 Molecular & Cellular Biomechanics
Molecular & Cellular Biomechanics
 Molecular & Cellular Biomechanics Molecular & Cellular Biomechanics |  |
DOI: 10.32604/mcb.2021.014071
ARTICLE
Cyclic Stretch Induces Inflammatory Cytokines via the Oxidative Stress and NF-ΚB Pathways Activation in Human Keratoconic Fibroblasts
1College of Biomedical Engineering, Taiyuan University of Technology, Taiyuan, 030024, China
2Department of Biomedical Engineering, State University of New York at Stony Brook, Stony Brook, NY 11794 5281, USA
3Keratonosus Department, Shanxi Eye Hospital, Taiyuan, 030002, China
4Excimer Laser Department, Shanxi Eye Hospital, Taiyuan, 030002, China
*Corresponding Authors: Xiaona Li. Email: lixiaona@tyut.edu.cn; Rui He. Email: he.r@163.com
Received: 02 September 2020; Accepted: 17 November 2020
Abstract: The cornea is a load-bearing tissue. Lower biomechanical properties in the local tissue of keratoconic cornea evoke mechanical stress increase. Inflammatory cytokines have been shown to be over-expressed in patients with keratoconus. However, how mechanical stimuli are involved in the production of inflammatory cytokines in keratoconus remains unclear. The objective of the study is to determine the role of mechanical stretch in the regulation of inflammatory cytokines and the underlying mechanisms in keratoconus. Human keratoconic fibroblasts (hKCFs) were subjected to 12% cyclic mechanical stretch at 0.1 Hz or in static conditions as controls. N-acetyl cysteine (NAC) and pyrrolidine dithiocarbamate and pyrrolidine dithiocarbamate (PDTC) were used to inhibit reactive oxygen species (ROS) production and NF-κB pathway respectively. ROS production was measured using 2’,7’-dichlorodihydrofluorescindiacetate probe. Conditioned media and cell lysates were collected for protein assessment. Cyclic stretch-induced a higher production of intercellular cell adhesion molecule-1 (ICAM-1), tumor necrosis factor α (TNF-α), interleukin (IL)-6, and IL-8 in hKCFs than static controls. ROS was also elevated in response to cyclic stretch. Inhibition of ROS or NF-κB attenuated stretch-induced ICAM-1, TNF-α, IL-6, and IL-8. Inhibition of stretch-induced ROS production by NAC also attenuated NF-κB activation. Our findings suggest that mechanical stretch may induce the release of inflammatory cytokines by activating oxidative stress and NF-kB pathway, and ROS may positively control NF-κB signaling. Over-expression of inflammatory cytokines induced by mechanical stretch may play a role in progression of keratoconus.
Keywords: Keratoconus; corneal fibroblasts; mechanical stretch; inflammatory cytokines; oxidative stress; signal pathway
Keratoconus (KC) is a progressive corneal ectasia disorder associated with corneal thinning and irregular astigmatism that leads to reduced visual performance [1]. Some risk factors that include genetic, mechanical, environmental factors, and their possible combinations are believed to be linked to the etiology of KC [2]. As a load-bearing tissue, the cornea is mainly subjected to biaxial tensile stress under the intraocular pressure (IOP) in vivo. It is well established that KC is characterized by a marked degradation of the extracellular matrix, resulting in a focal reduction in biomechanical properties. A decrease in thickness and the biomechanical properties of corneas will provoke larger tensile stress across the keratoconus corneas compared to healthy ones. This will force keratoconic corneas to deform to a great amount under IOP, and the stress across the cornea will be redistributed as the tissue bulges [3]. Therefore, it’s important to explore the role of mechanical stress during the development of KC.
Recent studies indicate that the pathogenesis of KC may involve complex chronic inflammatory events [4,5]. Inflammatory cytokines, such as interleukin-1β (IL-1β)/-4/-5/-6/-8, tumor necrosis factor alpha (TNF-α), and intercellular cell adhesion molecule-1(ICAM-1), have been shown to be over-expressed in patients with KC in the tear film and corneas [6,7]. Moreover, inflammatory cytokines can facilitate generation of proteolytic enzymes such as matrix metalloproteinases (MMPs) and lead to focal tissue destruction [8], e.g., TNF-α increased MMP-1 expression via IL-6 in human keratoconic fibroblasts (hKCFs) [9]. Ocular cells are capable of producing proinflammatory cytokine in response to mechanical stretch in vitro [10–12]. Our previous studies showed that cyclic stretch was involved in the regulation of MMPs and their inhibitors (tissue inhibitors of metalloproteinases, TIMPs) in corneal fibroblasts [13,14]. It is well accepted that wearing contact lenses, eye rubbing, and ultraviolet irradiation can induce inflammation features in KC [15]. However, the role of mechanical stimuli in the expression of inflammatory cytokines in KC remains unclear.
The effect of oxidative damage in corneal cells [16,17] and tissue [18,19], tear film [20] and serum [21,22] from KC patients suggested that oxidative stress was involved in KC. Oxidative stress in KC causes an increase in ROS and a decrease in antioxidants, which may result in subsequent degradation and thinning of the corneal tissue [23,24]. Previous studies showed that hKCFs have an inherent, hypersensitive response to oxidative stressors [16,17]. It is well accepted that ROS and the nuclear factor κ-light-chain-enhancer of activated B cells (NF-κB) regulate a wide variety of genes involved in inflammatory responses in many cell types by mechanical stretch [25–27]. However, the role of oxidative stress and NF-κB activation in the hKCFs has not explored.
The goal of this study was to investigate whether cyclic stretch could induce the release of inflammatory cytokines in hKCFs and its possible pathway. First, hKCFs was subjected to cyclic stretch, and ROS generation and the expression of inflammatory cytokines were determined. Then, we showed that the cyclic stretch-induced increase in inflammatory cytokines via ROS generation could be attenuated by ROS scavengers and NF-kB inhibitors. Our results may provide clues to understanding the mechanisms behind KC from mechanobiology view.
2.1 Isolation and Culture of KC Fibroblasts
The discarded KC cornea tissue was collected from the patients with KC defect who received corneal transplantation in Shanxi Eye Hospital (Taiyuan city, China). The study was conducted with the approval of the Ethics Committee of Shanxi University (No. SXULL2019041) after obtaining the consent from the patient. In brief, the epithelial sheet and endothelial layer of the cornea was removed mechanically; and the remaining tissue was treated with 1 mg/ml type II collagenase (Thermo scientific, USA) at 37°C until a single-cell suspension was obtained. Isolated cells were cultured in complete fibroblasts medium (FM) (consisting of 2% fetal bovine serum, 1% penicillin/streptomycin, and 1% fibroblasts growth supplement) (Gibico, USA) in a humidified atmosphere of 5% CO2/95% air at 37°C.
hKCFs (passages 2∼3) were harvested with 0.25% trypsin and EDTA, then seeded onto at a density of 30,000 per cm2 for further experiments.
Mechanical stretch was applied on several ocular cell types, such as corneal fibroblasts [13,14], scleral fibroblasts [28], trabecular meshwork cells [11] and retinal glial (Müller) cells [10]. Loading regimens varied from different research groups, with frequency from 0.03 Hz to 1 Hz, strain amplitude 0.45% to 20%, and duration from 30 min to 72 h. Considering the thickness and modulus of the cornea will decrease in the keratoconus, and IOP will increase during eye rubbing, the strain across the cornea may becaome larger than the normal one. In this study, hKCFs were seeded on six-well Bioflex® plates (Flexcell Int. Corp., Hillsborough, NC, USA) with silicon membrane bottoms coated with type I collagen. When the cell density reached 80% confluence, the medium was removed and replaced with basic FM medium. Then the cells were subjected to Flexcell® Tension Plus™ system (FX-4000, Flexcell Int. Corp., Hillsborough, NC, USA) with an elongation rate of 12%, 0.1 Hz, for 3 h and 9 h, respectively. Unstretched cells were treated as static controls.
For signaling pathway studies, cells were pretreated with the 0.5 mM N-acetyl cysteine (NAC) as a ROS scavenger, or NF-κB inhibitor pyrrolidine dithiocarbamate (PDTC; 20 μM, Sigma, USA) for 1 h prior to stretch. Conditioned medium and cells lysates were harvested after stretch.
2.3 Detection of Intracellular Reactive Oxygen Species (ROS)
Intracellular ROS production was measured using 2’,7’-dichlorodihydrofluorescindiacetate probe (DCFH-DA; Sigma, USA). After the stretch, cells were rinsed with phenol red-free DMEM and loaded with 10 μM DCFH-DA in phenol red-free DMEM for 40 min at 37°C. The cells were then washed three times with PBS, and changed to fresh phenol red-free DMEM. Images were captured on an inverted fluorescent microscope (IX71; Olympus, Japan), and analyzed using Image Pro Plus 5.2 Software (Media Cybernetics, USA).
The western blotting analysis was used to investigate the effects of cyclic stretch on the expression of inflammatory cytokines and signaling pathways. Briefly, equal amounts of cell lysates or conditioned medium were fractionated by SDS-PAGE and electrically transferred onto a polyvinylidene difluoride membrane. Non-special protein reaction was blocked with TBST containing 5% non-fat milk. The membranes were incubated overnight with primary antibodies against IL-1β, IL-6, IL-8, TNF-α, ICAM-1 (dilution ratio 1:200), NF-κB, p-NF-κB (dilution ratio 1:500), and β-actin (dilution ratio 1:1000) (Bio-High Technology, Shijiazhuang, Hebei, China) at 4°C followed by incubation with HRP-conjugated secondary antibodies (dilution ratio 1:1000) for 1 h at room temperature. The enhanced chemiluminescent substrate was used to visualize protein bands according to the manufacturer’s recommended instructions.
Statistical analysis was performed using SPSS 18.0. All data were analyzed using one-way ANOVA tests followed by turkey post hoc tests. P value below 0.05 was considered statistically significant. Results are presented as the mean ± standard deviation of three independent experiments.
3.1 Cyclic Stretch Increases the Expression of IL-6, IL-8, TNF-α, and ICAM-1 in hKCFs
In order to evaluate the effects of cyclic stretch on the expression of inflammatory cytokines in hKCFs, cells were subjected to 12% elongation, 0.1 Hz for 3 h and 9 h respectively. Compared with static controls, cyclic stretch alone significantly increased the protein production of ICAM-1, TNF-α, IL-6, and IL-8 (P < 0.05), while there were no significant changes in IL-1β for 3 h. The trend was similar in 9 h treatment groups (Fig. 1). These results indicated that cyclic stretch facilitated production of inflammatory cytokines in hKCFs in vitro.

Figure 1: Cyclic stretch induces inflammatory cytokines in hKCFs in vitro. Cells were subjected to cyclic stretch with an elongation rate of 12%, 0.1 Hz in the presence or absence of 0.5 mM NAC or 20 μM PDTC for 3 h or 9 h. Protein expression levels were then evaluated by western blotting. Unstretched cells were treated as static controls. β-Actin was used as a loading control for cell lysates or conditioned medium. The indicated quantitative data refer to ratio to static control for 3h. Data are presented as mean ± standard deviation of three independent experiments. *P < 0.05, vs. static control group; #P < 0.05 vs. cyclic stretch only. NAC, N-acetyl cysteine, a ROS scavenger; PDTC, pyrrolidine dithiocarbamate, a NF-κB inhibitor
3.2 Inflammatory Cytokines Induced by Cyclic Stretch are Down-Regulated by ROS Inhibition
Oxidative stress is demonstrated to be induced by mechanical stress in different types of cells, such as endothelial cells [25], epithelial cells [27], and mesenchymal stem cells [29]. In this study, we hypothesized that the effects of cyclic stretch on the expression of inflammatory cytokines might be mediated via ROS production. To test this hypothesis, the intracellular ROS generation was analyzed in hKCFs under cyclic stretch. Our results showed that the intracellular ROS generation increased significantly when hKCFs were subjected to cyclic stretch compared with static control (P < 0.05) (Fig. 2). To evaluate the role of ROS in the expression of inflammatory cytokines in hKCFs, the expression of inflammatory cytokines in hKCFs were detected in the presence of 0.5 mM NAC as a ROS scavenger under cyclic stretch. NAC significantly reduced the expression of ICAM-1, TNF-α, IL-6, and IL-8 in hKCFs exposed to cyclic stretch, but had no obvious effect on the expression of IL-1β (P > 0.05) (Fig. 1). These results indicate that up-regulation of inflammatory cytokines induced by a cyclic stretch is at least partly mediated via ROS generation in hKCFs.

Figure 2: Intracellular ROS generation increased in hKCFs under stretched condition. Cells were subjected to cyclic stretch with an elongation rate of 12%, 0.1 Hz for 3 h or 9 h. Representative images of cells stained with DCFH-DA probe. Scale bar: 100 μm. The indicated quantitative data refer to ratio to static control. Data are presented as mean ± standard deviation of three independent experiments. *P < 0.05, vs. static control group. DCFH-DA, 2’,7’-dichlorodihydrofluorescindiacetate
3.3 Cyclic Stretch Induces the Expression of Inflammatory Cytokines via NF‑κB Pathway
It’s well accepted that the NF‑κB pathway is one of major signaling pathways in mediating the expression of inflammatory cytokines [30]. In order to explore the role of NF‑κB activation in the expression of stretch‑induced inflammatory cytokines, phosphorylation of the NF‑κB protein p65 (p‑p65) was detected, and NF‑κB inhibitor, PDTC was used to confirm the results. As shown in Fig. 3, cyclic stretch increased the expression of p‑p65 compared with the static controls (P < 0.05). However, treatment with 20 μM PDTC partly inhibited the expression of p‑p65 (P < 0.05). Furthermore, treatment with PDTC could attenuate the protein production of ICAM-1, TNF-α, IL-6, and IL-8 induced by cyclic stretch (P < 0.05), but had no obvious effect on the expression of IL-1β (P > 0.05) (Fig. 1). These results indicate that the increase of inflammatory cytokines production induced by a cyclic stretch is at least partly mediated via the activation of NF‑κB pathway in hKCFs. Moreover, inhibition of stretch-induced ROS production by NAC attenuated NF-κB activation (Fig. 3), suggesting that ROS may positively control NF-κB signaling.
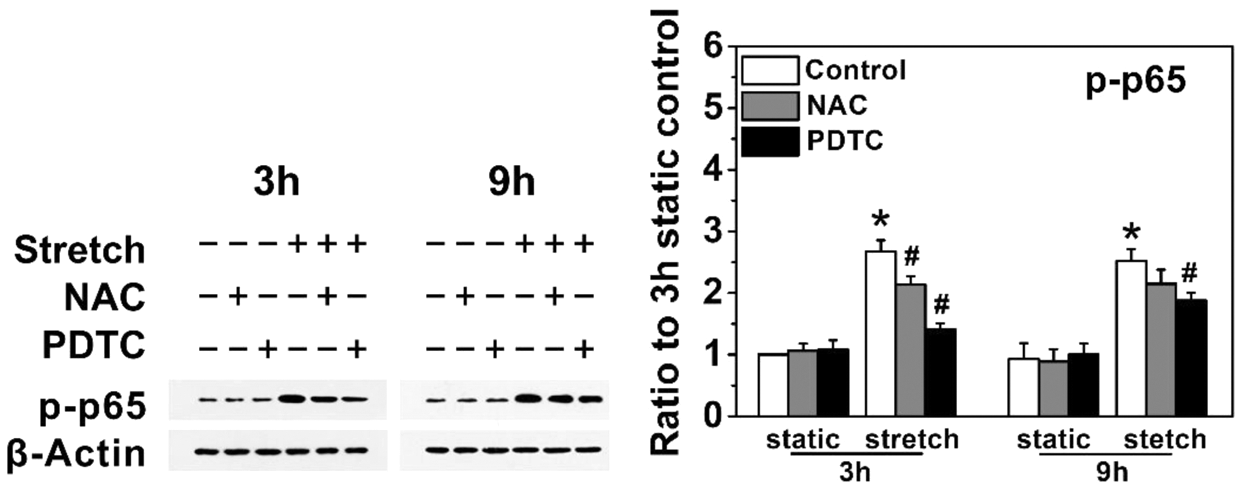
Figure 3: Cyclic stretch induces inflammatory cytokines of hKCFs via NF-κB pathway, and ROS positively control NF-κB activity hKCFs were subjected to cyclic stretch with an elongation rate of 12%, 0.1 Hz in the presence or absence of 0.5 mM NAC or 20 μM PDTC for 3 h or 9 h. Protein expression levels were then evaluated by western blotting. Unstretched cells were treated as static controls. β-Actin was used as a loading control for cell lysates. The indicated quantitative data refer to ratio to static control for 3 h. Data are presented as mean ± standard deviation of three independent experiments. *P < 0.05, vs. static control group; #P < 0.05 vs. cyclic stretch only. NAC, N-acetyl cysteine, a ROS scavenger; PDTC, pyrrolidine dithiocarbamate, a NF-κB inhibitor
Herein, we mainly focus on the generation of inflammatory cytokines because they have been shown to be over-expressed in the patients with KC [6,7,9], and inflammation is closely related with the focal tissue destruction [8]. During the progressive bulge of the diseased cornea, the corneal tissue is subjected to constant and increasing mechanical forces [3]. Mechanical stimuli have been demonstrated to play a key role in many diseases related to inflammatory events, such as glaucoma, atherosclerosis, and ventilator-induced lung injury [11–13,25,31]. Thus, we hypothesize that mechanical stimuli may be one of the activators of the inflammation cytokines production in the development of KC. The present study demonstrated that cyclic stretch significantly elevated levels of ICAM-1, TNF-α, IL-6, and IL-8 via oxidative stress and NF-κB pathways in hKCFs.
In our previous study, we found that the major difference between human normal corneal (NC) and KC fibroblasts is that basic levels of MMPs, inflammatory cytokines, and ROS were higher in KC fibroblasts than those of NC fibroblasts [9,31]. In addition, both NC (human and rabbit) and KC (human) fibroblasts showed almost similar respond in the expression of MMPs, inflammatory cytokines, TIMPs, collagen I, and LOXs in the presence of cyclic stretch [13,14], inflammatory cytokines [9,31], and H2O2 (data unpublished). Generally, such stimulus up-regulate MMPs and inflammatory cytokines in both NC and KC fibroblasts, while TIMPs and LOXs showed converse trend in NC and KC cells, with increase in NC cells, and decrease in KC ones [9,13,14,32]. Recent study showed that the oxidative stress imbalances found in KC were caused by defects in ROS/nitrogen species (ROS/RNS) removal rather than increased ROS/RNS production [33].
Cyclic stretch had a biphasic effect in regulating MMP-2, and IL-1β had combined effects on the regulation of MMP-2 and -9 expression with cyclic stretch in normal rabbit corneal fibroblasts [13,14]. Cyclic stretch was also applied on hKCFs in vitro, which increased levels of MMP‑1 and ‑3 and IL‑6, while reduced levels of TIMP‑1 and ‑2 [34]. In this study, we showed that cyclic stretch alone significantly increased the protein production of ICAM-1, TNF-α, IL-6, and IL-8 compared to static controls. Our previous study demonstrated that IL-6 could mediate the TNF-a-induced MMP-1 expression in hKCFs [9]. We confirmed that hKCFs could sense the mechanical stretch by releasing inflammatory cytokines, which may promote the expression of MMPs and contribute to degradation of extracellular matrix (ECM) components.
As important regulators of proinflammatory gene expression, NF-κB has been intensively studied in stretched cardiovascular and pulmonary cells [25–27]. However, the mechanisms of inflammatory cytokines production in hKCFs in response to mechanical stretch remain unclear. We found that mechanical stretch-activated pathway by enhancing phosphorylation of NF-κB p65. Moreover, inhibition of NF-κB by PDTC (NF-κB inhibitor) significantly blocked stretch-induced expression of TNF-a, IL-6, IL-8, and ICAM-1 in hKCFs. The activation of NF-κB has been found the critical linkage with a wide variety of human diseases related to the chronic inflammation [30]. Our findings highlight the important role of NF-κB in regulating the expression of inflammatory cytokines in hKCFs in response to mechanical stretch.
Data suggested that oxidative damage was involved in KC [16–22]. ROS are another key signaling molecule involved in the progression of inflammatory diseases [35,36]. In this study, we found that cyclic stretch promoted the generation of ROS in hKCFs in response to mechanical stretch. In our further experiment, NAC, a known ROS scavenger could reduce inflammatory cytokines production in the presence of cyclic stretch. These findings imply that cyclic stretch induces inflammatory responses in hKCFs via generation of ROS. ROS are central to the regulation of many inflammatory events. On the one hand, ROS can stimulate inflammatory cytokines production by activating NF-κB. On the other hand, ROS generation can be induced by inflammatory cytokines, which further promotes NF-κB activation [37]. It is worth mentioning that NAC has antioxidant and anti-inflammation roles, and has been reported to be beneficial in some chronic clinical diseases related with inflammation, such as obstructive pulmonary disease and other pulmonary diseases, hepatic diseases and injuries, cysticfibrosis, human immunodeficiency virus infection, and septic shock [37]. This finding indicates that the inhibition of ROS by NAC might provide a novel treatment strategy to reduce inflammatory cytokines for KC, and is worth application in clinical practice in the future.
Research in humans is limited because of ethical issues, therefore, it is important to establish animal model. Due to multiple etiologies of KC (biochemical, genetic, and environmental factors), it’s difficult to establish a standard animal model of KC. So far, keratoconus was experimentally induced in mice [38] or rabbits [39] by injecting collagenase in the cornea with special focus on the role of collagen in histopathological mechanisms of the disease. Liu et al. reported that collagenase type II caused a marked increase in mean keratometry and central cornea thickness in rabbit corneas, and induced an increase in ROS generation [38]. This study showed that the Nrf‑2/HO‑1 pathway may be activated by sulforaphane to resist the oxidative stress response of KC, and prevent the progression of KC. It would be interesting to investigate whether mechanical stress will be involved in ROS and inflammatory cytokines generation, and the possible signal pathway using KC animal model in the future study.
In summary, the results of our study suggest that mechanical stretch can activate the release of inflammatory cytokines in hKCFs. Stretch-induced inflammatory cytokines may be mediated through the generation of ROS and activation of the NF-κB signaling pathways. Over-expression of inflammatory cytokines induced by mechanical stretch may aggravate the cascade of extracellular matrix degenerative events and accelerate the development of keratoconus. Understanding the signal pathways mediating the induction of inflammatory cytokines in hKCFs will enable us to devise a potential therapeutic approach to KC.
Availability of Data and Materials: The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Funding Statement: This present study was supported by the National Natural Science Foundation of China [Grant No. 11872262].
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Davidson, A. E., Hayes, S., Hardcastle, A. J., Tuft, S. J. (2014). The pathogenesis of keratoconus. Eye, 28(2), 189–195. DOI 10.1038/eye.2013.278. [Google Scholar] [CrossRef]
2. Gordon-Shaag, A., Millodot, M., Shneor, E., Liu, Y. (2015). The genetic and environmental factors for keratoconus. BioMed Research International, 2015(10), 1–19. DOI 10.1155/2015/795738. [Google Scholar] [CrossRef]
3. Piñero, D. P., Alcón, N. (2015). Corneal biomechanics: A review. Clinical & Experimental Optometry Journal, 98, 107–116. DOI 10.1111/cxo.12230. [Google Scholar] [CrossRef]
4. McMonnies, C. W. (2015). Inflammation and keratoconus. Optometry & Vision Science Offcial Publication of the American Academy of Optometry, 92(2), e35–e41. DOI 10.1097/OPX.0000000000000455. [Google Scholar] [CrossRef]
5. Lei, X., Sun, Y., Song, J., Li, X., Chen, W. et al. (2020). Effect of mechanical stretch on the expression of aquaporin AQP1 in human keratocytes. Journal of Taiyuan University of Technology, 51(1), 144–149. DOI 10.16355/j.cnki.issn1007-9432tyut.2020.01.020. [Google Scholar] [CrossRef]
6. Sorkhabi, R., Ghorbanihaghjo, A., Taheri, N., Ahoor, M. H. (2015). Tear film inflammatory molecules in patients with keratoconus. International Ophthalmology, 35(4), 467–472. DOI 10.1007/s10792-014-9971-3. [Google Scholar] [CrossRef]
7. Ionescu, C., Corbu, C. G., Tanase, C., Jonescu-Cuypers, C., Nicula, C. et al. (2016). Inflammatory biomarkers profile as microenvironmental expression in keratoconus. Disease Markers, 2016(9), 1–8. DOI 10.1155/2016/1243819. [Google Scholar] [CrossRef]
8. Nissinen, L., Kähäri, V. M. (2014). Matrix metalloproteinases in inflammation. Biochimica & Biophysica Acta, 1840(8), 2571–2580. DOI 10.1016/j.bbagen.2014.03.007. [Google Scholar] [CrossRef]
9. Du, G., Liu, C., Li, X., Chen, W., He, R. et al. (2016). Induction of matrix metalloproteinase-1 by tumor necrosis factor-α is mediated by interleukin-6 in cultured fibroblasts of keratoconus. Experimental Biology and Medicine, 241(18), 2033–2041. DOI 10.1177/1535370216650940. [Google Scholar] [CrossRef]
10. Wang, X., Fan, J., Zhang, M., Sun, Z., Xu, G. (2013). Gene expression changes under cyclic mechanical stretching in rat retinal glial (Müller) cells. PLoS One, 8(5), e63467. DOI 10.1371/journal.pone.0063467. [Google Scholar] [CrossRef]
11. Liton, P. B., Luna, C., Bodman, M., Hong, A., Epstein, D. L. et al. (2005). Induction of IL-6 expression by mechanical stress in the trabecular meshwork. Biochemical and Biophysical Research Communications, 337(4), 1229–1236. DOI 10.1016/j.bbrc.2005.09.182. [Google Scholar] [CrossRef]
12. Križaj, D., Ryskamp, D. A., Tian, N., Tezel, G., Mitchell, C. H. et al. (2013). From mechanosensitivity to inflammatory responses: New players in the pathology of glaucoma. Current Eye Research, 39(2), 105–119. DOI 10.3109/02713683.2013.836541. [Google Scholar] [CrossRef]
13. Liu, C., Feng, P., Li, X., Song, J., Chen, W. (2014). Expression of MMP-2, MT1-MMP, and TIMP-2 by cultured rabbit corneal fibroblasts under mechanical stretch. Experimental Biology and Medicine, 239(8), 907–912. DOI 10.1177/1535370214536650. [Google Scholar] [CrossRef]
14. Feng, P., Li, X., Chen, W., Liu, C., Rong, S. et al. (2016). Combined effects of interleukin‐1β and cyclic stretching on metalloproteinase expression in corneal fibroblasts in vitro. BioMedical Engineering OnLine, 15(1), 1801. DOI 10.1186/s12938-016-0198-6. [Google Scholar] [CrossRef]
15. Gatzioufas, Z., Panos, G. D., Hamada, S. (2017). Keratoconus: Is it a non-inflammatory disease? Medical Hypothesis Discovery & Innovation in Ophthalmology, 6, 1–2. [Google Scholar]
16. Chwa, M., Atilano, S. R., Hertzog, D., Zheng, H., Langberg, J. et al. (2008). Hypersensitive response to oxidative stress in keratoconus corneal fibroblasts. Investigative Ophthalmology & Visual Science, 49(10), 4361–4369. DOI 10.1167/iovs.08-1969. [Google Scholar] [CrossRef]
17. Karamichos, D., Hutcheon, A. E., Rich, C. B., Trinkaus-Randall, V., Asara, J. M. et al. (2014). In vitro model suggests oxidative stress involved in keratoconus disease. Scientific Reports, 4, 1–7. DOI 10.1038/srep04608. [Google Scholar] [CrossRef]
18. Atilano, S. R., Coskun, P., Chwa, M., Jordan, N., Reddy, V. et al. (2005). Accumulation of mitochondrial DNA damage in keratoconus corneas. Investigative Ophthalmology & Visual Science, 46(4), 1256–1263. DOI 10.1167/iovs.04-1395. [Google Scholar] [CrossRef]
19. Arnal, E., Peris-Martínez, C., Menezo, J. L., Johnsen-Soriano, S., Romero, F. J. (2011). Oxidative stress in keratoconus? Investigative Opthalmology & Visual Science, 52(12), 8592–8597. DOI 10.1167/iovs.11-7732. [Google Scholar] [CrossRef]
20. Saijyothi, A. V., Fowjana, J., Madhumathi, S., Rajeshwari, M., Thennarasu, M. et al. (2012). Tear fluid small molecular antioxidants profiling shows lowered glutathione in keratoconus. Experimental Eye Research, 103, 41–46. DOI 10.1016/j.exer.2012.07.010. [Google Scholar] [CrossRef]
21. Toprak, I., Kucukatay, V., Yildirim, C., Kilic-Toprak, E., Kilic-Erkek, O. (2014). Increased systemic oxidative stress in patients with keratoconus. Eye, 28(3), 285–289. DOI 10.1038/eye.2013.262. [Google Scholar] [CrossRef]
22. Kilic, R., Cumurcu, T., Sancaktar, E., Evliyaoğlu, O., Sezer, H. (2015). Systemic prolidase activity and oxidative stress in keratoconus. Current Eye Research, 41(1), 28–33. DOI 10.3109/02713683.2015.1004717. [Google Scholar] [CrossRef]
23. Shetty, R., Sharma, A., Pahuja, N., Chevour, P., Padmajan, N. et al. (2017). Oxidative stress induces dysregulated autophagy in corneal epithelium of keratoconus patients. PLoS One, 12(9), e0184628. DOI 10.1371/journal.pone.0184628. [Google Scholar] [CrossRef]
24. Kenney, M. C., Chwa, M., Atilano, S. R., Tran, A., Carballo, M. et al. (2005). Increased levels of catalase and cathepsin V/L2 but decreased TIMP-1 in keratoconus corneas: Evidence that oxidative stress plays a role in this disorder. Investigative Opthalmology & Visual Science, 46(3), 823–832. DOI 10.1167/iovs.04-0549. [Google Scholar] [CrossRef]
25. Jufri, N. F., Mohamedali, A., Avolio, A., Bake, M. S. (2015). Mechanical stretch: Physiological and pathological implications for human vascular endothelial cells. Vascular Cell, 7(1), 8. DOI 10.1186/s13221-015-0033-z. [Google Scholar] [CrossRef]
26. Amma, H., Naruse, K., Ishiguro, N., Sokabe, M. (2005). Involvement of reactive oxygen species in cyclic stretch-induced NF-κB activation in human fibroblast cells. British Journal of Pharmacology, 145(3), 364–373. DOI 10.1038/sj.bjp.0706182. [Google Scholar] [CrossRef]
27. Davidovich, N., DiPaolo, B. C., Lawrence, G. G., Chhour, P., Yehya, N. et al. (2013). Cyclic stretch-induced oxidative stress increases pulmonary alveolar epithelial permeability. American Journal of Respiratory Cell Molecular Biology, 49(1), 156–164. DOI 10.1165/rcmb.2012-0252OC. [Google Scholar] [CrossRef]
28. Wang, G. H., Chen, W. Y. (2012). Effects of mechanical stimulation on viscoelasticity of rabbit scleral fibroblasts after posterior scleral reinforcement. Experimental Biology and Medicine, 237(10), 1150–1154. DOI 10.1258/ebm.2012.012196. [Google Scholar] [CrossRef]
29. Tan, J. L., Xu, X., Tong, Z. H., Lin, J., Yu, Q. J. et al. (2015). Decreased osteogenesis of adult mesenchymal stem cells by reactive oxygen species under cyclic stretch: A possible mechanism of age related osteoporosis. Bone Research, 3(1), 186. DOI 10.1038/boneres.2015.3. [Google Scholar] [CrossRef]
30. Lawrence, T. (2009). The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb Perspect Biology, 1(6), a001651. DOI 10.1101/cshperspect.a001651. [Google Scholar] [CrossRef]
31. Tian, Y., Gawlak, G., O’Donnell, J. J., Mambetsariev, I., Birukova, A. A. (2016). Modulation of endothelial inflammation by low and high magnitude cyclic stretch. PLoS One, 11, 1–13. DOI 10.1371/journal.pone.0153387. [Google Scholar] [CrossRef]
32. Feng, P. F., Li, X. N., Chen, W. Y., He, R., Wang, X. N. (2016). Effects of tumor necrosis factor alpha on the expression of matrix metalloproteinases and tissue inhibitors of matrix metalloproteinases in kereatoconus fibroblasts. Journal Biomedical Engineering, 33, 1139–1144. [Google Scholar]
33. Atilano, S. R., Lee, D. H., Fukuhara, P. S., Chwa, M., Nesburn, A. B. et al. (2019). Journal Ophthalmic and Vison Research, 14(1), 62–70. DOI 10.4103/jovr.jovr_80_18. [Google Scholar] [CrossRef]
34. Du, G., Chen, W., Li, X., He, R., Feng, P. (2017). Induction of MMP‐1 and ‐3 by cyclical mechanical stretch is mediated by IL‐6 in cultured fibroblasts of keratoconus. Molecular Medicine Reports, 15(6), 3885–3892. DOI 10.3892/mmr.2017.6433. [Google Scholar] [CrossRef]
35. Mittal, M., Siddiqui, M. R., Tran, K., Reddy, S. P., Malik, A. B. (2014). Reactive oxygen species in inflammation and tissue injury. Antioxid Redox Signal, 20(7), 1126–1167. DOI 10.1089/ars.2012.5149. [Google Scholar] [CrossRef]
36. Blaser, H., Dostert, C., Mak, T. W., Brenner, D. (2016). TNF and ROS crosstalk in inflammation. Trends in Cell Biology, 26(4), 249–261. DOI 10.1016/j.tcb.2015.12.002. [Google Scholar] [CrossRef]
37. de Andrade, K. Q., Moura, F. A., dos Santos, J. M., de Araújo, O. R., de Farias Santos, J. C. et al. (2015). Oxidative stress and inflammation in hepatic diseases: Therapeutic possibilities of N-Acetylcysteine. International Journal of Molecular Sciences, 16(12), 30269–30308. DOI 10.3390/ijms161226225. [Google Scholar] [CrossRef]
38. Moghadam, F. A., Jahromy, M. H., Fazelipour, S., Khakpour, S., Younesian, M. S. (2009). Induction of experimental keratoconus in mice using collagenase. Physiology Pharmacology, 13(3), 209–215. DOI 10.4196/kjpp.2009.13.3.209. [Google Scholar] [CrossRef]
39. Liu, R., Yan, X. (2018). Sulforaphane protects rabbit corneas against oxidative stress injury in keratoconus through activation of the Nrf-2/HO-1 antioxidant pathway. International Journal of Molecular Medicine, 42, 2315–2328. DOI 10.3892/ijmm.2018.3820. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |