

 | Journal of Renewable Materials |  |
DOI: 10.32604/jrm.2021.015663
ARTICLE
Covalent and Ionic Bonding between Tannin and Collagen in Leather Making and Shrinking: A MALDI-ToF Study
1LERMAB-ENSTIB, University of Lorraine, Epinal, 88000, France
*Corresponding Author: Antonio Pizzi. Email: antonio.pizzi@univ-lorraine.fr
Received: 03 January 2021; Accepted: 18 January 2021
Abstract: Collagen powder hydrolysates were reacted with a solution of commercial mimosa bark tannin extract. The mixture was prepared at ambient temperature and prepared at 80°C to determine what reactions, if any, did occur between the collagen protein through its amino acids and the polyphenolic condensed tannin. The reaction products obtained were analyzed by matrix assisted laser desorption ionization time-of-flight (MALDI ToF) mass spectrometry. Reactions between the two materials did appear to occur, with the formation of a relatively small proportion of covalent and ionic linkages at ambient temperature but a considerable proportion of covalent linkages tannin-protein amino acids and the disappearance of ionic bonds. The linkages between the two materials appeared to be by amination of the phenolic –OHs of the tannin by the amino groups of the non-skeletal side chains of arginine, and by esterification by the –COOH groups of glutamic and aspartic acid of the aliphatic alcohol-OH on the C3 site of the flavonoid units heterocycle of the tannin. The proportion of covalent linkages increases markedly and predominate with increasing temperatures. This tightening of the tannin-protein covalent network formed may be an additional contributing factor both to leather wear resistance and performance as well to leather shrinking when this is subjected to excessive temperatures.
Keywords: Leather; collagen; vegetal tannins; tanning; tannin-protein reactions; covalent bonds; leather cross-linking; leather shrinkage; MALDI
Vegetable tannins, both hydrolysable and condensed ones, are polyphenolic materials that have been used for a long time for the manufacture of heavy-duty leather. The structures of hydrolysable and condensed tannins are well studied too [1–9]. The interaction between any of the two types of vegetable tannins and the collagen of hides to prepare leather has always been known to depend on the strong complexation of the tannin with the collagen protein by the innumerable secondary forces acting between them. While this is age-old acquired and recognized knowledge, the effect of temperature on both vegetable tanning and on excessive temperature-induced shrinking of tanned leather has been ascribed to many existing effects, such as moisture loss and leather drying and others [10–15]. Such effects exist, are real and have been studied [10–15]. However, the possibility of the influence on these effects of the formation of temperature-induced covalent bonds between tannin and collagen has never really been considered.
Recently, research work on the interaction of hydrolysable and condensed tannins with soy protein in the field of wood adhesives [16–18] has brought such a hypothesis to the fore also for other proteins interaction with tannins. This work has shown that at ambient temperature tannins do appear to form both ionic and covalent bonds with soy protein [16–18]. While the use of the analyzed sample showed bonds formed at room temperature the subsequent use of the resins so formed as thermoset wood adhesives for wood panels would appear to indicate that such bonds might persist or even form in greater proportion even at higher temperatures.
The presence in leather of the combination of tannin with collagen, this being a proteic material, renders probable that also in this case covalent and ionic bonds between the two substances do occur at ambient and higher temperature. These might play a role in leather stability as well as in its shrinking under excessive temperature conditions. The latter might be due to the contribution of either the progressive increase as a function of temperature of covalent bonds protein/tannin or to the tightening due to the tannin structure rearrangement when this is covalently bonded to collagen..
The work presented here it is only aimed at, and limited to determine if covalently bonded structures occurs between collagen and tannin in view to render possible to understand in future their contribution to leather shrinking and stability as a function of the temperature.
Commercial Mimosa tannin extract (Acacia mearnsii, De Wild) was supplied by Silva Chimica (St. Michele Mondovi, Italy). Pure collagen powder hydrolysates were obtained from Myprotein (Chester, Cheshire, UK).
The pure collagen powder hydrolysates were added to water and mixed with a mechanical stirrer. The tannin solution (15 wt% based on collagen dry weight) was then added to the collagen hydrolysates slurry and stirred for 60 min. The tannin solution in water was prepared at 45 wt% concentration. The same procedure was followed for the preparation at 80°C, but the mixture was heated at 80°C for 60 min rather than at ambient temperature. The pH of the solutions were adjusted to 7 before analysis.
Samples for matrix assisted laser desorption ionization time-of-flight (MALDI-ToF) analysis were prepared by first dissolving 7.5 mg of the samples in 1 mL of a 50:50 v/v acetone/water solution. Then 10 mg of this solution was added to 10 µL of a 2,5-dihydroxy benzoic acid (DHB) matrix. The locations dedicated to the samples on the analysis sample holder were first covered with 2 µL of a NaCl solution 0.1M in 2:1 v/v methanol/water, and pre-dried. Then 1.5 µL of the sample solution was placed on its dedicated location and the plaque was dried again. Red phosphorous was used to standardize the MALDI equipment. MALDI-ToF spectra were obtained using an Axima-Performance mass spectrometer from Shimadzu Biotech (Kratos Analytical, Shimadzu Europe, Ltd., Manchester, UK) using a linear polarity-positive tuning mode. The measurements were carried out making 1000 profiles. The spectra were precise at +1 Da.
The experiment conducted were to examine by matrix assisted laser desorption ionization time of flight (MALDI ToF) mass spectrometry the products obtained by reaction of a condensed tannin with collagen at ambient temperature and at 80°C to determine if covalently co-reacted structures occur, in which cases and to what extent in the two cases. The tannin used is mainly composed of four different flavonoid units linked either C4-C6 or C4-C8, namely robinetinidin, fisetinidin, catechin and gallocatechin, with the first two being strongly predominant [19–22] their respective percentages in mimosa tannin being approximately 68%, 22%, 5%, 5% by weight [19–21]. The M.W. of robinetinidin and catechin is the same. Both flavonoids are present in mimosa tannin but robinetinidin is greatly predominant. Any structure in the Tab. 1 and Tab. B1 in which one of the two is mentioned could also be the other. Due to its predominance robinetinidin should be the favourite one for this tannin.

Table 1: Reaction products of condensed tannins with collagen aminoacids

The MALDI ToF spectrum in Fig. 1 of the reaction at ambient temperature between collagen and mimosa condensed tannin showed only a limited amount of covalent co-reaction between tannin and single amino acids. These are shown in Tab. 1. There are three considerations to keep in mind from the results in Tab. 1.

Figure 1: MALDI ToF spectrum of ambient temperature co-reacted collagen protein and mimosa bark tannin
There are flavonoid monomers linked to amino acids by covalent bonds and linked by ionic bonds. However, in the case of the covalent bonds that would count on linking the protein structure to a flavonoid tannin only the ones between amino acids possessing a side chain with a –NH2 [23–26] or a –COOH group will be capable to link to the body of the protein. Thus, linkages with amino acids such as arginine, glutamic acid and aspartic acid are the only ones to contribute in linking and cross-linking the body of the protein with the tannin. Thus, only the species at 390 Da, 408 Da, 412 Da, 426 Da, 440 Da, 442 Da, 454 Da, 458 Da, 469 Da and 484 Da are the only one that counts in this respect. The other amino acids are linked to flavonoid units either through their amino group or carboxylic acid group that will participate to form the polypeptide backbone of the protein. Thus, while they can react when alone, they will not contribute once they are inserted within the protein chain. Among the linked compounds listed above as valid for linking to the whole protein, only the couplings at 408 Da, 440 Da (mixed ionic/covalent) and 454 Da are ionic [16–18], with the 440 Da one belonging both to a covalently bound compound and an ionic bound one.

Valid covalent linkages between the protein and tannin flavonoid units are then the species at 390 Da, 412 Da 426 Da, 442 Da, 458 Da and 484 Da in Tab. 1. They can be linked through an ester bond (390 Da) or through the substitution of an –NH- for the OH group of a flavonoid unit (469 Da). In the first case the favorite reaction point is the aliphatic alcoholic –OH at C3 of the flavonoid, while in the second case the favorite linkage is at any one of the acid phenolic –OH groups.

It is then evident from Tab. 1 and Fig. 1 that at ambient temperature that valid covalent and ionic linkages between tannin units and the body of the protein do exist but their number appears to be relatively low. These strong linkages probably also contribute to the good performance and durability of vegetable tanned leather.
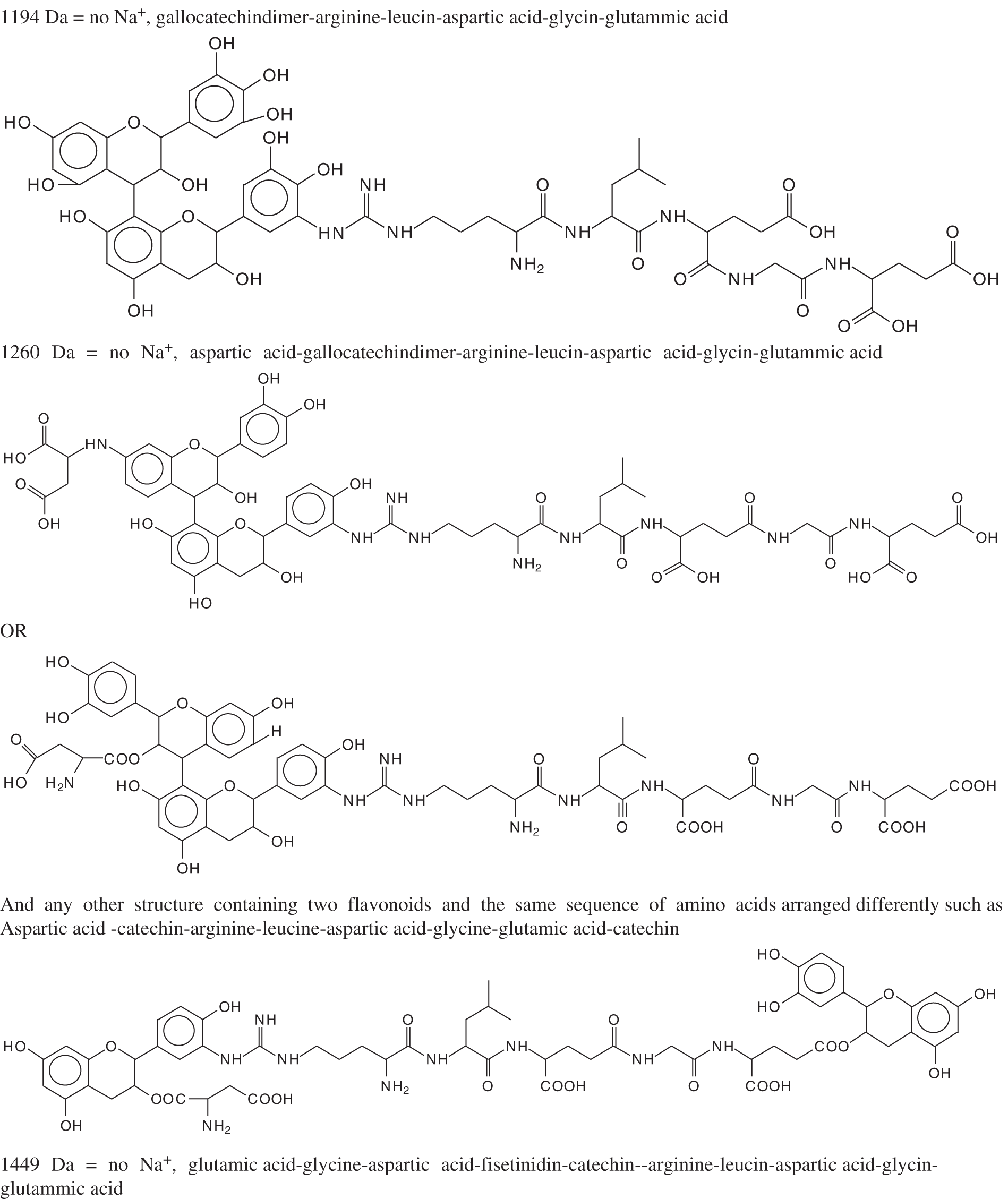
The situation appears to be rather different at the higher temperature of 80°C used. In Figs. A1a–A1h, and in Tab. B1 are shown the links formed between tannins monomers and oligomers with both amino acids and short peptide sequences of predominant amino acids present in collagen. Again, it is necessary to distinguish between two cases: (i) Amino acid monomers reacted with the tannin by the –COOH and the –NH2 groups of the amino acid that enter in the formation of the skeletal peptide chain of the protein, and (ii) –COOH and –NH2 groups of the amino acid side chains that are and will not be involved in the skeletal peptide sequence of the protein. The first of these will not contribute to the cross-linking of the protein, thus while formed they should not be considered. The second ones of these instead, if formed, will actively participate to cross-liking of the protein. Thus, only arginine, glutamic acid and aspartic acid, in relative proportions 6.1%, 12.2% and 7.3% respectively in collagen, belong to the second category, the first due to its –NH2 and =NH side chain groups capable of reacting with the acid phenolic –OH of the tannin [23–26], and the other two due to the presence in each of an extra –COOH side chain group leading to esterification of the aliphatic alcoholic –OH on the C3 site of tannin flavonoids. To the first group belong the peaks at 129 Da, 146 Da, 154 Da, 155 Da, 175 Da, 198 Da, 203 Da, 217 Da, 273 Da, 308 Da and 327 Da, all these belonging to either flavonoid monomers, or amino acid monomers or dimers, thus not generated by the reaction of tannin and protein. To the first group also belong the peaks at 643 Da, 908 Da and 925 Da, these types of reaction products not able to contribute to protein cross linking. Finally, the many species found by MALDI that can contribute to protein cross-linking, durability and shrinking are those assigned to the peaks at 391 Da, 413 Da, 425 Da, 441 Da, 467 Da, 483 Da, 575 Da, 601 Da, 632 Da, 688.8 Da, 705 Da, 857 Da, 873 Da, 889 Da, 1146 Da, 1162 Da, 1177 Da, 1194 Da, 1260 Da, 1449 Da, 1465 Da, 1481 Da, 1497 Da, and 1805 Da.
It is already evident from this latter list that a lot more reaction products between tannin and collagen amino acids do occur at 80°C (Tab. B1, Figs. A1a–A1h) than at ambient temperature (25°C) (Tab. 1, Fig. 1). It is equally evident that at 80°C, differently than at ambient temperature, no ionic bound reaction products are formed, the totality of the reaction products being covalently bound. This appear to indicate that as vegetal tanned leather is heated further covalent cross-linking by the tannin does occur tightening the network and contributing to the phenomenon of heat induced leather shrinkage.
As regards the reaction products found, a few are noticeable indicating the capacity of covalent cross-linking of the protein by the tannin. Thus the two species 601 Da and 632 Da, respectively being assigned to the sequence aspartic acid-gallocatechin-arginine and arginine-gallocatechin-arginine are the first ones indicating that cross-linking can occur.

As one considers the species formed at progressively larger molecular masses, the interpretation of the actual structure can be variable. An example of a flavonoid monomer linked to a long peptide chain, such as the species at 889 Da can be assigned to a peptide chain fragment of sequence-arginine-leucin-aspartic acid-glycin-glutammic acid having reacted with a gallocatechin yielding structures, among other possibilities, such as:

These types of structure are recurrent starting from 688 Da. The long peptide chains as in species at 889 Da have several useful reaction sites on which tannin chains can react to contribute to protein cross-linking. Thus, a species such the one at 1260 Da can be interpreted as belonging either to a peptide chain liked to a flavonoid dimer or as linking through different groups two flavonoid monomers, as follows:
A sequence of aspartic acid-gallocatechin dimer-arginine-leucin-aspartic acid-glycin-glutammic acid

and/or
A sequence of aspartic acid-catechin-arginine-leucine-aspartic acid-glycine-glutamic acid-catechin

The residual groups in the above amino acids sequence can also react, forming species of higher molecular weight indicating that extensive covalent cross-linking can in reality occur between tannin and protein as for example in the structure assigned to the peak at 1805 Da, this being an example of how the body of the protein is cross-linked by the tannin.

All the above indicates that in the tanning of hides with vegetal tannins a certain number of covalent and ionic bonds do occur between tannin and protein contributing to leather solidity and wear resistance. The proportion of these linkages increases with increasing temperatures of tanning, and in particular the proportion of tannin-protein covalent linkages does increase until these become the only one presents. While such covalent cross-linking may well be a contributing factor to the stability and performance of leather, when this becomes excessive as leather is subjected to higher temperatures, may well be also a strong additional, contributing factor to leather shrinking.
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Pasch, H., Pizzi, A., Rode, K. (2001). MALDI–TOF mass spectrometry of polyflavonoid tannins. Polymer, 42(18), 7531–7539. DOI 10.1016/S0032-3861(01)00216-6. [Google Scholar] [CrossRef]
2. Pasch, H., Pizzi, A. (2002). Considerations on the macromolecular structure of chestnut ellagitannins by matrix-assisted laser desorption/ionization-time-of-flight mass spectrometry. Journal of Applied Polymer Science, 85(2), 429–437. DOI 10.1002/app.10618. [Google Scholar] [CrossRef]
3. Pizzi, A., Pasch, H., Rode, K., Giovando, S. (2009). Polymer structure of commercial hydrolyzable tannins by matrix-assisted laser desorption/ionization-time-of-flight mass spectrometry. Journal of Applied Polymer Science, 113(6), 3847–3859. DOI 10.1002/app.30377. [Google Scholar] [CrossRef]
4. Navarrete, P., Pizzi, A., Pasch, H., Rode, K., Delmotte, L. (2010). MALDI-TOF and 13C NMR characterisation of maritime pine industrial tannin extract. Industrial Crops and Products, 32(2), 105–110. DOI 10.1016/j.indcrop.2010.03.010. [Google Scholar] [CrossRef]
5. Navarrete, P., Pizzi, A., Pasch, H., Rode, K., Delmotte, L. (2013). Characterisation of two maritime pine tannins as wood adhesives. Journal of Adhesion Science and Technology, 27(22), 2462–2479. [Google Scholar]
6. Ucar, M. M., Ucar, G., Pizzi, A., Gonultas, O. (2013). Characterisation of Pinus brutia bark tannin by MALDI-TOF MS and 13C NMR. Industrial Crops and Products, 49(8), 697–704. DOI 10.1016/j.indcrop.2013.06.010. [Google Scholar] [CrossRef]
7. Motillon, C., Schmitt, C., Grassl, B., Khouk, A., Pellerin, V. et al. (2014). The pH sensitive colloidal and chemical structure of tannins from barks of maritime pine in water. Journal of Colloid Science and Biotechnology, 3, 343–350. [Google Scholar]
8. Ricci, A., Lagel, M. C., Parpinello, G. P., Pizzi, A., Kilmartin, P. A. et al. (2016). Spectroscopy analysis of phenolic and sugar patterns in a food grade chestnut tannin. Food Chemistry, 203(4), 405–429. DOI 10.1016/j.foodchem.2016.02.105. [Google Scholar] [CrossRef]
9. Wedaina, G. A., Pizzi, A., Danwe, R., Nzie, W., Konai, N. et al. (2020). Performance of unidirectional biocomposite developed with Piptadeniastrum africanum tannin resin and Urena lobata fibers as reinforcement. Journal of Renewable Materials, 9(3), 477–493. DOI 10.32604/jrm.2021.012782. [Google Scholar] [CrossRef]
10. Chei, H., Shan, Z. H. (2008). Changes in hydrothermal stability of collagen with several catechin-metal compounds: A DSC study. Journal of the Society of Leather Technologist and Chemists, 92, 93–95. [Google Scholar]
11. O’Flaherty, F., Roddy, W. T., Lollar, R. M. (1978). The chemistry and technology of leather, volume ii, types of tannages. Malabar, Florida, USA: Robert E. Krieger Publishing Company. [Google Scholar]
12. Griyanitasari, G., Pahlawan, I. F., Kasmudjiastuti, E. (2018). Thermal stability of shoe upper leather: Comparison of chestnut and quebracho as vegetable tanning agent. IOP Conference Series: Materials Science and Engineering, 432, 012040. DOI 10.1088/1757-899X/432/1/012040. [Google Scholar] [CrossRef]
13. Covington, A. (1997). Modern tanning chemistry. Chemical Society Review, 26(2), 111–126. DOI 10.1039/cs9972600111. [Google Scholar] [CrossRef]
14. Carşote, C., Badea, E., Miu, L., Gatta, D. G. (2016). Study of the effect of tannins and animal species on the thermal stability of vegetable leather by differential scanning calorimetry. Journal of Thermal Analysis and Calorimetry, 124(3), 1255–1266. DOI 10.1007/s10973-016-5344-7. [Google Scholar] [CrossRef]
15. Liu, W. T., Li, G. Y. (2010). Non-isothermal kinetic analysis of the thermal denaturation of type I collagen in solution using isoconversional and multivariate non-linear regression methods. Polymer Degradation and Stability, 95(12), 2233–2240. DOI 10.1016/j.polymdegradstab.2010.09.012. [Google Scholar] [CrossRef]
16. Ghahri, S., Pizzi, A., Mohebby, B., Mirshoktaie, A., Mansouri, H. R. (2018a). Improving water resistance of soy-based adhesive by vegetable tannin. Journal of Polymers and the Environment, 26(5), 1881–1890. DOI 10.1007/s10924-017-1090-6. [Google Scholar] [CrossRef]
17. Ghahri, S., Pizzi, A., Mohebby, B., Mirshoktaie, A., Mansouri, H. R. (2018b). Soy-based, tannin-modified plywood adhesives. Journal of Adhesion, 94(3), 218–237. DOI 10.1080/00218464.2016.1258310. [Google Scholar] [CrossRef]
18. Ghahri, S., Pizzi, A. (2018). Improving soy-based adhesives for wood particleboard by tannins addition. Wood Science and Technology, 52(1), 261–279. DOI 10.1007/s00226-017-0957-y. [Google Scholar] [CrossRef]
19. Roux, D. G. (1965). Modern Applications of Mimosa Extract, pp. 34–41. Grahamstown, South Africa: Leather Industries Research Institute. [Google Scholar]
20. Pizzi, A. (1980). Tannin-based adhesives: A review. Journal of Macromolecular Science, Part C, 18(2), 247–294. DOI 10.1080/00222358008081043. [Google Scholar] [CrossRef]
21. Pizzi, A. (1983). Tannin-based adhesives, Chapter 4. In: Pizzi, A. (ed.Wood adhesives chemistry and technology, pp. 175–246. New York: Marcel Dekker. [Google Scholar]
22. Pizzi, A. (2019). Tannin-based biofoams. Journal of Renewable Materials, 7(5), 477–492. DOI 10.32604/jrm.2019.06511. [Google Scholar] [CrossRef]
23. Santiago-Medina, F. J., Pizzi, A., Basso, M. C., Delmotte, L., Celzard, A. (2017). Polycondensation resins by flavonoid tannins reaction with amines. Polymers, 9(2), 37. DOI 10.3390/polym9020037. [Google Scholar] [CrossRef]
24. Thebault, M., Pizzi, A., Santiago-Medina, F. J., Al-Marzouki, F. M., Abdalla, S. (2017). Isocyanate-free polyurethanes by coreaction of condensed tannins with aminated tannins. Journal of Renewable Materials, 5(1), 21–29. DOI 10.7569/JRM.2016.634116. [Google Scholar] [CrossRef]
25. Braghiroli, F., Fierro, V., Pizzi, A., Rode, K., Radke, W. et al. (2013). Reaction of condensed tannins with ammonia. Industrial Crops and Products, 44(4), 330–335. DOI 10.1016/j.indcrop.2012.11.024. [Google Scholar] [CrossRef]
26. Hashida, K., Makino, R., Ohara, S. (2009). Amination of pyrogallol nucleus of condensed tannins and related polyphenols by ammonia water treatment. Holzforschung, 63(3), 319–326. DOI 10.1515/HF.2009.043. [Google Scholar] [CrossRef]



Figure A1: MALDI ToF spectrum of collagen protein and mimosa bark tannin co-reacted at 80°C. (a) 100 Da–300 Da range. (b) 300 Da–500 Da range. (c) 400 Da–700 Da range. (d) 700 Da–1000 Da range. (e) 1000 Da–1500 Da range. (f) detail of the 1200 Da–1400 Da range. (g) 1400 Da–1600 Da range. (h) 1800 Da–2000 Da range
Table B1: Structure assignement of MALDI ToF spectrum peaks for the products of the reaction at 80°C of mimosa condensed tannin with powder collagen hydrolysates







 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |