

 | Journal of Renewable Materials |  |
DOI: 10.32604/jrm.2021.014555
ARTICLE
Healing Effect and Underlying Mechanism of Silver Nanoparticles Prepared Using Carboxymethyl Chitosan under Solar Irradiation
1College of Pharmacy, Guizhou University of Traditional Chinese Medicine, Guiyang, 550025, China
2Second Affiliated Hospital, Guizhou University of Traditional Chinese Medicine, Guiyang, 550002, China
3Department of Environmental Monitoring, Changsha Environmental Protection College, Changsha, 410004, China
4Guizhou Provincial Center for Disease Control and Prevention, Guiyang, 550004, China
*Corresponding Authors: Yi Long. Email: longyi621@gzy.edu.cn; Chunling Zhang. Email: zhangchunling016@gzy.edu.cn
Received: 08 October 2020; Accepted: 10 November 2020
#These authors contributed equally to this work
Abstract: In our previous study, silver nanoparticles were prepared using AgNO3 and carboxymethyl chitosan (CMCS) which is commercially available with solar irradiation. In this study, the efficacy and safety of silver nanoparticles prepared by this method were evaluated for healing wounds in rats with diabetes. We also attempted to determine the underlying mechanism and influencing factors of the silver nanomaterial preparation method. Compared with growth factors, silver nanoparticles exhibit better healing effects for rats with diabetes. No remnant silver ions were detected in the major organs of these rats after the application of silver nanoparticles. Silver nanoparticles prepared using CMCS are less toxic than those prepared from the conventional method, promote the proliferation of skin fibroblasts, and are promising as a topical medication for infected wounds. An obvious breakage process of the particles is observed during the growth of nanocrystalline silver in CMCS. In this study, we also attempted to determine whether this method is suitable for synthesizing silver nanoparticles using N-trimethyl chitosan chloride and sodium alginate were used in this particular experiment. The characteristic UV-vis absorbance peak of silver nanoparticles was found only in the reaction mixture containing N-trimethyl chitosan chloride. Our study demonstrates that free radicals are the key factor in this silver nanoparticle preparation method.
Keywords: Silver nanoparticle; carboxymethyl chitosan; low toxicity; healing; free radical
Metal nanoparticles have attracted considerable attention for their size dependent chemical and physical properties [1]. Nano silver particles are one of the most extensively investigated metal nanomaterials, and thus have been applied in medicine [2], catalyst chemistry [3], and biology [4]. Chemical reduction must be involved in the conventional preparing methods of silver nanoparticles as reducing and dispersing chemical agents. Although this method is simple and effective, the removal of these agents is time-intensive and costly, and the biological and environmental toxicities of residual agents are also problematic [5]. Researchers have therefore explored alternative preparation methods involving biomaterials to overcome these disadvantages [6].
In recent decades, the use of biopolymers in research and industry has significantly increased due to low cost, renewability of resources, and nontoxic, environmentally friendly processing. This has also been reflected in the metallic nanoparticle research domain, where polysaccharide biopolymers such as alginate [7], starch [8,9], or chitosan [10,11] is used as chemical dispersing agents “green” substitute [12] for controlling the growth of metal nanoparticles. Although it has been demonstrated that many polysaccharides or derivants act as dispersants or reductants of the preparation process [13,14], the preparing process must be initiated with either gamma [5] or UV [15] irradiation in addition to heat treatment [16].
In our previous study, an environmentally friendly and convenient method for preparing silver nanoparticles was proposed [17,18]. This method can be used to prepare silver nanoparticles using only AgNO3 and CMCS mixture. Due to trace chlorine, the suspension of silver chloride was produced in this mixture. The photolysis of AgCl is the key step in this method. Under solar irradiation, AgCl was reduced to silver nanoparticles and chloride radicals in the CMCS matrix.
In this study, the efficacy and safety of a silver nanomaterial prepared using the aforementioned method were evaluated for healing wounds in rats with diabetes. We also attempted to determine the underlying mechanism and the influencing factors of the silver nanomaterial preparation method.
Honghai Biotechnology Co. Ltd. (Qingdao, China) provided pharmaceutical grade CMCS (amino substituted ratio, 95.1%). N-trimethyl chitosan chloride was obtained from Golden-Shell Biochemical Co., Ltd. (Zhejiang, China). Food grade sodium alginate was obtained from Bright Moon Seaweed Group Co., Ltd. (Qingdao, China). 1,1-Diphenyl-2-picrylhydrazyl (DPPH) was obtained from Tokyo Chemical Industry (Japan).
High-sugar and high-lipid food was obtained from Tianqin Biotechnology Co., Ltd. (Changsha, China). Blood glucose test strips were obtained from Acon Laboratories, Inc. (Hangzhou, China). Streptozocin (STZ) was obtained from Sigma-Aldrich LLC (Germany). Imipenem and cilastatin sodium for injection were obtained from Merck & Co., Inc. (Germany). Recombinant Human Epidermal Growth Factor Hydro Gel was obtained from Pavay Gene Pharmaceutical Co., Ltd. (Guilin, China). A silver ion detection kit (WAK-Ag) was obtained from Kyoritsu Chemical-Check Lab., Corp. (Japan).
Kelong Chemical Reagent Factory (Chengdu, China) provided all other compounds.
2.2 Preparation of Silver Nanoparticles Using CMCS
Commercial CMCS was used in this experiment. 0.1% CMCS aqueous solution was prepared. It had been detected by ICS-3000 ion chromatography (Dionex, USA), the chloride ion concentration of this solution was 14 mg/L (≈ 0.4 mM). AgNO3 aqueous solution (0.6 M, 200 μL) and the CMCS stock solution (100 mL) was mixed. Then, the reaction solution was irradiated under sunlight for 8 h with continual stirring.
2.3 Preparation of Sponge Loading Silver Nanoparticles
CMCS solution (10 mL; 1% w/v) and the unpurified solution (10 mL; Ag+:Cl− = 3:1) were mixed. The mixture was then poured into a 24-well plate (1 mL in each well), frozen overnight at –50°C, and then freeze-dried overnight in an Edwards freeze dryer.
2.4 Wound Healing Experiment in Rats with Diabetes
2.4.1 STZ-Induced Diabetic Rats
After one week of adaptive feeding, eight clean grade male Wistar rats (Age: Six months; weight: 180–220 g) obtained from the Chongqing national biological industry base experimental animal center were selected and fed a high-sugar and high-fat diet (basic food 59%, sucrose 20%, lard oil 18%, and yolk powder 3%). After three weeks of feeding, the rats were fasted for 12 h prior to intraperitoneal injection with STZ (1%; 45 mg/kg) prepared in a fresh citric acid and sodium citrate buffer. The value of the fasting blood glucose (FBG) obtained after one week was monitored, with FBG ≥ 16.7 mmol/L indicating a successful diabetic model [19]. The high-sugar and high-fat diets were continued in diabetic rats throughout the course of the study. Research was conducted in accordance with the principles for laboratory animal use and care as found in “Regulation on the Administration of Laboratory Animals” (2017 Revision, State Scientific and Technological Commission, China) and approved by Animal Ethics Committees of Guizhou University of Traditional Chinese Medicine (animal ethics approval number: 20200005).
2.4.2 Establishment of Diabetic Rat Skin Injury Model and Administration
The rats with diabetes were anesthetized with 10% chloral hydrate (0.3 mL/100 g), their backs were shaved, and circles (1.5 cm diameter) were marked using gentian violet. A round incision was then made to the muscular and fascial layers using sterile scissors. Two rats were randomly allocated to a negative control group under saline administration, while the remaining rats were allocated to the experimental group and administered the recombinant human epidermal growth factor gel, imipenem-cilastatin sodium aqueous solution, and CMCS-silver nanoparticle sponge. All dressings were changed every day for 21 days.
After 3 weeks of wound healing, all animals were executed. Wound skins were obtained and fixed in saline of formol (10%) for 1 day 3 μm sections from paraffin blocks were subjected to hematoxylin and eosin (H & E) staining for determine structural changes.
2.4.4 Detection of Silver Ions in Rat Tissue
Two rats in the CMCS-silver nanoparticle sponge group were anesthetized with chloral hydrate and killed, the liver and brain tissue were removed immediately, and then 50 mg of each of these tissues was ground. Pool testing method and WAK-Ag kit were used for detecting the amount of silver ion in the tissue extracts.
2.5 Cell Culture and Cytotoxicity Evaluation
Human skin fibroblast cells (CRL-2522 ATCC, USA) were used in this study. The cell line was cultured in ATCC-recommended media with 10% FBS and 1% penicillin/streptomycin in a humidified incubator at 37°C with 5% CO2. The cells were plated in a 96-well plate at 5 × 103 cells per well in 100 mL medium, grown for 24 h, and then exposed to different concentrations of diluted silver nanoparticle solutions from the concentrated reaction solution for 48 h. Cell viability was measured using the CCK-8 assay, as described previously [20].
2.6 Silver Nanoparticles Preparation with N-Trimethyl Chitosan Chloride and Sodium Alginate
Aqueous polymer solutions (N-trimethyl chitosan chloride or sodium alginate; 0.1% (w/v)) were prepared. The mixture stirred overnight to obtain stock solutions for use in different silver nanoparticle preparations.
2.6.1 Silver Nanoparticles Preparation with N-Trimethyl Chitosan Chloride
An aqueous AgNO3 solution (1 mL, 0.6 M) was added dropwise into 100 mL of the N-trimethyl chitosan chloride stock solution and then irradiated under sunlight for 8 h with magnetic stirring.
2.6.2 Silver Nanoparticles Preparation with Sodium Alginate and Chloride Ions
An 0.6 M aqueous solution of AgNO3 (1 mL) and an water solution of sodium chloride (10 μL, 4 M) were added dropwise into a sodium alginate solution (100 mL). The mixture was stired with sunlight irradiated for 8 h.
2.6.3 Silver Nanoparticles Preparation with Sodium Alginate and N-Trimethyl Chitosan Chloride
An aqueous solution of AgNO3 (1 mL, 0.6 M) was added dropwise into the mixture of 100 mL sodium alginate and different amounts stock solution of N-trimethyl chitosan chloride. The mixture was stired with sunlight irradiated for 8 h.
2.7 Free Radical Activity Experimental Setup
Aqueous polymer solutions (N-trimethyl chitosan chloride or sodium alginate; 0.5%, w/v) were prepared and stirred overnight. A mixture of 60 μL polymer aqueous solution, 20 μL DPPH ethanol solution (0.05%, w/v), and 220 μL DMSO was monitored using a Varioskan™ LUX multimode microplate reader (Thermo Scientific, Finland).
2.8 Characterization of Silver Nanoparticles
Nanoparticles were purified by centrifugation and resuspension for three times before characterization. The silver nanoparticle sizes were measured using a Nano ZS90 Zetasizer (Malvern, UK). JEM-2100f TEM (JEOL, Japan) was used for TEM (transmission electron microscopy) and SAED (selected area electron diffraction) experiments. Rigaku X-ray diffractometer (D/MAX-2500PC, Japan) was used for XRD (X-ray diffraction) experiments (Cu Kα radiation, 40 kV, 150 mA). The films of silver nanoparticle were used for the two tests mentioned above.
3.1 Wound Healing in Rats with Diabetes
After three weeks of treatment, the rat wounds in all the experimental groups were significantly healed, except in the negative control group (Fig. 1, first group). Compared with growth factors, antibiotics and silver nanoparticles (Fig. 1, last two groups) exhibit better healing effects, probably because the wounds in the rats with diabetes are susceptible to infection; therefore, an anti-infective treatment is crucial.
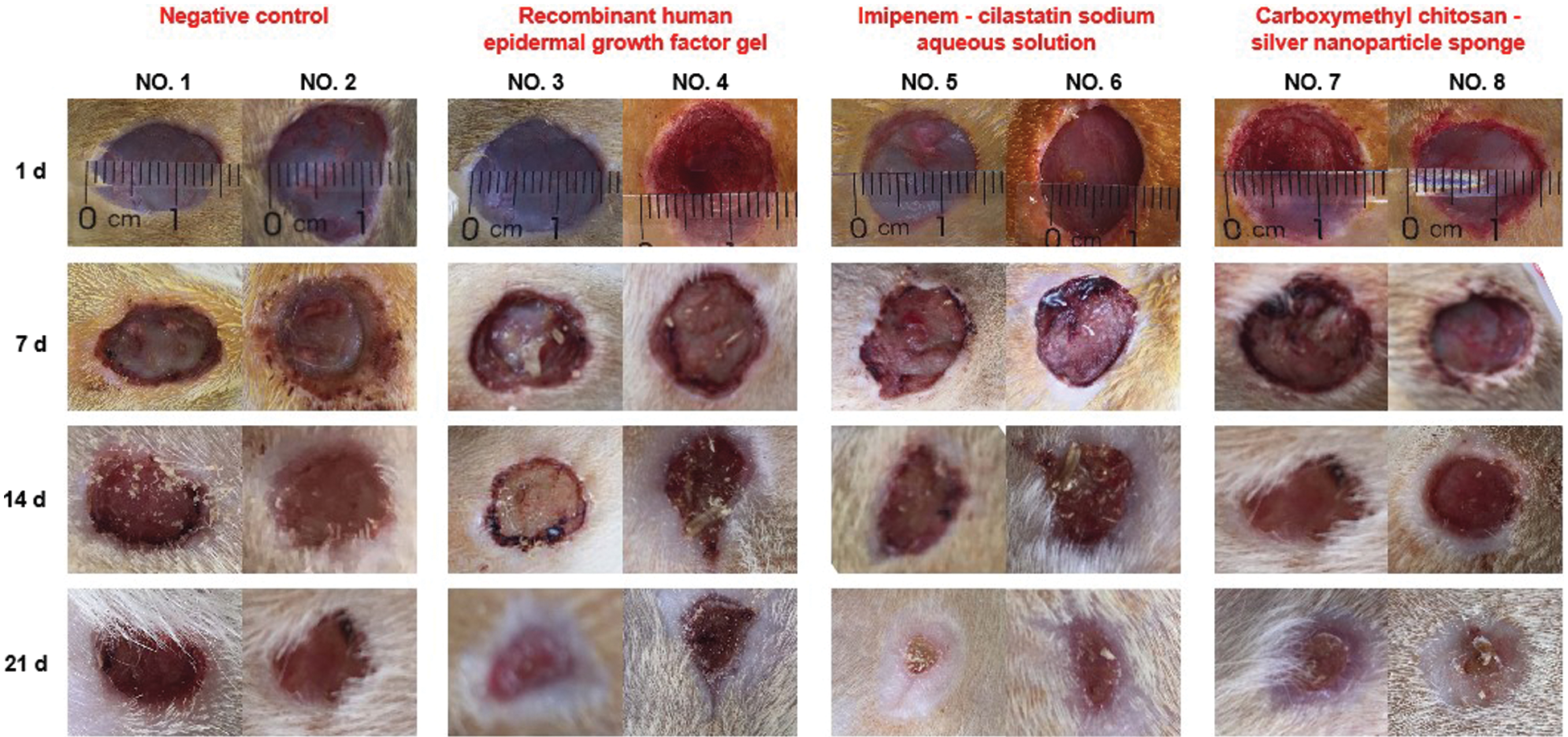
Figure 1: Wound treatment of rats with diabetes
The histological analysis of the healing process in negative control group demonstrated tissue necrosis of the wound (Fig. 2, first group). This phenomenon was not found in treatment group. Both fibrin and inflammatory cells were found in the growth factors group (Fig. 2, second group). These suggest that wound healing was accompanied by an inflammatory response in this group. By contrast, the inflammation in the last two groups was much less intense (Fig. 2, last two group). Significant granulation tissue was also found in the both of two groups.
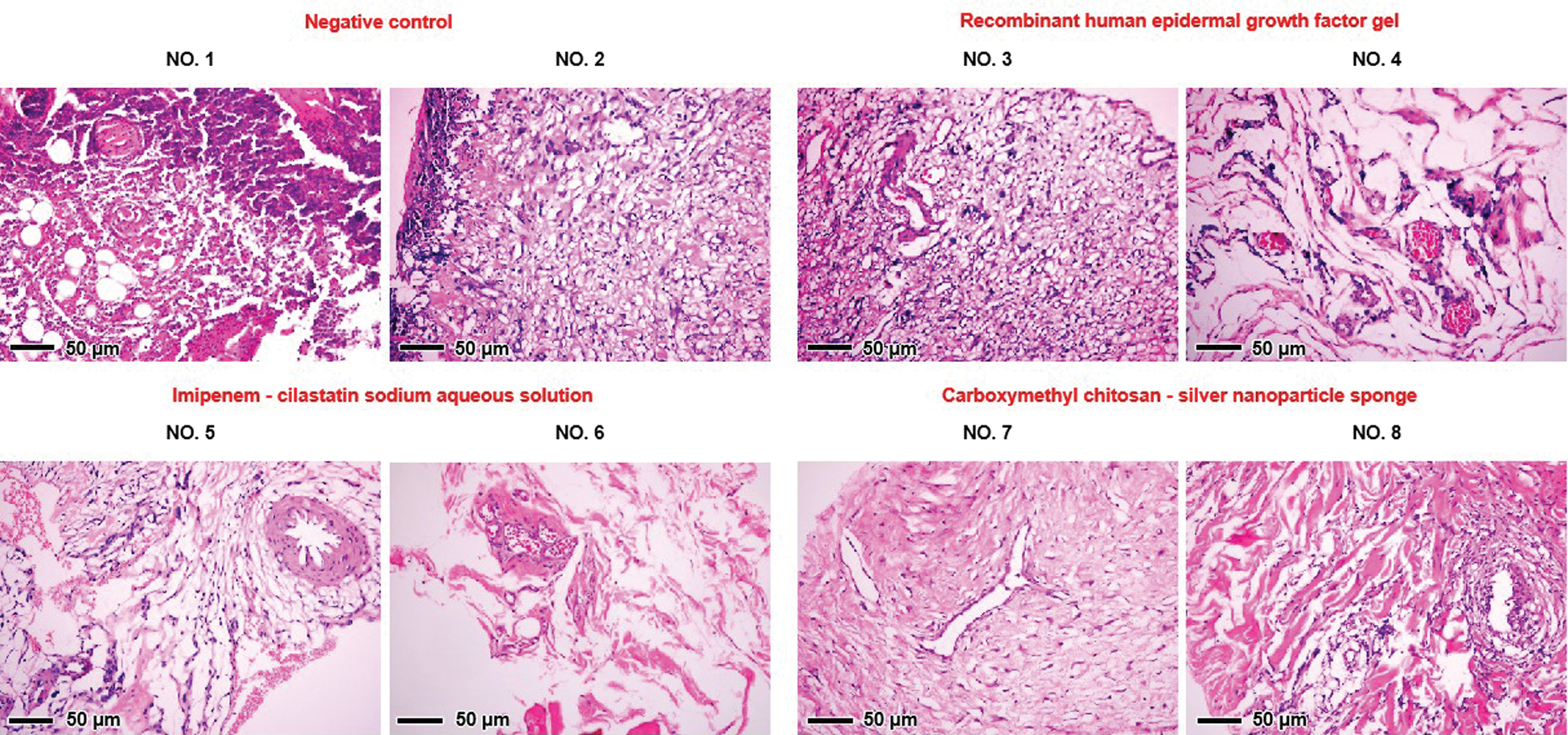
Figure 2: Photomicrograph of H&E stained sections (×200) of rat wound skin
Furthermore, no remnant silver ions were detected in the major organs of these rats (Fig. 3) after the application of silver nanoparticles (Fig. 2).
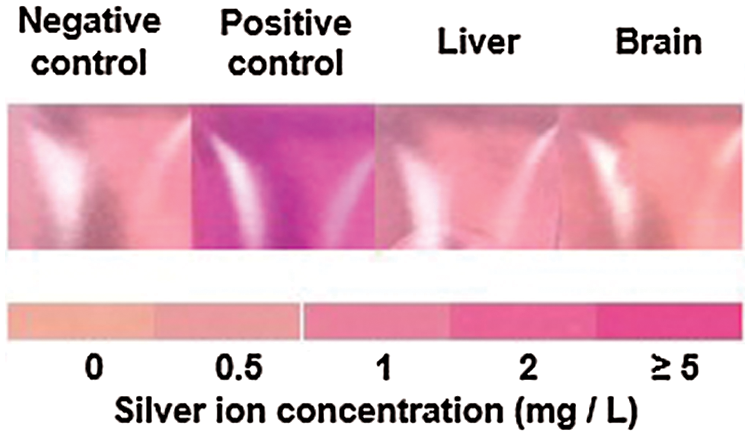
Figure 3: Silver ion detection from rat liver and brain when the wounds were treated with sponge-loaded silver nanoparticles
3.2 Cell Culture and Evaluation of Cytotoxicity
In our previous study, we proposed that silver nanoparticles prepared using CMCS can be used for downstream applications (e.g., bacteriostatic agents or dressings) without further purification. Accordingly, we intend to test the cellular toxicity of the unpurified reaction solutions containing the prepared silver nanoparticles. For this experiment, human skin fibroblast cells were used.
Based on cell viability, no toxicity to human skin fibroblast cells was observed in the diluted reaction solutions until the silver content was increased to 0.4 mM. However, sudden proliferation occurred when the human skin fibroblast cells were exposed to the silver nanoparticles (Fig. 4), suggesting a plausible explanation for the healing effect observed in the wounds of rats with diabetes.
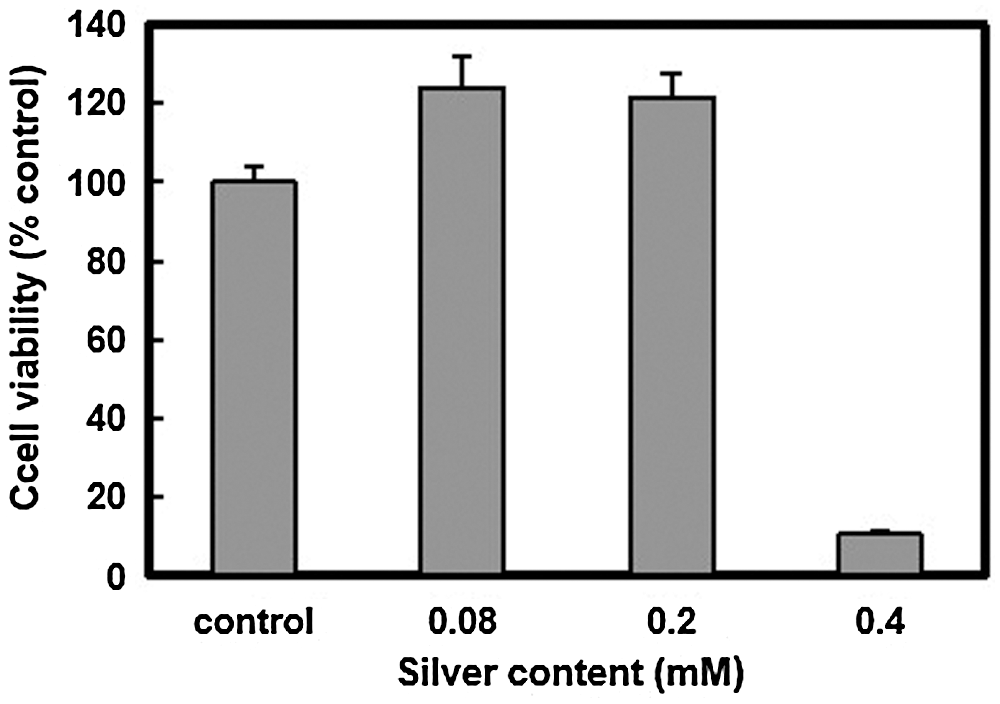
Figure 4: Cytotoxicity evaluation of silver nanoparticles prepared using CMCS (human skin fibroblast cells, n = 3)
3.3 Growth Kinetics of Silver Nanoparticles Prepared with CMCS
To determine the growth process of silver nanoparticles in CMCS, the reaction solutions were analyzed at different time intervals via TEM and XRD, as well as using a Zetasizer. An obvious breakage process of the particles is observed during the growth of nanocrystalline silver in CMCS (Fig. 5, 30 min and 1 h).
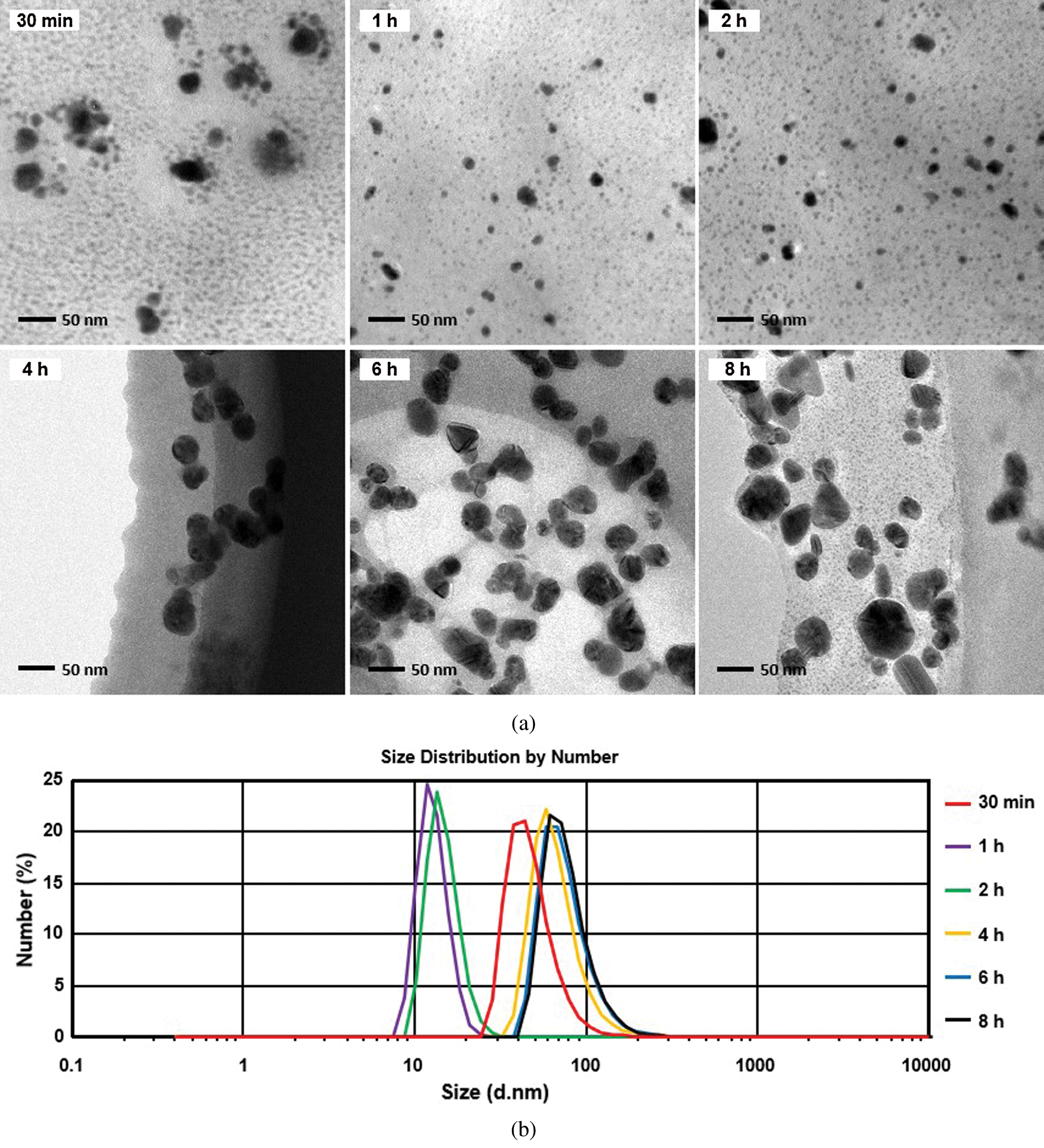
Figure 5: (a) TEM and (b) Zetasizer analyses of silver nanoparticles prepared using CMCS at different reaction times
Cl• are primarily responsible for the transformation of Ag+ to Ag0 [21]. Therefore, the above experimental phenomena may be attributed to the increasing concentration of Cl• in the crystalline core.
The XRD spectrum of the silver nanoparticle solution reaction indicates that prolonging the reaction time reduces the intensity of the AgNO3 diffraction peak until it disappears completely (Fig. 6a). Almost the entirety of the Ag+ is reduced to Ag0, despite the molar ratio of Ag+ and Cl- being 3:1. Since the molar ratio of Ag and Cl is 1:1 in AgCl, it can be assumed that Cl- was recycled after Ag+ was reduced to Ag0 in this reaction. Accordingly, the mechanism of the silver nanoparticle preparation method using CMCS is presumed to arise from the transformation of Ag+ to Ag0 (Fig. 6b) [20].
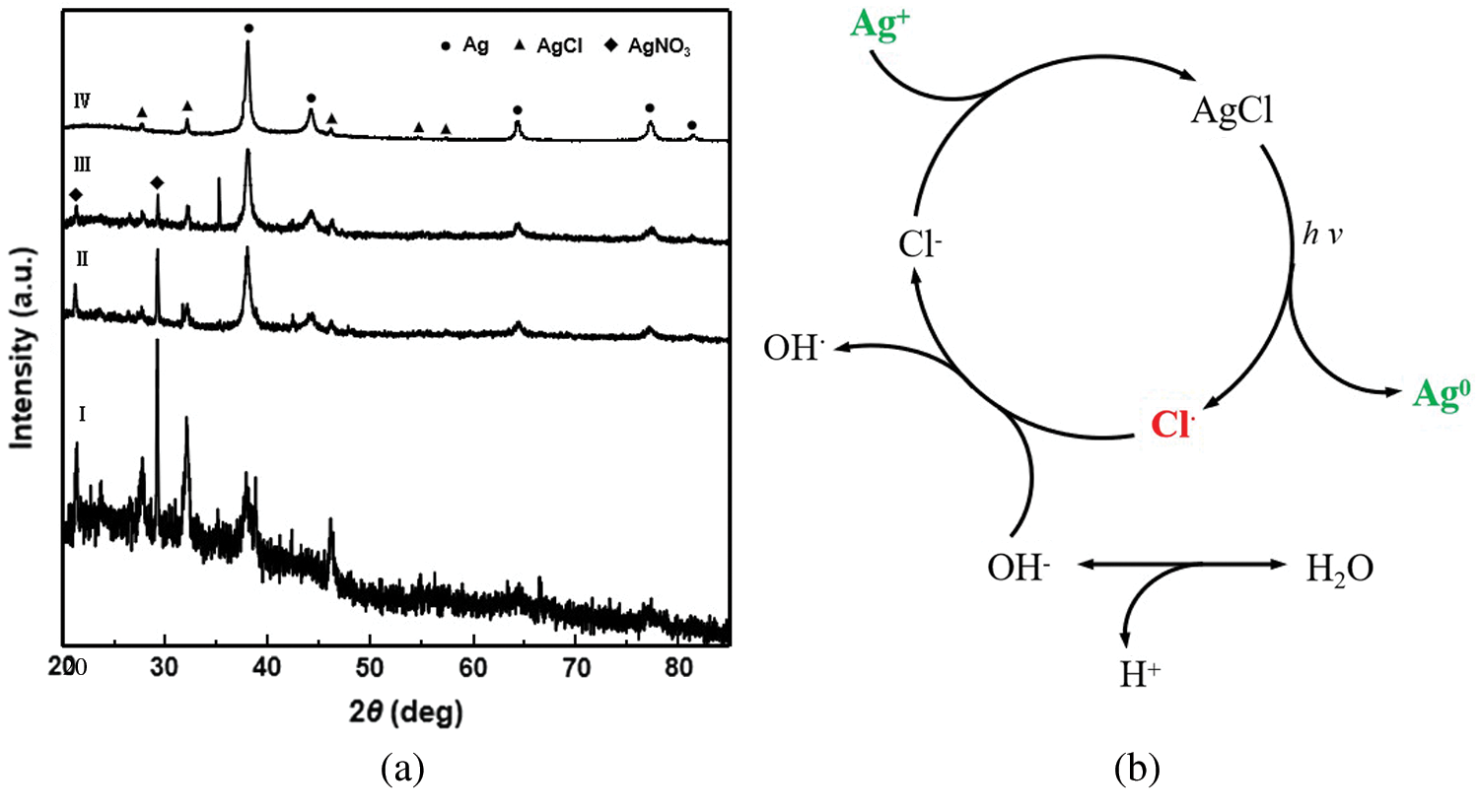
Figure 6: (a) XRD diffraction patterns of the dried films on glass plates from the original reaction solutions (I to IV: after 0.5, 2, 4, and 8 h solar irradiation). (b) Presumed mechanism of the silver nanoparticle preparation method using CMCS
3.4 Preparation of Silver Nanoparticles Using N-Trimethyl Chitosan Chloride and Sodium Alginate
In this study, we also attempted to determine whether this method is suitable for synthesizing silver nanoparticles using other similar polymers and halide ions. N-trimethyl chitosan chloride and sodium alginate were used in this particular experiment.
From the UV-vis spectra, the characteristic UV-vis absorbance peak of silver nanoparticles was found only in the reaction mixture containing N-trimethyl chitosan chloride (Fig. 7a), probably because of the free radical activities of the two polymers. There was a significant difference in this index between the two polymers (Fig. 7b). Notably, the silver nanoparticles were successfully prepared using N-trimethyl chitosan chloride; however, an increased amount of precipitation was observed.
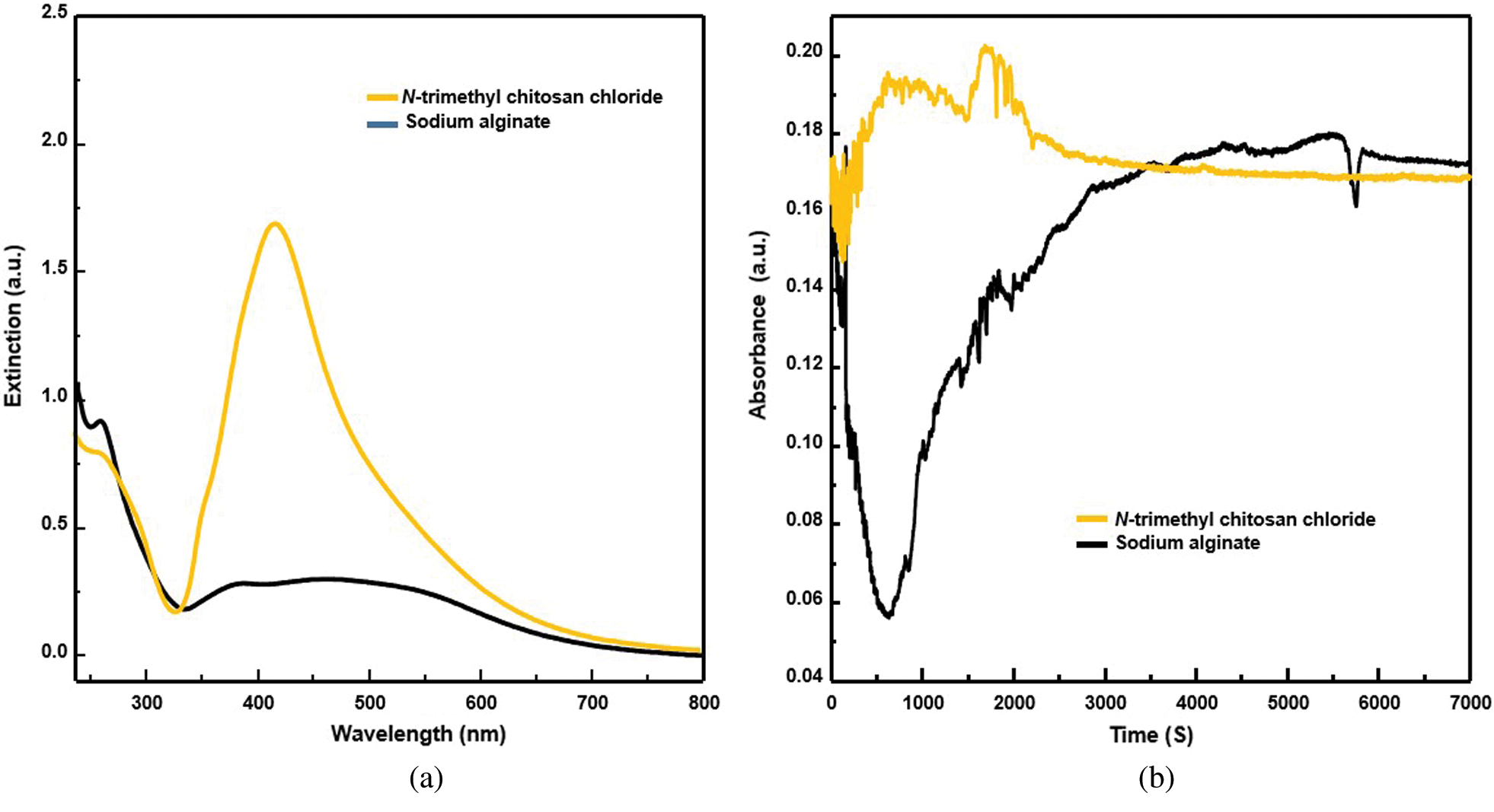
Figure 7: (a) UV-visible spectra of the reaction mixture containing N-trimethyl chitosan chloride (yellow) and sodium alginate (black). (b) Free radical activity of N-trimethyl chitosan chloride (yellow) and sodium alginate (black)
Interestingly, silver nanoparticles were synthesized successfully, and absolutely no precipitation was observed when both polymers were used together (Fig. 8). This may be due to the free radical activity compromising the mixed reaction system.
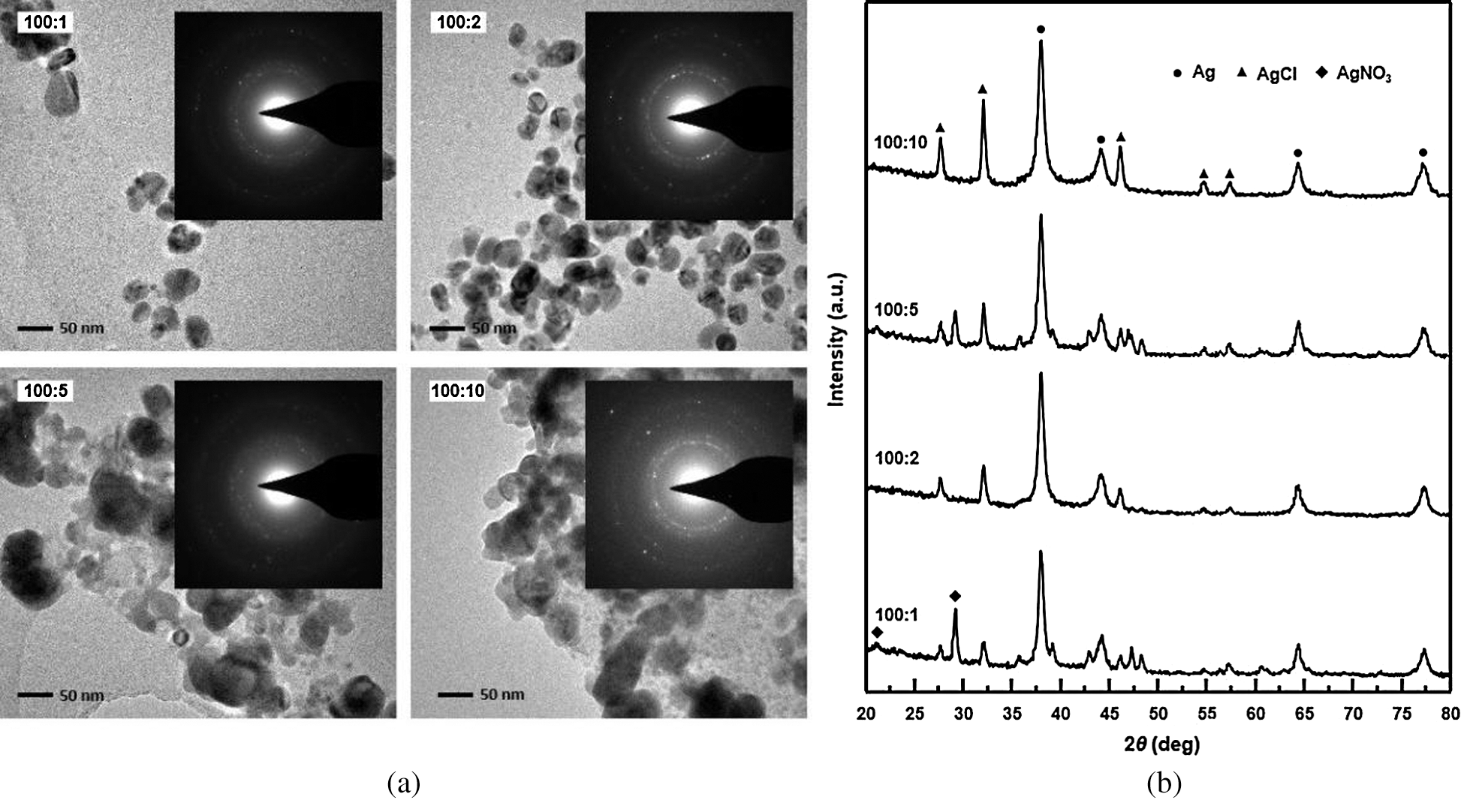
Figure 8: (a) TEM image and SAED pattern (inset) of silver nanoparticles prepared from solutions containing different ratios of N-trimethyl chitosan chloride and sodium alginate. (b) XRD spectra of nano silver particles which were prepared from solutions containing different ratios of N-trimethyl chitosan chloride and sodium alginate
To summarize, the silver nanoparticles prepared by CMCS are less toxic and promote the proliferation of skin fibroblasts. They are promising for potential applications such as in topical medications for treatment of infected wounds. Compared with antibiotics, silver nanoparticles have broad-spectrum antibacterial activity, are easily available, and do not cause drug resistance. These characteristics make silver nanoparticles attractive for a number of uses in biomedical applications. Moreover, our study demonstrates that free radicals are the key factor in this silver nanoparticle preparation method.
Author Contributions: This study was conceived by Yi Long and Chunling Zhang. Yi Long, Chunling Zhang and Lan Chen designed the experiments. Yi Long wrote the manuscript. Yi Long analyzed the data. Tietao Di and Lu Chen provided experimental materials. Lan Chen, Lisha Tang and Kaizhong Luo performed the experiments. Yu Yan, Pingzhen Tong and Jie Xiang performed histopathological experiment. All authors read and approved the final manuscript.
Funding Statement: This research was funded by the Science and Technology Fund of Guizhou Province, Grant No. (2016)7125; the National Natural Science Foundation of China, Grant No. 81660710; and the Natural Science Research Fund of Guizhou Education Department, Grant No. (2017)042.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Kamat, P. (2002). Photophysical, photochemical and photocatalytic aspects of metal nanoparticles. Journal of Physical Chemistry B, 106(32), 7729–7744. DOI 10.1021/jp0209289. [Google Scholar] [CrossRef]
2. Salata, O. (2004). Applications of nanoparticles in biology and medicine. Journal of Nanobiotechnology, 2(1), 3–4. DOI 10.1186/1477-3155-2-3. [Google Scholar] [CrossRef]
3. Lewis, L. (1993). Chemical catalysis by colloids and clusters. Chemical Reviews, 93(8), 2693–2730. DOI 10.1021/cr00024a006. [Google Scholar] [CrossRef]
4. Niemeyer, C. (2010). Nanoparticles, proteins, and nucleic acids: Biotechnology meets materials science. Angewandte Chemie International Edition, 40(22), 4128–4158. DOI 10.1002/1521-3773(20011119)40:22<4128::AID-ANIE4128>3.0.CO;2-S. [Google Scholar] [CrossRef]
5. Yoksan, R., Chirachanchai, S. (2009). Silver nanoparticles dispersing in chitosan solution: Preparation by γ-ray irradiation and their antimicrobial activities. Materials Chemistry and Physics, 115(1), 296–302. DOI 10.1016/j.matchemphys.2008.12.001. [Google Scholar] [CrossRef]
6. Mucic, R., Storhoff, J., Mirkin, C., Letsinger, R. (1998). DNA-directed synthesis of binary nanoparticle network materials. Journal of the American Chemical Society, 120(48), 12674–12675. DOI 10.1021/ja982721s. [Google Scholar] [CrossRef]
7. Yonezawa, Y., Takami, A., Sato, T., Yamamoto, K., Sasanuma, T. et al. (1990). Photochemical formation of silver metal films from silver salt of natural high molecular carboxylic acid. Journal of Applied Physics, 68(3), 1297–1302. DOI 10.1063/1.346731. [Google Scholar] [CrossRef]
8. Raveendran, P., Fu, J., Wallen, S. (2003). Completely “green” synthesis and stabilization of metal nanoparticles. Journal of the American Chemical Society, 125(46), 13940–13941. DOI 10.1021/ja029267j. [Google Scholar] [CrossRef]
9. Božani, D., Djokovi, V., Blanuša, J., Nair, P., Georges, M. et al. (2007). Preparation and properties of nano-sized Ag and Ag2S particles in biopolymer matrix. European Physical Journal E, 22(1), 51–59. DOI 10.1140/epje/e2007-00008-y. [Google Scholar] [CrossRef]
10. Shih, C., Shieh, Y., Twu, Y. (2009). Preparation of gold nanopowders and nanoparticles using chitosan suspensions. Carbohydrate Polymers, 78(2), 309–315. DOI 10.1016/j.carbpol.2009.04.008. [Google Scholar] [CrossRef]
11. Sun, C., Qu, R., Chen, H., Ji, C., Wang, C. et al. (2008). Degradation behavior of chitosan chains in the ‘green’ synthesis of gold nanoparticles. Carbohydrate Research, 343(15), 2595–2599. DOI 10.1016/j.carres.2008.05.027. [Google Scholar] [CrossRef]
12. Liu, J., Anand, M., Roberts, C. (2006). Synthesis and extraction of β-d-glucose-stabilized Au nanoparticles processed into low-defect, wide-area thin films and ordered arrays using CO2-expanded liquids. Langmuir, 22(9), 3964–3971. DOI 10.1021/la060450q. [Google Scholar] [CrossRef]
13. Murugadoss, A., Chattopadhyay, A. (2008). A ‘green’ chitosan-silver nanoparticle composite as a heterogeneous as well as micro-heterogeneous catalyst. Nanotechnology, 19(1), 015603. DOI 10.1088/0957-4484/19/01/015603. [Google Scholar] [CrossRef]
14. Cheng, D., Zhou, X., Xia, H., Chan, H. (2005). Novel method for the preparation of polymeric hollow nanospheres containing silver cores with different sizes. Chemistry of Materials, 17(14), 3578–3581. DOI 10.1021/cm0503230. [Google Scholar] [CrossRef]
15. Miyama, T., Yonezawa, Y. (2004). Photoinduced formation and aggregation of silver nanoparticles at the surface of carboxymethylcellulose films. Journal of Nanoparticle Research, 6(5), 457–465. DOI 10.1007/s11051-004-1716-1. [Google Scholar] [CrossRef]
16. Huang, H., Yang, X. (2004). Synthesis of chitosan-stabilized gold nanoparticles in the absence/presence of tripolyphosphate. Biomacromolecules, 5(6), 2340–2346. DOI 10.1021/bm0497116. [Google Scholar] [CrossRef]
17. Long, Y., Ran, X., Zhang, L., Guo, Q., Yang, T. et al. (2013). A method for the preparation of silver nanoparticles using commercially available carboxymethyl chitosan and sunlight. Materials Letters, 112, 101–104. DOI 10.1016/j.matlet.2013.09.035. [Google Scholar] [CrossRef]
18. Long, Y., Tong, T., Yang, W., Wang, S. (2015). Unique biological performance of the silver nanoparticles prepared by using carboxymethyl chitosan and sunlight. Optoelectronics and Advanced Materials–Rapid Communications, 9(3), 377–380. [Google Scholar]
19. Andrade, E., Silva, V., Moura, N., Foureaux, R., Orlando, D. et al. (2018). Physical exercise improves glycemic and inflammatory profile and attenuates progression of periodontitis in diabetic rats (HFD/STZ). Nutrients, 10(11), 1702. DOI 10.3390/nu10111702. [Google Scholar] [CrossRef]
20. Velupillai, P., Sung, C., Tian, Y., Dahl, J., Carroll, J. et al. (2010). Polyoma virus-induced osteosarcomas in inbred strains of mice: Host determinants of metastasis. PLoS Pathogens, 6(1), e1000733. DOI 10.1371/journal.ppat.1000733. [Google Scholar] [CrossRef]
21. Ashokkumar, M., Marignier, J. (1999). Hydrogen and oxygen evolution from water using Ag and AgCl colloids. International Journal of Hydrogen Energy, 24(1), 17–20. DOI 10.1016/S0360-3199(98)00028-7. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |