

 | Journal of Renewable Materials |  |
DOI: 10.32604/jrm.2021.014876
ARTICLE
Glycolysis Recycling of Waste Polyurethane Rigid Foam Using Different Catalysts
College of Materials Science and Engineering, Qiqihar University, Qiqihar, 161006, China
*Corresponding Author: Hongxiang Luo. Email: lhxiang2014@foxmail.com
Received: 05 November 2020; Accepted: 06 January 2021
Abstract: Dramatically increasing waste polyurethane rigid foam (WPRF) draws the attention of the world. A mixture of ethylene glycol (EG) and diethylene glycol (DEG) is used as glycolysis agents. WPRF was subjected to alcoholysis using different catalysts which are titanium ethylene glycol and potassium hydroxide to obtain recycled polyol, respectively. The effect of a different catalyst on the viscosity and hydroxyl value of recycled polyol is discussed. The regenerated polyurethane (RPU) is performed using the recycled polyol. Infrared spectrum, compressive strength, apparent density, water absorption, scanning electron microscope, and thermogravimetric analysis are carried out to investigate the effect of WPRF degradation using different catalysts. The results show that titanium glycol is more efficient than potassium hydroxide in almost all conditions. The viscosity of the recycled polyol is relatively low, and the hydroxyl value meets the requirements of industrial use. When the titanium glycol titanium addition amount is 0.05%, the prepared RPU has a compressive strength of 0.24 MPa, an apparent density of 41.75 kg/m3, and a good foam structure. Besides, the water absorption rate of the RPU under the two catalytic systems is not much different, and the thermal stability is good. The recycled polyol can generally partially replace traditional polyols to prepare polyurethane rigid foams with good comprehensive properties.
Keywords: Waste polyurethane; recycling; glycolysis; catalyst
Polyurethane refers to the general name of macromolecular compounds containing repeating carbamate groups on the main chain and is a polymer made from many raw materials [1]. It is a class of materials with a wide range of uses, which can be used in many different applications from the automotive industry to coatings or biological materials [2,3]. Polyurethane foam is widely used, inevitably leading to the production of a large amount of polyurethane foam scraps and waste. These wastes are insoluble and infusible and are usually disposed of by landfill and incineration. However, with the enhancement of people’s environmental awareness and the introduction of relevant laws and regulations, the two traditional polyurethane treatment methods of burying and incineration have been increasingly restricted. The current recovery methods include physical recovery, chemical recovery, and energy recovery methods [4,5]. Chemical recycling of polyurethane is mainly used to break the polyurethane chain segment at high temperature through the degradation agent and catalyst. Its degradation products are small molecules and oligomers containing alcoholic hydroxyl groups, which can react with isocyanate groups. Therefore, it can be reused as raw materials of polyurethane.
Many scholars have done many researches on the chemical recovery of waste polyurethane, including glycolysis, phosphate ester method, hydrolysis, amination, pyrolysis, and alcoholysis [6–9]. Kou et al. [10] used a single-component alcoholysis agent to degrade rigid polyurethane foam and proved that the decomposition process with alkanolamine is an alcoholysis reaction. Wang [11] used di propylene glycol to degrade and recycle polyurethane materials used in waste cars. Xu et al. [12] used diethanolamine as a de-crosslinking agent to chemically degrade waste polyurethane rigid foam in a HAAKE rheometer, and they studied the effect of processing conditions such as temperature and time on the degradation of the product. Lu et al. [13] used alcohol, phosphate ester, and alcohol-phosphate ester compound method to degrade polyurethane foam, and further explained and explored the mechanism of polyurethane degradation. Motokucho et al. [14] hydrolyzed polyurethane waste in a high-pressure CO2 atmosphere, and the degree of hydrolysis was up to 93%. At present, for the glycolysis research of waste polyurethane, most of the degradants used are a single component, and few studies are focusing on degradation catalysts. Ulrich et al. [15] and others used LiOH as a catalyst. DEG is a glycolytic agent to degrade waste polyurethane at 185~200°C, and the mass ratio of DEG/PU is between 50/50 and 60/40. Wu et al. [16] used DEG at 220°C and used potassium acetate (KAc) as a catalyst to degrade PU. The recovery rate of polyol was 55.4%. Carmen et al. [17] used stannous octoate as a catalyst for PU degradation and explored more optimal reaction conditions. Zhang [18] carried out innovations on waste polyurethane catalysts, using self-made magnetic solid catalyst CaO/MgO/SrFe12O19 to degrade polyurethane foam, which can be recycled and reused with magnets after the reaction. Morcillo-Bolaños et al. [19] prepared Zn/Sn/Al hydrotalcite heterogeneous catalyst and used DEG as an alcoholysis agent to recycle soft polyurethane foam.
In this study, ethylene glycol and diethylene glycol were used as glycolysis agents, and different added amounts of titanium glycol and potassium hydroxide were used as catalysts to degrade waste PU. Titanium is a typical transition metal element. Due to its empty d orbital, it can accept electrons or electron pairs to form complexes. Titanium glycol is an organic titanium-based compound with a highly effective degradation effect. Its usage so small that there are few metal ions in the recycled polyol, which effectively reduces the adverse effect of metal ions on the performance of the polyol. Potassium hydroxide is an alkali metal hydroxide with strong alkalinity and is used for comparative studies in this study. The effects of two kinds of catalysts on the viscosity and hydroxyl value of recycled polyol were investigated, respectively. The recycled polyol could replace part of traditional industrial polyol for the manufacture of rigid polyurethane foam. The ethylene glycol used in the research is a de-crosslinking agent with good performance. The ether bond in diethylene glycol helps to form hydrogen bonds between polymer molecular chains, which is beneficial to enhance the physical properties of RPU. Titanium ethylene glycol and the glycolytic agent have good compatibility, which is conducive to the dispersion of the catalyst in the system. This research is helpful to promote the industrialization process of waste polyurethane recycling.
Waste polyurethane rigid foam (WPRF), refrigerator disassembly, provided by Qingdao Haier Co., Ltd.; ethylene glycol (EG), diethylene glycol (DEG), potassium hydroxide (KOH), and triethanolamine (TEOA) are all analysis pure, purchased from Tianjin Branch Miou Chemical Reagent Co., Ltd., Tianjin, China; Ti(OCH2CH2O)2, analytically pure, Zibo Xiaoguang Chemical Materials Co., Ltd., Zibo, China; Dibutyltin dilaurate (DBTDL), chemically pure, Jinan Nuochuang Chemical Co., Ltd.; polyether polyol 4110 (PP4110), industrial-grade, Yantai Wanhua Chemical Group Co., Ltd., Yantai, China; Dimethicone (PDMS), analytical pure, Jinan Nuochuang Chemical Co., Ltd., Jinan, China; monofluorodichloroethane (HCFC-141B), industrial-grade, Shandong Binhai Chemical Co., Ltd., Binhai, China; Polymethylene polyphenyl polyisocyanate (PAPI), industrial-grade, Yantai Wanhua Chemical Group Co., Ltd., Yantai, China.
According to our previous study, the mass ratio of EG:DEG = 6:4 was the optimal proportion of glycolysis agents. 100 g WPRF, 60 g EG and 40 g DEG were added into a reactor respectively together with different ratio of KOH or Ti(OCH2CH2O)2 (0.01%, 0.05%, 0.1%, 0.5%, 1.0%). The mixtures were stirred for 2.5 h at 180°C to obtain the recycled polyol, the recycled polyol of WPRF. Under the effect of the small alcohols (EG and DEG), the -NCOO- is broken and replaced by a relatively short alcohol chain, thereby forming a recycled polyol of polyurethane to recover polyols. The glycolysis mechanism of polyurethane can generally be simplified as a transesterification reaction between carbamate linkage glycolysis agents [20], and the mechanism was shown in Fig. 1.
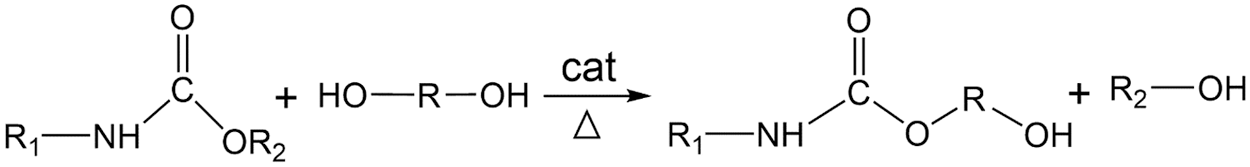
Figure 1: Degradation of WPRF
RPU was prepared by a one-step method, following the formula showed in Tab. 1. Firstly, predetermined amounts of recycled polyol, PDMS, HCFC-141b, and TEA and DBTDL were added to the polyether polyol 4110 in a polypropylene beaker and mixed under mechanical stirring at 500 rpm for 30 s to obtain a homogeneous polyol mixture. Then, a precalculated amount of PAPI was added to the polyol mixture and mixed under mechanical stirring at 1000 rpm until foaming. After curing at 60°C for 20 min, the RPU was let stand for at least 24 h at room temperature before performing the characterizations. Besides, the essential properties for commercial PU are showed in Tab. 2.
Table 1: List the formulations used in RPU

Table 2: The essential properties for the commercial polyurethane rigid foam

The viscosity of the recovered polyol was determined by a rotary viscometer (Model NDJ-5S, Wuxi Xigong Tools and Measuring Co., Ltd., China). This test was carried out in a glass beaker at 25°C. An appropriate amount of recycled polyol was placed in a 100 mL Erlenmeyer flask, and the hydroxyl value was determined according to GB/TQ 128.3-2009.
The Fourier Transform Infrared Spectrometer (FTIR; FTS-135, Perkin Elmer Co., USA), the analysis was done in a spectrometer. Preparation of samples using potassium bromide tableting method. FTIR spectra were collected in the region of 500 to 4000 cm−1, at room temperature.
Molecular weight distributions were determined by using Wyatt GPC/SEC-MALS. Tetrahydrofuran is used as a mobile phase.
The apparent density of the RPU was tested, following GB/T 6343-1986. The apparent density of five specimens per sample was measured, and the average values was calculated.
The compression strength of the RPU was determined in a universal testing machine (Model HKW 50 kn, Shanghai Huanke Measurement and Control Technology Co., Ltd., China) according to GB/T 8813-2008 with sample dimensions of 50 × 50 × 50 mm3. The compression strengths of five specimens per sample were measured, and the average values were obtained.
The microstructure of the RPU was observed through a scanning electron microscope (SEM; model S-4300, PE, USA) with an acceleration voltage of 20 kV. The samples were prepared by a brittle fracture with liquid nitrogen and coated with gold before observation.
The water absorption rate of the RPU was determined according to GB/T 8810-88.
The thermogravimetric analyze of the RPU was studied by a thermogravimetric (TG) analyser (Model STA449F3, NETZSCH Co., Germany). The characterization was performed from 25°C to 500°C at a heating rate of 30 °C/min under a nitrogen atmosphere, and the test was carried out at a gas flow rate of 50 ml/min.
The FTIR spectra of the WPRF is shown in Fig. 2. The spectrum revealed that the WPRF had some major peaks: C-O stretching vibration peak (1071 cm−1), -NH (amide II band, near 1517 cm−1), C=O (amide I band, near 1727 cm−1), CH2 stretching vibration peak (near 2925 and 2862 cm−1), and -NH stretching vibration peak (near 3314 cm−1) [21]. It can be seen that the WPRF is polyether polyurethane.

Figure 2: Infrared spectra of the WPRF
During the degradation process, the catalysts KOH and Ti(OCH2CH2O)2 were added in different addition amounts, and the recycled polyol obtained were regenerated polyols. It was characterized by infrared spectroscopy with polyether polyol 4110 as the raw material of polyurethane, and the results are shown in Fig. 3. It can be seen from Fig. 3 that the recycled polyol under the two catalyst systems are basically similar to the polyether polyol 4110 in the characteristic peak pattern, showing a clear and strong band at 3400 cm−1, which is the alcohol hydroxyl absorption peak. The characteristic absorption peaks of the sp3 C-H stretching vibration is at 2930 and 2870 cm−1 [22]. There are absorption bands in 1454 and 1374 cm−1 characteristic of bending vibrations of C-H in the polyol chain [23]. The absorption peak appearing at 1060 cm−1 corresponds to the vibration of C-O-C. It can be seen that under the action of glycolytic agents and catalysts, waste polyurethanes are successfully degraded into small molecular polyols similar in structure to polyether polyols, and their chemical properties are similar to polyether polyol 4110, which can be substituted for the preparation of RPU materials.

Figure 3: Infrared spectra of recycled polyol at different conditions and polyether polyol 4110
3.2 Viscosity of Recycled Polyol
Under the other same experimental conditions, the same added amount of catalyst KOH and Ti(OCH2CH2O)2 were used to degrade waste polyurethane, respectively, and the viscosity of the resulting recycled polyol was tested. The results are shown in Fig. 4a. The viscosity of the Ti(OCH2CH2O)2 degradation system is basically lower than that of the KOH degradation system, indicating that the Ti(OCH2CH2O)2 recycled polyol has better fluidity and a more complete degradation reaction. For the KOH system, the viscosity of the recycled polyol decreases with the increase of KOH addition, and the lowest is 5736.4 mPa•s. When the addition amount is small, the viscosity of the recycled polyol is higher and the difference is small. In the Ti(OCH2CH2O)2 degradation system, as the amount of catalyst added increases, the viscosity of the product basically decreases first and then increases, and the fluctuation is more obvious within a certain range. When the amount of Ti(OCH2CH2O)2 added was 0.05%, the viscosity of the recycled polyol reached the lowest, which was 4175.4 mPa•s, after which the viscosity of the system increased. To a certain extent, it can be seen that Ti(OCH2CH2O)2 is a highly active catalyst, and a small amount of addition can achieve an efficient degradation effect. The addition of the catalyst will promote the cleavage of the urethane bond in the polyurethane. In the glycolysis of WPRF, the side reaction is the conversion of the urea groups into urethane groups and amine groups [24,25], as shown in Fig. 5. The by-products are carbamates and amines, playing an effect on the viscosity [26]. When the catalyst is too much, it is not conducive to the glycolysis reaction of the polyurethane, and excessive by-products will increase the viscosity of the system.

Figure 4: The effect of different catalytic systems on (a) the viscosity of recycled polyol and (b) the compressive strength of RPU
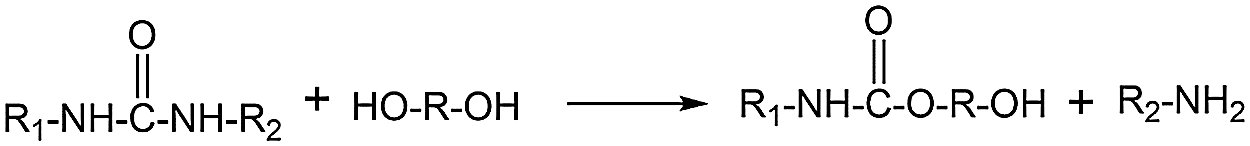
Figure 5: The side reaction: Glycolysis of the urea groups
3.3 Hydroxyl Value of Recycled Polyol
Under the other same experimental conditions, different addition amounts of catalysts KOH and Ti(OCH2CH2O)2 were used to degrade waste polyurethane, respectively, and the hydroxyl value of the obtained recycled polyol was tested. The results are shown in Tab. 3.
Table 3: Hydroxyl value of recycled polyol under different catalytic systems
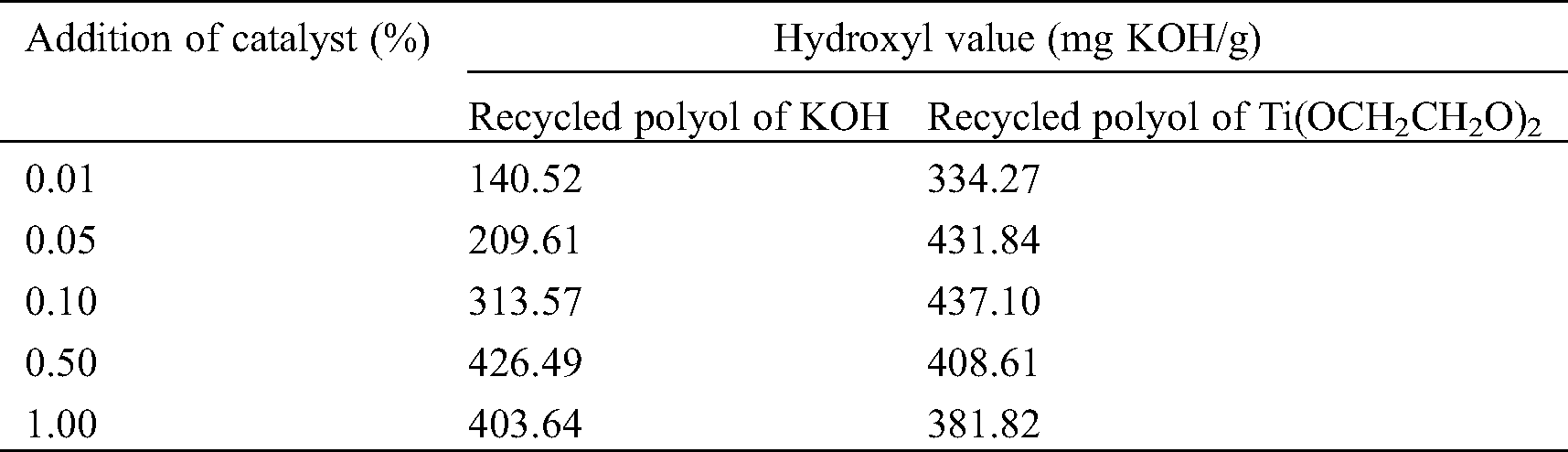
Within a certain range, the hydroxyl value of the recycled polyol of Ti(OCH2CH2O)2 system is larger than that of KOH system, which indicates that the catalytic activity of Ti(OCH2CH2O)2 is higher. The alcoholysis reaction in its degradation system is more intense, which can promote a lot of The carbamate bond breaks, the molecular chain of the recycled polyol polyol is relatively short, and the small alcohol produced contains a large number of terminal hydroxyl groups. For the recycled polyol under the two catalyst systems, the hydroxyl value of the recycled polyol increased greatly with the increase in the amount of catalyst at first, and then decreased slightly. The hydroxyl value of Ti(OCH2CH2O)2 system decreased more obviously, which was due to excessive amount of catalyst leads to recondensation of recycled polyol to reduce the hydroxyl value [27].
The Number-average molecular weights (Mn) of recycled polyol at different reaction conditions are shown in Fig. 6. The Mn of the recycled polyol of Ti(OCH2CH2O)2 is in the range of 3077–3574. The Mn of the recycled polyol under the two systems is viable for industrial use. The Mn of recycled polyol of Ti(OCH2CH2O)2 system is lower than KOH, and the glycolysis of polyurethane is more complete.
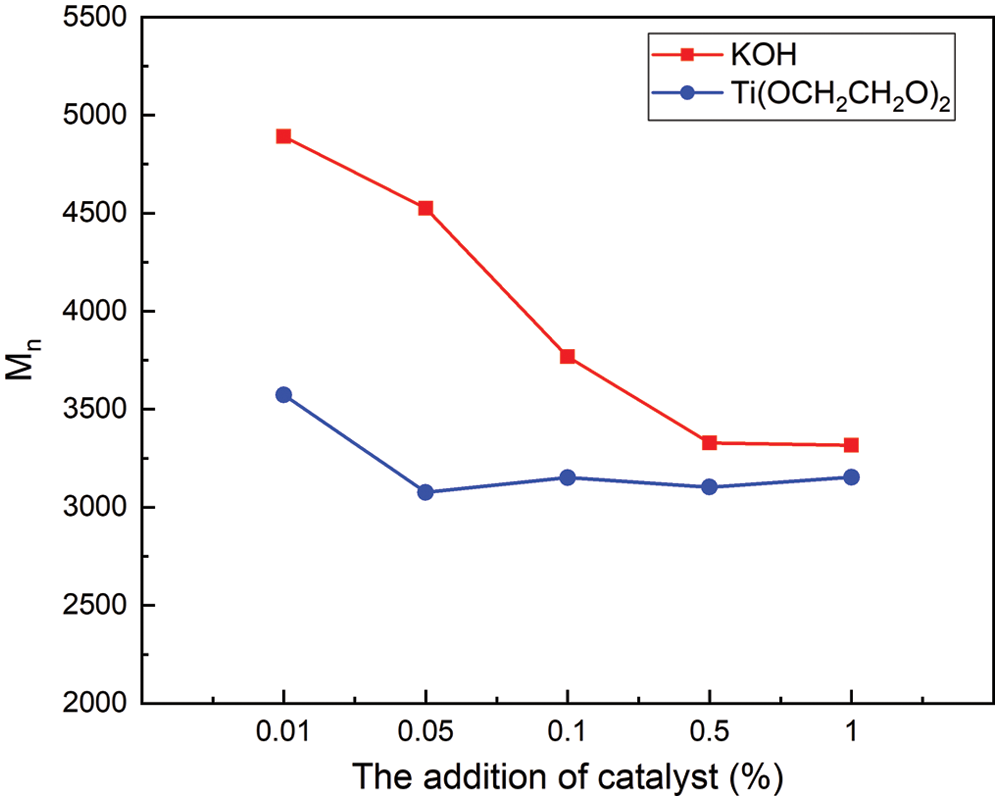
Figure 6: Mn of recycled polyol under different catalytic systems
3.5 Compressive Strength of RPU
The compressive strength test of the foams of Ti(OCH2CH2O)2 degradation system and KOH degradation system under different catalyst addition amounts is shown in Fig. 4b. The compressive strength of the foam under the two systems increases with the increase of the catalyst addition and gradually decreases after reaching the peak. When the amount of catalyst added is very small (only a few hundred ppm), the compression strength of polyurethane foam prepared from recycled polyol of Ti(OCH2CH2O)2 system is significantly higher than that of polyurethane foam prepared from recycled polyol of KOH system in most cases, and the catalytic activity is better. Ti(OCH2CH2O)2 is a chelating agent containing a five-membered ring, which has the structural stability of the ring. After the degradation reaction, it is uniformly dispersed in the obtained recycled polyol. Therefore, the mechanical properties of the polyurethane foam prepared by this are improved. Under this system, there are more active groups such as urea groups in the recycled polyol, which helps to form a rigid structure inside the foam and improve its compressive strength. When the catalyst addition amount is the same 0.05%, the compressive strength of the RPU of the Ti(OCH2CH2O)2 degradation system is 0.24 Mpa, which is 118.2% higher than that of the KOH system degradation foam (0.11 Mpa). When the two catalysts are added in the best amounts, the compressive strength of the polyurethane foam (0.24 Mpa) of the 0.05% Ti(OCH2CH2O)2 system is 9.1% higher than that of the 0.5% KOH system (0.22 Mpa).
Britain, JW and others believe that metal catalysts can catalyze the isocyanate-hydroxyl reaction and form metal coordination compounds. Similarly, the catalytic effect of Ti(OCH2CH2O)2 on polyurethane glycolysis is also due to the formation of metal coordination complexes [28,29]. As a new type of titanium-based catalyst alcoholate, ethylene glycol titanium has strong catalytic activity. It can greatly improve the polarity of the carbonyl group in the polyurethane molecule, effectively reduce the activation energy of the reaction [30], and promote the degradation of the polyurethane get on. Fig. 7 shows the possible mechanism of the catalytic degradation of PU by titanium glycol. The titanium ion on Ti(OCH2CH2O)2 is easy to interact with the hydroxyl oxygen on ethylene glycol and diethylene glycol and the ester bond oxygen in polyurethane. In the multi-ring structure with position function, the charge on the group will be redistributed, so that the ester carbonyl carbon has positive charge, which accelerates the nucleophilic reaction between the groups and improves the rate of polyurethane degradation reaction.
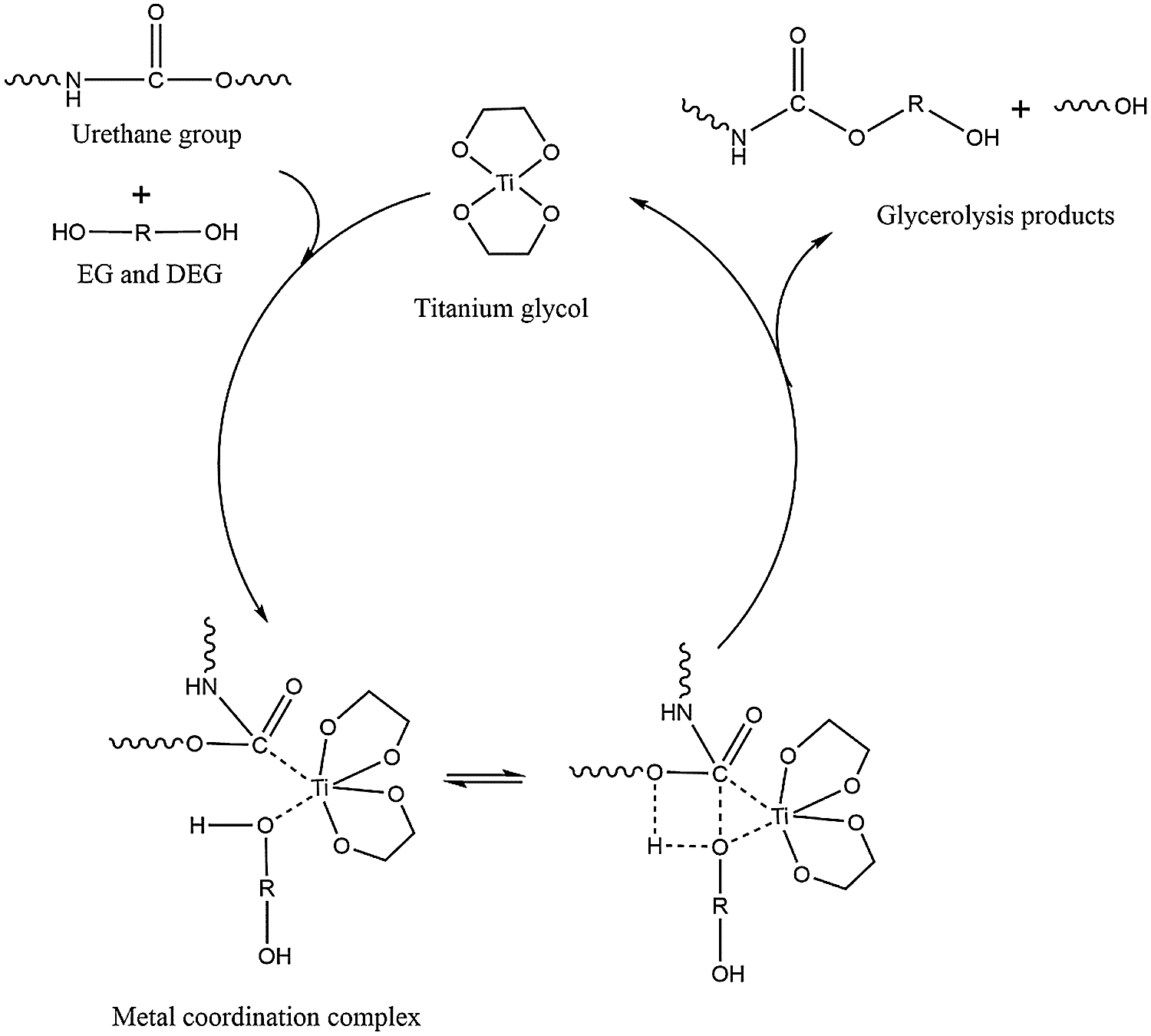
Figure 7: Possible mechanism of PU degradation catalyzed by titanium glycol
The measurement results of the apparent density of the foam prepared by the recycled polyol under the two catalyst systems are shown in Fig. 8a. As the amount of catalyst added increases, the density of the foam sample increases first and then decreases. Excessive catalyst will cause too many by-products in the recycled polyol, the hydroxyl value of the recycled polyol will decrease, and the viscosity will increase, which is not conducive to the formation of the cross-linked network structure of the polyurethane foam, resulting in the gradual decrease of its density and compressive strength. The density of RPU prepared from recycled polyol of Ti(OCH2CH2O)2 system is basically higher than that of KOH system foam. The optimal addition amount is 0.05%. At this time, the density of RPU with 0.05% Ti(OCH2CH2O)2 is 41.75 kg/m3, which is slightly higher than the maximum density of RPU with KOH. When KOH is used as a catalyst, there are many by-products in the recycled polyol regenerated polyol, which inhibits the formation of CO2 in the foaming reaction, and the number of cells formed is small, resulting in a low density of polyurethane foam in the KOH degradation system.
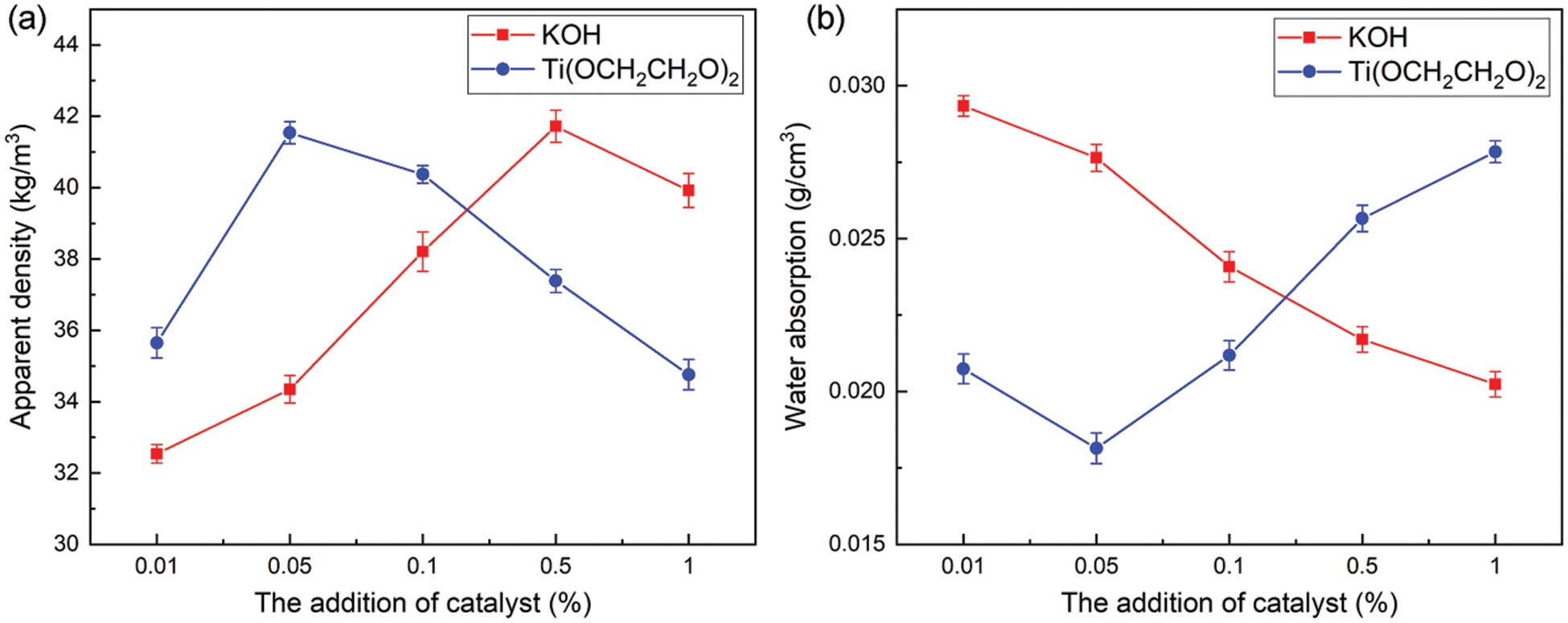
Figure 8: The effect of different catalytic systems on (a) the apparent density and (b) the water absorption of RPU
Based on the changes in the apparent density and compressive strength of the foam samples, the effect of different catalyst degradation systems on the water absorption of the RPU was further explored. The results are shown in Fig. 8b.
The water absorption rate of foam samples prepared from recycled polyol of different catalyst systems is quite different. Within a certain range, the water absorption rate of the RPU decreases with the increase of the catalyst addition. Overall, the water absorption rate of the RPU of the Ti(OCH2CH2O)2 system is lower than that of the KOH system foam. When the addition amount of Ti(OCH2CH2O)2 is 0.05%, the water absorption rate of the foam is the smallest (0.018 g/cm3). The water absorption rate of the foam reflects its water-blocking ability, which is closely related to the degree of closed-cell. The lower the water absorption rate, the more complete the pore structure of the RPU, the higher the closed-cell rate, and the more gas it can block, the better its thermal insulation performance. Also, after the polyurethane foam sample is immersed in water, the cell wall is damaged by external forces such as squeezing, which ruptures and opens the cells, causing the water absorption rate to increase. The greater the apparent density and compressive strength of the foam, the less likely the cell wall is to rupture, and the lower the water absorption rate.
Fig. 9 shows the SEM of RPU with KOH or Ti(OCH2CH2O)2 (0.05%, 0.5%). The results of pore size from the SEM images are shown in Fig. 10. As shown in Figs. 9b and 9c, when the catalyst is little, the polyurethane molecular chain will not be fully broken and the degradation reaction is not complete, which is not conducive to the application of regenerated polyol. When the catalyst is excessive, a large number of by-products will be generated in the reaction, which is harmful to the structure of the RPU. There are some cracks in the cell wall in the cell structure, and the cell size is not uniform. A proper amount of catalyst can improve the integrity of the cells and make the shape more regular, as shown in Figs. 9a and 9d. The polyurethane foam cell distribution is dense and the cell structure is more regular under 0.05% Ti(OCH2CH2O)2 and 0.5% KOH. In addition, the 0.05% Ti(OCH2CH2O)2 polyurethane sample has more closed cells, a more transparent pore membrane, and a strong skeleton, which reflects the better branching and crosslinking structure of the foam, and has good support, ring structure, and matrix. There is a good interaction to enhance the strength of the pore wall, which is consistent with the results of the compression strength test.
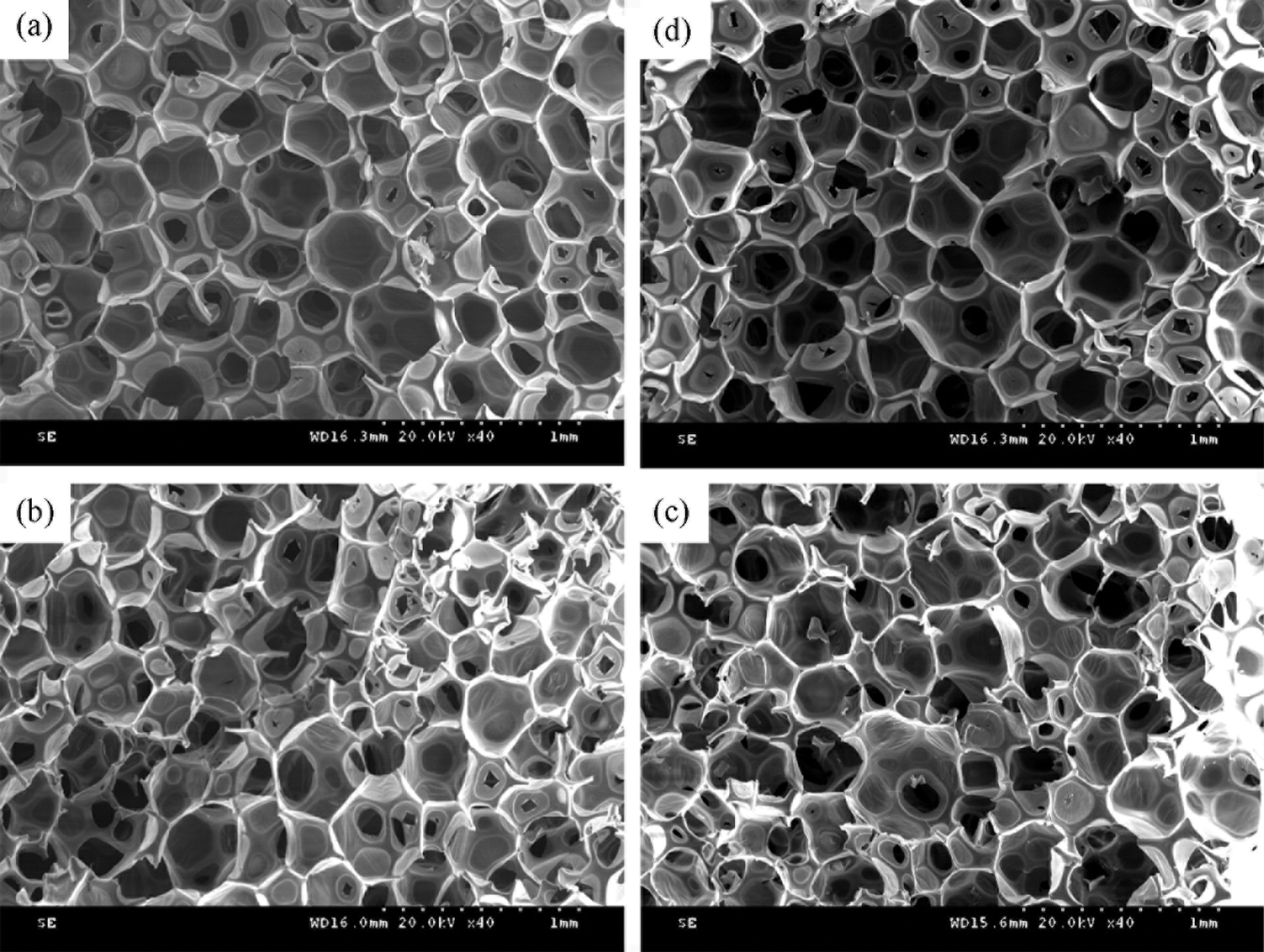
Figure 9: SEM images of RPU of different catalyst systems. (a) 0.05% Ti(OCH2CH2O)2 (b) 0.5% Ti(OCH2CH2O)2 (c) 0.05% KOH (d) 0.5% KOH
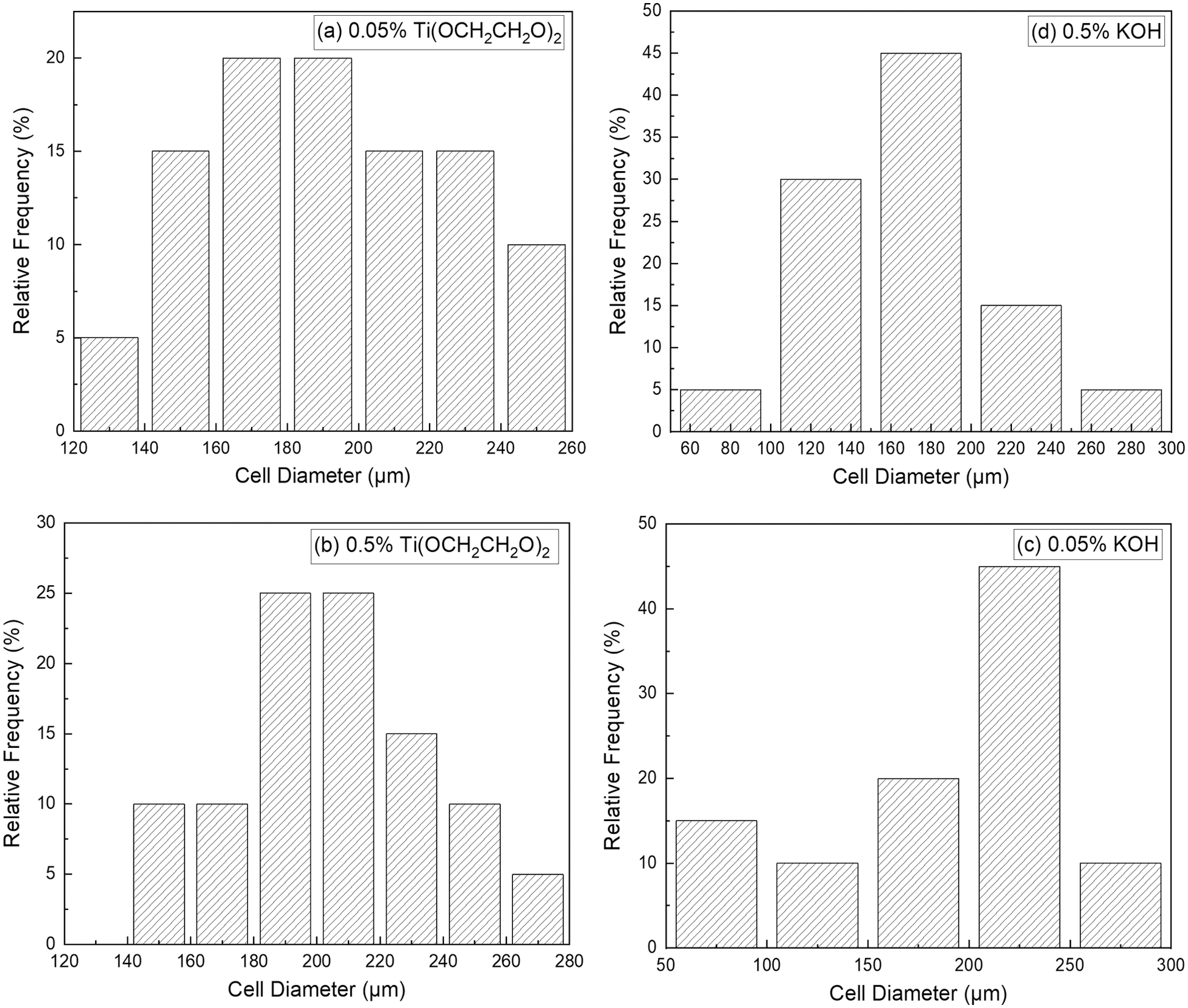
Figure 10: The pore size of RPU of different catalyst systems
3.9 Thermal Stability Analysis of RPU
The TG and DTG curves of polyurethane foam are shown in Fig. 11. The 5% weight loss temperature T5%, the 50% weight loss temperature T50%, and the maximum weight loss rate temperature Tmax are shown in Tab. 4. It can be seen from Fig. 10, the thermal weight loss of polyurethane can be divided into three stages. The first stage occurs at 80–186°C, which is mainly caused by hydrogen bond breaking and water loss; the thermal weight loss at 196–248°C is mainly due to polyurethane It contains urethane hard segment decomposition [31]; the third stage occurs at 248–443°C, which belongs to the soft segment decomposition of polyurethane [32,33]. With the increase of KOH addition, the 5% thermal decomposition temperature, 50% thermal weight loss temperature, and maximum thermal weight loss rate temperature of the polyurethane foam basically increase, indicating that the increase of the degradation catalyst KOH is beneficial to promote the degradation of waste polyurethane and the benzene ring derived from waste polyurethane can be more introduced into the molecular chain of RPU so that the thermal stability of RPU is improved. When the addition of Ti(OCH2CH2O)2 is 0.05%, the thermal weight loss temperatures of the polyurethane foam are almost higher than others.
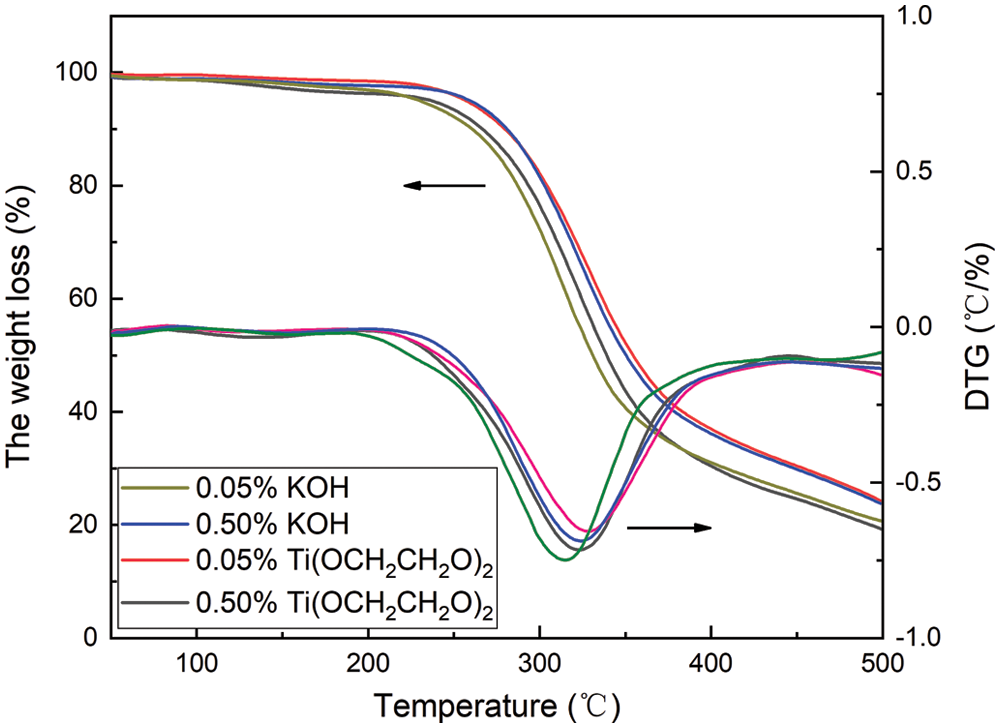
Figure 11: TG and DTG curves of RPU prepared from recycled polyol
Table 4: Thermal weight loss data of RPU samples

Glycolysis had been carried out to treat the WPRF. Ti(OCH2CH2O)2 and KOH were used as a catalyst. FTIR indicates that the WPRF has been degraded, and the new RPU was prepared using the recycled polyol, replacing partly traditional polyether. With the increase of Ti(OCH2CH2O)2 or KOH, the viscosity of the recycled polyol first decreases and then increases, and the hydroxyl value first decreases and then increases. The addition of a suitable amount of catalyst can make the degradation of waste polyurethane more thorough, while excessive catalyst will lead to an increase in side reactions. When the addition amount of Ti(OCH2CH2O)2 is 0.05%, the compressive strength reaches the maximum value of 0.26 Mpa, which is an increase of 18.2% compared with the maximum value of 0.22 Mpa of the KOH (0.5%). TG shows the thermal stability of RPUs of 0.05% Ti(OCH2CH2O)2 and 0.5% KOH is good. Consequently, the results demonstrate that Ti(OCH2CH2O)2 can achieve a better degradation effect than KOH. An industrial application of this research will be the subject of future papers.
Funding Statement: This work was supported by the 2019 Graduate Student Innovative Research Project of Qiqihar University Heilongjiang Province, China (YJSCX2019063), Qiqihar Science and Technology Bureau Project (GYGG-201902), and Heilongjiang Provincial Department of Education Project (135409301).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Ge, Z. Q., Xu, H. X., Li, Z. Y., Cheng, X. L. (2008). Treatment and recycling of polyurethane waste. Chemical Propellants and Polymer Materials, 6(1), 65–68. [Google Scholar]
2. Nelson, A. M., Long, T. E. (2014). Synthesis, properties, and applications of ion-containing polyurethane segmented copolymers. Macromolecular Chemistry and Physics, 215(22), 2161–2174. DOI 10.1002/macp.201400373. [Google Scholar] [CrossRef]
3. Engels, H. W., Pirkl, H. G., Albers, R., Albach, R. W., Krause, J. et al. (2013). Polyurethanes: Versatile materials and sustainable problem solvers for today’s challenges. Angewandte Chemie International Edition, 52(36), 9422–9441. DOI 10.1002/anie.201302766. [Google Scholar] [CrossRef]
4. Wang, J. R., Chen, D. J. (2004). The chemical degradation mechanism of polyurethane wastes. Polymer Bulletin, 17(2), 85–90. [Google Scholar]
5. Xu, P. L., Zhang, S. Q. (2002). Handbook of polyurethane materials, pp. 7. Beijing: Chemical Industry Press. [Google Scholar]
6. Troev, K., Grancharov, G., Tsevi, R., Tsekova, A. (2000). A novel approach to recycling of polyurethanes: Chemical degradation of flexible polyurethane foams by triethyl phosphate. Polymer, 41(19), 7017–7022. DOI 10.1016/S0032-3861(00)00054-9. [Google Scholar] [CrossRef]
7. Dai, Z. Y., Hatano, B., Kadokawa, J., Tagaya, H. (2002). Effect of diaminotoluene on the decomposition of polyurethane foam waste in superheated water. Polymer Degradation and Stability, 76(2), 179–184. DOI 10.1016/S0141-3910(02)00010-1. [Google Scholar] [CrossRef]
8. Datta, J. (2010). Synthesis and investigation of glycolysates and obtained polyurethane elastomers. Journal of Elastomers & Plastics, 42(2), 117–127. DOI 10.1177/0095244309354368. [Google Scholar] [CrossRef]
9. Schué, F. 2000. Polymer recycling John Scheirs John Wiley and Sons Ltd. Polymer International, 49(2), 235–236. [Google Scholar]
10. Kanaya, K., Takahashi, S. (1994). Decomposition of polyurethane foams by alkanolamines. Journal of Applied Polymer Science, 51(4), 675–682. DOI 10.1002/app.1994.070510412. [Google Scholar] [CrossRef]
11. Wang, Y. W. (1999). Recycling of polyurethane foam for automobile by alcoholysis. Zhejiang Chemical Industry, 30(4), 58–59. [Google Scholar]
12. Xu, Q. W., Wang, J. H., Li, G. M. (2011). Research on the de-crosslinking of waste polyurethane rigid foam. Engineering Plastics Application, 39(3), 14–18. [Google Scholar]
13. Lu, G. F., Ding, Y. B., Zhao, C. S., Cui, D. S. (2004). Research development of chemical method recovery waste polyurethane at home and aboard. Chemical Engineer, 109(10), 45–48. [Google Scholar]
14. Motokucho, S., Yamaguchi, A., Nakayama, Y., Morikawa, H., Nakatani, H. (2017). Hydrolysis of aromatic polyurethane in water under high pressure of CO2. Journal of Polymer Science Part A: Polymer Chemistry, 55(12), 1–7. DOI 10.1002/pola.28576. [Google Scholar] [CrossRef]
15. Ulrich, H., Odinak, A., Tucker, B., Sayigh, A. (1978). Recycling of polyurethane and polyisocyanurate foam. Polymer Engineering and Science, 18(11), 844–848. DOI 10.1002/pen.760181103. [Google Scholar] [CrossRef]
16. Wu, C. H., Chang, C. Y., Cheng, C. M., Huang, H. C. (2003). Glycolysis of waste flexible polyurethane foam. Polymer Degradation and Stability, 80(1), 103–111. DOI 10.1016/S0141-3910(02)00390-7. [Google Scholar] [CrossRef]
17. Molero, C., de Lucas, A.,Romero, F., Rodriguez, J. F. (2009). Glycolysis of flexible polyurethane wastes using stannous octoate as the catalyst. Journal of Material Cycles and Waste Management, 11(2), 130–132. DOI 10.1007/s10163-008-0224-2. [Google Scholar] [CrossRef]
18. Zhang, L. (2012). Preliminary study on polyols recovered by alcoholysis of waste polyurethane rigid foam. Chongqing University, China. [Google Scholar]
19. Morcillo-Bolaños, Y. D., Malule-Herrera, W. J., Ortiz-Arango, J. C., Villa-Holguín, A. L. (2018). Polyurethane flexible foam recycling via glycolysis using Zn/Sn/Al hydrotalcites as heterogeneous catalyst. Revista Facultad de Ingenieria, 35(87), 77–85. [Google Scholar]
20. Zhu, P., Cao, Z. B., Chen, Y., Zhang, X. J., Qian, G. R. (2014). Glycolysis recycling of rigid waste polyurethane foam from refrigerators. Environmental Technology, 35(21), 2676–2684. DOI 10.1080/09593330.2014.918180. [Google Scholar] [CrossRef]
21. Li, J. W., Lee, H. T., Tsai, H. A., Suen, M. C., Chiu, C. W. (2018). Synthesis and properties of novel polyurethanes containing long-segment fluorinated chain extenders. Polymers, 10(11), 1292. DOI 10.3390/polym10111292. [Google Scholar] [CrossRef]
22. Shin, S. R., Kim, H. N., Liang, J. Y. (2019). Sustainable rigid polyurethane foams based on recycled polyols from chemical recycling of waste polyurethane foams. Journal of Applied Polymer Science, 136(35), 47916. DOI 10.1002/app.47916. [Google Scholar] [CrossRef]
23. Molero, C., de Lucas, A., Romero, F., Rodriguez, J. F. (2005). Recovery of polyols from flexible polyurethane foam by “split-phase” glycolysis: Glycol influence. Polymer Degradation and Stability, 91(2), 221–228. DOI 10.1016/j.polymdegradstab.2005.05.008. [Google Scholar] [CrossRef]
24. Diao, Q. (2012). Research on alcoholysis of waste polyurethane materials. Fudan University, China. [Google Scholar]
25. Modesti, M., Simioni, F. (1996). Chemical recycling of reinforced polyurethane from the automotive industry. Polymer Engineering & Science, 36(17), 2173–2178. DOI 10.1002/pen.10614. [Google Scholar] [CrossRef]
26. Datta, J., Kopczyńska, P. (2016). From polymer waste to potential main industrial products: Actual state of recycling and recovering. Critical Reviews in Environmental Science and Technology, 46(10), 905–946. DOI 10.1080/10643389.2016.1180227. [Google Scholar] [CrossRef]
27. Cao, Y. (2018). Development of green technology for degradation of rigid polyurethane materials. Changchun University of Technology, China. [Google Scholar]
28. Britain, J. W., Gemeinhardt, P. G. (1960). Catalysis of the isocyanate-hydroxyl reaction. Journal of Applied Polymer Science, 4(11), 207–211. DOI 10.1002/app.1960.070041112. [Google Scholar] [CrossRef]
29. Murai, M., Sanou, M., Fujimoto, T., Baba, F. (2003). Glycolysis of rigid polyurethane foam under various reaction conditions. Journal of Cellular Plastics, 39(1), 15–27. DOI 10.1177/002195503031021. [Google Scholar] [CrossRef]
30. Zhang, X. L. (2017). Industrial application of environmentally friendly titanium-based polyester catalyst. Tianjin University, China. [Google Scholar]
31. Zhou, W., Zheng, K. M., Zhou, Y. H., Zhang, M. (2019). Preparation and characterization of novel phosphorus-containing flame retardant tung oil-based rigid polyurethane foam. Chemical Industry and Engineering Progress, 38(7), 3285–3290. [Google Scholar]
32. Petrović, Z. S., Zavargo, Z., Flyn, J. H., Macknight, W. J. (1994). Thermal degradation of segmented polyurethanes. Journal of Applied Polymer Science, 51(6), 1087–1095. DOI 10.1002/app.1994.070510615. [Google Scholar] [CrossRef]
33. Cinelli, P., Anguillesi, I., Lazzeri, A. (2013). Green synthesis of flexible polyurethane foams from liquefied lignin. European Polymer Journal, 49(6), 1174–1184. DOI 10.1016/j.eurpolymj.2013.04.005. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |