

 | Journal of Renewable Materials |  |
DOI: 10.32604/jrm.2021.015268
REVIEW
Biodegradable Materials as Nanocarriers for Drugs and Nutrients
1Key Laboratory of Textile Science & Technology, Ministry of Education, College of Textiles, Donghua University, Shanghai, 201620, China
2School of Perfume and Aroma Technology, Shanghai Institute of Technology, Shanghai, 201418, China
3R&D Center of China Tobacco Yunnan Industrial Co., Ltd., Kunming, 650231, China
*Corresponding Author: Kai Wang. Email: 27922573@163.com
Received: 05 December 2020; Accepted: 13 January 2021
Abstract: Several important drugs and nutritional supplements are limited by their lack of bioavailability. Nanomaterials display unique beneficial properties that might help improve the bioavailability of drugs and nutritional supplements. Unfortunately, nanomaterials produced from synthetic polymers and metals may have similar difficulties with bioavailability and toxicity. Naturally occurring biopolymers are biodegradable and non-toxic and are adaptable to the synthesis of nanoparticles. Drugs and other substances can be encapsulated or embedded in such particles with an increase in bioavailability. The search for biodegradable nanomaterials is an active research field. This review summarizes the research on nanocrystalline cellulose, starch, lignin, and other biological and environment-friendly nanocomposites which are commonly used as nanocarriers for drugs and nutrients. Further, prospects for the use of biodegradable nanomaterials in targeted therapy, including environmentally responsive therapy, are discussed.
Keywords: Nanomaterials; biodegradation; classification; application
1 Overview of Nanoencapsulation of Drugs and Nutrients
Many oral drugs, especially anticancer agents, have limitations such as poor chemical stability and low water solubility and bioavailability in the gastrointestinal tract [1], which greatly restrict their clinical application. Additionally, various nutrients such as polyphenols, polyunsaturated fatty acids which are promoted to help prevent chronic disease and prolong lifespan also show low water solubility and stability, resulting in limited bioavailability [2].
Various new carrying systems are now available to overcome these shortcomings and increase the efficacy of drugs and nutrients, and nanoencapsulation is a relatively recent and promising technology [3,4]. Nanoparticles (NPs) exhibit unique surface mass ratios and quantum properties and can be effective carriers for bioactive substances [5,6]. NPs significantly the improve bioavailability of various substances, especially insoluble drugs and nutrients [7−10]. Several modes of entry of nanoparticles into cells are illustrated (Fig. 1). The uptake mechanisms of nanoparticles include phagocytosis, macropinocytosis, and endocytosis. Nanoparticles are very small and are mainly absorbed through endocytosis which can be divided into subgroups according to various specific protein-receptor interactions, such as clathrin- and caveolae-mediated endocytosis. Successful transformation of clinical drug delivery systems from macro to micro to nano promotes the development of controlled drug release and surmounts pharmacological limitations of traditional dosage forms [11].

Figure 1: Schematic representation of NPs’ uptake mechanisms [12]
1.2 Traditional Wall Materials for Nano-Encapsulation
Traditional capsule wall materials used to embed drugs and nutrients are divided into three main categories: semisynthetic polymer materials, fully synthetic polymer materials, and inorganic materials. Fully synthetic polymer materials display good film-forming properties, high mechanical strength, and stability and are the most widely used capsule wall materials. Fully synthetic polymer materials, such as polyvinyl chloride, polyethylene, polyurea, polyester, and polyurethane, are typically non-biodegradable. Natour et al. [13] utilized interfacial polycondensation between amine and isocyanate monomers in inverse nanoemulsion (water-in-oil). These authors encapsulated ionic liquid-modified magnetite nanoparticles (MNPs) with polyurea nanocapsules (PU NCs) with an average size of 5 nm–20 nm. Hasani-Sadrabadi et al. [14] used self-assembly technology to synthesize nanocapsules composed of a dendritic polyethylene core and Pluronic copolymer shell. The nanocapsules enclosed several hydrophobic anticancer drugs, such as paclitaxel, in the size range of 50 nm–200 nm. However, most polymer monomers in these preparation processes are toxic. Moreover, organic solvents required in these processes are costly and may cause environmental pollution.
Studies on nanoembedded drugs and nutrients using semisynthetic polymer materials are relatively few. Such studies have mainly used cellulose derivatives, such as methylcellulose, ethylcellulose, and cellulose acetate butyrate, and oils, such as 1,3-distearin, and hydroxystearic alcohol. El-Habashy et al. [15] used a solvent evaporation method to prepare ethyl cellulose-based NPs containing piroxicam. These NPs were spherical, with slightly porous surface, and the average particle size was 240 nm.
Inorganic materials are often used in phase-change energy storage materials and are rarely used for the encapsulation of drugs or nutrients. The inorganic materials used for encapsulation include calcium carbonate, phosphate, aluminum, silicate, glass, and clay. Ma et al. [16] prepared sea urchin-shaped hollow CaCO3 nanospheres (HCNS) mediated with soybean trypsin inhibitor. These particles displayed a layered porous hollow structure and were used as anticancer drug carriers. The hierarchically porous hollow structure of the carrier realized high loading and continuous release of doxorubicin (DOX).
1.3 Shortcomings of Traditional Nanomaterials in Nanoencapsulation of Drugs and Nutrients
Epidemiological studies on urban air pollution suggest that finer particles are more harmful than larger particles. Humans continuously inhale millions of pollutant particles without serious harm. However, an increase of only 10 µg/m3 can increase the mortality caused by cardiac dysfunction by 1% [17]. The impact of smaller particle size on human health is increasing, suggesting that particles in the nanometer range also produce additional biological effects. The possible use of nanomaterials in food applications increases concerns regarding toxicity. Toxicity assessments need to consider relevant parameters [18], such as materials, structure, surface area, charge, solubility, stability, and aggregation state [19]. Toxic effects of nanomaterials may include inflammatory reactions that worsen airway disease, cardiovascular events caused by hypercoagulability or plaque instability [20], and chronic effects in animal models [21,22]. Nanomaterials used in various fields might eventually enter the human body through the food chain and affect human health, although this possibility is highly speculative. A primary concern among consumers is how nanomaterials are transferred from packaging materials to food products and how the NPs might affect human digestive tract [23].
1.4 Advantages of Biodegradable Materials as Nanocarriers
Biodegradable materials are widely used for encapsulating drugs and nutrients owing to their characteristics such as degradability, biocompatibility, and low toxicity. Biodegradable materials refer to polymers that are degraded by the action of microorganisms, such as bacteria and fungi, as per the American Society for Testing Material definition [24]. The definition of biodegradation in medical fields refers to changes in medical devices or biological materials, including loss of integrity or performance under physiological or simulated conditions. The use of biodegradable polymer materials may efficiently solve the problems associated with synthetic polymers and raw vegetable wastes. Thus, the development of biodegradable composites using novel natural polymers that can be degraded into harmless compounds is a priority [25]. Biodegradable nanomaterials are both feasible and safe for use in drug and nutrient encapsulation, with advantages such as high loading efficiency, good stability, biocompatibility, biodegradability, and controlled release [26,27]. This article systematically classifies biodegradable nanomaterials based on source and summarizes their application. Biodegradable nanomaterials exhibit high potential for use in the field of nanomaterials in the future.
2 Sources and Preparation of Natural Biodegradable Nanomaterials
Natural biodegradable materials can be divided into the following six categories: (1) Cellulose, chitin, starch, lignin, alginate, and other natural polysaccharide materials; (2) Collagen, fibrinogen, and other natural protein materials; (3) Vegetable oil, animal fat, and other lipid materials; (4) Polyhydroxyalkanoates [PHA, including polyhydroxybutyrate (PHB), polyhydroxybutyrate valerate (PHBV)], polyesters synthesized by bacteria; (5) Polyester, such as polylactic acid (PLA), synthesized from monomers produced by microbial fermentation; and (6) CO2 copolymers, such as polypropylene carbonate (PPC) prepared from CO2 and propylene oxide or ethylene oxide (Tab. 1).
Table 1: Forms and applications of different natural nanomaterials

Cellulose is the most abundant natural biopolymer in nature. All plants and some tunicates, algae, and bacteria synthesize cellulose. Cellulose is obtained through selecting material with naturally high cellulose content or extracting pure cellulose from wood and woody plants. In industry, wood pulp and cotton linter are the main cellulose sources.
Biodegradation of cellulose can be complete biodegradation or destructive. For example, cellulose macromolecules are substrates for biological enzymes, and polysaccharide chains are hydrolyzed to produce xylose, glucose, cellobiose, and other simple sugars. The latter are mineralized to carbon dioxide and water [46].
Nanocellulose can be divided into three types based on preparation method, material, and structure: Nanocrystalline cellulose (NCC); bacterial nanocellulose (BNC); and microfibrillated cellulose (MFC). Nanocellulose is primarily prepared from plant materials through controllable chemical, physical, or biological methods. Further, some microorganisms and tunicates are useful sources for preparing nanocellulose. Microfibrillated cellulose (MFC) is mainly prepared by a combination of mechanical methods and enzymatic/chemical treatments, and acid hydrolysis is the main method for preparing nanocrystalline cellulose (NCC). Bacterial nanocellulose (BNC) is prepared by bacterial synthesis [47]. Compared with macro-scale cellulose, nanocellulose displays high specific surface area, high tensile strength, high Young’s modulus, high hydrophilicity, and high crystallinity [48]. Nanoscale cellulose exhibits advantageous characteristics of both natural cellulose and nanomaterials. Such properties indicate a new degradable, functional, and sustainable nanomaterial.
Starch is a natural polymer found widely in plant tissues. It is highly abundant in nature and has advantages such as low price, high biocompatibility, and biodegradability. However, starch displays poor mechanical properties and water resistance. Starch is produced from α-D-glucose formed via photosynthesis and converted into the polymer in plant tissues by dehydration and condensation. Starch is a natural biodegradable polymer that is decomposed into glucose by microorganisms and finally metabolized into water and carbon dioxide. Degradation mechanisms of starch remain unclear. Aminabhavi et al. [49] and Maddever et al. [50] suggest that degradation of starch-based polymers occurs via two mechanisms: (1) Starch gradually disappears after being attacked by fungi, bacteria, and other microorganisms to form a hollow structure in the polymer that decreases its mechanical strength and increases its surface area; increased access to the polymer surface favors additional natural decomposition; (2) Starch degradation triggers the release of pro-oxidants and self-oxidants. Long polymer chains are cleaved, causing a decrease in molecular weight; it can then be attacked by microorganisms and eventually mineralized. These two processes are mutually reinforcing.
Application of nanotechnology to starch can improve thermal and mechanical properties of starch without affecting biodegradable and non-toxic properties. Nanostarch constructs include starch nanocrystals and starch nanoparticles [51]. Nanocrystals are typically prepared by acid hydrolysis using starch granules as starting materials. Several methods are used for preparing starch nanoparticles, including precipitation of amorphous starch, combining complex formation and enzymatic hydrolysis, microfluidization and chemical precipitation [51].
Lignin is a complex natural polymer produced by higher plants and is a central component of conifers, broad-leaved trees, and grasses. Lignin exhibits environmentally friendly and degradable characteristics. Lignin is primarily extracted from plants by ionic liquids, bases, organic solvents, or ozone [52]. Denatured lignin is divided into four categories, lignosulfonate, sulfate lignin, alkali lignin (KL) and hydrolyzed lignin, based on the production process. In nature, complete degradation of lignin depends on both fungi and bacteria, with fungi playing a major role [53].
Compared with general lignin, nanolignin is a more active adsorbent and surfactant. Further, nanolignin exhibits antibacterial and noncytotoxic properties. Nanolignin is usually classified by lignin processing, as indicated above. Several methods are used for preparing nanolignin, including adding water to a lignin solution as non-solvent and ultrasound-assisted mechanical treatments by high shear homogenization [54].
Chitosan (CS) is formed by deacetylation of chitin after treatment with concentrated alkali. Chitosan is defined as chitin after ≥55% deacetylation. Chitin is produced by molluscs, crustaceans, insects, and fungi. It is a rare alkaline polysaccharide among natural polymers and is insoluble in water and organic solvents [55]. Chitosan has properties such as biodegradability, low toxicity, good biocompatibility, and mucosal adhesion. This latter property makes chitosan a new type of nanodrug carrier. Chitosan exhibits anti-acid, anti-ulcer, and wound healing properties.
The extraction of chitosan differs with the source. For example, Kumari et al. [56] obtained chitosan from the scales of the fish via treatment with acid and alkali. The chitosan was analyzed by Fourier transform infrared spectroscopy (FTIR), X-ray diffraction (XRD), and scanning electron microscope (SEM). Habibi et al. [57] studied submerged fermentation with Aspergillus terreus with apple pomace extract as the sole carbon source to produce chitosan. The chitosan produced under optimal condition was characterized and analyzed by FTIR, thermogravimetric analysis (TGA), and differential scanning calorimetry (DSC), and the degree of deacetylation was 88.2%.
Chitosan is easily biodegradable by several microorganisms. Compared with chitosan, chitosan nanoparticles are non-toxic, have good biocompatibility, and are biodegradable. Chitosan NPs as carriers improve the stability of drugs and improve their bioavailability. Chitosan nanoparticles can be prepared by complex coacervation, solvent evaporation [58], covalent cross-linking [59], and ion gelation [60].
Collagen is a white, opaque, unbranched fibrous protein. It is the basic structural component of the extracellular matrix and accounts for 30% of the body protein in humans. Collagen molecules are connected by hydrogen and covalent bonds and can self-assemble into stable fibers. This highly ordered structure imparts high mechanical strength, thus supporting organs and protecting body function [61−63]. Extraction and preparation of collagen from aquatic animals are accomplished by acid extraction, alkaline extraction, enzymatic extraction, and hot water extraction. The basic principle is to alter the external environment of the protein according to its characteristics and then separate collagen from other proteins [24].
Collagen is a family of proteins, divided into Type I (skin, tendon, and bone), type II (cartilage), and type III (skin and vasculature). All three types are essential to the structure and integrity of the fiber structure. The most common collagen is type I, which is widely used in various applications [64]. Collagen is biodegradable and biocompatible, can improve cell permeability and wound healing, and provide a template for cellular attachment, migration, and proliferation. These properties are the basis for its use as a biomedical matrix material [65−67].
Collagen is made up of several amino acids connected by peptide bonds and is initially degraded into short peptides by extracellular proteolytic enzymes produced and secreted by microorganisms. These peptides are further decomposed into amino acids under the action of peptidases. Nanocollagen is a biologically active nanoscale molecule comprising mixed oligopeptide. The biological activity and skin permeability of nanocollagen are superior to those of collagen. Nanocollagen is mainly produced as nanoparticles.
PLA, also known as polylactide, is a biodegradable polyester with good biocompatibility that also shows good processability and mechanical properties. The material is produced by polymerization of lactic acid. Starting material is obtained from fermented starch, such as corn and rice. It can also be obtained from waste materials, such as cellulose, kitchen garbage, or fish. Currently, PLA is obtained by direct polymerization and ring-opening polymerization of lactide [68]. Direct polymerization method employed melt, melt-solid phase, and solution methods. Ring-opening polymerization is achieved by anionic ring-opening, cationic ring-opening, and coordination opening polymerization. PLA can be decomposed by adding water, and degradation in the human body can be independent of enzymes. It can also be degraded by microbial enzymes in soil and seawater [69]. The intermediate product of degradation is lactic acid that can be mineralized. This polymer is one of a few biomedical materials approved by the US Food and Drug Administration (FDA) [70].
PLA nanomaterials mainly exist in the form of nanoparticles, i.e., PLA block copolymers are directly produced as nanosized particles. Commonly used preparation methods for PLA nanoparticles include solvent evaporation, solvent replacement, and salting out.
3 Nanocarriers for Drugs and Nutrients Prepared from Biodegradable Materials
Drug Delivery Systems (DDS) emerged at the end of the 20th century. Such systems rely on the preparation of encapsulated drugs in a carrier that improves efficacy. Advantages of DDS include improved bioavailability and targeting, reduced toxic side-effects, and controlled drug release. The term “nanocarrier” generally refers to carriers with a particle size in the range of 1 nm–1000 nm. Commonly used nanocarriers include liposomes, nanoparticles, micelles, microemulsions, and solid lipid nanoparticles.
Liposomes are phospholipid double-layer enclosed structures with a hydrophilic core and lipophilic properties. The structures bind/encapsulate hydrophobic, hydrophilic, and amphiphilic drugs and nutrients. Hydrophilic substances are encapsulated in the aqueous core; lipophilic drugs and nutrients can disperse in the phospholipid bilayer. This DDS shows strong encapsulation ability. Liposomes can be made to alter the encapsulation efficiency of drugs and nutrients through adjustment of surface charge of phospholipid molecules. Sterically stable liposomes, cationic liposomes, and active targeting liposomes have been developed. Li et al. [71] synthesized thiol derivatized chitosan (CSSH) NPs and utilized them to embed curcumin liposomes. The encapsulation efficiency of CSSH-coated curcumin liposomes was 93.95%, the drug load was 7.95%, and the average particle size was 406.0 nm. Compared with curcumin liposomes, proper liposome coating can improve the stability. Han et al. [72] used d-α-tocopherol polyethylene glycol 1000 succinate (TPGS) as an inhibitor to prepare a surface-modified liposome (TPGS-PTX liposome) to load paclitaxel. The experimental results showed that the average particle size of the delivery system was 282.6 ± 20.41 nm and affected sustained release and inhibition of P-glycoprotein. Hsieh et al. [73] indicated that liposome incorporating lecithin/cholesterol or stearic acid was effective in protecting entrapped α-amylase from suffering acid (pH 2.8) and pepsin (15 mg/mL). Ghorbanzade et al. [74] used nano-liposomes to encapsulate fish oil and found that yogurt with nano-encapsulated fish oil contented a higher content of DHA and EPA. Essential oil can also be encapsulated in liposome nanoparticles. Khosravi-Darani et al. [75] successfully produced liposomal Zataria multiflora Boiss essential oil and found the antibacterial activity was maintained well.
Nanoparticles are a class of solid polymer colloidal particles with a particle size of <1 μm. Drugs and nutrients can be dissolved, coated, or adsorbed on the surface or inside of the particles. Loaded NPs exhibit high carrying capacity and sustained drug release. NPs can be synthesized and modified using various materials for high structural flexibility; however, large amounts of organic solvents are used in the preparation of nanoparticles, and complete removal of these solvents to ensure safety is an ongoing issue.
Nanoparticles can be divided into nanocapsules and nanospheres based on preparation methods and means employed for encapsulating drugs and nutrients (Fig. 2). The characteristics of nanocapsules are membrane wall structure, and aqueous or oil core as a reservoir to accommodate bioactive substances. Differently, nanospheres exhibit a matrix system in which bioactive materials are dispersed in the particles. Simi et al. [33] cross-linked modified starch nanoparticles with sodium tripolyphosphate and used indomethacin as a model drug to observe the morphology of grafted starch nanoparticles and to examine loading and controlled release by scanning electron and atomic force microscopy. The authors used diclofenac sodium (DS) as a model drug to study medical applications of cross-linked starch nanoparticles for transdermal administration [34]. Starch nanoparticles can be used as a DS drug carrier and provide a promising nano-connected system for transdermal delivery of non-steroidal anti-inflammatory drugs. Janes et al. [38] prepared doxorubicin (DOX) chitosan nanoparticles for use in chemotherapy. In vitro controlled release was achieved, with stable release maintained for 5 days. Mitra et al. [39] connected low molecular weight dextran (DEX) with the DOX complex to reduce the adverse effects, such as cardiotoxicity and bone marrow suppression, and then produced chitosan nanoparticles. These DOX-loaded NPs showed a significant inhibitory effect on tumor growth. Sriram et al. [40] added naphthalene acetate (NAA) to the chitosan amine group to form a hydrophobic chitosan–NAA drug carrier. Calcium ferrite nanoparticles (CFNP) were embedded in a chitosan–NAA matrix to form a superparamagnetic hybrid nanocarrier for the controlled release of curcumin. Hybrid nanocarrier was successfully employed for targeted drug delivery to tumor cell. Vijayan et al. [76] used chitosan, PLA, and poly aluminum chloride to prepare high-quality biodegradable polymer nanoparticles loaded with repaglinide by solvent extraction. Prepared nanoparticles were spherical, with size ranging from approximately 108.6 ± 3.4 nm to (220.6 ± 1.2) nm. Encapsulation efficiency (EE%) was 81.4% ± 1.8% to 92.7% ± 1.4%. A transdermal patch containing repaglinide nanoparticles was 76 times more effective than conventional oral patches.

Figure 2: Drug loading methods for nanocapsules (reservoir system) and nanospheres (matrix system) [82]
Besides, nanoparticles are suitable for encapsulating various nutrients. Yuan et al. [77] loaded lutein in zein nanoparticles coated with sophorolipid (ZSLNPs). ZSLNPs were spherical, with a particle size of about 200 nm, and the encapsulation efficiency and loading capacity were 90.04% and 0.82%, respectively. ZSLNPs showed great stability, redispersibility, and increased water solubility of lutein. Luo et al. [78] prepared zein nanoparticles coated with carboxymethyl chitosan (CMCS) to encapsulate vitamin D3. The nanoparticles with CMCS coating had a spherical structure with a particle size of 86 to 200 nm. The encapsulation efficiency after CMCS coating was greatly increased to 87.9%. This method was a promising method to enhance chemical stability and controlled release performance. Alishahi et al. [79] fabricated chitosan nanoparticles by adding vitamin C to the tripolyphosphate solution and gradually mixing it into the chitosan solution under stirring. The prepared nanoparticles had a spherical shape, a smooth surface, and maintain the immune-inducing properties of vitamin C. The controlled release of vitamin C was achieved.
PLA requires the use of higher-priced medicinal grade material. PLA currently used in pesticides formulations is prepared with industrial injection molding grade chemical [80]. Yao et al. [45] used an oil/water (O/W) emulsion solvent evaporation to prepare azoxystrobin-PLA microspheres (MS) and showed good dispersibility and spheroidization.
The method of oral delivery of active substances by polymer nanoparticles modified with collagen peptides (CPs) is attractive in Chinese medicine. Tang et al. [43] studied the use of ion-exchange resins to simply separate collagen cation CPs from bovine CPs and modified the surface of mixed nanomicelles (MMs) to improve the oral bioavailability of Cucurbitacin B (CuB). Successful modification of nanomicelles was confirmed. The inhibitory effect of CuB-MMs-CPs on tumors was significantly enhanced. Thus, nanomicelles, co-modified with isolated CPs, could be attractive as carriers for the oral delivery of CuB. Cao-Hoang et al. [81] used the water-in-oil solvent displacement method to encapsulate synthetic and natural β-Carotene into PLA nanoparticles, which offers better protection against oxidation.
Micelles show particle sizes of >10 nm in a narrow size distribution. These structures self-assemble via microphase separation of amphiphilic block copolymers in selected solvents. The micelles with hydrophilic shells and hydrophobic cores can spontaneously form polymer micelles after dissolving in water (Fig. 3). Commonly used hydrophilic blocks include polyvinylpyrrolidone (PVP), polyethylene glycol (PEG), poly(N-(2-hydroxypropyl) methacrylamide) (PHPMA), and poly (n-isopropyl acrylamide) (PNIPAM). Micelles are characterized by the solubilization of hydrophobic drugs, good stability, ease of modification, and significant drug loading capacity. Zhang et al. [83] prepared transferrin-modified paclitaxel-loaded polyphosphoester hybrid micelles (TPM). These micelles formed a core-shell structure with high encapsulation efficiency (89.9% ± 3.4%) in an aqueous medium. Chen et al. [84] attached paclitaxel to the C-6 position of the N-acetyl-D-glucosamine (GlcNAc) residue of hyaluronic acid (HA) using hexamethylene diamine as a linker to produce a new type of drug conjugate, HA-6-PTX. With a drug loading capacity of 21.8%, increased drug release was observed. Harada et al. [85] used PEG polyglutamate block copolymers to encapsulate granulocyte colony-stimulating factor (G-CSF); G-CSF encapsulated in polymeric micelles of diameter 60 nm–70 nm displayed a longer half-life and enhanced efficacy. Tang et al. [86] enclosed paclitaxel (PTX) into a dual pH-sensitive micelle with an acid-cleavable anionic shell, a pH-sensitive core, and a PEG corona. Inhibition of tumor growth and lung metastasis with these particles was achieved, with values of 77.7% and 88.3%, respectively, without significant toxicity. Wang et al. [87] designed and prepared an amphiphilic carboxymethyl chitosan–quercetin (CQ) conjugate that increased water solubility of paclitaxel (PTX) to improve oral bioavailability. The critical micelle concentration of the CQ conjugate was low, and it was self-assembled into polymer micelles in an aqueous solution. Drug loading and encapsulation efficiency were 33.62% ± 1.34% and 85.63% ± 1.26%, respectively. The system was a good carrier for oral non-water-soluble anticancer drugs. Ghasemi et al. [88] fabricated natural casein micelle nanocapsule to encapsulate fish oil by means of pH changes and ultrasound. The encapsulation efficiency was 92%–97%. The technology proposed in this study can be used as an effective method for wrapping and transporting unsaturated fatty acids, oils and other hydrophobic compounds in milk, other foods, and medicines to improve the health of the general population. Levinson et al. [89] prepared VD3 loaded re-assembled casein micelles to solubilize VD3 in a fat-free food system and protect it from degradation throughout production, shelf life and digestion. The experimental results showed that VD3 re-assembled casein micelles successfully delivered VD3 and improved the rheology of yogurt without affecting the taste.

Figure 3: Drug-loaded polymeric micelles formed from self-assembly of amphiphilic block copolymers in aqueous media [90]
Nanoemulsions are transparent or semi-transparent dispersion systems with a particle size of 20 nm–200 nm. These emulsions are produced by mixing water phase, oil phase, surfactants, and co-surfactants in an appropriate ratio (Fig. 4). Emulsions display low viscosity and isotropic thermodynamic and dynamic stability. Nanoemulsions generally require the use of higher concentrations of surfactants, which may limit their application. For example, clinical applications may cause toxicity. Further, the high energy requirement for the preparation of nanoemulsions involves more expensive equipment, resulting in higher production costs. Severino et al. [91] compared minimum inhibitory concentrations of carvacrol, mandarin, bergamot, and lemon Eos nanoemulsions. Carvacrol nanoemulsion was selected for incorporation into modified chitosan to form a bioactive coating. This modified chitosan-based coating increased the radiosensitivity of Escherichia coli and Salmonella typhimurium by 1.32 times and 1.30 times, respectively, without modified atmosphere packaging. Lin et al. [92] prepared positively charged nanoemulsion particles with amoxicillin, chitosan, and heparin using water-in-oil emulsification. Nanoemulsion particles were spherical and displayed controlled release of amoxicillin for targeting Helicobacter pylori infection sites. Najlah et al. [93] used two nanoemulsion preparations (Clinoleic 20% and Intralipid 20%) as drug carriers for paclitaxel (PX) and characterized particle size, zeta potential, loading rate, and pH. Particle sizes were uniform and stable, and the pH range of the monoclinic oil was slightly higher than that of the lipid formulation. Chaiyana et al. [94] used essential oil from Zingiber cassumunar rhizome (EO)/Tween 20 and propylene glycol (2:1)/water system to develop an EO nanoemulsion. Internal droplet size displayed a range of 211.5 ± 63.3 nm to 366.7 ± 77.8 nm. The nanoemulsion significantly enhanced the anti-inflammatory effects of the EO without obvious toxicity in human peripheral blood mononuclear cells. As for nutrients, polyphenols, such as procyanidins, have many desired biological effects, their use in oral preparations is also limited by some structural features. Cerda-Opazo et al. [95] fabricated O/W nanoemulsion and used it to encapsulate avocado peel extract, which was a promising source of procyanidins. The resulting spherical nanoemulsion had a particle size of about 160 nm and had good stability. Costa et al. [96] developed lipid-based nanoemulsions collected from de microalga Spirulina sp. LEB18, and incorporated with biopeptides obtained from this same microalga. The antioxidant activity was evaluated. The average droplet diameter of nanoemulsions was at the range of 222.9 ± 3.4 nm to 466.9 ± 5.3 nm. Raikos et al. [97] used oil-in-water beverage emulsions for lycopene encapsulation, and the most favorable formulation containing a mixture of long-to short-chain triglyceride at a ratio 75:25.

Figure 4: Schematic diagram of water-in-oil (W/O) and oil-in-water (O/W) nanoemulsions composed of surfactant micelles [98]
Solid lipid nanoparticles were first reported in the 1990s as natural/synthetic lipids or lipids as carriers encapsulated or embedded in a lipid core. This nano-DDS shows a particle size of 50 nm–1000 nm (Fig. 5). Solid lipid nanoparticles improve bioavailability, reduce toxicity, and increase drug stability. However, their biggest advantages are that organic solvents are not required and they can be produced on a large scale [99]. This nanocarrier is suitable for delivering fat-soluble drugs and nutrients and has a high encapsulation rate; however, hydrophilic drugs and nutrients are generally not suitable. Further, this DDS is susceptible to drug leakage and increased particle size during storage. Wang et al. [100] prepared tilmicosin-loaded hydrogenated castor oil solid lipid nanoparticles (SLNs) using hot homogenization and ultrasound to enhance the antibacterial activity of SLNs. Particle size was 343 ± 26 nm, and encapsulation efficiency was 60.4% ± 3.3%. Shi et al. [101] used soybean lecithin as a solid lipid to prepare an aqueous dispersion of SLNs encapsulating frankincense and myrrh essential oils using high-pressure homogenization. SLNs exhibited a core with a lower ordered structure. The particle size was 113.3 ± 3.6 nm, and the drug encapsulation efficiency was 80.60% ± 1.11%. Shtay et al. [102] used cocoa butter as a lipid core and sodium stearoyl-2-lactate (SSL) and mono- and diglycerides of fatty acids (MDG) as emulsifiers and surfactants to prepare SLN. Particle characteristics, stability in suspension, and effects of different cooling conditions on the performance and stability of SLNs during storage were reported. Cocoa butter SLN showed a particle size of 112.7 nm, which is suitable for a food-grade SLN. Yang et al. [103] prepared fish oil-loaded hollow solid lipid micro- and nanoparticles according to atomization of the CO2-expanded lipid mixture. The received particles were spherical and free-flowing. Fish oil loading efficiency was accomplished at 92.3% (w/w). Through this method, the bioavailability of EPA and DHA was significantly increased from 9.7% to 18.2%. Shtay et al. [104] prepared Epigallocatechin-3-gallate-loaded solid lipid nanoparticles (EGCG-SLN) made of a mixture of cocoa butter and food-grade surfactants by a hot homogenization method. This system can introduce EGCG into food. The average particle size of EGCG nanoparticles was 108 nm–122 nm and the maximum packaging rate was 68.5%. Zardini et al. [105] loaded lycopene on nanostructured lipid carriers and solid lipid nanoparticles by high shear homogenization and ultrasonic treatment. The particle size of the developed nanocarriers was between 74.93 nm and 183.40 nm.
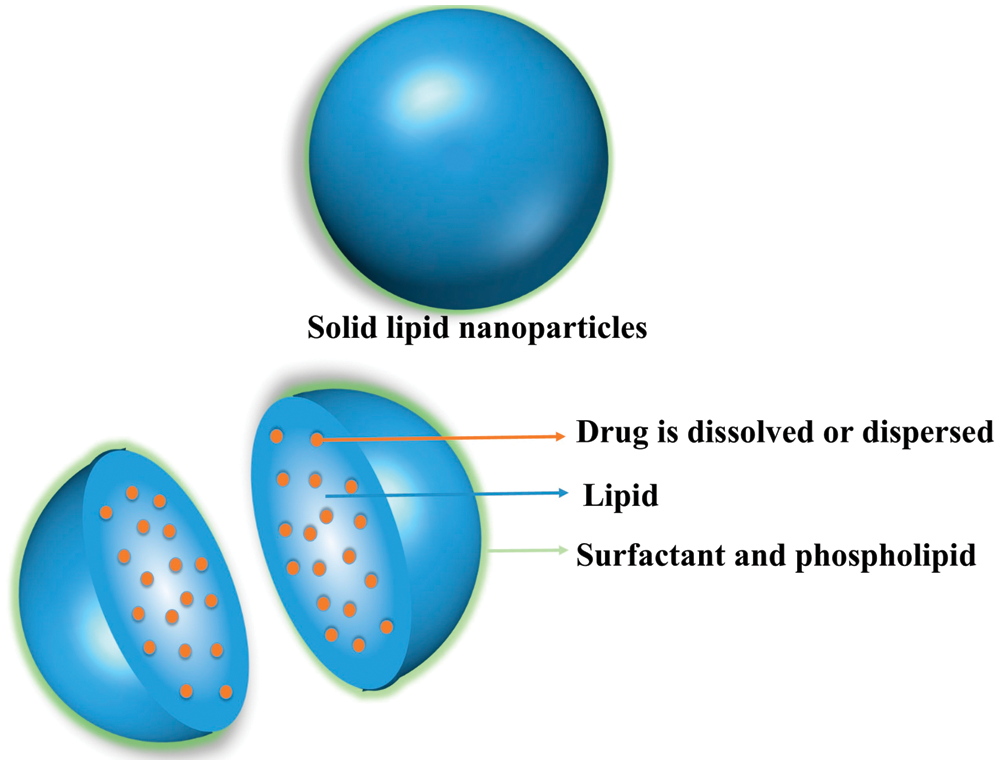
Figure 5: Drug loading methods for SLNs [106]
4 Development Trends of the Use of Biodegradable Nanomaterials as Drug and Nutrient Carriers
4.1 Green Preparation Technology
Public demand is increasing for environmental protection and sustainable development. Researchers now consider both development of biodegradable nanomaterials and environmentally friendly methods for the preparation of these materials. Green preparation of nanomaterials focuses on renewable solvents, pollution-free reducing agents, and non-toxic protective agents to avoid adverse environmental impacts.
Traditional nanomaterial preparation methods use either top-down or bottom-up methods (Fig. 6). Top-down methods transform materials into nanoparticles through external mechanical forces. Bottom-up refers to the formation of nuclei through chemical changes with gradual generation of nanomaterials, and it is preferred to produce nanoparticles [107,108]. Green preparation methods mainly involve plant and microbial synthesis. Plant preparation refers to the use of plants as reducing agents for the synthesis of nanomaterials or plant extracts, such as proteins, amino acids, phenols, saponins, terpenes, and vitamins, to be used as reducing and capping agents for synthesis. Microbial synthesis [109] refers to the use of microalgae, bacteria, and other microorganisms to adsorb and reduce metal ions in situ to obtain nanoparticles. Roychoudhury et al. [110] used Lyngbya majuscula as a biological agent for the synthesis of NPs. These cyanobacteria were first mixed with gold and silver ions in solution, and a bimetallic gold-silver nano-alloy was formed under appropriate conditions. This method is similar to plant synthesis method and directly uses extracts of microorganisms, such as polysaccharides, proteins, polyphenols, etc., as reducing agents and stabilizers for the synthesis of nanoparticles. For example, Netala et al. [111] used a water extract of endophytic fungi to reduce AgNO3 and obtain spherical silver nanoparticles with a particle size of 3 nm–40 nm. In addition, environmentally friendly and sustainable processing technology is also the focus of research. Water and supercritical carbon dioxide are seen as interesting alternatives to traditional organic solvents. As for conventional heating, hydrothermal approaches, microwave energy and focused sunlight are popular green strategies.

Figure 6: Preparation methods of nanomaterials [107]
4.2 Environmentally Responsive Nanomaterials
Environmentally responsive materials release active ingredients in response to changes in environmental stimuli, such as enzymes, pH, redox, and ionic strength. Such materials are of great interest in medicine, food processing, and environmental engineering [112−116]. Application of environmentally responsive materials in medicine focuses on the development of tumor microenvironmental stimulus-responsive nanodrug delivery systems and is a core technology for tumor-targeted therapy [117−119]. Reported tumor microenvironmental stimulus–response signals include low pH, tumor tissue, endosomes and lysosomes; lysosomal enzymes; tumor extracellular matrix metalloproteinases; reactive oxygen species generated by mitochondria; and reduced glutathione (GSH) in tumor cells. These stimuli allow targeting of drug release from nanoparticles to kill cancer cells (Fig. 7) [120].
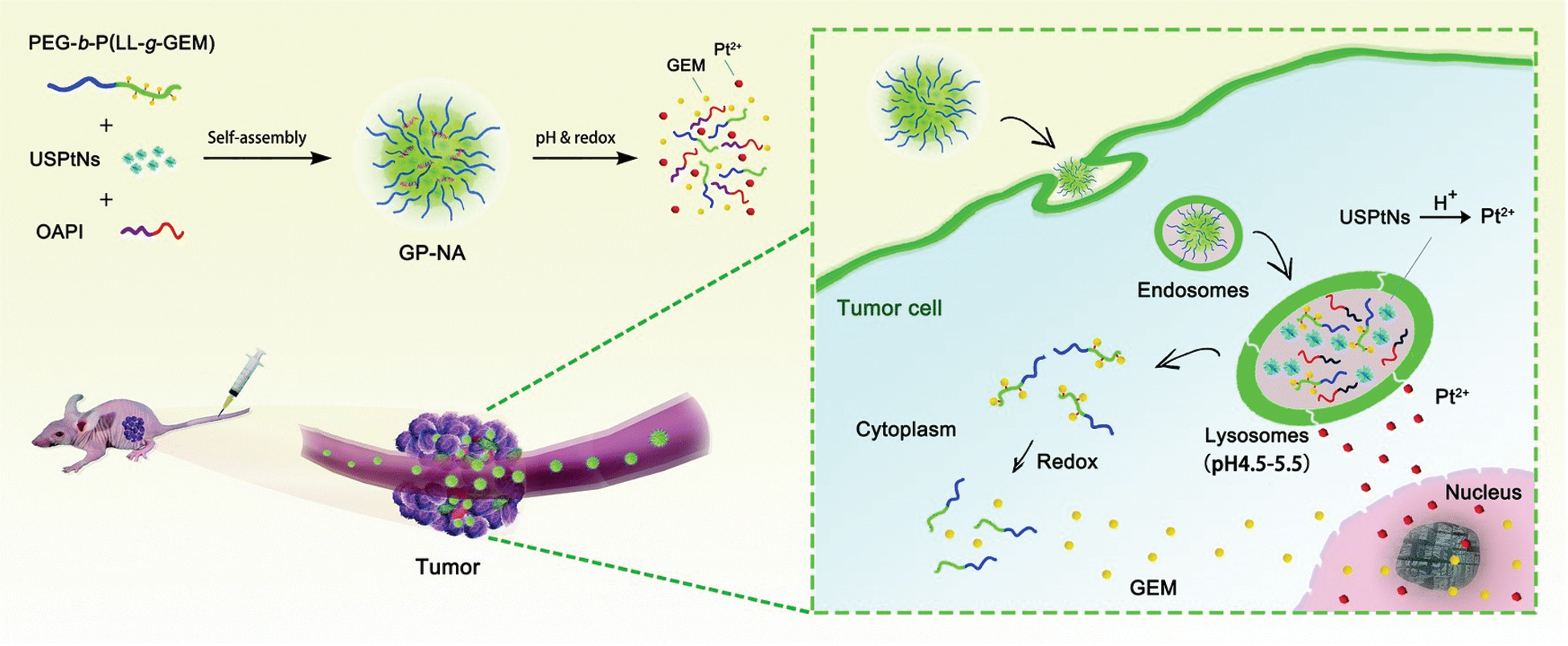
Figure 7: Tumor microenvironmental stimuli in response to biological signals [120]
Five categories of stimuli are recognized: Enzyme-responsive, pH-responsive, redox-responsive, light-responsive, and temperature-responsive. For example, Wang et al. [121] developed a hydrogen peroxide (H2O2)-triggered nanomaterial (LV–TAX/Au@Ag) for combined chemo–photothermal therapy. When LV–TAX/Au@Ag reaches the tumor, endogenous H2O2 in the tumor microenvironment triggers combination therapy by etching silver on the surface of AuNRs. It was observed that these NPs caused extensive necrosis and apoptosis in tumor tissues and greatly inhibited the proliferation of solid tumor cells. Xu et al. [122] developed an original strategy for simultaneous pesticide encapsulation based on emulsion and surface modification of mesoporous silica nanoparticles with carboxymethyl chitosan. pH-responsive release was controlled by carboxymethyl chitosan, and a satisfactory loading (21%) was achieved. Yu et al. [123] first synthesized polylactic acid-graft-acetaldehyde/PEG (PLA-g-ALD/PEG) through a chemical reaction and then synthesized a polymer-drug conjugate nanodrug delivery system. The nano-DDS has remarkable pH sensitivity.
Use of nanotechnology to target tumor stem cells is currently an active field of research that seeks controlled release from nanoparticles for targeted cancer treatment (Fig. 8) [124]. Targeted DDS are generally divided into passive and active targeting, and more finely categorized into passive [125], physical [126], active [127], and bionic targeting [128] based on the underlying targeting principle (Fig. 9) [129]. Passive targeting is based on the physiological and pathological characteristics of the treatment site and intrinsic properties of the nanodrug delivery system to achieve accumulation of the DDS at the treatment site. Passive targeting does actively identify specific sites. Current passive targeted drug delivery strategies include EPR effect [130], intelligent regulation of particle size to decrease or increase aggregation [131,132], intelligent adjustment of surface properties [133], reduction of interstitial tumor pressure [134], and drug release in response to reaching the target site [135]. Li et al. [136] modified PLGA with PEG to prepare two types of biodegradable nanoparticles loaded with salinomycin and docetaxel for sustained release of drugs. Tumor suppression is enhanced compared with single and dual drugs.

Figure 8: Controlled release nanoparticles for the targeted treatment of cancer [124]

Figure 9: Schematic representation of smart drug targeted strategies [129]
Active targeting is the combination of specific molecules, such as proteins, peptides, nucleic acids, antibodies, and small molecules, on the surface of the nanodrug delivery system that interact at the target site to increase effects in target tissues. Currently, active targeted drug delivery strategies involve [130] targeting tumor cells, tumor stem cells, tumor neovascularization, tumor-associated macrophages, other stromal cells, and various other cells. Yang et al. [137] prepared PLGA biodegradable nanoparticles loaded with curcumin and paclitaxel and included modified HA on the surface of the nanoparticles to interact with CD44 receptor on the surface of breast cancer stem cells to stimulate release specifically in breast cancer stem cells and breast cancer cells.
Nature provides abundant materials to meet various needs. The use of natural polymers as described above to synthesize degradable nanomaterials from natural resources and novel nanocomposite materials are widely studied in various research fields. Such research has important practical significance in the context of establishing an environment-friendly society. Development of biodegradable nanomaterials is challenging in terms of scaling production from patents to markets [138]. Challenges come from several aspects. The mechanical properties of degradable materials are still insufficient, and chemical modification is usually required. The application scenarios of biodegradable materials are limited. The current main applications in the biomedical and food fields are drug delivery and food packaging materials/storage containers. In biomedicine, through the chemical modification of polymers, degradable materials are expected to be used in bio-imaging, targeted drug delivery, implantation and tissue engineering. In vitro research and clinical trials are also areas that need to be expanded. In terms of food, degradable materials are expected to be used in new foods, food and feed additives, biocides, pesticides, and food contact materials. The production cost is related to the manufacturing process. The most widely studied silicon nanostructures used in biomedical applications are porous silicon particles and nanosilicon quantum dots, which usually require a multi-step preparation process and lack a convenient preparation process. Black phosphorus, which has recently attracted attention, can be prepared by liquid phase exfoliation to prepare several layers of black phosphorus, which is relatively simple and low in cost and is very advantageous for the production of layers and the biological applications of several layers of two-dimensional black phosphorus nanoparticles [139]. Thus, exploring new nanomaterials is a feasible solution strategy. It is important to show sufficient cost savings to make up for the large amount of early cost investment.
Further, with the development of nanomaterials, human beings are increasingly exposed to nano-level exogenous objects. Even biodegradable nanomaterials need to be evaluated for their safety. Development of packaging materials is one the main applications of nanotechnology in food industry. However, nanoparticles may migrate to food through the mass transfer process, thereby affecting human health. Further research is needed to clarify the migration mechanism. In addition to paying attention to the impact of nanomaterials on human health, it is also necessary to evaluate its impact on ecology. Nano packaging materials or products added with nanoparticles may undergo various modifications such as photochemical conversion, oxidation, reduction, and biodegradation after microbial metabolism. Therefore, attention should be paid to their potential accumulation in the environment. The antibacterial activity of various nanoparticles has been confirmed, but the effect of the antimicrobial effect after treatment on the microbial community in soil and water is still unclear. This requires us to conduct ecotoxicity testing and find more powerful analysis techniques [140]. Lack of standards for nanomaterials introduces the issues of quality control and supervision. These problems place high demands on researchers to move innovations with these materials from the laboratory to general use for the benefit of society. Identification and development of non-toxic, sustainable, and environment-friendly biodegradable nanomaterials will continue to attract substantial interest in the field of polymer materials.
Funding Statement: This research was supported by National Natural Science Foundation of China (31901618), China Postdoctoral Science Foundation (2020M681125), Open Project Fund from Shanghai Collaborative Innovation Center for Aroma, Perfume and Cosmetics Research, and the Estabalishment of the Tabocco Product and Technology Integrated Innovation System for the Southeastern Asia Tobacco Market (2018IA057).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Chen, H., Zheng, Y., Tian, G., Tian, Y., Zeng, X. et al. (2010). Oral delivery of DMAB-modified docetaxel-loaded PLGA-TPGS nanoparticles for cancer chemotherapy. Nanoscale Research Letters, 55(1), 72. DOI 10.1007/s11671-010-9741-8. [Google Scholar] [CrossRef]
2. Gonçalves, R. F. S., Martins, J. T., Duarte, C. M. M., Vicente, A. A., Pinheiro, A. C. (2018). Advances in nutraceutical delivery systems: From formulation design for bioavailability enhancement to efficacy and safety evaluation. Trends in Food Science & Technology, 78(1–2), 270–291. DOI 10.1016/j.tifs.2018.06.011. [Google Scholar] [CrossRef]
3. Li, C., Wang, J., Wang, Y., Gao, H., Wei, G. et al. (2019). Recent progress in drug delivery. Acta Pharmaceutica Sinica B, 9(6), 1145–1162. DOI 10.1016/j.apsb.2019.08.003. [Google Scholar] [CrossRef]
4. Suganya, V., Anuradha, V. (2017). Microencapsulation and nanoencapsulation: A review. International Journal of Pharmaceutical and Clinical Research, 9(3), 233–239. DOI 10.25258/ijpcr.v9i3.8324. [Google Scholar] [CrossRef]
5. Ferrari, M. (2005). Cancer nanotechnology: Opportunities and challenges. Nature Reviews Cancer, 5(3), 161–171. DOI 10.1038/nrc1566. [Google Scholar] [CrossRef]
6. de Jong, W. H., Borm, P. J. (2008). Drug delivery and nanoparticles: Applications and hazards. International Journal of Nanomedicine, 3(2), 133–149. DOI 10.2147/IJN.S596. [Google Scholar] [CrossRef]
7. Janes, K. A., Calvo, P., Alonso, M. J. (2001). Polysaccharide colloidal particles as delivery systems for macromolecules. Advanced Drug Delivery Reviews, 47(1), 83–97. DOI 10.1016/S0169-409X(00)00123-X. [Google Scholar] [CrossRef]
8. Mishra, N., Goyal, A., Khatri, K., Vaidya, B., Paliwal, R. et al. (2008). Biodegradable polymer based particulate carrier(s) for the delivery of proteins and peptides. Anti-Inflammatory & Anti-Allergy Agents in Medicinal Chemistry (Formerly Current Medicinal Chemistry-Anti-Inflammatory and Anti-Allergy Agents), 7(4), 240–251. DOI 10.2174/187152308786847816. [Google Scholar] [CrossRef]
9. Avnesh, K., Sudesh, K. Y., Subhash, C. (2010). Biodegradable polymeric nanoparticles based drug delivery systems. Colloids and Surfaces B: Biointerfaces, 75(1), 1–18. DOI 10.1016/j.colsurfb.2009.09.001. [Google Scholar] [CrossRef]
10. Lu, J. M., Wang, X., Marin-Muller, C., Wang, H., Lin, P. H. et al. (2014). Current advances in research and clinical applications of PLGA-based nanotechnology. Expert Review of Molecular Diagnostics, 9(4), 325–341. DOI 10.1586/erm.09.15. [Google Scholar] [CrossRef]
11. Kamaly, N., Yameen, B., Wu, J., Farokhzad, O. C. (2016). Degradable controlled-release polymers and polymeric nanoparticles: Mechanisms of controlling drug release. Chemical Reviews, 116(4), 2602–2663. DOI 10.1021/acs.chemrev.5b00346. [Google Scholar] [CrossRef]
12. Saadat, M., Zahednezhad, F., Zakeri-Milani, P., Heidari, H. R., Shahbazi-Mojarrad, J. et al. (2019). Drug targeting strategies based on charge dependent uptake of nanoparticles into cancer cells. Journal of Pharmacy & Pharmaceutical Sciences, 22, 191–220. DOI 10.18433/jpps30318. [Google Scholar] [CrossRef]
13. Natour, S., Levi-Zada, A., Abu-Reziq, R. (2019). Magnetic polyurea nano-capsules synthesized via interfacial polymerization in inverse nano-emulsion. Molecules, 24(14), 2663. DOI 10.3390/molecules24142663. [Google Scholar] [CrossRef]
14. Hasani-Sadrabadi, M. M., Karimkhani, V., Majedi, F. S., Van Dersarl, J. J., Dashtimoghadam, E. et al. (2014). Microfluidic-assisted self-assembly of complex dendritic polyethylene drug delivery nanocapsules. Advanced Materials, 26(19), 3118–3123. DOI 10.1002/adma.201305753. [Google Scholar] [CrossRef]
15. El-Habashy, S. E., Allam, A. N., El-Kamel, A. H. (2016). Ethyl cellulose nanoparticles as a platform to decrease ulcerogenic potential of piroxicam: Formulation and in vitro/in vivo evaluation. International Journal of Nanomedicine, 11, 2369–2380. DOI 10.2147/IJN.S93354. [Google Scholar] [CrossRef]
16. Ma, X. M., Zhu, Y. C., Yang, P., Wei, Z. P., Liu, P. et al. (2016). Bio-inspired fabrication and potential applications of hierarchically porous CaCO3 hollow nanospheres. New Journal of Chemistry, 40(8), 6874–6880. DOI 10.1039/C6NJ00558F. [Google Scholar] [CrossRef]
17. Dowling, A. P. (2004). Development of nanotechnologies. Materials Today, 7(12), 30–35. DOI 10.1016/S1369-7021(04)00628-5. [Google Scholar] [CrossRef]
18. Lai, D. Y. (2012). Toward toxicity testing of nanomaterials in the 21st century: A paradigm for moving forward. Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology, 4(1), 1–15. DOI 10.1002/wnan.162. [Google Scholar] [CrossRef]
19. Podila, R., Brown, J. M. (2013). Toxicity of engineered nanomaterials: A physicochemical perspective. Journal of Biochemical and Molecular Toxicology, 27(1), 50–55. DOI 10.1002/jbt.21442. [Google Scholar] [CrossRef]
20. Pope, C. A., Burnett, R. T., Thurston, G. D., Thun, M. J., Calle, E. E. et al. (2004). Cardiovascular mortality and long-term exposure to particulate air pollution: Epidemiological evidence of general pathophysiological pathways of disease. Circulation, 109(1), 71–77. DOI 10.1161/01.CIR.0000108927.80044.7F. [Google Scholar] [CrossRef]
21. Driscoll, K. E., Carter, J. M., Howard, B. W., Hassenbein, D. G., Pepelko, W. et al. (1996). Pulmonary inflammatory, chemokine, and mutagenic responses in rats after subchronic inhalation of carbon black. Toxicology and Applied Pharmacology, 136(2), 372–380. DOI 10.1006/taap.1996.0045. [Google Scholar] [CrossRef]
22. Borm, P. J., Schins, R. P., Albrecht, C. (2004). Inhaled particleas and lung cancer, Part B: paradigms and risk assessment. International Journal of Cancer, 110(1), 3–14. DOI 10.1002/ijc.20064. [Google Scholar] [CrossRef]
23. Silvestre, C., Duraccio, D., Cimmino, S. (2011). Food packaging based on polymer nanomaterials. Progress in Polymer Science, 36(12), 1766–1782. DOI 10.1016/j.progpolymsci.2011.02.003. [Google Scholar] [CrossRef]
24. Castro-Ceseña, A. B., Novitskaya, E. E., Phadke, A., Varghese, S., McKittrick, J. (2013). Isolation of collagen from cortical bovine bone for preparation of porous collagen sponges. New York: Springer. [Google Scholar]
25. Rogovina, S. Z., Aleksanyan, K. V., Vladimirov, L. V., Berlin, A. A. (2019). Biodegradable polymer materials based on polylactide. Russian Journal of Physical Chemistry B, 13(5), 812–818. DOI 10.1134/S1990793119050099. [Google Scholar] [CrossRef]
26. McClements, D. J., Decker, E. A., Park, Y., Weiss, J. (2009). Structural design principles for delivery of bioactive components in nutraceuticals and functional foods. Critical Reviews in Food Science and Nutrition, 49(6), 577–606. DOI 10.1080/10408390902841529. [Google Scholar] [CrossRef]
27. Shin, G. H., Kim, J. T., Park, H. J. (2015). Recent developments in nanoformulations of lipophilic functional foods. Trends in Food Science & Technology, 46(1), 144–157. DOI 10.1016/j.tifs.2015.07.005. [Google Scholar] [CrossRef]
28. Abd-Elhalem, S. S., El-Shinnawy, N. A., Abu-El Magd, E. E., El Zawawy, W. K., Haggag, N. Z. (2020). Application of either nano fibrillated cellulose methotrexate or nano silicon dioxide methotrexate composites against renal fibrosis in leukemia rat model. International Journal of Biological Macromolecules, 157(11), 329–339. DOI 10.1016/j.ijbiomac.2020.04.110. [Google Scholar] [CrossRef]
29. Zikmundova, M., Vereshaka, M., Kolarova, K., Pajorova, J., Svorcik, V. et al. (2020). Effects of bacterial nanocellulose loaded with Curcumin and its degradation products on human dermal fibroblasts. Materials, 13(21), 4759. DOI 10.3390/ma13214759. [Google Scholar] [CrossRef]
30. Müller, A., Ni, Z., Hessler, N., Wesarg, F., Müller, F. A. et al. (2013). The biopolymer bacterial nanocellulose as drug delivery system: Investigation of drug loading and release using the model protein albumin. Journal of Pharmaceutical Sciences, 102(2), 579–592. DOI 10.1002/jps.23385. [Google Scholar] [CrossRef]
31. Mohanta, V., Madras, G., Patil, S. (2014). Layer-by-layer assembled thin films and microcapsules of nanocrystalline cellulose for hydrophobic drug delivery. ACS Applied Materials & Interfaces, 6(22), 20093–20101. DOI 10.1021/am505681e. [Google Scholar] [CrossRef]
32. Qing, W. X., Wang, Y., Wang, Y. Y., Zhao, D. B., Liu, X. H. et al. (2016). The modified nanocrystalline cellulose for hydrophobic drug delivery. Applied Surface Science, 366, 404–409. DOI 10.1016/j.apsusc.2016.01.133. [Google Scholar] [CrossRef]
33. Simi, C. K., Emilia Abraham, T. (2007). Hydrophobic grafted and cross-linked starch nanoparticles for drug delivery. Bioprocess and Biosystems Engineering, 30(3), 173–180. DOI 10.1007/s00449-007-0112-5. [Google Scholar] [CrossRef]
34. El-Naggar, M. E., El-Rafie, M. H., El-sheikh, M. A., El-Feky, G. S., Hebeish, A. (2015). Synthesis, characterization, release kinetics and toxicity profile of drug-loaded starch nanoparticles. International Journal of Biological Macromolecules, 81(2), 718–729. DOI 10.1016/j.ijbiomac.2015.09.005. [Google Scholar] [CrossRef]
35. Yang, J., Huang, Y., Gao, C., Liu, M., Zhang, X. (2014). Fabrication and evaluation of the novel reduction-sensitive starch nanoparticles for controlled drug release. Colloids and Surfaces B: Biointerfaces, 115, 368–376. DOI 10.1016/j.colsurfb.2013.12.007. [Google Scholar] [CrossRef]
36. Rai, S., Singh, B. K., Bhartiya, P., Singh, A., Kumar, H. et al. (2017). Lignin derived reduced fluorescence carbon dots with theranostic approaches: Nano-drug-carrier and bioimaging. Journal of Luminescence, 190, 492–503. DOI 10.1016/j.jlumin.2017.06.008. [Google Scholar] [CrossRef]
37. Choi, D., Heo, J., Park, J. H., Jo, Y., Jeong, H. et al. (2016). Nano-film coatings onto collagen hydrogels with desired drug release. Journal of Industrial and Engineering Chemistry, 36, 326–333. DOI 10.1016/j.jiec.2016.02.023. [Google Scholar] [CrossRef]
38. Janes, K. A., Fresneau, M. P., Marazuela, A., Fabra, A., Alonso, M. J. (2001). Chitosan nanoparticles as delivery systems for doxorubicin. Journal of Controlled Release, 73(2−3), 255–267. DOI 10.1016/S0168-3659(01)00294-2. [Google Scholar] [CrossRef]
39. Mitra, S., Gaur, U., Ghosh, P. C., Maitra, A. N. (2001). Tumour targeted delivery of encapsulated dextran-doxorubicin conjugate using chitosan nanoparticles as carrier. Journal of Controlled Release, 74(1−3), 317–323. DOI 10.1016/S0168-3659(01)00342-X. [Google Scholar] [CrossRef]
40. Sriram, K., Maheswari, P. U., Begum, K. M. M. S., Arthanareeswaran, G. (2018). Functionalized chitosan with super paramagnetic hybrid nanocarrier for targeted drug delivery of curcumin. Iranian Polymer Journal, 27(7), 469–482. DOI 10.1007/s13726-018-0624-7. [Google Scholar] [CrossRef]
41. Farhangi, M., Kobarfard, F., Mahboubi, A., Vatanara, A., Mortazavi, S. A. (2018). Preparation of an optimized ciprofloxacin-loaded chitosan nanomicelle with enhanced antibacterial activity. Drug Development and Industrial Pharmacy, 44(8), 1273–1284. DOI 10.1080/03639045.2018.1442847. [Google Scholar] [CrossRef]
42. Kim, H. S., Kim, S. J., Kang, J. H., Shin, U. S. (2018). Positively and negatively charged collagen nanohydrogels: pH-responsive Drug-releasing characteristics. Bulletin of the Korean Chemical Society, 39(4), 477–482. DOI 10.1002/bkcs.11412. [Google Scholar] [CrossRef]
43. Tang, L., Fu, L., Zhu, Z., Yang, Y., Sun, B. et al. (2018). Modified mixed nanomicelles with collagen peptides enhanced oral absorption of Cucurbitacin B: Preparation and evaluation. Drug Delivery, 25(1), 862–871. DOI 10.1080/10717544.2018.1425773. [Google Scholar] [CrossRef]
44. Roussaki, M., Gaitanarou, A., Diamanti, P. C., Vouyiouka, S., Papaspyrides, C. et al. (2014). Encapsulation of the natural antioxidant aureusidin in biodegradable PLA nanoparticles. Polymer Degradation and Stability, 108(3), 182–187. DOI 10.1016/j.polymdegradstab.2014.08.004. [Google Scholar] [CrossRef]
45. Yao, J., Cui, B., Zhao, X., Zhi, H., Zeng, Z. et al. (2018). Antagonistic effect of Azoxystrobin Poly (Lactic Acid) microspheres with controllable particle size on Colletotrichum higginsianum Sacc. Nanomaterials, 8(10), 857. DOI 10.3390/nano8100857. [Google Scholar] [CrossRef]
46. Castro-Ceseña, A. B., Novitskaya, E. E., Phadke, A., Varghese, S., Mckittrick, J. (2013). Isolation of collagen from cortical bovine bone for preparation of porous collagen sponges. New York: Springer. [Google Scholar]
47. Thomas, B., Raj, M. C., Athira, K. B., Rubiyah, M. H., Joy, J. et al. (2018). Nanocellulose, a versatile green platform: From biosources to materials and their applications. Chemical Reviews, 118(24), 11575–11625. DOI 10.1021/acs.chemrev.7b00627. [Google Scholar] [CrossRef]
48. Dufresne, A. (2013). Nanocellulose: A new ageless bionanomaterial. Materials Today, 16(6), 220–227. DOI 10.1016/j.mattod.2013.06.004. [Google Scholar] [CrossRef]
49. Aminabhavi, T. M., Balundgi, R. H., Cassidy, P. E. (1990). A review on biodegradable plastics. Polymer-Plastics Technology and Engineering, 29(3), 235–262. DOI 10.1080/03602559008049843. [Google Scholar] [CrossRef]
50. Maddever, W. J., Chapman, G. (1989). Modified starch-based biodegradable plastics. Plastics Engineering, 45(7), 31–33. [Google Scholar]
51. Le Corre, D., Bras, J., Dufresne, A. (2010). Starch nanoparticles: A review. Biomacromolecules, 11(5), 1139–1153. DOI 10.1021/bm901428y. [Google Scholar] [CrossRef]
52. Lupoi, J. S., Singh, S., Parthasarathi, R., Simmons, B. A., Henry, R. J. (2015). Recent innovations in analytical methods for the qualitative and quantitative assessment of lignin. Renewable and Sustainable Energy Reviews, 49(7), 871–906. DOI 10.1016/j.rser.2015.04.091. [Google Scholar] [CrossRef]
53. Rahul, D., Aditi, K., Divyashri, B., Ali, M., Amitava, M. et al. (2017). Enzymatic degradation of lignin in soil: A review. Sustainability, 9(7), 1163. DOI 10.3390/su9071163. [Google Scholar] [CrossRef]
54. Camargos, C. H. M., Silva, R. A. P., Csordas, Y., Silva, L. L., Rezende, C. A. (2019). Experimentally designed corn biomass fractionation to obtain lignin nanoparticles and fermentable sugars. Industrial Crops and Products, 140(80), 111649–111658. DOI 10.1016/j.indcrop.2019.111649. [Google Scholar] [CrossRef]
55. Kumar, M. N. V. R. (2000). A review of chitin and chitosan applications. Reactive and Functional Polymers, 46(1), 1–27. DOI 10.1016/S1381-5148(00)00038-9. [Google Scholar] [CrossRef]
56. Kumari, S., Rath, P. K. (2014). Extraction and characterization of Chitin and Chitosan from (Labeo rohit) fish scales. Procedia Materials Science, 6, 482–489. DOI 10.1016/j.mspro.2014.07.062. [Google Scholar] [CrossRef]
57. Habibi, A., Karami, S., Varmira, K., Hadadi, M. (2020). Key parameters optimization of chitosan production from Aspergillus terreus using apple waste extract as sole carbon source. Bioprocess and Biosystems Engineering, 1–13. DOI 10.1007/s00449-020-02441-2. [Google Scholar] [CrossRef]
58. Bozkir, A., Saka, O. M. (2008). Chitosan nanoparticles for plasmid DNA delivery: Effect of chitosan molecular structure on formulation and release characteristics. Drug Delivery, 11(2), 107–112. DOI 10.1080/10717540490280705. [Google Scholar] [CrossRef]
59. Bodnár, M., Hartmann, J. F., Borbély, J. (2005). Nanoparticles from Chitosan. Macromolecular Symposia, 227(1), 321–326. DOI 10.1002/masy.200550932. [Google Scholar] [CrossRef]
60. Rampino, A., Borgogna, M., Blasi, P., Bellich, B., Cesaro, A. (2013). Chitosan nanoparticles: Preparation, size evolution and stability. International Journal of Pharmaceutics, 455(1−2), 219–228. DOI 10.1016/j.ijpharm.2013.07.034. [Google Scholar] [CrossRef]
61. Eyre, D. R., Wu, J. J. (2005). Collagen cross-links. Collagen: Primer in structure, processing and assembly. Berlin: Springer Berlin Heidelberg. [Google Scholar]
62. Kielty, C. M., Grant, M. E. (2002). The collagen family: Structure, assembly, and organization in the extracellular matrix. Connective tissue and its heritable disorders. New York: John Wiley & Sons. [Google Scholar]
63. Fullana, M. J., Wnek, G. E. (2012). Electrospun collagen and its applications in regenerative medicine. Drug Delivery and Translational Research, 2(5), 313–322. DOI 10.1007/s13346-012-0087-x. [Google Scholar] [CrossRef]
64. Sionkowska, A., Skrzynski, S., Smiechowski, K., Kolodziejczak, A. (2017). The review of versatile application of collagen. Polymers for Advanced Technologies, 28(1), 4–9. DOI 10.1002/pat.3842. [Google Scholar] [CrossRef]
65. Akturk, O., Tezcaner, A., Bilgili, H., Deveci, M. S., Gecit, M. R. et al. (2011). Evaluation of sericin/collagen membranes as prospective wound dressing biomaterial. Journal of Bioscience and Bioengineering, 112(3), 279–288. DOI 10.1016/j.jbiosc.2011.05.014. [Google Scholar] [CrossRef]
66. Hayashi, Y., Yamada, S., Yanagi Guchi, K., Koyama, Z., Ikeda, T. (2012). Chapter 6–Chitosan and fish collagen as biomaterials for regenerative medicine. Advances in food and nutrition research. US: Academic Press. [Google Scholar]
67. Kruger, T. E., Miller, A. H., Wang, J. (2013). Collagen scaffolds in bone sialoprotein-mediated bone regeneration. Scientific World Journal, 2013(2), 1–6. DOI 10.1155/2013/812718. [Google Scholar] [CrossRef]
68. Norazlina, H., Kamal, Y. (2015). Graphene modifications in polylactic acid nanocomposites: A review. Polymer Bulletin, 72(4), 931–961. DOI 10.1007/s00289-015-1308-5. [Google Scholar] [CrossRef]
69. Lee, S. H., Kim, I. Y., Song, W. S. (2014). Biodegradation of polylactic acid (PLA) fibers using different enzymes. Macromolecular Research, 22(6), 657–663. DOI 10.1007/s13233-014-2107-9. [Google Scholar] [CrossRef]
70. Pastorino, L., Dellacasa, E., Petrini, P., Monticelli, O. (2017). Stereocomplex poly(lactic acid) nanocoated chitosan microparticles for the sustained release of hydrophilic drugs. Materials Science and Engineering: C, 76(8), 1129–1135. DOI 10.1016/j.msec.2017.03.170. [Google Scholar] [CrossRef]
71. Li, R. W., Deng, L., Cai, Z. W., Zhang, S. Y., Wang, K. et al. (2017). Liposomes coated with thiolated chitosan as drug carriers of curcumin. Materials Science and Engineering: C, 80, 156–164. DOI 10.1016/j.msec.2017.05.136. [Google Scholar] [CrossRef]
72. Han, S. M., Baek, J. S., Kim, M. S., Hwang, S. J., Cho, C. W. (2018). Surface modification of paclitaxel-loaded liposomes using d-α-tocopheryl polyethylene glycol 1000 succinate: Enhanced cellular uptake and cytotoxicity in multidrug resistant breast cancer cells. Chemistry and Physics of Lipids, 213, 39–47. DOI 10.1016/j.chemphyslip.2018.03.005. [Google Scholar] [CrossRef]
73. Hsieh, Y. F., Chen, T. L., Wang, Y. T., Chang, J. H., Chang, H. M. (2002). Properties of liposomes prepared with various lipids. Journal of Food Science, 67(8), 2808–2813. DOI 10.1111/j.1365-2621.2002.tb08820.x. [Google Scholar] [CrossRef]
74. Ghorbanzade, T., Jafari, S. M., Akhavan, S., Hadavi, R. (2017). Nano-encapsulation of fish oil in nano-liposomes and its application in fortification of yogurt. Food Chemistry, 216(3), 146–152. DOI 10.1016/j.foodchem.2016.08.022. [Google Scholar] [CrossRef]
75. Khosravi-Darani, K., Khoosfi, M. E., Hosseini, H. (2016). Encapsulation of Zataria multiflora Boiss. Essential oil in Liposome: Antibacterial activity against E. Coli O157: H7 in Broth Media and Minced Beef. Journal of Food Safety, 36(4), 515–523. DOI 10.1111/jfs.12271. [Google Scholar] [CrossRef]
76. Vijayan, V., Reddy, K. R., Sakthivel, S., Swetha, C. (2013). Optimization and charaterization of repaglinide biodegradable polymeric nanoparticle loaded transdermal patchs: in vitro and in vivo studies. Colloids and Surfaces B: Biointerfaces, 111(1), 150–155. DOI 10.1016/j.colsurfb.2013.05.020. [Google Scholar] [CrossRef]
77. Yuan, Y. K., Li, H., Liu, C. Z., Zhang, S. Z., Xu, Y. et al. (2019). Fabrication and characterization of Lutein-loaded nanoparticles based on Zein and Sophorolipid: Enhancement of water solubility, stability, and bioaccessibility. Journal of Agricultural and Food Chemistry, 67(43), 11977–11985. DOI 10.1021/acs.jafc.9b05175. [Google Scholar] [CrossRef]
78. Luo, Y., Teng, Z., Wang, Q. (2012). Development of zein nanoparticles coated with carboxymethyl chitosan for encapsulation and controlled release of Vitamin D3. Journal of Agricultural and Food Chemistry, 60(3), 836–843. DOI 10.1021/jf204194z. [Google Scholar] [CrossRef]
79. Alishahi, A., Mirvaghefi, A., Tehrani, M. R., Farahmand, H., Koshio, S. et al. (2011). Chitosan nanoparticle to carry vitamin C through the gastrointestinal tract and induce the non-specific immunity system of rainbow trout (Oncorhynchus mykiss). Carbohydrate Polymers, 86(1), 142–146. DOI 10.1016/j.carbpol.2011.04.028. [Google Scholar] [CrossRef]
80. Liu, B. X., Wang, Y., Yang, F., Wang, X., Shen, H. et al. (2016). Construction of a controlled-release delivery system for pesticides using biodegradable PLA-based microcapsules. Colloids and Surfaces B: Biointerfaces, 144, 38–45. DOI 10.1016/j.colsurfb.2016.03.084. [Google Scholar] [CrossRef]
81. Cao-Hoang, L., Fougère, R., Waché, Y. (2011). Increase in stability and change in supramolecular structure of β-carotene through encapsulation into polylactic acid nanoparticles. Food Chemistry, 124(1), 42–49. DOI 10.1016/j.foodchem.2010.05.100. [Google Scholar] [CrossRef]
82. Ibrahim, K., Alan, B., Iza, R., Marek, K., Tamara, K. et al. (2017). Bacterial-derived polymer Poly-y-Glutamic Acid (y-PGA)-based micro/nanoparticles as a delivery system for Antimicrobials and other biomedical applications. International Journal of Molecular Sciences, 18(2), 313. DOI 10.3390/ijms18020313. [Google Scholar] [CrossRef]
83. Zhang, P., Hu, L., Yin, Q., Zhang, Z., Feng, L. et al. (2012). Transferrin-conjugated polyphosphoester hybrid micelle loading paclitaxel for brain-targeting delivery: Synthesis, preparation and in vivo evaluation. Journal of Controlled Release, 159(3), 429–434. DOI 10.1016/j.jconrel.2012.01.031. [Google Scholar] [CrossRef]
84. Chen, Y., Peng, F., Song, X., Wu, J., Yao, W. et al. (2018). Conjugation of paclitaxel to C-6 hexanediamine-modified hyaluronic acid for targeted drug delivery to enhance antitumor efficacy. Carbohydrate Polymers, 181(2), 150–158. DOI 10.1016/j.carbpol.2017.09.017. [Google Scholar] [CrossRef]
85. Harada, M., Ohuchi, M., Hayashi, T., Kato, Y. (2011). Prolonged circulation and in vivo efficacy of recombinant human granulocyte colony-stimulating factor encapsulated in polymeric micelles. Journal of Controlled Release, 156(1), 101–108. DOI 10.1016/j.jconrel.2011.06.024. [Google Scholar] [CrossRef]
86. Tang, S., Meng, Q., Sun, H., Su, J., Yin, Q. et al. (2017). Dual pH-sensitive micelles with charge-switch for controlling cellular uptake and drug release to treat metastatic breast cancer. Biomaterials, 114, 44–53. DOI 10.1016/j.biomaterials.2016.06.005. [Google Scholar] [CrossRef]
87. Wang, X., Chen, Y., Dahmani, F. Z., Yin, L., Zhou, J. et al. (2014). Amphiphilic carboxymethyl chitosan-quercetin conjugate with P-gp inhibitory properties for oral delivery of paclitaxel. Biomaterials, 35(26), 7654–7665. DOI 10.1016/j.biomaterials.2014.05.053. [Google Scholar] [CrossRef]
88. Ghasemi, S., Abbasi, S. (2014). Formation of natural casein micelle nanocapsule by means of pH changes and ultrasound. Food Hydrocolloids, 42(1), 42–47. DOI 10.1016/j.foodhyd.2013.10.028. [Google Scholar] [CrossRef]
89. Levinson, Y., Ish-Shalom, S., Segal, E., Livney, Y. D. (2016). Bioavailability, rheology and sensory evaluation of fat-free yogurt enriched with VD 3 encapsulated in re-assembled casein micelles. Food & Function, 7(3), 1477–1482. DOI 10.1039/C5FO01111F. [Google Scholar] [CrossRef]
90. Jhaveri, A. M., Torchilin, V. P. (2014). Multifunctional polymeric micelles for delivery of drugs and siRNA. Frontiers in Pharmacology, 5, 994. DOI 10.3389/fphar.2014.00077. [Google Scholar] [CrossRef]
91. Severino, R., Ferrari, G., Vu, K. D., Donsì, F., Salmieri, S. et al. (2015). Antimicrobial effects of modified chitosan based coating containing nanoemulsion of essential oils, modified atmosphere packaging and gamma irradiation against Escherichia coli O157: H7 and Salmonella Typhimurium on green beans. Food Control, 50, 215–222. DOI 10.1016/j.foodcont.2014.08.029. [Google Scholar] [CrossRef]
92. Lin, Y. H., Chiou, S. F., Lai, C. H., Tsai, S. C., Chou, C. W. et al. (2012). Formulation and evaluation of water-in-oil amoxicillin-loaded nanoemulsions using for Helicobacter pylori eradication. Process Biochemistry, 47(10), 1469–1478. DOI 10.1016/j.procbio.2012.05.019. [Google Scholar] [CrossRef]
93. Najlah, M., Kadam, A., Wan, K. W., Ahmed, W., Taylor, K. M. et al. (2016). Novel paclitaxel formulations solubilized by parenteral nutrition nanoemulsions for application against glioma cell lines. International Journal of Pharmaceutics, 506(1–2), 102–109. DOI 10.1016/j.ijpharm.2016.04.027. [Google Scholar] [CrossRef]
94. Chaiyana, W., Anuchapreeda, S., Leelapornpisid, P., Phongpradist, R., Viernstein, H. et al. (2017). Development of Microemulsion delivery system of essential oil from Zingiber cassumunar Roxb. Rhizome for improvement of stability and anti-inflammatory activity. AAPS PharmSciTech, 18(4), 1332–1342. DOI 10.1208/s12249-016-0603-2. [Google Scholar] [CrossRef]
95. Cerda-Opazo, P., Gotteland, M., Oyarzun-Ampuero, F. A., Garcia, L. (2021). Design, development and evaluation of nanoemulsion containing avocado peel extract with anticancer potential: A novel biological active ingredient to enrich food. Food Hydrocolloids, 111(8), 106370. DOI 10.1016/j.foodhyd.2020.106370. [Google Scholar] [CrossRef]
96. Costa, A. M., Bueno, K. T. L., Rosa, A. P. C. D., Costa, J. A. V. (2019). The antioxidant activity of nanoemulsions based on lipids and peptides from Spirulina sp. LEB18. LWT, 99(109), 173–178. DOI 10.1016/j.lwt.2018.09.069. [Google Scholar] [CrossRef]
97. Raikos, V., Hayward, N., Hayes, H., Meroni, E., Ranawana, V. (2019). Optimising the ratio of long- to short-chain triglycerides of the lipid phase to enhance physical stability and bioaccessibility of lycopene-loaded beverage emulsions. International Journal of Food Science & Technology, 54(4), 1355–1362. DOI 10.1111/ijfs.14024. [Google Scholar] [CrossRef]
98. Marzuki, N. H. C., Wahab, R. A., Hamid, M. A. (2019). An overview of nanoemulsion: Concepts of development and cosmeceutical applications. Biotechnology & Biotechnological Equipment, 33(1), 779–797. DOI 10.1080/13102818.2019.1620124. [Google Scholar] [CrossRef]
99. Erik, B., Jason, C., Anthony, L. (2011). Emerging technologies of polymeric nanoparticles in cancer drug delivery. Journal of Nanomaterials, 2011(9), 1–10. DOI 10.1155/2011/408675. [Google Scholar] [CrossRef]
100. Wang, X. F., Zhang, S. L., Zhu, L. Y., Xie, S. Y., Dong, Z. et al. (2012). Enhancement of antibacterial activity of tilmicosin against Staphylococcus aureus by solid lipid nanoparticles in vitro and in vivo. Veterinary Journal, 191(1), 115–120. DOI 10.1016/j.tvjl.2010.11.019. [Google Scholar] [CrossRef]
101. Shi, F., Zhao, J. H., Liu, Y., Wang, Z., Zhang, Y. T. et al. (2012). Preparation and characterization of solid lipid nanoparticles loaded with frankincense and myrrh oil. International Journal of Nanomedicine, 7, 2033–2043. DOI 10.2147/IJN.S30085. [Google Scholar] [CrossRef]
102. Shtay, R., Tan, C. P., Schwarz, K. (2018). Development and characterization of solid lipid nanoparticles (SLNs) made of cocoa butter: A factorial design study. Journal of Food Engineering, 231(39), 30–41. DOI 10.1016/j.jfoodeng.2018.03.006. [Google Scholar] [CrossRef]
103. Yang, J. S., Ciftci, O. N. (2020). In vitrobioaccessibility of fish oil-loaded hollow solid lipid micro- and nanoparticles. Food & Function, 11(10), 8637–8647. DOI 10.1039/D0FO01591A. [Google Scholar] [CrossRef]
104. Shtay, R., Keppler, J. K., Schrader, K., Schwarz, K. (2019). Encapsulation of (─)-epigallocatechin-3-gallate (EGCG) in solid lipid nanoparticles for food applications. Journal of Food Engineering, 244(5), 91–100. DOI 10.1016/j.jfoodeng.2018.09.008. [Google Scholar] [CrossRef]
105. Zardini, A. A., Mohebbi, M., Farhoosh, R., Bolurian, S. (2018). Production and characterization of nanostructured lipid carriers and solid lipid nanoparticles containing lycopene for food fortification. Journal of Food Science and Technology-Mysore, 55(1), 287–298. DOI 10.1007/s13197-017-2937-5. [Google Scholar] [CrossRef]
106. Mishra, V., Bansal, K. K., Verma, A., Yadav, N., Thakur, S. et al. (2018). Solid lipid nanoparticles: Emerging colloidal nano drug delivery systems. Pharmaceutics, 10(4), 191. DOI 10.3390/pharmaceutics10040191. [Google Scholar] [CrossRef]
107. Patra, J. K., Baek, K. H. (2014). Green nanobiotechnology: Factors affecting synthesis and characterization techniques. Journal of Nanomaterials, 2014(6), 1–12. DOI 10.1155/2014/417305. [Google Scholar] [CrossRef]
108. Devatha, C. P., Thalla, A. K. (2018). Green synthesis of nanomaterials. Synthesis of inorganic nanomaterials. UK: Woodhead Publishing. [Google Scholar]
109. Rafique, M., Sadaf, I., Rafique, M. S., Tahir, M. B. (2017). A review on green synthesis of silver nanoparticles and their applications. Artificial Cells, Nanomedicine, and Biotechnology, 45(7), 1272–1291. DOI 10.1080/21691401.2016.1241792. [Google Scholar] [CrossRef]
110. Roychoudhury, P., Ghosh, S., Pal, R. (2016). Cyanobacteria mediated green synthesis of gold-silver nanoalloy. Journal of Plant Biochemistry and Biotechnology, 25(1), 73–78. DOI 10.1007/s13562-015-0311-0. [Google Scholar] [CrossRef]
111. Netala, V. R., Kotakadi, V. S., Bobbu, P., Gaddam, S. A., Tartte, V. (2016). Endophytic fungal isolate mediated biosynthesis of silver nanoparticles and their free radical scavenging activity and anti microbial studies. 3 Biotech, 6(2), 144. DOI 10.1007/s13205-016-0433-7. [Google Scholar] [CrossRef]
112. Eswaramma, S., Rao, K. S. (2017). Synthesis of dual responsive carbohydrate polymer based IPN microbeads for controlled release of anti-HIV drug. Carbohydrate Polymers, 156(3), 125–134. DOI 10.1016/j.carbpol.2016.09.023. [Google Scholar] [CrossRef]
113. Manatunga, D. C., de Silva, R. M., de Silva, K. M. N.,de Silva, N.,Bhandari, S. et al. (2017). pH responsive controlled release of anti-cancer hydrophobic drugs from sodium alginate and hydroxyapatite bi-coated iron oxide nanoparticles. European Journal of Pharmaceutics and Biopharmaceutics, 117(81), 29–38. DOI 10.1016/j.ejpb.2017.03.014. [Google Scholar] [CrossRef]
114. Shao, L., Hua, B., Sun, J. F., Li, Q., Yang, J. et al. (2017). A cucurbit[7]uril-based supra-amphiphile: Photo-responsive self-assembly and application in controlled release. Tetrahedron Letters, 58(19), 1863–1867. DOI 10.1016/j.tetlet.2017.03.091. [Google Scholar] [CrossRef]
115. Zhang, C., Pan, D., Li, J., Hu, J., Bains, A. et al. (2017). Enzyme-responsive peptide dendrimer-gemcitabine conjugate as a controlled-release drug delivery vehicle with enhanced antitumor efficacy. Acta Biomaterialia, 55, 153–162. DOI 10.1016/j.actbio.2017.02.047. [Google Scholar] [CrossRef]
116. Zhou, Y., Jie, K., Huang, F. (2017). A redox-responsive selenium-containing pillar[5]arene-based macrocyclic amphiphile: Synthesis, controllable self-assembly in water, and application in controlled release. Chemical Communications, 53(59), 8364–8367. DOI 10.1039/C7CC04779G. [Google Scholar] [CrossRef]
117. Sukhorukov, G., Fery, A., Möhwaldb, H. (2005). Intelligent micro- and nanocapsules. Progress in Polymer Science, 30(8−9), 885–897. DOI 10.1016/j.progpolymsci.2005.06.008. [Google Scholar] [CrossRef]
118. Esser-Kahn, A. P., Odom, S. A., Sottos, N. R., White, S. R., Moore, J. S. (2011). ChemInform abstract: Triggered release from polymer capsules. ChemInform, 42(39). DOI 10.1002/chin.201139276. [Google Scholar] [CrossRef]
119. Kost, J., Langer, R. (1991). Responsive polymeric delivery systems. Advanced Drug Delivery Reviews, 6(1), 19–50. DOI 10.1016/0169-409X(91)90030-G. [Google Scholar] [CrossRef]
120. Shi, H., Xu, M., Zhu, J., Li, Y., He, Z. et al. (2020). Programmed co-delivery of platinum nanodrugs and gemcitabine by a clustered nanocarrier for precision chemotherapy for NSCLC tumors. Journal of Materials Chemistry B, 8(2), 332–342. DOI 10.1039/C9TB02055A. [Google Scholar] [CrossRef]
121. Wang, K. Y., Cai, Z. Y., Fan, R., Yang, Q., Zhu, T. et al. (2020). A tumor-microenvironment-responsive nanomaterial for cancer chemo-photothermal therapy. RSC Advances, 10(37), 22091–22101. DOI 10.1039/D0RA04171H. [Google Scholar] [CrossRef]
122. Xu, C. L., Cao, L. D., Zhao, P. Y., Zhou, Z. L., Cao, C. et al. (2018). Emulsion-based synchronous pesticide encapsulation and surface modification of mesoporous silica nanoparticles with carboxymethyl chitosan for controlled azoxystrobin release. Chemical Engineering Journal, 348, 244–254. DOI 10.1016/j.cej.2018.05.008. [Google Scholar] [CrossRef]
123. Yu, Y., Chen, C. K., Law, W. C., Sun, H., Prasad, P. N. et al. (2015). A degradable brush polymer–drug conjugate for pH-responsive release of doxorubicin. Polymer Chemistry, 6(6), 953–961. DOI 10.1039/C4PY01194E. [Google Scholar] [CrossRef]
124. Liu, C., Ewert, K. K., Yao, W., Wang, N., Li, Y. et al. (2019). A multifunctional lipid incorporating active targeting and dual-control release capabilities for precision drug delivery. ACS Applied Materials & Interfaces, 12(1), 70–85. DOI 10.1021/acsami.9b14470. [Google Scholar] [CrossRef]
125. Guan, X., Guo, Z., Wang, T., Lin, L., Chen, J. et al. (2017). A pH-responsive detachable PEG shielding strategy for gene delivery system in cancer therapy. Biomacromolecules, 18(4), 1342–1349. DOI 10.1021/acs.biomac.7b00080. [Google Scholar] [CrossRef]
126. Tay, Z. W., Chandrasekharan, P., Chiu-Lam, A., Hensley, D. W., Dhavalikar, R. et al. (2018). Magnetic particle imaging-guided heating in vivo using gradient fields for arbitrary localization of magnetic hyperthermia therapy. ACS Nano, 12(4), 3699–3713. DOI 10.1021/acsnano.8b00893. [Google Scholar] [CrossRef]
127. Yang, R., Xu, J., Xu, L., Sun, X., Chen, Q. et al. (2018). Cancer cell membrane-coated adjuvant nanoparticles with mannose modification for effective anticancer vaccination. ACS Nano, 12(6), 5121–5129. DOI 10.1021/acsnano.7b09041. [Google Scholar] [CrossRef]
128. Li, J., Zhen, X., Lyu, Y., Jiang, Y., Huang, J. et al. (2018). Cell membrane coated semiconducting polymer nanoparticles for enhanced multimodal cancer phototheranostics. ACS Nano, 12(8), 8520–8530. DOI 10.1021/acsnano.8b04066. [Google Scholar] [CrossRef]
129. Dhanasekaran, S., Chopra, S. (2016). Getting a handle on smart drug delivery systems–A comprehensive view of therapeutic targeting strategies. Smart Drug Delivery System, 1, 31–62. [Google Scholar]
130. Perez-Herrero, E., Fernandez-Medarde, A. (2015). Advanced targeted therapies in cancer: Drug nanocarriers, the future of chemotherapy. European Journal of Pharmaceutics and Biopharmaceutics, 93(2010), 52–79. DOI 10.1016/j.ejpb.2015.03.018. [Google Scholar] [CrossRef]
131. Ruan, S., Cao, X., Cun, X., Hu, G., Zhou, Y. et al. (2015). Matrix metalloproteinase-sensitive size-shrinkable nanoparticles for deep tumor penetration and pH triggered doxorubicin release. Biomaterials, 60(4), 100–110. DOI 10.1016/j.biomaterials.2015.05.006. [Google Scholar] [CrossRef]
132. Nam, J., Won, N., Jin, H., Chung, H., Kim, S. (2009). pH-Induced aggregation of gold nanoparticles for photothermal cancer therapy. Journal of the American Chemical Society, 131(38), 13639–13645. DOI 10.1021/ja902062j. [Google Scholar] [CrossRef]
133. Lee, D. J., Oh, Y. T., Lee, E. S. (2014). Surface charge switching nanoparticles for magnetic resonance imaging. International Journal of Pharmaceutics, 471(1–2), 127–134. DOI 10.1016/j.ijpharm.2014.05.029. [Google Scholar] [CrossRef]
134. Zhang, L., Wang, Y., Yang, Y., Liu, Y., Ruan, S. et al. (2015). High tumor penetration of paclitaxel loaded pH sensitive cleavable liposomes by depletion of tumor collagen I in breast cancer. ACS Applied Materials & Interfaces, 7(18), 9691–9701. DOI 10.1021/acsami.5b01473. [Google Scholar] [CrossRef]
135. Li, L., Sun, W., Zhong, J. J., Yang, Q. Q., Zhu, X. et al. (2015). Multistage nanovehicle delivery system based on stepwise size reduction and charge reversal for programmed nuclear targeting of systemically administered anticancer drugs. Advanced Functional Materials, 25(26), 4101–4113. DOI 10.1002/adfm.201501248. [Google Scholar] [CrossRef]
136. Li, L., Cui, D. J., Ye, L. M., Li, Y., Zhu, L. Y. et al. (2017). Codelivery of salinomycin and docetaxel using poly(D,L-lactic-co-glycolic acid)- poly(ethylene glycol) nanoparticles to target both gastric cancer cells and cancer stem cells. Anti-Cancer Drugs, 28(9), 989–1001. DOI 10.1097/CAD.0000000000000541. [Google Scholar] [CrossRef]
137. Yang, Z., Sun, N., Cheng, R., Zhao, C., Liu, J. et al. (2017). Hybrid nanoparticles coated with hyaluronic acid lipoid for targeted co-delivery of paclitaxel and curcumin to synergistically eliminate breast cancer stem cells. Journal of Materials Chemistry B, 5(33), 6762–6775. DOI 10.1039/C7TB01510K. [Google Scholar] [CrossRef]
138. Raghvendra, K., Kyu, H. S., Kartikey, V., Tiwari, S. K. (2018). Recent progress in selected bio-nanomaterials and their engineering applications: An overview. Journal of Science: Advanced Materials and Devices, 3(3), 263–288. DOI 10.1016/j.jsamd.2018.05.003. [Google Scholar] [CrossRef]
139. Qiu, M., Singh, A., Wang, D., Qu, J., Swihart, M. et al. (2019). Biocompatible and biodegradable inorganic nanostructures for nanomedicine: Silicon and black phosphorus. Nano Today, 25, 135–155. DOI 10.1016/j.nantod.2019.02.012. [Google Scholar] [CrossRef]
140. Souza, V. G. L., Fernando, A. L. (2016). Nanoparticles in food packaging: Biodegradability and potential migration to food—A review. Food Packaging and Shelf Life, 8(2), 63–70. DOI 10.1016/j.fpsl.2016.04.001. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |