
Materials

 | Journal of Renewable Materials |  |
DOI: 10.32604/jrm.2021.015346
ARTICLE
Visualizing Complex Anatomical Structure in Bamboo Nodes Based on X-ray Microtomography
1Bamboo and Rattan Science and Technology Laboratory, International Center for Bamboo and Rattan, Beijing, 100102, China
2Key Laboratory of Wood Science and Technology of State Forestry and Grassland Administration, Research Institute of Wood Industry, Chinese Academy of Forestry, Beijing, 100091, China
3Carl Zeiss (Shanghai) Co., Ltd., Shanghai, 200131, China
4Shanghai Institute of Applied Physics, Chinese Academy of Sciences, Shanghai, 201204, China
*Corresponding Author: Shumin Yang. Email: yangsm@icbr.ac.cn
Received: 11 December 2020; Accepted: 31 December 2020
Abstract: In recent years, bamboo has been widely used in a broad range of applications, a thorough understanding of the structural characteristics of bamboo nodes is essential for better processing and manufacturing of biomimetic materials. This study investigated the complex anatomical structure for the nodes of two bamboo species, Indocalamus latifolius (Keng) McClure and Shibataea chinensis Nakai, using a high-resolution X-ray microtomography (μCT). The results show that the vascular bundle system in the nodal region of I. latifolius and S. chinensis is a net-like structure composed of horizontal and axial vascular bundles. Furthermore, the fiber sheath surrounding metaxylem vessels tended to be shorter in the tangential direction. This structure of bamboo nodes facilitates the tangential and axial transport of moisture and nutrients. The anatomical structure of I. latifolius and S. chinensis nodes has obvious differences, especially in the arrangement of vascular bundles. Vascular bundle frequency was significantly higher in S. chinensis nodes than in I. latifolius nodes. These findings indicate that μCT is a non-destructive three-dimensional imaging method that can used to examine the anatomical structure of bamboo nodes.
Keywords: Anatomical structure; bamboo nodes; I. latifolius; S. chinensis; X-ray microtomography (μCT)
Bamboo is one of the most important forest resources, especially in Asian countries. With its excellent mechanical properties, fast-growth rate, high-yield, and renewable nature, bamboo is widely used for various products, such as paper, furniture, and structural composites. A bamboo culm consists of a linear series of hollow internodes and solid nodes [1]. In the internode region, all cells are strictly aligned in the axial direction, resulting in the bamboo culms being easily split by force [2−4]. In the nodal region, some horizontal vascular bundles appear on the diaphragm [5,6]. The presence of horizontal vascular bundles not only strengthens the uprightness of bamboo culms [3,7] but also plays a key role in the transversal transport of water and nutrients [5,6,8,9]. The unique anatomical structure of bamboo is well-known to determine its physical and mechanical properties. Therefore, a better understanding of the structural characteristics of bamboo, especially the bamboo nodes, will lead to a better understanding of its function and performance, and inspire the design of biomimetic materials.
Due to the complex arrangement of vascular bundles in the nodal region, the anatomical structure of the nodes has been less studied than that of the internode. Bamboo nodes consist of a sheath scar, diaphragm, nodal ridge, and intra-node. Grosser et al. first investigated the anatomical characteristics of bamboo nodes. This study reported that the most significant difference between the internodes and the nodes occurred in the horizontal vascular bundles of the nodes [10]. Since, several other studies have focused on the complex arrangement of vascular bundles in the nodal region. These studies showed that most vascular bundles that passed into the diaphragm were derived from the inner part of the bamboo culm, and the other part came from the periphery [3,11,12]. Also, Ding et al. [6] reconstructed a three-dimensional (3D) image of the nodal region based on the serial sections of a sample to visualize the internal structure of the nodes. In the above-mentioned studies, the structural characteristics of bamboo nodes were studied by serial sectioning methods. However, such serial sectioning methods have disadvantages, e.g., they are invasive and time-consuming, thus non-invasive and high-resolution approaches are needed.
X-ray-computed microtomography (μCT), a high-resolution non-invasive imaging technique, has been increasingly used in wood and bamboo research to explore internal structure [9,13−15], density [16,17], knots [18,19], and fracture characteristics [20,21] of plant culms. Peng et al. [5] first used μCT to examine the complex vascular system in the nodal region [3]. Palombini et al. [9] presented one of the first 3D microstructural reconstructions of the complex nodal vascular system of monocots, identifying its arrangement within the node and tracking the lateral movement of the vascular bundles. The above-cited studies found that branching types were a major factor that strongly influences the anatomy structure of bamboo nodes. However, there has not been research on the anatomical structure of different branch types in the nodal region.
The study presents 2D and 3D characterization of the morphology and the structural analysis of a single-branched type (Indocalamus latifolius (Keng) McClure) and multiple-branched type (Shibataea chinensis Nakai) bamboo nodes using a high-resolution μCT. I. latifolius is a shrubby bamboo species, up to 2 m tall and 0.5–1.5 cm diameter, commonly distributed in eastern China. Its culm is used as raw materials for chopsticks and penholder. The species S. chinensis is one ornamental bamboo, up to 1 meter height and 2–3 millimeter diameter, mainly distributed in Jiangsu, Anhui, and Fujian province, China. This study will provide critical data for further construction of a bamboo nodes database.
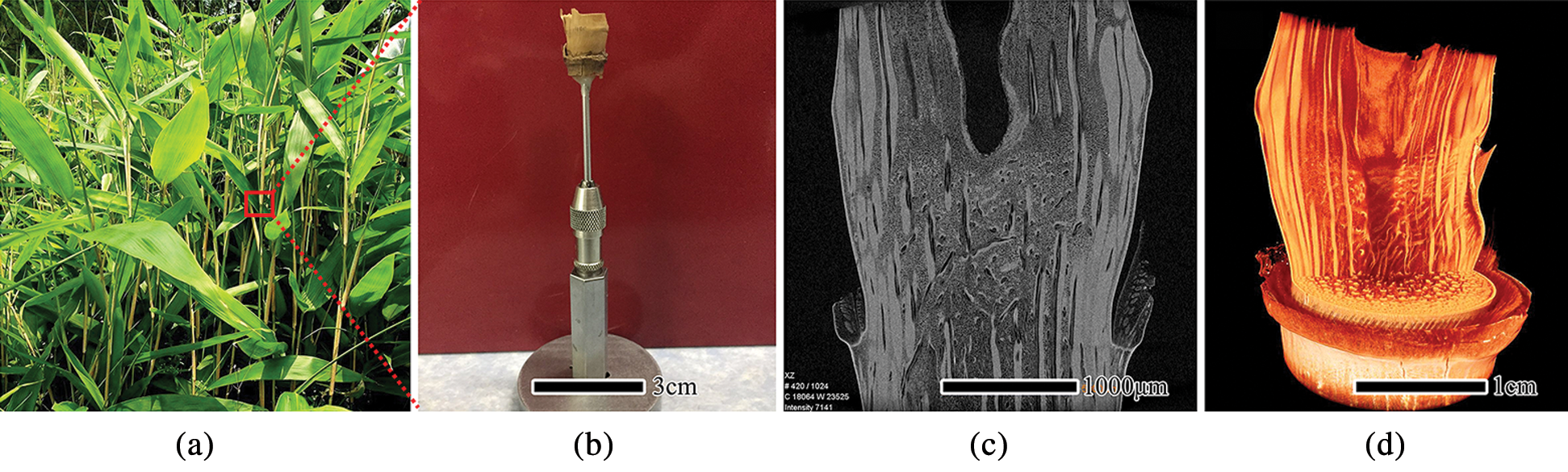
Figure 1: The sample preparation process of bamboo nodes for μCT, mature bamboo stem (a), the dried specimens of the bamboo nodes (b), μCT image of axial section (c), 3D reconstruction image (d)
To characterize the anatomical structure of the bamboo nodes, samples of I. latifolius and S. chinensis were collected from a bamboo plantation of Taiping Lake Base in Anhui Province, China. I. latifolius culm belongs to the single-branched type, and S. chinensis culm belongs to the multiple-branched type [22]. Mature bamboo plants (3-year-olds) were used for all experiments (Fig. 1a). The complete specimen of bamboo nodes was cut from the 10th nodes of the bamboo culm and then brought back to the laboratory. The air-dried specimens were mounted on the holder with an aluminum tube as an adapter (Fig. 1b) and stored at room temperature until the imaging experiments were carried out.
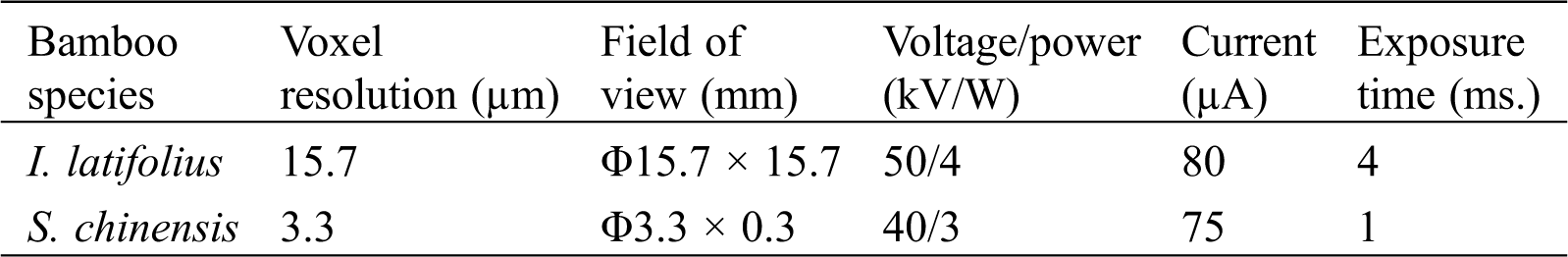
As a non-invasive and non-destructive detection method, μCT can obtain a 2D slice imaging of a sample based on the attenuation of the radiation. The scans were performed with a laboratory scaled X-ray-microscope (Xradia510 Versa; Carl Zeiss (Shanghai) Co., Ltd., Shanghai, China) entailing geometric and optical magnification. It was possible to place the specimens entirely within the equipment’s field of view because of their relatively small sizes. The 510 Versa has a sealed transmission source that reaches 160 kV and 10 W, with a voxel resolution ranging from 3 μm to 20 μm, depending on the size of the region of interest (ROI). The detailed parameters of scanning for the bamboo nodes are listed in Tab. 1. The sample was mounted on a stage and rotated 360° around its central axis to produce a series of XY-, YZ-, and XZ-projected images. After scanning, the background image was subtracted from the projected image. Then, the tomographic image was obtained by using an FDK reconstruction algorithm which is based on the GPU acceleration (Fig. 1c). Finally, the tomographic images were imported into ZEISS XM 3DViewer software for 3D reconstruction (Fig. 1d).
Table 1: Scanning parameters for the bamboo nodes
The number of vascular bundle in the bamboo nodes was counted using the image-processing software Image J. The vascular bundle frequency was calculated according the following formula, where
3.1 Structure of I. latifolius Nodes
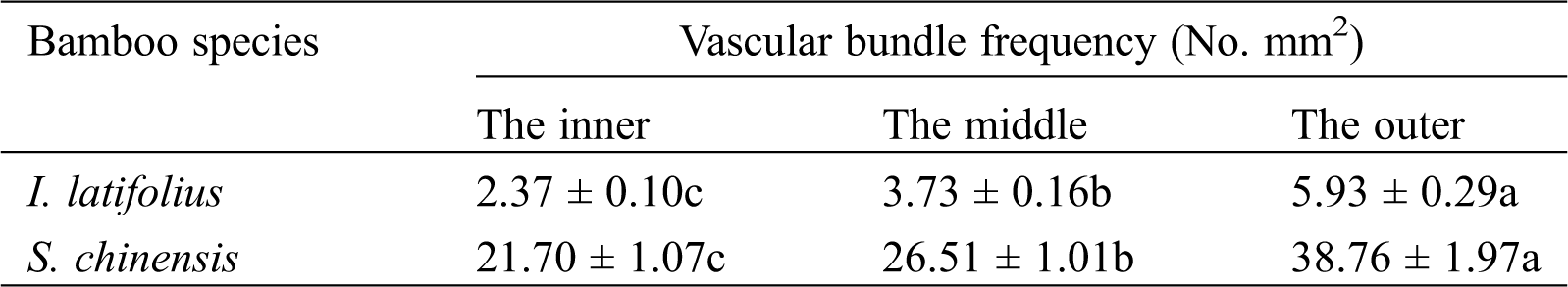
Fig. 2 shows the scanning area and the transverse section μCT slices of I. latifolius nodes. The nodes of I. latifolius are convex with an undeveloped branch on the outside of the culm (Fig. 2a). In the sheath scar, the unshed sheath leaf encloses the bamboo culm. The vascular bundles within the sheath leaf were arranged in 1–2 rounds with a larger fibrous cap near the dorsal side of the sheath leaf (Fig. 2b, white arrow). According to the vascular bundle classification method proposed by Liese in 1998 [1], the typical vascular bundle in the internodes of I. latifolius was classified as double broken-waist type, which consists of a central vascular strand and two fiber strands (outside and inside the central vascular strand) [22]. However, similar typical vascular bundles were not observed in the nodal region. The morphology of the vascular bundle in the nodal region was observed at high voxel resolution (Fig. 2c). It was found that the fiber sheath close to intercellular space was longer in the radial direction, while the fiber sheath surrounding metaxylem vessels tended to be shorter in the tangential direction. This finding is consistent with Grosser and Liese (1971) [10]. In the diaphragm, the frequency of vascular bundle (Tab. 2) and the proportion of fiber surrounding vessels decrease from the outer to the inner of the culm, while the size of the vessel becomes larger. Many horizontal tubular vascular bundles were observed in the transverse section (Fig. 2d, white arrow). In the nodal ridge, the thickness of the bamboo wall and the size of the vascular bundle at the nodal ridge were larger than those at other parts of the node (Fig. 2e). Such behavior is consistent with the description of bamboo nodal structure reported by Shao et al. [4].
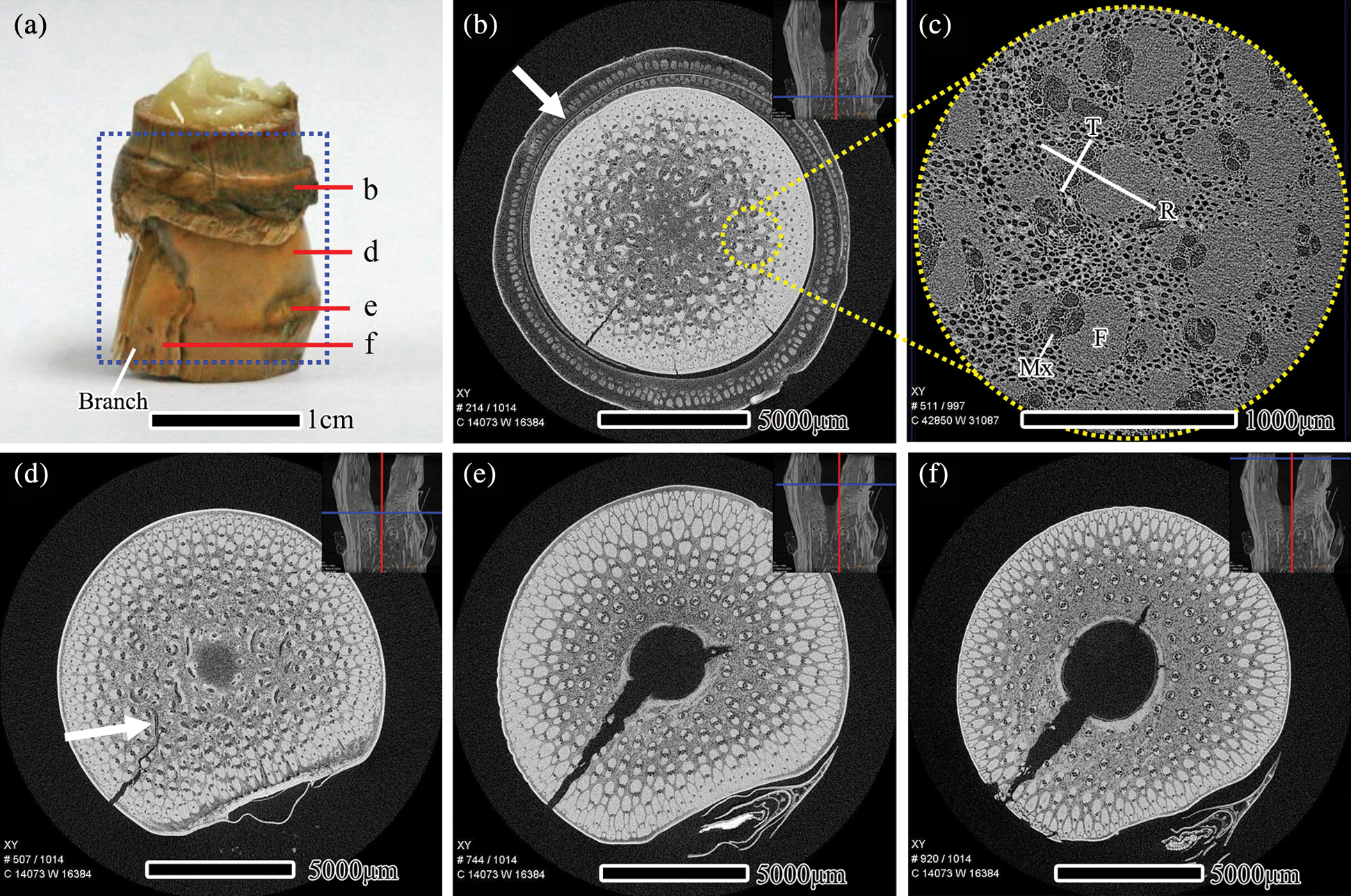
Figure 2: The scanning area and the transverse section μCT slices of I. latifolius nodes, (a) scanning area of the specimen, scale bars = 1 cm. μCT slices is located at the sheath scar (b), diaphragm (d), nodal ridge (e), the top region of the nodal ridge (f), scale bars = 5000 μm. (c) The vascular bundles in the nodal region with high magnification, scale bars = 1000 μm. Mx: Metaxylem, F: Fiber, R: Radial direction, T: Tangential direction
Table 2: Statistical results from vascular bundle frequency of bamboo diaphragm
The axial slices of the I. latifolius node are depicted in Fig. 3. The horizontal and curved vascular bundles (Fig. 3a, white arrow) were observed at the inner of the bamboo culm along the bottom of the sheath scar, which illustrated that the vascular bundle in the nodal region begins to bifurcate below the sheath scar [6]. Therefore, the study of node anatomical structure cannot be limited between the nodal ridge and the sheath scar. Vascular bundles originating in the leaf sheaths insert into the outer area of the culm, merging with the vascular bundles in the outer area (Fig. 3a, dark arrow). In the diaphragm, the vascular bundles are numerous and larger near the periphery, and tended to be sparser and smaller toward the middle of the culm center (Fig. 3a). At the upper edge of the diaphragm, many small vascular bundles turn horizontally and twist repeatedly (Fig. 3b, yellow dotted box). Further observation of the small bundles at high voxel resolution reveals that the fibrous sheath surrounding the vessel is well-developed (Fig. 3c). Between the nodal ridge and the upper edge of the diaphragm, there are more fibers around the vessel than on the diaphragm.
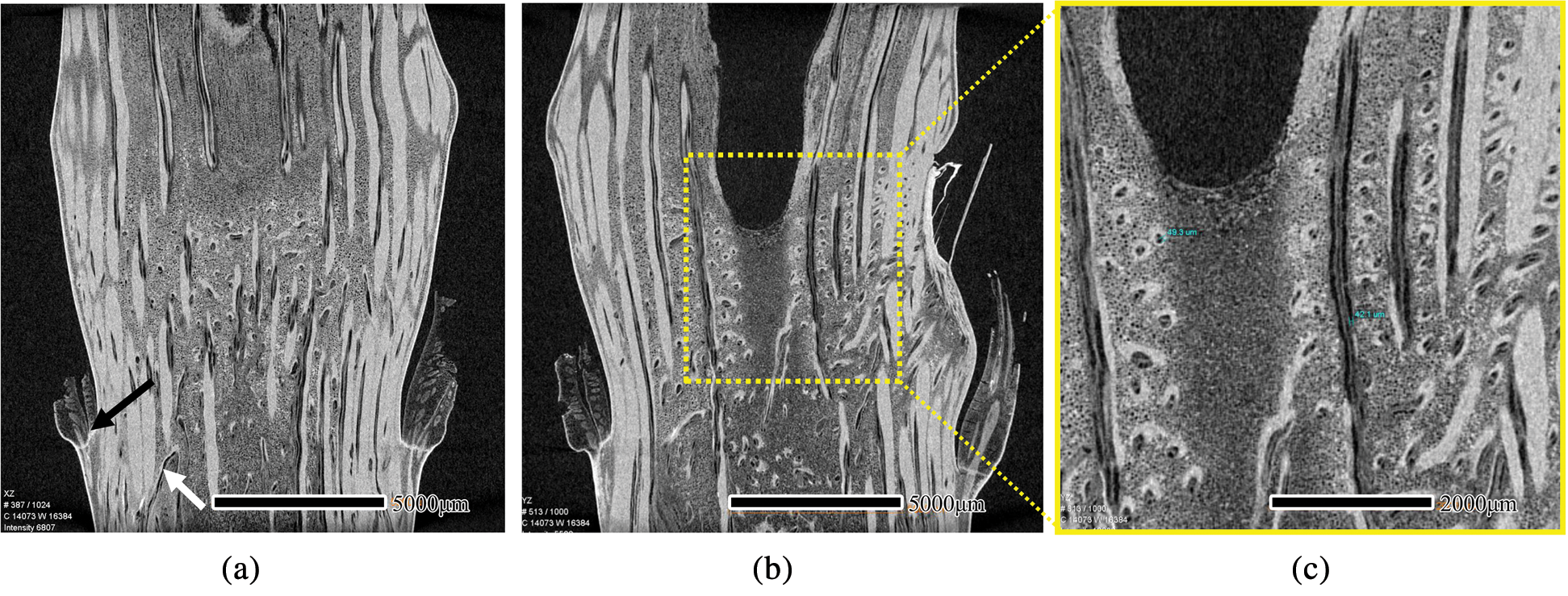
Figure 3: μCT scanning images of an axial section of I. latifolius. XZ (a) and YZ (b) of μCT images, scale bars = 5000 μm. Small vascular bundle at high voxel resolution (c), scale bars = 2000 μm
The 3D structure of I. latifolius nodes was reconstructed by continuous transverse and axial slices (Fig. 4). The different tissues are distinguished by color. The bright areas represent vascular bundles, whereas the dark areas represent parenchyma cells. Most vertically arranged vascular bundles near the peripheral of the nodal regions pass directly through the node, while those near the inner of the nodal regions pass into the diaphragm in the form of “Γ”, and turn horizontally and twist repeatedly within the diaphragm (Fig. 4d). The finding is consistent with previous studies [3,10]. In the diaphragm, vascular bundle systems are a net-like structure composed of horizontal and axial vascular bundles. This network structure not only contributes to the mechanical properties, such as flex resistance and split resistance [3,7,23], but also facilitates the tangential and axial transport of moisture and nutrients [5,8,11].
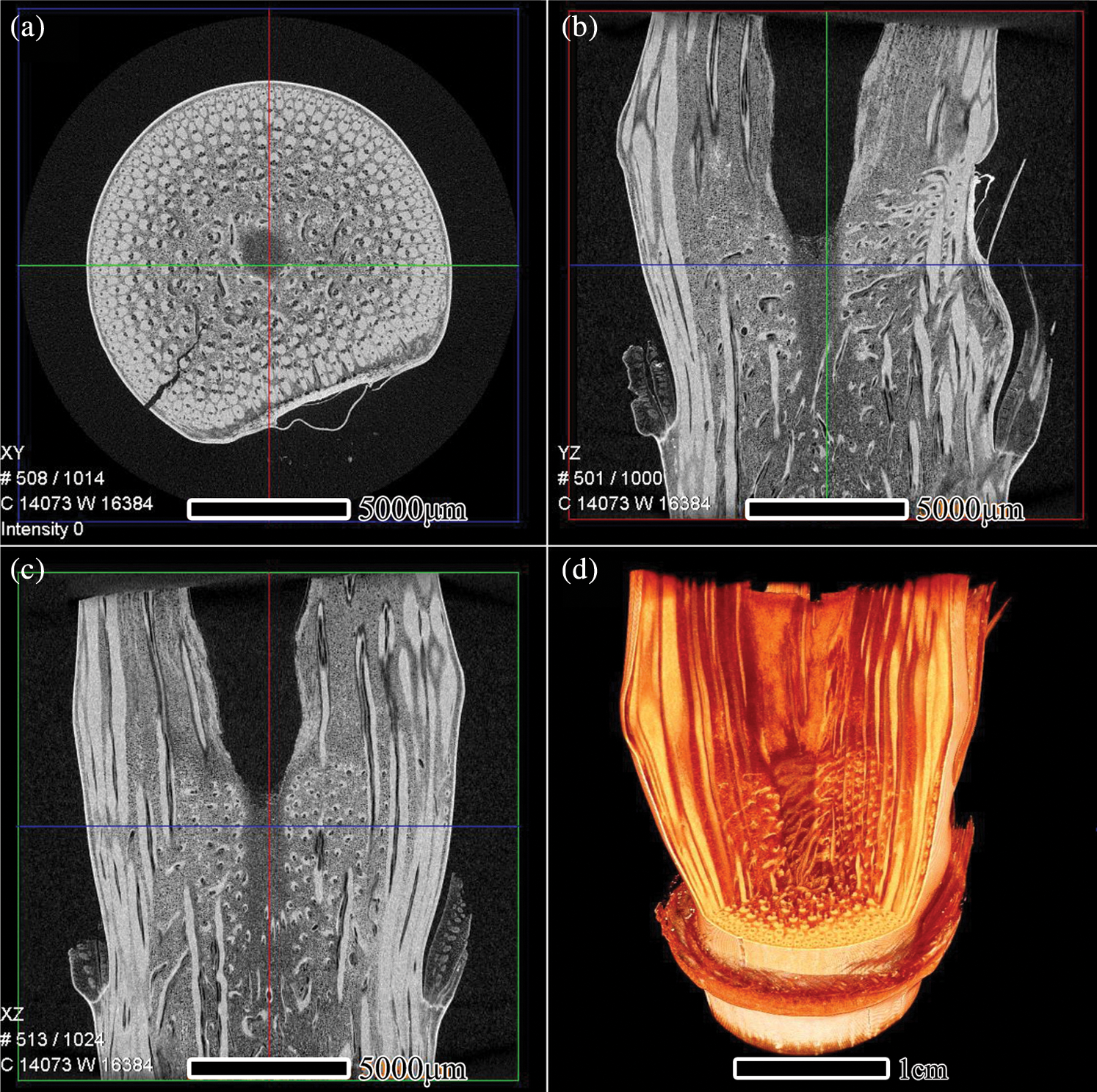
Figure 4: μCT reconstruction of the nodal region of I. latifolius, XY (a), YZ (b), and XZ (c) of μCT images, 3D reconstructed image (d)
3.2 Structure of S. Chinensis Nodes
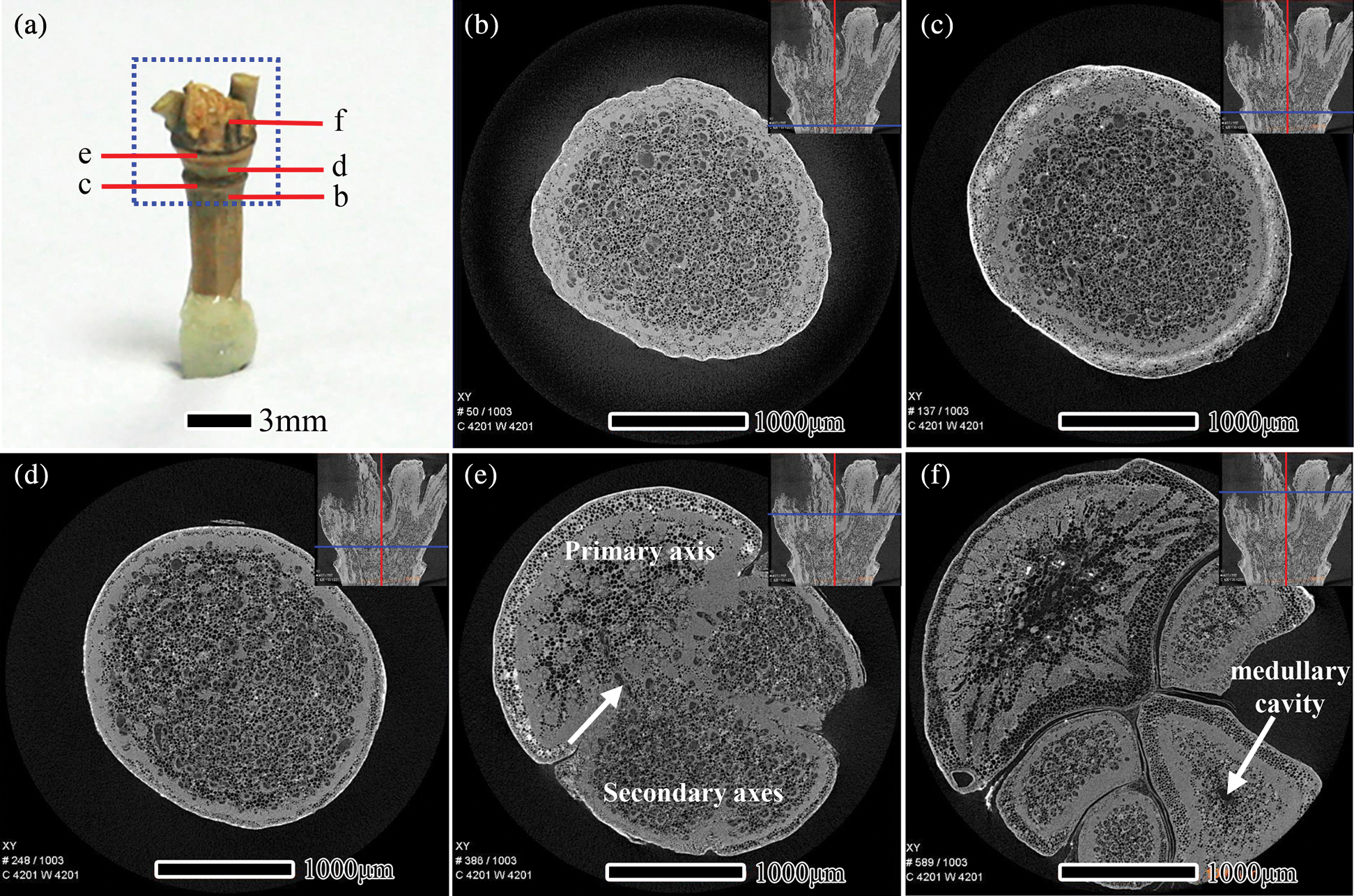
Figure 5: The scanning area and the transverse section μCT slices of S. chinensis nodes, scanning area of the sample (a), Scale bars = 3 mm. μCT slices located at the bottom region of the sheath node (b), sheath scar (c), diaphragm (d), nodal ridge (e), and the top region of the nodal ridge (f), Scale bars = 1000 μm
The scanning area and transverse section of S. chinensis nodes are presented in Fig. 5. S. chinensis culm belongs to a multiple-branch type (Fig. 5a). By the transverse section from the bottom to the top of the nodal region, Figs. 5b−5e illustrates the development of branches into secondary axes. In Fig. 5b, the transverse section was taken close to the lower part of the sheath scar. The vascular bundle frequency of the vascular bundle of S. chinensis nodes decreases from the outer to the inner area of the culm (Tab. 2), which is similar to the results of the nodes of I. latifolius. A higher proportion of fiber surrounding vessels was observed in the outer of the culm than that in the inner (Figs. 5b−5d). In the diaphragm, vascular bundle frequency was significantly higher in S. chinensis nodes than in I. latifolius nodes (Tab. 2). In sections closer to the nodal ridge, bamboo culm was divided into two parts by the primary axis and secondary axes. Horizontal tubular vascular bundles and fibrous cells were observed in the junction between the primary axis and secondary axes (Fig. 5e, white arrow). After distinctively separated into new axes in the top part of the nodal ridge, the size of the vascular bundle in the primary axis and secondary axes with a medullary cavity is larger than that without a medullary cavity (Fig. 5f).
The axial section of S. chinensis nodes is shown in Figs. 6a and 6b. The sheath leaf drops off the culm leaving the sheath scar at the sheath node. Many horizontal vascular bundles were observed in the diaphragm. This anatomical structure of the diaphragm may result from its biological functions, such as the transverse transport of moisture and nutrients within the bamboo node [6]. Some of the vascular bundles in the primary axis move transversally and bend into the secondary axes, and fiber cells were visible at the boundary between the primary axis and secondary axes. This structure helps strengthen the connection strength between the primary axis and secondary axes. Another study also postulated that stressed regions occurred mostly in the lower regions of secondary axes and in the interconnecting zones among other branches during compression [9]. Horizontal vascular bundles were observed on the bamboo wall without a medullary cavity in the primary axis or secondary axes; all cells in the bamboo wall recovered the axial alignment when there was a medullary cavity in the primary axis or secondary axes. This is consistent with results of Peng et al. for nodes of Pleioblastus gozadakensis [5].
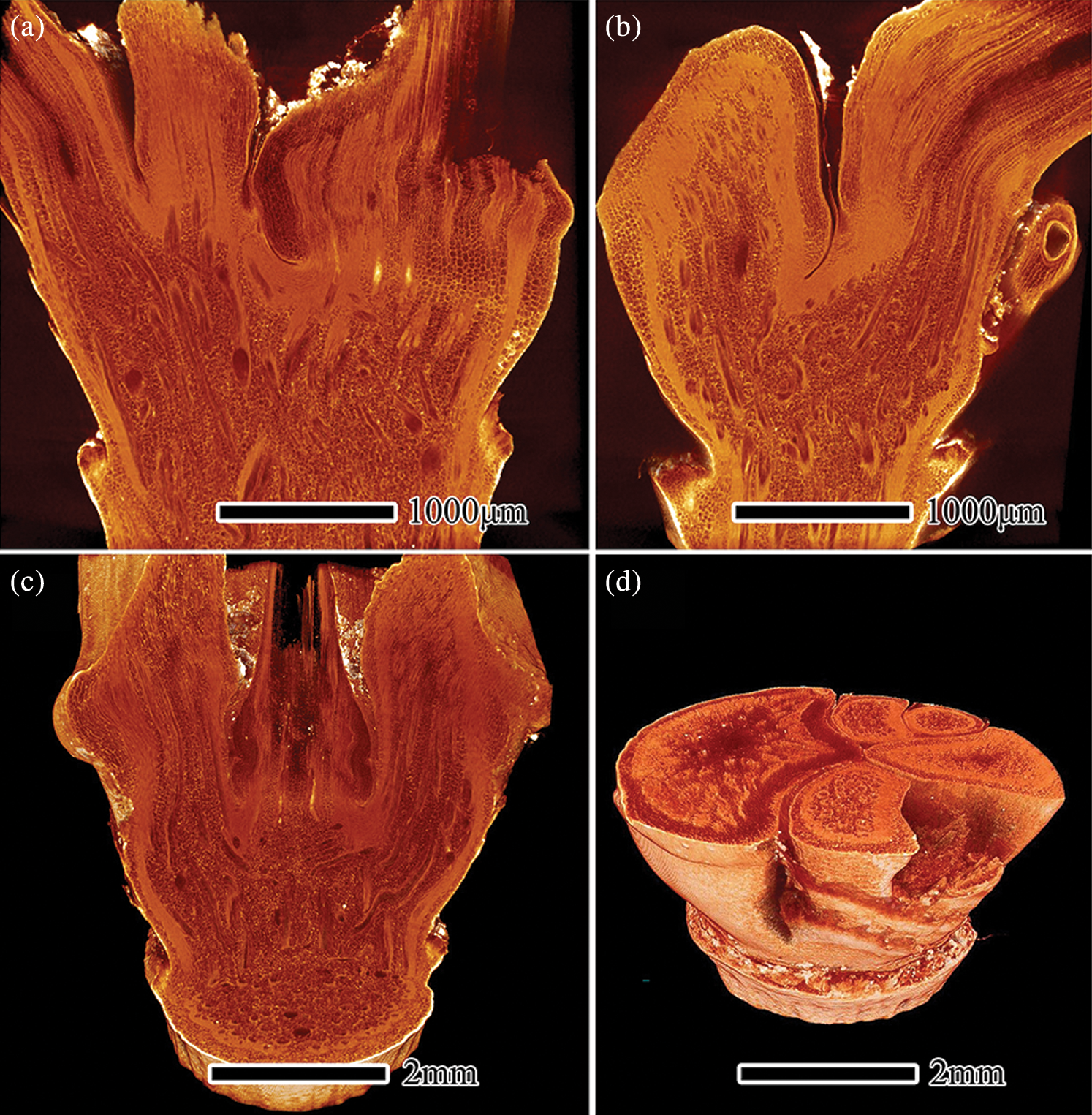
Figure 6: μCT image of axial section (a, b) and the 3D reconstruction (c, d) of S. chinensis nodes
Figs. 6c and 6d present the 3D reconstruction of S. chinensis nodes by continuous transverse and axial μCT imaging, and show the internal anatomical structure of bamboo nodes. The anatomical structure of the bamboo node, especially in the arrangement of vascular bundles, was affected by branch type. This results in the arrangement of vascular bundles in the S. chinensis node being more complex than that in the I. latifolius node. Most of the vascular bundles in the secondary axes are derived from the relative position of the diaphragm, and the other part came from the primary axis (Fig. 6c). The results are largely consistent with the finding of Palombini et al. on vascular bundle arrangement in new branches [9]. The nodes of S. chinensis comprise a primary axis and five secondary axes, and the secondary axes are arranged in a whorl (Fig. 6d).
The anatomical structure and 3D visual characterization of I. latifolius and S. chinensis nodes were investigated by high-resolution μCT.
1. The vascular bundle system in the nodal region of I. latifolius and S. chinensis is a net-like structure composed mainly of horizontal and axial vascular bundles. Furthermore, the fiber sheath surrounding metaxylem vessels tended to be shorter in the tangential direction. This structure of bamboo nodes facilitates the tangential and axial transport of moisture and nutrients.
2. The anatomical structure of bamboo nodes, especially in the arrangement of vascular bundles, was affected by branch type. In the nodal region of I. latifolius, most vertically arranged vascular bundles near the peripheral of the nodal regions pass directly through the node, while those near the inner of the nodal regions pass into the diaphragm in the form of “Γ”. In the nodal region of S. chinensis, some of the vascular bundles in the primary axis move transversally and bend into the secondary axes, and fiber cells were visible at the boundary between the primary axis and secondary axes. Vascular bundle frequency was significantly higher in S. chinensis nodes than in I. latifolius nodes.
3. μCT was indicated be a feasible and reliable method to characterize the anatomical structure of bamboo nodes nondestructively.
Acknowledgement: The authors gratefully acknowledge the support of this research from Carl Zeiss (Shanghai) Co., Ltd., Shanghai, China.
Funding Statement: This research was funded by the Nature Science Foundation of China (Grant No. 31670565) and the National Key Research & Development Program (No. 2016YFD0600904).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Liese, W. (1998). The anatomy of bamboo culms. INBAR Technical Report, No. 18, 12. China. [Google Scholar]
2. Wang, F. L., Shao, Z. P., Wu, Y. J., Wu, D. (2014). The toughness contribution of bamboo node to the Mode I interlaminar fracture toughness of bamboo. Wood Science and Technology, 48(6), 1257–1268. DOI 10.1007/s00226-013-0591-2. [Google Scholar] [CrossRef]
3. Huang, X. Y., Li, F., De Hoop, C. F., Jiang, Y. Z., Xie, J. L. et al. (2018). Analysis of Bambusa rigida bamboo culms between internodes and nodes: Anatomical characteristics and Physical-mechanical properties. Forest Products Journal, 68(2), 157–162. DOI 10.13073/FPJ-D-17-00035. [Google Scholar] [CrossRef]
4. Shuo, Z. P., Huang, S. X., Wu, F. S., Zho, L., Clement, A. (2008). A study on the difference of structure and strength between internodes and nodes of Moso bamboo. Journal of Bamboo Reasearch, 27(2), 48–52. [Google Scholar]
5. Peng, G. Y., Jiang, Z. H., Liu, X. E., Fei, B. H., Yang, S. M. et al. (2014). Detection of complex vascular system in bamboo node by X-ray μCT imaging technique. Holzforschung, 68(2), 223–227. DOI 10.1515/hf-2013-0080. [Google Scholar] [CrossRef]
6. Ding, Y. L., Liese, W. (1995). On the nodal structure of bamboo. Journal of Bamboo Reasearch, 14(1), 24–32. [Google Scholar]
7. Zou, M., Xu, S. C., Wei, C. G., Wang, H. X., Liu, Z. Z. (2016). A bionic method for the crashworthiness design of thin-walled structures inspired by bamboo. Thin-Walled Structures, 101, 222–230. DOI 10.1016/j.tws.2015.12.023. [Google Scholar] [CrossRef]
8. Hsiung, W. Y., Qiao, S. Y., Li, Y. F. (1980). The anatomical structure of culms of Phyllostachys Pubescens Mazel EX H.De Lehaie. Acta Botanica Sinica, 22(4), 344–348. [Google Scholar]
9. Palombini, F. L., Nogueira, F. M., Junior, W. K., Paciornik, S., Mariath, J. E. D. A. et al. (2020). Biomimetic systems and design in the 3D characterization of the complex vascular system of bamboo node based on X-ray microtomography and finite element analysis. Journal of Materials Research, 35(8), 842–854. DOI 10.1557/jmr.2019.117. [Google Scholar] [CrossRef]
10. Grosser, D., Liese, W. (1971). On the anatomy of Asian bamboos, with special reference to their vascular bundles. Wood Science and Technology, 5(4), 290–312. DOI 10.1007/BF00365061. [Google Scholar] [CrossRef]
11. Liese, W., Tang, T. K. H. (2015). Properties of the bamboo culm, pp. 227–256. Switzerland: Springer International Publishing. [Google Scholar]
12. Jiang, Z. H. (2007). Bamboo and Rattan in the world, pp. 127–131. China: China Forestry Publishing House. [Google Scholar]
13. Trtik, P., Dual, J., Keunecke, D., Mannes, D., Niemz, P. et al. (2007). 3D imaging of microstructure of spruce wood. Journal of Structural Biology, 159(1), 46–55. DOI 10.1016/j.jsb.2007.02.003. [Google Scholar] [CrossRef]
14. Mayo, S. C., Chen, F., Evans, R. (2010). Micron-scale 3D imaging of wood and plant microstructure using high-resolution X-ray phase-contrast microtomography. Journal of Structural Biology, 171(2), 182–188. DOI 10.1016/j.jsb.2010.04.001. [Google Scholar] [CrossRef]
15. Brodersen, C. R. (2013). Visualizing wood anatomy in three dimensions with high-resolution X-ray micro-tomography (μCT)–A review. IAWA Journal, 34(4), 408–424. DOI 10.1163/22941932-00000033. [Google Scholar] [CrossRef]
16. Huang, P. X., Chang, W. S., Ansell, M. P., Chew, Y. M. J., Shea, A. (2015). Density distribution profile for internodes and nodes of Phyllostachys edulis (Moso bamboo) by computer tomography scanning. Construction and Building Materials, 93(15), 197–204. DOI 10.1016/j.conbuildmat.2015.05.120. [Google Scholar] [CrossRef]
17. Wang, Q. P., Liu, X. E., Zhang, G. L., Yang, S. M., Tian, G. L. et al. (2015). Rapidly detection for Moso bamboo density under different moisture condition based on X-CT Technology. Spectroscopy and Spectral Analysis, 36(6), 1899–1903. DOI 10.3964/j.issn.1000-0593(2016)06-1899-05. [Google Scholar] [CrossRef]
18. Roussel, J. R., Mothe, F., Krähenbühl, A., Kerautret, B., Debled-Rennesson, I. et al. (2014). Automatic knot segmentation in CT images of wet softwood logs using a tangential approach. Computers and Electronics in Agriculture, 104, 46–56. DOI 10.1016/j.compag.2014.03.004. [Google Scholar] [CrossRef]
19. Breinig, L., Brüchert, F., Baumgartner, R., Sauter, U. H. (2012). Measurement of knot width in CT images of Norway spruce (Picea abies [L.] Karst.)—Evaluating the accuracy of an image analysis method. Computers and Electronics in Agriculture, 85, 149–156. DOI 10.1016/j.compag.2012.04.013. [Google Scholar] [CrossRef]
20. Fei, B. H., Zhao, Y., Qin, D. C., Yang, Z., Hou, Z. Q. et al. (2007). Applying computerized tomography (CT) to study the feature of wood fracture. Scientia Silvae Sinicae, 43(4), 137–140. DOI 10.11707/j.1001-7488.20070425. [Google Scholar] [CrossRef]
21. Forsberg, F., Mooser, R., Arnold, M., Hack, E., Wyss, P. (2008). 3D micro-scale deformations of wood in bending: Synchrotron radiation uCT data analyzed with digital volume correlation. Journal of Structural Biology, 164(3), 255–262. DOI 10.1016/j.jsb.2008.08.004. [Google Scholar] [CrossRef]
22. Fu, J. (2014). Preliminary study on the application and on the basic properties of Indocalamus latifolius (Ph.D. Thesis). Anhui Agricultural University, China. [Google Scholar]
23. Zeng, Q. Y., Li, S. H., Bao, X. R. (1992). Effect of bamboo nodal on mechanical properties of bamboo wood. Scientia Silvae Sinicae, 28(3), 247–252. [Google Scholar]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |