

 | Molecular & Cellular Biomechanics |  |
DOI: 10.32604/mcb.2021.015596
ARTICLE
Study of Hepatocytes Polyploidization Peculiarities in Cholestatic Liver of Adult Rats
1Faculty of Exact and Natural Sciences, Ivane Javakhishvili Tbilisi State University, Tbilisi, Georgia
2Faculty of Medicine, Ivane Javakhishvili Tbilisi State University, Tbilisi, Georgia
*Corresponding Author: Salome Kiparoidze. Email: skiparoidze@newvision.ge
Received: 30 December 2020; Accepted: 15 June 2021
Abstract: According to the literature, different mechanisms and kinetics proceeding of regenerative growth has been established using the basic models of liver regeneration (after resection or chemically induced). Hence, in order to determine general regularities of the adaptive-compensatory processes in various pathological conditions, the processes taking place in the cholestatic liver of adult white rats during the first four days after common bile duct ligation have been studied. It has been shown that in cholestatic liver, compensatory-adaptive processes take place with different kinetics compared to those after resection. In particular, in response to the increased functional load caused by destructive processes during cholestasis, the liver, at an early stage, responds by simple division of high ploidy (binuclear tetraploid) cells and further provides their quantitative increase. The difference between the processes taking place in cholestatic and resected liver is more expressed on the third and fourth day after common bile duct ligation. In particular, 4c cells are still highest in cholestatic liver, while all ploidy cells are present in equal numbers in the regenerated liver after resection. This fact of compensatory growth characteristic for reparative regeneration was not detected in cholestatic liver at the mentioned date.
Keywords: Liver; cholestasis; polyploidization; white rats
The physiological regeneration of the liver starting from mollusks and including mammals has been well studied by numerous works conducted for many years. Especially, the large amount of information is obtained about an ability to restore liver (reparative regeneration) in case of damage or loss of mass by using 2/3 resection of rodent liver and other models (compensatory hyperplasia). It has been established that compensatory and adaptive growth of the liver is governed by strictly regulated sequential processes, such as: proliferation, hypertrophy and polyploidization (respectively 50%, 20% and 30%) [1,2].
However, recent studies have not provided much information about the regeneration of liver at the initial stage of reparative growth that proceeds differently during various pathologies and especially during chronic diseases (chronic viral hepatitis, alcohol intoxication and concomitant pathologies, e.g., cirrhosis). For example, it has been established that during alimentary dyslipidemia, the mechanism of regeneration used for organ reparation depends on the duration of hepatogenic ration usage and the degree of damage. In particular, it has been shown that tissue renewal at the initial stage is achieved mainly by increasing the ploidy of liver parenchymal cells [3]. Increasing of the number of high ploidy hepatocytes in the liver has also been shown in a model of Wilson disease in rats [4]. It has been established that the increase in the degree of polyploidy takes place on the background of radiation and oxidative stress [5]. The number of polyploid cells is increased in the destructive liver parenchyma at 4th day after common bile duct ligation [6]. Based on the above, during some pathology, quantitative changes of high ploidy cells in the liver are achieved by stimulation of the polyploidy at the initial stage. So, it is clear that the study of cellular and molecular mechanisms of liver regenerative processes still remains a topical problem and therefore, special importance is attached to the study of the peculiarities of the adaptive-compensatory processes of the liver in various pathological conditions.
The goal of this study was the establish the hepatocytes polyploidization peculiarities in cholestatic liver of adult rats during the first four days after ligation of the common bile duct (CBDL). It was found that 48 h after CBDL, the number of highly ploidy cells and the mitotic activity of liver parenchymal cells increases sharply. Against the background of a subsequent decrease in mitotic activity, the number of binuclear tetraploid and octaploid cells again increases. From our results follows that in cholestatic liver at the initial stage (the first four days) the compensatory-adaptive processes proceed with a different kinetics compared to the resected one.
Experiments were carried out on adult (130–150 g) white rats. Model of cholestatic liver with common bile duct ligation was used [7]. Animals were housed under controlled conditions at a temperature of 25 ± 2°C, relative humidity of 60% ± 10%, with room air changes 12–18 times/hour, and a dark/light ratio = 14/10. During the experiment, the animals were provided with unrestricted access to water and food. The animals were decapitated under ether anesthesia. The study was approved by a board of professors from the Biology Department, Faculty of Exact and Natural Sciences, at Ivane Javakhishvili Tbilisi State University, according to the legal and statutory acts extant in Georgia under the Laws on Health Care and the Protection of Experimental Animals.
For studying the peculiarities of hepatocytes polyploidization in cholestatic liver rats were divided into 3 groups (seven rats per group; total-42 rats) as follows: 1) intact, 2) rats with sham operation (opening and closing of the peritoneal cavity), 3) rats with common bile duct ligation (CBDL). Observations in dynamics were performed to assess changes in the mitotic index and ploidy of liver cells. The liver tissue (study material) was taken at 24 h, 48 h, 72 h, and 96 h after CBDL. The adequacy of the experiment during the sampling was confirmed by the presence of expanded common bile duct.
2.3 Determination of Mitotic Index
1 mg/kg of colchicine (Sigma, USA) was injected into the animals of all, the control and the test groups. For each sample 5000 cells were counted and the number of mitotic cells per 1000 cells (‰) was determined.
2.4 Fixation and Embedding in Paraffin of Liver Tissue
Liver tissue was fixed in buffered formalin 4% solution pH 7.2–7.4 for 2 days. After fixation, the tissue sections were dehydrated by passing through in increasing concentration of alcohol baths (70%–30 min, 80%–30 min, 96%–30 min). Afterwards, tissues were placed in acetone three times for 20 min each, acetone-benzene (1:1) for 30 min, benzene three times for 20 min each. Then samples were placed in Paraffin wax (58–60°C), with three changes for 1 h per each. Tissues were embedded into paraffin blocks, sectioned by Leica microtome (thickness of sections −5 µm) and stained using standard protocol of hematoxylin and eosin (H&E) [8].Tissue samples were studied under the light microscope (Zeiss Primo Star, Germany).
2.5 Preparation of Schiff’s Reagent and Smears Staining
Hepatocytes smears were stained by Schiff reagent (Feulgen staining). Schiff’s reagent was prepared as follows: 200 ml of boiling, distilled water was poured on 1 g of powdered basic fuchsine. After cooling to 50°C, the solution was filtered and 20 ml of 1 N HCl was added, cooled to 25°C and 2 g of K2S2O5 was added; vacate overnight in a dark place. The bisulfite washing solution was composed of 10 ml 10% K2S2O5, 10 ml 1 N HCl and 100 ml of distilled water.
After fixation of methyl alcohol, the smear was fixed in 5% sulfosalicylic acid for 10 min, rinsed in distilled water and placed in solution (LiCl 9 M + HCl 0.2 M) for 20 min, rinsed in 0.01 M HCl, placed in the dye (Schiff reagent) for 30 min–1 h; moved in bisulfite washing solution three times for 5 min each; rinsed in 0,01 M HCl; then alcohol baths (80°−5 min, 96°−5 min); placed in xylol (two changes, 20 min per each) and mount in mounting media.
2.6 Nuclear DNA Content Measurement
Nuclear DNA content was detected by using computer software ImageJ 1.36b. Photos of Schiff’s reagent-stained slides were photographed (Canon) under a light microscope (immersion objective X100) with an interference filter (wave length–570 nm). Each image was processed in the computer software ImageJ 1.36b. Photos were switched to 32 bits, inverted (the background fades, and the nuclei take a light color). Staining intensity (absorption) was measured by software. 300 cells were measured for each sample. Totally 42 samples were analyzed.
Conventional light microscope, ocular micrometer and stage micrometer were used for morphometry. The ocular micrometer was calibrated using a stage micrometer-the size of the divisions on the ocular micrometer was determined at the appropriate magnification. Immersion objective (X100) and H&E stained preparations were used for the measurement. For each structure (hepatocytes and their nuclei) height and width were measured, received numbers were multiplied to obtain the area. 300 cells were measured for each sample [9,10]. Totally 42 samples were analyzed.
Data are expressed as mean SD. Student’s t test was used for comparison among the different groups. P < 0.05 was considered statistically significant.
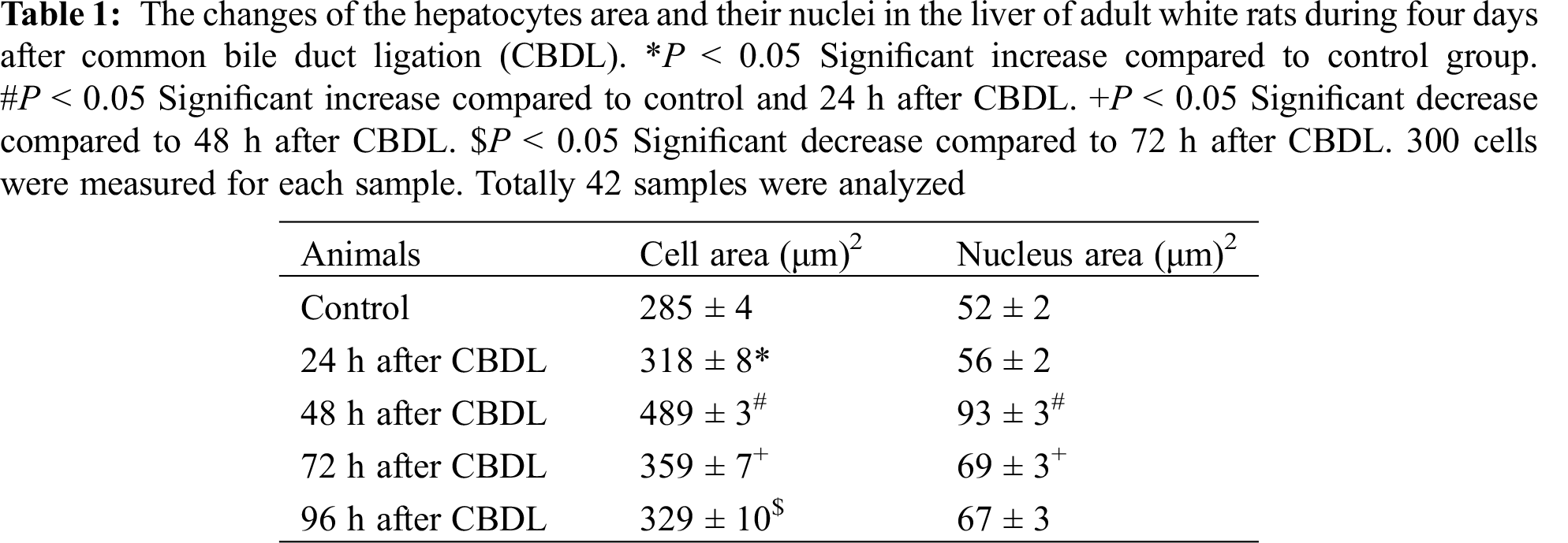
Validation of animal model of cholestatic liver was assessed by the presence of expanded common bile duct and histological changes in the liver tissue. Changes in the liver hystoarchitectonic of experimental group animals after CBDL were assessed at the initial stage of the study. Within 24 h after surgery, changes in tissue building in the cholestatic liver of adult white rats were detected. It appeared that in 24 h after the operation, slight damage was observed in the liver histoarchitecture of experimental animals, namely the plates of the liver are partially disrupted, the spaces of Disse are noticeably expanded (Figs. 1C and 1D). These destructive changes persist late after ligation (48, 72 h) (Figs. 1E–1H), although there is no significant increase in the degree of destruction mitotic figures in the liver parenchyma (Figs. 1D, 1F, 1H).
Within 24 h after surgery, mitotic activity of liver parenchymal cells increased slightly, but was reliable compared to control animals and amounted to 5‰ approximately (Fig. 2). At the same time, the number of diploid cells in the cholestatic liver was increased statistically reliably by approximately 20% and the number of tetraploid hepatocytes (4c and 2c × 2) was decreased (Fig. 3).

Figure 1: The liver tissue histoarchitecture of control (intact) and experimental animal groups. H&E. Intact group (A, B); 24 h after CBDL (C, D); 48 h after CBDL (E, F); 72 h after CBDL (G, H); 96 h after CBDL (I, J). In 24 h after the operation, slight damage was observed in the liver histoarchitecture of experimental animals. The plates of the liver are partially disrupted; the spaces of Disse are noticeably expanded. These destructive changes persist late after ligation (48, 72 h), although there is no significant increase in the degree of destruction (A, C, G, I–x 200; B, D, F, J–x 400). Arrows indicate mitotic cells
The area of hepatocytes was slightly increased over this period, although the sizes of their nuclei have not changed (Fig. 3, Tab. 1). Changes in the liver hystoarchitectonic were more sharply expressed at the 48 h after CBDL. Disruption of the plates structure and increase of the distance by disconnection of the links between the hepatocytes was clearly seen (Figs. 1E and 1F).
With all these changes, high proliferative activity of liver parenchymal cells was revealed. The mitotic index of hepatocytes reaches 45‰ at 48 h after CBD ligation (Fig. 2). It should be noted that at the same time, the number of high ploidy cells also increases dramatically.

Figure 2: The changes of the hepatocytes mitotic activity of the adult white rats during four days after common bile duct ligation (CBDL). Mitotic activity of cells in the liver is significantly increased compared to control (intact and sham operated animal) group within 24 h after CBDL. The maximum mitotic activity (45‰) is observed at 48 h after CBDL. At 72 h after CBDL mitotic index is decreased compared to 48 h after CBDL and remains unchangeable at 96 h after CBDL.*P < 0.05 Significant increase compared to control (intact and sham operated animal) group. #P < 0.05 Significant increase compared to control (intact and sham operated animal) and 24 h after CBDL animal group. +P < 0.05 Significant decrease compared to 48 h after CBDL animal group. For each sample 5000 cells were counted and the number of mitotic cells per 1000 cells (‰) was determined
In particular, the number of 4c cells was increased and mononuclear and binuclear octaploid (8c, 4c × 2) cells appeared (Fig. 3). Simultaneously, areas of liver parenchymal cells and their nuclei were significantly increased (Tab. 1). The mitotic index of hepatocytes remained high compared to the control at 72 h after CBDL, although it is was significantly lower than the corresponding data recorded at 48 h (Fig. 2). The number of high ploidy cells (4c, 2c × 2, 8c) did not change either. The mitotic index decreased at 96 h after CBDL (Fig. 2), but the number of binuclear tetraploid (2c × 2), and octaploid (4c × 2) cells increased (Fig. 3).
The basic models (resection and chemically induced) used to study liver regeneration have been established and showed that despite the similarity of the main signaling pathways, regeneration in different cases takes place through different mechanisms and kinetics [11]. It should also be noted, that liver regeneration is significantly related to the rate of bile flow and excretion of bile constituent lipids and, consequently, enterohepatic circulatory disorders are considered to be a significant inhibitor of liver regeneration [12,13].

Figure 3: Changes in hepatocyte ploidy profile in the liver of adult white rats during four days after common bile duct ligation (CBDL). Within 24 h after surgery, the number of tetraploid cells decreases and the number of diploid hepatocytes increases. The number of high ploidy cells (4c, 4c × 2, and 8c) increases reliably only 48 h after surgery. *P < 0.05 Significant increase compared to control group. +P < 0.05 Significant decrease compared to control group. #P < 0.05 Significant decrease compared to 24 h after CBDL animal group. %P < 0.05 Significant increase compared to 24 h after CBDL animal group. &P < 0.05 Significant decrease compares to 48 h after CBDL animal group. @P < 0.05 Significant increase compared to 72 h after CBDL animal group. 300 cells were measured for each sample. Totally 42 samples were analyzed
At the same time, after common bile duct ligation, the concentration of hepatocyte growth factor (HGF) (peak on the 2nd day) and the mitotic activity of parenchymal cells increases very rapidly [14,6]. Based on the above in conditions of liver regeneration delay, the concentration of hepatocyte growth factor and mitotic activity of hepatocytes is increased [12,13]. Considering that any liver pathology may be present in complex with cholestasis, in order to determine the compensatory-adaptive mechanisms of the organ under various pathology conditions, it is especially important to know the peculiarities of the processes taking place in the cholestatic liver at the initial stage (during the first four days).
Upon the alteration of liver architecture of experimental animal groups, diploid hepatocytes are quantitatively increased already within 24 h after common bile duct ligation (Fig. 3). According to the literature, two mononuclear hepatocytes can be created by simple division from binuclear hepatocytes [15]. The number of diploid cells can also be increased by the transition of 4c cells being in G2-0 into mitosis phase [16,17]. Hence, a small but reliable increase in mitotic index and quantitative increase in diploid hepatocytes is achieved by division of tetraploid hepatocytes (4c and 2c × 2) within 24 h after common bile duct ligation. An increase in the number of diploid hepatocytes by simple division of tetraploid hepatocytes has also been reported within 0.5 h after large-scale resection of the liver in white adult rats [16,17]. Compared to resection, the increased number of diploid cells in cholestatic liver about 24 h later can be explained by the fact that during this period, the destructive changes caused by CBDL do not lead to a significant increase of the liver functional load.
Reinforcement of destructive processes in the liver tissue within 48 h after surgery supposedly has to cause an increase in functional load on the organ. It is interesting how the parenchymal organ respond to the functional load in this case.
The analysis of the histograms presented in Fig. 2 showed that during this period, the percentage cells with different ploidy in the liver parenchyma changes dramatically. In particular, upon the modification of the decreased number of diploid cells, tetraploid and octaploid cells (4c, 4c × 2 and 8c) have been noticeably increased. This indicates that at this time (48 h) the liver responds to functional load by increasing the number of high ploidy cells. For this purpose, the liver can use endoreduplication mechanism characteristic of hepatocytes, which allows increasing the functional activity of the organ only by polyploidization without increasing the number of cells [18]. At the same time, the liver parenchymal cells and nuclei areas are significantly increased (Fig. 2, Tab. 1). Similar growth of high ploidy cells in the liver of adult rats within 2–18 h after partial hepatectomy have been reported [16,17].
A different picture was revealed according to quantitative changes of high ploidy cells as well as the mitotic activity of hepatocytes in cholestatic liver compared to the resected liver data obtained from literature. In particular, within 42 h after liver resection, 4c cells decrease and only the number of octaploid (8c) cells increases dramatically [16,17]. In cholestatic liver, as described above, upon the modification of the decreased number of diploid cells, tetraploid and octaploid cells (4c, 4c × 2 and 8c) has been noticeably increased. After resection, the mitotic index of hepatocytes decreases to 4% [16,17], while the mitotic index in cholestatic liver reaches 45%.
The difference between the processes going on in the cholestatic and resected liver is clearly expressed at 72 h after CBDL. In particular, 4c cells (Fig. 3) is still the highest in cholestatic liver, while all ploidy cells are present in equal numbers in the regenerated liver after resection [16,17]. This fact of compensatory growth characteristic for reparative regeneration was not detected in cholestatic liver at a later date. Upon the decreasing of mitotic index at 96 h after CBDL (Fig. 2) mononuclear tetraploid cells still remain higher. However, the number of binuclear octaploid (4c × 2) cells also increases slightly, but reliably (Fig. 3).
The obtained result let us conclude that:
• In response to the increased functional load caused by destructive processes during cholestasis, the liver, at an early stage, uses mainly high ploidy cells. In particular, the increase of mass and functional activity is provided primarily by the simple division of binuclear tetraploid cells and their further quantitative increases.
• In cholestatic liver, compensatory-adaptive processes take place with different kinetics compared to the resected one.
Funding Statement: The research was supported by the Shota Rustaveli National Science Foundation of Georgia, No. DP2016_22. New Interfaculty Interdisciplinary Structured Doctoral Programme “Translational Biomedicine” (Direction–“Hepatology”).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Sakuta, G. A., Kudriavtsev, B. N. (2005). Cellular mechanisms of cirrhotic rat liver regeneration. II. Proliferation, polyploidization and hypertrophy after partial hepatectomy. Tsitologia, 47(5), 379–387. [Google Scholar]
2. Eidi, A., Mortazavi, P., Moghadam, J. Z., Mardani, P. M. (2015). Hepatoprotective effects of Portulacaoleracea extract against CCl4-induced damage in rats. Pharmaceutical Biology, 53(7), 1042–1051. DOI 10.3109/13880209.2014.957783. [Google Scholar] [CrossRef]
3. Bivalkevich, N. V., Karaman, Yu, K. (2009). Mechanisms of rat liver regeneration in alimentary dyslipidemia. Health Medical Ecology Science, 4–5, 39–40. [Google Scholar]
4. Yamada, T., Sogawa, K., Kim, J. K., Izumi, K., Suzuki, Y. et al. (1998). Increased polyploidy, delayed mitosis and reduced protein phosphatase-1 activity associated with excess copper in the Long Evans Cinnamon rat. Research Communications in Molecular Pathology and Pharmacology, 99(3), 283–304. [Google Scholar]
5. Gentric, G., Maillet, V., Paradis, V., Couton, D., L’Hermitte, A. et al. (2015). Oxidative stress promotes pathologic polyploidization in nonalcoholic fatty liver disease. Journal of Clinical Investigation, 125(3), 981–992. DOI 10.1172/JCI73957. [Google Scholar] [CrossRef]
6. Bakuradze, E. (2006). Charachteristic of liver regeneration in codition of cholestasis (Ph.D. Thesis). [Google Scholar]
7. Tag, C. G., Sauer-Lehnen, S., Weiskirchen, S., Borkham-Kamphorst, E., Tolba, R. H. et al. (2015). Bile duct ligation in mice: Induction of inflammatory liver injury and fibrosis by obstructive cholestasis. Journal of Visualized Experiments, 96, 52438. DOI 10.3791/52438. [Google Scholar] [CrossRef]
8. Cardiff, R. D., Miller, C. H., Munn, R. J. (2014). Manual hematoxylin and eosin staining of mouse tissue sections. Cold Spring Harbor Protocols, 6, 655–658. DOI 10.1101/pdb.prot073411. [Google Scholar] [CrossRef]
9. Hunter, K. L., Hunter, R. B. (2004). Marigold cell size and polyploidy. In: M. A. O’Donnell (Ed.Tested studies for laboratory teaching, vol. 25, no. 5, pp. 125–133. Proceedings of the 25th Workshop/Conference of the Association for Biology Laboratory Education. [Google Scholar]
10. Adili, N., Melizi, M., Bennoune, O. (2013). The influence of age, sex and altitude on the morphometry of red blood cells in bovines. Veterinary World, 6(8), 476–478. [Google Scholar]
11. Mehendale, H. M., Chilakapati, J. (2010). Hepatic toxicology. Comprehensive Toxicology, 9, 627–638. [Google Scholar]
12. Chijiiwa, K., Mizuta, A., Ueda, J., Takamatsu, Y., Nakamura, K. et al. (2002). Relation of biliary bile acid output to hepatic adenosine triphosphate level and biliary indocyanine green excretion in humans. World Journal of Surgery, 26(4), 457–461. DOI 10.1007/s00268-001-0249-3. [Google Scholar] [CrossRef]
13. Yokoyama, Y., Nagino, M., Nimura, Y. (2007). Mechanism of impaired hepatic regeneration in cholestatic liver. Journal of Hepato-Biliary-Pancreatic Surgery, 14(2), 159–166. DOI 10.1007/s00534-006-1125-1. [Google Scholar] [CrossRef]
14. Yu, Y., Yao, A. H., Chen, N., Pu, L. Y., Fan, Y. et al. (2007). Mesenchymal stem cells over-expressing hepatocyte growth factor improve small-for-size liver grafts regeneration. Molecular Therapy: The Journal of the American Society of Gene Therapy, 15(7), 1382–1389. DOI 10.1038/sj.mt.6300202. [Google Scholar] [CrossRef]
15. Sigal, S. H., Rajvanshi, P., Gorla, G. R., Sokhi, R. P., Saxena, R. et al. (1999). Partial hepatectomy-induced polyploidy attenuates hepatocyte replication and activates cell aging events. Americal Jounal of Physiology, 276(5), G1260–72. DOI 10.1152/ajpgi.1999.276.5.G1260. [Google Scholar] [CrossRef]
16. Romanova, L. K. (1984). The regulation of rehabilitation processes, pp. 176. Publishing House of Moscow University. [Google Scholar]
17. Ryabinina, Z. A., Benyush, V. A. (1973). Poliploidiya i gipertrofiya kletok v protsessakh rosta i vosstanovleniya (Polyploidy and Hypertrophy of Cells during Growth and Recovery). Moscow: Meditsina. [Google Scholar]
18. Celton-Morizur, S., Merlen, G., Couton, D., Desdouets, C. (2010). Polyploidy and liver proliferation: Central role of insulin signaling. Cell Cycle, 9(3), 460–466. DOI 10.4161/cc.9.3.10542. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |