
Materials

 | Journal of Renewable Materials |  |
DOI: 10.32604/jrm.2021.015514
ARTICLE
Effect of Doped Alkali Metal Ions on the SO2 Capture Performance of MnO2 Desulfurization Materials at Low Temperature
1Guangzhou Institute of Energy Conversion, Chinese Academy of Science, Guangzhou, 510640, China
2School of Mechanical Engineering, College of Science and Engineering, Kanazawa University, Kanazawa, 9201192, Japan
3School of Mechanical and Power Engineering, Nanjing Tech University, Nanjing, 211800, China
*Corresponding Author: Yugo Osaka. Email: y-osaka@se.kanazawa-u.ac.jp
Received: 24 December 2020; Accepted: 27 January 2021
Abstract: Sulfur dioxide (SO2) emissions from diesel exhaust pose a serious threat to the environment and human health. Thus, desulfurization technology and the performance of desulfurization materials must be improved. In this study, MnO2 was modified with various alkali metal ions using the impregnation method to enhance its SO2 capture performance. The composites were characterized intensively by scanning electron microscopy, energy-dispersive X-ray spectroscopy, X-ray diffraction spectroscopy, and Brunauer-Emmett-Teller theory. The SO2 capture performance of these composites were measured via thermogravimetry, and the effect of doping with alkali metal ions on the SO2 capture performance of MnO2 was investigated. Results showed that the SO2 capture performance of MnO2 could be enhanced by doping with alkali metal ions, and the MnO2 composite doped with LiOH (2.0 mol/L) had the best SO2 capture capacity (124 mgSO2/gMaterial), which was 18% higher than that of pure MnO2. Moreover, the type and concentration of alkali metal ions had varying effects on the SO2 capture performance of MnO2. In our experiment, the SO2 capture performance of the MnO2 doped with NaOH, LiCl, Na2CO3, K2CO3, and Li2CO3 composites were worse than that of pure MnO2. Therefore, the influences of the type and concentration of alkali metal ions to be doped into desulfurization materials must be considered comprehensively.
Keywords: Sulfur dioxide capture; desulfurization materials; manganese dioxide; alkali metal ions doped
Over the past few decades, environmental pollution has threatened human health and survival [1]. Sulfur dioxide (SO2), which is a major source of atmospheric pollution, leads to acid rain and acid smog formation. This pollutant is strongly harmful toward animals, plants and human beings, causes asthma and respiratory diseases, and is associated with increased mortality and morbidity [2–4]. Therefore, the World Bank and many countries have enacted increasingly stringent regulations to limit SO2 emissions [5].
Diesel cycle engines, which have good thermal efficiency and high power, are used as the power source of automotive vehicles and ships. However, the type and content of sulfur compounds in diesel actually used in diesel engines vary according to operating conditions, with sulfur content ranging from 300 ppm to 5000 ppm. The diesel cycle is inferior to the Otto cycle in terms of emission feature [6]. The SO2 from diesel cycle combustion exhaust is a common air pollutant that can have harm for human health and living environment even at levels lower than 100 ppm. In addition, the SO2 from diesel exhaust has been reported to reduce the efficiency of exhaust denitration greatly [7,8]. The International Marine Organization regulates the total NOx emissions, whereas sulfur concentrations are only regulated within the fuel. Therefore, the SO2 from diesel exhaust must be effectively removed. This process is usually achieved through fuel upgrades, fuel catalytic combustion, exhaust gas filtration (i.e., dry desulfurization) and so on [9]. However, finding appropriate solutions to prevent the NOx removal catalyst from being exposed to SO2 remains challenging because in most cases the SO2 content of diesel exhaust still varies from tens of ppm to hundreds of ppm [10].
Dry flue gas desulfurization (FGD) is a major industrial method to regulate SO2 emissions in the atmosphere environment. Desulfurization materials are the core of FGD technology and the main research direction at present. Metal oxides, which are commonly used as dry desulfurization materials, can remove SO2 effectively and have widely used, good reliability, good activity, high stability and so on [11]. Metal doping into desulfurization materials can improve desulfurization performance [12]. However, studies on the effect of alkali metal doping into desulfurization materials for FGD are limited. Liu et al. [13] studied the effects of adding NaCl on the SO2 capture by CaCO3 during the coal combustion porcess, and found that adding NaCl can improve the SO2 capture performance of CaCO3. Kim et al. [14] prepared a K2CO3/Al2O3 sorbent and investigated the effect of SO2 on CO2 sorption capacities. They found that the SO2 in flue gas can react with K2CO3 to form K2SO4 which is quite stable at the temperatures between 180°C and 550°C. Osaka et al. [15] synthesized a new type of Na-doped CaCO3 material for the SO2 adsorption and found that the Na-doped CaCO3 material improved the SO2 absorption capacity better than the pure CaCO3 material. Pittalis et al. [16] found that the addition of active alkali metals (Na and/or Li) could promote the reductive desulfurization of dibenzothiophene and its hindered analogs at room temperature. Wang et al. [17] prepared an alkali metal-doped CaO composite for SO2 adsorption. They found that the number of active sites on the surface of CaO for SO2 adsorption increased after doping with alkali metal, and SO2 showed stronger adsorption on the alkali metal-doped CaO compared with that on pristine CaO.
Existing researches have proven that doping with alkali metal ions could improve the performance of materials to capture SO2, but these studies have focused mainly on the modification of CaO, CaCO3, K2CO3, MgO and so on as conventional FGD materials. The studies that focus on the modification of manganese dioxide (MnO2), which is a new generation of dry desulfurization material for diesel exhaust, are few. In the previous studies [18,19], MnO2 with a simple sulfate reaction process (MnO2+SO2→MnSO4) was reported as a kind of good desulfurization material, and we found that MnO2 could effectively remove SO2 in the middle and low temperature regions (200°C–450°C) and was suitable for application in diesel exhaust conditions. The SO2 capture performance of MnO2 could reach more than 400 mgSO2/gMnO2 at 450°C, but its performance at 200°C is approximately 170 mgSO2/gMnO2, which is 43% lower than the performance at 450°C [19]. Reaction temperature is a very important factor for the SO2 capture performance of desulfurization materials. Generally speaking, the higher the reaction temperature is, the better the SO2 capture performance of desulfurization materials will be. Many existing studies have also indicated that the same desulfurization material can show higher SO2 capture performance at high temperature (above 200°C), but its desulfurization performance is insufficient at low temperature (below 200°C) [20]. The desulfurization reaction at low temperature (below 200°C) requires the physical and chemical properties of desulfurization materials more. Therefore, enhancing the SO2 capture performance of desulfurization materials at low temperature is very necessary and meaningful.
In this work, the SO2 capture performance of the MnO2 composites doped with various alkali metal ions were investigated via thermogravimetry (TG) method at 200°C. The structural characteristics of the composites before and after desulfurization reaction were studied using the N2 adsorption–desorption isotherm method, energy dispersive X-ray (EDX) spectroscopy, and scanning electron microscopy (SEM). Finally, the effect of doping with alkali metal ions on the desulfurization performance of MnO2 was analyzed, and the results provided a reference for the improvement of desulfurization performance of metal oxides at low temperature.
The MnO2 with a high specific surface area (HSSA MnO2, 98%) was supplied by the Japan Metals and Chemicals Co., Ltd., Tokyo, Japan. The HSSA MnO2 was produced by treating raw materials with acid, and its specific surface area was about 275 m2/g, its particle size distribution was shown in Fig. 1. Analytical grade LiOH (98%), NaOH (96%), LiCl (99%), Li2CO3 (99%), Na2CO3 (99%), and K2CO3 (99%) were purchased from the Kanto Chemical Co., Inc., Japan.
In this study, the MnO2 materials doped with alkali metal ions were prepared using the impregnation method [21]. The synthesis proportion of the MnO2 composites doped with different alkali metal ions are shown in Tab. 1. First, different quantities of alkali metal ions were dissolved in 100 mL deionized water. Then, a certain quality of HSSA MnO2 was added into the alkali metal ionic solution, and the mixture was magnetically stirred and ultrasonically oscillated (40 kHz) at room temperature for more than 30 min each. Then the solid and liquid mixtures were dried in an oven at 120°C for 5 h, after drying the dried solids were ground into powder. The products were denoted as the MnO2 doped with alkali metal ions.
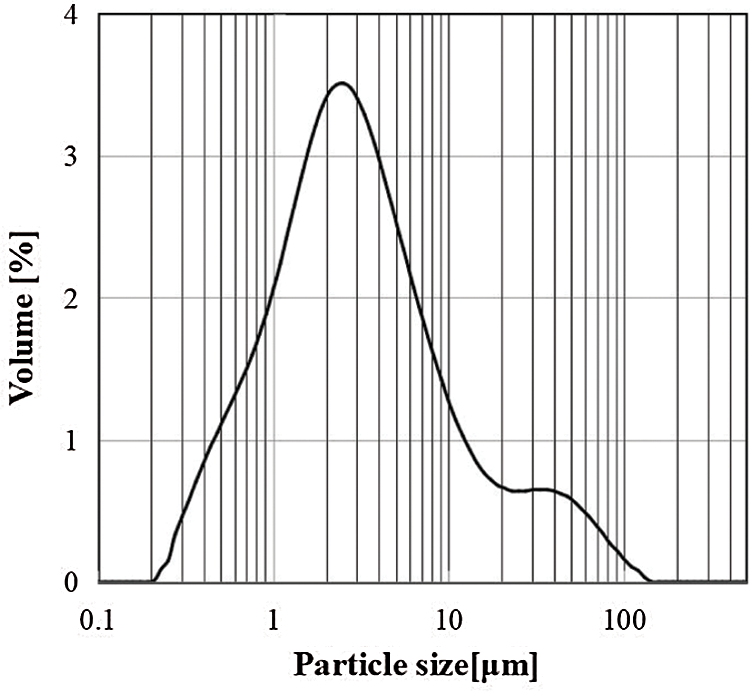
Figure 1: The particle size distribution of MnO2
Table 1: The MnO2 doped with alkali meta ions composites synthesis proportion

2.2 Structural Characteristics
In this study, the morphology of the MnO2 doped with alkali meta ions composites were observed via scanning electron microscope (SEM, HITACHI SU1510, Japan). The crystal structures of the composites were tested by X-ray diffraction (XRD) spectroscopy (X’ Pert Pro MPD, Cu Kα radiation). Data were collected in the 2θ range from 5° to 80° at a scanning velocity of 10°/min. The specific surface areas and pore size distributions of the composites were analyzed by the N2 adsorption-desorption isotherms using an Automatic Specific Surface and Pore Distribution Analyzer (Autosorb-iQ, Quanta Co., USA).
2.3 Desulfurization Performance Measurement
The SO2 capture performance of the alkali metal ion doped MnO2 samples were measured using a thermogravimetry (TG) method [22]. The TG device was self-made because the samples after TG measurement needed to be measured using various physical property evaluation devices. Therefore, the structure of the original TG device that can measure a large amount of sample mass and minimize the diffusion inhibition in stacked samples was designed. A schematic of the TG method for our experiment is shown in Fig. 2. The test samples (approximately 100 mg) placed on quartz crucibles were slowly heated (10 K/min) to a target temperature in nitrogen atmosphere. This condition was maintained for approximately 2 h. In order to eliminate the influence of other interfering gases on the sample desulfurization reaction, the reaction gas in this experiment only used SO2 and N2. The reactant gas, which contained 500 ppm SO2 in base N2, was controlled by the flow controller, and the total flow gas rate was 2 L/min. During the SO2 adsorption, the reactant gas was passed over the sample at the target temperature for 2 h. The reaction temperature of the TG tests was 200°C. The SO2 capture performance of the samples was measured by the TG device. The SO2 capture performance of the test samples could be calculated using the following equation:
where P [gSO2/gMaterial] is the SO2 capture performance per unit mass, s0 [mg] is the initial weight, and st [mg] is the weight after t seconds.
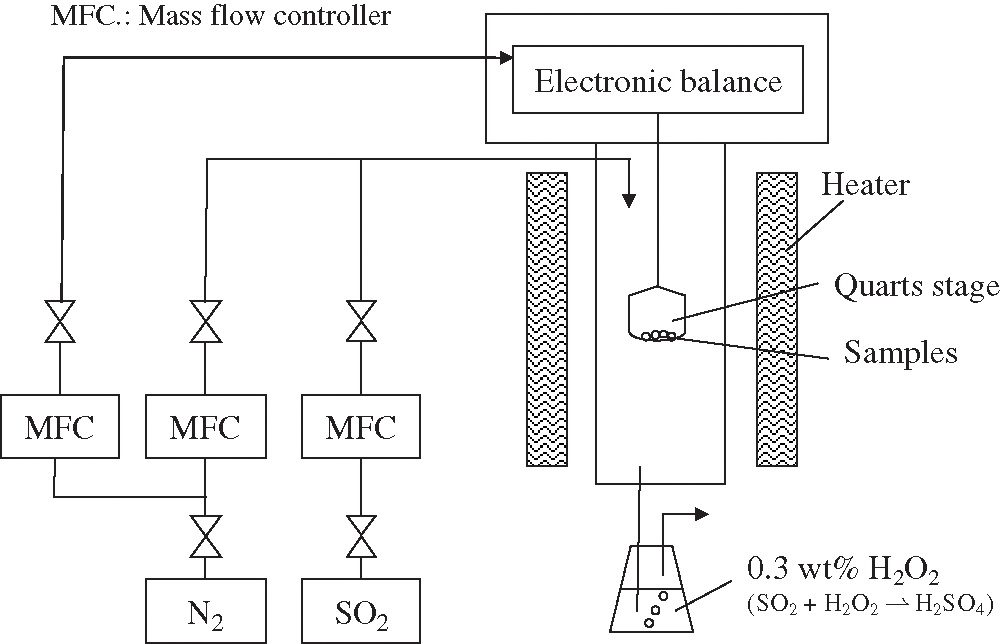
Figure 2: The schematic drawing of the thermogravimetry method for testing the SO2 capture performance
3.1 Morphological Characteristics of the MnO2 Doped with Alkali Metal Ions composites
The SEM micrographs of pure MnO2 and the MnO2 doped with LiOH/NaOH samples are shown in Fig. 3. Pure MnO2 was composed of nonuniform, smooth, spherical particles with a size of approximately 1 µm, and the morphology of the MnO2 doped with LiOH did not change remarkable after LiOH addition. Moreover, the agglomeration and particle size did not change much. Meanwhile, the morphology of the MnO2 doped with NaOH did not change significantly after NaOH addition, but the agglomeration and particle size increased. In addition, increasing the concentration of alkali metal ions did not result in a significant change in the morphology of the MnO2 doped with LiOH/NaOH composites but resulted in slightly increased agglomeration.
The EDX elemental analysis photographs of MnO2 doped with NaOH (2.0 mol/L) are shown in Fig. 4. After NaOH addition, many evenly dispersed Na elements were observed on the surface of MnO2 particles. Photographs of MnO2 doped with LiOH (2.0 mol/L) were not attached because Li elements were not detected by the EDX detector.
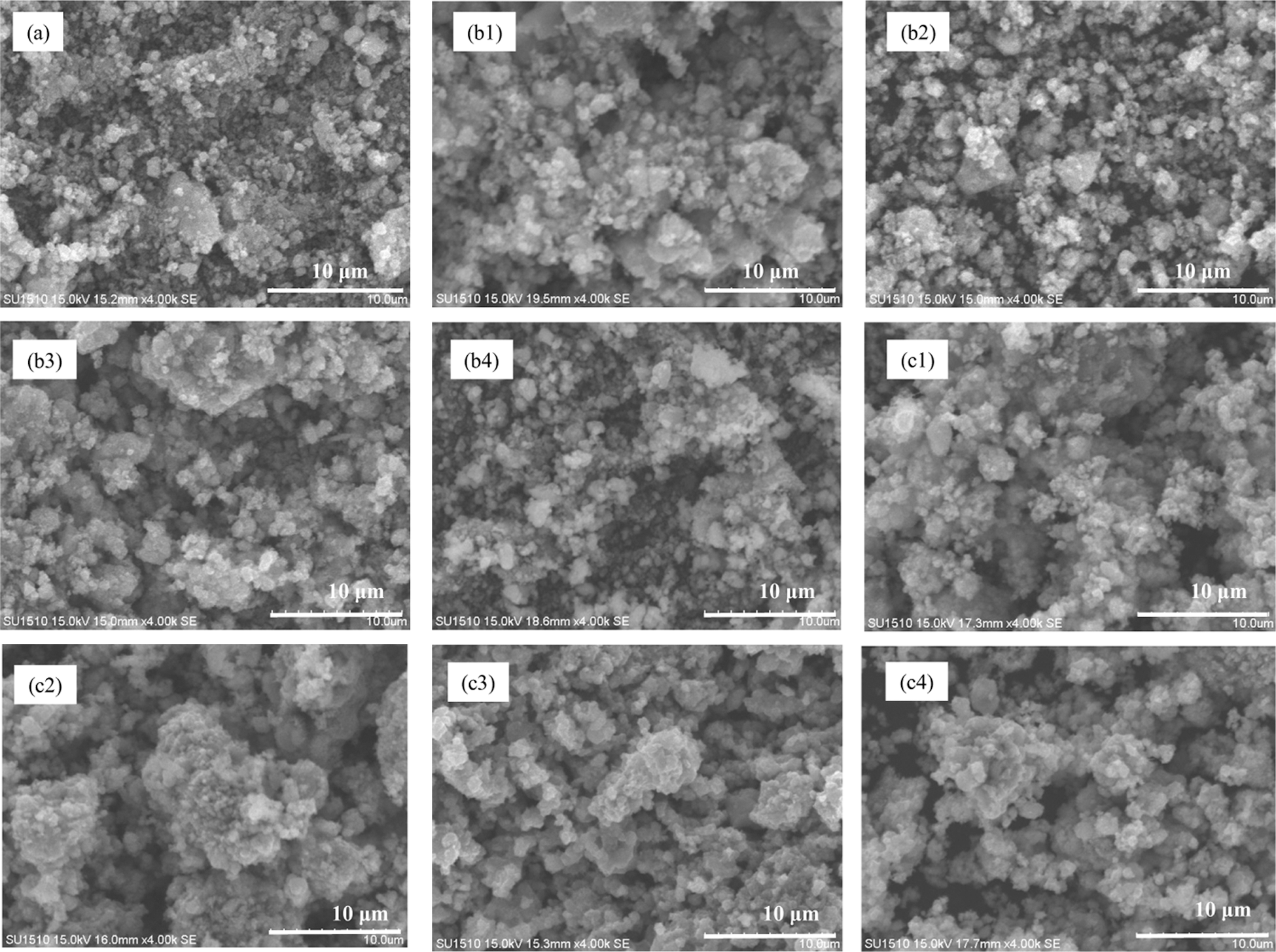
Figure 3: SEM images of MnO2 and MnO2 doped with alkali metal ions (a) MnO2, (b1) MnO2 doped with LiOH (0.5 mol/L), (b2) MnO2 doped with LiOH (1.0 mol/L), (b3) MnO2 doped with LiOH (1.5 mol/L), (b4) MnO2 doped with LiOH (2.0 mol/L), (c1) MnO2 doped with NaOH (0.5 mol/L), (c2) MnO2 doped with NaOH (1.0 mol/L), (c3) MnO2 doped with NaOH (1.5 mol/L), (c4) MnO2 doped with NaOH (2.0 mol/L)
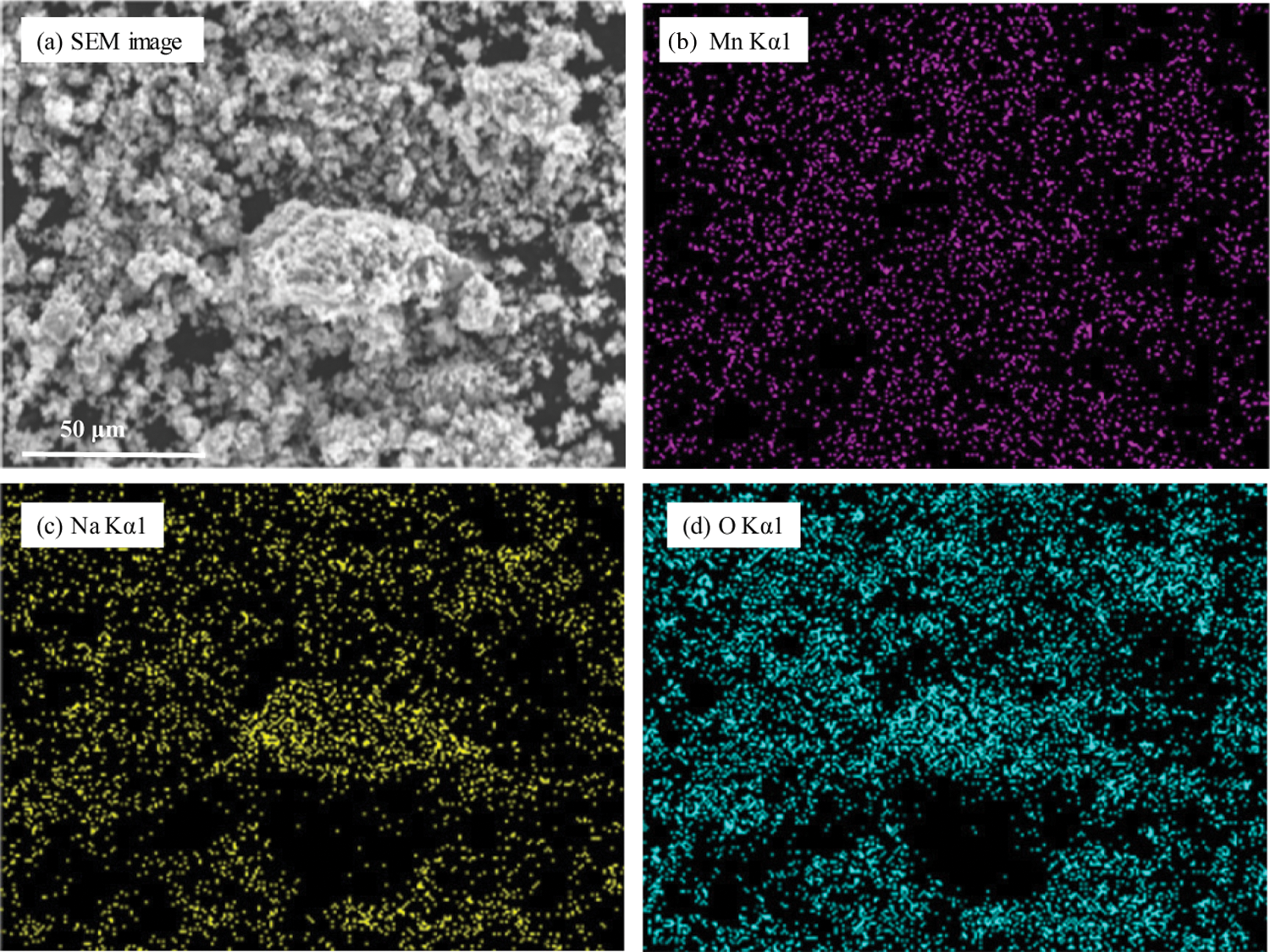
Figure 4: EDX element analysis photographs of MnO2 doped with NaOH (2.0 mol/L) (a) SEM image, (b) Mn Kα1, (c) Na Kα1, (d) O Kα1
The XRD patterns of MnO2 doped with LiOH (2.0 mol/L) are shown in Fig. 5. After doping with LiOH, many diffraction peaks were consistent with those of pure MnO2, and there was no characteristic peak of LiOH in the composite. One of the characteristic peaks of Li2CO3 probably occurred due to the reaction of LiOH with air (the Li2CO3 may be produced when the sample was dried in an oven). These results were obtained because LiOH was highly dispersed on the surface of MnO2 particles, and LiOH particles were small and amorphous, resulting in no LiOH diffraction peaks.
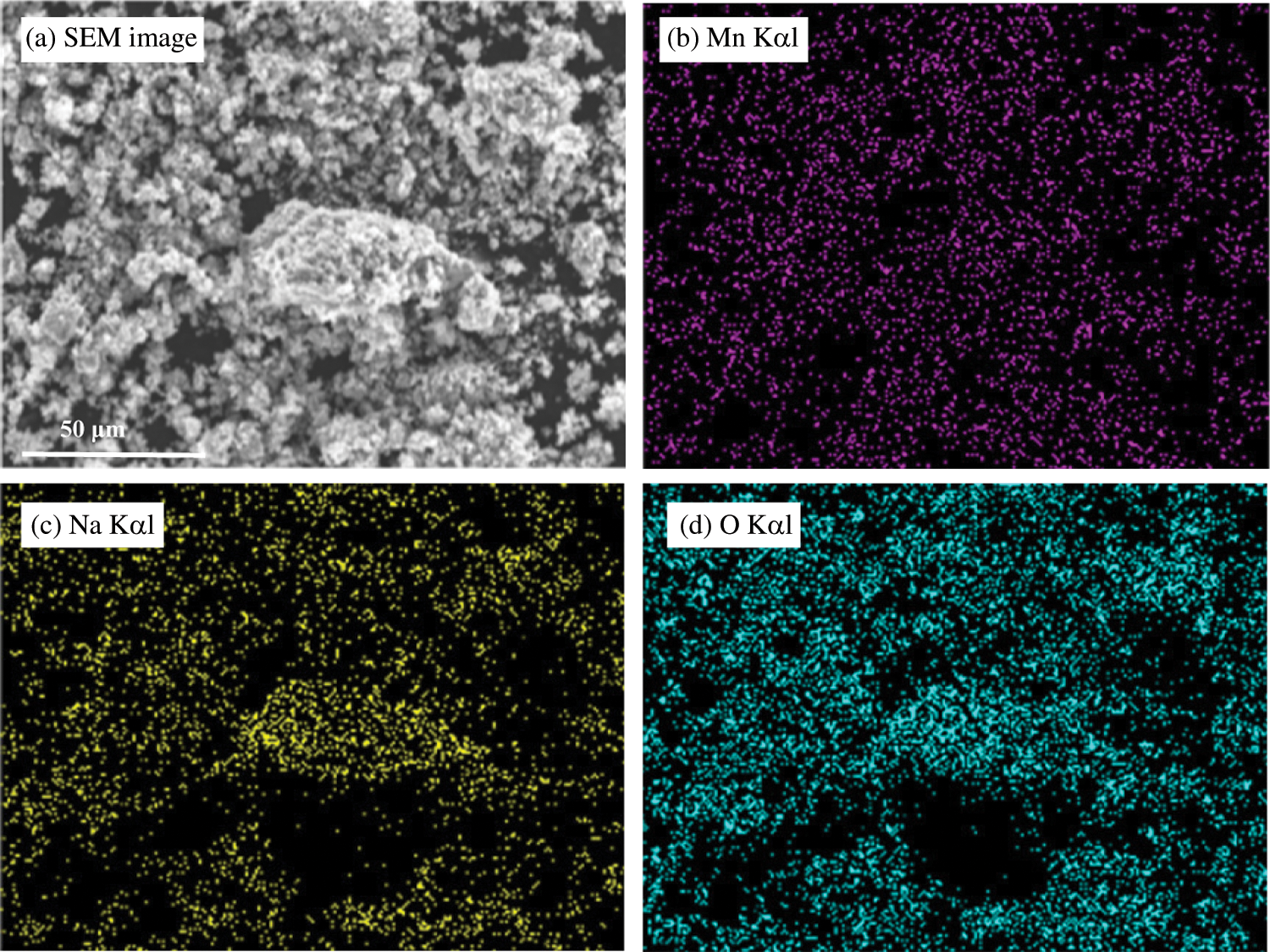
Figure 5: XRD patterns of pure MnO2 and MnO2 doped with LiOH (2.0 mol/L)
3.2 Physical and Chemical Characterization of the MnO2 Doped with Alkali Metal Ions Composites
The specific surface area and the pore size distribution of pure MnO2 and MnO2 doped with LiOH/NaOH samples were measured using the N2 adsorption-desorption instruments, as shown in Figs. 6 and 7. The specific surface area of the pure MnO2 sample was 275 m2/g before impregnation with alkali metal ions (LiOH and NaOH). In turn, the specific surface area of the MnO2 doped with LiOH or NaOH decreased gradually, and the specific surface area of MnO2 doped with NaOH decreased more than that doped with LiOH. Moreover, different concentrations of alkali metal ions had different effects on the specific surface area of the MnO2 doped with LiOH or NaOH composites.
As shown in Fig. 7, the pore size distribution of pure MnO2 and MnO2 doped with LiOH/NaOH samples were mainly mesoporous and macroporous. The number of mesopores and macropores of the MnO2 doped with LiOH/NaOH decreased gradually with an increasing concentration of alkali metal ions. In particular, the number of mesopores and macropores of the MnO2 doped with NaOH samples dropped rapidly. Impregnation with alkali metal ions did not change the pore size distribution of MnO2 material, but only reduced the number of pores in MnO2 material.
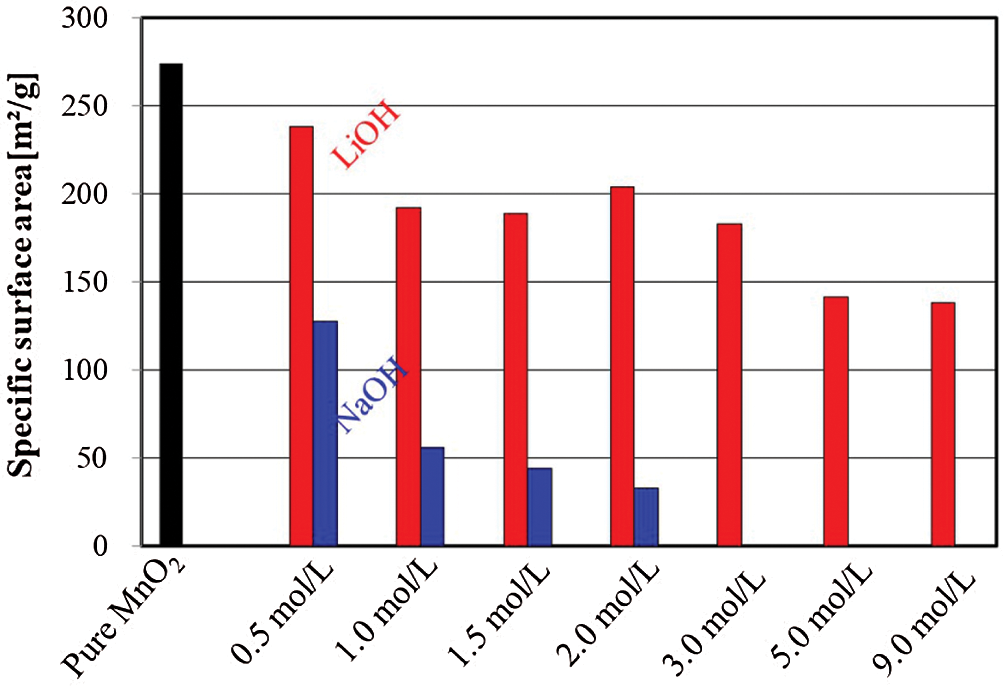
Figure 6: The specific surface area of MnO2 doped with LiOH and NaOH
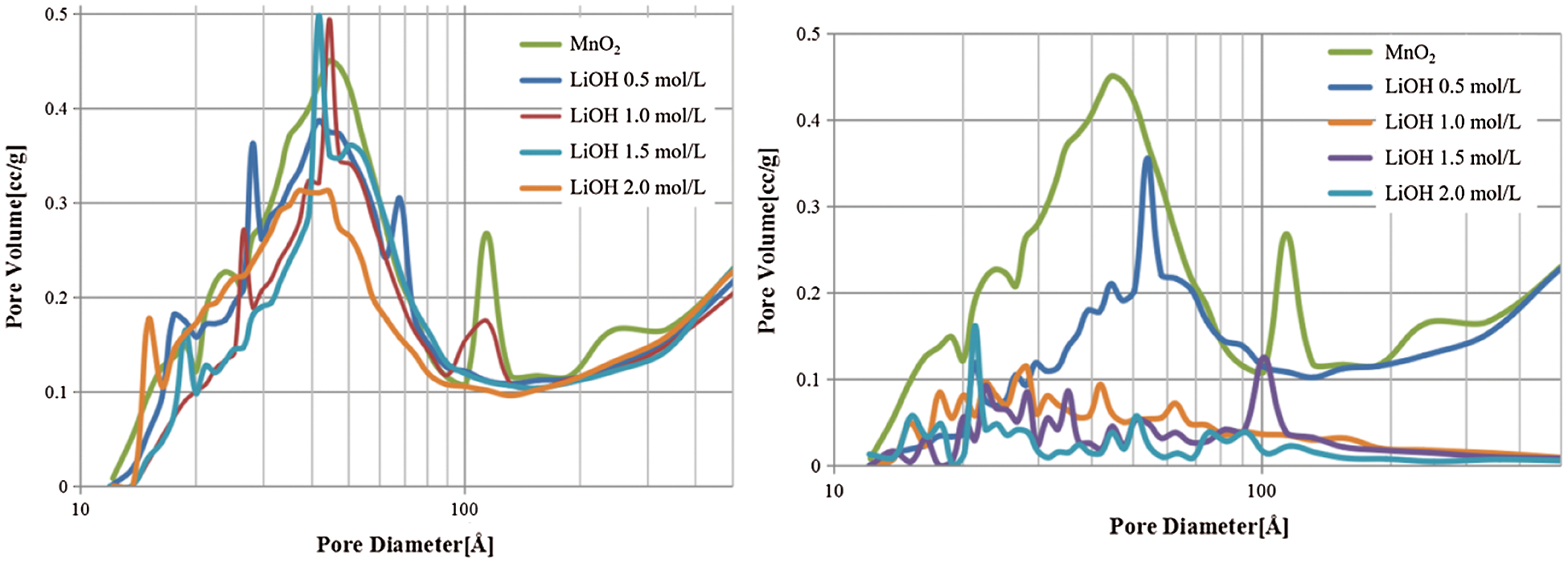
Figure 7: The pore size distribution of MnO2 doped with LiOH (left) and NaOH (right)
The decrease in the specific surface area and the number of mesopores and macropores of the MnO2 doped with LiOH/NaOH composites were due to the alkali metal ions that were embedded into the pore channels of MnO2 during impregnation. As a result, the pore volume, the number of mesopores and macropores, and the specific surface area of the MnO2 doped with alkali metal ions composites decreased. Fig. 8 shows a schematic of the MnO2 material after doping with alkali metal ions. NaOH was easier blocked the pore channels of MnO2 than that of LiOH, because the diameter of the NaOH molecule was larger than that of the LiOH molecule. Thus, the surface area and the number of mesopores and macropores of the MnO2 doped with NaOH composites dropped further. Moreover, as shown in Figs. 3 and 7, the MnO2 doped with NaOH samples were much agglomerated and less pores number.

Figure 8: The conceptual diagram of MnO2 surface after doping with alkali metal ions
3.3 SO2 Capture Performance of the MnO2 Doped with Alkali Metal Ions Composites
The SO2 capture performance of the prepared MnO2 doped with alkali metal ions samples were measured using a TG method at 200°C and the gas flow rate of 2 L/min, which contained 500 ppm SO2 in base N2 for 2 h. Fig. 9 shows the time history of the SO2 capture performances of pure MnO2 and the MnO2 doped with LiOH samples. The results in Fig. 9 show that the SO2 capture rate and capacity of MnO2 doped with LiOH (2.0 mol/L) were significantly higher than those of pure MnO2, and the SO2 capture capacity (2 h) of pure MnO2 was 105 mgSO2/gMaterial. MnO2 doped with LiOH (2.0 mol/L) had the best SO2 capture capacity in these prepared samples and captured 124 mgSO2/gMaterial, which was 18% higher than that of pure MnO2. Moreover, different specific surface area and different concentrations of LiOH had different effects on the SO2 capture performance of the MnO2 composite (Fig. 10). Increased LiOH concentration increased the SO2 capture capacity of the MnO2 doped with LiOH/NaOH composite. However, after taking 2.0 mol/L as the maximum value, increasing the concentration of LiOH aqueous solution resulted in a decreased SO2 capture performance. In this experiment, the desulfurization performance of the composite was better than that of pure MnO2 only at a concentration of 2.0 mol/L LiOH. The SO2 capture capacity was related to the surface properties of the desulfurization materials. An increase in the concentration of LiOH, which was doped on the surface of MnO2, decreased the specific surface area and the number of mesopores and macropores of the MnO2 composite (as mentioned above). The decrease in the specific surface and the number of mesopores and macropores of the MnO2 composite led to decreased SO2 capture capacity [11]. Meanwhile, alkali metal ions may be formed when alkali metal ions were doped on the surface of MnO2 because alkali metal ions react with SO2 to form sulfates [21]. As their additive amounts increased, the alkali metal ions reacted with more SO2, increasing the desulfurization capacity of the composite. Therefore, the concentration of LiOH doped into MnO2 had an optimum value.
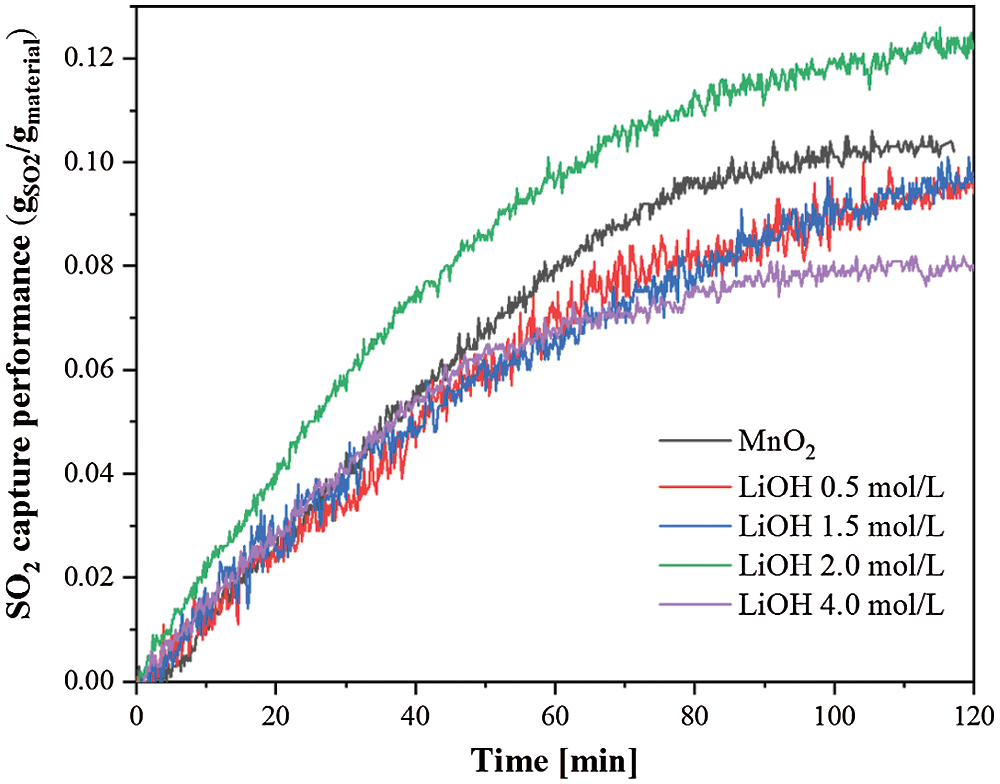
Figure 9: Time history on the SO2 capture performance of MnO2 and MnO2 doped with LiOH at 200°C, 500 ppm SO2 in base N2 for 2 h
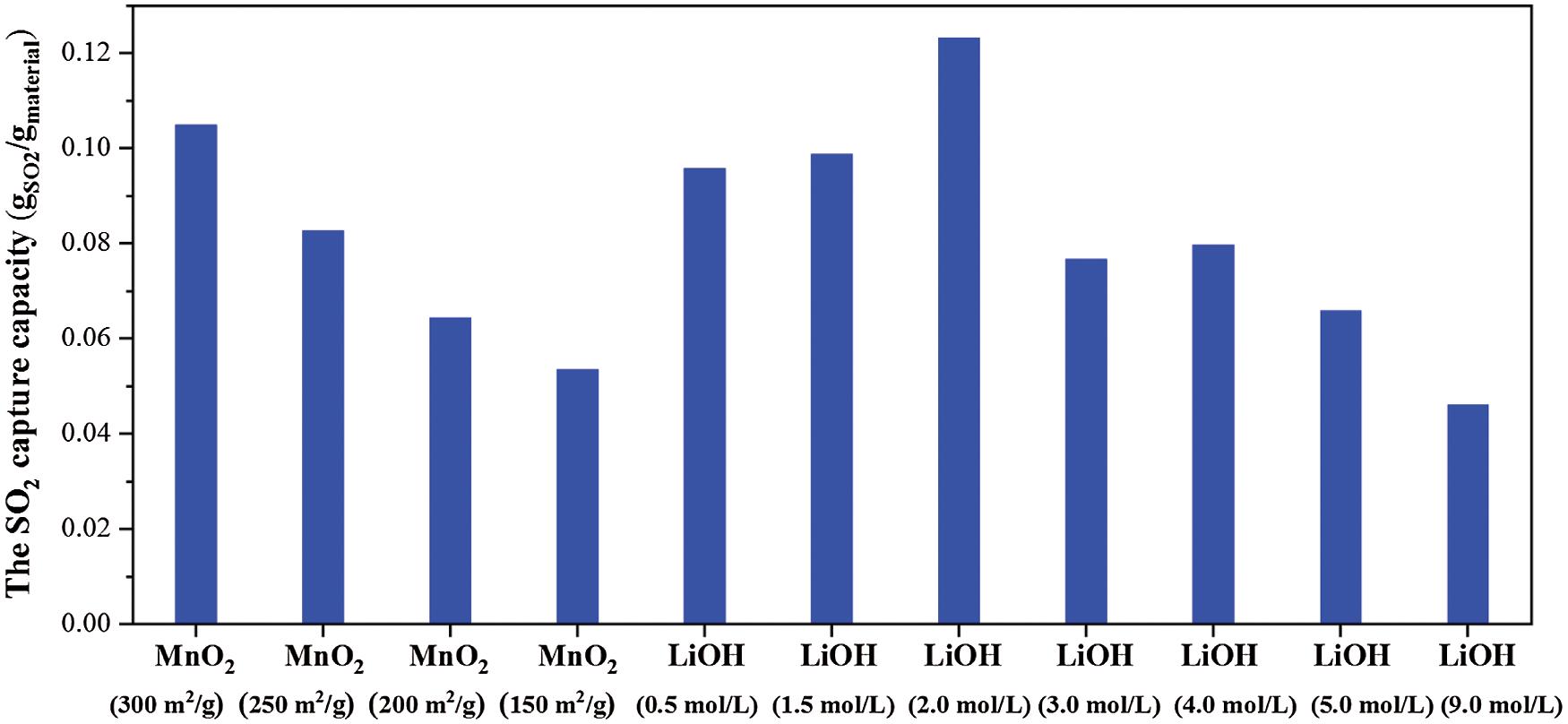
Figure 10: SO2 capture capacity of pure MnO2 and MnO2 doped with LiOH at 200°C, 500 ppm SO2 in base N2 for 2 h
Fig. 11 shows the SO2 capture performance (2 h) of pure MnO2 and the MnO2 composites doped with LiOH (2.0 mol/L), NaOH (2.0 mol/L), LiCl (2.0 mol/L), Na2CO3 (2.0 mol/L), K2CO3 (2.0 mol/L), and Li2CO3 (2.0 mol/L). Na2CO3, K2CO3, Li2CO3 are carbonates that can be produced when alkali metals are mixed. The results in Fig. 11 show that the desulfurization performance of carbonate was low, indicating that the use of carbonates, such as Na2CO3 and Li2CO3, which were converted from LiOH and NaOH via thermal synthesis, could not improve desulfurization performance. Compared with different alkali metal-doped MnO2, the MnO2 doped with LiOH had the best SO2 capture capacity (2 h) at the same concentration. This result may be due to the strong alkalinity of the MnO2 doped with LiOH composite. In our early experiments, the pH of MnO2 doped with LiOH aqueous solution was highest when the above several composites were dispersed in water. Thus, according to the reaction mechanism of acid-base neutralization, the higher the alkalinity of the composite material, the easier it is to react with SO2 (acidic gas) [23]. Moreover, the agglomeration of the MnO2 doped with alkali metal ions composites also affected the SO2 capture capacity. As mentioned above, the MnO2 doped with NaOH had more agglomerations compared with the MnO2 doped with LiOH, and the former’s specific surface area and proes number were smaller, leading to lower SO2 capture performance.
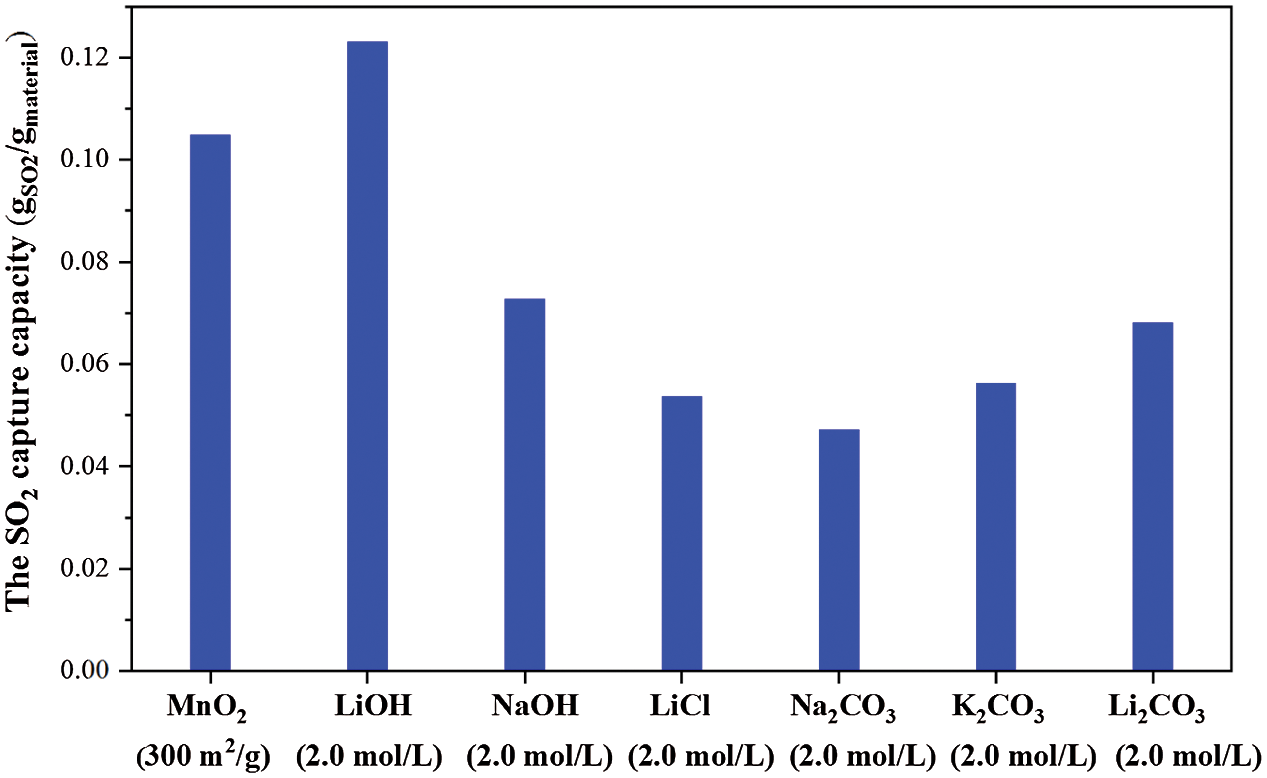
Figure 11: SO2 capture capacity of pure MnO2 and the MnO2 doped with different alkali metal salts at 200°C, 500 ppm SO2 in base N2 for 2 h
3.4 Effect of Doping with Alkali Metal Ions on the Desulfurization Performance of MnO2 at Low Temperature
The effect of doping with alkali metal ions on the desulfurization performance of MnO2 was a comprehensive process. The SEM and EDX elemental analysis photographs of the MnO2 samples doped with NaOH (2.0 mol/L) after desulfurization are shown in Fig. 12. Fig. 12 shows that many S elements were observed on the surface of the composite, and their distribution was similar to that of Na and O elements. Thus, the Na elements in the composite could possibly capture the S elements in the SO2. Moreover, after impregnation and NaOH doping into MnO2, Na ions formed in the composite and reacted with SO2 during the desulfurization process. The desulfurization reaction between the MnO2 doped with NaOH and the SO2 can be described by two reaction processes, and the equations are as follows:
Therefore, the effect of doping with alkali metal ions was similar to that of doping with NaOH. When different alkali metal ions were doped into MnO2 by impregnation, the surface of MnO2 formed alkali metal ions, which may react with SO2 to form sulfate during the desulfurization process and promote the performance of SO2 capture. As shown in Fig. 8, the reaction of alkali metal ions and SO2 to form sulfate would also close the internal pores of the MnO2 particles, which would affect the flow and mass transfer of SO2 gas in the MnO2 particles, and make it difficult to maintain the desulfurization reaction. Meanwhile, the reaction of MnO2 and SO2 to form MnSO4, which would also cause the blockage of the internal pores of the MnO2 particles, thus increasing the mass transfer resistance of desulfurization reaction [24]. On the other hand, doping with different alkali metal ions at different concentrations enhanced the agglomeration, reduced the specific surface area and pore quantity of the composite, leading to the degradation of the SO2 capture performance of the composites. Thus, doping with different alkali metal ions at different concentrations had different effects on the desulfurization performance of the composites, and the effect of all aspects should be considered comprehensively.
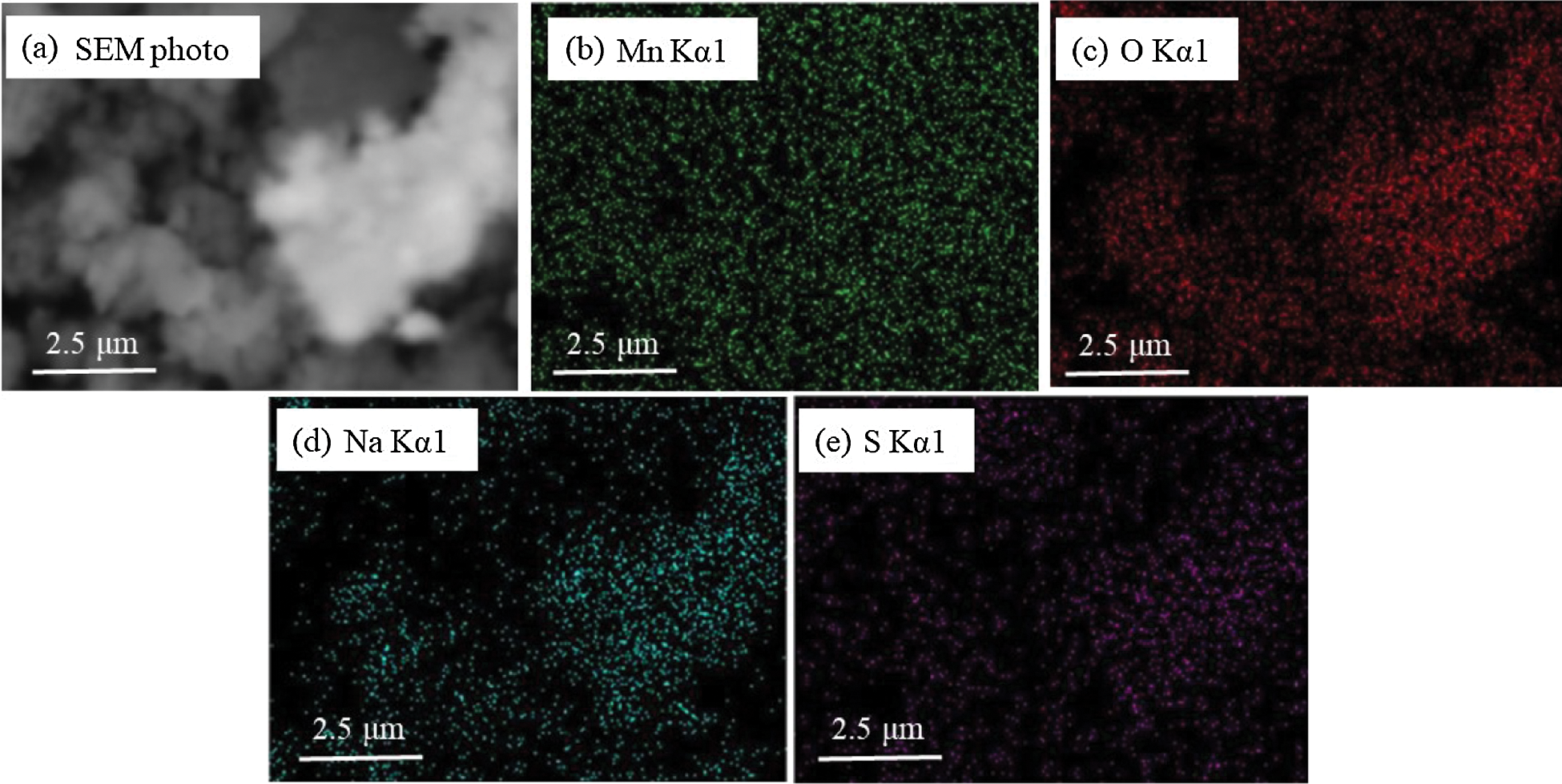
Figure 12: SEM and EDX element analysis photographs of MnO2 doped with NaOH (2.0 mol/L) sample after desulfurization: (a) SEM image, (b) Mn Kα1, (c) O Kα1, (d) Na Kα1, (e) S Kα1
MnO2 modified with alkali metal ions was used as SO2 capture material for dry desulfurization technology in a diesel exhaust system. The SO2 capture performance of the MnO2 doped with alkali metal ions composites were measured using a TG method at 200°C and 2 L/min gas flow, which contained 500 ppm SO2 in base N2 for 2 h. Results revealed the following:
The SO2 capture performance of MnO2 could be enhanced by doping with alkali metal ions. In our experiment, the SO2 capture performance of MnO2 doped with LiOH (2.0 mol/L) was significantly higher by 18% compared with that of pure MnO2 at 200°C. However, doping with alkali metal ions at different concentrations did not always improve the SO2 capture performance of MnO2. The SO2 capture performance of the MnO2 composites doped with NaOH, LiCl, Na2CO3, K2CO3, and Li2CO3 were lower than that of pure MnO2.
MnO2 desulfurization materials doped with alkali metal ions at low temperature formed alkali metal ions on the surface of MnO2. These alkali metal ions could react with SO2 to form sulfate during the desulfurization process, then increase the active components of the composite, and promote the SO2 capture performance.
Doping with different alkali metal ions at different concentrations had different effects on the SO2 capture performance of the composite. Doping with alkali metal ions resulted in a decrease in specific surface area and number of mesopores and macropores and increase in agglomeration and active components of the composite. These aspects comprehensively affected the SO2 capture performance of the composite.
Funding Statement: This work was financially supported by the Key Program of Frontier Science of Chinese Academy of Sciences (QYZDY-SSW-JSC038), the Natural Science Foundation of Guangdong Province (2017A030310185) and the Science and Technology Planning Project of Guangzhou, China (201704030040).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Yu, F., Liu, C., Xie, P., Yu, S. (2015). Oxidative-extractive deep desulfurization of gasoline by functionalized heteropoly acid catalysts. RSC Advances, 5(104), 85540–85546. DOI 10.1039/C5RA16013H. [Google Scholar] [CrossRef]
2. Kan, H., Wong, C. M., Vichit-Vadakan, N., Qian, Z. (2010). Short-term association between sulfur dioxide and daily mortality: The Public Health and Air Pollution in Asia (PAPA) study. Environmental Research, 110(3), 258–264. DOI 10.1016/j.envres.2010.01.006. [Google Scholar] [CrossRef]
3. Van-Thriel, C., Schäper, M., Kleinbeck, S., Kiesswetter, E., Blaszkewicz, M. et al. (2010). Sensory and pulmonary effects of acute exposure to sulfur dioxide (SO2). Toxicology Letters, 196(1), 42–50. DOI 10.1016/j.toxlet.2010.03.013. [Google Scholar] [CrossRef]
4. Weerasinghe, S. (2010). A missing values imputation method for time series data: An efficient method to investigate the health effects of sulphur dioxide levels. Environmetrics, 21, 162–172. [Google Scholar]
5. Mathieu, Y., Tzanis, L., Soulard, M. (2013). Adsorption of SOx by oxide materials: A review. Fuel Processing Technology, 114(3), 81–100. DOI 10.1016/j.fuproc.2013.03.019. [Google Scholar] [CrossRef]
6. Osaka, Y., Takahashi, F., Tsujiguchi, T., Kodama, A. (2014). Development of SO2 absorption materials having low temperature activity by base adducted complex method. Advanced Materials Research, 4, 960–961. [Google Scholar]
7. Choi, J. S., Partridge, W. P., Pihl, J. A., Daw, C. S. (2008). Sulfur and temperature effects on the spatial distribution of reactions inside a lean NOx trap and resulting changes in global performance. Catalysis Today, 136(1-2), 173–182. DOI 10.1016/j.cattod.2008.01.008. [Google Scholar] [CrossRef]
8. Zhang, M., Huang, B., Jiang, H. (2017). Research progress in the SO2, resistance of the catalysts for selective catalytic reduction of NOx. Chinese Journal of Chemical Engineering, 25(12), 1695–1705. DOI 10.1016/j.cjche.2017.03.030. [Google Scholar] [CrossRef]
9. Zhou, J., Zhou, S., Zhu, Y. (2017). Experiment and prediction studies of marine exhaust gas SO2 and particle removal based on NaOH solution with a U-Type scrubber. Industrial & Engineering Chemistry Research, 56(43), 12376–12384. DOI 10.1021/acs.iecr.7b02397. [Google Scholar] [CrossRef]
10. Ying, X., Jin, Z. L., Ying, C. (2011). Study on the properties of Pt/Ba/TiCeO catalyst for NOx storage and resistance to SO2. Journal of Fuel Chemistry and Technology, 39(4), 300–306. [Google Scholar]
11. Liu, X., Osaka, Y., Huang, H., Kodama, A., He, Z. et al. (2016). Development of high-performance SO2, trap materials in the low-temperature region for diesel exhaust emission control. Separation and Purification Technology, 162(2), 127–133. DOI 10.1016/j.seppur.2016.02.010. [Google Scholar] [CrossRef]
12. Gao, X., Liu, S., Zhang, Y., Luo, Z., Cen, K. (2011). Physicochemical properties of metal-doped activated carbons and relationship with their performance in the removal of SO2 and NO. Journal of Hazardous Materials, 188(1–3), 58–66. DOI 10.1016/j.jhazmat.2011.01.065. [Google Scholar] [CrossRef]
13. Liu, Y., Che, D., Xu, T. (2006). Effects of NaCl on the capture of SO by CaCO during coal combustion. Fuel, 85(4), 524–531. DOI 10.1016/j.fuel.2005.08.001. [Google Scholar] [CrossRef]
14. Kim, K., Yang, S., Lee, J. B. (2012). Analysis of K2CO3 /Al2O3, CO2, sorbent tested with coal-fired power plant flue gas: Effect of SOx. International Journal of Greenhouse Gas Control, 9(4), 347–354. DOI 10.1016/j.ijggc.2012.04.004. [Google Scholar] [CrossRef]
15. Osaka, Y., Kurahara, S., Kobayashi, N., Hasatani, M., Matsuyama, A. (2014). Study on SO2 absorption behavior of composite materials for DeSOx filter from diesel exhaust. Heat Transfer Engineering, 36(3), 325–332. DOI 10.1080/01457632.2014.916162. [Google Scholar] [CrossRef]
16. Pittalis, M., Azzena, U., Pisano, L. (2013). Active-alkali metal promoted reductive desulfurization of dibenzothiophene and its hindered analogues. Tetrahedron, 69(1), 207–211. DOI 10.1016/j.tet.2012.10.044. [Google Scholar] [CrossRef]
17. Wang, W., Fan, L., Wang, G., Li, Y. (2017). CO2 and SO2 sorption on the alkali metals doped CaO (100) surface: A DFT-D study. Applied Surface Science, 425, 972–977. DOI 10.1016/j.apsusc.2017.07.158. [Google Scholar] [CrossRef]
18. Liu, X., Osaka, Y., Huang, H., Li, J., He, Z. et al. (2017). Development of a compact MnO2 filter for removal of SO2 from diesel vehicle emissions. RSC Advances, 7(30), 18500–18507. DOI 10.1039/C7RA00096K. [Google Scholar] [CrossRef]
19. Osaka, Y., Kito, T., Kobayashi, N., Kurahara, S., Huang, H. et al. (2015). Removal of sulfur dioxide from diesel exhaust gases by using dry desulfurization MnO2 filter. Separation and Purification Technology, 150(26), 80–85. DOI 10.1016/j.seppur.2015.02.001. [Google Scholar] [CrossRef]
20. Mathieu, Y., Tzanis, L., Soulard, M., Patarin, J., Vierling, M. et al. (2013). Adsorption of SOx by oxide materials: A review. Fuel Processing Technology, 114, 81–100. DOI 10.1016/j.fuproc.2013.03.019. [Google Scholar] [CrossRef]
21. Chen, Y., He, J., Tian, H., Wang, D., Yang, Q. (2014). Enhanced formaldehyde oxidation on Pt/MnO2, catalysts modified with alkali metal salts. Journal of Colloid and Interface Science, 428, 1–7. DOI 10.1016/j.jcis.2014.04.028. [Google Scholar] [CrossRef]
22. Li, X., Chen, L., Osaka, Y., He, Z., Deng, L. et al. (2020). Preparation and desulfurization performance of various MnOx materials for ship exhaust emissions control. Separation and Purification Technology, 253(3), 117182. DOI 10.1016/j.seppur.2020.117182. [Google Scholar] [CrossRef]
23. Koralegedara, N. H., Pinto, P. X., Dionysiou, D. D., Al-Abed, S. R. (2019). Recent advances in flue gas desulfurization gypsum processes and applications-a review. Journal of Environmental Management, 251(5), 109572. DOI 10.1016/j.jenvman.2019.109572. [Google Scholar] [CrossRef]
24. Li, X., Osaka, Y., Chen, L., Deng, L., Huang, H. (2020). Preparation of various manganese dioxide composites and their desulfurization performance. Journal of the Energy Institute, 93(4), 1495–1502. DOI 10.1016/j.joei.2020.01.011. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |