

 | Molecular & Cellular Biomechanics |  |
DOI: 10.32604/mcb.2021.017750
REVIEW
A Review of the Role of ERp57 in Cancerous and Non-Cancerous Cell Physiology and its Potential as a Therapeutic Target
1Islamic Azad University, Tehran Medical Branch, Tehran, Iran
2Tehran University of Medical Sciences, Tehran, Iran
3Department of Pharmacology, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran
4Department of Neurosurgery, University Medical Center Tuebingen, Tuebingen, Germany
*Corresponding Author: Siavash Shariatzadeh. Email: siavash.shariatz@gmail.com
Received: 03 June 2021; Accepted: 16 September 2021
Abstract: The protein ERp57 is a stress-responsive protein, mainly exists in the endoplasmic reticulum (ER), and a small amount in the cell membrane, cytoplasm, nucleus and mitochondria, which is involved in the signal transduction from the cell surface, the regulation process that occurs in the nucleus, and the formation of polymer protein complexes involved in DNA repair. Various degrees of ERp57 dysregulation has been observed in many types of non-communicable diseases especially in cancers. Previous studies showed that the expression of ERp57 could play a key role in occurrence and development of cancers such as breast cancer, gastric cancer, ovarian cancer, etc.; in addition, it has been suggested to play a pivotal role in disease progression of non-cancerous diseases such as neurodegeneration, liver disease, kidney disease, intestinal irritability syndrome and airway hypersensitivity. Thus, abnormal expression of ERp57 could be used as promising biomarker for cancer diagnosis and prognosis based on the previous studies. In this regard, current study was aimed to review the literature, which have been elucidate the role of ERp57 protein expression in both non-cancer and cancer disease. Overall, most studies have shown that inhibiting/knocking out of ERp57 could inhibit the cell proliferation and also induce apoptosis in both human cancerous and non-cancerous cells. Also, it has been suggested that the overexpression of ERp57 could intensify the cancer development. Therefore, it could be hypothesized that targeting of ERp57 might be a potential treatment in cancerous and non-cancerous diseases.
Keywords: Cancer; ERp57; dysregulation; prognostic; diagnostic; target therapy
The ERp57/GRP58 protein is a stress-responsive protein mainly exists in the endoplasmic reticulum (ER) [1]; and encoded by the gene (protein disulfide isomerase A3 precursor) PDIA3 located on chromosome 15 [2–4]. Based on the previous studies, ERp57 has been classified as a member of protein disulfide isomerase (PDI); although PDI family have two or three active sites, ERp57 structure is composed of four domains (called a, b, a’ and b’), with a molecular weight of 58 KD [5]. Unlike typical PDI, ERp57 can specifically interact with newly synthesized glycoprotein interaction [6]. ERp57 is found in many different subcellular locations and has been shown to be involved in a variety of biological processes and diseases [7]. Among them, the ER is the main place where ERp57 exists. The function of ERp57 in the ER is to correct folding, secretion or quality control of new glycoproteins synthesized in the cell membrane. In this process, ERp57 interacts with calreticulin or calnexin, which is responsible for recognizing and binding mono-glycosylated proteins [6,8,9].
On the other hand, studies have confirmed that ERp57 also exists outside the ER and could contributed to DNA repair. For example, a study showed the association of ERp57 with bone-related genes in osteoblast MC3T3-E1 [10]. They observed that after entering ERp57 to the nucleus, ERp57 participate in target gene transcription and DNA repair [10]. In addition, ERp57 in mitochondria can also resist the degradation of mitochondria and participate in cell apoptosis. Overall, it could be concluded that ERp57 possibly mediate the occurrence and development of diseases through various functions inside and outside the endoplasmic reticulum [3,7]. Although the basic implications of ERp57 in cancer has been reviewed recently [7], this manuscript comprehensively reviewed the literature. The current review summarizes the effects of ERp57 on cell survival and apoptosis after gene overexpression or knockout in cancerous cells (Table 1, Fig. 1) and non-cancerous cells (Table 2, Fig. 1), which will help us to better understand the pathological, molecular processes, and regulation pathway, which ERp57 is involved.
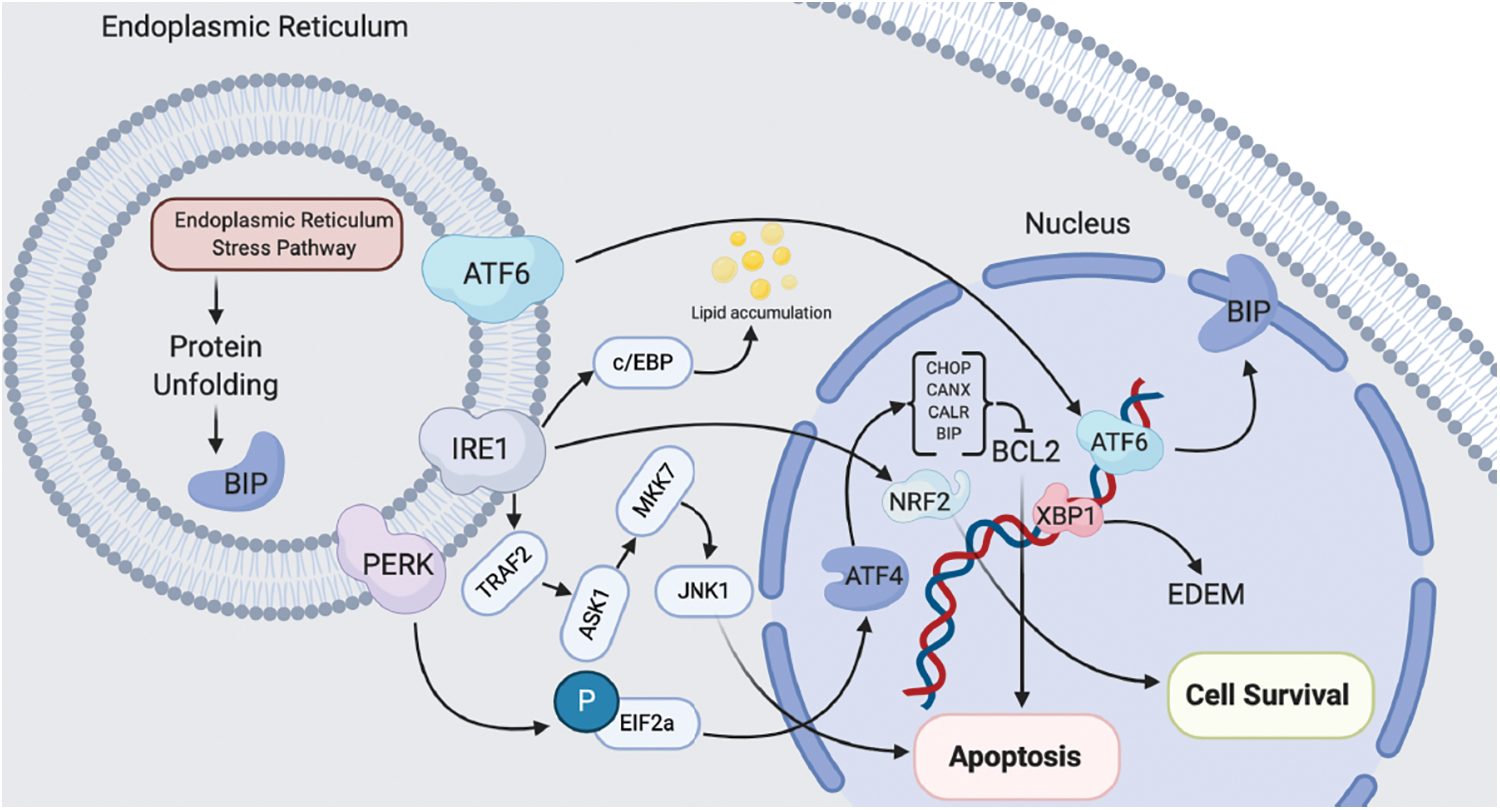
Figure 1: Schematic pathway of ER-induced cell apoptosis/survival in both normal and cancerous cells
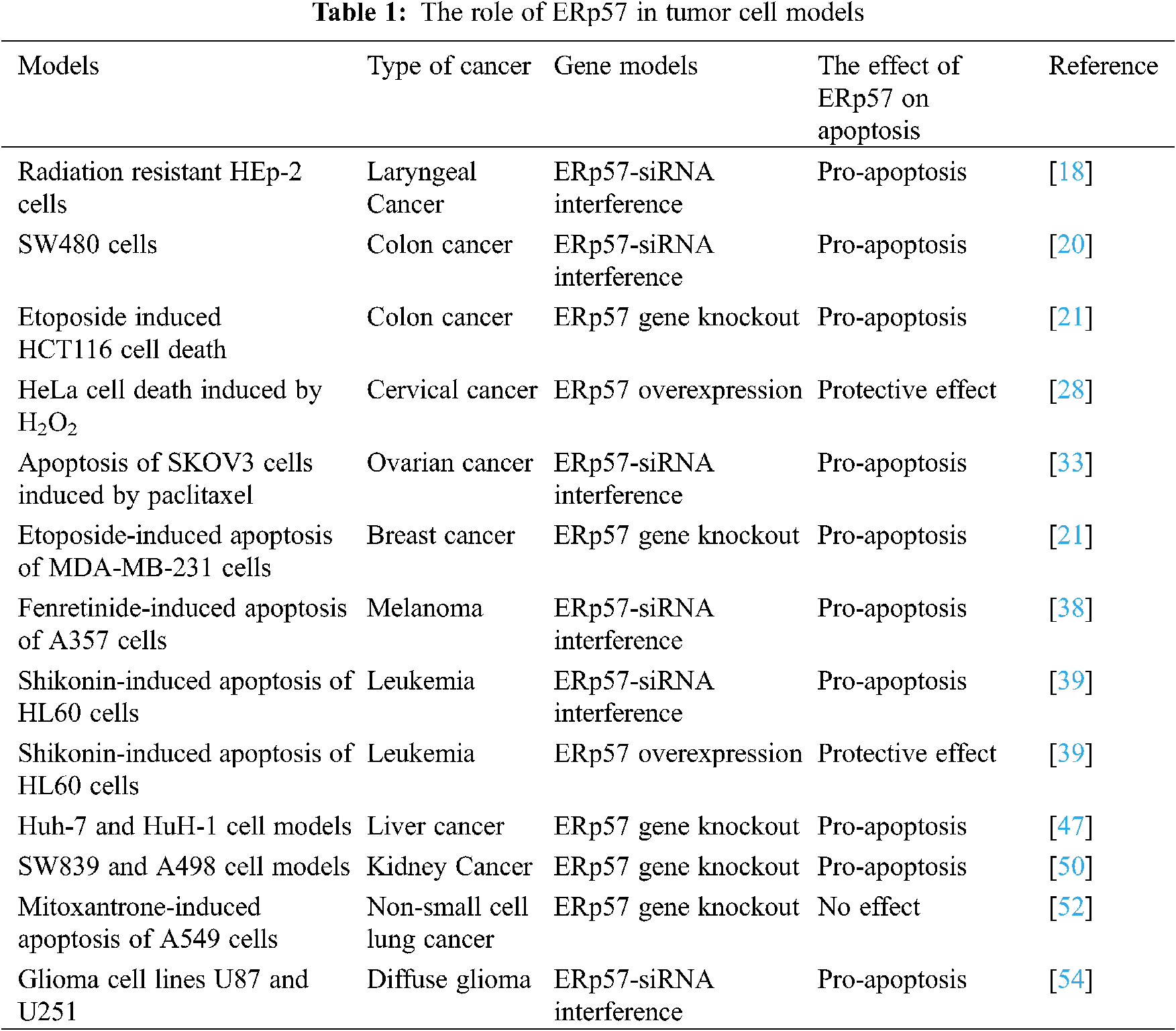
2 Role of ERp57 in Cell Survival and Apoptosis of Cancerous Cells
Various degrees of ERp57 dysregulation has been observed in many types of cancer, depending on the affected tissues and cells [11–15]. At present, the abnormal expression of ERp57 has been clinically evaluated as a marker for cancer diagnosis and prognosis [16–19]. The relationship between the changes in ERp57 expression and the occurrence, development and prognosis of cancers clearly indicates that ERp57 might be a possible cause of cancers through a complex cellular and molecular processes.
Laryngeal cancerous cells can spread directly to the adjacent tissue structure, or spread through the local lymph nodes of the neck, or spread to more distant places through the bloodstream, of which the most common metastasis site is lung [18]. A study explored the effect of ERp57 on radiation therapy (as the standard treatment of laryngeal cancer). They showed that ERp57 enhances the anti-radiation resistance of laryngeal cancer through ERp57-STAT3 (signal converter and transcription activator 3)-Mcl-1(anti-apoptotic protein) axis regulation [18], and provided an evidence for the carcinogenic role of ERp57 in regulating of STAT3 activation, tumor development and resistance. Therefore, it could be concluded that ERp57 might be a clinical marker of radiation resistance and poor prognosis of laryngeal cancer.
Colorectal cancer (CRC) is the third most common malignant tumor in developing countries and the second most common cause of cancer-related death in men [20]. Tumor resection is the standard treatment of CRC. For patients without lymph node metastasis, the 5-year survival rate of CRC is as high as 90%, so early detection is essential for a good prognosis of cancer treatment. A study showed that ERp57 could be a candidate CRC biomarker [20]. They observed that ERp57-siRNA expression in SW480 cells and HCT116 cells can cause changes in subcellular morphology, decreased cell proliferation, and increased apoptosis. Their results showed that ERp57-siRNA was positively correlated with SW480 cell apoptosis, and thus ERp57 may be a potential therapeutic target for CRC [20,21].
Gastric cancer (GC) is the third leading cause of cancer-related deaths in Japan. Early diagnosis and surgical treatment can improve the prognosis, but the prognosis of advanced GC is still not ideal. Studies have shown that ERp57 is located on the tubular membrane at the top of gastric parietal cells, which regulates the H+ and K+-ATPase activities in gastric acid secretion [22], and plays an important role in energy homeostasis [23]. ERp57 is down-regulated in GC tissues, and its expression is closely related to the occurrence and development of GC [24]. A study showed that the expression of MHC class I was increased by stimulation of interferon gamma in GC cells. Co-immunoprecipitation analysis showed that ERp57 and MHC class I may have an ability to transport antigens to the cell membrane. These findings suggest that ERp57 may be involved in the immune response of cancer cells and play an important role in the pathobiology of GC. Therefore, the expression of ERp57 may be a useful biomarker for predicting the prognosis of GC [25].
Studies have found that ERp57 expression is closely related to the occurrence and development of cervical cancer [26–28]. From normal epithelium to cervical intraepithelial neoplasia and subsequently to cancer, the expression of ERp57 gradually decreases [26–28]. There is an important correlation between the down-regulation of ERp57 and clinicopathological parameters. Therefore, the down-regulation of ERp57 expression is considered to be an independent predictor of poor prognosis in early cervical cancer [26]. Other studies have shown that ERp57 overexpression is an effective prognostic factor for cervical adenocarcinoma, and ERp57 regulates the stability of β-catenin protein in cervical adenocarcinoma cell lines [27]. The overexpression of ERp57 in HeLa cells has a significant protective effect on cell damage induced by hydrogen peroxide [28]. Also, it has been suggested that the combination of ERp57 index and lymph node metastasis index may increase the specificity of the prognosis of cervical adenocarcinoma. Thus, it could be concluded that up-regulation of ERp57 could inhibit the invasion and metastasis of cervical adenocarcinoma cancer cells [16].
Prostate cancer (PC) is the most common type of cancer among men over the age of 50, and it is also the second most common cause of cancer-related deaths in men. Oxidative stress may increase cell viability and anti-stress by upregulating ERp57, so oxidative stress cascade may be a potential target for the treatment of prostate disease [29]. Based on the previous studies the presence of glutathionylated STAT3 and the high expression of ERp57 protein suggests that there is an imbalance in oxidative stress components in prostate tissues [30]. Studies have shown that the overexpression of LEDGF/p75 (lens epithelium-derived growth factor 75 kd, LEDGF confers cellular protection against stress-induced apoptosis via transcriptional activation of stress-related genes) in PC can lead to the up-regulation of ERp57 expression [31].
Ovarian cancer is the second most commonly diagnosed malignant tumor of the reproductive system and the leading cause of death from gynecological cancer. Most advanced ovarian cancers are initially sensitive to taxane chemotherapy, but they are resistant to such chemotherapy drugs and are highly prone to drug resistance, which lead to decrease in the 5year-survival rate of patients. Research data shows that ERp57 has a significant regulatory role in paclitaxel resistance, and it may be one of the biomarkers representing chemoresistance ovarian cancer. The main reason is that nuclear ERp57 complex participates in the mechanism related to chromosome segregation, where the specific conformational state of actin plays a key role in the paclitaxel resistance pathway [32]. ERp57 not only contributes to paclitaxel resistance, but also promotes the growth of ovarian cancer cells. Studies have shown that ERp57-siRNA can significantly inhibit proliferation and promote paclitaxel-induced apoptosis of ovarian cancer cells, while overexpression of ERp57 has shown to induce the opposite effect [33]. In another study, ERp57 was considered to be a potential target protein of Dicer. In vivo and in vitro studies showed that ERp57-siRNA could significantly reduce the proliferation and migration effects mediated by Dicer exhaustion [11]. Members of the PDI family (PDIR, ERp72, ERp57, and AGR3) are potential prognostic markers for ovarian cancer [34]. Studies have found that PACMAs (propynoic acid carbamoyl methyl amide), a kind of irreversible PDI small molecule inhibitor, shows a targeted anti-tumor ability, which can significantly inhibit the growth of ovarian tumors without affecting the normal tissues [35].
Breast cancer is one of the most common cancers among women in the world and most cases are not diagnosed until the advanced stage. Based on the previous reports, the expression of PDIA3 and PDIA6 genes can be used as a marker of the aggressiveness of breast duct disease [34]. Knocking-out of ERp57 in breast cancer cells confirmed that ERp57 has a specific metastasis-promoting effect in the breast [36]. However, another study showed that ERp57-siRNA could not affect the expression of EGFR in MDA-MB-468 breast adenocarcinoma cells with overexpressing of EGFR [14]. Overall other studies have confirmed that the deletion of ERp57 in MDA-MB-231 cells inhibits the proliferation of breast cancer cells and increases the sensitivity to ionizing radiation and chemotherapeutic drugs [21].
Melanoma, a cancer formed by melanocytes, is poor prognosis and highly aggressive, with a frequent metastasis to lymphatic and blood tract even in the early stages of tumor. Studies have shown that ERp57 interacts with DNA fragments in melanoma cells and may be involved in gene transcription regulation [37]. It has been suggested that decreasing in the ER-stress response might play a key role in apoptosis induced by oxidative stress. In this regard, a study showed that administration of ERp57-siRNA increased the apoptosis response after treating cells with Fenretinide (N-(4-hydroxyphenyl) retinamide; 4-HPR) [38]. In addition, it has been observed that ERp57 has a high affinity for binding to Ref-1 (Ref-1 is a protein involved in DNA repair and the reduction and activation of transcription factors). Overall, it could be conducted that ERp57 seems to co-regulate gene expression mediated by redox-sensitive transcription factors with Ref-1 in melanoma [28].
Both leukemia and lymphoma are very serious diseases and are difficult to treat with high mortality rate. A study on the anti-leukemia activity of Shikonin (suppresses proliferation and induces apoptosis in human leukemia) showed that overexpression of ERp57 has a protective effect on Shikonin-induced apoptosis of HL-60 cells, and knocking out ERp57 will increase Shikonin-induced apoptosis [39,40]. In addition, they observed that ERp57 can recognize DNA structural changes and phosphorylate H2AX complexes in Nalm6 (human b-line leukemia cell line) cells. On the other hand, clinical studies have shown that ERp57 plays a role in the sensitivity of cells to chemotherapy drugs [41].
Hepatocellular carcinoma (HCC) is the most common type of liver tumor with the poor prognosis outcome clinically. HCC patients often have liver cirrhosis, thrombocytopenia, ascites, neutropenia and other diseases, which are often ineffective in treatment, coupled with control of cell proliferation and survival [42]. Previous studies showed that the expression of ERp57 in HBV-HCC (hepatitis B virus-associated hepatocellular carcinoma) is increased. Interestingly, highly expressed ERp57 may lead to poor prognosis of HBV-HCC patients, which may be related to hepatitis B virus infection [43]. Changes in the expression of calreticulin, GRP78, PDI and ERp57 in hepatocellular carcinoma may have important implications for MHC class I (major histocompatibility complex) assembly, peptide loading, and presentation on tumor cell surfaces, and participate in the immune system [44]. A study in HepG2 cells found that after ERp57-siRNA down-regulated the expression of MMP-2 and MMP-9 proteins, down-regulation of ERp57 by siRNA could reduce the migration of liver cancer cells [45]. Other study has reported that the expression of ERp57 is related to the reduction of tumor proliferation and apoptosis in HCC, and it has been shown that higher ERp57 levels were correlate with poor prognosis and poor outcome [46]. The down-regulation of ERp57 significantly inhibits the cell proliferation and induces cell apoptosis of liver cancer cell lines. It indicates that ERp57 can regulate cell proliferation and apoptosis in HCC [47]. Therefore, ERp57 may be a key molecule for targeting therapy of hepatocellular carcinoma.
Renal cancer is the sixth most common cancer among men and the eighth most common cancer among women. According to the latest cancer statistics in the United States in 2019, it is predicted that there are 73,820 new cases of renal cancer [48]. Among them, clear cell renal cell carcinoma (ccRCC) is the main subtype of renal cell carcinoma, accounting for 70% of renal cancer. Studies have pointed out that the secretion of ERp57 is of great significance to the accumulation of extracellular matrix and the progression of renal fibrosis, and the secretion of ERp57 is one of the earliest signs of the beginning and development of renal fibrosis [49]. A study has evaluated the role of ERp57 in ccRCC both in in-vivo and in-vitro [50]. The results of recently mentioned study showed that the overexpression of ERp57 enhanced the proliferation of ccRCC cells, while the loss of ERp57 inhibited the proliferation of ccRCC cells. ERp57 positively regulates the expression of ILF3 in ccRCC cells, which is related to poor prognosis. ILF3 can bind to ERp57 and positively regulate its expression by enhancing its mRNA stability [51]. The ERp57/STAT3 (transcription activator 3 complex)/ILF3 (interleukin-enhancing binding factor 3) feedback loop plays a key role in the tumorigenesis of ccRCC and provides a potential therapeutic target for ccRCC therapy [51].
According to World Cancer Research Fund, lung cancer is the most common malignant tumor globally [52]. Studies have shown that CALR (calreticulin) and ERp57 are overexpressed in non-small cell lung cancer compared with adjacent non-tumor lung tissue [52]. The low expression of CALR and ERp57 is positively correlated with poor overall survival [52]. Therefore, it could be concluded that ERp57 can be used as an effective biomarker for diagnosis and prediction of the prognosis of non-small cell lung cancer [53,54]. In IAV (influenza A virus) infection in vivo, the deletion of ERp57 in lung epithelial cells significantly reduces the overall viral load. ERp57 knockout can significantly reduce IAV-mediated airway inflammation, airway hyperresponsiveness, and mice peripheral airway resistance [54].
Diffuse gliomas are the most common type of primary brain and central nervous system (CNS) tumors. Previously the role of P4HB and ERp57 has been studied the in diffuse glioma [55]. The analysis results showed that in glioma, the expression levels of P4HB and ERp57 were up-regulated. The high expression of P4HB and ERp57 is significantly associated with high Ki-67 and high frequency of TP53 mutations. P4HB and ERp57 may be independent prognostic biomarkers of diffuse glioma. The inhibition of ERp57 in vitro can inhibit cell proliferation, induce apoptosis, and reduce the migration of glioma cells. In addition, down-regulation of P4HB and ERp57 may help improve the survival of patients receiving chemotherapy and radiation therapy. ERp57 can be used as a prognostic biomarker and treatment target for diffuse glioma [55].
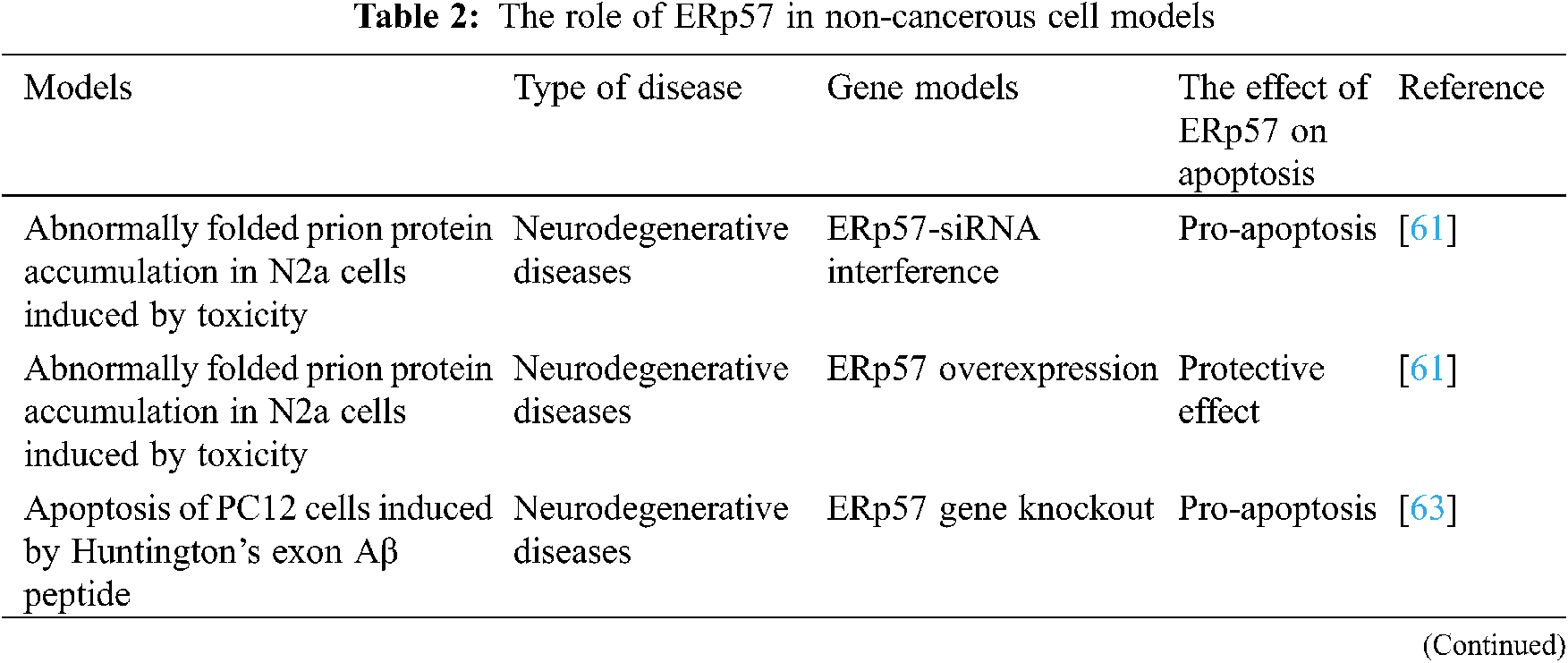
Past studies have shown that ERp57 is closely related to the occurrence and development of various diseases such as neurodegeneration, reproduction disease, liver disease, kidney disease, intestinal irritability syndrome, and airway hypersensitivity [49,55,56]. Therefore, the detection or intervention of ERp7 protein expression level in related parts is expected to become a new strategy for early diagnosis and treatment of disease.
3.1 Neurodegenerative Diseases
The accumulation of misfolded proteins in neurons is a hallmark of many neurodegenerative diseases, which can lead to cell dysfunction and cell death. It has been reported that GFAP antibody interacts with ERp57 and also has a protective effect on retinal nerve cells under oxidative stress [57]. Prion-related diseases are also a lethal and rare neurodegenerative disease. Protein disulfide isomerase regulates the endoplasmic reticulum stress and apoptosis process during prion infection [58], of which ERp57 is a kind of Disulfide isomerase, which is up-regulated in prion-related diseases. In a study, mice overexpressing ERp57 showed increased levels of prion protein in the nervous system. Therefore, ERp57 is involved in regulating prion protein levels. The steady state of [59,60]. ERp57 is a protective factor against prion neurotoxicity. In vitro studies using N2a neuroblasts have shown that inhibiting ERp57 expression with small interfering RNA can significantly enhance PrP SC (abnormally folded prion protein accumulation) toxicity, and the overexpression of ERp57 protects cells from PrP SC toxicity and reduces the activation rate of Caspase-12 (apoptotic protease 12). Studies have shown that the expression of ERp57 is an early cellular response to prion replication and is a nerve against prion neurotoxicity protection factor [61]. In the transgenic mouse model overexpressing ERp57, the overexpression of ERp57 does not affect the loss of dopaminergic neurons and the innervation of the striatum after the injection of Parkinson’s disease-induced neurotoxin. ERp57 contributes to the regeneration of peripheral nerves and is the center. Nervous system is essential for the survival of specific neuron groups [62]. A study using small molecule screening methods found five compounds with different structures, and identified PDI as the molecular target of these small molecules, which can prevent cell apoptosis induced by huntingtin mutein. In PC12 cells, overexpression of PDIA3 (ERp57) can simulate the MOMP (mitochondrial outer membrane permeability) experiment in the cell environment, and block the catalytic activity of PDI to induce apoptosis through small molecule inhibitors [63]. In experiments related to ERp57 and some neurodegenerative diseases, such as Alzheimer’s disease, prion protein and Parkinson’s disease, ERp57 either acts as a protective agent or acts as a harmful molecule on the disease, and specifically the mode of action needs to be confirmed by further experiments.
Type 2 diabetes is characterized by insulin resistance and pancreatic cell dysfunction. Type 2 diabetes is caused by insufficient pancreatic islet cell volume expansion and insufficient insulin secretion, so that pancreatic islet cells gradually lose their ability to compensate for insulin resistance. In this process, autophagy plays an important role in the homeostasis of pancreatic β cells. In Atg7 combined INS-1 cells, siRNA inhibition of ERp57 expression resulted in an increase in the level of lysed Caspase-3 protein and a decrease in the number of live cells, while the increase in ERp57 expression may help prevent β-cell death caused by autophagy failure [64]. Another study showed that GRp58 gene knockout interferes with the proliferation of insulin or serum, which may be related to the interaction of mTOR. Therefore, it was found that GRp58 is a new type of mTOR interacting protein that can promote the assembly and activation of mTORC1 [65].
Non-alcoholic fatty liver (NAFLD) is a common chronic metabolic syndrome characterized by accumulation of fat within liver. Previously it has been reported that NAFLD has a higher prevalence in Eastern countries. The expression of ERp57 has shown to be up-regulated in the human fetal hepatocyte line (L-02) [66]. In this study, ERp57-siRNA significantly attenuates FFA (free fatty acid)-induced cell fat accumulation, so the expression level of ERp57 was observed to have significantly correlated with inflammation grade and fibrosis stage [66]. Another study pointed out that knocking down of ERp57 by using siRNA in L-02 cells not only increased the accumulation of cell lipids, but also aggravated the hepatocyte apoptosis induced by sodium palmitate [67]. Also, it has been shown that fatty acid synthase (FAS) is a key enzyme involved in fatty acid synthesis; and ERp57 knocking down could up-regulate the protein expression of FAS, so ERp57 is involved in elevated circulating free fatty acids (FFA) induced hepatocyte steatosis and apoptosis [66].
3.4 Bronchopulmonary Dysplasia
Premature infants have a great risk of developing bronchopulmonary dysplasia (BPD). The pathogenesis of BPD is not yet fully understood. Oxygen poisoning caused by oxygen therapy/hyperoxia is considered to be one of the main pathogenic factors. Under hyperoxic conditions, ERp57 is down-regulated in the lungs of newborn rats and cultured human endothelial cells. Studies have shown that transient transfection of ERp57-siRNA can significantly reduce the expression of ERp57 and reduce the apoptosis of human endothelial cells induced by hyperoxia or tunicamycin, while the overexpression of ERp57 aggravates the human endothelium induced by hyperoxia or tunicamycin apoptosis. Therefore, ERp57 can regulate the apoptosis of human endothelial cells, and the down-regulation of ERp57 protects the body from hyperoxia and tunicamycin-induced apoptosis by increasing the induction of BiP/GRP78 [68].
Cardiovascular disease (CVD) is the leading cause of global morbidity and mortality. The root cause of CVD is complex and multifactorial, involving many risk factors, such as abnormal blood lipids, high cholesterol levels, and overweight. Previous studies reported that a new derivative called danshensu/tetramethylpyrazine derivative (ADTM) could exhibit strong cardioprotective effects in both in vitro and in vivo acute myocardial infarction, and can resist cell damage induced by oxidative stress through ERp57 signaling pathway [69]. Chemical proteomics methods identified ERp57 as the main target of ADTM [70]. Then the biotin-conjugated ADTM analog (BAA) is used as a molecular probe to identify its protein target. The results also show that BAA has a protective effect on cell damage induced by oxidative stress in H9c2 cardioblasts. Various methods such as chemical proteomics have determined that ERp57 is a specific target of BAA [71]. Later studies have found that BAA has a synergistic effect. The anti-breast cancer and reduction of cardiotoxicity may be closely related to the endoplasmic reticulum stress [72].
The structure and function of ERp57 are complex and widely distributed, mainly in the ER, and a small amount in the cell membrane, cytoplasm, nucleus and mitochondria. Studies have shown that ERp57 is involved in the signal transduction from the cell surface, the regulation process that occurs in the nucleus, and the formation of polymer protein complexes involved in DNA repair. It could be concluded that ERp57 gene knock-out/knock-down might result in pro-apoptotic effect in most tumor cell models, while increasing in ERp57 expression inhibited the apoptosis. In non-small cell lung cancer, gene silencing of ERp57 has no effect on cell survival. In non-cancerous cell models, pro-apoptotic effects were observed after using siRNA against ERp57 or ERp57gene knockout, while ERp57 overexpression has a protective effect on cells; however, in the bronchopulmonary dysplasia the opposite conclusion was obtained. Overall, most studies have shown that inhibiting/knocking out of ERp57 could inhibit the cell proliferation and also induce apoptosis, and the overexpression of ERp57 could intensify the cancer development. Therefore, the effects of ERp57 on cell survival in normal or cancerous cells are in wide range and it is highly depending on the type of the cells. Further studies should focus on the role of ERp57 as a stress response protein and a protein involved in the recognition of DNA damage. The mechanism by which ERp57 is involved in a variety of pathological conditions is not fully understood, and further work is needed to optimize the broad therapeutic potential of targeting ERp57.
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Turano, C., Coppari, S., Altieri, F., Ferraro, A. (2002). Proteins of the PDI family: Unpredicted non-eR locations and functions. Iournal of Cellular Physiology, 193(2), 154–163. DOI 10.1002/(ISSN)1097-4652. [Google Scholar] [CrossRef]
2. Khanal, R. C., Nemere, I. (2007). The ERp57/GRp58/1,25d3-mARRS receptor: Multiple functional roles in diverse cell systems. Current Medicinal Chemistry, 14(10), 1087–1093. DOI 10.2174/092986707780362871. [Google Scholar] [CrossRef]
3. Kozlov, G., Maattanen, P., Schrag, J. D., Pollock, S., Cygler, M. et al. (2006). Crystal structure of the bb’ domains of the protein disulfide isomerase ERp57. Structure, 14(8), 1331–1339. DOI 10.1016/j.str.2006.06.019. [Google Scholar] [CrossRef]
4. Bennett, C. F., Balcarek, J. M., Varrichio, A., Crooke, S. T. (1988). Molecular cloning and complete amino-acid sequence of form-i phosphoinositide-specific phospholipase C. Nature, 334(6179), 268–270. DOI 10.1038/334268a0. [Google Scholar] [CrossRef]
5. Kozlov, G., Maattanen, P., Schrag, J. D., Pollock, S., Cygler, M. et al. (2006). Crystal structure of the bb’ domains of the protein disulfide isomerase ERp57. Structure, 14(8), 1331–1339. DOI 10.1016/j.str.2006.06.019. [Google Scholar] [CrossRef]
6. Oliver, J. D., Roderick, H. L., Llewellyn, D. H., High, S. (1999). ERp57 functions as a subunit of specific complexes formed with the ER lectins calreticulin and calnexin. Molecular Biology of the Cell, 10(8), 2573–2582. DOI 10.1091/mbc.10.8.2573. [Google Scholar] [CrossRef]
7. Song, D., Liu, H., Wu, J., Gao, X., Hao, J. et al. (2021). Insights into the role of ERp57 in cancer. Journal of Cancer, 12(8), 2456. DOI 10.7150/jca.48707. [Google Scholar] [CrossRef]
8. Molinari, M., Helenius, A. (1999). Glycoproteins form mixed disulphides with oxidoreductases during folding in living cells. Nature, 402(6757), 90–93. DOI 10.1038/47062. [Google Scholar] [CrossRef]
9. Oliver, J. D., van Der Wal, F. J., Bulleid, N. J., High, S. (1997). Interaction of the thiol-dependent reductase ERp57 with nascent glycoproteins. Science, 275(5296), 86–88. DOI 10.1126/science.275.5296.86. [Google Scholar] [CrossRef]
10. Hirano, N., Shibasaki, F., Sakai, R., Tanaka, T., Nishida, J. et al. (1995). Molecular cloning of the human glucose-regulated protein ERp57/GRP58, a thiol-dependent reductase. identification of its secretory form and inducible expression by the oncogenic transformation. European Journal of Biochemistry, 234(1), 336–342. DOI 10.1111/j.1432-1033.1995.336_c.x. [Google Scholar] [CrossRef]
11. Zhu, Y., Cai, L., Guo, J., Chen, N., Yi, X. et al. (2016). Depletion of dicer promotes epithelial ovarian cancer progression by elevating PDIA3 expression. Tumor Biology, 37(10), 14009–140023. DOI 10.1007/s13277-016-5218-4. [Google Scholar] [CrossRef]
12. Wise, R., Duhachek-Muggy, S., Qi, Y., Zolkiewski, M., Zolkiewska, A. (2016). Protein disulfide isomerases in the endoplasmic reticulum promote anchorage-independent growth of breast cancer cells. Breast Cancer Research and Treatment, 157(2), 241–252. DOI 10.1007/s10549-016-3820-1. [Google Scholar] [CrossRef]
13. Ogino, T., Bandoh, N., Hayashi, T., Miyokawa, N., Harabuchi, Y. et al. (2003). Association of tapasin and HLA class I antigen down-regulation in primary maxillary sinus squamous cell carcinoma lesions with reduced survival of patients. Clinical Cancer Research, 9(11), 4043–4051. [Google Scholar]
14. Gaucci, E., Altieri, F., Turano, C., Chichiarelli, S. (2013). The protein ERp57 contributes to EGF receptor signaling and internalization in MDA-mB-468 breast cancer cells. Journal of Cellular Biochemistry, 114(11), 2461–2470. DOI 10.1002/jcb.24590. [Google Scholar] [CrossRef]
15. Celli, C. M., Jaiswal, A. K. (2003). Role of GRP58 in mitomycin C-induced DNA cross-linking. Cancer Research, 63(18), 6016–6025. [Google Scholar]
16. Liao, C. J., Wu, T. I., Huang, Y. H., Chang, T. C., Wang, C. S. et al. (2011). Glucose-regulated protein 58 modulates cell invasiveness and serves as a prognostic marker for cervical cancer. Cancer Science, 102(12), 2255–2263. DOI 10.1111/j.1349-7006.2011.02102.x. [Google Scholar] [CrossRef]
17. Leys, C. M., Nomura, S., LaFleur, B. J., Ferrone, S., Kaminishi, M. et al. (2007). Expression and prognostic significance of prothymosin-alpha and ERp57 in human gastric cancer. Surgery, 141(1), 41–50. DOI 10.1016/j.surg.2006.05.009. [Google Scholar] [CrossRef]
18. Choe, M. H., Min, J. W., Jeon, H. B., Cho, D. H., Oh, J. S. et al. (2015). ERp57 modulates STAT3 activity in radioresistant laryngeal cancer cells and serves as a prognostic marker for laryngeal cancer. Oncotarget, 6(5), 2654–2666. DOI 10.18632/oncotarget.3042. [Google Scholar] [CrossRef]
19. He, Y., Shao, F., Pi, W., Shi, C., Chen, Y. et al. (2016). Largescale transcriptomics analysis suggests over-expression of BGH3, MMP9 and PDIA3 in oral squamous cell carcinoma. PLoS One, 11(1), 1–13. DOI 10.1371/journal.pone.0146530. [Google Scholar] [CrossRef]
20. Yang, Z., Liu, J., Shi, Q., Chao, Y., Di, Y. et al. (2018). Expression of protein disulfide isomerase A3 precursor in colorectal cancer. Oncotargets and Therapy, 11, 4159–4166. DOI 10.2147/OTT. [Google Scholar] [CrossRef]
21. Hussmann, M., Janke, K., Kranz, P., Neumann, F., Mersch, E. et al. (2015). Depletion of the thiol oxidoreductase ERp57 in tumor cells inhibits proliferation and increases sensitivity to ionizing radiation and chemotherapeutics. Oncotarget, 6(36), 39247–39261. DOI 10.18632/oncotarget.5746. [Google Scholar] [CrossRef]
22. Fujii, T., Awaka, S. Y., Takahashi, Y., Fujita, K., Tsuji, H. et al. (2013). Modulation of H+, K+-ATPase activity by the molecular chaperone ERp57 highly expressed in gastric parietal cells. FEBS Letters, 587(24), 3898–3905. DOI 10.1016/j.febslet.2013.10.030. [Google Scholar] [CrossRef]
23. Bravo, S. B., Caminos, J. E., González, C. R., Vázquez, M. J., Garcés, M. F. et al. (2011). Leptin and fasting regulate rat gastric glucose-regulated protein 58. International Journal of Peptide Research and Therapeutics, 2011, 1–11. DOI 10.1155/2011/969818. [Google Scholar] [CrossRef]
24. Leys, C. M., Nomura, S., LaFleur, B. J., Ferrone, S., Kaminishi, M. et al. (2007). Expression and prognostic significance of prothymosin-α and ERp57 in human gastric cancer. Surgery, 141(1), 41–50. DOI 10.1016/j.surg.2006.05.009. [Google Scholar] [CrossRef]
25. Shimoda, T., Wada, R., Kure, S., Ishino, K., Kudo, M. et al. (2019). Expression of protein disulfide isomerase A3 and its clinicopathological association in gastric cancer. Oncology Reports, 41(4), 2265–2672. DOI 10.3892/or. [Google Scholar] [CrossRef]
26. Chung, H., Cho, H., Perry, C., Song, J., Ylaya, K. et al. (2013). Downregulation of ERp57 expression is associated with poor prognosis in early-stage cervical cancer. Biomarkers, 18(7), 573–579. DOI 10.3109/1354750X.2013.827742. [Google Scholar] [CrossRef]
27. Liao, C. J., Wu, T. I., Huang, Y. H., Chang, T. C., Lai, C. H. et al. (2014). Glucose-regulated protein 58 modulates beta-catenin protein stability in a cervical adenocarcinoma cell line. BMC Cancer, 14, 55–63. DOI 10.1186/1471-2407-14-555. [Google Scholar] [CrossRef]
28. Grillo, C., D’Ambrosio, C., Scaloni, A., Maceroni, M., Merluzzi, S. et al. (2006). Cooperative activity of Ref-1/APE and ERp57 in reductive activation of transcription factors. Free Radical Biology and Medicine, 41(7), 1113–1123. DOI 10.1016/j.freeradbiomed.2006.06.016. [Google Scholar] [CrossRef]
29. Roumeguère, T., Sfeir, J., El Rassy, E., Albisinni, S., van Antwerpen, P. et al. (2017). Oxidative stress and prostatic diseases. Molecular and Clinical Ooncology, 7(5), 723–728. DOI 10.3892/mco.2017.1413. [Google Scholar] [CrossRef]
30. Cocchiola, R., Romaniello, D., Grillo, C., Altieri, F., Liberti, M. et al. (2017). Analysis of STAT3 post-translational modifications (PTMs) in human prostate cancer with different gleason score. Oncotarget, 8(26), 42560–42570. DOI 10.18632/oncotarget.17245. [Google Scholar] [CrossRef]
31. Basu, A., Cajigas-Du Ross, C. K., Rios-Colon, L., Mediavilla-Varela, M., Daniels-Wells, T. R. et al. (2016). LEDGF/p75 overexpression attenuates oxidative stress-induced necrosis and upregulates the oxidoreductase ERP57/PDIA3/GRP58 in prostate cancer. PLoS One, 11(1), 1–25. DOI 10.1371/journal.pone.0146549. [Google Scholar] [CrossRef]
32. Cicchillitti, L., Della Corte, A., Di Michele, M., Donati, M. B., Rotilio, D. et al. (2010). Characterisation of a multimeric protein complex associated with ERp57 within the nucleus in paclitaxel-sensitive and -resistant epithelial ovarian cancer cells: The involvement of specific conformational states of beta-actin. International Journal of Oncology, 37(2), 445–454. DOI 10.3892/ijo. [Google Scholar] [CrossRef]
33. Zhao, S., Wen, Z., Liu, S., Liu, Y., Li, X. et al. (2015). Microrna-148a inhibits the proliferation and promotes the paclitaxel-induced apoptosis of ovarian cancer cells by targeting PDIA3. Molecular Medicine Reports, 12(3), 3923–3929. DOI 10.3892/mmr.2015.3826. [Google Scholar] [CrossRef]
34. Samanta, S., Tamura, S., Dubeau, L., Mhawech-Fauceglia, P., Miyagi, Y. et al. (2017). Expression of protein disulfide isomerase family members correlates with tumor progression and patient survival in ovarian cancer. Oncotarget, 8(61), 103543–103556. DOI 10.18632/oncotarget.21569. [Google Scholar] [CrossRef]
35. Xu, S., Butkevich, A. N., Yamada, R., Zhou, Y., Debnath, B. et al. (2012). Discovery of an orally active small-molecule irreversible inhibitor of protein disulfide isomerase for ovarian cancer treatment. Proceedings of the National Academy of Sciences of the United States of America, 109(40), 16348–16353. DOI 10.1073/pnas.1205226109. [Google Scholar] [CrossRef]
36. Santana-Codina, N., Carretero, R., Sanz-Pamplona, R., Cabrera, T., Guney, E. et al. (2013). A transcriptome-proteome integrated network identifies endoplasmic reticulum thiol oxidoreductase (ERp57) as a hub that mediates bone metastasis. Molecular & Cellular Proteomics, 12(8), 2111–2125. DOI 10.1074/mcp.M112.022772. [Google Scholar] [CrossRef]
37. Aureli, C., Gaucci, E., Arcangeli, V., Grillo, C., Eufemi, M. et al. (2013). ERp57/PDIA3 binds specific DNA fragments in a melanoma cell line. Gene, 524(2), 390–395. DOI 10.1016/j.gene.2013.04.004. [Google Scholar] [CrossRef]
38. Corazzari, M., Lovat, P. E., Armstrong, J. L., Fimia, G. M., Hill, D. S. et al. (2007). Targeting homeostatic mechanisms of endoplasmic reticulum stress to increase susceptibility of cancer cells to fenretinide-induced apoptosis: The role of stress proteins ERdj5 and ERp57. British Journal of Cancer, 96(7), 1062–1071. DOI 10.1038/sj.bjc.6603672. [Google Scholar] [CrossRef]
39. Trivedi, R., Müller, G. A., Rathore, M. S., Mishra, D. P., Dihazi, H. (2016). Anti-leukemic activity of shikonin: Role of ERP57 in shikonin induced apoptosis in acute myeloid leukemia. Cellular Physiology and Biochemistry, 39(2), 604–616. DOI 10.1159/000445652. [Google Scholar] [CrossRef]
40. Hwang, D., Kim, M., Park, H., Jeong, M. I., Jung, W. et al. (2019). Natural products and acute myeloid leukemia: A review highlighting mechanisms of action. Nutrients, 11(5), 1010–1032. DOI 10.3390/nu11051010. [Google Scholar] [CrossRef]
41. Hettinghouse, A., Liu, R., Liu, C. J. (2018). Multifunctional molecule ERp57: From cancer to neurodegenerative diseases. Pharmacology & Therapeutics, 18(1), 34–48. DOI 10.1016/j.pharmthera.2017.07.011. [Google Scholar] [CrossRef]
42. Huang, B. P., Lin, C. S., Wang, C. J., Kao, S. H. (2017). Upregulation of heat shock protein 70 and the differential protein expression induced by tumor necrosis factor-alpha enhances migration and inhibits apoptosis of hepatocellular carcinoma cell HepG2. International Journal of Medical Sciences, 14(3), 284–293. DOI 10.7150/ijms.17861. [Google Scholar] [CrossRef]
43. Liu, M., Du, L., He, Z., Yan, L., Shi, Y. et al. (2017). Increased ERp57 expression in HBV-related hepatocellular carcinoma: Possible correlation and prognosis. Biomed Research International, 12(5), 26–47. DOI 10.1155/2017/1252647. [Google Scholar] [CrossRef]
44. Chignard, N., Shang, S., Wang, H., Marrero, J., Bréchot, C. et al. (2006). Cleavage of endoplasmic reticulum proteins in hepatocellular carcinoma: Detection of generated fragments in patient sera. Gastroenterology, 130(7), 2010–2022. DOI 10.1053/j.gastro.2006.02.058. [Google Scholar] [CrossRef]
45. Chen, Y. Y., Liu, F. C., Wu, T. S., Sheu, M. J. (2015). Antrodia cinnamomea inhibits migration in human hepatocellular carcinoma cells: The role of ERp57 and PGK-1. American Journal of Chinese Medicine, 43(8), 1671–1696. DOI 10.1142/S0192415X15500950. [Google Scholar] [CrossRef]
46. Takata, H., Kudo, M., Yamamoto, T., Ueda, J., Ishino, K. et al. (2016). Increased expression of PDIA3 and its association with cancer cell proliferation and poor prognosis in hepatocellular carcinoma. Oncology Letters, 12(6), 4896–4904. DOI 10.3892/ol.2016.5304. [Google Scholar] [CrossRef]
47. Kondo, R., Ishino, K., Wada, R., Takata, H., Peng, W. X. et al. (2019). Downregulation of protein disulfideisomerase A3 expression inhibits cell proliferation and induces apoptosis through STAT3 signaling in hepatocellular carcinoma. International Journal of Oncology, 54(4), 1409–1421. DOI 10.3892/ijo.2019.4710. [Google Scholar] [CrossRef]
48. Siegel, R. L., Miller, K. D., Jemal, A. (2019). Cancer statistics 2019. A Cancer Journal for Clinicians, 69, 7–34. DOI 10.3322/caac.21551. [Google Scholar] [CrossRef]
49. Dihazi, H., Dihazi, G. H., Bibi, A., Eltoweissy, M., Mueller, C. A. et al. (2013). Secretion of ERP57 is important for extracellular matrix accumulation and progression of renal fibrosis, and is an early sign of disease onset. Journal of Cell Science, 126(16), 3649–3463. DOI 10.1242/jcs.125088. [Google Scholar] [CrossRef]
50. Zou, H., Wen, C., Peng, Z., Shao, Y. Υ., Hu, L. et al. (2018). P4HB and PDIA3 are associated with tumor progression and therapeutic outcome of diffuse gliomas. Oncology Reports, 39(2), 501–510. DOI 10.3892/or.2017.6134. [Google Scholar] [CrossRef]
51. Liu, Y., Wang, J. X., Nie, Z. Y., Wen, Y., Jia, X. J. et al. (2019). Upregulation of ERp57 promotes clear cell renal cell carcinoma progression by initiating a STAT3/ILF3 feedback loop. Journal of Experimental & Clinical Cancer Research, 38(1), 439–456. DOI 10.1186/s13046-019-1453-z. [Google Scholar] [CrossRef]
52. Wang, K., Li, H., Chen, R., Zhang, Y., Sun, X. X. et al. (2017). Combination of CALR and PDIA3 is a potential prognostic biomarker for non-small cell lung cancer. Oncotarget, 8(57), 96945–96957. DOI 10.18632/oncotarget.18547. [Google Scholar] [CrossRef]
53. Perez, R. E., Truog, W. E., Navarro, A., Xu, D. (2007). ERp57, an endoplasmic reticulum (ER) protein, reduces hyperoxia-Induced ER stress in lung epithelial cells. The FASEB Journal, 21(6), A818. DOI 10.1096/fasebj.21.6.A818. [Google Scholar] [CrossRef]
54. Chamberlain, N., Korwin-Mihavics, B. R., Nakada, E. M., Bruno, S. R., Heppner, D. E. et al. (2019). Lung epithelial protein disulfide isomerase A3 (PDIA3) plays an important role in influenza infection, inflammation, and airway mechanics. Redox Biology, 22(10), 11–29. DOI 10.1016/j.redox.2019.101129. [Google Scholar] [CrossRef]
55. Perri, E., Parakh, S., Atkin, J. (2017). Protein disulphide isomerases: Emerging roles of PDI and ERp57 in the nervous system and as therapeutic targets for ALS. Expert Opinion on Therapeutic Targets, 21(1), 37–49. DOI 10.1080/14728222.2016.1254197. [Google Scholar] [CrossRef]
56. Wang, J., Wang, Z., Zhou, T., Chen, K., Gu, Y. et al. (2017). Effect of PDIA3 gene silence on colonic mast cells and visceral sensitivity of rats with irritable bowel syndrome. International Journal of Clinical and Experimental Pathology, 10(10), 10666. [Google Scholar]
57. Wilding, C., Bell, K., Funke, S., Beck, S., Pfeiffer, N. et al. (2015). GFAP antibodies show protective effect on oxidatively stressed neuroretinal cells via interaction with ERP57. Journal of Pharmacological Sciences, 127(3), 298–304. DOI 10.1016/j.jphs.2014.12.019. [Google Scholar] [CrossRef]
58. Xu, H., Luo, X., Qian, J., Pang, X., Song, J. et al. (2012). Protein disulfide isomerase regulates endoplasmic reticulum stress and the apoptotic process during prion infection and PrP mutant-induced cytotoxicity. PLoS One, 7(6), 1–12. DOI 10.1371/journal.pone.0038221. [Google Scholar] [CrossRef]
59. Torres, M., Medinas, D. B., Matamala, J. M., Woehlbier, U., Cornejo, V. H. et al. (2015). The protein-disulfide isomerase ERp57 regulates the steady-state levels of the prion protein. Journal of Biological Chemistry, 290(39), 23631–23645. DOI 10.1074/jbc.M114.635565. [Google Scholar] [CrossRef]
60. Sepulveda, M., Rozas, P., Hetz, C., Medinas, D. B. (2016). ERp57 as a novel cellular factor controlling prion protein biosynthesis: Therapeutic potential of protein disulfide isomerases. Prion, 10(1), 50–56. DOI 10.1080/19336896.2015.1129485. [Google Scholar] [CrossRef]
61. Hetz, C., Russelakis-Carneiro, M., Wälchli, S., Carboni, S., Vial-Knecht, E. et al. (2005). The disulfide isomerase grp58 is a protective factor against prion neurotoxicity. Journal of Neuroscience, 25(11), 2793–2802. DOI 10.1523/JNEUROSCI.4090-04.2005. [Google Scholar] [CrossRef]
62. Castillo, V., Oñate, M., Woehlbier, U., Rozas, P., Andreu, C. et al. (2015). Functional role of the disulfide isomerase ERp57 in axonal regeneration. PLoS One, 10(9), 1–23. DOI 10.1371/journal.pone.0136620. [Google Scholar] [CrossRef]
63. Hoffstrom, B. G., Kaplan, A., Letso, R., Schmid, R. S., Turmel, G. J. et al. (2010). Inhibitors of protein disulfide isomerase suppress apoptosis induced by misfolded proteins. Nature Chemical Biology, 6(12), 900–906. DOI 10.1038/nchembio.467. [Google Scholar] [CrossRef]
64. Yamamoto, E., Uchida, T., Abe, H., Taka, H., Fujimura, T. et al. (2014). Increased expression of ERp57/GRP58 is protective against pancreatic beta cell death caused by autophagic failure. Biochemical and Biophysical Research Communications, 453(1), 19–24. DOI 10.1016/j.bbrc.2014.09.040. [Google Scholar] [CrossRef]
65. Ramírez-Rangel, I., Bracho-Valdés, I., Vázquez-Macías, A., Carretero-Ortega, J., Reyes-Cruz, G. et al. (2011). Regulation of mTORC1 complex assembly and signaling by GRp58/ERp57. Molecular and Cellular Biology, 31(8), 1657–1671. DOI 10.1128/MCB.00824-10. [Google Scholar] [CrossRef]
66. Wang, H., Chan, P. K., Pan, S. Y., Kwon, K. H., Ye, Y. et al. (2010). ERp57 is up-regulated in free fatty acids-induced steatotic L-02 cells and human nonalcoholic fatty livers. Journal of Cellular Biochemistry, 110(6), 1447–1456. DOI 10.1002/jcb.22696. [Google Scholar] [CrossRef]
67. Zhang, X. Q., Pan, Y., Yu, C. H., Xu, C. F., Xu, L. et al. (2015). PDIA3 knockdown exacerbates free fatty acid-induced hepatocyte steatosis and apoptosis. PLoS One, 10(7), e0133882. DOI 10.1371/journal.pone.0133882. [Google Scholar] [CrossRef]
68. Xu, D., Perez, R. E., Rezaiekhaligh, M. H., Bourdi, M., Truog, W. E. (2009). Knockdown of ERp57 increases BiP/GRP78 induction and protects against hyperoxia and tunicamycin-induced apoptosis. American Journal of Physiology-Lung Cellular and Molecular Physiology, 297(1), 44–51. DOI 10.1152/ajplung.90626.2008. [Google Scholar] [CrossRef]
69. Cui, G., Shan, L., Chu, I. K., Li, G., Leung, G. P. H. et al. (2015). Identification of disulfide isomerase ERp57 as a target for small molecule cardioprotective agents. RSC Advances, 5(91), 74605–74610. DOI 10.1039/C5RA08551A. [Google Scholar] [CrossRef]
70. Cui, G., Shan, L., Guo, L., Chu, I. K., Li, G. et al. (2015). Novel anti-thrombotic agent for modulation of protein disulfide isomerase family member ERp57 for prophylactic therapy. Scientific Reports, 5, 10353–10363. DOI 10.1038/srep10353. [Google Scholar] [CrossRef]
71. Cui, G., Shan, L., Chu, I. K., Li, G., Leung, G. P. H. et al. (2015). Identification of disulfide isomerase ERp57 as a target for small molecule cardioprotective agent. RSC Advances, 5(91), 74605–74610. DOI 10.1039/C5RA08551A. [Google Scholar] [CrossRef]
72. Zhang, Y., Deng, H., Zhou, H., Lu, Y., Shan, L. et al. (2019). A novel agent attenuates cardiotoxicity and improves antitumor activity of doxorubicin in breast cancer cells. Journal of Cellular Biochemistry, 120(4), 5913–5922. DOI 10.1002/jcb.27880. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |