
 | Oncologie |  |
DOI: 10.32604/Oncologie.2021.015503
ARTICLE
Mir-612 Inhibits Proliferation and Invasion of Urothelial Carcinoma of Bladder Cells through Activating Hippo Pathway via Targeting PMEPA1
Department of Urology, Shandong Provincial Hospital, Cheeloo College of Medicine, Shandong University, Jinan, 250021, China
*Corresponding Authors: Xunbo Jin. Email: wp1202000@163.com; Dong Zhang. Email: zd_sdu@163.com
Received: 23 December 2020; Accepted: 02 April 2021
Abstract: Urothelial carcinoma of bladder (UCB) is a common urological malignancy in the world, but its progression mechanism remains unclear. MiR-612 was found as an anti-tumor factor in multiple types of cancer, while few studies have revealed its functions in UCB cells. Based on this, UCB cells such as HTB-9 and HTB-4, and normal urothelial of bladder cells such as SV-Huc1, were used as subjects in this study. Western blot, qRT-PCR, CCK-8 assay and transwell assay were used to assess functions of miR-612 in UCB cells. The database, miRWalk, was used to search for potential targets of miR-612, and dual-luciferase reporter assay was used to verify the accuracy of the forecasting results. Besides, PMEPA1 overexpressed vector and miR-612 mimics were co-transfected into HTB-4 to observe the regulation mechanism of miR-612. It was found that miR-612 was significantly downregulated in HTB-9 and HTB-4 cells rather than in SV-Huc1 cells, and overexpressed miR-612 reduced the proliferation and invasion of HTB-4 cells. The expression level of YAP1 was negatively related with miR-612 while LATS1 was positively related with miR-612. Besides, the results of miRWalk and dual-luciferase reporter assay indicated that PMEPA1 was a target of miR-612, and overexpression of PMEPA1 could reverse the effects of miR-612 on UCB including weakened proliferation and invasion abilities, low expression level of YAP1 and high expression level of LATS1. Therefore, this study suggests that miR-612 inhibits the proliferation and invasion of urothelial carcinoma of bladder cells through activating Hippo pathway via targeting PMEPA1.
Keywords: UCB; miR-612; Hippo; YAP1; PMEPA1
Urothelial carcinoma of bladder (UCB) is one of the most dangerous urological malignancy, and more than 80,000 people have been diagnosed with this disease in the United States annually [1–3]. At present, several studies have indicated that the abnormal expression of some oncogenes or anti-oncogenes may cause the tumorigenesis of UCB, and some tumor factors have been found to play a crucial role in formation and development of UCB [4,5]. For instance, it has been found that TRIM65 can enhance the invasion ability of UCB via promoting ANXA2 ubiquitination and degradation [6]. Although many studies have revealed that some tumor factors take part in the formation and invasion of cancer cells via regulating some signal pathways such as wnt/β-catenin and PI3K/AKT, the mechanism of UCB development remains unclear [7,8]. Therefore, more comprehensive researches on UCB are necessary.
MicroRNAs (miRNAs), a class of noncoding RNAs, which have short chains with 20–24 nucleotides [9,10]. In recent years, miRNAs have attracted increasing attention from researchers in the world, and lots of studies have indicated that miRNAs play important roles in life activities of cells [11]. Besides, it is also found that some miRNAs have intimate connection with the formation and development of tumor cells, and their abnormal expression is confirmed as the major reason of cancer deterioration, including tumor malignant proliferation, invasion and so on. Some miRNAs such as miR-143, miR-410 and miR-34a-3p have been confirmed to be related with UCB development [12–14]. Generally, the deterioration of cancer cells is related with the inhibition or activation of some signal pathways, and some miRNAs may play key roles in inhibiting or activating the pathways. For instance, miR-98 can regulate the malignancy of bladder cancer through STAT3/Wnt/β-catenin pathway axis, and miR-125b-5p can target HK2 and suppress PI3K/AKT pathway to inhibit the proliferation of bladder cancer [15,16].
MiR-612, an anti-tumor factor, has negative correlation with some cancers and takes part in many signal pathways, while few studies have revealed its regulatory mechanism on UCB [17]. In this study, we explored the functions of miR-612 in UCB cells, and illuminated its functions and regulation mechanism in UCB, aiming to provide some reference for the research and treatment of UCB.
Bladder urothelial carcinoma cell lines (HTB-9 and HTB-4) and normal bladder epithelial cell line (SV-HUC-1) were used as subjects in this study. All frozen cells were melted in 37°C water bath, and then were washed with serum-free DMEM. After that, all cells were resuspended with DMEM containing 10% foetal bovine serum and then were cultured in an incubator (37°C and 5% CO2). All cells were subcultured at 80% confluence.
When cells were at 70% confluence in each well, the culture fluid was replaced with DMEM without FBS (fetal bovine serum). After that, the miR-612 mimic, miR-NC, PMEPA1 overexpression plasmid (pCMV-PMEPA1) and empty pCMV plasmid (NC) (RiboBio, Guangzhou, China) were transfected into the wells with Lipofectamine 2000 (Invitrogen, California, USA), and then the cells were incubated for 48 h. 4 μg DNA, 100 pmol RNA or 10 μl Lipofectamine 2000 diluted by 250 μl serum-free medium, respectively. After incubation for 5 min, the diluted DNA/RNA were mixed with diluted Lipofectamine 2000 (total volume = 500 μl), and then the mixtures were incubated for 20 min at room temperature. After that, each well was added with the 500 μl of the mixture, and then the cell were cultured for 24~48 h.
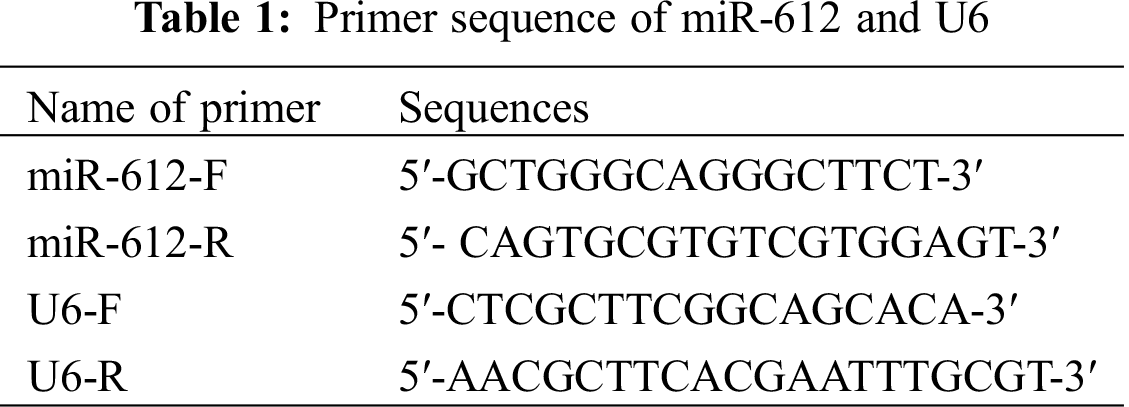
Total RNAs of the cells were extracted by Trizol reagent, and then were transcribed into cDNA by PrimeScript® RT reagent Kit (Thermo Fisher, Massachusetts, USA). The primers of miR-612 were synthesized and purified by RiboBio, Guangzhou, China. The qRT-PCR reaction system (10 μL) was prepared according to the operating instructions of SYBR Fluorescent Quantitative Premix Kit. The PCR reaction conditions were pre-denaturation at 95°C for 30 s, 95°C for 5 s, and 60°C for 30 s for a total of 40 cycles. U6 was used as the control of miR-612. The forward and reverse primer sequences of miR-612 and U6 are shown in Tab. 1 [18].
The total proteins of cells were extracted with RIPA buffer, and 1% PMSF (Beyotime, Shanghai, China) was instantly added to keep the activity of proteins. The concentration of extracts was measured by a Pierce BCA protein assay kit (Beyotime, Shanghai, China). After that, the proteins of the extracts were separated by 10% SDS-PAGE, and then were transferred from SDS-PAGE onto the PVDF membranes. The membranes were blocked with 5% non-fat milk for 1 h. After that, the membranes were incubated with the primary antibodies of target proteins at 4°C over night and β-actin was used for the quantification of target proteins. After washing three times with TBST, the membranes were incubated with secondary antibodies for 1.5 h at 25°C. Finally, chemiluminescence detection system was used to observe protein expression level. The antibodies were used as follow: anti-YAP1 (1:1000, ab2219137, ThermoFisher, Massachusetts, USA); anti-LATS1(1:1000, ab2736084, ThermoFisher, Massachusetts, USA); anti-PMEPA1 (1:2000, ab2850672, ThermoFisher, Massachusetts, USA); anti-β-actin (1:1000, sc-47,778, Santa Cruz, Argentina).
2.5 Dual-Luciferase Reporter Assay
The 3’-UTR of PMEPA1 containing the predicted wild-type (Wt) or mutant-type (Mut) miR-612 binding sequences were inserted into pmirGLO luciferase reporter vector (Promega, Wisconsin, USA). The luciferase vectors and miR-612 were co-transfected into HEK-293T cells, and then the cells were incubated for 48 h. Finally, the luciferase activity of the HEK-293T cells was observed by a dual-luciferase reporter assay system.
The invasion of the cells was detected by transwell assay. The cell suspension (5 × 104 cell/mL) was prepared at first. Transwell chambers coated with Matrigel were used to assess invasion capability of cells. 300 μL of serum-free cell suspension was added to the upper chamber, and 500 μL of medium (containing 10% FBS) was added into the bottom chamber. All cells were incubated for 24 h, and the cells in the upper chamber were removed and the cells in the bottom chamber were fixed by 10% formaldehyde. After that, 0.05% crystal violet was used to stain the cells. Finally, the number of cells on the bottom was observed and counted under the microscope.
UCB cells were seeded into 96-well plates and cultured with DMEM (1% FBS) for 24 to 96 h. Then miR-612 mimic, miR-NC, PMEPA1 overexpression plasmid (pCMV-PMEPA1) and empty pCMV plasmid (NC) were transfected into cells with their density at 70% in each well. After incubating for 48 h, all wells were added with 10 μL of CCK-8 solution (Amyjet, Wuhan, China). After incubation for 4 h, the absorbance value of the cells was measured by a microplate reader (Flash, Shanghai, China) at 450 nm, and the absorbance value of the wells with CCK-8 solution was used as controls.
All the experiments were performed at least three times, and the data were analyzed by SPSS 20.0, and the figures were charted by Graphpad Prism 8.0. The difference between the groups was calculated through Chi-squared test or ANOVA with Tukey’s post hoc-test. *P < 0.05 means that the difference of the data in two groups is statistically significant.
3.1 MiR-612 was Downregulated in UCB Cell Lines
The miR-612 expression level in UCB cell lines was detected by qRT-PCR. As shown in Fig. 1, compared with normal urothelial cells, miR-612 was significantly downregulated in HTB-9 and HTB-4 cells (Fig. 1. P < 0.05).
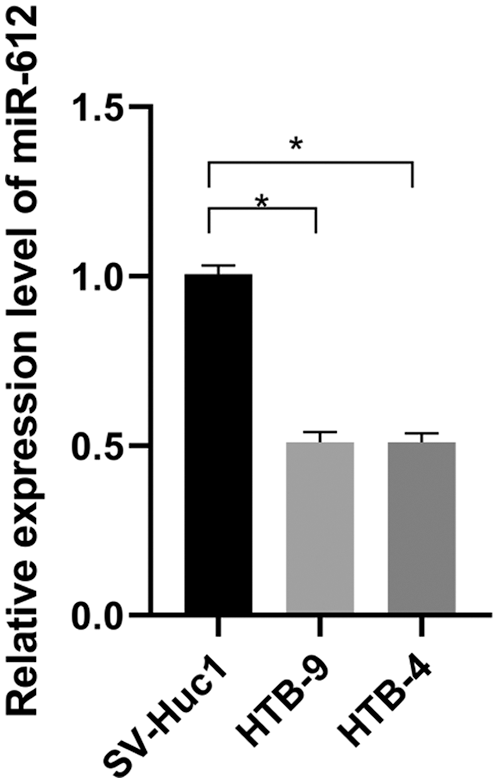
Figure 1: MiR-612 was downregulated in urothelial carcinoma of bladder cells compared with normal cell lines. * means P < 0.05
3.2 Overexpression of miR-612 Inhibited the Proliferation and Invasion of HTB-4 Cells
To analyze the functions of miR-612 in UCB cells, miR-612 mimics were transfected into HTB-4 cells, and CCK-8 assay and transwell assay were used to observe the proliferation and invasion abilities of HTB-4 cells. The results (Fig. 2B, P < 0.05) showed that miR-612 mimics effectively enhanced expression level of miR-612, and then the proliferation of HTB-4 cells was inhibited notably. The results showed that the number of invasion cells decreased when miR-612 was upregulated (Fig. 2C. P < 0.05).
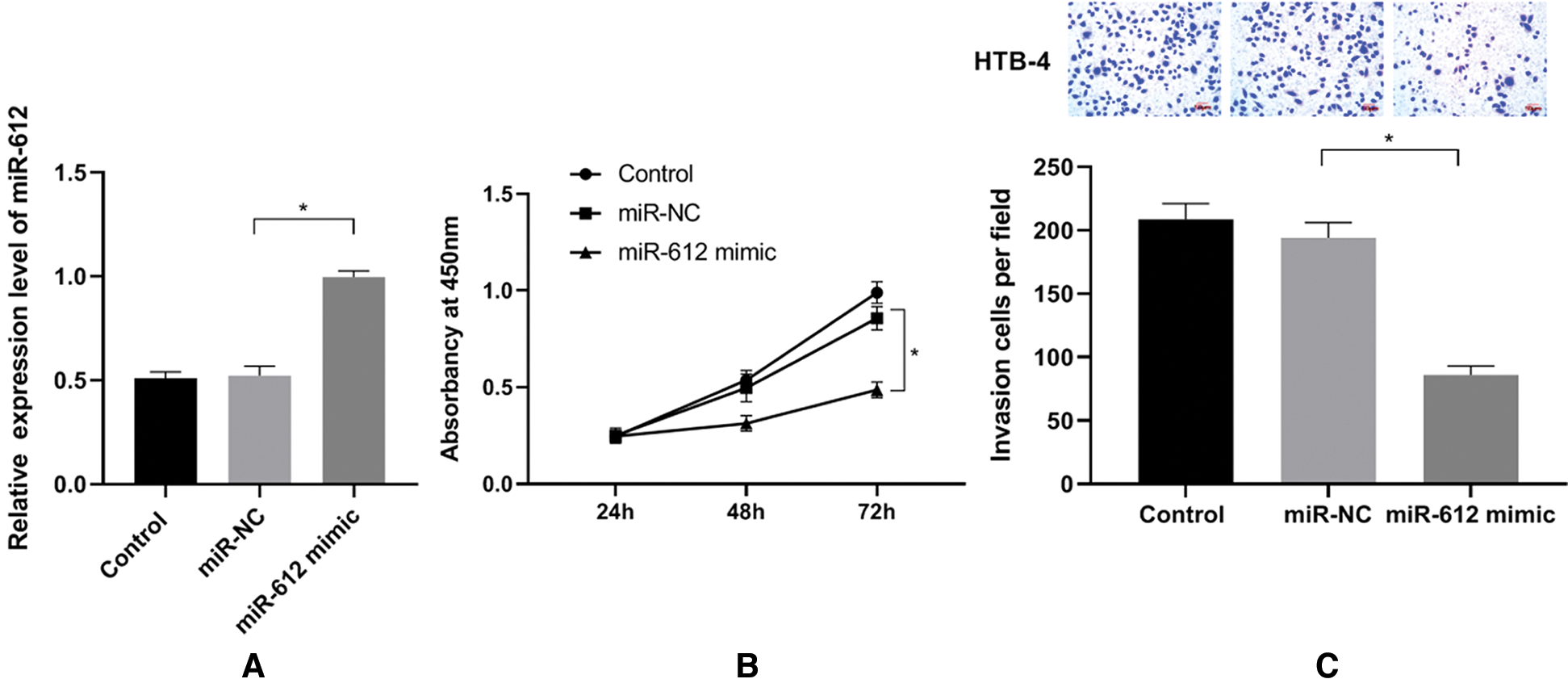
Figure 2: Overexpression of miR-612 inhibited proliferation and invasion abilities of HTB-4. (A) The relative expression level of miR-612 was measured by qRT-PCR. (B) The viability of HTB-4 was observed by CCk-8 assay. (C) The invasion of HTB-4 was observed by transwell assay. * means P < 0.05
3.3 YAP1 was Downregulated and LATS1 was Upregulated in HTB-4 Cells Induced by miR-612
To explore the mechanism of miR-612 on HTB-4 cells, the western blot was used to measure the expression level of YAP1 when miR-612 mimics were transfected into HTB-4 cells. The results showed that YAP1 was downregulated but LATS1 was upregulated when miR-612 was overexpressed in HTB-4 cells (Figs. 3A–3C. P < 0.05).
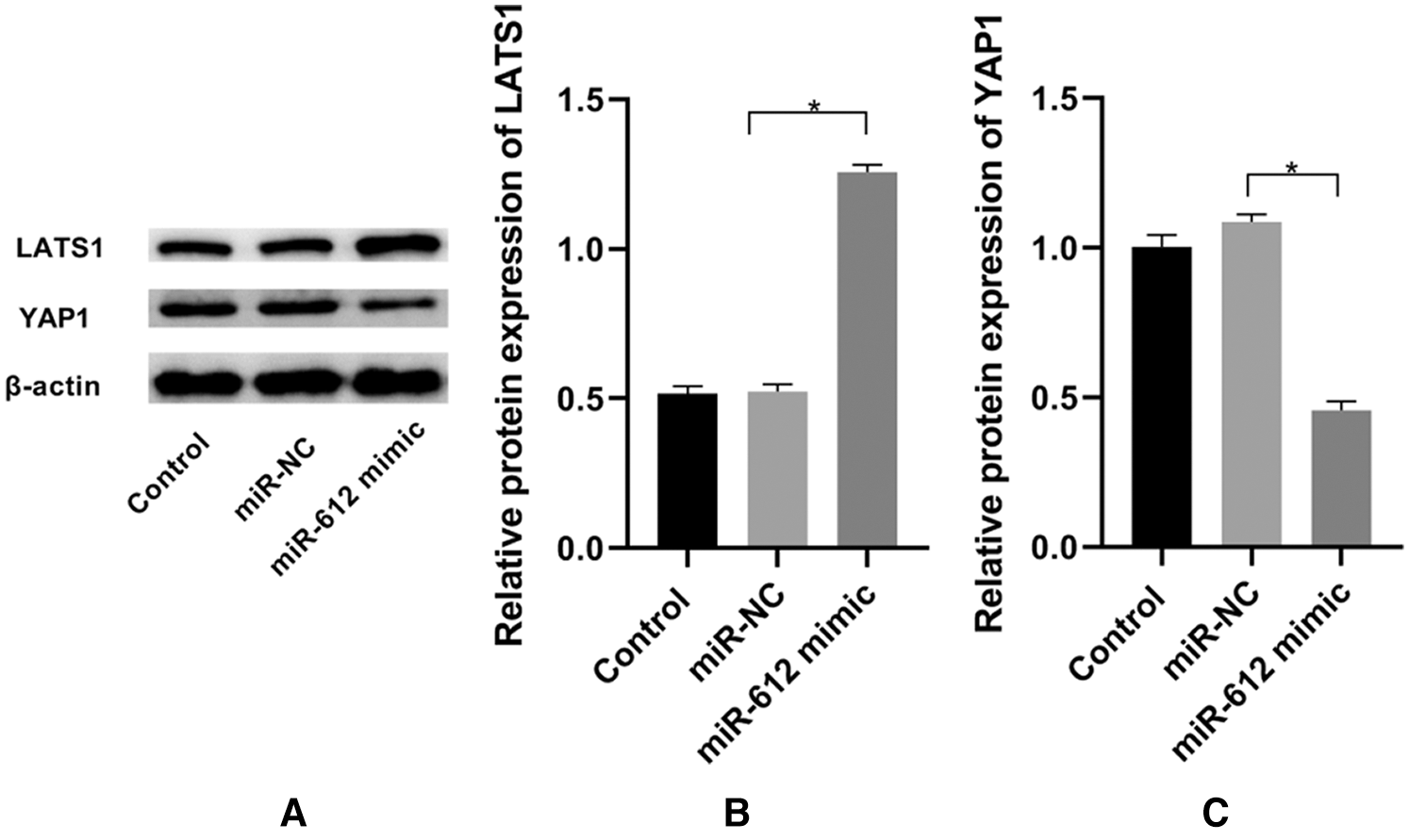
Figure 3: Overexpressed miR-612 promoted the expression of LATS1 and inhibited the expression of YAP1. (A) The relative expression levels of LATS1 and YAP1 was measured by western blot. (B) The relative protein expression level of LATS1. (C) The relative protein expression level of YAP1. * means P < 0.05
3.4 PMEPA1 is a Direct Target of miR-612
According to miRWalk, an online prediction database, PMEPA1 is one of the potential candidates, which has lower binding energy with miR-612 compared with other factors. To further validate the accuracy of this forecasting result, dual-luciferase reporter assay was used to detect the effect of miR-612 on luciferase activities of PMEPA1-wt and PMEPA1-mut. The results showed that the luciferase activity of the cells decreased significantly when co-transfected with PMEPA1-wt and miR-612. However, the luciferase activity of PMEPA1-mut did not show any notable changes whether miR-612 or miR-NC was co-transfected with PMEPA1-mut into HEK-293T cells (Fig. 4A, P < 0.05). Besides, PMEPA1 was significantly upregulated in HTB-9 and HTB-4 cells rather than in SV-Huc1 (Figs. 4B and 4C, P < 0.05).
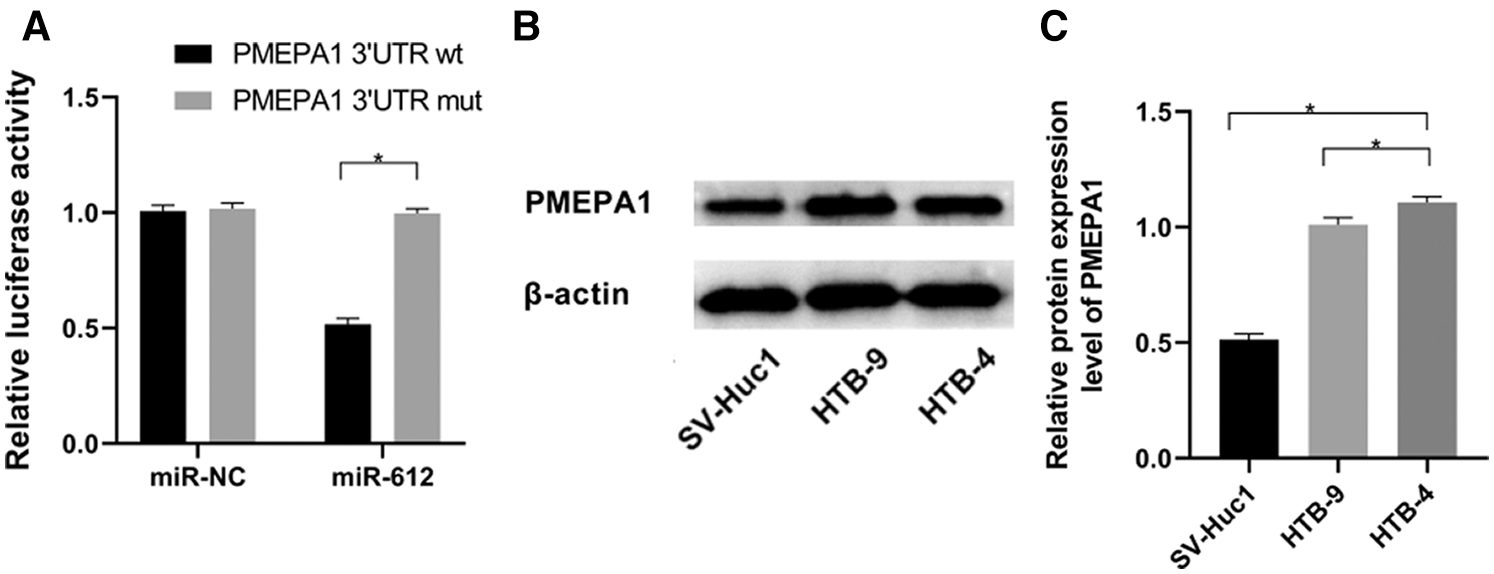
Figure 4: MiR-612 directly targeted PMEPA1-wt but didn’t have effect on PMEPA1-mut. PMEPA1 was significantly upregulated in HTB-9 and HTB-4 cells. (A) The binding effect of miR-612 and PMEPA1 was observed by dual-luciferase reporter assay. (B) The relative protein expression level of PMEPA1 was measured by western blot. (C) The relative protein expression level of PMEPA1 was measured by western blot. * means p < 0.05
3.5 PMEPA1 Reversed the Effects of miR-612 on HTB-4 Cells
To confirm whether the carcinostasis effect of miR-612 has direct connection with PMEPA1, miR-612 mimic and pCMV-PMEPA1 were co-transfected into HTB-4 cells to observe the proliferation and invasion capacities of HTB-4 cells. The results showed that miR-612 inhibited the expression level of PMEPA1. The results of western blot showed that LATS1 was upregulated but YAP1 was downregulated significantly when transfected with miR-612 mimics, while those phenomena were reversed in the cells which were co-transfected with miR-612 mimic and pCMV-PMEPA1 (Figs. 5A–5D, all P < 0.05). HTB-4 cells showed poor proliferation and invasion abilities when transfected with miR-612 mimics, and the effects of miR-612 on HTB-4 cells were reversed when the cells were co-transfected with miR-612 mimics and pCMV-PMEPA1 (Figs. 5E–5G, all P < 0.05).
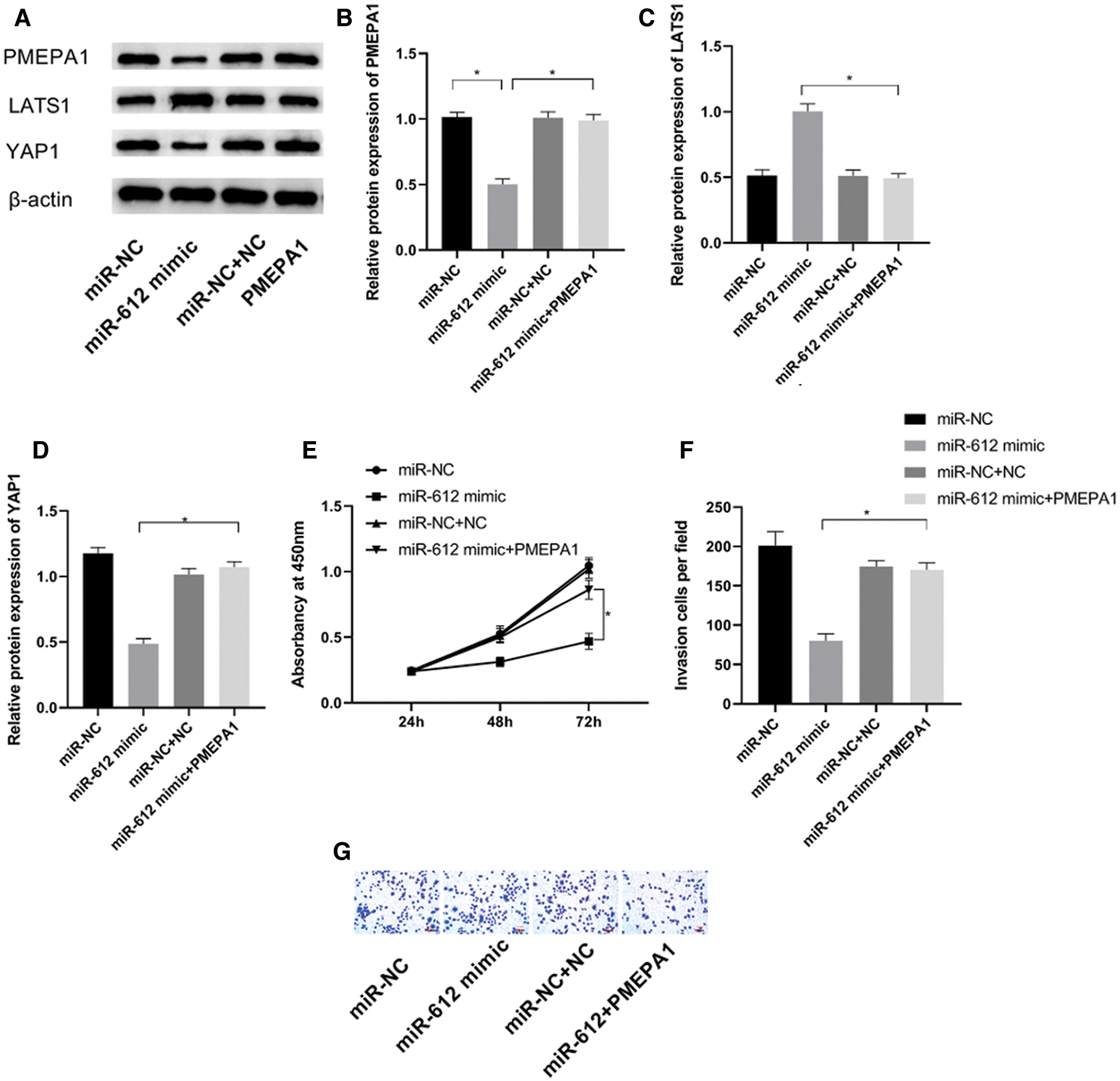
Figure 5: PMEPA1 reversed the effects of miR-612 on HTB-4 cells. (A) The protein expression level of PMEPA1, LATS1 and YAP1 were measured by western blot. (B) The relative protein expression level of PMEPA1. (C) The relative protein expression level of LATS1. (D) The relative protein expression level of YAP1. (E) The proliferation ability of HTB-4 was observed by CCK-8. (F, G) The invasion ability of HTB-4 was observed by transwell assay. * means P < 0.05
Recently, although many studies have focused on researching the formation and development of UCB, the pathogenic mechanism of UCB remains unclear [19]. MiRNAs involve in many life activities of cells, and increasing researches have indicate that the abnormal expression of some miRNAs contributes the proliferation and invasion of UCB [20]. Latest studies have showed that miR-612 plays an anti-tumor role in UCB cells, which inhibits UCB progression via targeting FOXK1 and ME1 [21,22]. In this study, it was found that miR-612 was downregulated in UCB cells such as HTB-9 and HTB-4 compared with SV-Huc1, indicating that miR-612 is related with the development of UCB to a large extent. Although, multiple studies have indicated that the expression level of miR-612 is related with the development of some tumors, while few studies revealed the functions of miR-612 in UCB cells.
In this study, it was found that miR-612 overexpression could effectively inhibit the proliferation and invasion of UCB cells, suggesting that miR-612 played an anti-tumor role in UCB cells. In hepatocellular carcinoma, miR-612 is downregulated and could inhibit the invasion ability of cancer cells through reprogramming lipid and blocking the function of their pseudopods via reducing the expression level of HADHA [23]. YAP1 is a key factor of downstream of Hippo pathway, which takes great part in cell life activities. In this study, the upregulation of miR-612 significantly reduced expression level of YAP1 in UCB cells, which may be one of the direct reasons for weakened proliferation and invasion abilities of UCB cells. Recently, YAP1 has been confirmed as a tumor promoter which is significantly upregualted in multiple types of cancer, and it has direct the relationship with proliferation and invasion of cancer cells [24,25]. The research have indicated that the invasion ability of prostate and bladder cancer can be significantly inhibited when YAP1 was downregulated [26,27]. In colorectal cancer, high expression level of YAP1 can promote the proliferation of cancer cells, which is related with upregulation of LncRNA RP11-757G1.5 and downregualtion of miR-139-5p [28]. Although miR-612 has been confirmed to regulate the development of cancer cells via activating or inactivating several pathways, its effects on Hippo pathway remains unclear. In our study, LATS1 was also found to have positive relationship with the expression level of miR-612. Several studies have indicated that LATS1 is an upstream factor of YAP1, which could mediate the ubiquitin process and further reduce the expression level of YAP1 via phosphorylating YAP1 [29,30]. Therefore, miR-612 inhibits the expression of Yap1 via regulating the stability of LATS1. With the help of miRWalk, PMEPA1 was confirmed as a downstream target which has lower binding energy with miR-612, and the luciferase activity of the cells transfected with the wild type of PMEPA1 can be decreased by miR-612. PMEPA1 is considered as a carcinogen to induce EMT procession via activating TGF-β pathway in colorectal cancer [31]. In glioblastoma cells, PMEPA1 can promote cancer cells progression via combining with LATS1 and then recruiting NEDD4, an E3 ligase, to mediate the ubiquitin progression of PMEPA1 [32]. In this study, compared with normal cell line, PMEPA1 was regulated in HTB-9 and HTB-4, indicating that PMEPA1 may take part in the formation and development of UCB. Besides, the overexpression of PMEPA1 could reverse some effects of miR-612 on UCB cells, suggesting that PMEPA1 is connected with the effects of miR-612 on inhibiting the proliferation and invasion of UCB cells.
In summary, this study demonstrated that miR-612 deficiency was a key reason to directly promote the upregulation of PMEPA1, PMEPA1 further inhibited the expression of LATS1 and increased the expression of YAP1, and finally promoted the proliferation and invasion abilities of UCB cells. Considering YAP1 is beneficial to promote the proliferation and invasion of UCB cells, miR-612 should be taken seriously in the research, diagnosis and treatment of UCB. However, more credible evidence of miR-612/PMEPA1/LATS1/Yap1 axis in UCB should be obtained from animal model experiments.
In summary, our study suggests that miR-612 is a tumor suppressor which is downregulated in UCB cells, and it can inhibit the proliferation and invasion of cancer cells through activating Hippo pathway via targeting PMEPA1.
Funding Statement: National Natural Science Foundation of China (Grant Nos. 81202016, 81572534 and 81300629) and the 59-class General Financial Grant from the China Postdoctoral Science Foundation (Grant No. 2016M590638).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Chism, D. D. (2017). Urothelial carcinoma of the bladder and the rise of immunotherapy. Journal of the National Comprehensive Cancer Network, 15(10), 1277–1284. DOI 10.6004/jnccn.2017.7036. [Google Scholar] [CrossRef]
2. Hanna, K. S. (2020). Clinical overview of enfortumab vedotin in the management of locally advanced or metastatic urothelial carcinoma. Drugs, 80(1), 1–7. DOI 10.1007/s40265-019-01241-7. [Google Scholar] [CrossRef]
3. Wang, L., Smith, B. A., Balanis, N. G. (2020). A genetically defined disease model reveals that urothelial cells can initiate divergent bladder cancer phenotypes. Proceedings of the National Academy of Sciences USA, 117(1), 563–572. DOI 10.1073/pnas.1915770117. [Google Scholar] [CrossRef]
4. Kuang, S., Li, H., Feng, J., Xu, S., Le, Y. (2019). Correlation of BRCA2 gene mutation and prognosis as well as variant genes in invasive urothelial carcinoma of the bladder. Cancer Biomarkers, 25(2), 203–212. DOI 10.3233/CBM-182379. [Google Scholar] [CrossRef]
5. Li, G., Yu, J., Song, H., Zhu, S., Sun, L. et al. (2017). Squamous differentiation in patients with superficial bladder urothelial carcinoma is associated with high risk of recurrence and poor survival. BMC Cancer, 17(1), 69. DOI 10.1186/s12885-017-3520-1. [Google Scholar] [CrossRef]
6. Wei, W. S., Chen, X., Guo, L. Y., Li, X. D., Deng, M. H. et al. (2018). TRIM65 supports bladder urothelial carcinoma cell aggressiveness by promoting ANXA2 ubiquitination and degradation. Cancer Letters, 435(1), 10–22. DOI 10.1016/j.canlet.2018.07.036. [Google Scholar] [CrossRef]
7. Garg, M., Maurya, N. (2019). WNT/β-catenin signaling in urothelial carcinoma of bladder. World Journal of Nephrology, 8(5), 83–94. DOI 10.5527/wjn.v8.i5.83. [Google Scholar] [CrossRef]
8. Sun, C. H., Chang, Y. H., Pan, C. C. (2011). Activation of the PI3K/Akt/mTOR pathway correlates with tumour progression and reduced survival in patients with urothelial carcinoma of the urinary bladder. Histopathology, 58(7), 1054–1063. DOI 10.1111/j.1365-2559.2011.03856.x. [Google Scholar] [CrossRef]
9. Zhou, L., Zheng, S. J. (2019). The roles of microRNAs (miRNAs) in avian response to viral infection and pathogenesis of avian immunosuppressive diseases. International Journal of Molecular Sciences, 20(21), 5454. DOI 10.3390/ijms20215454. [Google Scholar] [CrossRef]
10. Shirjang, S., Mansoori, B., Asghari, S., Duijf, P. H. G., Mohammadi, A. et al. (2019). MicroRNAs in cancer cell death pathways: Apoptosis and necroptosis. Free Radical Biology and Medicine, 139(5), 1–15. DOI 10.1016/j.freeradbiomed.2019.05.017. [Google Scholar] [CrossRef]
11. Cortez, M. A., Anfossi, S., Ramapriyan, R., Menon, H., Atalar, S. C. et al. (2019). Role of miRNAs in immune responses and immunotherapy in cancer. Genes, Chromosomes Cancer, 58(4), 244–253. DOI 10.1002/gcc.22725. [Google Scholar] [CrossRef]
12. Luo, J., Chen, J., Li, H., Yang, Y., Yun, H. et al. (2017). LncRNA UCA1 promotes the invasion and EMT of bladder cancer cells by regulating the miR-143/HMGB1 pathway. Oncology Letters, 14(5), 5556–5562. DOI 10.3892/ol.2017.6158. [Google Scholar] [CrossRef]
13. Shan, G., Tang, T., Xia, Y., Qian, H. J. (2020). Long non-coding RNA NEAT1 promotes bladder progression through regulating miR-410 mediated HMGB1. Biomedicine & Pharmacotherapy, 121(2), 109248. DOI 10.1016/j.biopha.2019.109248. [Google Scholar] [CrossRef]
14. Feng, F., Chen, A., Huang, J., Xia, Q., Chen, Y. et al. (2018). Long noncoding RNA SNHG16 contributes to the development of bladder cancer via regulating miR-98/STAT3/Wnt/β-catenin pathway axis. Journal of Cellular Biochemistry, 119(11), 9408–9418. DOI 10.1002/jcb.27257. [Google Scholar] [CrossRef]
15. Liu, S., Chen, Q., Wang, Y. (2020). MiR-125b-5p suppresses the bladder cancer progression via targeting HK2 and suppressing PI3K/AKT pathway. Human Cell, 33(1), 185–194. DOI 10.1007/s13577-019-00285-x. [Google Scholar] [CrossRef]
16. Xie, X., Xiong, G., Wang, Q., Ge, Y., Cui, X. (2019). Long non-coding RNA LINC00460 promotes head and neck squamous cell carcinoma cell progression by sponging miR-612 to up-regulate AKT2. American Journal of Translational Research, 11(10), 6326–6340. [Google Scholar]
17. Agrawal, A., Sahni, S., Vulisha, A. K., Gumpeni, R., Shah, R. et al. (2017). Pulmonary manifestations of urothelial carcinoma of the bladder. Respiratory Medicine, 128(1), 65–69. DOI 10.1016/j.rmed.2017.05.006. [Google Scholar] [CrossRef]
18. Xie, X., Xiong, G., Wang, Q., Ge, Y., Cui, X. (2019). Long non-coding RNA LINC00460 promotes head and neck squamous cell carcinoma cell progression by sponging miR-612 to up-regulate AKT2. American Journal of Translational Research, 11(10), 6326–6340. [Google Scholar]
19. Liu, D., Zhou, B., Liu, R. (2020). An RNA-sequencing-based transcriptome for a significantly prognostic novel driver signature identification in bladder urothelial carcinoma. PeerJ-Life & Environment, 8, e9422. DOI 10.7717/peerj.9422. [Google Scholar] [CrossRef]
20. Li, W. T., Zheng, H., Nguyen, V., Wang-Rodriguez, J., Ongkeko, W. M. (2018). Functional genomics profiling of bladder urothelial carcinoma microRNAome as a potential biomarker. Neoplasia, 20(4), 364–373. DOI 10.1016/j.neo.2018.01.008. [Google Scholar] [CrossRef]
21. Li, J., Huang, S., Zhang, Y., Zhuo, W., Tong, B. et al. (2021). LINC00460 enhances bladder carcinoma cell proliferation and migration by modulating miR-612/FOXK1 axis. Pharmacology, 106(1-2), 79–90. DOI 10.1159/000509255. [Google Scholar] [CrossRef]
22. Liu, M., Chen, Y., Huang, B., Mao, S., Cai, K. et al. (2018). Tumor-suppressing effects of microRNA-612 in bladder cancer cells by targeting malic enzyme 1 expression. International Journal of Oncology, 52(6), 1923–1933. DOI 10.3892/ijo.2018.4342. [Google Scholar] [CrossRef]
23. Liu, Y., Lu, L. L., Wen, D., Liu, D. L., Dong, L. L. et al. (2020). MiR-612 regulates invadopodia of hepatocellular carcinoma by HADHA-mediated lipid reprogramming. Journal of Hematology & Oncology, 13(1), 7. DOI 10.1186/s13045-019-0841-3. [Google Scholar] [CrossRef]
24. Isfort, I., Elges, S., Cyra, M., Berthold, R., Renner, M. et al. (2019). Prevalence of the Hippo effectors YAP1/TAZ in tumors of soft tissue and bone. Scientific Reports, 9(1), 491. DOI 10.1038/s41598-019-56247-8. [Google Scholar] [CrossRef]
25. Hsu, H. T., Estarás, C., Huang, L., Jones, K. A. (2018). Specifying the anterior primitive streak by modulating YAP1 levels in human pluripotent stem cells. Stem Cell Reports, 11(6), 1357–1364. DOI 10.1016/j.stemcr.2018.10.013. [Google Scholar] [CrossRef]
26. Shen, T., Li, Y., Zhu, S., Yu, J., Zhang, B. et al. (2020). YAP1 plays a key role of the conversion of normal fibroblasts into cancer-associated fibroblasts that contribute to prostate cancer progression. Journal of Experimental & Clinical Cancer Research, 39(1), 7. DOI 10.1186/s13046-020-1542-z. [Google Scholar] [CrossRef]
27. Xu, M., Gu, M., Zhou, J., Da, J., Wang, Z. (2019). Interaction of YAP1 and mTOR promotes bladder cancer progression. International Journal of Oncology, 56(1), 232–242. DOI 10.3892/ijo.2019.4922. [Google Scholar] [CrossRef]
28. Zhu, X., Bu, F., Tan, T., Luo, Q., Zhu, J. et al. (2020). Long noncoding RNA RP11-757G1.5 sponges miR-139-5p and upregulates YAP1 thereby promoting the proliferation and liver, spleen metastasis of colorectal cancer. Journal of Experimental & Clinical Cancer Research, 39(1), 207. DOI 10.1186/s13046-020-01717-5. [Google Scholar] [CrossRef]
29. Eder, N., Roncaroli, F., Domart, M. C., Horswell, S., Andreiuolo, F. et al. (2020). YAP1/TAZ drives ependymoma-like tumour formation in mice. Nature Communications, 11(1), 491. DOI 10.1038/s41467-020-16167-y. [Google Scholar] [CrossRef]
30. Li, D., Ji, J. X., Xu, Y. T., Ni, H. B., Rui, Q. et al. (2018). Inhibition of Lats1/p-YAP1 pathway mitigates neuronal apoptosis and neurological deficits in a rat model of traumatic brain injury. CNS Neuroscience & Therapeutics, 24(10), 906–916. DOI 10.1111/cns.12833. [Google Scholar] [CrossRef]
31. Zhang, L., Wang, X., Lai, C., Zhang, H., Lai, M. (2019). PMEPA1 induces EMT via a non-canonical TGF-β signalling in colorectal cancer. Journal of Cellular and Molecular Medicine, 23(5), 3603–3615. DOI 10.1111/jcmm.14261. [Google Scholar] [CrossRef]
32. Ji, J., Ding, K., Luo, T., Xu, R., Zhang, X. et al. (2020). PMEPA1 isoform a drives progression of glioblastoma by promoting protein degradation of the Hippo pathway kinase LATS1. Oncogene, 39(5), 1125–1139. DOI 10.1038/s41388-019-1050-9. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |