatment, 18(5), 153303381983285. DOI 10.1177/1533033819832856. [Google Scholar] [CrossRef]
27. Sung, J., Kim, N., Lee, J., Hwang, Y. J., Kim, H. W. et al. (2018). Associations among gastric juice pH, atrophic gastritis, intestinal metaplasia and Helicobacter pylori infection. Gut and Liver, 12(2), 158–164. DOI 10.5009/gnl17063. [Google Scholar] [CrossRef]
28. Hong, C. S., Cui, J., Ni, Z., Su, Y., Puett, D. et al. (2011). A computational method for prediction of excretory proteins and application to identification of gastric cancer markers in urine. PLoS One, 6(2), e16875. DOI 10.1371/journal.pone.0016875. [Google Scholar] [CrossRef]
29. Xiao, C., Hong, H., Yu, H., Yuan, J., Guo, C. et al. (2018). MiR-340 affects gastric cancer cell proliferation, cycle, and apoptosis through regulating SOCS3/JAK-STAT signaling pathway. Immunopharmacology and Immunotoxicology, 40(4), 278–283. DOI 10.1080/08923973.2018.1455208. [Google Scholar] [CrossRef]
30. Dong, X. Q., Zhang, Y. H., Shang, X. Q., Zeng, Y. J. (2019). Effects of miR-101 on the proliferation and apoptosis of gastric mucosal epithelial cells via Nrf2/ARE signaling pathway. European Review for Medical and Pharmacological Sciences, 23(12), 5187–5194. DOI 10.26355/eurrev_201906_18183. [Google Scholar] [CrossRef]
31. Pernot, S., Voron, T., Perkins, G., Lagorce-Pages, C., Berger, A. et al. (2015). Signet-ring cell carcinoma of the stomach: Impact on prognosis and specific therapeutic challenge. World Journal of Gastroenterology, 21(40), 11428–11438. DOI 10.3748/wjg.v21.i40.11428. [Google Scholar] [CrossRef]
32. Yang, W., Ma, J., Zhou, W., Cao, B., Zhou, X. et al. (2017). Molecular mechanisms and theranostic potential of miRNAs in drug resistance of gastric cancer. Expert Opinion on Therapeutic Targets, 21(11), 1063–1075. DOI 10.1080/14728222.2017.1389900. [Google Scholar] [CrossRef]
33. Fagoonee, S., Pellicano, R. (2019). Helicobacter pylori: molecular basis for colonization and survival in gastric environment and resistance to antibiotics. A short review. Infectious Diseases, 51(6), 399–408. DOI 10.1080/23744235.2019.1588472. [Google Scholar] [CrossRef]
34. Zhang, Y., Xu, W., Ni, P., Li, A., Zhou, J. et al. (2016). MiR-99a and MiR-491 regulate cisplatin resistance in human gastric cancer cells by targeting CAPNS1. International Journal of Biological Sciences, 12(12), 1437–1447. DOI 10.7150/ijbs.16529. [Google Scholar] [CrossRef]
35. Xu, S., Li, D., Li, T., Qiao, L., Li, K. et al. (2018). miR-494 sensitizes gastric cancer cells to TRAIL treatment through downregulation of survivin. Cellular Physiology and Biochemistry, 51(5), 2212–2223. DOI 10.1159/000495867. [Google Scholar] [CrossRef]
36. Cao, H. Y., Xiao, C. H., Lu, H. J., Yu, H. Z., Hong, H. et al. (2019). MiR-129 reduces CDDP resistance in gastric cancer cells by inhibiting MAPK3. European Review for Medical and Parmacological Sciences, 23(15), 6478–6485. DOI 10.26355/eurrev_201908_18531. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |