

 | Molecular & Cellular Biomechanics |  |
DOI: 10.32604/mcb.2022.019080
ARTICLE
Injectable Collagen/CMC Soft Tissue Filler with Developed Flow Properties
1Department of Tissue Engineering and Applied Sciences, School of Advanced Technologies in Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran
2Department of Research and Development Iranian Tissue Product Co., Tehran, Iran
3Department of Bio-Computing, Faculty of Interdisciplinary Sciences and Technologies, Tarbiat Modares University, Tehran, Iran
4Iranian Tissue Bank & Research Center, Gene, Cell, and Tissue Research Institute, Tehran University of Medical Sciences, Tehran, Iran
*Corresponding Author: Amirhossein Tavakoli. Email: Dr.amirtavakoli@regen.ir
Received: 01 September 2021; Accepted: 30 December 2021
Abstract: Based on the remarkable demand for facial reconstitute or reshape fillers due to the dermal defects arising from specific diseases, trauma, or aging, several natural or synthetic materials have been investigated. Among the evaluated materials, decellularized dermis is one of the most biocompatible choices for the aim of skin tissue regenerative approaches. On the other hand, Carboxymethyl Cellulose (CMC), a synthetic polysaccharide, with the desirable degradability, biomechanical stability, and nontoxicity seems to be an acceptable reinforcement agent for decellularized dermis. Thus, in this research, an injectable soft tissue filler contained of human-derived decellularized collagen and CMC was fabricated. The cell-removal approving was performed utilizing H&E staining assay. The biocompatibility of the prepared samples was confirmed by MTT assay. The rheology examination demonstrated the increased storage modulus and enhanced elastic property as a consequence of CMC presence. Furthermore, the required flow force of the collagen/CMC filler was decreased as a consequence of decreasing the viscosity and its injectability was improved. According to the provided biomechanical and biological results, it could be claimed that the collagen/CMC hydrogel is a suitable substitute filler for skin tissue engineering.
Keywords: Collagen; CMC; injectable filler; soft tissue regeneration; rejuvenation
There is a growing demand to employ soft tissue fillers for reconstructive, injection laryngoplasty, and aesthetic purposes. Therefore, considering the exceeding market size, injectable fillers used as soft tissue augmentation are extensively required in dermal collagen and fat loss due to aging, trauma, and disease. Several natural and synthetic fillers have been used clinically in the field of skin regenerative medicine for facial correction wrinkle to restore tissue structure and enhance facial appearance [1]; However, no entirely efficient component for satisfying the market requirements have been achieved. Hence, the development of dermal fillers which have become an integral part of any aesthetic physician’s intervention, is an essential desired approach [2].
Ideally, temporary filler material should be easily injectable, biocompatible, resorbable, and mechanically stable over the long term [3]. The importance of the temporary dermal filler industry is reflected by several weeks to one year which they are eventually reabsorbed through macrophage activation. Stimulatory fillers are fabricated from medical grade synthetic materials such as polylactic acid and calcium hydroxyapatite which are more durable and lasting up to years that are appropriate for satisfying of the increasing growth in aesthetic demand during the past years and introduction of natural fillers, which have come to market [1,4]. Hyaluronic acid, Collagen, Biological fillers, CMC, PLLA, PCL, Calcium hydroxyapatite are the most common fillers. Collagen and Hyaluronic acid are the dominant components of native tissue, making them susceptible to enzymatic degradation and significantly reducing their lifespan to a few weeks or months [2]. CMC is an FDA-approved anionic water-soluble cellulose-derived polysaccharide, which has several biomedical applications due to its nontoxicity, degradability, acceptable biological compatibility, with no protein residues or bacterial endotoxins and low cost [1]. CMC is less likely to elicit an immune response due to plant-based origin, which is a key advantage over other animal-derived natural fillers [5,6]. Moreover, the absence of the cellulose-digesting enzyme in the body affords suitable mechanical stability of CMC in vivo compared to other natural biomaterial fillers [7]. CMC is also used as a gel carrier in injectable dermal fillers and volumizers in combination with other biodegradable and biocompatible materials to enhance characteristics of existing dermal fillers such as Sculptra, Radiesse and ELLANSÉ. Furthermore, there are several studies in medical science and plastic surgery department of the universities for fabricating CMC-based injectable hydrogels [2,8]. The aim of this study is to develop the performance of the human derive collagen filler by using CMC as an affordable alternative gel carrier which can result in better handling, injection, and maintaining the homogeneity over its shelf-life in addition to biocompatibility and safety especially for the treatment of facial defects and imperfections [9].
Decellularization of the human dermis was performed by the previously introduced method [10]. In summary, human skin samples of age 15 to 60 deceased donors was supplied by Iranian Tissue Product company in Tehran, Iran. Skin tissue subjected to decellularization process with chemical protocol followed by enzymatic techniques to remove cells. The lyophilized and freeze-dried acellular dermis membrane micronized to the particle size of 125 to 500 μm through milling. The micronized collagen fibrils were sterilized by 25 kGy gamma radiation. Moreover, the collagen gel has been investigated in clinical studies; safety, efficacy, and effectiveness of the filler for the correction of the nasolabial folds approved by an independent investigator [11].
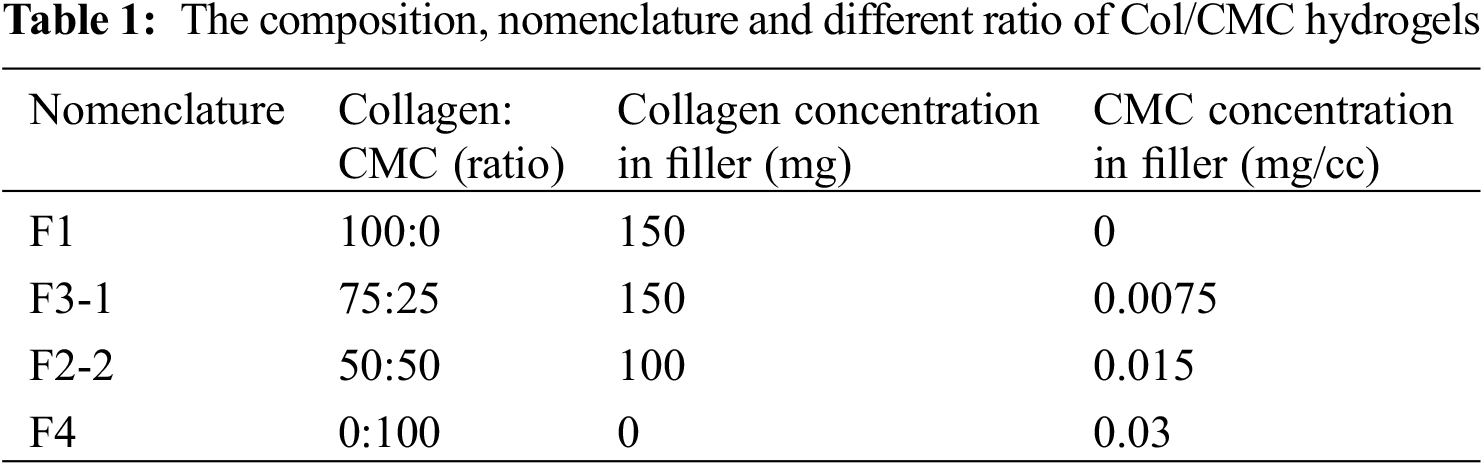
CMC (Sigma-Aldrich) which meets USP test specification was also sterilized by gamma irradiation as a dry powder. CMC was dissolved in deionized water in 90°C for 60 min to obtain 3% (wt.) solution. The collagen fibrils were rehydrated in sterile water and the samples with different ratios of CMC: Collagen fibrils were produced according to the data provided Table 1.
2.1 Observation of H&E Staining
Pathological specimens of the acellular dermis and cellular skin tissue were fixed in formalin, sectioned, and stained with hematoxylin and eosin to demonstrate the effectivity of the cell removal procedure and microstructure of the acellular membrane.
2.2 Rheological Characterization
Rheological analysis was employed for investigating the gelation characteristic via a rotational rheometer (Haake MARS III, Germany) equipped with a 20 mm diameter cone (1° angle) and plate geometry. The temperature was managed at 20°C utilizing a cooling and heating system (Phoenix II, Germany). The samples were embedded cautiously on the surface of plate and the cone was pulled down to a 0.052 mm gap distance. An amplitude strain sweep was performed at frequency of 1 Hz and deformation from 0.001 to 10 for the oscillatory assay. The frequency sweep was carried out in a frequency of 0.1 to 1000 rad/s and in a controlled deformation (fixed from the linear viscoelasticity region where the storage and loss modulus are independent of the frequency and strain). It should be noted that prior to testing, the samples were maintained equilibrating for 5 min to reach the temperature and mechanical equilibrium. Also, for further investigation, the viscosity (η) of each prepared sample was reported at 1 Hz using a digital viscometer (Anton Paar, Austria).
The force of the filler’s extrusion from 1 ml syringe (needle has a 24 G/0.55 mm diameter) was carried out by uniaxial tensile testing utilizing a universal testing machine with a speed rate of 10 mm/min Force vs. movement of syringe plunger. Immediately after observation of the initial filler droplet, the test was stopped (Fig. 1).

Figure 1: Universal testing machine apparatus for flow test
2.4 Cytotoxicity Analysis (MTT Assay)
To estimate the cytocompatibility, fibroblast cells proliferation was assessed by MTT assay according to the international standard of ISO 10993-5. Briefly, 1 ml of media was added to 0.2 g of the dried filler incubated in 5% CO at 37°C for 72 h. L929 mouse fibroblast cells prepared by Pasteur Institute of Iran were cultured in RPMI medium supplemented with 10% fetal calf serum (FCS), 100 Unit/mL penicillin and 100 μg/mL streptomycin. The cell suspensions were prepared enzymatically after detaching the cells by incubation in 0.05% (w/v) trypsin with 0.03% (w/v) ethylene-diamine-tetra acetic acid (EDTA) in Earl’s balanced salt solution from the tissue culture plates. Cells were seeded in 96-well microplates at a density of 1 × 10 cells/well and incubated for 24 hours to allow attachment. The medium of each plate was replaced with 50 μl of MTT solution (Sigma, 5 mg/ml) and 500 μl of fresh medium, then incubated for 3 hours at 37°C. Afterwards, the medium was removed, and blue-violet insoluble formazan in living cells was dissolved using 500 μl dimethyl sulfoxide (DMSO). Resulting-colored solutions were directly proportionate to cell viability and were measured employing an Elisa plate reader set at a wavelength of 570 nm. Toxicity of the samples measured by (1):
The results were reported as means. At least three samples were utilized for each experiment and the experiments were reported at least twice.
Based on the H&E staining results, apparently, no fibroblast cells were observed, hematoxylin binded to acidic structures and stained nuclei of the cells. Fig. 2 shows the lack of blue-stained areas (comprising the cells) after decellularization, which indicates that dermal fibroblasts have been removed successfully by the decellularization process and basic dermal architecture of the collagen remained in the three-dimensional of the native dermis [12].

Figure 2: Light microscopy of the H&E staining of the human dermis; a) before, b) after decellularization, the points indicated by arrows are showing the cells. The lighter parts after decellularization represents the complete cell removal of the matrix
The gelation properties of the fillers were examined by rheology test. Fig. 3 demonstrates the results of oscillometery assay. As can be observed, the higher amount of G” than the G’ (loss modulus) indicated the viscoelastic gel behavior of both samples. Considering the trends of storage modulus, it could be claimed that the Collagen/CMC sample maintained its structural integrity and stability as the frequency increased. It may be associated with the potential interaction between collagen and CMC. Also, the amount of G” for Collagen/CMC sample was approximately 10 times more than the collagen sample due to its notable elastic property. Choi et al. evaluated a levan-based hydrogel with CMC for soft tissue engineering and revealed that the reason of improvement of the elastic characteristics by the presence of CMC could be the relatively strong hydrogen binding between the polymers [3].
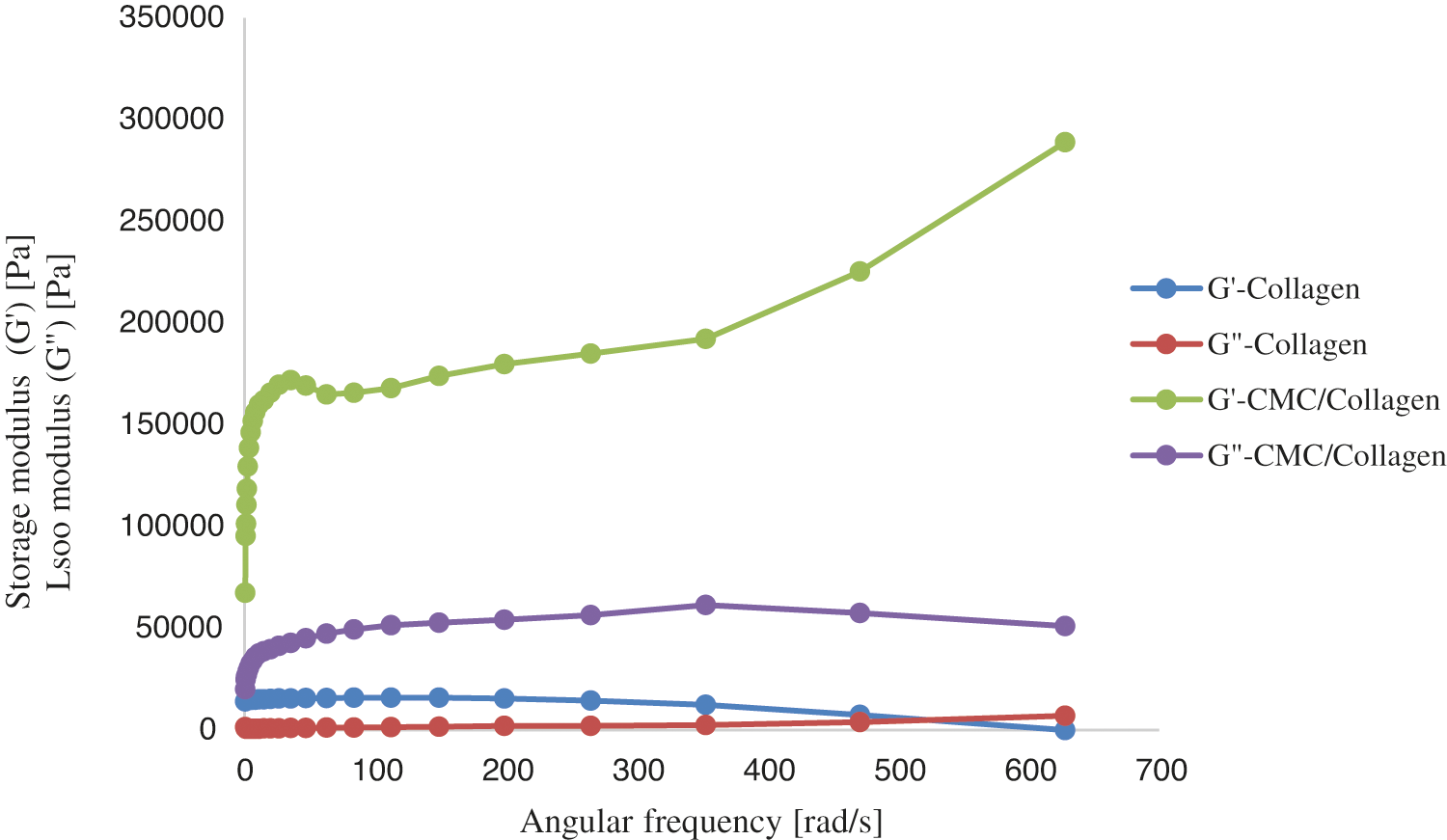
Figure 3: Oscillometry assay of the prepared hydrogels
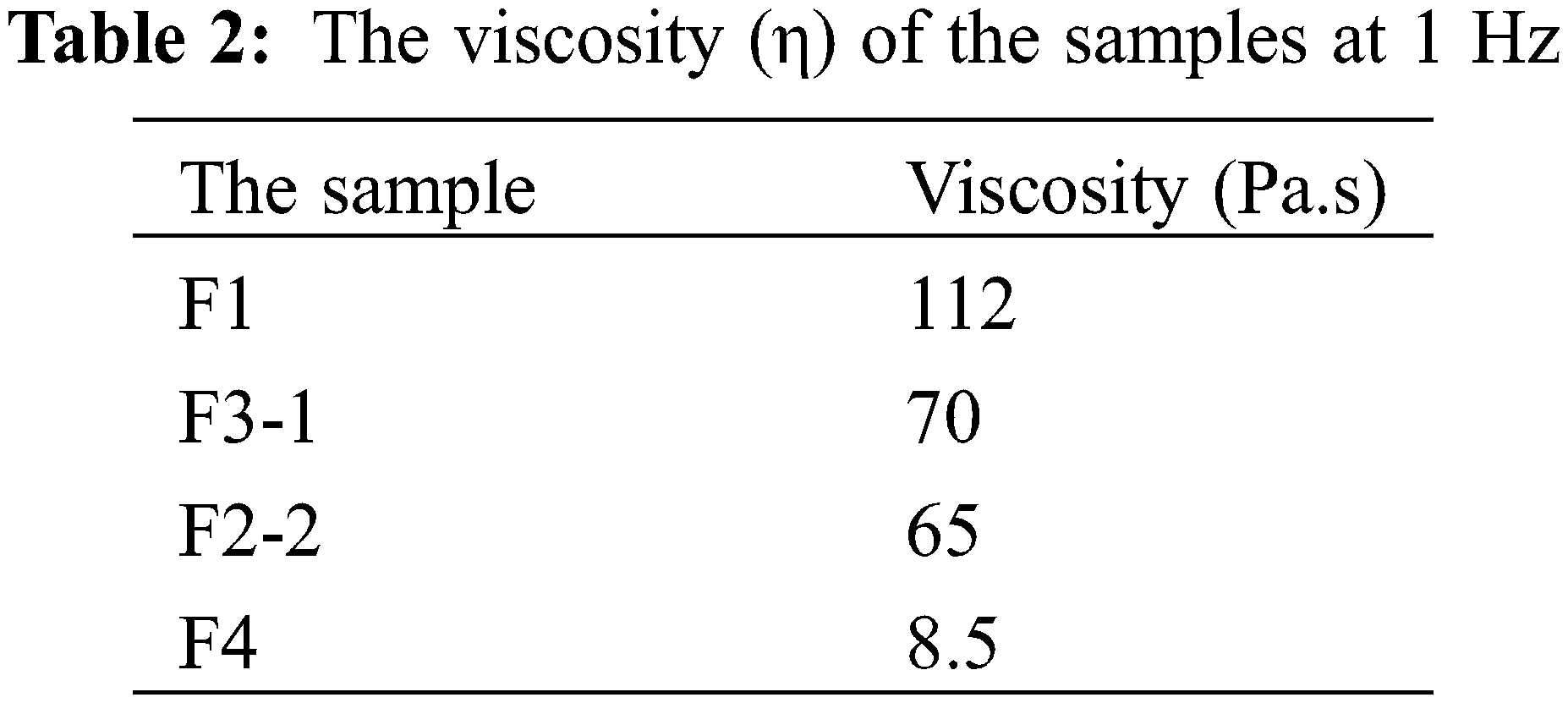
Table 2 shows the reported viscosity of each sample. As can be observed, the viscosity of the fillers was decreased by increasing the ratio of CMC. The behind reason may be due to the avoidance of aggregated messes formation by CMC addition as an appropriate fluid. This occurrence would lead to the enhancement of the filler injectability as well as the improvement of maintaining its stability as an integrated filament that could be placed in the fine facial defects properly during the injecting procedure and further massaging by the clinician. Thus, according to the previous literatures, it could be evidenced that F3-1 and F2-2 fillers contained the acceptable viscosity for facilitating the injection process [13].
There are different variants which impact on the flow force of the filler during the injection such as particle size, uniformly distribution of collagen fibrils, and the applied carrier rheology. We measured the force required to inject the filler in the air through mechanical test analyzer by modelling the filler injection. As shown in Fig. 4, the maximum force (Fmax) for CMC gel was the lowest measured amount (0.87 N). For collagen filler without CMC carrier, it was determined to be 4.5 N. There was a remarkable difference between the required employed force of these two injectable fillers. The Fmax of F3-1 and F2-2 were 2.35 and 1.26 N, respectively. It shows that the increase of the CMC ratio in the filler induces to decrease flow force and better injectability which was in accordance with the viscosity assay. However, all the data were in the range of recommended expelling force for an injectable material, the better injectability can cause better handling for clinicians and uniform injection in the surgical site [13].
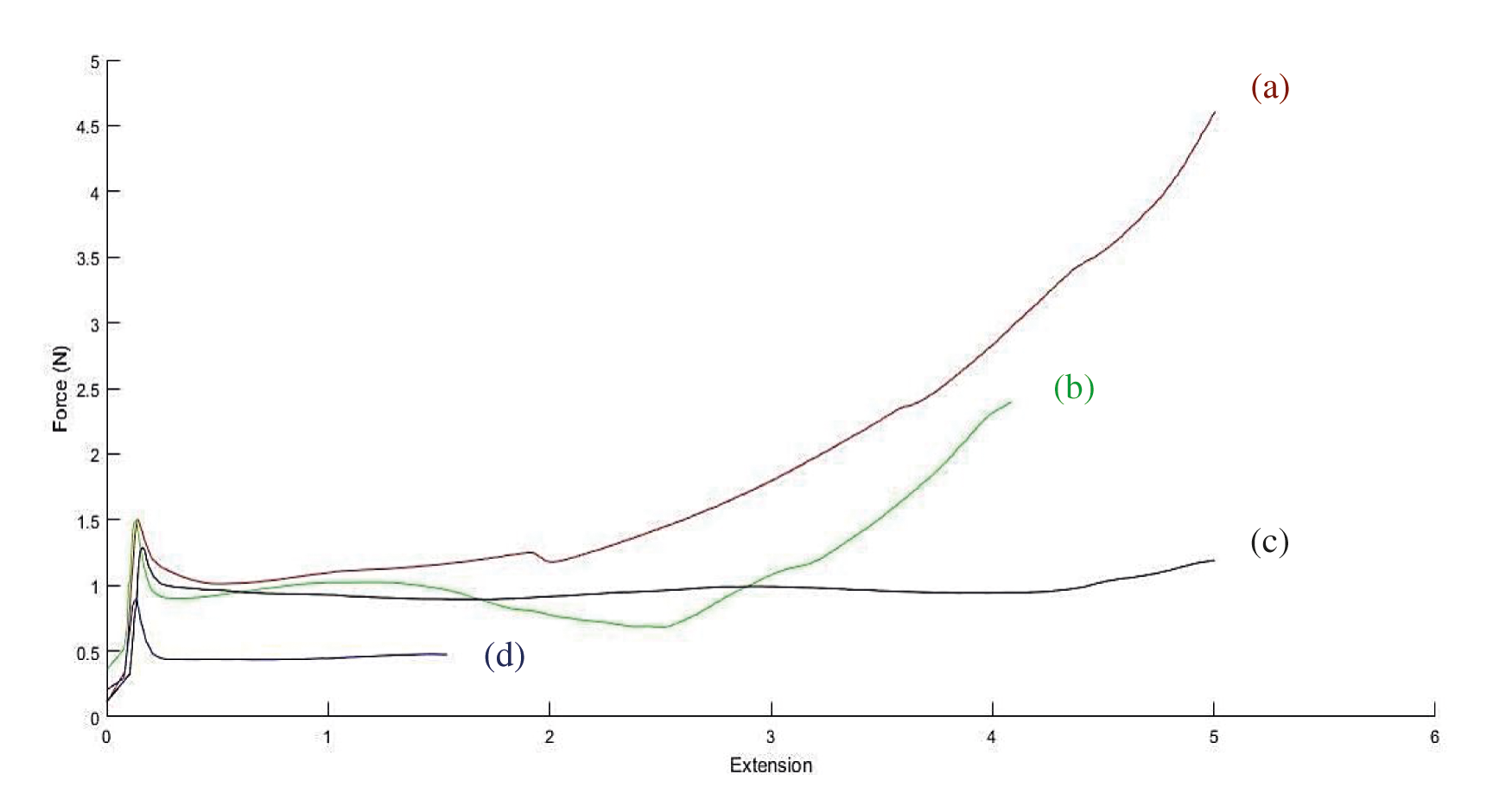
Figure 4: Injectability test; Force-plunger extension measured by the compression testing machine: a) F1; b) F3-1; c) F2-2; d) F4
MTT assay indicated that the samples are biocompatible, and cell viability for L929 fibroblasts for all the species after 72 h is more than 70% (Fig. 5). In comparison to the control group, cell viability of the injectable collagen gel was approximately 92%. Although by using CMC as a gel carrier, the viability slightly decreased, yet it was not meaningful. None of the samples presented signs of cytotoxicity demonstrating their potential application in soft tissues. It is noteworthy that the obtained results were in accordance with the previous researches; Basu et al. [14] reported the biocompatibility of an electrospun bioengineered soft tissue substitute consisted of poly ethylene oxide (PEO) and CMC. Therefore, it can be evidenced that the fabricated filler provides an appropriate environment for cells to grow and proliferate [14].
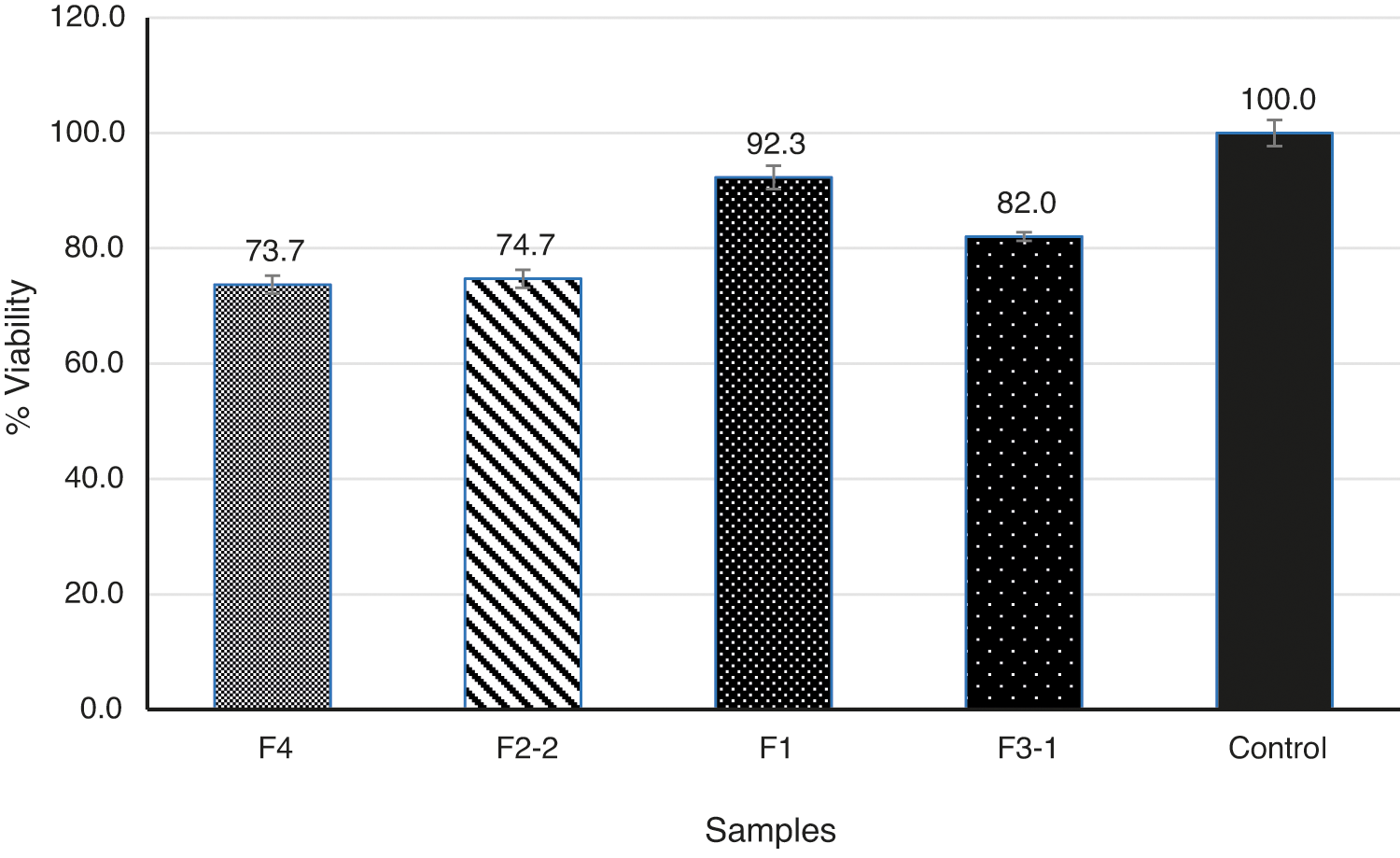
Figure 5: Results of MTT cell viability assay using fibroblast cells culture on filler structures
The purpose of this project was to fabricate an appropriate filler for soft tissue engineering. The most important required criteria for this filler were biocompatibility, injectability, and enhanced flow properties which was leading to improved handling. Among the 4 samples with different ratio of collagen and CMC, Both F1 and F3-1 species have the same collagen fibrils concentration of 150 mg, but the injectability, stability, and integrity of the F3-1 was better due to the presence of CMC as a gel carrier. As an outcome, CMC avoided the formation of aggregated masses that were visible as beads or lumps. Considering that the physician may massage the injection site in an attempt to distribute the filler, adding the CMC can ameliorate the injection of smaller mass injection for fine wrinkles and generally, develop the clinician’s control during injection. Moreover, the non-cytotoxicity of the human-derived collagen fibrils and CMC projected to a nontoxic filler with sufficient biocompatibility. Therefore, regarding the MTT assay, rheology, and gel injectability results, it could be concluded that the F3-1 sample with 75:25 collagen and CMC ratio is the optimum biocompatible soft tissue filler with enhanced flow characteristics suitable for soft tissue regeneration.
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Cheng, L.-Y., Sun, X. M., Tang, M. Y., Jin, R., Cui, W. G. et al. (2016). An update review on recent skin fillers. Plastic and Aesthetic Research, 3, 92–99. DOI 10.20517/2347-9264.2015.124. [Google Scholar] [CrossRef]
2. Varma, D. M., Gold, G. T., Taub, P. J., Nicoll, S. B. (2014). Injectable carboxymethylcellulose hydrogels for soft tissue filler applications. Acta Biomaterialia, 10(12), 4996–5004. DOI 10.1016/j.actbio.2014.08.013. [Google Scholar] [CrossRef]
3. Choi, W. I., Hwang, Y., Sahu, A., Min, K., Sung, D. et al. (2018). An injectable and physical levan-based hydrogel as a dermal filler for soft tissue augmentation. Biomaterials Science, 6(10), 2627–2638. DOI 10.1039/C8BM00524A. [Google Scholar] [CrossRef]
4. Liu, B., Ma, X., Zhu, C., Mi, Y., Fan, D. et al. (2013). Study of a novel injectable hydrogel of human-like collagen and carboxymethylcellulose for soft tissue augmentation. e-Polymers, 13(1380–390. DOI 10.1515/epoly-2013-0135. [Google Scholar] [CrossRef]
5. Kim, E. J., Choi, J. S., Kim, J. S., Choi, Y. C., Cho, Y. W. (2016). Injectable and thermosensitive soluble extracellular matrix and methylcellulose hydrogels for stem cell delivery in skin wounds. Biomacromolecules, 17(1), 4–11. DOI 10.1021/acs.biomac.5b01566. [Google Scholar] [CrossRef]
6. Zhang, M., Ding, C., Yang, J., Lin, S., Chen, L. et al. (2016). Study of interaction between water-soluble collagen and carboxymethyl cellulose in neutral aqueous solution. Carbohydrate Polymers, 137, 410–417. DOI 10.1016/j.carbpol.2015.10.098. [Google Scholar] [CrossRef]
7. Leonardis, M., Palange, A., Dornelles, R. F., Hund, F. (2010). Use of cross-linked carboxymethyl cellulose for soft-tissue augmentation: Preliminary clinical studies. Clinical Interventions in Aging, 5, 317. DOI 10.2147/CIA. [Google Scholar] [CrossRef]
8. Leonardis, M., Palange, A. (2015). New-generation filler based on cross-linked carboxymethylcellulose: Study of 350 patients with 3-year follow-up. Clinical Interventions in Aging, 10, 147. DOI 10.2147/CIA. [Google Scholar] [CrossRef]
9. Wallace, D. G., Rhee, W., Reihanian, H., Ksander, G., Lee, R. et al. (1989). Injectable cross-linked collagen with improved flow properties. Journal of Biomedical Materials Research, 23(8), 931–945. DOI 10.1002/(ISSN)1097-4636. [Google Scholar] [CrossRef]
10. Mahdavi-Mazdeh, M., Heshmati, B. N., Tavakoli, S., Ayaz, M., Ardalan, F. A. et al. (2013). Human split-thickness skin allograft: Skin substitute in the treatment of burn. International Journal of Organ Transplantation Medicine, 4(3), 96. [Google Scholar]
11. Nassiri, K. M., Rajabi, E. A., Zartab, H., Khoshpouri, P., Khoshpouri, P. et al. (2015). Evaluation of human allogeneic collagen gel for correction of nasolabial folds using non-invasive measurement techniques, Iranian Journal of Dermatology, 18(3), 97–103. [Google Scholar]
12. Sclafani, A. P., Romo, T., III, Jacono, A. A., McCormick, S., Cocker, R. et al. (2000). Evaluation of acellular dermal graft in sheet (AlloDerm) and injectable (micronized AlloDerm) forms for soft tissue augmentation: Clinical observations and histological analysis. Archives of Facial Plastic Surgery, 2(2), 130–136. DOI 10.1001/archfaci.2.2.130. [Google Scholar] [CrossRef]
13. Kouhi, M., Varshosaz, J., Hashemibeni, B., Sarmadi, A. (2020). Injectable gellan gum/lignocellulose nanofibrils hydrogels enriched with melatonin loaded forsterite nanoparticles for cartilage tissue engineering: Fabrication, characterization and cell culture studies. Materials Science and Engineering: C, 115, 111114. DOI 10.1016/j.msec.2020.111114. [Google Scholar] [CrossRef]
14. Basu, P., Repanas, A., Chatterjee, A., Glasmacher, B., NarendraKumar, U. et al. (2017). PEO-CMC blend nanofibers fabrication by electrospinning for soft tissue engineering applications. Materials Letters, 195, 10–13. DOI 10.1016/j.matlet.2017.02.065. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |