

 | Journal of Renewable Materials |  |
DOI: 10.32604/jrm.2021.014909
ARTICLE
The Preparation and Properties of Starch Based Shape Memory Hydrogel
1School of Textile Materials and Engineering, Wuyi University, Jiangmen, 529000, China
2Key Laboratory of Polymer Ecomaterials, Changchun Institute of Applied Chemistry, Chinese Academy of Science, Changchun, 130022, China
3Wenzhou Institute, University of Chinese Academy of Sciences, Wenzhou, 325001, China
*Corresponding Authors: Kun Xu. Email: xukun@ciac.ac.cn; Ying Tan. Email: tanying@ciac.ac.cn
Received: 07 November 2020; Accepted: 31 December 2020
Abstract: In this work, the –catechol and –thiol modified starch was prepared by the esterification and amino condensation reaction, then a fully starch based hydrogel was prepared via the thiol-catechol Michael addition reaction. The starch hydrogel gained shape memory behaviors by coordinate with Fe3+ ions at alkaline condition. 1H-NMR had been used to character the structure of the starch derivatives and its character peaks. The hydrogel’s modulus had also been measured before and after coordinating with Fe3+ ions in linear area and the result showed that both the hydrogel’s storage modulus and loss modulus kept constant in linear area from 0.1 rad/s to 100 rad/s, which demonstrated a good network was formed inside the hydrogel. Furthermore, the shape memory behaviors had been tested by changing the pH value in solution. The result showed that the hydrogel can keep its temporary shape in high pH condition and recover to its original state after the shaped hydrogel immersed into acidic solution. This hydrogel might have great application prospects in the field of biomedical and engineering.
Keywords: Starch; thiol-catechol Michael addition reaction; shape memory
Shape-memory polymers (SMPs) which can retain two or three shapes are featured by the ability to return from a deformed state (temporary shape) to their original shape (permanent) induced by an external stimulus, such as pH, light, electric or magnetic field, solution and temperature [1–6]. As polymeric material, SMPs can be used in various industrial application (e.g., adaptive grip, automobile fenders and auto-choke element for engines) and medical applications (e.g., glaucoma shunts, intravenous cannula, self-adjusting orthodontic and intraocular lenses). Generally, SMPs contained thermoplastic polymeric materials, thermoset polymeric materials and hydrogels. Among them, the shape memory hydrogels (SMHs) had attracted more and more attention because of its unique properties such as flexibility, significant water content, responsive to specific molecules and small molecule diffusion [7–9]. For example, Wu’s group had reported a shape memory hydrogel by incorporating gold nanorods (AuNRs) into the glassy gel matrix of poly(methacrylic acid-co-methacrylamide) without compromising the excellent mechanical properties, where the near-infrared light could control this hydrogel’s shape memory behaviors [10]. Zhang’s group reported a hierarchically macro-micro-nanoporous structure’s hydrogel constructs which can readily recover to their original shapes, and sustain high cell viability, proliferation, spreading, and differentiation after compression and injection [11]. David Diaz Diaz’g group reported a new series shape memory membranes composed of trialkyne deribatives of glycerol ethoxylate and bisphenol A diazide (BA-diazide) or diazide-terminated PEG600 monomer via a Cu(I)-catalyzed photoclick reaction. These hydrogel exhibit a reversible shape-memory effect in response to temperature through a defined phase transition [12]. Recently, the hot topic for the SMHs was mainly in supra-molecular interactions, host-guest interaction, micellar copolymerizaion, metal ions and phosphate, imidazole-zinc ion coordination and reversible phenylboronic acid-diol ester bonds [13–20].
Injectable hydrogels can translate its condition immediately from sol to gel. It is preferable for biomedical applications because they can be deployed by injection instead of surgical implantation. Based on different non-covalent and covalent interaction, lots of injectable SMHs hydrogel has been synthesized containing dual- or triple- shape memory effect triggered by pH, light, thermal, sugar, magnetic or salt [21–24]. For example, Gao’s group reported a dual physically cross-linked shape memory hydrogel sensor via blending poly vinyl alcohol (PVA), cationic chitosan and grapheme oxide in water and glycerol, and this hydrogel can be used as a thermo-responsive shape memory sensor by virtue of orientation of PVA molecular chains under force and disorientation after heating [25]. Shi’s group reported an injectable composite hydrogel based on a modified gelatin matrix integrated with shape-memory polymer fibers, where the gelatin matrix creates a local microenvironment for cell assembly and acts as a lubricant during injection through a fine catheter and shape memory fiber scaffolds are able to recover to maintain the microstructures even after dramatic deformation from injection operation, providing the necessary support and guidance for motor neuron differentiation [26]. In addition, in consideration of the practical application of SMHs in biomedical field, the introducing of natural components in such system is of interesting due to their biodegradability, biocompatibility, and sustainable ability [27–33].
Starch is a natural polysaccharide that has been widely investigated for many years in the biomedical field. We have shown recently that starch-based injectable hydrogel with well mechanical strength can be fabricated through a Schiff-base reaction and thiol-ene click reaction [34,35]. Here, a full starch based injectable SMHs is synthesized via the Thiol-catechol Michael Addition reaction. Dopamine and thiol group is separately grafted into the starch macromolecular backbone. And the injectable hydrogel formed by simply mixing the starch-dopamine (St-DA) and starch-catechol (St-CS) solution. The obtained hydrogel presents shape memory behaviors by coordinate with Fe3+ ions at alkaline condition. Moreover, its mechanical properties and shape memory behaviors ware also investigated in detail and this shape memory hydrogel might find applications in the biomedical and engineering fields [36,37].
Corn starch purchased from Dacheng Corn Development Co., Ltd. (China). N-Hydroxysuccinimide (NHS), Succinic anhydride, Ethylenediaminetetraacetic acid (EDTA) and 1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC) were purchased from Aladdin Chemistry Co., Ltd., Shanghai, China, Dopamine hydrochloride and L-Cysteine hydrochloride was achieved from Alfa Aesar Co., Ltd., Shanghai, China, All the other chemicals were obtained from Beijing Chemical Reagent and used as received.
This step was manipulated as follow [35]: The starch (6.52 g) was put into 100 ml DMSO at 70°C. When the solution became clear, the temperature was adjusted to 60°C. Ten minutes later, imidazole (5.44 g), to be used as catalyst and provide a good alkaline environment, and succinic anhydride (2.40 g), to be used as esterifying agent, were added and kept for 2 h. After that, the solution was kept for 24 h at room temperature and then was poured into 500 ml isopropanol solution. The precipitate was collected after washed for several times and dried in at 45°C in vacuum oven.
The degree of –COOH’s substitution was about 0.67 (obtained by area integration from 1H-NMR spectra). 1H-NMR (D2O): δ = 4.84–5.70 ppm, 3.20–4.65 ppm (AGU, H; where AGU represents the anhydroglucose unit in starch and the H represents the hydrogen atom), δ = 2.45–2.67 ppm (succinic H).
St-CS’s synthesis was as follow [35]: St-COOH (2.00 g) was added at a concentration of 25 mg/ml in PBS (0.1 M) solutions at 8°C. Then NHS (1.42 g) and EDC (2.40 g) were added under stirring; after that, 2.18 g L-Cysteine hydrochloride was put (COOH/L-Cysteine hydrochloride approximately 1/2 (mol/mol)) and the solution’s pH kept 5.5 by dropping 1 M NaOH solution. After 12 hours, the crude product dialyzed at pH = 5.0 for three times against 0.01 M PBS solutions and then for other three times against distilled water. Finally, the polymers were collected after freeze-drying.
The degree of St-CS’s substitution was about 0.16 (obtained by area integration from 1H-NMR spectra). 1H-NMR (D2O): δ = 3.10–3.22 ppm, 4.85–5.70 ppm (AGU H); δ = 3.10–3.22 ppm (cysteine methylene H); δ = 3.00–2.3 ppm (succinic H).
St-DA was prepared as follow: St-COOH (2.00 g) was added at a concentration of 25 mg/ml in PBS (0.1 M) solutions at 8°C. Then NHS (1.42 g) and EDC (2.40 g) were added under stirring; after that, 2.18 g Dopamine hydrochloride was put (COOH/ Dopamine hydrochloride approximately 1/2 (mol/mol)) and the solution’s pH kept 5.5 by dropping 1 M NaOH solution. After 12 h, the crude product dialyzed at pH = 5.0 for three times against 0.01 M PBS solutions and then for other three times against distilled water. Finally, the polymers were collected after freeze-drying.
The degree of St-DA’s substitution was about 0.19 (obtained by area integration from 1H-NMR spectra). 1H-NMR (D2O): δ = 2.13–3.00 ppm (succinic H); δ = 5.68–4.94 ppm, 3.37–4.46 ppm (AGU H); δ = 5.68–7.55 ppm (benzene H).
2.5 Preparation of Starch Based Hydrogel
Typically, 20wt% St-DA Tris-HCL buffer solutions (10 mM, pH = 8.5) and 20 wt% St-CS Tris-HCL buffer solutions (10 mM, pH = 8.5) were mixing together through a co-extrusion needle to form hydrogel. Then we used inverted method to confirm the formation of the hydrogel and the gelation time.
The degree of substitution of St-COOH, St-CS and St-DA were determined by 1H-NMR where the deuterium oxide was used as solvent. The modulus of the hydrogel was tested by rheometer (Ta Rheometer Discovery HR3) at 25°C. Firstly, the strain was changing from 0.1% to 100% to select the linear viscoelastic region (oscillation model) at fixed frequencies of 1 Hz. Then, the hydrogel’s modulus was tested by changing the frequency from 100 rad/s to 0.01 rad/s at linear viscoelastic region.
2.7 Evaluation of Shape Memory Performance
The shape memory behaviors of the hydrogels were evaluated at room temperature. A straight columned of hydrogel (30 mm × 1 mm) was immersed into 0.05 M FeCl3 Tris-HCL solution (10 mM, pH ≈ 11.0) and bent it into a V-form shape for 3 h to fix the temporary “V” shape. The shape recovery of the hydrogel was finally investigated through the immersing of the hydrogel into Tris-HCL buffer solutions (10 mM, pH = 11), Tris-HCL buffer solutions (10 mM, pH = 2.0) and 0.1 M EDTA solutions, respectively.
2.8 Evaluation of Shape Memory Cycle
The quantitative shape memory cycle was determined according to the reported method. The shape fixity ratio (Rf) and shape recovery ratio (Rr) were defined by the following equation:
where
3.1 Synthesis of St-CS and St-DA
In our approach, the succinic anhydride was successfully grafted to the starch backbone using imidazole as catalyst agent, offering carboxyl groups to the starch’s backbone (Fig. 1). As shown in Fig. 2, 1H-NMR showed resonance characteristic of St-COOH signals at δ = 2.67–2.45 ppm (a, b) attributable to the methylene protons of succinic anhydride, indicating the succinic groups had grafted into the starch backbone successfully and the imidazole was an effective catalyst agent. The degree of substitution of succinic anhydride (DS, defined as the number of succinic anhydride per glucose unit) could be estimated by comparing the integrals area of signal at δ = 2.67–2.45 ppm and δ = 5.70–4.84 ppm, 4.65–3.20 ppm (AGU H) which was calculated to ≈0.67. It was noted that if the degree of substitution was beyond 0.67, the yield of the product would reduce significantly. This phenomenon can be explained as follows: The -COOH group can change starch’s solubility, especially when the –COOH’s degree of substitution beyond 0.67, the St-COOH’s solubility increased in isopropanol.
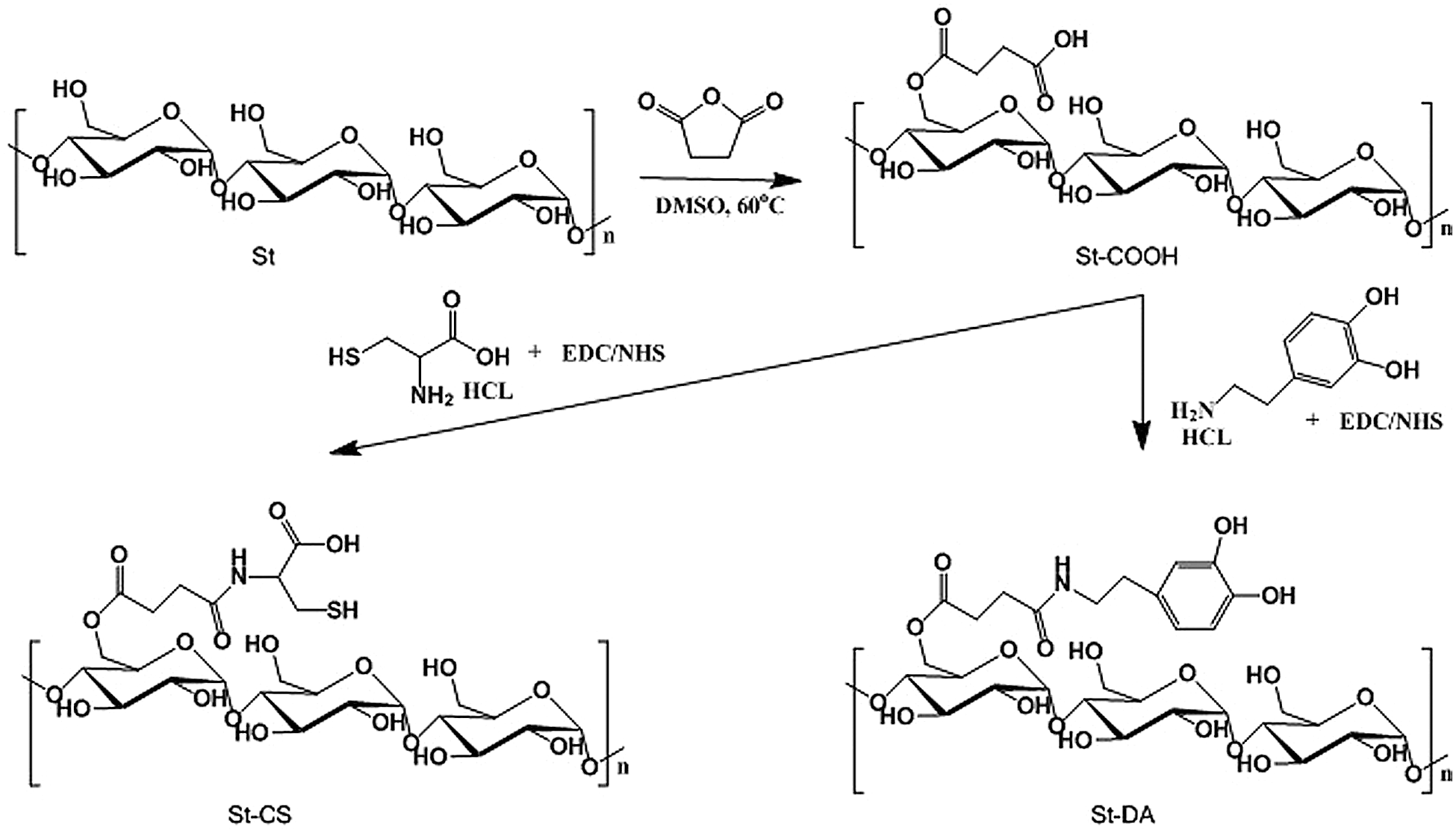
Figure 1: The process to prepare St-CS and St-DA
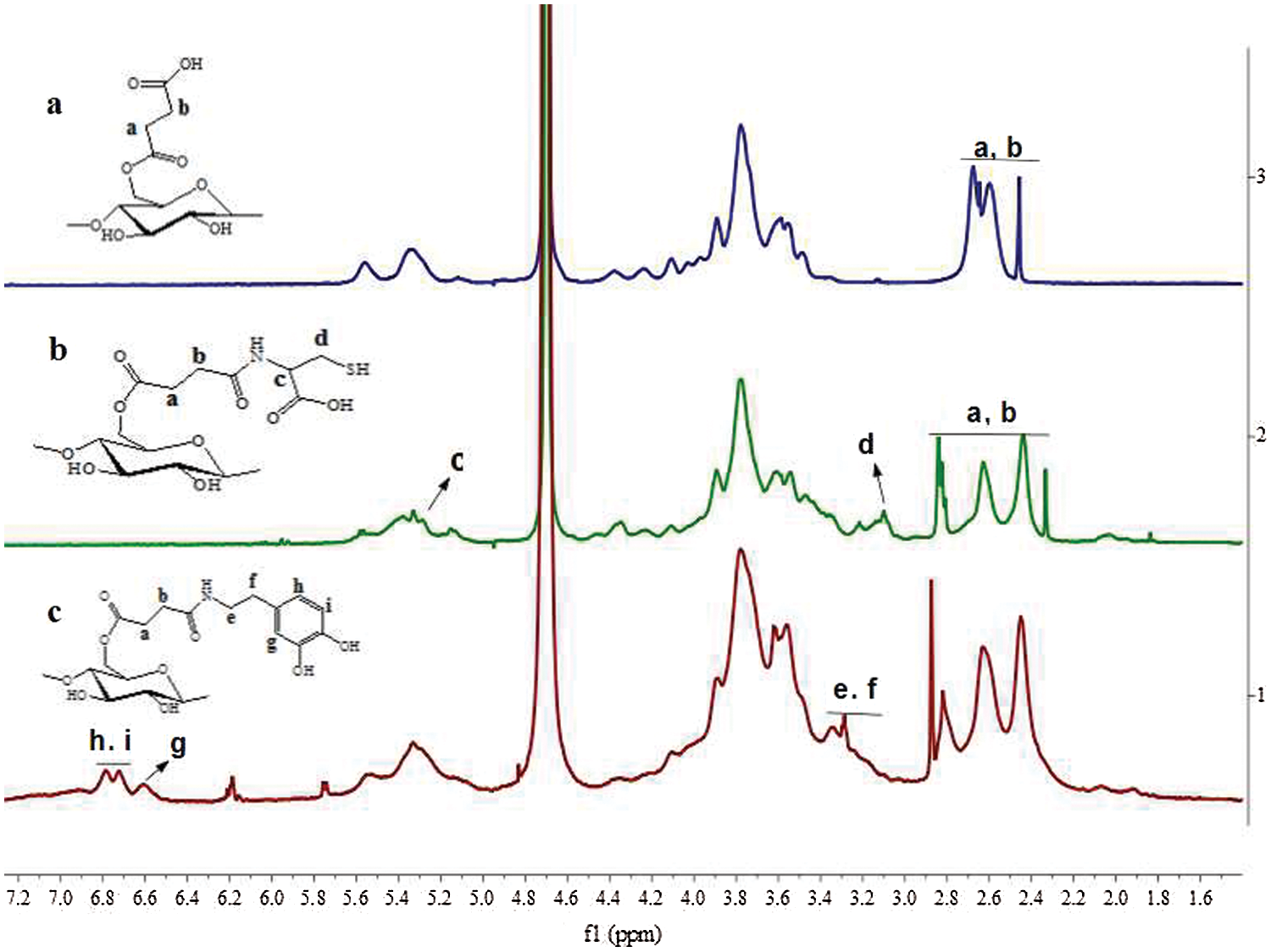
Figure 2: The 1H-NMR (D2O) of St-COOH (a), St-CS (b) and St-DA (c)
Furthermore, the carboxyl groups were reacted with L-cysteine hydrochloride to gain the thiol groups using EDC/NHS as coupling agent to form St-CS. The signals appeared at δ = 3.22–3.10 ppm (c, d) might be the character peaks for L-cysteine (Fig. 2). The degree of substitution of L-cysteine was about 0.16 calculated by comparing the integrals area of signal δ = 3.22–3.10 ppm and δ = 5.70–4.85 ppm, 4.65–3.20 ppm. On the other side, the dopamine hydrochloride was also reacted with carboxyl groups as mentioned above. As showed in Fig. 2, the signals, appeared at δ = 7.55–5.68 ppm, were attributed to character peaks for protons of the benzene groups. It demonstrated that the dopamine groups have been successfully introduced to the starch backbone. The substitution degree was also calculated by comparing the integrals area of δ = 7.55–5.68 ppm (benzene H) and δ = 5.68–4.94 ppm, 4.46–3.37 ppm (AGU H) which was calculated to ≈0.19.
3.2 Preparation of the Hydrogel and its Derivation
The hydrogel was formed by simple mix St-CS and St-DA in Tris-HCl solutions (10 mM, pH = 8.5) through a co-extrusion needle. As is reported, the catechol structure of the dopamine could become quinone structure at alkaline condition [38,39]. And the quinone groups could react with thiol or amine groups via Michael addition reaction. Herein, when the St-CS and St-DA were mix together at alkaline condition, the catechol groups in St-DA would react with thiol groups in St-CS via Michael addition reaction resulting in the formation of the hydrogel (Fig. 3). Here a series of hydrogel was prepared by adjusting the molar ratio between the St-CS and the St-DA, and these hydrogel’s gelation time had been measured by inverted method. The data was collected in Tab. 1 and the result showed that the gelation time become longer from 3 min to 20 min when the St-DA’s molar reduced from 0.2 mmol to 0.02 mmol while kept the St-CS’s molar as 0.20 mmol. As a comparison, the pure St-DA solution and pure St-CS solution had also been investigated to confirm if they could form hydrogel. The result demonstrated pure St-DA cannot form the hydrogel ever for a long time. On the other side, for St-CS, it can form hydrogel due to the air oxidation of free SH groups to S-S bridges among the thiols groups [40,41], but for a very long time, almost 48 h. Here, we took Gel-C1D1 as target sample to investigate the property of the hydrogel because of its short gelation time.
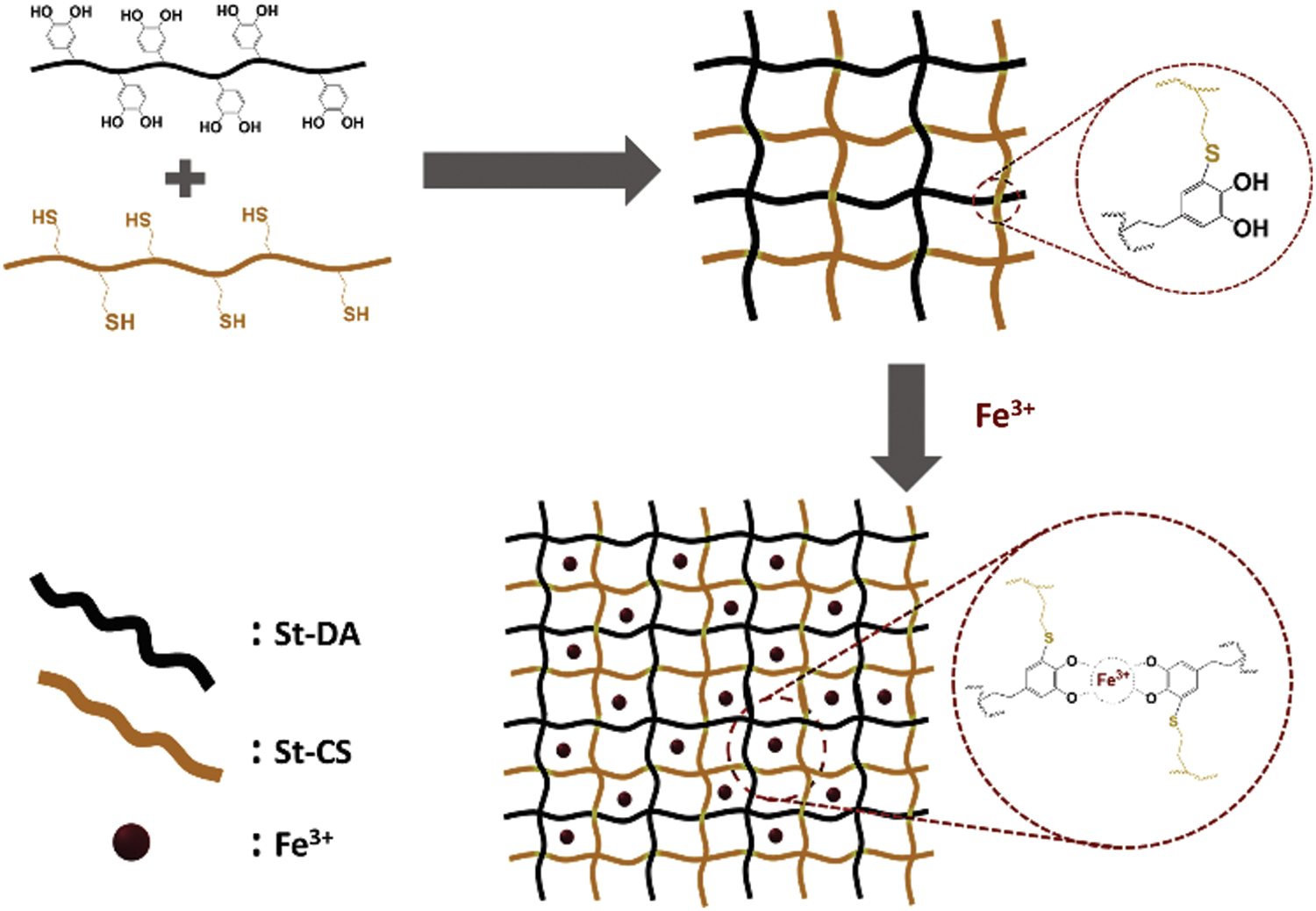
Figure 3: The process to prepare the hydrogel with or without Fe3+ ions
Table 1: The component of the hydrogel and its gelation time

In addition, the Gel-C1D1 was immersed into 0.05 M FeCl3 Tris-HCL solutions (10 mM, pH ≈ 11) to form the second network between Fe3+ ions and catechol groups via metal-coordination interaction as is shown in Fig. 3. We called it as Gel-C1D1&Fe. It is worth noting that the first network was formed via covalent coupling in alkaline condition. The pH value in 0.05 M FeCl3 Tris-HCL solutions (10 mM) was about 11, in this case, the tris-complex was the major component in the second network. Many groups had reported that Fe3+ ions could coordinate with catechol to form mono-complex, bis-complex and tris-complex at different pH condition [42]. The relationship among these complexes was significantly depending on the pH condition. Generally speaking, the mono-, bis-, and tris-complex were found to dominate in solutions in pH ranges 1–5, 5–10.8 and 10.8–12, respectively [43]. So based on that description, it could be speculated that the major component in the second network was tris-complex.
The hydrogel’s mechanical property was investigated by rheometer. As shown in Fig. 4a, when the frequency was fixed at 1 Hz, the Gel-C1D1 can keep its network structure before the strain got 45%. It demonstrated that the Gel-C1D1 can bear about 45% strain without yielding. In addition, if Gel-C1D1 reacted with Fe3+ via the chelate effect to form another network, the hydrogel will become rigid and its strength will increase accordingly. It can be seen from Fig. 4b, both the storage and loss modulus of the hydrogel, Gel-C1D1&Fe, became larger compared with the original state. The Gel-C1D1&Fe’s storage modulus has increased from 2000 Pa to 9000 Pa, which is an increase by 4.5 times. On the other side, the Gel-C1D1&Fe’s maximum strain is getting smaller, about 12%. So, it can be concluded from Fig. 4a that the Gel-C1D1 could react with Fe3+ via metal-catechol interaction to form the second network to enhance the hydrogel’s mechanical property. Furthermore, both hydrogel’s modulus had been tested in linear viscoelastic region (in oscillation). As shown in Fig. 4b, both hydrogel’s modulus preformed almost constant in the whole frequency area. It further confirmed that a uniformed network had formed inside the hydrogel, even treated with Fe3+ irons.
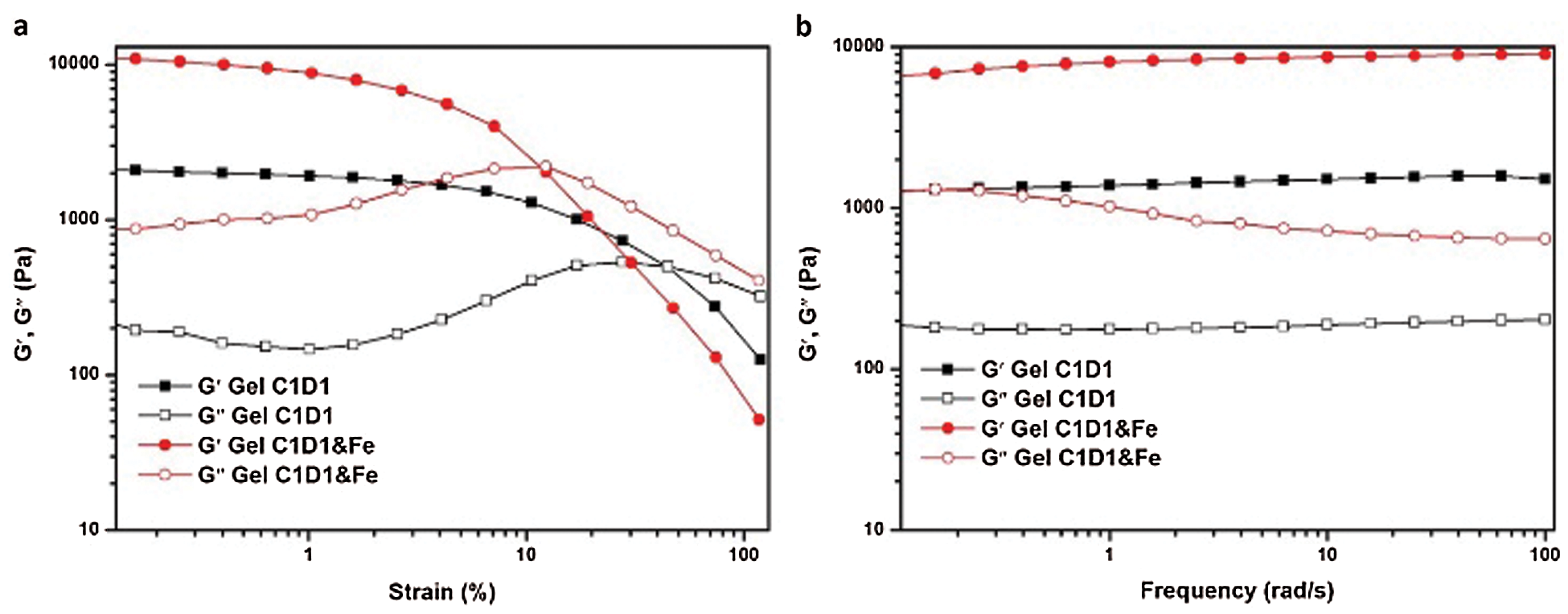
Figure 4: The Gel-C1D1 and Gel-C1D1&Fe’s stress-strain and stress-frequency curve
In our system, the covalent crosslink between thiol and quinone formed the permanent network meanwhile the complexation between Fe3+ and catechol groups formed the temporary network. Because of the reversibility of the Fe3+-catechol complex, the hydrogel could obtain pH responsive ability. Here, we immersed the Gel-C1D1 into 0.05 M FeCl3 Tris-HCL solutions (10 mM, pH ≈ 11) with a fixed “V” shape to get the temporary shape base on the Fe3+-catechol tris-complex interaction. After the shape was fixed, the hydrogel, Gel-C1D1&Fe, was washed by distilled water several times and then put into pH = 2.0 Tris-HCL buffer solution (10 mM) and pH = 11 Tris-HCL buffer solution (10 mM) respectively, to observe the shape recover behaviors. As seen in Fig. 5a, the Gel-C1D1&Fe’s recover behaviors with “V” shape was performed to study the shape recover behaviors.
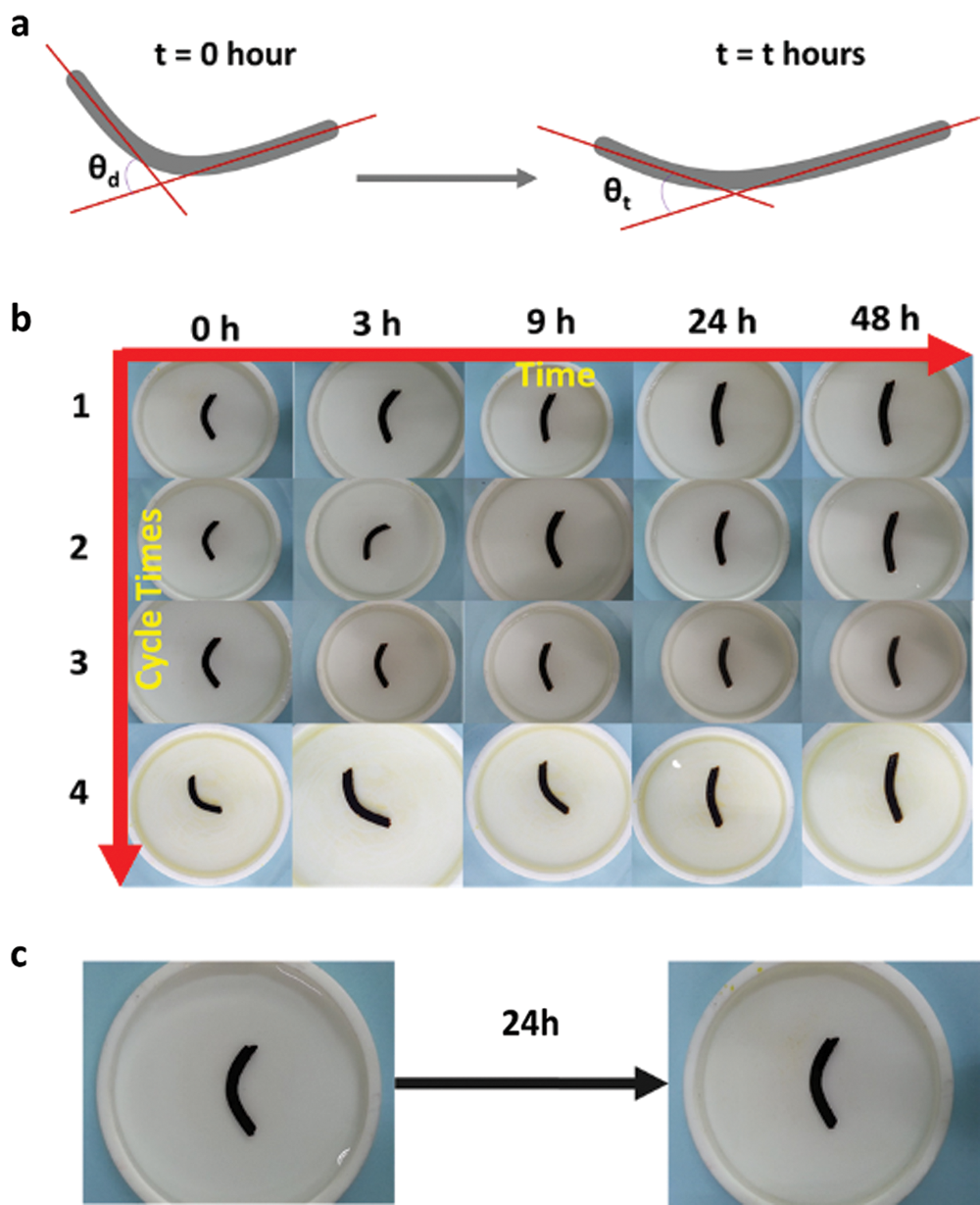
Figure 5: a) The angle change at different time; b) The Gel-C1D1&Fe’s cycle shape recovery behaviours at different time in pH = 2.0 Tris-HCL buffer solution; c) The Gel-C1D1&Fe’s shape recovery behaviours after 24 h in pH = 11 Tris-HCL buffer solution
Then we used protractor to measure the angle change at different time interval and calculated the shape fixing efficiency. The data was listed in Tab. 2. As seen in Fig. 5b, the Gel-C1D1&Fe’s shape was changing from “V” shape to “⎯” shape slowly. After 24 h later, the shape recovery got equilibrium and then even if the time was extended to 48 hours, there was no obvious changing of Gel-C1D1&Fe’s shape. The Gel-C1D1&Fe’s shape recovery behaviors had been totally tested four times to calculate the shape fixing efficiency and shape recover ratio. As seen in Tab. 2, there were almost no change for the result of the Gel-C1D1&Fe’s shape recover ratio, that confirmed the hydrogel own the shape recover ability in acidic condition. Further, the shape recover ability in alkaline condition was also tested in Tris-HCL buffer solution (10 mM, pH = 11) for comparison. The result showed that there was almost no changing for the Gel-C1D1&Fe’s shape in Fig. 4c. So, the Gel-C1D1&Fe could not get shape recover ability in alkaline condition.
Table 2: The Gel-C1D1&Fe’s shape fixing efficiency and shape recovery ratio

The mechanism of the shape memory behaviors of the Gel-C1D1&Fe was shown in Fig. 6, exhibiting that the tris-complex account for the majority of the hydrogel when the pH value was beyond 10.8, the bis-complex explained for the majority of the hydrogel when the pH value was between 5.0 and 10.8, and the mono- complex accounted for the majority of the hydrogel when the pH value was ranging from 1 to 5. So, in this case, when the hydrogel was fixed at “V” shape at high pH condition (pH = 11), tris- complex were the driving force to form temporary shape. When the “V” shape hydrogel was immersed into alkaline condition (pH = 11), there was no shape changing because of no bonds had been broken in that condition. On the other side, if the hydrogel was immersed into acidic pH condition (e.g., pH = 2.0), under this circumstances, the bond interaction between the Fe3+ and catechol groups would change from tris-complex to mono-complex, resulting the shape changed from “V” shape to “⎯” shape.
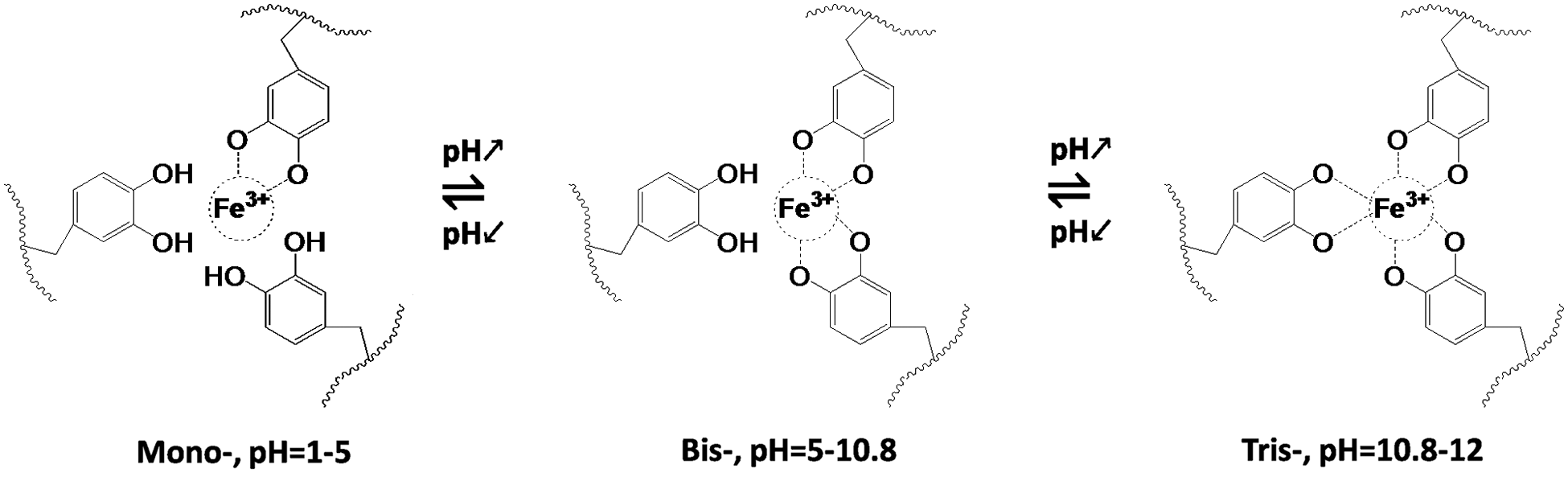
Figure 6: The relationship between Fe3+ and catechol at different pH conditions
Here, it should be noted that the Gel-C1D1&Fe’s shape could not recover completely due to the existing of mono-complex. To eliminate the influence of the mono- complex and get fully shape recover ratio, the EDTA was introduced into the system at the last stage. Because the EDTA can competitive with catechol groups to form chelation with Fe3+ ions, the Kf = 1.7 × 1024 ([Fe(EDTA)]-). As showed in Fig. 7, the hydrogel could recover to its original shape within 2 h with the help of 0.1 M EDTA solution.

Figure 7: The shape recovery behaviors of mono-complex C1D1&Fe hydrogel in 0.1 M EDTA solution at different time
A fully starch base shape memory hydrogel was prepared via the thiol-catechol Michael addition reaction. The hydrogel can keep its temporary shape at alkaline condition for a long time, and change its shape to original state if the pH switched to acidic condition. This shape recover behaviours could repeat several times while there was almost no changing for the shape recover ratio. The starch hydrogel could not recover completely because of the mono-complex interaction between the Fe3+ and catechol in acidic condition. But the EDTA can make the hydrogel’s shape recover completely due to the strong interaction between the Fe3+ and EDTA. It demonstrated that the interaction between Fe3+-catechol was a weak bond compared with the interaction between Fe3+and EDTA. This method might provide a new candidate for application in biomedical and engineering fields.
Funding Statement: This work was supported by the National Natural Science Foundation of China (Grant No. 51673191) and Wuyi University’s special fund (Grant No. 5041700128).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Guo, W., Lu, C. H., Orbach, R., Wang, F., Qi, X. J. et al. (2015). pH-stimulated DNA hydrogels exhibiting shape-memory properties. Advanced Materials, 27(1), 73–78. DOI 10.1002/adma.201403702. [Google Scholar] [CrossRef]
2. Lendlein, A., Jiang, H., Jünger, O., Langer, R. (2005). Light-induced shape-memory polymers. Nature, 434(7035), 879–882. DOI 10.1038/nature03496. [Google Scholar] [CrossRef]
3. Deng, J., Chang, Z., Zhao, T., Ding, X., Sun, J. et al. (2016). Electric field induced reversible phase transition in Li doped phosphorene: Shape memory effect and superelasticity. Journal of the American Chemistry Society, 138(14), 4772–4778. DOI 10.1021/jacs.5b13274. [Google Scholar] [CrossRef]
4. Liu, Y., Xu, K., Chang, Q., Darabi, M. A., Lin, B. et al. (2016). Highly flexible and resilient elastin hybrid cryogels with shape memory, injectability, conductivity, and magnetic responsive properties. Advanced Materials, 28(35), 7758–7767. DOI 10.1002/adma.201601066. [Google Scholar] [CrossRef]
5. Cui, Y., Tan, M., Zhu, A., Guo, M. (2014). Mechanically strong and stretchable PEG-based supramolecular hydrogel with water-responsive shape-memory property. Journal of Materials Chemistry B, 2(20), 2978–2982. DOI 10.1039/C4TB00315B. [Google Scholar] [CrossRef]
6. Jeon, S., Hauser, A., Hayward, R. (2017). Shape-morphing materials from stimuli-responsive hydrogel hybrids. Accounts of Chemical Research, 2(50), 161–169. DOI 10.1021/acs.accounts.6b00570. [Google Scholar] [CrossRef]
7. Zhang, X., Cai, J., Liu, W., Qiu, X. (2019). Synthesis of strong and highly stretchable, electrically conductive hydrogel with multiple stimuli responsive shape memory behavior. Polymer, 188, 122–147. DOI 10.1016/j.polymer.2019.122147. [Google Scholar] [CrossRef]
8. Koga, T., Tomimori, K., Higashi, N. (2020). Transparent, high-strength, and shape memory hydrogels from thermo-responsive amino acid-derived vinyl polymer networks. Macromolecular Rapid Communications, 41(7), 1900650. DOI 10.1002/marc.201900650. [Google Scholar] [CrossRef]
9. Cera, L., Gonzalez, G. M., Liu, Q., Choi, S., Parker, K. K. (2020). A bioinspired and hierarchically structured shape-memory material. Nature Materials, 1–8, DOI 10.1038/s41563–020-0789-2. [Google Scholar] [CrossRef]
10. Dai, C. F., Du, C., Xue, Y., Zhang, X. N., Zheng, Q. (2019). Photo-directed morphing structures of nanocomposite shape memory hydrogel with high stiffness and toughness. ACS Applied Materials & Interfaces, 11(46), 43631–43640. DOI 10.1021/acsami.9b16894. [Google Scholar] [CrossRef]
11. Ying, G., Jiang, N., Parra-Cantu, C., Tang, G., Zhang, J. et al. (2020). Bioprinted injectable hierarchically porous gelatin methacryloyl hydrogel constructs with shape-memory properties. Advanced Functional Materials, 30(46), 2003740. DOI 10.1002/adfm.202003740. [Google Scholar] [CrossRef]
12. Maiti, B., Abramov, A., Franco, L., Puiggalí, J., Enshaei, H. et al (2020). Thermoresponsive shape-memory hydrogel actuators made by phototriggered click chemistry. Advanced Functional Materials, 30(24), 2001683. DOI 10.1002/adfm.202001683. [Google Scholar] [CrossRef]
13. Meng, H., Xiao, P., Gu, J., Wen, X., Xu, J. et al. (2014). Self-healable macro-/microscopic shape memory hydrogels based on supramolecular interactions. Chemical Communications, 50(82), 12277–12280. DOI 10.1039/C4CC04760E. [Google Scholar] [CrossRef]
14. Le, X., Lu, W., Zheng, J., Tong, D., Zhao, N. et al. (2016). Stretchable supramolecular hydrogels with triple shape memory effect. Chemical Science, 7(11), 6715–6720. DOI 10.1039/C6SC02354A. [Google Scholar] [CrossRef]
15. Miyamae, K., Nakahata, M., Takashima, Y., Harada, A. (2015). Self-healing, expansion-contraction, and shape-memory properties of a preorganized supramolecular hydrogel through host-guest interactions. Angewandte Chemie International Edition, 54(31), 8984–8987. DOI 10.1002/anie.201502957. [Google Scholar] [CrossRef]
16. Bilici, C., Okay, O. (2013). Shape memory hydrogels via micellar copolymerization of acrylic acid and n-Octadecyl acrylate in aqueous media. Macromolecules, 46(8), 3125–3131. DOI 10.1021/ma400494n. [Google Scholar] [CrossRef]
17. Zhao, T., Tan, M., Cui, Y., Deng, C., Huang, H. et al. (2014). Reactive macromolecular micelle crosslinked highly elastic hydrogel with water-triggered shape-memory behaviour. Polymer Chemistry, 5(17), 4965–4973. DOI 10.1039/C4PY00554F. [Google Scholar] [CrossRef]
18. Yasin, A., Li, H., Lu, Z., Rehman, S., Siddiq, M. et al. (2014). A shape memory hydrogel induced by the interactions between metal ions and phosphate. Soft Matter, 10(7), 972–977. DOI 10.1039/C3SM52666F. [Google Scholar] [CrossRef]
19. Nan, W., Wang, W., Gao, H., Liu, W. (2013). Fabrication of a shape memory hydrogel based on imidazole–zinc ion coordination for potential cell-encapsulating tubular scaffold application. Soft Matter, 9(1), 132–137. DOI 10.1039/C2SM26918J. [Google Scholar] [CrossRef]
20. Meng, H., Zheng, J., Wen, X., Cai, Z., Zhang, J. et al. (2015). pH- and sugar-induced shape memory hydrogel based on reversible phenylboronic Acid-Diol ester bonds. Macromolecular Rapid Communications, 36(6), 533–537. DOI 10.1002/marc.201400648. [Google Scholar] [CrossRef]
21. Han, X. J., Dong, Z. Q., Fan, M. M., Liu, Y., Wang, Y. F. et al. (2012). pH-induced shape-memory polymers. Macromolecular Rapid Communications, 33(12), 1055–1060. DOI 10.1002/marc.201200153. [Google Scholar] [CrossRef]
22. Feng, W., Zhou, W., Zhang, S., Fan, Y., Yasin, A. et al. (2015). UV-controlled shape memory hydrogels triggered by photoacid generator. RSC Advances, 5(100), 81784–81789. DOI 10.1039/C5RA14421C. [Google Scholar] [CrossRef]
23. Hao, J., Weiss, R. (2013). Mechanically tough, thermally activated shape memory hydrogels. ACS Macro Letters, 2(1), 86–89. DOI 10.1021/mz3006389. [Google Scholar] [CrossRef]
24. Gulyuz, U., Okay, O. (2014). Self-healing poly(acrylic acid) hydrogels with shape memory behavior of high mechanical strength. Macromolecules, 47(19), 6889–6899. DOI 10.1021/ma5015116. [Google Scholar] [CrossRef]
25. Yang, T. Y., Wang, M., Jia, F., Ren, X. Y., Gao, G. H. (2020). Thermo-responsive shape memory sensors based on tough, remolding and anti-freezing hydrogels. Journal of Materials Chemistry C, 8(7), 2326–2335. DOI 10.1039/C9TC05804D. [Google Scholar] [CrossRef]
26. Wang, C., Yue, H. B. (2018). Injectable nanoreinforced shape-memory hydrogel system for regenerating spinal cord tissue from traumatic injury. ACS Applied Materials & Interfaces, 10(35), 29299–29307. DOI 10.1021/acsami.8b08929. [Google Scholar] [CrossRef]
27. Bencherif, S. A., Sands, R. W., Bhatta, D., Arany, P., Verbeke, C. S. et al. (2012). Injectable preformed scaffolds with shape-memory properties. Proceedings of the National Academy of Sciences of the United States of America, 109(48), 19590–19595. DOI 10.1073/pnas.1211516109. [Google Scholar] [CrossRef]
28. Izawa, H., Kaneko, Y., Kadokawa, J. I. (2009). Unique gel of xanthan gum with ionic liquid and its conversion into high performance hydrogel. Journal of Materials Chemistry, 19(38), 6969–6972. DOI 10.1039/b916864h. [Google Scholar] [CrossRef]
29. Yu, F., Cao, X., Du, J., Wang, G., Chen, X. (2015). Multifunctional hydrogel with good structure integrity, self-healing, and tissue-adhesive property formed by combining Diels–Alder click reaction and acylhydrazone bond. ACS Applied Materials & Interfaces, 7(43), 24023–24031. DOI 10.1021/acsami.5b06896. [Google Scholar] [CrossRef]
30. Ren, H. W., Wang, L., Bao, H., Xia, Y. Y., Xu, D. et al. (2020). Improving the antibacterial property of chitosan hydrogel wound dressing with licorice polysaccharide. Journal of Renewable Materials, 8(10), 1343–1355. DOI 10.32604/jrm.2020.010903. [Google Scholar] [CrossRef]
31. Thakur, S., Sharma, B., Verma, A., Chaudhary, J., Tamulevicius, S. (2018). Recent progress in sodium alginate based sustainable hydrogels for environmental applications. Journal of Cleaner Production, 198, 143–159. DOI 10.1016/j.jclepro.2018.06.259. [Google Scholar] [CrossRef]
32. Burhan, A., Suleyman, K., Ahmet, U., Canbolat, G., Vijay, K. T. (2020). Chemistry, structures, and advanced applications of nanocomposites from biorenewable resources. Chemical Reviews, 120(17), 9304–9362. DOI 10.1021/acs.chemrev.9b00553. [Google Scholar] [CrossRef]
33. Bhawna, S., Sourbh, T., Gcina, M., Prateek, Raju, K. G. et al. (2020). Titania modified gum tragacanth based hydrogel nanocomposite for water remediation. Journal of Environmental Chemical Engineering, 9(1), 104608. DOI 10.1016/j.jece.2020.104608. [Google Scholar] [CrossRef]
34. Li, Y., Liu, C., Tan, Y., Xu, K., Lu, C. et al. (2014). In situ hydrogel constructed by starch-based nanoparticles via a Schiff base reaction. Carbohydrate Polymers, 110(6), 87–94. DOI 10.1016/j.carbpol.2014.03.058. [Google Scholar] [CrossRef]
35. Li, Y., Tan, Y., Xu, K., Lu, C., Liang, X. et al. (2015). In situ crosslinkable hydrogels formed from modified starch and O-carboxymethyl chitosan. RSC Advances, 5(38), 30303–30309. DOI 10.1039/C4RA14984J. [Google Scholar] [CrossRef]
36. Korde, J. M., Kandasubramanian, B. (2020). Naturally biomimicked smart shape memory hydrogels for biomedical functions. Chemical Engineering Journal, 379, 122430. DOI 10.1016/j.cej.2019.122430. [Google Scholar] [CrossRef]
37. Delaey, J., Dubruel, P., van, S. (2020). Shape-memory polymers for biomedical applications. Advanced Functional Materials., 30(44), 1909047. DOI 10.1002/adfm.201909047. [Google Scholar] [CrossRef]
38. Cencer, M., Liu, Y., Winter, A., Murley, M., Meng, H. et al. (2014). Effect of pH on the rate of curing and bioadhesive properties of dopamine functionalized poly(ethylene glycol) hydrogels. Biomacromolecules, 15(8), 2861–2869. DOI 10.1021/bm500701u. [Google Scholar] [CrossRef]
39. Lee, Y. K., Lee, S. Y. (2014). A colorimetric alginate-catechol hydrogel suitable as a spreadable pH indicator. Dyes and Pigments, 108(1), 1–6. DOI 10.1016/j.dyepig.2014.04.014. [Google Scholar] [CrossRef]
40. Palumbo, F. S., Pitarresi, G., Albanese, A., Calascibetta, F., Giammona, G. (2010). Self-assembling and auto-crosslinkable hyaluronic acid hydrogels with a fibrillar structure. Acta Biomaterialia, 6(1), 195–204. DOI 10.1016/j.actbio.2009.06.014. [Google Scholar] [CrossRef]
41. Shu, X. Z., Liu, Y., Luo, Y., Roberts, M. C., Prestwich, G. D. (2002). Disulfide cross-linked hyaluronan hydrogels. Biomacromolecules, 3(6), 1304–1311. DOI 10.1021/bm025603c. [Google Scholar] [CrossRef]
42. Holten-Andersen, N., Harrington, M. J., Birkedal, H., Lee, B. P., Messersmith, P. B. et al. (2011). pH-induced metal-ligand cross-links inspired by mussel yield self-healing polymer networks with near-covalent elastic moduli. Proceedings of the National Academy of Sciences of the United States of America, 108(7), 2651–2655. DOI 10.1073/pnas.1015862108. [Google Scholar] [CrossRef]
43. Krogsgaard, M., Behrens, M. A., Pedersen, J. S., Birkedal, H. (2013). Self-healing Mussel-inspired multi-pH-responsive hydrogels. Biomacromolecules, 14(2), 297–301. DOI 10.1021/bm301844u. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |