

 | Journal of Renewable Materials |  |
DOI: 10.32604/jrm.2021.014683
ARTICLE
Extraction Hydrolysates from Larimichthys Polyactis Swim Bladder Using Enzymatic Hydrolysis
1College of Material Science and Engineering, Northeast Forestry University, Harbin, 150040, China
2State Key Laboratory of Biobased Material and Green Papermaking, Qilu University of Technology, Shandong Academy of Sciences, Jinan, 250353, China
*Corresponding Authors: Yubo Tao. Email: taoyubo@qlu.edu.cn; Peng Li. Email: lipeng@qlu.edu.cn
Received: 20 October 2020; Accepted: 30 November 2020
Abstract: As a kind of biopolymer, hydrolysates of fish swim bladder, safer than those of land mammals, are widely used in food, cosmetics as well as pharmaceutical and biomedical fields for their biocompatibility, biodegradability, and weak antigenicity. To enhance hydrolysate production, in this paper, the papain and alcalase hydrolysis processes of larimichthys polyactis swim bladder were optimized with orthogonal experiments. With 89.5% hydrolysate yield, the optimal processing conditions for alcalase were solid-liquid ratio of 1:30, enzyme concentration of 0.7%, and extraction time of 6 h. As for papain, under the optimal processing conditions: solid-liquid ratio of 1:20, enzyme concentration of 0.5%, and extraction time of 8 h, the hydrolysate yield was 65.1%. To obtain higher hydrolysate yields, the ultrasonic pretreatments were implemented before the optimal enzyme hydrolysis processes. With ultrasonic waves of 100 W for 50 min, the hydrolysate yields were increased 2.1% (alcalase) and 4.5% (papain), respectively. The Fourier Transform Infrared (FTIR) spectroscopic analysis revealed that the hydrolysates extracted by papain exist in triple-helical forms. The Ultra-Violet (UV) absorption spectra indicated that the aromatic amino acids in the hydrolysates had strong absorptions in the wavelength range of 240 nm–300 nm. The results of this research demonstrate that the alcalase hydrolysates have better solubility in water and the solution is more stable under ambient temperature. However, the hydrolysates extracted by papain have a gel property and are insoluble in weak acid at room temperature, which is more suitable for applications in feedstock of biomedical.
Keywords: Swim bladder; enzymatic hydrolysis; ultrasonic-assisted extraction; hydrolysates analysis
As a kind of natural water-soluble biopolymer material, collagen has been used in biomedical materials [1,2] and pharmaceutical products [3] for its biocompatibility, biodegradability, and weak antigenicity [4]. On the one hand, collagen can be directly used in tissue engineering, it provides the biological activity of cell adhesion and interactions with signal molecules to meet the needs of cell transport and tissue regeneration. On the other hand, collagen can also be integrated into many composites for recycling applications [5], such as musculoskeletal tissue engineering. Traditionally, collagen is isolated from the skins and bones of terrestrial mammals, such as cows and pigs [4]. However, in recent years, due to the outbreaks of bovine sponge encephalopathy, foot-and-mouth disease, and avian influenza [6] as well as religious reasons [7], its applications have been restricted. Without above mentioned restrictions, fish collagen is widely used in various biomedical fields, such as wound healing, tissue engineering, drug delivery, and cell and tissue culture [8].
Swim bladder, a low fat biological material, is often used in medicine and food fields because it is especially rich in natural animal protein (generally over 80%) and amino acid [9,10]. The previous researches have shown that the hydrolysates extracted from swim bladder have high digestibility, thermal stability, and biocompatibility [11]. However, the extraction method is one of factors that affect the swim bladder collagen characteristics and the hydrolysate yields. The previous researches have stated that enzymatic hydrolysis, with short time, no environmental pollution, stable collagen property, and etc., is a primary approach for collagen extraction from fish and swim bladder [12]. Fish waste is abundant worldwide, and much of it is thrown away and underused, causing fundamental problems for the environment. Therefore, the waste from the production and processing of fish products is developed as an environmentally friendly and sustainable source of collagen. Not only can it increase the economic value of by-products from fish processing and create a rich collagen supply, but it can also reduce environmental pollution problems in contemporary society [8].
In recent years, the enzyme used to extract collagen from swim bladder was mainly pepsin [12,13]. However, compared to pepsin, papain decomposed protein under mild conditions, cleaving only the non-helical terminal peptide, which endowed the soluble hydrolysates much stable helical structure and benefitted the purification of later products as well as the treatment of waste liquid [11,14]. In addition, the efficiency of alcalase on the hydrolysis of swim bladder is also worth investigating. The previous researches showed that alcalase is efficient on the hydrolysis of fish, with high hydrolysate yield (70.6%) and polypeptide content [15]. Low lipid content as well as significantly functional properties endowed alcalase-treated hydrolysates with many potential uses [16].
Since it is difficult to fully extract collagen using a single method, a combination of multiple methods is often adopted to improve the yield of collagen, such as ultrasonic assistance on enzymatic hydrolysis and acid hydrolysis [17]. Ultrasonic extraction has advantages of short extraction time, low energy consumption, less impurities, and less damage to effective components. In the process of ultrasonic-assisted extraction, which produces cell disruption and particle size reduction, the ultrasonic jet towards solid’s surfaces creates a greater contact area between solid and liquid phase, better access of solvent to valuable components. Thus, ultrasonic treatment can significantly improve the extraction efficiency of the target material compared to the traditional methods [18]. In recent years, there have been reports on ultrasonic-assisted extraction of collagen. The yield of collagen from skins of the seabass (Lateolabrax japonicus) was increased by 116.65% compared to that of traditional acid method. The study by Cuevas-Acuña et al. [19] showed that the high-intensity ultrasonic pulse treatments is useful to improve fish gelatin antioxidant properties. This indicated that the moderate ultrasonic treatment could increase the yield of collagen, but it is worth optimizing processing parameters to minimize the collagen degradation.
Nonetheless, the extraction and characterization of collagen from swim bladder of larimichthys polyactis (yellow croaker) are limited so far, extractions accompanied by ultrasonic pretreatment are also rare. In the present work, the effects of two kinds of enzyme on the hydrolysate yield and characterization were compared. In this paper, the optimal process parameters of the solid-liquid ratio, enzyme concentration, and the extraction time were obtained. The effects of ultrasonic time and power on the hydrolysate yield were also discussed.
Farmed yellow croaker swim bladders (Larimichthys polyactis), about 5 cm long, with water content around 5% were supplied by Gao Shun Hang Co., Ltd., (Guangdong, China). The enzymatic hydrolysis was performed on swim bladders using papain (Papain from papaya latex, P-3250, 0.5 units/mg to 2.0 units/mg solid, Sigma-Aldrich) and alcalase (Novozymes 3.0 T, YiNuo Biotechnology Co., Ltd., Beijing, China). Sodium hydroxide (analytical grade, 96.0%, TianLi Chemical Reagent Co., Ltd., Tianjin, China) was prepared into 1.0 M NaOH solution with distilled water. 0.5 M acetic acid was diluted with glacial acetic acid (analytical grade, 99.7%, TianLi Chemical Reagent Co., Ltd., Tianjin, China).
2.2 Enzymatic Hydrolysis of Swim Bladder
The swim bladder was soaked in distilled water for 24 h to remove ash and grease on the surface, during which the water was changed at least 3–4 times. After drying and smashing, the swim bladder powder was obtained by screening through a 2 mm mesh sieve. According to different solid-liquid ratio (1:10, 1:20, and 1:30), the swim bladder powder and distilled water were added to three beakers, respectively. The mixture was placed in a thermostatic water bath under 60°C, adding papain of different mass ratios (0.3, 0.5 and 0.7, w/w %) and stirring continuously. The enzymatic hydrolysis reaction was carried out when the inner temperature was stable. After a certain time, the mixture was filtered by a 0.09 mm aperture screen to filter out all the insoluble materials. Filtered substance was dried under 40°C until the moisture content is 8%. Compared to papain, the only difference of alcalase extraction was that the pH value of the mixture needed to be maintained around 7.5. The other extraction conditions and methods are the same as above.
The yield of hydrolysates [20] was calculated using Eq. (1).
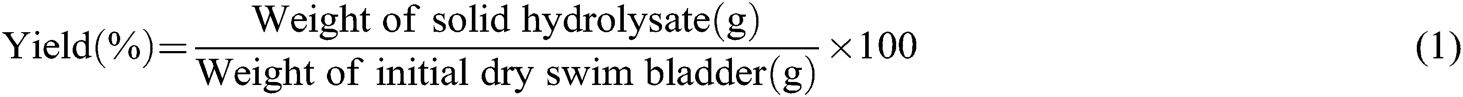
The optimized process conditions of enzymatic extraction were determined by the orthogonal experiment. Based on enzymatic hydrolysis process, the solid-liquid ratio, enzyme concentration, and extraction time were used as experimental factors. The hydrolysate yield was used as the evaluation index. The orthogonal experimental arrangements, the factor levels of solid-liquid ratio, enzyme concentration, and extraction time were shown in Tab. 1.
Table 1: Orthogonal experiment scheme
2.5 Ultrasonic-Assisted Extraction
On the basis of the experimental scheme of the highest hydrolysate yield, dried swim bladder powder was evenly mixed according to a certain solid-liquid ratio (papain 1:20, alcalase 1:30). Then, the mixture was pretreated in an ultrasonic cleaner (KH-100SPV, Kunshan Hechuang ultrasonic instrument Co., Kunshan, China). The effects of ultrasonic time (10, 30, 50, 70, and 90 min) and ultrasonic power (60 W, 80 W, and 100 W) on the yield were studied. Each group was repeated three times, whose data were averaged.
2.6 Fourier Transform Infrared (FTIR) Spectroscopic Analysis
The FTIR spectra of hydrolysates were obtained using a FT-IR spectrometer (Frontier, PerkinElmer Inc., USA) Nicolette 6700. The samples of solid hydrolysates (ca. 1.0 mg) and potassium bromide (150 mg) were grounded in an agate mortar, which were pressed after drying. The samples were measured in the range of 4000 cm-1~450 cm-1.
The UV absorption investigations were performed with a UV-VIS spectrophotometer (UV-2600, Shimadazu, Japan). The dried extracted collagen was dissolved in 0.5 M acetic acid. To obtain papain hydrolyate solution, the mixture was placed in 50°C thermostatic water bath. The UV absorption analysis was conducted in the wavelength range between 400 nm and 190 nm.
3.1 Optimal Process Analysis of Enzymatic Hydrolysis
3.1.1 Optimal Process Analysis of Enzymatic Hydrolysis
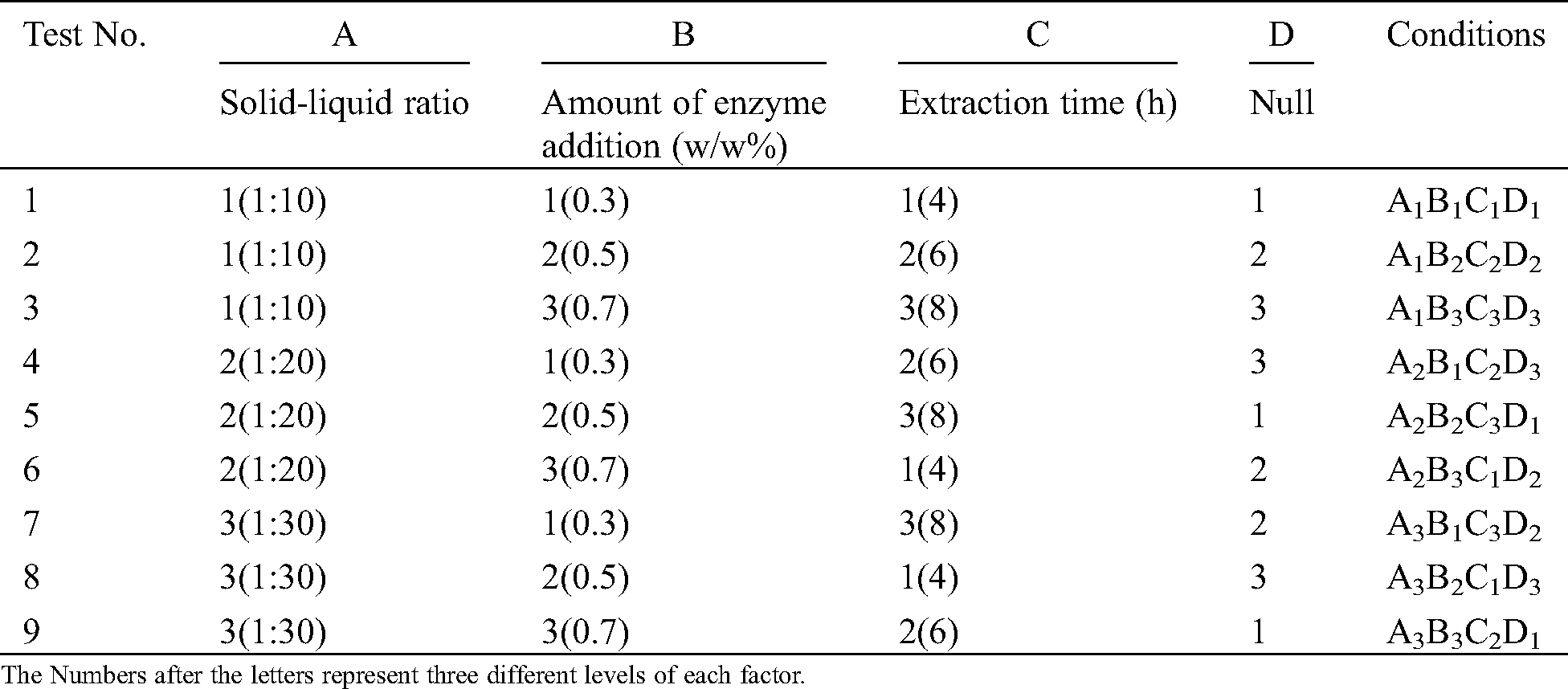
Under the optimal process condition, papain hydrolysates of swim bladder reached a yield of 65.1% (solid-liquid ratio of 1:20, enzyme concentration of 0.5%, and extraction time of 8 h) and alcalase reached a yield of 89.5% (solid-liquid ratio of 1:30, enzyme concentration of 0.7%, and extraction time of 6 h), as shown in Tab. 2. The hydrolysate yield of alcalase is significantly higher than that of papain. The orthogonal test revealed that the optimal processing conditions for papain hydrolysis and alcalase hydrolysis of yellow croaker swim bladder are A2B2C3D1 and A3B3C2D1, respectively.
Table 2: Test results of extraction yield
3.1.2 The Relationship between Variables and Responses
To illustrate the influence of each factor on the response, the hydrolysate yields of yellow croaker swim bladder extracted with papain and alcalase were compared. As shown in Fig. 1, A1, A2, and A3 correspond to three levels of solid-liquid ratio (A factor: 1:10, 1:20, and 1:30). The corresponding papain hydrolysate yield were 56.3%, 60.7%, and 56.1%, respectively. As for alcalase, the solid-liquid ratio of 1:30 (A3) could result in higher hydrolysate yield. Similarly, a concentration of 0.5% (B2) and extraction time of 8 h (C3) contributed higher papain yield, whereas, a concentration of 0.7% (B3) and extraction time of 6 h (C2) were beneficial for higher alcalase yield. The results are consistent with the optimal process conditions (papain: A2B2C3, alcalase: A3B3C2) obtained by the orthogonal experiment.
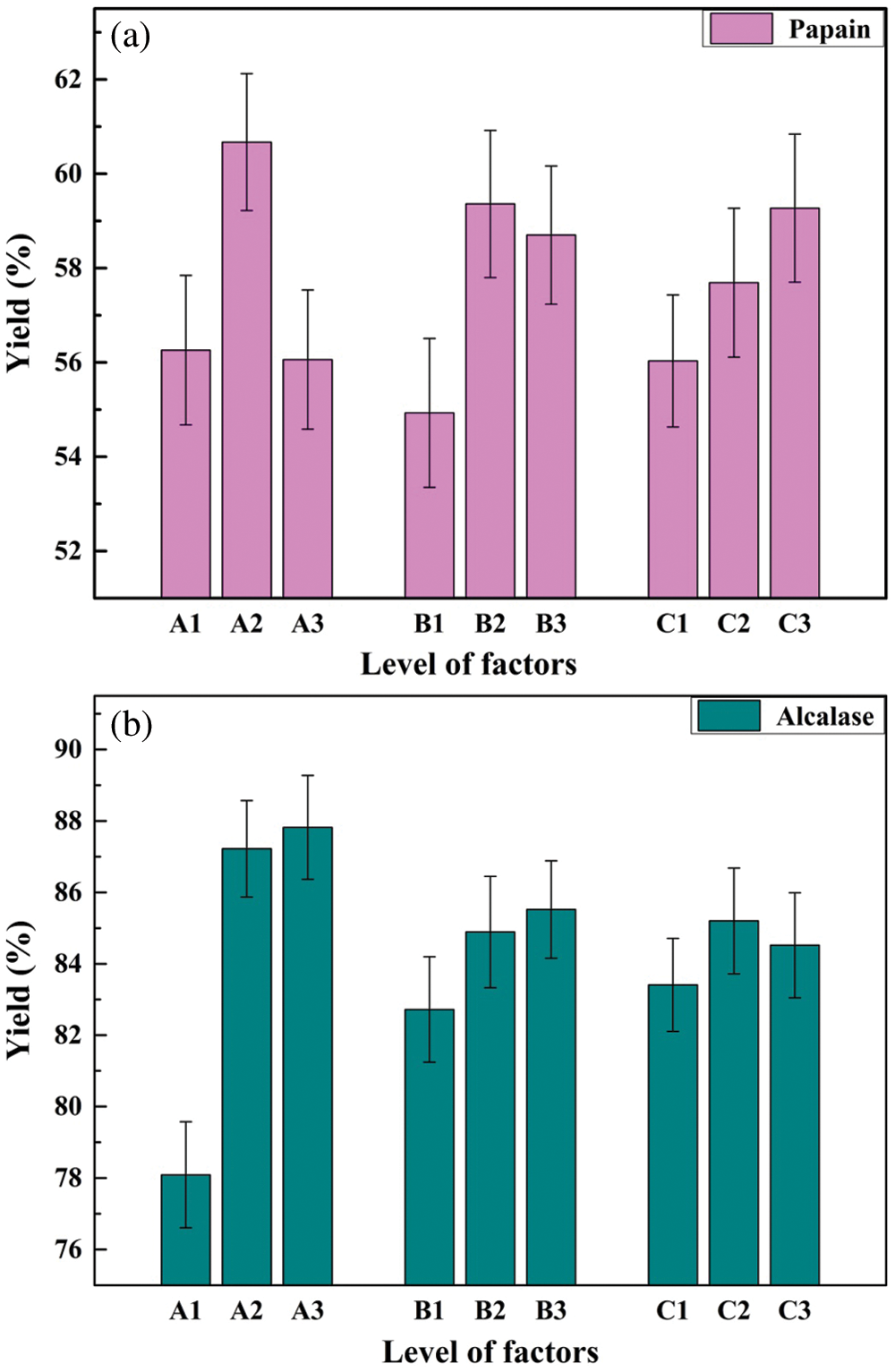
Figure 1: The relationship between variables and responses
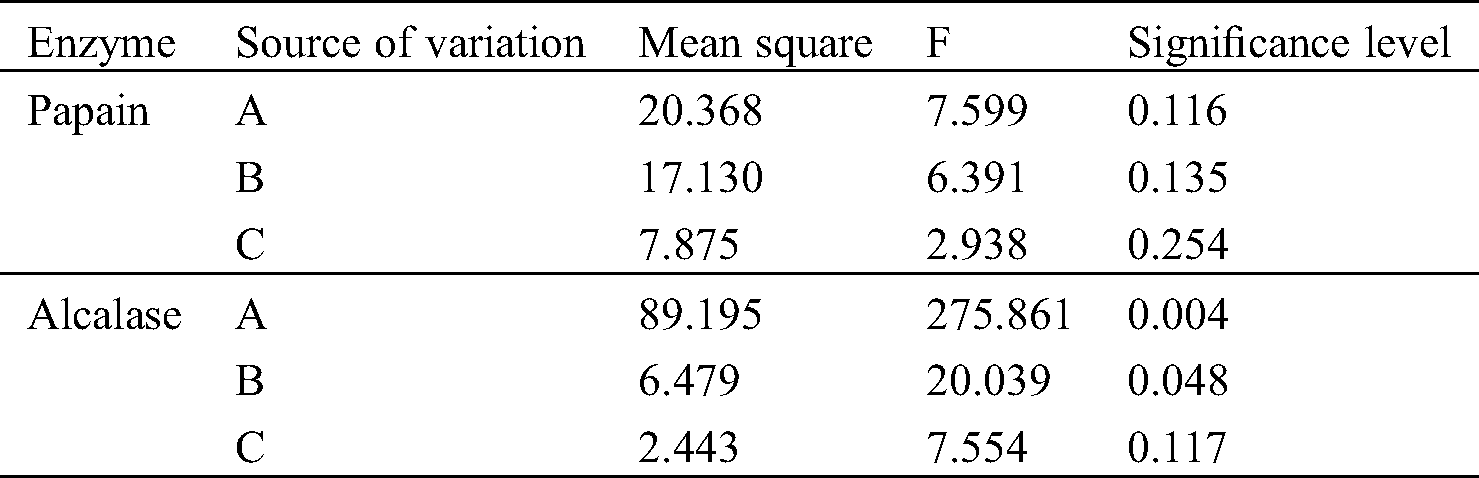
The effects of each factor on the hydrolysate yield using papain and alcalase are shown in Tab. 3. When using alcalase as an enzymatic hydrolysis catalyst, it was worth noting that the significance level of factor A (solid-liquid ratio) was less than 0.01, which indicated that the impact of solid-liquid ratio on the yield of hydrolysates was significant. The significance level of factor B (enzyme concentration) was less than 0.05, indicating that the amount of alcalase addition also had a noteworthy effect on the yield. The significance level of factor C (extraction time) was greater than 0.05, which demonstrated that the extraction time with alcalase had no significant effect on the hydrolysate yield compared to the factor A and B. However, the significance levels of three factors were all higher than 0.05 when the papain was used as catalyst to hydrolyze the yellow croaker swim bladder. Therefore, the significance of the three factors were not significant, which were consistent with research of Mei et al. [21]. However, according to the value of each factor, the relative order of their influence on the extraction yield was A, B and C, which was also consistent with the results of alcalase.
Table 3: Variance analysis of extraction yield
3.2 The Effect of Ultrasonic Pretreatment on the Hydrolysate Yield
3.2.1 The Effect of Ultrasonic Time on the Hydrolysate Yield
On the basis of the optimum process, the effect of ultrasonic time on the yield of the papain and alcalase hydrolysates was studied under the conditions of ultrasonic power of 80 W and ultrasonic frequency of 25 kHz. As shown in Fig. 2a, during 0 min–50 min, the hydrolysate yield using both papain and alcalase as catalyst increased with the increase of ultrasonic time and reached the maximum at 50 min. Then the yield gradually decreased with the increase of the processing time. Therefore, the appropriate ultrasonic time is 50 min.
Initially, the cavitation and mechanical effects produced by ultrasonic pretreatment can not only facilitate the disruption of swim bladder tissue cells and make the collagen fibers loose or broken, but also expose the active points buried inside the protein to better response to enzymes, which contributes to the improvement of the protein hydrolysis [22]. Nonetheless, the hydrolysate yield of swim bladder decreased gradually with the increase of ultrasonic time. This might be caused by the thermal and mechanical effects produced by long time ultrasonic treatment that destroyed the structure of collagen and decreased the solubility of the hydrolysates. This suggested that appropriate ultrasonic treatment can improve the yield of hydrolysates, but the thermal and mechanical effects can degrade collagen, which is consistent with the research conducted by Wang et al. [17].
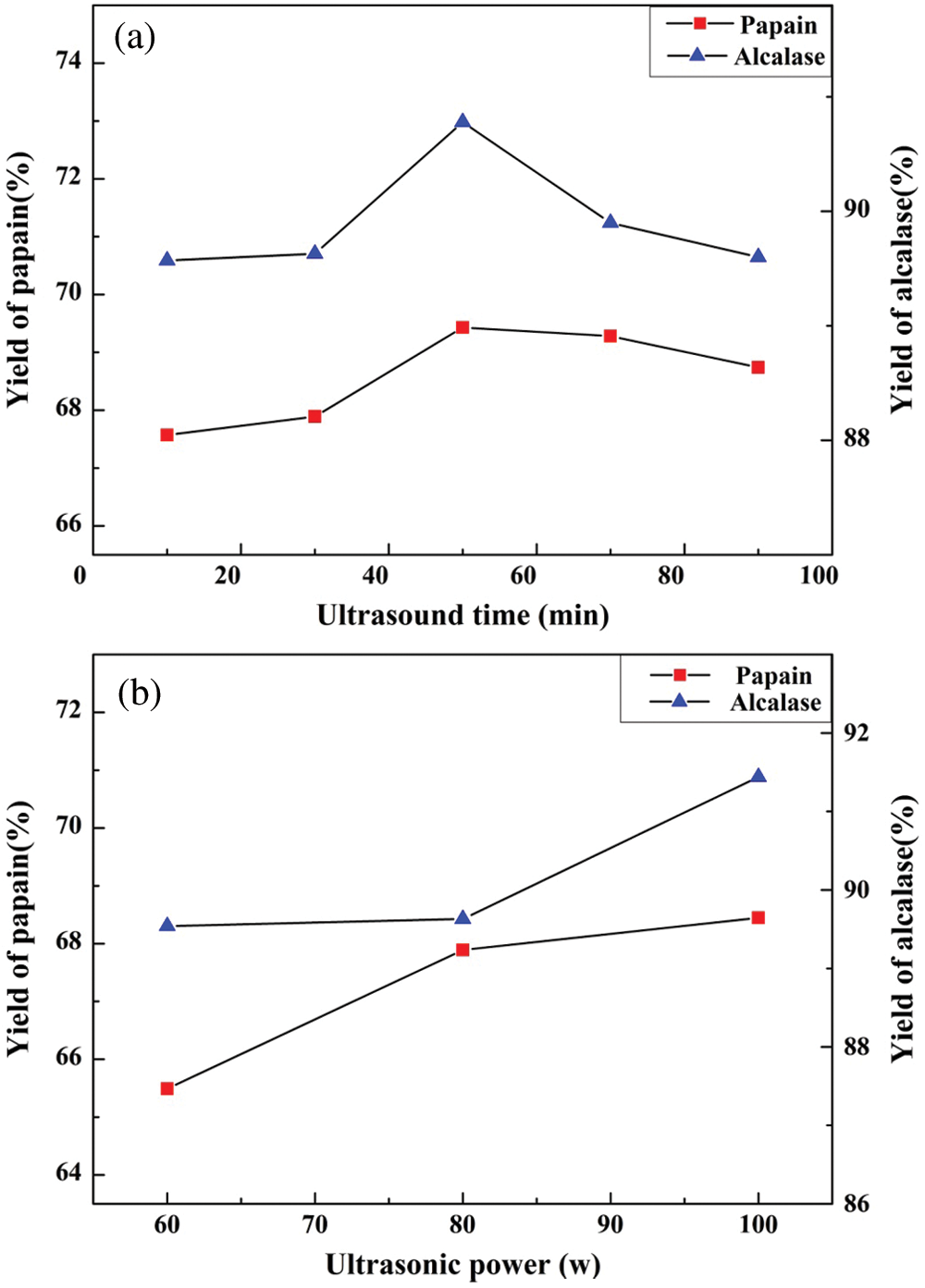
Figure 2: The effect of ultrasonic pretreatment on the yield of larimichthys polyactis hydrolysates
3.2.2 The Effect of Ultrasonic Power on the Hydrolysate Yield
The effect of ultrasonic power on the hydrolysate yield was studied under the condition of the optimal hydrolysis process for swim bladder with the ultrasonic frequency of 25 kHz and an ultrasonic time of 30 min. The yield increased with the increase of ultrasonic power in the range of 60 W–100 W, and reached the maximum at 100 W, as shown in Fig. 2b. The greater ultrasonic intensity is, the more apparent the cavitation effects are. In addition, the mechanical effects of ultrasonic wave can also promote the rupture of swim bladder, which benefits higher hydrolysate yield. Therefore, it can be proposed that the enzymatic hydrolysate yield from swim bladder is related to the cavitation as well as mechanical effects produced by ultrasonic vibration. The appropriate ultrasonic power was determined to be 100 W during enzymatic hydrolysis.
In summary, the optimal process for ultrasonic-assisted extraction was the ultrasonic frequency of 25 kHz, ultrasonic power of 100 W, and ultrasonic time of 50 min. The yield produced was 69.6% (papain) and 91.6% (alcalase), respectively. In comparison with enzymatic methods, the yields of papain and alcalase hydrolysate were all improved through ultrasonic pretreatment. However, the increases were not significant, which were mainly due to the low power of ultrasonic cleaner used in the experiment.
3.3 Fourier Transform Infrared (FTIR) Spectroscopic Analysis
The FTIR spectra and main absorption peaks of the hydrolysates prepared by different processes were shown in Fig. 3. The strength and wavenumber of some typical peaks differ from each other based on the enzyme used and ultrasonic pretreatment. This revealed that differences exist in the secondary structure of collagen protein produced by different methods [23].
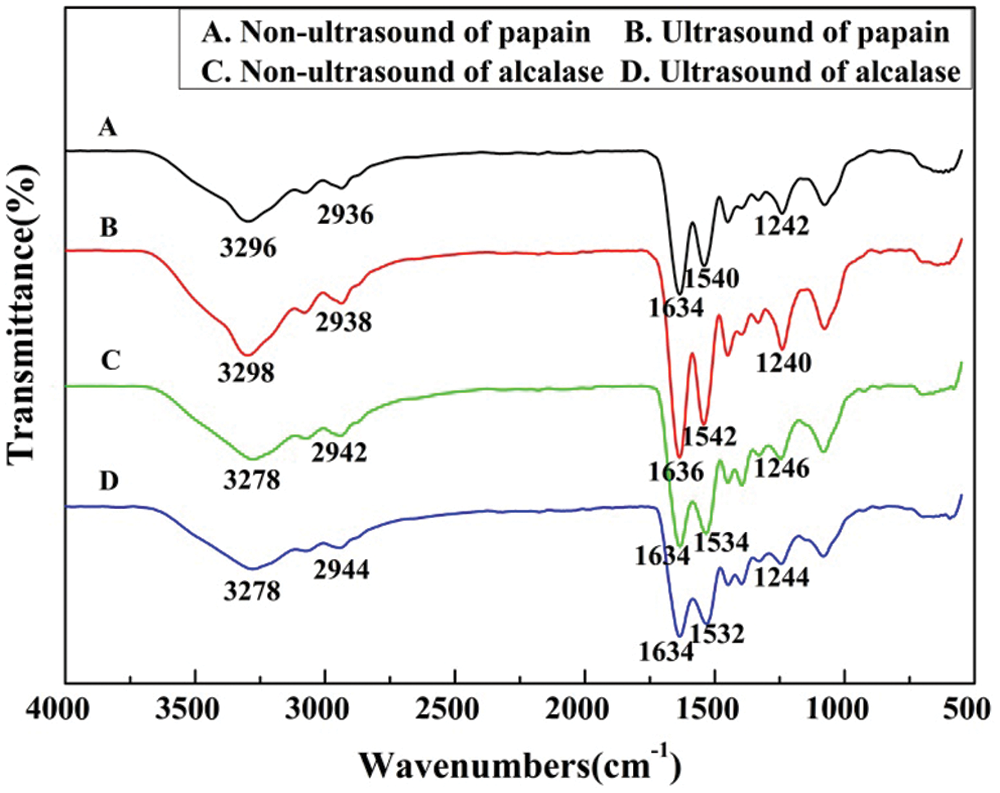
Figure 3: FTIR spectra of larimichthys polyactis hydrolysates
In the infrared spectrum, amide I, II, and III are frequently used as characteristic bands for the identification of secondary structures of collagen, which can reflect the skeleton structure of peptide chains [24]. The strong absorption for all samples that appeared around 3300 cm-1 indicates the existence of amide-A band coupled with hydrogen bonds. A free N-H stretching vibration usually exhibits strong absorption in the range of 3400 cm-1–3440 cm-1 [25]. However, when the N-H and O-H group of a peptide is coupled by a hydrogen bond, the band position would be shifted to smaller wavenumbers [25,26]. The Fig. 3 demonstrated that the characteristic absorption bands of amide-A appeared at 3296, 3298, 3278, and 3278 cm-1 for four samples, showing that the N-H is involved in maintaining the special triple helical structure of collagen and the formation of hydrogen bonds. The results also are in accordance with the collagen from swim bladder of seabass (Lates calcarifer). Compared to papain hydrolysate, the N-H stretching vibration shifted to a lower frequency. The amide-I band is related to the stretching vibration of C=O and bend of N-H, which commonly occurs in the range of 1600 cm-1–1700 cm-1 [27]. The amide-I bands appeared at 1634, 1636, 1634, and 1634 cm-1 for the four kinds of hydrolysates, respectively. Bending vibration absorption band of the N-H of amide-II band appeared at 1540, 1542, 1534, and 1532 cm-1, respectively. The bending vibration amplitude of the N-H of amide-II was intensified by the ultrasonic pretreatment. The existence of amide-III band corresponds to the integrity of triple helix structure of collagen [28] and its absorption band is usually ranged from 1220 to 1320 cm-1. The amide-III band was observed at 1242, 1240, 1246, and 1244 cm-1 for four kinds of hydrolysates. The amplitude of amide II and III band that appeared on the hydrolysates of papain were stronger than those of alcalase. Thus, the hydyolysates obtained with papain could maintain more stable collagen secondary structures.
3.4 Ultraviolet Absorption Spectrum Analysis
Protein molecules contain chromophores, which can absorb a certain wavelength ultraviolet light. The peptide groups have light absorption in the range of 180 nm–230 nm. The aromatic amino acids, such as tyrosine (Tyr), tryptophan (Trp), phenylalanine (Phe) also showed absorption in this region, but the largest absorptions appear in the range of 240 nm–300 nm. In twenty kinds of basic amino acids, Tyr, Trp, and Phe usually have conjugate double bonds, all of which have absorption peaks at 275 nm, 280 nm, and 258 nm, respectively [29].
As can be seen from Fig. 4, the hydrolysates of the yellow croaker swim bladders (Larimichthys polyactis) demonstrated strong absorption in the wavelength range of 250 nm–280 nm. The four species of hydrolysates had absorptions at 253 nm and 275 nm. The amplitude of papain hydrolysates are stronger at 253 nm than that of acalase hydrolysates, whereas, the acalase hydrolysates showed stronger absorption at 275 nm, which indicates that more Phe in papain hydrolysates and more Tyr in acalase hydrolysates. This result agrees with the different solubility of two kinds of hydrolysates in the 0.5 M acetic acid. In which, the papain hydrolysates swell, but the acalase hydrolysates solve thoroughly under the ambient temperature. Compared to Tyr, Phe has better hydrophobic ability.
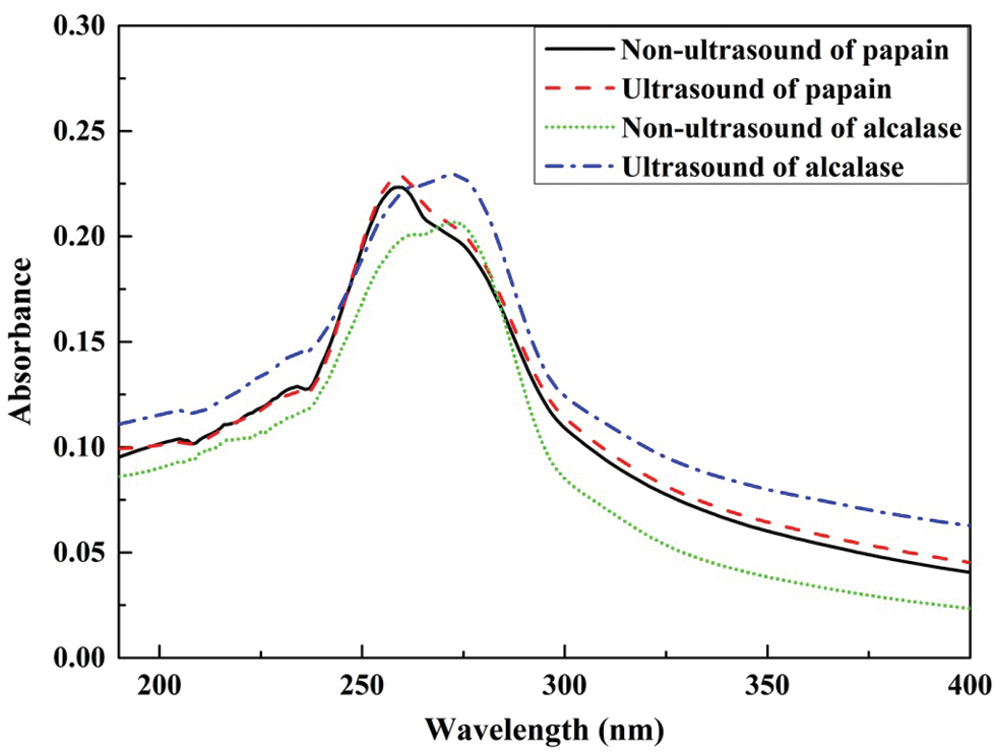
Figure 4: UV-visible spectra of larimichthys polyactis hydrolysates
The hydrolysate yield of alcalase hydrolysis was higher than that of papain under the same processing conditions. With ultrasonic waves of 100 W for 50 min, the hydrolysate yields were increased 2.1% (alcalase) and 4.5% (papain), respectively. During the enzymatic hydrolysis process, although the molecular structures of the hydrolysates of swim bladder undergo minor changes, the triple-helical structures still dominate due to the quantity of hydrogen bonds. The UV absorption spectrum indicates that the hydrolysates are composed of aromatic amino acids. The alcalase hydrolysates have better solubility in water and the solution is more stable under ambient temperature. However, the hydrolysates extracted by papain have a gel property and are insoluble in weak acid at room temperature, which is more suitable for applications in biomedical materials.
Funding Statement: This research was funded by the Fundamental Research Funds for the Central Universities, Grant No. 2572018AB10 and the Fundamental Research Funds for the Central Universities, Grant No. 2572019BB07.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Lee, C. H., Singla, A., Lee, Y. (2001). Biomedical applications of collagen. International Journal of Pharmaceutics, 221(1-2), 1–22. DOI 10.1016/S0378-5173(01)00691-3. [Google Scholar] [CrossRef]
2. Tolano-Villaverde, I. J., Ocano-Higuera, V., Ezquerra-Brauer, J., Santos-Sauceda, I. Santacruz-Ortega, H. et al. (2018). Physicochemical characterization of actomyosin−Paramyosin from giant squid mantle (Dosidicus gigas). Journal of the Science of Food and Agriculture, 98(5), 1787–1793. DOI 10.1002/jsfa.8653. [Google Scholar] [CrossRef]
3. Veeruraj, A., Arumugam, M., Ajithkumar, T., Balasubramanian, T. (2012). Isolation and characterization of drug delivering potential of type−I collagen from eel fish Evenchelys macrura. Journal of Materials Science: Materials in Medicine, 23(7), 1729–1738. DOI 10.1007/s10856-012-4650-2. [Google Scholar] [CrossRef]
4. Liu, W. T., Li, G. Y., Miao, Y. Q., Wu, X. H. (2009). Preparation and characterization of pepsin−Solubilized type I collagen from the scales of snakehead (Ophiocephalus argus). Journal of Food Biochemistry, 33(1), 20–37. DOI 10.1111/j.1745-4514.2008.00207.x. [Google Scholar] [CrossRef]
5. Pan, L., Li, P., Tao, Y. (2020). Preparation and properties of microcrystalline cellulose/fish gelatin composite film. Materials, 13(19), 4370. DOI 10.3390/ma13194370. [Google Scholar] [CrossRef]
6. Jongjareonrak, A., Benjakul, S., Visessanguan, W., Nagai, T., Tanaka, M. (2005). Isolation and characterisation of acid and pepsin−Solubilised collagens from the skin of Brownstripe red snapper (Lutjanus vitta). Food Chemistry, 93(3), 475–484. DOI 10.1016/j.foodchem.2004.10.026. [Google Scholar] [CrossRef]
7. Ahmad, M., Benjakul, S. (2010). Extraction and characterisation of pepsin−Solubilised collagen from the skin of unicorn leatherjacket (Aluterus monocerous). Food Chemistry, 120(3), 817–824. DOI 10.1016/j.foodchem.2009.11.019. [Google Scholar] [CrossRef]
8. Coppola, D., Oliviero, M., Vitale, G. A., Lauritano, C., D’Ambra, I. et al. (2020). Marine collagen from alternative and sustainable sources: Extraction, processing and applications. Marine Drugs, 18(4), 214. DOI 10.3390/md18040214. [Google Scholar] [CrossRef]
9. Xu, D., Ding, G. F., Fang, Y., Yan, Z. Y., Huang, L. Y. et al. (2018). Nutritional analysis of different parts of seven marine products. Acta Nutrimenta Sinica, 40(4), 409–411. DOI 10.13325/j.cnki.acta.nutr.sin.2018.04.025. [Google Scholar] [CrossRef]
10. Subhan, F., Hussain, Z., Tauseef, I., Shehzad, A., Wahid, F. (2020). A review on recent advances and applications of fish collagen. Critical Reviews in Food Science and Nutrition, 26(181), 1–11. DOI 10.1080/10408398.2020.1751585. [Google Scholar] [CrossRef]
11. Duan, R., Zhang, J., Du, X., Yao, X., Konno, K. (2009). Properties of collagen from skin, scale and bone of carp (Cyprinus carpio). Food Chemistry, 112(3), 702–706. DOI 10.1016/j.foodchem.2008.06.020. [Google Scholar] [CrossRef]
12. Gaurav, K. P., Nidheesh, T., Govindaraju, K., Jyoti, Suresh, P. V. (2017). Enzymatic extraction and characterisation of a thermostable collagen from swim bladder of rohu (Labeo rohita). Journal of the Science of Food and Agriculture, 97(5), 1451–1458. DOI 10.1002/jsfa.7884. [Google Scholar] [CrossRef]
13. Kaewdang, O., Benjakul, S., Kaewmanee, T., Kishimura, H. (2014). Characteristics of collagens from the swim bladders of yellowfin tuna (Thunnus albacares). Food Chemistry, 155, 264–270. DOI 10.1016/j.foodchem.2014.01.076. [Google Scholar] [CrossRef]
14. Zhang, J. J., Tang, J. S., Wang, H. B., Li, C. Z., Feng, X. R. (2014). Extraction of collagen from grass carp scale with papain by response surface methodology. China Brewing, 10, 99–103. DOI 10.11882/j.issn.0254−5071.2014.10.024. [Google Scholar] [CrossRef]
15. Kristinsson, H. G., Rasco, B. A. (2000). Kinetics of the hydrolysis of Atlantic salmon (Salmo salar) muscle proteins by alkaline proteases and a visceral serine protease mixture. Process Biochemistry, 36(1−2), 131–139. DOI 10.1016/S0032-9592(00)00195-3. [Google Scholar] [CrossRef]
16. Shahidi, F., Han, X., Synowiecki, J. (1995). Production and characteristics of protein hydrolysates from capelin (Mallotus villosus). Food Chemistry, 53(3), 285–293. DOI 10.1016/0308-8146(95)93934-J. [Google Scholar] [CrossRef]
17. Wang, J. M., Bao, J. Q. (2016). Study on ultrasonic-assisted solvent extraction and purification of pepsin-soluble collagen from grass carp skin. Science and Technology of Food Industry, 37(18), 236–240. DOI 10.13386/j.issn1002−0306.2016.18.036. [Google Scholar] [CrossRef]
18. Li, H. (2005). Application of ultrasonic technique for extracting chlorogenic acid from Eucommia ulmodies Oliv. (E. Ulmodies). Ultrasonics Sonochemistry, 12(4), 295–300. DOI 10.1016/j.ultsonch.2004.01.033. [Google Scholar] [CrossRef]
19. Cuevas-Acuña, D. A., Arias-Moscoso, J. L., Torres-Arreola, W., Cadena-Cadena, F. Valdez-Melchor, R. G. et al. (2020). High−Intensity ultrasound pulses effect on physicochemical and antioxidant properties of tilapia (Oreochromis niloticus) skin gelatin. Applied Sciences, 10(3), 1004. DOI 10.3390/app10031004. [Google Scholar] [CrossRef]
20. Zheng, X., Liu, J., Pei, Y., Li, J., Tang, K. (2012). Preparation and properties of sisal microfibril/gelatin biomass composites. Composites Part A: Applied Science and Manufacturing, 43(1), 45–52. DOI 10.1016/j.compositesa.2011.08.024. [Google Scholar] [CrossRef]
21. Mei, X. D., Zeng, J. N., Jiang, B. Q. (2014). Process optimization of enzyme-extracted collagen from fish scale. Food and Machinery, 30(6), 156–159. DOI 10.13652/j.issn.1003−5788.2014.06.039. [Google Scholar] [CrossRef]
22. Kim, H. K., Kim, Y. H., Park, H. J., Lee, N. H. (2013). Application of ultrasonic treatment to extraction of collagen from the skins of sea bass Lateolabrax japonicus. Fisheries Science, 79(5), 849–856. DOI 10.1007/s12562-013-0648-z. [Google Scholar] [CrossRef]
23. Wang, L., An, X., Xin, Z., Zhao, L., Hu, Q. (2007). Isolation and characterization of collagen from the skin of deep−Sea redfish (sebastes mentella). Journal of Food Science, 72(8), E450–E455. DOI 10.1111/j.1750−3841.2007.00478.x. [Google Scholar] [CrossRef]
24. Li, H., Liu, B. L., Gao, L. Z., Chen, H. L. (2004). Studies on bullfrog skin collagen. Food Chemistry, 84(1), 65–69. DOI 10.1016/S0308-8146(03)00167-5. [Google Scholar] [CrossRef]
25. Doyle, B. B., Bendit, E. G., Blout, E. R. (1975). Infrared spectroscopy of collagen and collagen−like polypeptides. Biopolymers, 14(5), 937–957. DOI 10.1002/bip.1975.360140505. [Google Scholar] [CrossRef]
26. Matmaroh, K., Benjakul, S., Prodpran, T., Encarnacion, A. B., Kishimura, H. (2011). Characteristics of acid soluble collagen and pepsin soluble collagen from scale of spotted golden goatfish (Parupeneus heptacanthus). Food Chemistry, 129(3), 1179–1186. DOI 10.1016/j.foodchem.2011.05.099. [Google Scholar] [CrossRef]
27. Payne, K. J., Veis, A. (1988). Fourier transform IR spectroscopy of collagen and gelatin solutions: Deconvolution of the amide I band for conformational studies. Biopolymers, 27(11), 1749–1760. DOI 10.1002/bip.360271105. [Google Scholar] [CrossRef]
28. Liu, D., Liang, L., Regenstein, J. M., Zhou, P. (2012). Extraction and characterisation of pepsin−Solubilised collagen from fins, scales, skins, bones and swim bladders of bighead carp (Hypophthalmichthys nobilis). Food Chemistry, 133(4), 1441–1448. DOI 10.1016/j.foodchem.2012.02.032. [Google Scholar] [CrossRef]
29. Wu, X., Cai, L., Cao, A., Wang, Y., Li, T. et al. (2016). Comparative study on acid−soluble and pepsin−Soluble collagens from skin and swim bladder of grass carp (Ctenopharyngodon idella). Journal of the Science of Food and Agriculture, 96(3), 815–821. DOI 10.1002/jsfa.7154. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |