 Journal of Renewable Materials
Journal of Renewable Materials
 Journal of Renewable Materials Journal of Renewable Materials |  |
DOI: 10.32604/jrm.2021.014286
REVIEW
Biomolecules of Interest Present in the Main Industrial Wood Species Used in Indonesia-A Review
1Université de Lorraine, Institut National de Recherche pour l’Agriculture, l’Alimentation et l’Environnement, Laboratoire d'Etudes et de Recherche sur le Matériau Bois, Nancy, France
2Department of Forest Products, Faculty of Forestry and Environment, Institut Pertanian Bogor, Bogor University, Bogor, Indonesia
*Corresponding Author: Philippe Gérardin. Email: philippe.gerardin@univ-lorraine.fr
Received: 17 September 2020; Accepted: 20 October 2020
Abstract: As a tropical archipelagic country, Indonesia’s forests possess high biodiversity, including its wide variety of wood species. Valorisation of biomolecules released from woody plant extracts has been gaining attractive interests since in the middle of 20th century. This paper focuses on a literature review of the potential valorisation of biomolecules released from twenty wood species exploited in Indonesia. It has revealed that depending on the natural origin of the wood species studied and harmonized with the ethnobotanical and ethnomedicinal knowledge, the extractives derived from the woody plants have given valuable heritages in the fields of medicines and pharmacology. The families of the bioactive compounds found in the extracts mainly consisted of flavonoids, stilbenes, stilbenoids, lignans, tannins, simple phenols, terpenes, terpenoids, alkaloids, quinones, and saponins. In addition, biological or pharmacological activities of the extracts/isolated phytochemicals were recorded to have antioxidant, antimicrobial, antifungal, anti-inflammatory, anti-diabetes, anti-dysentery, anticancer, analgesic, anti-malaria, and anti-Alzheimer activities. Aside from these remarkable characteristics of woody plant extractives, further studies concerning the valorisation of these extractives in the fields of nutraceutical, cosmetic, bio-control, bio-stimulation, and other advanced applications would be of interest.
Keywords: Bark; by-product; bioactivity; extractive; Indonesia; industry; molecule; valorization; wood species
The use of bioactive compounds derived from nature to promote human health and treat various diseases has been attracting considerable attention. Based on numerous ethnomedicines that commonly used for centuries, intensive research to uncover some bioactive chemicals responsible for these green medicines have been developed since the middle of the 20th century. Inherently separated from their sources, a large part of these bioactive compounds come from forests. In Asia-Pacific, forests and trees are an indispensable part of its regional economies due to their diverse economic, cultural, environmental, and social values. The relationship between the society and the forests encounter important changes, especially in terms of demand for forests. The total forest area in the Asia-Pacific region was estimated at 740 million ha or approximately 18.3% of the global forest area. Indonesia, Australia, China, and India, as the four largest countries in Asia-Pacific, contributed for about 71% of the forest area (Fig. 1). Myanmar, Papua New Guinea, Japan, Malaysia, Lao PDR and Thailand collectively contributed for another 18%, with the final 11% located in the remaining 23 countries and territories [1]. Trade is an important factor of forestry change in the Asia-Pacific region due to the enormous value traded. The import values of primary wood products increased from about US$ 27.1 billion in 1990 to US$ 63.3 billion in 2008. Meanwhile, Asia and the Pacific’s share of global forestry exports grew from US$ 12.6 billion in 1990 to US$ 33.7 billion in 2008. A major forest product exporting subregion in Asia–Pacific came from Southeast Asia, with the total export values of the forest products reported in 2008 was US$13.7 billion. Contributing for 90% of these exports, Thailand (13.4%), Malaysia (27.9%), and Indonesia (48.7%) are the largest exporting countries in this subregion [1].
Indonesia as one of the countries with the largest forest area remains a major contributor to export activities in the forestry sector. Indonesian forests cover more than 49.81% of the national territory with an area of approximately 93.52 million ha. The export value of forest products in Indonesia increased from about US$ 6.61 billion in 2014 to US$ 12.13 billion in 2018 [2]. Log productions of timber culture establishments are spread throughout the islands of Indonesia. In 2018, Sumatra island is the largest region log production with production volume reaching 33,733.64 thousand m3, equivalent to 81.56% of total production. After Sumatra, Borneo island is placed as the second highest producer of logs from these culturing activities [3]. However, these highest log production capacities would be consistent or even increase if sustainable management of forest resources is well implemented, realizing action towards sustainable forestry. As an effort to achieve this, the development of forest products by means of practicing the whole-tree utilization or recycling the wood wastes derived particularly from industrial activities should be well established and executed.
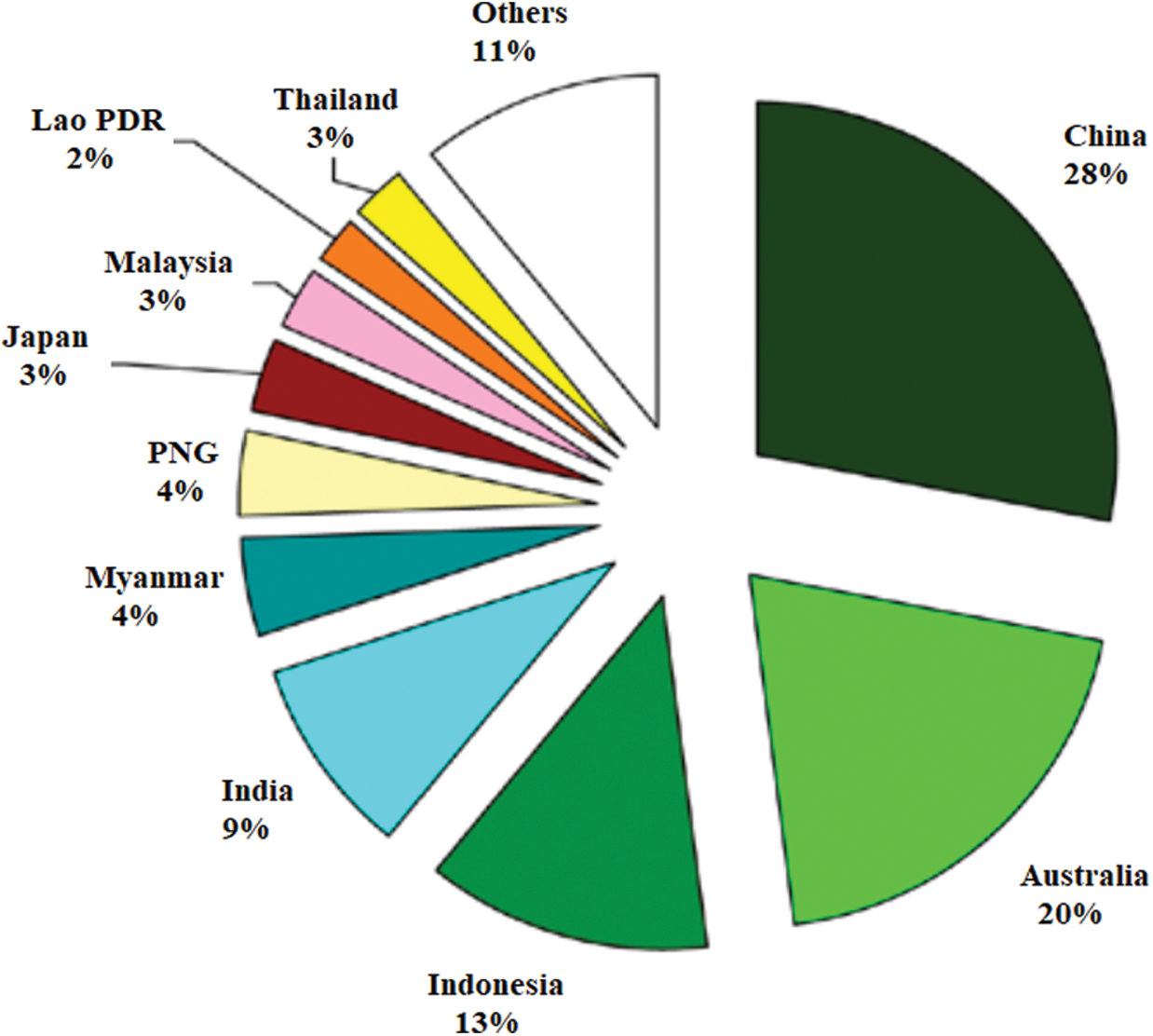
Figure 1: Distribution of forest area by country [1]
Being a tropical archipelagic country, Indonesia’s forests possess high biodiversity, bestowing the nation to have a wide variety of wood species. Around 4000 species of wood have been registered, consisting of 785 genera in 106 families [4]. Among these species, 400 species are commercially used. Indonesia’s forests are also known to have more than 400 species (70%) of the largest meranti (family Dipterocarpaceae) in the world which then recognized as excellent tropical wood species. The wealth of plant diversity can also be shown particularly by the wealth of the Borneo forests [5]. In order to maintain and protect the ecosystem and its biodiversity, Indonesian Ministry of Forestry have established a number of units of marine and terrestrial conservation, consisting of national parks, nature tourism parks, grand forest parks, game hunting parks, natural reserves, and wildlife sanctuaries [6].
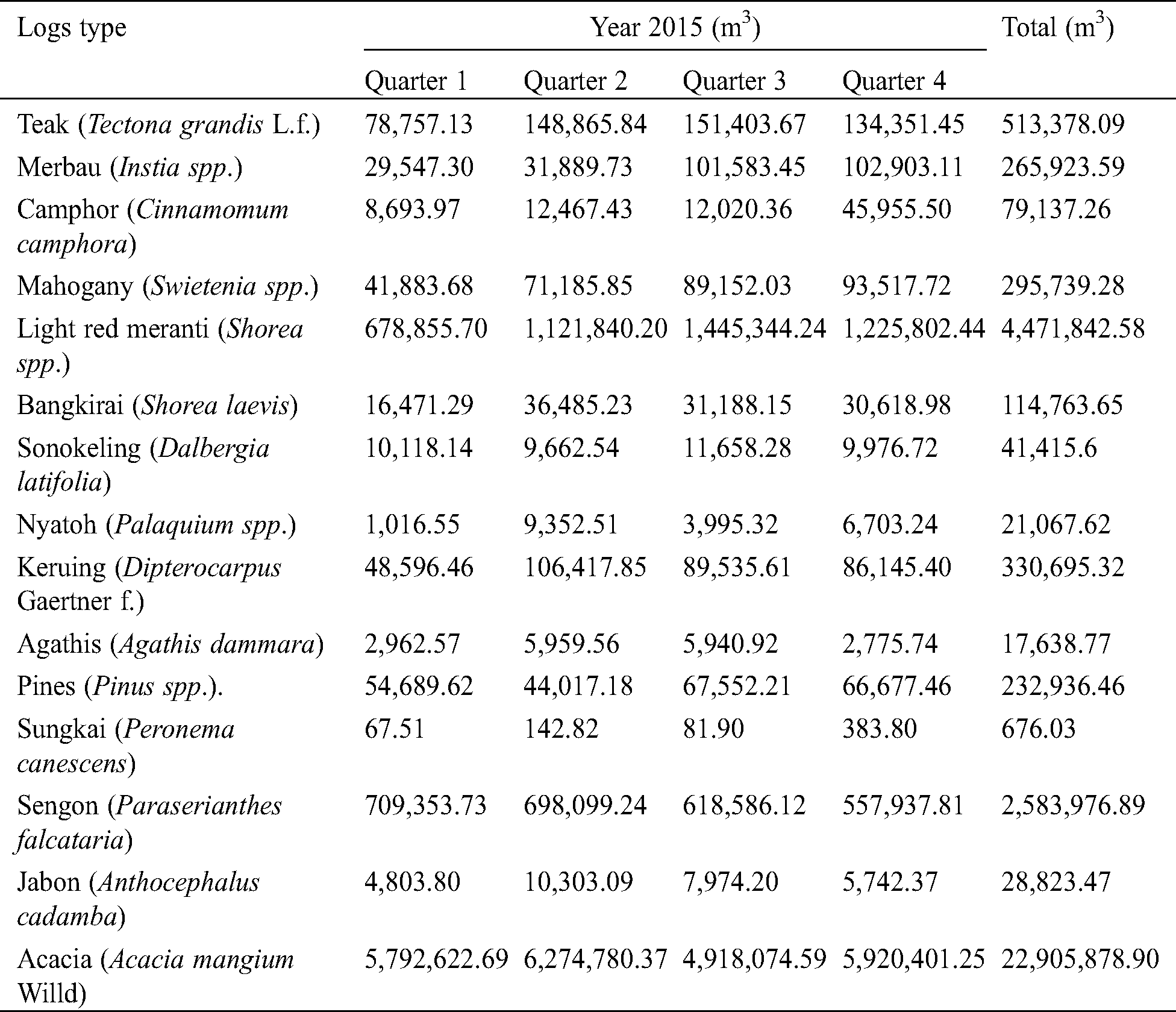
The Indonesian Forestry Law Number 41 Year 1999 (Article 6) stated that based on the main functions, Indonesia’s forest areas are classified into three categories: production forest, conservation forest, and protection forest [7]. In terms of the production forest, its total areas consisted of primary forest, secondary forest, plantation forest, non-forested land, and area with no data. The primary forests were located in Papua and Kalimantan islands, while the secondary forests were located in Sumatra and Kalimantan islands. Plantation forest was dominantly located in Java, followed in Sumatra and Kalimantan islands. Among various wood species that already existed in the production forest, there are twenty wood species either from natural or from plantation forest that widely commercialized for various wood products. These wood species consist mainly of teak (Tectona grandis L.f.), merbau (Instia spp.), camphor (Cinnamomum camphora), mahogany (Swietenia spp.), ebony (Diospyros spp.), light red meranti (Shorea spp.), bangkirai (Shorea laevis), sonokeling (Dalbergia latifolia), ulin (Eusideroxylon zwagery Teijsm. & Binnend.), kempas (Koompassia malaccensis Maingay ex Benth), nyatoh (Palaquium spp.), keruing (Dipterocarpus Gaertner f.), kapur (Dryobalanops. spp), agathis (Agathis dammara / A. loranthifolia / A. celebica / A. alba), and jelutung (Dyera costulata Hook) from the natural forest, whereas the wood species from plantation forest consist of pines (Pinus spp.), sungkai (Peronema canescens), sengon (Paraserianthes falcataria / Albizia falcataria), jabon (Anthocephalus cadamba), and acacia (Acacia mangium Willd). The annual share production of the wood logs of some of them has also been recorded in Tab. 1, according to the statistics of forestry production [8]. The teak as most popular species also comes from plantation forest. With relatively good physical, mechanical, and/or biological durability properties, the wood species from natural forest are commonly used for construction materials, whereas the wood species from plantation forest are commonly used for light construction, panel wood, pulp paper, and fuel wood. The teak, either comes from natural or plantation forest, is usually used for construction and furniture materials.
Although the utilization/processing of these wood species has been optimally practiced following the destined applications, the utilization of forest product residues, such as leaves, barks, roots, flowers, to become highly value-added by-products remains very limited. As an effort to solve this matter, numerous extensive researches have been conducted or even applied, such as valorisation of bark [9−11], leaves [12−14], including intensive analysis of the woody plant extracts for their chemical constituents as well as their biological and pharmacological properties. Correspondingly, it is known that some wood extractives have been reported to present several biological properties like antimicrobials, antibacterial, antifungals, insecticidal, antioxidants [15−17], and could be used as traditional or alternative medicines [18]. In terms of medicinal plant, there were around 1300 kinds of medicinal plants existed in Indonesian tropical forest [19]. As a country with rich plant diversity, Indonesia has become an exporter for the medicinal plants, aromatics, and herbs with the increased revenue from about US$ 299.79 million in 2012 to US$ 601.23 million in 2018 [3]. A lot of traditional knowledge, relating to the use of medicinal plants from various ethnicities living in the forest ecosystem, has been adopted and developed by the herbal and pharmaceutical industries into herbal products. Accordingly, related to the whole-tree utilization, this later case could become a precious contributor in developing the value-added by-products as a source of bioactive molecules for pharmaceutic, cosmetic, nutraceutical, or other advantageous applications.
The objective of this review is to present the knowledge already available in the literature on the structure and properties of the existing bio-molecules of wood extracts from tropical wood species exploited industrially in Indonesia.
Table 1: Annual production of the wood logs by type per quarter in 2015 [8]
2 Chemical Composition and Properties of Some Woody Plant Extracts Derived from the of Main Industrial Wood Species in Indonesia
2.1 Teak (Tectona grandis L.f.)
Teak (Tectona grandis L.f.), a well-known tropical hardwood species in Indonesia, belongs to the family Verbenaceae. Teak is one of the most valuable tropical timber species. Due to its excellent properties (texture, physical and biological durability), it is commonly used industrially for shipbuilding, outdoor equipment, and furniture. Teak grows naturally in South and South-East Asia, mainly in Bangladesh, India, Laos, Myanmar, Indonesia, and Thailand. The Indonesian teak mostly comes from plantation forests in Java island, and the others come from natural or coppice forest on Sulawesi island. The heartwood colour is golden brown and clearly distinct from the yellowish-white sapwood. Darmawan et al. [20] reported that heartwood portion of the teak aged of 10 years was 40%, whereas the teak at the age of 40 years was 80%. The specific gravity of the wood is 0.62–0.75 [4]. Rizanti et al. [21] reported that a long rotation teak (contains less juvenile wood and higher heartwood content) contained slightly more holocellulose, cellulose, and hemicellulose contents than a short rotation teak (contains more juvenile wood and higher sapwood content) (68.53%, 49.18%, and 19.35%; 67.50%, 48.80%, and 18.70%, respectively), but the lignin content was lower (32.19%; 35.53%, respectively). This lignin content is much higher compared to the lignin content in hardwood in general. In general, heartwood contains more lignin compared to sapwood [22]. Rizanti et al. [21] also reported that long rotation teak contained higher contents total of extractives than short rotation teak (8.0% and 3.7%, respectively). Long rotation teak has higher extractives contents than short rotation teak due to higher heartwood content and also has less proportion of juvenile wood. In the previous study, Miranda et al. [23] reported that the extractive content of teak aged 50–70 years was 12.7%. Later on, Moya et al. [24] reported that some influential factors such as growth location, the type of solvent, and extraction techniques could affect differences in this extractive content.
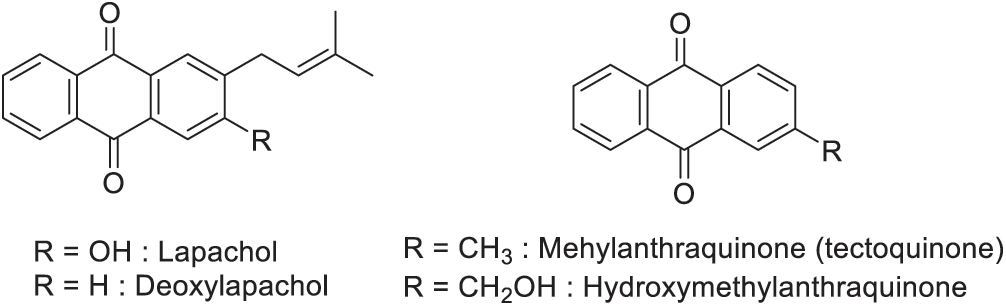
Figure 2: Predominantly quinone derivates of teak wood
Teak wood contains anthraquinones (tectoquinone, 2-methyl quinizarin, 1-hydroxy-2-methylanthraquinone, pachybasin), naphthoquinones (lapachol, deoxylapachol, 5-hydroxylapachol), naphthoquinone derivatives (tectol, dehydrotectol, α-dehydrolapachone, β-dehydrolapachone), and, squalene, β-sitosterol, betulinic acid, trichione, and obtusifolin [25]. Several extractives of teak wood improved teak wood’s ability to resist against biological attacks. These are predominantly quinone derivatives, like lapachol and tectoquinone (Fig. 2). Tectoquinone is the main component responsible for the wood natural durability against termites. Meanwhile, deoxylapachol has been found to have a strong anti-termite activity compared to lapachol reported to exibit a weak anti-termite activity [26]. Tectoquinone and deoxylapachol are active compounds against Aspergillus niger [27]. GC-MS analysis of dichloromethane extract (yield: 5.7%–9.06%) of the heartwood shows that squalene is the major component for this solvent [23,28]. The high nonpolar fraction in the heartwood was reported responsible for this teak durability [23]. The dichloromethane, acetone, and toluene-ethanol extracts of long rotation teak wood contain tectoquinone as the main substance identified with a percentage ranging from 4.5 to 14.5% [21]. Windeisen et al. [29] reported that some other substances were found in the acetone extract such as 2-hydroxymethylanthraquinone, and 2-tert-butylanthraquinone. Lukmandaru et al. [26] also reported that extraction using toluene-ethanol and ethanol-benzene solvent has allowed them to extract palmitic acid and squalene, respectively. Squalene might contribute towards durability in the form of a hydrophobic barrier to toxic triterpene compounds [29]. In pharmacology, Goswami et al. [30] reported that many parts of teak wood have been used for several treatments. Root contained lapachol, tectoquinone, β-sitosterol, tectol, 1-hydroxy-2-methyl anthraquinone, dehydrotectol, β-lapachone, dehydro-α-lapachone, new diterpene, tectograndinol, non-structural carbohydrates, pachybasin, obtusifolina, and betulinic acid that are used in the treatment of anurea and urine retention. Bark contained tannin (7.14%), 5-hydroxy-1,4-napthalenedione (juglon), obtusifolina, desidro-α-lapachona as astringent and useful for treatment of bronchitis. The wood is acrid, sedative, anthelmintic, expectorant, and useful in the treatment of gravid uterus, dysentery, headache, piles, leucoderma, and burning pain over the liver region. Meanwhile, the ashes of the wood are applied to swollen eyelids.
Merbau (Intsia spp.) is a tropical hardwood belonging to the family Caesalpiniaceae. The wood has a coarse-textured and a yellowish to orange-brown until reddish brown colour [4]. Merbau consists of nine species with different local names in different countries. Merbau is also commonly known as “kwila” or “ipil” in Papua New Guinea, “moluccan ironwood” in Philippines, and “Borneo teak” in United Kingdom. The most widespread species are Intsia bijuga and Intsia palembanica. Other known species are Intsia plurijuga, Intsia bakeri, Intsia puberula, Intsia amboinensis, Intsia retusa, and Intsia rhomboidei. The range of specific gravity is 0.63–1.04 for Intsia bijuga and 0.52–0.97 for Intsia palembanica. The chemical compositions of merbau consist of cellulose 46.9%, lignin 22.6%, pentosan 17.1%, ash 0.9%, and silica 0.2% [4]. The wood is generally used for structural furnishings such as windows, framing, flooring and doors due to its favourable physical and mechanical properties (especially excellent tensile strength) and its termite resistance properties [31].
Figure 3: Robinetin
In the heartwood of merbau, robinetin is the main polyphenol compound with smaller amounts of 3,5,4’-tri and 3,5,3’,4’ tetra-hydroxystilbene, dihydroxymyricetin, myricetin and naringenin. According to Hillis et al. [32], the yellow crystalline in the heartwood of merbau is identified as pure robinetin (Fig. 3). Intsia palembanica has a higher proportion of robinetin than Intsia bijuga. The pure robinetin is formed throughout the length of vessels [33]. Merbau also contains large amounts of water-soluble extractives, including leucocyanidin. The high-water solubility of these extractives is responsible for some disadvantageous discolouration, which is manifest as blackish-lined, dark-coloured flecks on the surface. Even though robinetin is the principal compound in heartwood, it does not have high fungal toxicity [34]. Bark contains leucocyanidins, polyphenols, stilbenes, polysaccharides, and water-soluble polymers [35]. Hasan et al. [36] reported that the ethyl acetate extract of Merbau has a potent antioxidant characteristic. The effective concentrations (EC50) for DPPH scavenging are 7.9 ± 0.01 µg/mL and 4.5 ± 0.02 µg/mL for the extract and standard antioxidant (Trolox), respectively. Whilst, the inhibitory concentrations (IC50) for inhibition of tertbutylhydroperoxide is 1.26 µg/mL, in comparison to the standard antioxidants, Trolox (IC50 = 3.98 µg/mL) and alpha-tocopherol acetate (IC50 = 1.58 µg/mL). The extract of Merbau has potent activity to be used for human health to prevent some diseases involving free radical and oxidative damage. In Madagascar, a decoction of the bark of Intsia bijuga is used in traditional medicine for diarrhea [37]. In Vanuatu, the inner bark of Intsia bijuga is used as remedy for asthma, meanwhile the leave of inner bark is used as remedy for diabetes and to relieve infections [38].
2.3 Camphor (Cinnamomum camphora)
The commercial Camphor comes only from Cinnamomum camphora (fam. Lauraceae) and Dryobalanops camphora (fam. Dipterocarpacaea). Camphor is widely planted in southern China, Vietnam, Japan, and Borneo. It is an evergreen tree species that grows to a great size, many-branched, flowers white, small, and clustered. Camphor wood is rigid and insect-resistant structural material that commonly used for luxury architecture and furniture. A considerable amount of valuable volatile chemical compounds such as campherenone, camphor, campherenol, nerolidol, and 1,8-cineole are contained in camphor trees [39]. Pandey et al. [40] also reported that camphor extracts contain α-terpineol, linalool, eucalyptol, safrole, etc. Camphor is a typical marker of camphor wood (Fig. 4). Camphor is commonly detected from all the camphor wood. Camphor is a natural product obtained through a steam distillation and purification by sublimination [41]. Camphor has been used in traditional and modern medicines, such as for the treatment of fibrous tissue inflammation, neuralgia, pruritic skin diseases, and influenza [42].
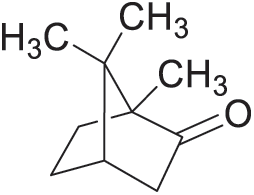
Figure 4: Camphor
Camphor is a colourless or white crystalline powder with a strong aromatic odour and volatile at room temperature. Camphor is slightly soluble in water (solubility of 0.12 g/100 mL at 25°C) and soluble in ethanol, chloroform, carbon disulphide, ether, and volatile or non-volatile oils [43]. The volatile gas of camphor can kill many types of harmful bacteria. The chemical components of extractives in camphor including their insecticidal and antimicrobial activities have been reported [44,45]. On the other hand, Kamariah et al. [46] investigated the oils from the leaves and seed of Dryobalanops aromatica. The leaf oil contains 84 compounds representing 92% of the total oil. α-terpineol (16%), terpinen-4-ol (15%), globulol (8%) and α-pinene (7%) are the major constituents. Meanwhile, the seed oil contains 31 compounds representing 100% of the total oil, with the major constituents being α-pinene (41%), α-thujene and β-pinene (13% each), sabinene, limonene and bicyclogermacrene (6% each), and myrcene (5%). The camphor extractives have been more commonly used in the form of the essential oil, which can be obtained from the trunk, leaves, and twigs by steam distillation or extraction using various solvents [47]. The camphor extract is used not only for medicinal application but also as an antibacterial agent for animals and plants against pathogenic bacteria, such as Escherichia coli, Staphylococcus aureus, Bacillus subtilis, etc. Camphor can also be used for food flavorings or preservatives due to its bacteriostatic and insecticidal activities that could restrain the growth of microbes [15,17].
Mahogany is a tropical hardwood species from the family Meliaceae. It consists of three species, Swietenia macrophylla king, Swietenia mahogany L. Jacq. and Swietenia humulis Zucc. The most widespread species are S. macrophylla and S. mahogany. S. macrophylla is native species to Central and South America and distributed from Mexico to Peru, Bolivia et al. [48]. S. macrophylla is used for many purposes such as furniture, ship building, panelling, etc. S. mahogany is mainly planted in southern Asia (Sri Lanka, India, and Bangladesh), in the Pasific (Indonesia, Malaysia, Philippines, and Fiji), and presented into cultivation in West Africa. S. mahogany is prospective to be applied for large scale timber production plantations, especially in dry areas, due to the excellent timber quality. Moreover, the plant is also used for soil improvement in agroforestry [49]. S. macrophylla and S. mahogany have specific gravity of 0.53-0.67 and 0.56-0.72, respectively. Mahagony contains cellulose 46.8%, lignin 26.9%, pentosan 16.4%, ash 0.6%, and silica 0.1% [4].
S. macrophylla contains alkaloids, flavonoids, saponins, phenols, triterpenoids, anthraquinones, phospholipid, tannins, sterols, glycoside, resins, volatile oils, and long chain unsaturated fatty acid [50]. The major fatty acids are linoleic (37.50%-39.21%), oleic (18.82%-22.03%), stearic (16.75%-17.65%), and palmitic (14.62%-15.47%) [51]. Mootoo et al. [52] reported there are fifteen limonoids isolated from S. macrophylla: 7-deacetoxy-7-oxogedunin, andirobin, and thirteen bicyclononanolides. Eleven of the latter are the known compounds, swietenine, proceranolide, swietenolide, 6-O-acetylswietenolide, 3,6-O,O-diacetylswietenolide, khayasin T, and swietemahonins E-G that all recently reported from S.mahagoni, 2-hydroxyswietenine, and 6-deoxyswietenine (febrifugin). On the other hand, study on the leaves of the plant has successfully isolated limonoid of phragmalin type named swietenine J with nine compounds such as methyl-6- -hydroxy angolensate, 1-O-acetylkhayanolide A, khayanolide E, khayalactone, khayanone, 1-O-acetylkhayanolide B, 1-O-deacetylkhayanolide E, khayanolide A, khayanolide B [13]. Fig. 5 shows the new limonoid, swietemacrophin. S. macrophylla is used for treating wound infection and skin condition [53]. In addition, S. macrophylla can also be used for treatment or prevention of various inflammatory diseases [54].
-hydroxy angolensate, 1-O-acetylkhayanolide A, khayanolide E, khayalactone, khayanone, 1-O-acetylkhayanolide B, 1-O-deacetylkhayanolide E, khayanolide A, khayanolide B [13]. Fig. 5 shows the new limonoid, swietemacrophin. S. macrophylla is used for treating wound infection and skin condition [53]. In addition, S. macrophylla can also be used for treatment or prevention of various inflammatory diseases [54].
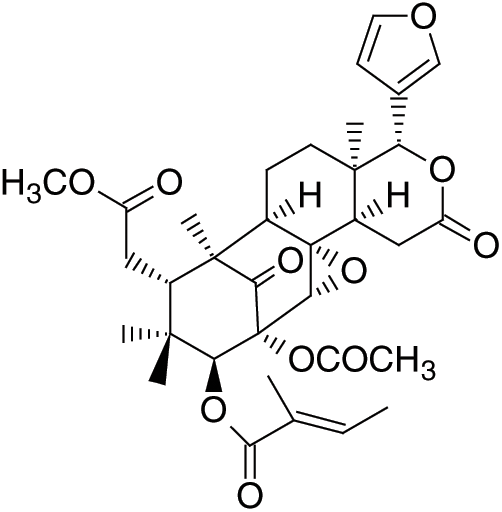
Figure 5: Swietemacrophin
S. mahagoni contains alkaloids, saponins, tannins, polyphenols, steroid, and triterpenoids [55]. Chen et al. [56] found two novels limonoids from S. mahagoni (Fig. 6).

Figure 6: Swiemahogins A (I) and B (II)
Limonoids have been isolated from twigs and leaves. Swiemahogins A (1) and B (2) are the first examples of andirobin and phragmalin types of limonoids with a rare  -lactone ring fused to the C-ring at C-8 and C-14 instead of the usual D- ring
-lactone ring fused to the C-ring at C-8 and C-14 instead of the usual D- ring  -lactone. Saad et al. [57] also reported that the extract obtained from the stem bark of the plant has three novel ring-D opened limonoids corresponding to the phragmalin 8,9,14-orthoacetate with the addition of methyl 2,30-orthoacetate or a propionate, swietenialides A, B, C and two ring-D opened phragmalin type 1,8,9-orthoacetates, swietanialides D and E are isolated together with one known mexicanolide, 2-hydroxyswietenin. The extract from the seed contains 18 tetranortriterpenoids consisting of five swietenins (B-F), three acylswietenolides, seven swietemahonins (A-G), swietemahonolide, mahonin, and secomahoganin [58]. The seeds are traditionally used for leishmaniasis and abortion medicine by the Amazonian Bolivian ethnic group [59] and for the treatment of hypertension, diabetes, and malaria in Indonesia [58].
-lactone. Saad et al. [57] also reported that the extract obtained from the stem bark of the plant has three novel ring-D opened limonoids corresponding to the phragmalin 8,9,14-orthoacetate with the addition of methyl 2,30-orthoacetate or a propionate, swietenialides A, B, C and two ring-D opened phragmalin type 1,8,9-orthoacetates, swietanialides D and E are isolated together with one known mexicanolide, 2-hydroxyswietenin. The extract from the seed contains 18 tetranortriterpenoids consisting of five swietenins (B-F), three acylswietenolides, seven swietemahonins (A-G), swietemahonolide, mahonin, and secomahoganin [58]. The seeds are traditionally used for leishmaniasis and abortion medicine by the Amazonian Bolivian ethnic group [59] and for the treatment of hypertension, diabetes, and malaria in Indonesia [58].
The genus Diospyros belongs to the family Ebenaceae. Brummitt [60] has divided the family into three genera: Diospyros, Euclea, and Tetraclis. Euclea and Tetraclis are small numbers of species, existing only in eastern and southern Africa and Madagascar. The most widespread species is Diospyros. Diospyros contains about 400 species mostly native to the tropics (Madagascar, Africa and Malaysia), and two natives to the United States. Ebony is a member of genus Diospyros. Macassar ebony (Diospyros celebica) which natives to Indonesia, Ceylon ebony (Diospyros ebenum) which natives to Sri Lanka and southern India, and Gabon ebony (Diospyros crassiflora) which natives to western Afrika are certain species of ebony. The heartwood is dark brown to black, while sapwood is white to a greyish brown. The average specific gravity of Macassar ebony is 1.09. Chemically, the wood contains cellulose 46.5%, lignin 28.5%, pentosan 18.4%, and ash 1.7% [4]. Diospyros spp. has many sources for naphtol and napthoquinones. The structural elucidation of Diospyros spp. produced diospyrol (I), mamegakinone (II), isodiospyrin (III) (Fig. 7) and bisisodiospyrin [61].
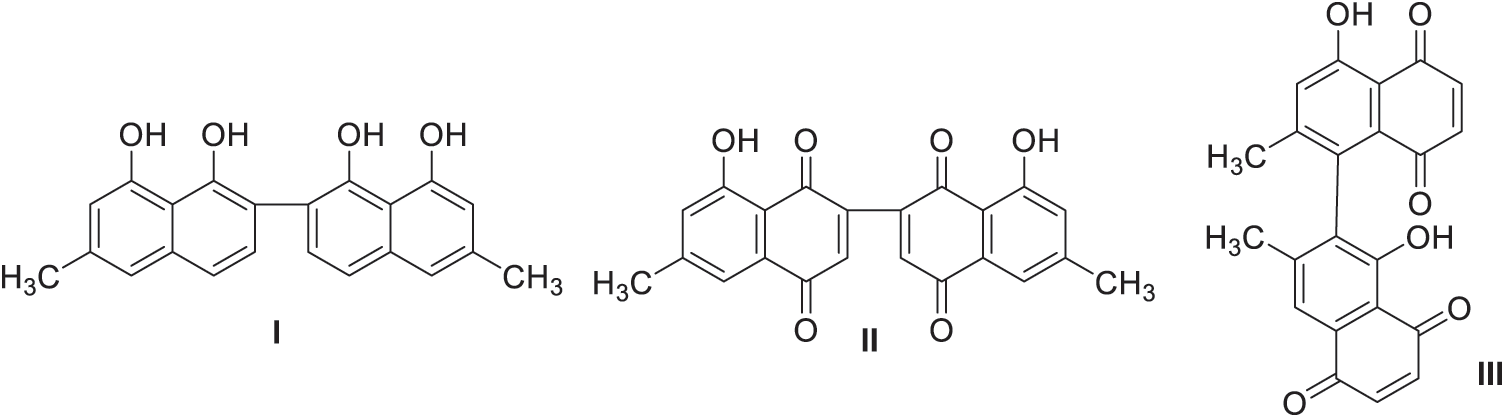
Figure 7: Compounds elucidated from Diospyros spp.
The extract obtained from the bark of the plant contains diosquinone, crassiflorone, plumbagin, cyclocanaliculatin and gerberinol [62,63]. In Macassar ebony (D. celebica), the heartwood contained Macassar II, Macassar III (the principal aromatic extractive derived from naphthol), and betulin [64]. Further, two β-naphthaldehydes and betulinic acid were isolated from the Diospyros ebenum [65]. On the other hand, other compounds such diospyrin and isodiospyrin are isolated from the heartwood of D. mespiliformis. Betulin, lupeol, plumbagin (2-methyljuglone), elliptinone are isolated from the bark of D. elliptifolia [66]. Diomelquinone, isocelebaquinone, o-naphthoquinone, celebaquinone, diosindigo B and its dihydro-derivative are isolated from the heartwood of D. celebica [67]. Dinaphthofuran 3,5´-O-cyclodiospyrin, 8´-hydroxydiospyrin, 2´- and 3´-chlorodiospyrin, 3´-chloro-2´-hydroxydiospyrin, the chromenone ester and acid are isolated from the heartwood and the bark of D. montana [68]. 4-hydroxy-5,6- dimethoxy-2-naphthaldehyde, 5,6,8-trimethoxy-3-methyl-1-naphthol, 4,8-dihydroxy-5-methoxy-2-naphthaldehyde, 4-hydroxy-5,8-dimethoxy-2-naphthaldehyde, and 4-hydroxy-5-methoxy-2-naphthaldehyde are isolated from the heartwood of D. kaki [69]. Isodiospyrin, bisisodiospyrin, 7-methyljuglone, shinanolone, taraxerol, lupeol, betulin, and betulinic acid are isolated from the root of D. Japonica [70]. Ehretione is isolated from the heartwood of D. ehretiodes [71].
In pharmacology, the anticancer activity of diospyrin, a bisnaphtoquinone, which presents in the heartwood of many species of Diospyros plants, has been investigated including its derivatives/analogues [72]. An antibacterial activity of a n-hexane, ethyl acetate, and ethanol extracts of the ebony bark against Staphylococcus aureus and Escherichia coli bacteria has also been investigated by Sumitriasih et al. [73]. This n-hexane extract contains only steroid compounds, the ethyl acetate extract contains alkaloids and tannins, while the ethanol extract contains flavonoids, alkaloids, and tannins. The ethyl acetate extract provides the highest inhibition towards the gram-positive bacteria (S. aureus), while ethanol extract provides the highest inhibition against the gram-negative bacteria (E. coli). Another study reported that the aqueous extract of D. mespiliformis bark has potential benefit for a neuropharmacological activity as a natural depressant. It is evidence that the extract can promote pentobarbital-induced sleep [74]. In addition, human health components analysis of the D. celebica extract by means of PY-GC-MS, TDS-GC-MS, and GC-MS has been investigated as well as the medicinal functions related to the obtained molecules through reviewing the literature. The study revealed that some of the obtained compounds have medicinal value as antiangiogenic, antioxidant, anti-inflammatory, anti-thrombosis and hypolipidemic. These compounds can protect the pancreatic B cells against the toxicity of alloxan, playing a role in endothelial dysfunction in uremic patients as well as repairing wound and reducing endothelial progression. The compound also has a protective effect on acetaminophen-induced necrosis of renal tissue, and antimicrobial activity against NCIM 2501 and NCIM 5021 [75]. On the other hand, anti-termite activities of the root extract of D. sylvatica and its four quinones (plumbagin, isodiospyrin, microphyllone, diospyrin) isolated from the chloroform extract of the root has been inquired, revealing that the toxic property of the extract as well as the tested quinones and showing high mortality of the subterranean termite Odontotermes obesus workers after 48 h on forced exposure [76].
Pine, a trade name of Pinus spp., is a conifer in the family Pinaceae. Pinaceae contains eleven recognized genera and 225 species. Pine is classified into two major lineages, subgenus Pinus and subgenus Strobus [77]. Pine is mainly distributed in the Northern Hemisphere and widely distributed across many forest types in Europe, Asia, North Africa, North America, and Central America. Pine species are important components of boreal, temperate, sub-tropical and tropical forests. Pine species are highly valuable for the industry due to their fast-growing, easily cultivated, and suitable for industrial plantations, agroforestry, and community forestry. P. merkusii is the most widely spreading across the south equator. Pinus merkusii is an important plant used on plantation Indonesia. The main purpose of pine plantation forest is to produce oleoresin. The unproductive pine trees and then felled to produce wood. Its wood is used for the wood-working and pulp-paper industries. Its oleoresin, obtained through tree-tapping, contains high quality of rosin and turpentine oil [78]. Martawijaya et al. [4] reported the range of specific gravity of P. merkusii wood was 0.40–0.75. P. merkusii is chemically composed of cellulose 54.9%, lignin 24.3%. This lignin content is lower compared to the lignin content in softwood in general. pentosan 14.04%, ash 1.1%, and silica 0.2%.

Figure 8: α-pinene of Pinus merkusii
From the genus Pinus, there are more than 280 compounds have been isolated, including phenols, terpenoids, lignans, flavonoids, and some other compounds. Li et al. [18] reported there are 35 triterpenoids isolated from Pinus plants, 18 seratane-types were mainly obtained from P. armandii and P. monticola, and 17 lanostane-types obtained from P. monticola. Diterpenoids are the main metabolites of Pines. Diterpenoids divided into three types, i.e., pimarane, labdane, and abietane types. Isodextropimaric acid type is an example of diterpenoids in P. armandii. Sesquiterpenoids and monoterpenoids are the main compounds found in oleoresins.
Chemical compositions of Pinus merkusii turpentine, oils, gum oleoresins, and rosins have been investigated by means of GC-MS. The neutral fraction of gum oleoresins and turpentine oils dominantly consists of α-pinene (Fig. 8), ∆-3-carene, and β-pinene [78,79], whereas the major constituent of the acidic fraction and rosins are identified as sandaracopimeric acid, isopimeric acid, palustric acid, dehydroabietic acid, abietic acid, neoabietic acid, and merkusic acid [78]. Similar study was also performed by Song [80] identifying the chemical components of several Pines oleoresins by GC-MS method on different pines. They find two sesquiterpenoids (longifolene and caryophyllene) and six common monoterpenoids (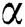 -pinene camphene,
-pinene camphene, 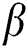 -pinene,
-pinene, 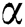 -myrcene, limonene, and
-myrcene, limonene, and 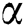 -terpinene). Flavonoids mainly occur in P. morrisonicola and P. armandii, while biflavones and triflavone are found in P. Sylvestris. Type of lignans are also found in Pines such as benzodioxanes, lignanolides, tetrahydrofurans, benzofurans, oligomerics diepoxylignan, arylnaphthalenes, and dibenzocyclooctene. Related to this study, steroid (e.g., stigmast-4-en-3-one; β-sitosterol) and triterpenoid (e.g., 3β-methoxyserratt-14-en-21-one; 3-α,21β- dimethoxy-∆14-serratene; serrate-14-en-3β,21β-diol) are identified as dominant lipophilic compounds in the inner bark of the P. merkusii whereas monoterpenes and sesquiterpenes are recorded in minor quantities either in inner or in outer bark [81,82].
-terpinene). Flavonoids mainly occur in P. morrisonicola and P. armandii, while biflavones and triflavone are found in P. Sylvestris. Type of lignans are also found in Pines such as benzodioxanes, lignanolides, tetrahydrofurans, benzofurans, oligomerics diepoxylignan, arylnaphthalenes, and dibenzocyclooctene. Related to this study, steroid (e.g., stigmast-4-en-3-one; β-sitosterol) and triterpenoid (e.g., 3β-methoxyserratt-14-en-21-one; 3-α,21β- dimethoxy-∆14-serratene; serrate-14-en-3β,21β-diol) are identified as dominant lipophilic compounds in the inner bark of the P. merkusii whereas monoterpenes and sesquiterpenes are recorded in minor quantities either in inner or in outer bark [81,82].
Pinus merkusii knots and stemwood have also been investigated for their phenolic and lipophilic extractive constituents as well as their antioxidant properties. The study has disclosed that knotwood extract contain mainly lignans, nortrachelogenin, stilbenes and resin acids, whereas heartwood extracts are constituted mainly of stilbenes, particularly pinosylvin monomethyl ether, pinosylvin and pinosylvin dimethyl ether, and of flavonoid such as pinocembrin. Acetone knotwood extract presents higher antioxidant activity using DPPH method, while acetone heartwood extract possesses higher antifungal activities against the two tested fungi Trametes versicolor and Poria placenta [83]. Another study reported that the leave extract of the plant also presents significant antioxidant property using the similar method [84]. The leaf oil of P. merkusii mainly contains α-pinene, ∆-3-carene, β-pinene, limonene, camphene, and β-phellandrene [85].
Pines have been used in traditional medicine, especially in China. P. tabulieformis and P. massoniana in Chinese medicine are used as herbal antirheumatic, analgesic, and anticancer [18]. Antibacterial activity of the essential oil obtained by means of hydro-distillation of P. merkusii leaves from Samosir, Indonesia, has been studied, presenting potent activity against S. aureus at concentration 0.25% and Pseudomonas aeruginosa at concentration 0.1% [86]. Meanwhile, antibacterial activity of the leaves extract of P. merkusii against Enterococcus faecalis has given minimum inhibitory concentration at 1.56% [87]. Antibacterial and antioxidant activities of the resin and essential oil of P. merkusii have also been investigated, disclosing that the resin-derived essential oil and the resin extracts could inhibit S. aureus, but not for E. coli. All the samples used has less potential antioxidant activity [88]. On the other hand, larvicidal activity of ethanol leaf or bark extract of Pinus merkusii on Aedes aegypti larvae has also been studied, revealing that this ethanol extract presents highest larvae mortality, promoting this extract can used for new bioinsecticides [89,90]. In addition, P. merkusii grown naturally in Indian Himalayas has various pharmacological values as follows: The alkaloids/phenolic such as stilbene, pinocembrin, lignans, miserotoxin, tannin, xanthotoxin have anti-cancerous properties, whereas, the essential oils have been used for antiseptic, diuretic, rubefacient, and vermifuge [91].
2.7 Sungkai (Peronema canescens)
Sungkai, a local name of Peronema canescens, belongs to the family Verbenaceae. Sungkai is one of the important types of wood in plantation forest. Sungkai has very diverse potential in the furniture industry (furniture), plywood with a beautiful and smooth texture. P. canescens is common in secondary forests. In industrial operation, the supply of sungkai has not been able to exceed teak, although both types are from the same family (Verbenaceae). Sungkai is distributed across Malaysia, Sumatra, Thailand, Java and Kalimantan [92]. The specific gravity of P. canescens is 0.52–0.73. P. canescens contains cellulose 48.6%, pentosan 16.5%, ash 1.6%, and silica 0.4%. The leaves of P. canescens contain β-sitosterol, phytol, β-amyrin, and several diterpenoids, namely peronemin compounds. Fig. 9 shows the structure of peronemins, consisting of peronemin B2 (1, 0.04% from the dried leaves), A2 (2, 0.005%), B1 (3, 0.01%), C1 (4, 0.04%), B3 (5, 0.03%), A3 (6, 0.01%), and D1 (7, 0.003%) [12].

Figure 9: Structure of Peronemins
The plant of the family Verbenaceae contains essential oils, lantaden terpenoid A compounds, lantaden B, lanthanolic acid, lantat acid, and class of lantonin alkaloids [93]. The crude extracts of methanol and ethyl acetate fractions are reported to contain alkaloid, terpenoids-steroids, flavonoids, and tannin compounds [94]. In addition, P. canescens bark contains several compounds: Quinic acid, guaiacol, hydroquinone, isovanillic acid, genkwanin, catechol, and benzoic acid that are categorized as phenolic compounds and owning strong antioxidant activity [95]. In traditional medicine, the young leaves of P. canescens are used for treatment of colds, fever, and ringworms. It was also used as a water for taking a bath of the woman after delivery and as a gum toothache prevention [96]. Whilst, the decoction of the leaves has been used for treatment of malaria [97].
2.8 Light Red Meranti (Shorea spp.)
Shorea is the largest and economically most important genus in the family Dipterocarpaceae. Shorea is classified into the white meranti, yellow meranti, red meranti, and balau. Light red meranti, a trade name for red meranti, is the most commercially available. The light red meranti consists of 70 species of the genus Shorea. The main sources of light red meranti species are Shorea parvifolia and Shorea leprosula. Light red meranti is used mainly for plywood, while the heavier one is used for light construction purposes. It is one the major export products of Malaysia and Indonesia. The average specific gravity of Shorea leprosula was 0.52. The chemical compositions of Shorea parvifolia and Shorea leprosula consist of cellulose 50.70% and 50.76%, lignin 33.0% and 30.6%, pentosan 12.16% and 12.74%, ash 0.24% and 0.68%, and silica 0.19% and 0.29%, respectively [4].
The active compounds contained in the extratcs of family Dipterocarpaceae include phenolic compounds such as oligostilbenoid, flavonoids, phenylpropanoid, and phenolic acid derivatives, as well as non-phenolic compounds, i.e., triterpenoids [98,99]. The heartwood extractives of light red meranti contain of resveratrol oligomers (Fig. 10) and a small amount of phenolic acids gallic and ellagic acids (Fig. 11). The chemical structures of resveratrol oligomers are very complex and have many isomers.
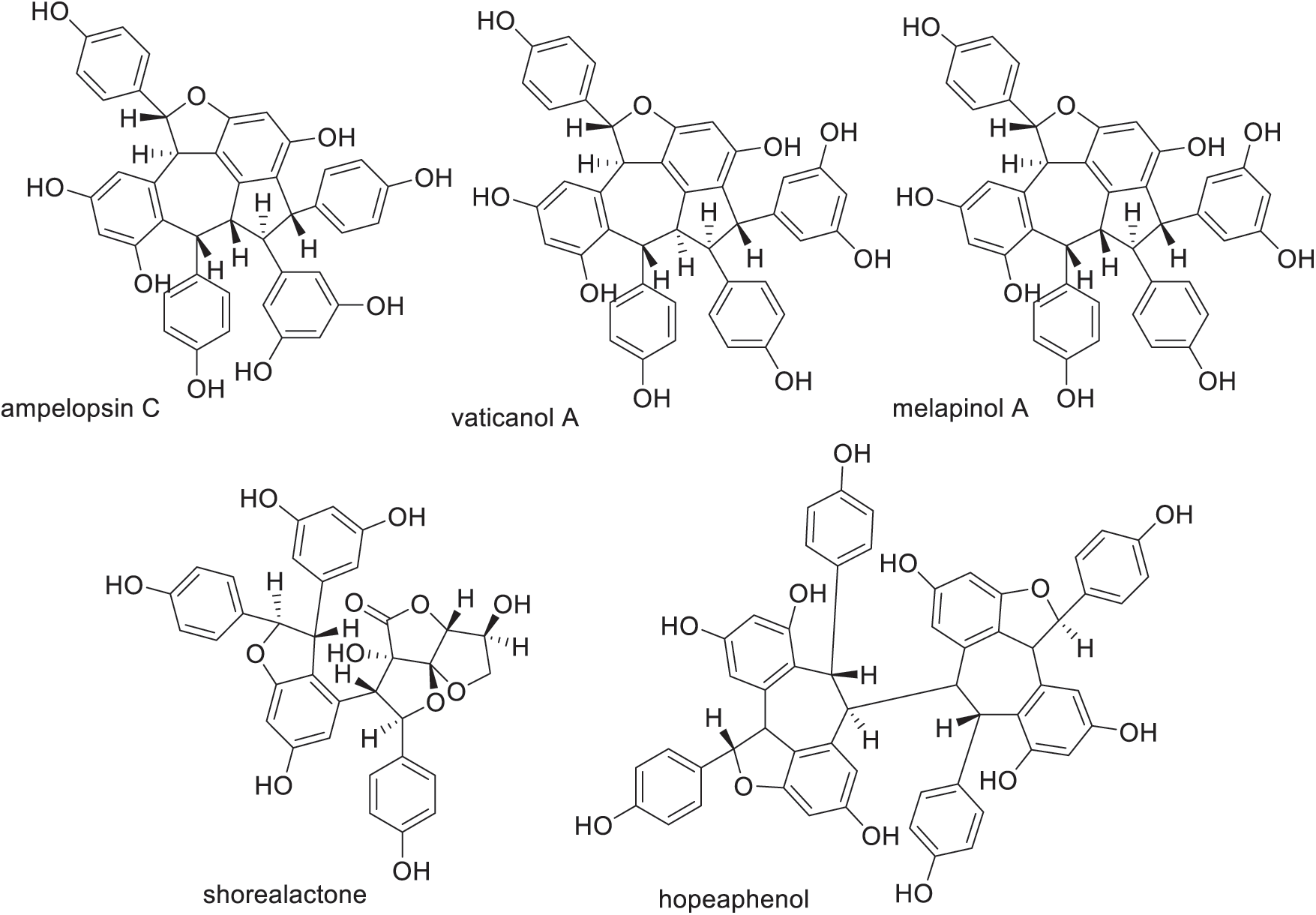
Figure 10: Resveratrol oligomers isolated from the heartwood of meranti Gallic acid (3,4,5-trihydroxybenzoic acid) is a simple phenolic acid which commonly found in tea leaves, oak bark, and other plants as a constituent of tannins
Figure 11: Phenolic acids isolated from the heartwood
The wood and resin of Dipterocarpaceae contain stilbenoid compound [100], monomers and oligomers of resveratrol [101−103]. The leaves of Shorea leprosula have potent activity as antibacterial agents against pathogenic bacteria. GC-MS analysis has identified the methanol fraction of the leaves extract, composing of 2-benzenedicarboxylic acid (65.77%), eicosanoic acid (9.82%), 2-pentadecenone (6.53%), tricosane (5.86%) and hexanedioic acid (4.13%) [94]. The derivative of oligomeric stilbene compound which is identified as α-viniferin (trimer stilbene) has been isolated from acetone extract of stem bark of Shorea ovalis Blume [104]. Acuminatol, a new resveratrol dimer, and other antioxidative resveratrol oligomers (laevifonol, (+)-α-viniferin, shoreaketone, vaticanol B and (−)-hopeaphenol), have also been identified from the acetone extract of the stem bark of Shorea acuminate [105]. In addition, it was also reported that stilbenoid compounds and resveratrol have effective biological activities such as their cytotoxic to cancer cells, anti-fungal, anti-inflammatory, antioxidant, therapy of skin allergies, diarrhea, dysentery, and astringency [16,106].
Bangkirai, a local name of Shorea laevis, is a hardwood species of the family Dipterocarpaceae. Bangkirai is widely distributed in Burma, Thailand, Sumatra, Peninsular Malaysia, and Borneo. Bangkirai is classified as high-quality wood suitable for construction due to its high mechanical and biological durability properties. The range of specific gravity of bangkirai was 0.60–1.16. The wood is composed of cellulose 52.9%, lignin 24.0%, pentosan 16.8%, ash 1.0%, and silica 0.4% [4]. This family of Dipterocarpaceae contains terpenoids, flavonoids, phenylpropanoids and oligomer resveratrol. Similar to the light red meranti, bangkirai contains oligomer resveratrol compound that can be isolated from the bark or the stem of the plant. The basic structural unit of resveratrol is trans-3,5,4’-trihydroxystilbene (Fig. 12).
Figure 12: The basic monomer of resveratrol
The resveratrol units are joined together by phenolic oxidative coupling reactions resulting in the formation of oligoresveratrol to form a resveratrol derivative [99]. Resveratrol (trans-3,4',5-trihydroxystilbene), a phytoalexin presents in grapes, has been reported to have cytotoxic activity against cancer cell, possessing chemo preventive and chemo therapeutic activities. Resveratrol has potential use for many chronic diseases such as inflammation. It was also reported that resveratrol has antioxidant activity and can be effective for diet treatment [107].
2.10 Ulin (Eusideroxylon zwagery Teijsm. & Binnend.)
Ulin/Belian, also known as Ironwood, belongs to the family Lauraceae. Ironwood forest is a characteristic type of forest in the lowlands of Borneo, Sumatra, and Southern Philippines [108,109]. Due to very slow growth, the Bornean ironwood is known as the hardest and most durable timber in South-East Asia and commonly used in marine work, boatbuilding, heavy construction, etc. [110]. It is also used for making a blowpipe of dart poison, poles, and beam by local “Dayak” in Borneo [111,112]. Due to high demands on this wood species and depletion of its availability in nature, Indonesia and the state of government of Sarawak has restricted this majestic wood for export trading. In terms of main chemical constituent of this plant extract, Hobbs et al. [113] found a small quantity (0.05%–0.08%) of a crystalline solid, namely eusiderin A (C22H26O6), as a fractionation product of oily material extracted from the shredded timber with boiling light petroleum. Eusiderin, a neolignane compound, is a possible by-product of lignin synthesis in E. zwageri. Further study reported that there were found two other lignans isolated with other known compounds, eusiderin A and eusiderin from Ulin wood. These two new lignans were determined to be (2R,3R,4S)-2,3-dimethyl-6,7-dimethoxy-4-ethoxy (3′,4′,5′-trimethoxybenzene)-1,5-dihydroxytetralin and (2R,3S,4S)-2,3-dimethyl-6,7-dimethoxy-4-ethoxy(3′,4′,5′-trimethoxybenzene)-1,5-dihydroxytetralin (Fig. 13) [114].

Figure 13: Eusiderin A, Eusiderin I and new lignans
Later on, eusiderin A, which can be found out in leaf, bark and root, has potential activity as an organic insecticide due to its antifungal and antifeedant properties towards some horticultural pests [115]. Study on the modification of allylic moiety of this compound via hydroboration-oxidation, Dess Martin, and Osmium tetraoxide oxidation have increased its hydrophilicity, enhancing its antifeedant activity, 3–4 times higher than the original compound [115]. Another study reported that eusiderin I (at 3, 4, and 5 ppm concentration in chloroform as solvent) has potent antifungal acitivity against three plant pathogenic fungi, Fusarium oxysporum f.sp. lycopersici, Sclerotium roefsii, and Rhizoctonia solani [116]. Other antifungal study using dichloromethane and methanol crude extract of E. zwageri revealed that both crude extracts are toxic to Trametes versicolor, Gloeophyllum trabeum and Chaetomium globosum. GC-MS analysis showed that hexanedeconic acids, 2-4-di-ter-butylphenol, methyl hexadecanoate, methyl octadeconate, γ-muurolene, α-cadinol and myristicin are among the expected compounds responsible to this fungal activity [117]. Antifungal activity against white-rot fungi, namely Pycnoporus coccineus and Schizophyllum commune, is also confirmed using acetone crude extract. 5- octadecene, palmitic acid and 4-tetradecanol, vanillin, 1-nitro-3,5-dimethoxyphenyl-ethylene, 2,4-dimethoxy-5,6-dimethylbenzaldehyde, benzenamine and 5-allyl-1,2,3-trimethoxybenzene are the compounds found after fractionation of this acetone crude extract. Further, cytotoxicity test against brine-shrimp, Artemia salina, disclosed that the extractives are toxic, giving a LC50 value at 0.8 µg/mL [118].
Due to variation of phytochemical compounds (alkaloid, flavonoid, saponin, tannin, sterol-triterpenoid) contained in E. zwageri, this plant has been used as traditional medicine by Kutai people in East Kalimantan [119]. The seed of E. zwageri is used as traditional medicine for rheumatism, diabetes, gout, and hair treatment [120]. Further, scientific study disclosed that methanol extract of E. zwageri (yield: 33.8%) has potential effects as anti-melanogenesis to reduce hyperpigmentation in the skin [120]. The study showed that this methanol extract can inhibit (100%) melanin formation at 300 µg/mL extract and has DPPH radical-scavenging activity (80%) at 100 µg/mL extract. Further, an ethanolic extract of the stem bark (yield 8.62%) have given the IC50 values of antioxidant activity of the extract in DPPH and superoxide radical scavenging mechanisms of 44.90 µg/mL and 30.47 µg/mL, respectively. With the same extract, phytochemical analysis revealed that the extract has the total phenolic, total proanthocyanidin, and total flavonoid contents of about 31.28 GAE/g extract (mg), 183.3 PE/g extract (mg), and 30.48 CE/g extract (mg), respectively. In antidiabetic assay, this extract has IC50 value of 58.45 µg/mL in α-glucosidase inhibition, and 9.04 µg/mL in α-amylase inhibition. Quercetin, an antidiabetic activity-having flavonoid, presents IC50 values 2.00 µg/mL and 4.04 µg/mL in α-glucosidase and α-amylase inhibitory assays, respectively [121]. Besides these medical uses, E. zwageri wood powder extract can also be used as a dying agent for fabric [122].
2.11 Kempas (Koompassia malaccensis Maingay ex Benth)
Kempas belongs to the family Leguminosae. There are three known species of Koompassia wood, K. malaccensis (kempas), K. excelsa (tualang), and K. grandiflora. Kempas is widely distributed in the southern parts of Thailand and Malaysia, and in Sumatra and Borneo. The heartwood of kempas is reddish-brown to light yellowish-brown sapwood, grain interlocked, texture coarse to very coarse, and parenchyma bands visible to the naked eye. Kempas is used for charcoal, fuelwood, rafters, pallets, window frames, furniture, while some preservative-treated ones can be used for railway sleepers, beams, joists, piling, etc. Kempas is chemically composed of 47% cellulose, 29% lignin, 17% pentosan, 0.7% ash and 0.1% silica. The solubility is 3.1% in alcohol-benzene, 1.1% in cold water, 2.4% in hot water and 9.0% in a 1% NaOH solution [123]. Extraction of the stem part with methanol (yield, 1.9%) provides total phenol content ± 38% µg gallic acid per mg dry extract, flavonoid ± 40% µg quercetin per mg dry extract, and tannin ± 77% µg BSA per mg dry extract [124]. On the other hand, kempas has been studied for its termite resistance in-ground and the result has supported for its moderately durable wood species according to in-ground natural durability ratings [125]. However, this species is classified as a high resistance class against white-rot fungus [126]. Kempas wood belongs to durability class III–IV (low class, non-durable). Its resistance to dry-wood termites belongs to class (low class, non-durable), while the resistance to wood-rotting fungi belongs to class II–III (moderate class) [127].

Figure 14: Kompasinol A
In medicinal application, kempas has been used traditionally for treatment of dysentery [128]. A stilbeno-phenylpropanoid namely Kompasinol A (Fig. 14), together with betulinic acid (a triterpene), 4-hydroxy-2’,4’-dimethoxychalcone (a phenylpropanoid), vincoside lactam (an indole alkaloid glycoside), and (+)-cathechin 3-O-α-L-rhamnopyranoside (a phenolic glycoside) have been isolated [129]. Kompasinol A (yield, 0.002%), a pale-yellow amorphous solid, is isolated from the ethyl acetate-soluble portion of the bark, partitioned from the methanol extract of the bark. An antimicrobial activity against Streptococcus sobrinus and an inhibitory activity against glucosyltransferase (GTase) of 50% ethanol extract of the heartwood are also investigated, providing a natural anticariogenic agent for dental caries [130]. The study reported that taxifolin and three flavanonol rhamnoside isomers, neoastilbin, astilbin, and isoastilbin are isolated and identified as its bioactive compounds in this case. Study on anti-acne and tyrosinase inhibition properties of these bioactive compounds is also conducted, reporting that there is no antimicrobial activity against Propionibacterium acnes from these four compounds. However, P. acnes lipase inhibitory activity is only worked on isoastilbin, with IC50 about 1.36 µg/mL. At the concentration of 10 µg/mL, taxifolin, neoastilbin, astilbin, and isoastilbin showantioxidant activity for about 31.16, 25.64%, 28.47%, and 31.01% respectively. All compounds provide tyrosinase inhibition at concentration 1 mg/mL for about 11%–24% (monophenolase) and 5%–9% (diphenolase) [131].
Nyatoh, a trade name for Palaquium species, belongs to Sapotaceae family. The genus Palaquium comprises of about 110 species, distributed from western India and Sri Lanka to southern China and east to Polynesia (Samoa). Nyatoh is a light to medium-weight timber, moderately hard to hard timber, and rated as moderately durable wood against fungi, but susceptible against termites. The heartwood is pinkish-brown. Nyatoh is generally used for furniture, decorative doors, veneers, panelling, flooring, partition, household appliance, musical instruments, etc. Chemically, P. microphyllum contains 52% cellulose, 22% lignin, 17% pentosan, 1.0% ash and 0.05% silica. The solubility is 3.2% in alcohol-benzene, 1.4% in cold water, 5.4% in hot water and 14.8% in a 1% NaOH solution. Gutta-percha, a latex from Palaquium trees, is used as material for insulation and its imperviousness to water [123]. In medicinal application, gutta-percha is the best material for root canal filling [132]. The dominant chemical components of gutta-percha (from P. Oblongifolum) consists of 2-methyl-1,3 butadiene (24.61%) (Fig. 15), geranyl linalool isomer, solanesol (polyisoprene), limonene (16%), β-elemene, γ-elemene (monoterpenes), farnesene, and nerolidol (sesquiterpene and its derivatives) based on pyrolysis-GC analysis [133]. In addition, previous chemical identification from Mariposa gutta obtained from Palaquium leiocarpum has been conducted by Heilbron et al. [134], isolating a compound “lupeol”, a monohydric alcohol, C20H50O.

Figure 15: 2-methyl-1,3 butadiene
Extraction of the bark and timber of some identified Palaquium sp using benzene and methanol followed with separation using chromatography columns and tin layer chromatography (TLC), and analysed by means of infra-red and NMR, yield various main chemical components, such as β-amyrin (Fig. 16), β-amyrin acetate, β-amrenone, α-spinasterol, ursonic acid, and betulinic acid [135]. On the other hand, analysis of a dichloromethane extract from the leaves of P. luzoniense, results indicated the following compounds: lupenone, lupeol acetate, lupeoyl-3β-O-cinnamate or lupeol cinnamate, oleanone, β-amyrin, β-amyrin cinnamate, ursenone, α-amyrin, spinasterol, squalene, and lutein [136].

Figure 16: β-amyrin
In addition, the analysis of methanol extracts from the bark (yield: 10.30%), heartwood (yield: 9.24%), and sapwood (yield: 4.73%) of P. hispidium revealed that the extracts have antioxidant activity as 50% inhibition concentration (IC50) and total phenol content of 9.64 µg/mL, 27.86 µg/mL, 23.81 µg/mL and 37.09%, 24.48%, 31.73%, respectively. Antifungal activities against Gloephyllum trabeum and Pycnoporus sanguineus disclosed that inhibition activities toward both fungi are only effective at the heartwood extract [137]. On the other hand, antifungal activities against Schizophyllum commune and Pleurotus ostreatus from a methanol extract of the heartwood of Palaquium sp. have shown that the chloroform partition (yield: 5.12%) presents higher inhibition activity against those fungi with IC50 of 56 ppm (S. commune) and 55 ppm (P. ostreatus). Further, a bio-active molecule found from this chloroform partition is identified as 2,3-dihydroxypentadecanoate [138].
2.13 Keruing (Dipterocarpus Gaertner f.)
Keruing belongs to the family Dipterocarpaceae. Dipterocarpus comprises of about 70 species, spreading from Sri Lanka, India and Burma, through Indo-China, southern China and Thailand towards western Malesia (found in Peninsular Malaysia, Borneo, Sumbawa, Bali, Java, Sumatra, the Philippines and intervening islands). It is a moderately heavy to heavy hardwood [139]. The heartwood is greyish-brown to red-brown, while the sapwood is yellowish to greyish-brown. D. gracilis contains 51% cellulose, 19% lignin, 17% pentosan, 0.9% ash and 0.6% silica. The solubility is 11.7% in a 1% NaOH solution, 3.9% in alcohol-benzene, 3.2% in hot water, and 0.3% in cold water. In addition, the essential oil fraction (yield: 38%–40%) obtained from the water distillation of D. gradiflorus results in the chemical compositions, as follows: copaene, α-gurjunene, β-gurjunene, β-elemene, caryophyllene, allo-aromadendrene, α-humulene, germacrene D, and γ-gurjunene [140]. Keruing is classified as moderately durable wood, and generally used for construction, furniture, tools, board, household materials, etc. [123].
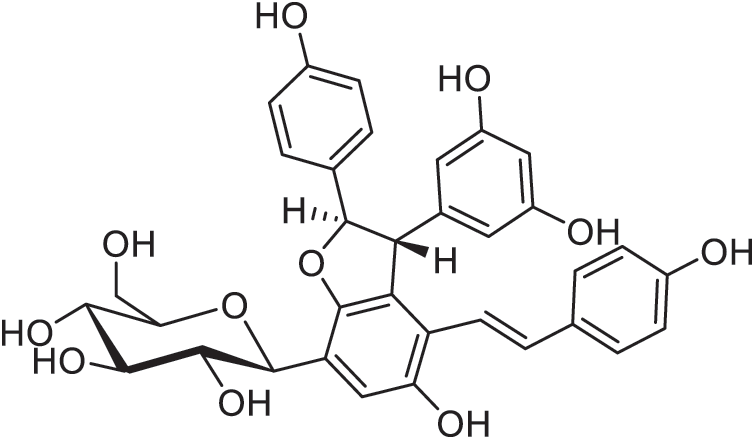
Figure 17: Diptoindonesin A
In medicinal use, Dipterocarpus alatus crude extracts (bark, leaves, twig, wood, and oleo-resin) were examined for antibacterial activity and wound healing effect against methicillin-resistant Staphylococcus aureus in a superficial skin infection in mice. The study revealed that oleo-resin, D. alatus wood extract, as well as α,β-gurjunene and dipterocarpol isolated from the oleo-resin significantly reduce the number of MRSA. Moreover, D. alatus dipterocarpol and its twig extract can be used to heal the infected wounds at rates comparable to the non-infected control group [141]. Other medicinal uses of Dipterocarpus species have been reviewed such as for treating a rheumatism, liver diseases, diaphoretic and antiseptic, hysteria, dysmenorrhoea, ulcer treatment, antiseptic for gonorrhoea and urinary disease, ringworm and skin diseases, anti-inflammatory. D. obtusifolius Teijsm ex Miq is one of the important species for its therapeutic function against AIDS. The bark of Dipterocarpus is presumed to be the most active for these medicinal functions. Moreover, it has also been described, to contain sesquiterpenes, triterpenes, oligostilbenoids, coumarin compounds, resveratrol compounds [e.g., diptoindonesin A (Fig. 17)], phytosterol [142]. In addition, the hexane extract of D. costatus wood shows cytotoxicity activity against breast cancer (MCF-7) and small cell lung cancer (NC-H187), in conjunction with potent anti-malarial activity against Plasmodium falciparum K1 strain). With the same extract, some 30 terpenoids are found which contain 12 triterpenes, such as 2 nordammaranes, 5 norlupanes, 3 dammaranes, and 2 seco-dammaranes. Cytotoxicity of these isolated compounds against four human cancer cell lines (PC3, MDA-MB-231, HT-29 and HCT116) was evaluated. Among these compounds, norlupane, a molecule possessing an endoperoxide group, provides a strong anti-plasmodial activity associated with low cytotoxicity [143]. Inhibition activity against acetylcholinesterase (AChE), a potential target for treating Alzheimer’s disease, has also been investigated using a stem wood extract of D. alatus. Four new oligostilbenoids, as dipterocarpols, and two known resveratrol oligomers, hopeahainol and hopeafuran, has been separated and identified. Dipterocarpol A and hopeahainol A present moderate AChE inhibitory acitivity, with IC50 8.28 µM and 11.28 µM, respectively [144].
Kapur, a trade name of Dryobalanops wood species, belongs to the family Dipterocarpeceae. Dryobalanops comprises of 7 species distributed in Peninsular Malaysia, Sumatra, Borneo, and intervening islands. Kapur is moderately heavy timber. The colour of the sapwood is yellowish-brown, whereas the heartwood is rose-red to dark reddish-brown. Kapur is used for heavy and light construction in the location free of termites, furniture, joints and beam, and extensively used for plywood. The fruits of kapur is edible. D. sumatrensis contains 60% cellulose, 27% lignin, 16% pentosan, 0.8% ash and 0.6% silica. The solubility is 2.7% in alcohol-benzene, 2.6% in cold water, 3.9% in hot water and 12.9% in a 1% NaOH solution [123]. Camphor, a crystalline solid yielded from the wood cavities or as an oil from holes cut in the trunk, has been used for medicinal purposes [145].
The chemical components of the hexane or diethylether extracts from the heartwood and sapwood of D. aromatica contains some compounds as follows: hydroxydammarenones-11 (dipterocarpol), kapurone, β-sitosterol, dryobalanone, terpinylhydrate, w-hydroxyfatty acid ferulate, and unresolved fraction [146]. Malaysianol A, a new trimer resveratrol oligomer from the acetone extract of the stem bark of D. aromatica has been isolated, along with five known resveratrol oligomers: laevifonol, ampelopsin, α-viniferin, ε-viniferin, diptoindonesin A, and bergenin. Cytotoxic activities of the compounds against several cell lines showed that α-viniferin strongly inhibits the growth of HL-60 cell line (human leukemia) [147]. Further, methanol extract of the stem bark of Dryobalanops beccarii provides a new trimeric oligostilbene, malaysianol D and galloylglucoside, malaysin A, together with 12 known compounds. Among these compounds, vaticanols C is found to be moderately active against human lung adenocarcinoma epithelial (A549) cell line while other compounds show weak or not active [148]. On the other hand, a new oligostilbenoid tetramer, malaysianol B is found from the acetone extract of the stem bark of D. lanceolata, together with other oligostilbenoids tetramers; hopeaphenol, stenophyllol, nepalensinol B, vaticanol B, vaticanol C, upunaphenol D, and flexuoson A. It was suggested that resveratrol in upunaphenol D and flexuoson A is the active site of the compounds to have antibacterial activities against Gram-positive strains, Staphylococcus epidermidis, S. aureus and S. xylosus [149]. Further, with different fractionation techniques of the same acetone extract from the bark of D. lanceolata, another study found a new tetramer oligostilbenoid possessing tetrahydrofuran ring, malaysianol C, together with four known oligostilbenoids nephalensinol E, ε-viniferin (Fig. 18), laevifonol, and ampelopsin. Among these compounds, only ε-viniferin and laevifonol show cytotoxic activity against human lung adenocarcinoma epithelial (A549) and breast cancer (MCF7) cell lines [150].

Figure 18: ε-viniferin of D. lanceolate
An essential oil obtained by steam distillation method (Yield: 0.12%) from the leaves of D. lanceolata showed that the extract inhibits the growth of microorganism Staphylococcus aureus and Candida albican and has a potency to inhibit the free radical (DPPH) at concentration 25–100 ppm [151]. Further, with the same essential oil, it is also active against Streptococcus sobrinus and Streptococcus mutans. Through GC-MS analysis, the major compounds identified in this oil are eugenol (28.73%), γ-terpinene (15.60%), 2-β-Pinene (9.80%) and 1-Limonene (8.09%) [152]. Other study used hydro-distillation to obtain the essential oil of the leaves and seed of D. aromatica. Eighty-three compounds are identified from the leaf oil with the main compounds composed of oxygenated bicyclic sesquiterpene (globulol 8%), bicyclic monoterpene (α-pinene 7%), and oxygenated monocyclic monoterpenes (α-terpineol 16%, terpinen-4-ol 15%,). Whilst, there are 31 components identified in the seed oil with the major compounds consist of acyclic monoterpene (myrcene 5%), monocyclic monoterpene (limonene 6%), bicyclic monoterpenes (α-pinene 41%, α-thujene and β-pinene 13% each, sabinene 6%), and bicyclic sesquiterpene (bicyclogermacrene 6%) [46]. Fractional distillation method to obtain essential oil (yield: 7.58%) from the exudate of D. aromatica was also demonstrated, yielding 30 compounds that were identified by GC-MS. About 97.56% of total essential oil compositions are identified as sesquiterpenes, monoterpenes, oxygenated monoterpenes, and oxygenated sesquiterpenes, as well as borneol [153].
2.15 Sengon (Paraserianthes falcataria/Albizia falcataria)
Sengon/Jeung jing (Indonesia) is local name of Paraserianthes falcataria L. Nielsen belongs to the family Fabaceae. It is native to Indonesia, Papua New Guinea, Solomon Islands and Australia. Due to its fast-growing and can grow on a wide range of soils, its preferable silvicultural characteristic and has acceptable quality of wood for the panel and plywood industries, it is one of the tree species favoured for industrial forest plantations in Indonesia. Sengon is a lightweight and soft to moderately soft. The heartwood colour ranges from whitish to a pale pinkish-brown or a light-yellowish to reddish brown. It is not durable. It is commonly used as material for light constructions, matches, musical instrument, toys, and plywood [154]. Since the specific gravity of this wood is very low, there is no pulp and paper mill in Indonesia using sengon as raw material. The chemical compositions of sengon comprise of cellulose 52.5%, lignin 26.5%, hemicellulose 21.0% [155], while for alcohol-toluene solubility 1.3%–1.7%, 1% NaOH solubility ±15%, hot-water solubility ±3.7%, cold-water solubility ±3.6%, ash content 0.86%–0.96% [156].
Preliminary study towards secondary metabolites/extractive of this species was performed by means of GC-MS and 1H NMR analysis to methanol extracts of the bark, sapwood, and heartwood of P. falcataria. The study identified that glycoside of syringaresinol is presented in the methanol extract of bark and sapwood, while syringaresinol (Fig. 19) is present in the methanol extract of the heartwood [157].

Figure 19: Syringaresinol
Other study reported that the hexane extract of the bark of P. falcataria affords some chemical groups rich with alkaloid, flavonoid, and triterpenoid. The study was then revealed that the purified triterpenoid group compounds are able to lower glucose concentration through in vivo test [158]. In addition, the acetone extract (yield: 0.74%) and the toluene/ethanol extract (yield: 1.16%) from the bark of this wood species show antioxidant (DPPH and Oxygen uptake inhibition method) and antifungal (Coriolus versicolor and Poria placenta) activities [159].
2.16 Agathis (Agathis dammara/A. loranthifolia/A. celebica/A. alba)
Agathis (Indonesia) belongs to the Araucariaceae family. The local name of A. dammara/A. lorathifolia/A. alba is damar, but it is usually called as agathis. It is native to Indonesia and Philippines, distributed in the Philippines (Palawan and Samar), Sulawesi island, and Java island. This species is an important source for its copal resin. There are two kinds of resin produced by agathis, a solid and a liquid resin. The solid resin is the evaporation product of the liquid resin. The solid resin does not have characteristic odour like the liquid resin. The wood is commonly used as material for pulp and paper with a high quality, besides it is used for construction. A very poor termite resistance against Coptotermes curvignathus of A. dammara obtained from the plantation sites, in West Java Indonesia has been reported [160]. Meanwhile, its resin products can be used as a material in plastic, textile, paint, ink, and matches industries. An adhesive property of the copal resin has been investigated, suggesting its potential to be used as a substitute agent owing biodegradable property for plastic-based coating for aluminium [161]. The copal resin contains agathic acid, agathalic acid, agatholic acid, and sandaracopimaric acid [162]. In addition, water distillation of the resinous exudate of A. philippinensis, known as Almaciga resin or Manila copal, yields oil rich in limonene (72%) and other minor compounds greater than 1% included α-pinene, p-cymene, terpinen-4-ol and α-terpineol [163]. Further, the acetone-methanol (9 : 1) extract of the copal from Sukabumi, a district in the West Java Indonesia, yields limonene (44%), ethylene oxide hexamer (11%), cis-limonene oxide (7%), toluene (5,25%), trans-carveol (5%), 2- sikloheksan-1-on (4%), trans-limonene oxide (3%), α pinene (2%), dan 2-pentanone (2%) [164].
On the other hand, the chemical component of the methanol extract from the leaves of A. alba contains a yellowish brown solid as a mixture of biflavones compounds, such as 7-O-methylagathisflavone, 7-O-methylcupressulflavone, 4´´´´,7-di-O-methylagathisflavone, 7,7´´-di-O-methylcupressuflavone [165]. Previously, it was also reported that the acetone extract of the light petrol extract from the leaves of A. alba contains 7-O-methylagathisflavone and 7,4”-di-O-methylagathisflavone [166,167].
In medicinal application, the methanol extract of A. dammara plant leaves has antimicrobial activity with the minimum inhibitory concentration (MICs) against Escherichia coli (250 µg/mL), Bacillus subtilis (62.5 µg/mL), Staphyloccocus aureus (62.5 µg/mL) and Proteus vulgaris (250 µg/mL) [168]. Meanwhile, the essential oil obtained from the hydro-distilled fresh leaves of A. dammara has antibacterial activities with the lowest MIC against S. aureus and B. substilis of about 1.25 mg/mL. The major components from 19 compounds identified are limonene (36.81%), β-bisabolene (33.43%), and β-myrcene (25.48%) [54]. A recent study reported that the bioflavonoids obtained from the leaves of A. dammara has an inhibitory activity against amyloid β40 aggregation in the brain that can cause Alzheimer diseases. These bioflavonoids of amyloid β consist of kayaflavone (Fig. 20); 7,4′,7″,4″′-tetra-O-methylamentoflavone; 7-O-methylagathisflavone; 7,7″-di-O-methylagathisflavone; 7,4″′-di-O-methylagathisflavone; 7,7″,4″′-tri-O-methylagathisflavone; 7,7″-di-O-methylcupressuflavone; 7,4′,7″-tri-O-methylcupressuflavone; 7,4′,7″,4″′-tetra-O-methylcupressuflavone; and hinokiflavone [169]. On the other hand, antiplasmodial activity of the methanolic extract from the leaves of A. borneensis (A. alba/A. dammara) has been investigated, revealing that the obtained IC50 is 11.00 ± 1.41 µ/mL [170].

Figure 20: Chemical structure of kayaflavone
2.17 Jelutung (Dyera costulata Hook)
Jelutung (Indonesia)/jelutong (Malaysia), a local name of Dyera costulata Hook, belongs to the family Apocynaceae. It grows in Borneo, Sumatra, Malaysia, and Peninsular Thailand. It is grown commercially for timber production; besides it yields latex when it is tapped, used as material for chewing-gum. Aged heartwood colour is yellowish-brown, and it is not clearly distinguished from the sapwood. Jelutung wood is a nondurable regarding decay resistance [171], and susceptible against insect attack [172]. The jelutung wood is mostly used for pencil production. Besides its wood and latex products, jelutung plant can be an efficient phytoremediator for copper-contaminting soils [173]. In traditional medicine, leaves and bark have been used for treating pain, inflammation, and fever [174].
Alkaloid content isolated from the leaves of the Dyera costulata has been carried in 1982, yielding six alkaloids consisting of 18-dehydroochrolifuanine A (4% of total), ochrolifuanine A (9%) (Fig. 21), 18-dehydroochrolifuanine E(3%), ochrolifuanine E(5%), ochrolifuanine F (1.6%), 18-dehydroochrolifuanine F (2.7%) [175]. Pharmacologically, chloroform and n-butanol extract from the leaves of the plant exhibit EC50 values for DPPH radical scavenging activity of 79.8 ± 0.2 and 12.0 ± 0.1 µg/mL, respectively. Quercetin-3-O-α-L-rhamnopyranoside is isolated from the n-butanol extract, whereas, β-Amyrin and rhamnazin were isolated from the chloroform extract. Antioxidant activity (DPPH method) of quercetin-3-O-α-L-rhamnopyranoside shows EC50 value of 9.37 ± 0.02 µM, about 8 times higher than the common antioxidant, BHT (EC50 value of 80.78 ± 0.01 µM) (Subhadhirasakul et al. 2003). On the other hand, 5-O-caffeoylquinic acid (5-CQA) (253 ± 32 mg CGA/100g samples fresh) is obtained from the methanolic extract of the plant leaves, giving an alternative source of chlorogenic acid [176].

Figure 21: Ochrolifuanine A
In medicinal application, a butanol fraction obtained from the methanolic extract of jelutung latex has been examined for its allergic inhibitory activity in guinea pigs. Dimethylmyoinositol (inhibition rate: 34.2%) is identified as the main compound in the jelutung butanol extract (inhibition rate: 55.0%) having this allergic inhibitory activity [177]. Later on, an in vitro test of the methanolic extract from the leaves of the D. costulata revealed that the extract has a potent as anti-trypanosomal activity with IC50 values of 0.58 ± 0.01 µg/mL, promoting this plant as candidate for treatment of sleeping sickness [178,179]. In addition, leaf extracts of D. costulata does not have antiproliferative activity, but its DCM/MeOH and MeOH extracts has positive antiplasmodial activity against K1 (EC50 < 10 µg/mL) and 3D7 (EC50 < 5 µg/mL) strains of Plamosdial falciparum. The results also reported that the methanol crude extract of D. costulata has higher total phenolic content (319 ± 25 mg gallic acid/g) and radical-scavenging activity (377 ± 25 mg ascorbic acid/g) [180,181]. The effects of the chloroform extract from the leaves of the plant on nociceptive response using writhing, hot plate and formalin tests in mice and the antipyretic activity in yeast-induced fever in rats have also been investigated. Based on the results, the study suggested that plant extract has a marked analgesic but no antipyretic effect [182].
2.18 Jabon (Anthocephalus cadamba)
Anthocephalus cadamaba, also known as kadam, jabon (Java, Indonesia), belongs to the family Rubiacea. It is native to the South Asia and Southeast Asia, including Indonesia. Jabon grows naturally in Australia, China, Indonesia, Malaysia, Papua New Guinea, Philippines, Singapore, and Vietnam [183]. In Indonesia, there are two species of jabon, namely kadamba [Anthocephalus cadamba (Roxb) Miq] dan samama (Anthocephalus macrophyllus (Roxb). However, only kadamba species that would be explained in detail in this review due to its most frequently planted in Indonesia. Jabon has several advantages including fast-growing, resistant to pests and diseases, and the wood has several uses. Jabon is lightweight hardwood. The heartwood is white and not clearly distinguished from the sapwood. The wood can be used for light construction materials, toys, pencils, beams, ceiling boards, material for the pulp and paper industry [184]. Besides its wood, its barks, fruits, leaves, roots, and seeds are commonly used for various medicinal applications.
The bioactive phytochemicals of A. cadamba have been reported and summarized by many review studies [185,186]. These phytochemicals are constituted of indole alkaloids, terpenoids, terpenes, steroids, saponins, sapogenins, fats and reducing sugars, glycoside, and flavonoids. Besides tannins, the stem bark of A. cadamba contains a new pentacyclic triterpenic acid, namely cadambagenic acid (18α-olean-12ene-3β-hydroxy-27,28-dioic acid). Quinovic acid and β-sitosterol are also isolated in the bark. The leaves contain glycosidic indole alkaloids; 3α-dihydrocadambine (C27H34N2O10), cadambine (C27H32N2O10) (Fig. 22) [187], isodihydrocadambine (C37H44N2O15), and two related non-glycosidic alkaloids; cadamine (C23H23N3O4) and isocadamine.
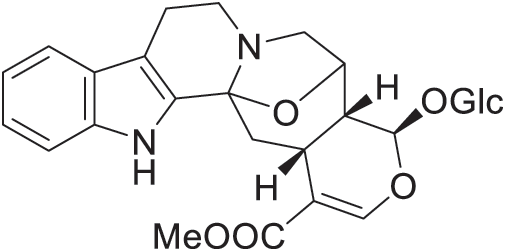
Figure 22: Cadambine
In addition, 3β-dihydrocadambine and 3β-isodihydrocadambine with molecular formula (C37H44N15O2) have been isolated from the leaves of the plant. A new saponin, saponin B (C48H76O17) has also been identified in the stem bark of A. cadamba [188]. Chlorogenic acid (CGA) obtained from the leaves of the plant has been tested with in vitro and in vivo methods for its hepatoprotective activity [189]. In the bark of the plant, it was disclosed that two novel triterpenoid saponins, namely phelasin A and phelasin B, have been isolated [190]. The bark was also identified, containing two triterpenoid glycosides, glycosides A and B which are defined as 3-O-(α-L-rhamnopyranosyl)-quinovic acid-28-O-(β-D-glucopyranosyl) ester and 3-O-(β-D- glucopyranosyl)-quinovic acid-28-O-(β-D-glucopyranosyl) ester, respectively [191]. Later on, it was also reported that the flower of the plant contains an essential oil that composes mainly of linalool, geraniol, geranyl acetate, linalyl acetate, α-selinene, 2-nonanol, β-phellandrene, α-bergamottin, p-cymol, curcumene, terpinolene, camphene and myrcene [185].
In pharmacology, A. cadamba is the well-known plant for its widely used as ethnomedicine in the treatment of anaemia, fever, uterine complaints, blood diseases, skin diseases, leprosy, dysentery, and for improvement of semen quality [192]. Numerous scientific studies about its medicinal potential have been conducted to date, among them, it was reported that the hydroethanolic extract of the flowering tops of A. cadamba at concentration of 400 mg/kg body mass demonstrates an effective antidiabetic (hypoglycaemic) property in alloxan-induced diabetic rats and is able to protect liver and brain from the oxidative damages caused by diabetes [193]. This antidiabetic efficacy can also be generated from the methanolic and aqueous extract from the root of the plant, causing significant reduction in the blood glucose level in both normoglycaemic and alloxan induced diabetic rats [194]. The study also reported that this methanol extract contains alkaloids, flavonoid, tannins, saponins, and sugars. Whilst, the aqueous extract also contains these compounds except alkaloids. Another study reported that the defatted ethanolic extract from the leaves of the plant possess the satisfactory significant activity as analgesic and anti-inflammatory agent in wistar rats, in comparison with aspirin and pentazocine as standards [195]. This anti-inflammatory activity can also be generated from the methanolic extract of the plant’s bark which its anti-inflammatory activity is possibly backed by its antihistaminic activity [196]. In addition, an anti-diarrhoeal activity study of the hydroethanolic extract from the flowering tops of the plants have revealed that the extract generates not only a dose-dependent decrease in the total number of faecal dropping in castor oil-induced diarrhoea in mice but also a dose-dependent reduction in intestinal fluids accumulation and in the gastrointestinal transit [197]. Equally important, the chlorogenic acid (CGA) obtained from the leaves of the plant has been reported to have hepatoprotective activity tested by in vitro and in vivo methods using carbon tetrachloride as a model of liver injury [189]. Microbial and antioxidant activities of the A. cadamba has also been investigated using the whole part of the plant using successively different solvents, petroleum ether, acetone, chloroform, ethanol, and water. Results have revealed that the alcoholic and aqueous extract of the plant presented significant activity against almost all the organisms tested: Micrococcus luteus, Bacillus subtilis, Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, Klebsiella pneumoniae, Proteus mirabilis, and four Candida albicans fungi known as the systemic fungi (Aspergillus flavus, Aspergillus niger, and Aspergillus nidulans) and the dermatophyte fungi (Trichophyton rubrum). Besides having potent wound healing capacity, the extract also has potent antioxidant activity by inhibiting lipid peroxidation and increase in the superoxide dismutase (SOD) and catalase activity [198]. Moreover, the development of antimicrobial drug based on the fruit extract of the A. cadamba is also suggested as a potent antimicrobial activity of the extract towards gram-positive (S. aureus, B. cereus) and gram-negative (E. coli, Salmonella abony, Shigella boydii) bacteria [199]. Similar antimicrobial study plus antioxidant activity of the hot aqueous extract from the leaves of the plant has also been demonstrated by Khandelwal et al. [200]. Another study reported that the ethanolic extract from the flowering tops of the plant possess both analgesic and gastroprotective activity on treated mice [201]. This similar extract is also reported to have remarkable antioxidative function towards several testing methods [202]. Comparatively, similar antioxidant study including analysis of the existed bioactive compounds was also reported by using different extract fractions from the leaves of the plants [203]. Furthermore, an anticancer study was also investigated using bark extract of the plant and revealed that cadambine is the most potent inhibitor of cell line HCT116 (human colorectal) and 3-O-[α-L-rhamnopyranosyl]-quinovic acid 28-O-[β-D-glucopyranosyl] ester showed the maximum inhibitory activity against HepG2 (hepatocellular carcinoma) [204].
2.19 Acacia (Acacia mangium Willd)
Acacia mangium, also known as mangium, belongs to the family Fabaceae (the new family name of Leguminoseae). It is native to Queensland in Australia, Papua New Guinea (PNG), and the Molucca island in the eastern Indonesia. Its rapid growth, good quality, and tolerance to a wide range of soils and environments might become alternative solution for the rampant deforestation in tropical areas. The heartwood of A. mangium is medium-brown coloured, hard, strong, and durable in well-ventilated conditions, but not in a ground-contact area. Its sapwood is narrow and light coloured. Its specific gravity ranges of 0.40–0.45 for timber obtained from the plantation and it is around 0.60 for the ones obtained in natural stands [205]. Chemically, A. mangium obtained from the Queensland has the highest cellulose (60.29%) content and the lowest lignin (21.29%) and extractive (3.36%) content than the ones obtained from PNG or eastern Indonesia provenance, supporting its potential as material for pulp and paper industry [206]. Besides pulp and paper, it is also prospective as material for particle board, wood chips, moulding, furniture, and veneer [207].
The heartwood extract of the A. mangium is dominated by three flavonoids, 2,3- trans-3,4´,7,8-tetrahydroxyflavanone, teracacidin (Fig. 23), and 4´,7,8,-trihydroxyflavanon [208]. Further, it was reported that 3,4´,7,8-tetrahydroxyflavanone and teracacidin show antifungal activities against P. noxius and P. badius, which might contribute to the heart rot resistance commonly happened towards the heartwood. In addition, these compounds have strong DPPH radical scavenging activity and laccase inhibition, giving suggestion that the antifungal mechanism occurrs through quenching the free radical produced from the extracellular fungal enzyme laccase [209]. On the other hand, the leaves of the plant are reported to contain several flavonol glycosides, consisting of 3-glucoside of quercetin, quercetin-3-diglucoside, kaempferol-3,7-dirhamnoside, kaempferol-7,4’-digalactoside, kaempferol-7-glucoside, and myricetin-3,7-diglucoside [210].

Figure 23: Teracacidin
In pharmacology, the plant is used traditionally for treating high fever [211]. The most recent study reported that the proanthocyanidins obtained from the ethanolic extract of the barks of A. mangium has a carbolytic enzymes inhibition activity towards α-amylase and α-glucosidase through in-vitro method, giving a potential as antidiabetic agent. In addition, the degree of polymerization of these proanthocyanidins enacts dominant role in this carbolytic enzymes inhibition activity [212]. On the other hand, the saponin compounds obtained from the pods extract of A. mangium has been reported can reduce the haemoglobin and haematocrit values of the Oreochromis niloticus, a wild fish competitor commonly found in the aquaculture of shrimp [213].
2.20 Sonokeling (Dalbergia latifolia)
Sonokeling (Dalbergia latifolia) belongs to the family Fabaceae (sub-family: Papilionaceae) and commonly known as East Indian rosewood or black wood. Sonokeling is native to tropical Asia, from Nepal to India and in Java (Indonesia). Sonokeling is an important and highly valuable commercial timber, nationally and internationally. Due to its strength and durability, it is suitable for all kinds of construction works. The range of its specific gravity is 0.77–0.86. The wood composes of cellulose 53.8%, lignin 27.3%, pentosan 10.1%, ash 1.0%, and silica 0.6% [4]. The extract from the seed of the plant contains dalbinol as a new 12α-hydroxyrotenoid and sisafolin coumarin [214]. Further, the bark contains dalbergichromene, lupeol, latifolin, and dalbergin (Fig. 24) [215]. The heartwood contains latinone, neoflavonoid dalcriodon and latinone, a substituted phenanthrene-1, 4-quinone isolated from Dalbergia latifolia [216]. Sonokeling is a fragrant wood, rich in aromatic oils. In traditional medicine, sonokeling has various advantages such as for aphrodisiac, abortifacient, expectorant, anthelmintic, reducing obesity, treating leucoderma, dyspepsia, dysentery, eye and nose diseases, syphilis, stomach troubles, leprosy, antipyretic, appetizer, allays thirst, vomiting, burning sensation, skin diseases, ulcers, the blood disease, scabies, and ringworm [217].

Figure 24: The structure of dalbergin
The use of bioactive compounds derived from nature to promote human health and treat various diseases has been attracting considerable attention. Based on numerous ethnomedicines that commonly used for centuries, intensive research to uncover some bioactive chemicals responsible for these green medicines has been developped since the middle of the 20th century. According to this literature review, numerous bioactive chemicals have been discovered in the Indonesian commercial wood species investigated in this study. These bioactive chemicals were in most case associated to ethnobotanical and ethnomedicinal knowledges. Summaries of the main compounds found in each wood species studied as well as their chemical families are presented in Tab. 2. Variabilities of these phytochemicals mostly depend on the type of plant species, part/sampling location within the plant, area/location where the plants grow, time/season of sampling, and the mode of extraction used. Among the varying extraction methods, water or organic solvent extraction was commonly practised. The use of different solvents for the extraction resulted in different yields and phytochemical constituents as well as their biological/pharmacological activities. Due to all these parameters used, phytochemicals in Tab. 2 might not represent totaly all biomolecules potentially available in the plant, but at least some prominent biomolecules were identified. Among the common solvents used for the extractions (water, ethanol, methanol, chloroform, dichloromethane, acetone), methanol seemed the most effective solvent and was frequently chosen for the extraction in the works described in this review. Although a supporting study also confirmed that methanol was identified as the most effective solvent for obtaining higher extract yields as well as the highest content of phenolic, flavonoids, alkaloids, and terpenoids, which are known as the main chemical families of the phytochemicals [218], different solubilities of bioactive constituents might remain unextractable. Hence, exploration and identification for unknown bioactive compounds in the reviewed plant species are still challenging to date. According to Tab. 2, among the chemical families recorded in this review, phenolic-based compounds (e.g., flavonoids, stilbene, stilbenoid, lignans, phenylpropanoids) were the most abundant phytochemicals in the wood species studied followed with terpenes, terpenoids and alkaloids. Similar results were also reported in a other literature study indicating that phenolic-based compounds dominated the plant secondary metabolites [219]. Data of the main chemical families with their corresponding wood species studied are presented in Tab. 3. Among the reviewed wood species, it seems that jabon has almost all phytochemical families, supporting its advantages in the ayurvedic remedies that encouraged numerous intensive research since a couple of decades ago [185]. Even though not all of the reviewed wood species contained all phytochemical families, it is possible that other phytochemicals of interest exist in other parts of the plant not investigated up to now encouraging further research. Intra and inter specific variabilities have also been the subject of relatively few research works necessitating additional studies in case of valorization of given extracts or molecules.
In terms of biological properties of the reviewed plant extracts or its isolated phytochemicals, Tab. 4 presents data of the biological or pharmacological characteristics of the reviewed wood species. Among the wood species studied, the whole part of jabon possessed advantageous biological/pharmacological activities, confirming its ethnomedicinal functions. Even though there have been many studies conducted to investigate the biological/pharmacological effects of the reviewed wood species, not all of these studies isolated the main bioactive compounds related to their biological/pharmacological activities. Therefore, further specific inquiries for isolation and analysis of the corresponding bioactive compounds remain challenging to investigate. Related to this pharmacognosy, the main biological/pharmacological properties classification of the wood species as well as their phytochemical families is presented in Tab. 5. According to Tab. 5, it seems that the phenolic-based compounds roughly dominated almost all of these main biological/pharmacological properties of the plants; terpene/terpenoid roughly dominated as anti-diabetes, anti-malaria, antibacterial, anti-fever; and alkaloids acted as an analgesic, anti-diabetes, anti-fever. Although all wood species have their own biological/pharmacological activities, some corresponding specific phytochemical families, as well as their compositions, have not been extensively investigated. In all cases, based on this preliminary study, further studies which concern on analysing the chemical compositions and quantities of the potential bio-sourced molecules would be crucial to be implemented. In addition, very limited studies of their valorisation in the fields of nutraceutical, cosmetic, bio-control, bio-stimulation, and other advanced applications have given attractive challenges towards green technology nowadays and perhaps in the future.
Table 2: Main families and molecules of interest identified from industrial wood species used in Indonesia





Table 3: Main chemical families with their corresponding wood species

Table 4: Main biological/pharmacological properties of the woody plant extracts or its isolated phytochemicals




Table 5: Main biological/wpharmacological properties classification of the wood species with their phytochemical families



Based on this literature study, it appears that the Indonesian forest, through its secondary species exploited for timbers production, provides an abundant source of valuable bioactive molecules/phytochemicals as demonstrated by their application as ethnomedicines. All phytochemical families such as phenolic-based compounds (flavonoids, stilbenes, stilbenoids, lignans, tannins, simple phenols), terpenes, terpenoids as well as alkaloids, quinones, or saponins are potentially available. Although almost all the reviewed wood species have these phytochemical families in their structures, some characteristic phytochemicals have been identified in each wood species which support their bioactive functions, such as Teak has tectoquinone (quinone), Merbau has robinetin (flavonoid), Camphor has camphor (terpenoids), Mahogany has swietemahonins (triterpenoids), Ebony has Macassar II & III (napthol) and diospyrin (napthoquinones), Pine has seratane and pinene (terpenoids), Sungkai has peronemin compounds (diterpenoids), Light red meranti has ampelopsin C, vaticanol A, melapinol A, shorealactone, hopeaphenol (resveratrol oligomers), Bangkirai has trans-3,5,4’-trihydroxystilbene (resveratrol oligomer), Ulin has eusiderin A, eusiderin I (neolignane compounds), Kempas has kompasinol A (stilbenophenylpropanoid), Nyatoh has 2-methyl-1,3 butadiene, limonene, β-elemene (terpenes), Keruing has diptoindonesin A (resveratrol), Kapur has dipterocarpols (terpenoids) and malaysianol (resveratrol), Sengon has syringaresinol (lignan), Agathis has agathic acid (terpenoid), 7-O-methylagathisflavone (biflavones compound), Jelutung has ochrolifuanine A, E, F (bisindole-alkaloids), Jabon has cadambine, isocadamine, isodihydrocadambine (indole alkaloids), phelasin A, phelasin B (triterpenoid saponins), Acacia has 3-trans-3,4´,7,8-tetrahydroxyflavanone, teracacidin, and 4´,7,8-trihydroxyflavanon (flavonoids), while Sonokeling has dalbinol and dalbergin (flavonoids). In general, most of these extractives contributed remarkably to the technological properties of wood, especially its natural durability. Bactericidal, fungicidal, and insecticidal properties of these extractives can be used in the formulation of biopesticides which will be characteristically more environmentally friendly than synthetic biocides. According to the nature of the wood species, their particular activity properties as antioxidant, antimicrobial, antifungal, anti-inflammatory, anti-diabetes, anti-dysentery, anticancer, analgesic, anti-malaria, and anti-Alzheimer have given special interests in the fields of medicines and pharmacology. After all, as a part of the efforts towards the implementation of the whole-tree utilization, additional studies that concern on the valorisation of these extractives in the fields of nutraceutical, cosmetic, bio-control, bio-stimulation, and other advanced applications would be of interests.
Funding Statement:The authors thank the Lorraine Université d’Excellence (LUE) initiative for the Grant to the first author to spend her Master 2 internship in France at Laboratoire d’Etudes et de Recherche sur le Matériau Bois (LERMAB). LERMAB is supported by a grant overseen by the French National Research Agency (ANR) as part of the “Investissements d’Avenir” program (ANR-11-LABX-0002-01. Lab of Excellence ARBRE).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. FAO. (2010). Asia−Pasific forests and forestry to 2020: Report for the Pacific.Asia−Pacific outlook study. Bangkok: FAO. [Google Scholar]
2. Ministry of Environment and Forestry Republic of Indonesia. (2018). Ministry of environment and forestry statistics. Jakarta: Ministry of Environment and Forestry Republic of Indonesia. [Google Scholar]
3. BPS Statistics Indonesia. (2018). Ekspor tanaman obat, aromatik, dan rempah−rempah menurut negara tujuan ttama 2012−2018. Jakarta: Subdirectory Ministry of Environment and Forestry Republic of Indonesia. [Google Scholar]
4. Martawijaya, A., Kartasujana, I., Kadir, K., Prawira, S. A. (2005). Indonesian wood atlas vol I. Bogor: Forestry Department. [Google Scholar]
5. Kusmana, C., Hikmat, A. (2015). The biodiversity of flora in Indonesia. Journal of Natural Resources and Environmental Management, 5(2), 187–198. [Google Scholar]
6. Ministry of Forestry. (2006). Strategic plan of the ministry of forestry 2005-2009. Jakarta: The Ministry of Forestry. [Google Scholar]
7. Muladi. (1999). The indonesian forestry law number 41 year 1999. Jakarta: State gazette of the Republic of Indonesia. [Google Scholar]
8. BPS Statistics Indonesia. (2015). Statistics of forestry production. Jakarta: Sub−directorate of Forestry Statistics of Indonesia. [Google Scholar]
9. Corneliu, T., Irina, B., Sanda, C., Ioana, R., Mariana, T. et al. (2017). Valorization of wastes from forestry industry in the culture of sage (Salvia officinalis L.). Studia Universitatis Vasile Goldiş Seria Ştiinţele Vieţii, 27(1), 27–31. [Google Scholar]
10. Feng, S., Cheng, S., Yuan, S., Leitch, M., Xu, C. (2013). Valorization of bark for chemicals and materials: A review. Renewable and Sustainable Energy Reviews, 26, 560–578. DOI 10.1016/j.rser.2013.06.024.
11. Silva, F. S., Guerra, A. R., Duarte, M. F., Soares, B., Freire, S. R. et al. (2015). New valorization strategies for Eucalyptus spp. bark extracts. Wastes: Solutions, Treatments and Opportunities-Selected Papers from the 3rd Edition of the International Conference on Wastes: Solutions, Treatments and Opportunities, pp. 14–16. Portugal. [Google Scholar]
12. Kitagawa, I., Simajuntak, P., Hori, K., Nagami, N., Mahmud, T. et al. (1994). Indonesian medicinal plants. VII. Seven new clerodane−type diterpenoids, peronemins A2, A3, B1, B2, B3, C1, and D1, from the leaves of Peronema canescens (Verbenaceae). Chemical and Pharmaceutical Bulletin, 42(5), 1050–1055. DOI 10.1248/cpb.42.1050. [Google Scholar] [CrossRef]
13. Liu, J., Wang, C., Chen, J., Qiu, M. (2012). Limonoids from the leaves of Swietenia macrophylla. Natural Product Research, 26(20), 1887–1891. DOI 10.1080/14786419.2011.625499. [Google Scholar] [CrossRef]
14. Sudrajat, Susanto, D., Kartika, R. (2016). Phytochemicals analysis and antibacterial activity of the leaf of red meranti, Shorea leprosula (Dipterocarpaceae). Nusantara Bioscience, 8(1), 111–116. DOI 10.13057/nusbiosci/n080119. [Google Scholar] [CrossRef]
15. Liu, C. H., Mishra, A. K., Tan, R. X., Tang, C., Yang, H. et al. (2006). Repellent and insecticidal activities of essential oils from Artemisia princeps and Cinnamomum camphora and their effect on seed germination of wheat and broad bean. Bioresource Technologyogy, 97(15), 1969–1973. DOI 10.1016/j.biortech.2005.09.002. [Google Scholar] [CrossRef]
16. Manjang, Y. Y., Abdi, D., Djaswir, D., Edison, M. (2015). Steroids from N−Hexane fraction of the stem bark of Shorea singkawang Mig and anticancer activity as tested with murin leukemia P−388 Cells. Research Journal of Pharmaceutical, Biological, and Chemical Science, 6(2), 1315–1320. [Google Scholar]
17. Walch, S. G., Kuballa, T., Stühlinger, W., Lachenmeier, D. W. (2011). Determination of the biologically active flavour substances thujone and camphor in foods and medicines containing sage (Salvia officinalis L.). Chemistry Central Journal, 5(1), 437. DOI 10.1186/1752-153X-5-44. [Google Scholar] [CrossRef]
18. Li, B., Sen, Y. H., Hea, Y. R., Zhang, W. D. (2013). Chemical constituents and biological activities of pinus species. Chemistry & biodiversity, 10(12), 2133–2160. DOI 10.1002/cbdv.201100373. [Google Scholar] [CrossRef]
19. Sangat, H. M. (2006). The role of local knowledge in developing indigenous indonesian medicine. Media Konservasi, 6(1), 29– 31. [Google Scholar]
20. Darmawan, W., Nandika, D., Sari, R. K., Sitompul, A., Rahayu, I. et al. (2015). Juvenile and mature wood characteristics of short and long rotation teak in Java. IAWA Journal, 36(4), 429–443. DOI 10.1163/22941932-20150112. [Google Scholar] [CrossRef]
21. Rizanti, D. E., Darmawan, W., George, B., Merlin, A., Dumarcay, S. et al. (2018). Comparison of teak wood properties according to forest management: Short versus long rotation. Annals of Forest Science, 75(2), 241. DOI 10.1007/s13595-018-0716-8. [Google Scholar] [CrossRef]
22. Fengel, D., Wegener, G. (1995). Wood: Chemistry, ultrastructure, reaction. Yogyakarta: Gadjah Mada University Press. [Google Scholar]
23. Miranda, I., Sousa, V., Pereira, H. (2011). Wood properties of teak (Tectona grandis) from a mature unmanaged stand in East Timor. Journal of Wood Science, 57(3), 171–178. DOI 10.1007/s10086-010-1164-8. [Google Scholar] [CrossRef]
24. Moya, R., Bond, B., Quesad, H. (2014). A review of heartwood properties of Tectona grandis trees from fast−growth plantations. Wood Science and Technology, 48(2), 411–433. DOI 10.1007/s00226-014-0618-3. [Google Scholar] [CrossRef]
25. Sumthong, P., Romero−Gonzalez, R. R., Verpoorte, R. (2008). Identification of anti−wood rot compounds in teak (Tectona grandis L.f.) sawdust extract. Journal of Wood Chemistry and Technology, 28(4), 247–260. DOI 10.1080/02773810802452592. [Google Scholar] [CrossRef]
26. Lukmandaru, G., Takahashi, K. (2009). Radial distribution of quinones in plantation teak (Tectona grandis Linn.fil.). Annals of Forest Science, 66(6), 605. DOI 10.1051/forest/2009051. [Google Scholar] [CrossRef]
27. Sumthong, P., Damveld, R. A., Choi, Y. H., Arentshorst, M., Ram, A. F. J. et al. (2006). Activity of quinones from teak (Tectona grandis) on fungal cell wall stress. Planta Medica, 72(10), 943–944. DOI 10.1055/s-2006-946676. [Google Scholar] [CrossRef]
28. Wijayanto, A., Dumarc, S., Gérardin−charbonnier, C. (2015). Phenolic and lipophilic extractives in Pinus merkusii Jungh. et de Vries knots and stemwood. Industrial Crops and Products, 69, 466–471. DOI 10.1016/j.indcrop.2015.02.061. [Google Scholar] [CrossRef]
29. Windeisen, E., Klassen, A., Wegener, G. (2003). On the chemical characterisation of plantation teakwood from Panama. Holz als Roh− und Werkstoff, 61(6), 416–418. DOI 10.1007/s00107-003-0425-2. [Google Scholar] [CrossRef]
30. Goswami, D. V., Nirmal, S. A., Patil, M. J., Dighe, N. S., Laware, R. B. et al. (2009). An Overview of Tectona grandis: Chemistry and Pharmacological profile. Pharmacognosy Reviews, 3(5), 181–185. [Google Scholar]
31. Tong, P. S., Chen, H. K., Hewitt, J., Affre, A. (2009). Review of trade in Merbau from major state range. Malaysia: Traffic Southeast Asia. [Google Scholar]
32. Hills, W. E., Yazaki, Y. (1973). Polyphenols of Intsia heartwoods. Phytochemistry, 12(10), 2491–2495. DOI 10.1016/0031-9422(73)80461-3. [Google Scholar] [CrossRef]
33. Hillis, W. E. (1996). Formation of robinetin crystals in vessels of Intsia species. IAWA Journal, 17(4), 405–419. DOI 10.1163/22941932-90000637. [Google Scholar] [CrossRef]
34. Rudman, P. (1963). The causes of natural durability in timber part XI. Some tests on the fungi toxicity of wood extractives and related compounds. Holzforschung, 17(2), 54–57. DOI 10.1515/hfsg.1963.17.2.54. [Google Scholar] [CrossRef]
35. Bandarayanake, W. M. (2002). Bioactivities, bioactive compounds and chemical constituents of mangrove plants. Wetlands Ecology and Management, 10(6), 421–452. DOI 10.1023/A:1021397624349. [Google Scholar] [CrossRef]
36. Hasan, M. H., Wahab, I. A., Adam, A. (2019). Antioxidant properties of the ethyl acetate fraction of Intsia palembanica (Merbau, Fabaceae). Archives of Pharmacy & Pharmacology Research, 2(4), 1–8. [Google Scholar]
37. Norscia, I., Borgognini−Tarli, S. M. (2006). Ethnobotanical reputation of plant species from two forests of Madagascar: A preliminary investigation. South African Journal of Botany, 72(4), 656–660. DOI 10.1016/j.sajb.2006.04.004. [Google Scholar] [CrossRef]
38. Bradacs, G. (2008). Ethnobotanical survey and biological screening of medicinal plants from Vanuatu (Ph.D. Dissertation). Germany: Universitat Regensburg. [Google Scholar]
39. Zhu, L., Yan, J., Zhu, Z., Ouyang, Y., Zhang, X. et al. (2013). Differential analysis of camphor wood products by desorption atmospheric pressure chemical ionization mass spectrometry. Journal Agricultural and Food Chemistry, 61(3), 547–552. DOI 10.1021/jf303793t. [Google Scholar] [CrossRef]
40. Pandey, A. K., Bora, H. R., Deka, S. C., Rastaji, R. C., Banch, A. K. S. (1997). Composition of the essential oil of the bark of Cinnamomum camphora. International Journal of Medical and Aromatic Plants, 19(2), 408–409. [Google Scholar]
41. Zuccarini, P. (2009). Camphor: Risks and benefits of a widely used natural product. Journal of Applied Sciences and Environmental Management, 13(2), 69–74. [Google Scholar]
42. Song, J. K., Du, L. D., Qiang, G. F., Du, G. H. (2018). Camphor. Natural Small Molecule Drugs from Plants, Singapore: Springer. 205–208. DOI 10.1007/978-981-10-8022-7_33 [Google Scholar] [CrossRef]
43. Rivera, H. L., Barrueto, F. (2014). Camphor. Encyclopedia of Toxicology, 1, 382–383. [Google Scholar]
44. Kim, D. I., Park, J. D., Kim, S. G., Kuk, H., Jang, M. S. et al. (2005). Screening of some crude plant extracts for their acaricidal and insecticidal efficacies. Journal of Asia−Pacific Entomology, 8(1), 93–100. DOI 10.1016/S1226-8615(08)60076-X. [Google Scholar] [CrossRef]
45. Wu, C., Wu, Z., Wu, Y. (2000). Study on antibacterial activity of ethanol extract of Cinamomum camphora. Amino Acids and Biotic Resources, 22(2), 41–42. [Google Scholar]
46. Kamariah, A. S., Ozek, T., Demirci, B., Baser, A. H. C. (2012). Chemical composition of leaf and seed oils of Dryobalanops aromatica Gaertn. (Dipterocarpaceae). ASEAN Journal on Science and Technology for Development, 29(2), 105–114. DOI 10.29037/ajstd.57. [Google Scholar] [CrossRef]
47. Chua, M. T., Tung, Y. T., Chang, S. T. (2008). Antioxidant activities of ethanolic extracts from the twigs of Cinnamomum osmophloeum. Bioresource Technology, 99(6), 1918–1925. DOI 10.1016/j.biortech.2007.03.020. [Google Scholar] [CrossRef]
48. Falah, S., Suzuki, T., Katayama, T. (2008). Chemical constituents from Swietenia macrophylla bark and their antioxidant. International Journal of Biological Science, 11(16), 2007–2012. [Google Scholar]
49. Schmidt, L., Joker, D. (2000). Swietenia mahagoni. Seed Leaflet, 18. Humlebaek, Denmark. https://sl.ku.dk/rapporter/seed-leaflets/filer/swietenia-mahagoni-18.pdf. [Google Scholar]
50. Arumugasamy, K., Latha, K. V., Kumar, N. H. S. (2004). Studies on some pharmacognostic profile of Swietenia macrophylla King. Ancient Science of Life, 2, 97–102. [Google Scholar]
51. Suliman, M., Nour, A., Yusoff, M., Nour, A., Kuppusamy, P. et al. (2013). Fatty acid composition and antibacterial activity of Swietenia macrophylla king seed oil. International Journal of Engineering Research & Technology, 2(6), 2207–2216. [Google Scholar]
52. Mootoo, B., Ali, A., Motilal, R., Pingal, R., RamLal, A. et al. (1999). Limonoids from Swietenia macrophylla and S. aubrevilleana. Journal of Natural Product, 62(11), 1514–1517. DOI 10.1021/np990199x. [Google Scholar] [CrossRef]
53. Chen, J. J., Huang, S. S., Liao, C. H., Wei, D. C., Sung, P. J. et al. (2010). A new phragmalin−type limonoid and anti−inflammatory constituents from the fruits of Swietenia macrophylla. Food Chemistry, 120(2), 379–384. DOI 10.1016/j.foodchem.2009.09.093. [Google Scholar] [CrossRef]
54. Chen, L., Liao, H., Chen, P., Kuo, W., Chang, T. et al. (2015). Limonoids from the Seeds of Swietenia macrophylla and Their Anti−Inflammatory Activities. Molecules, 20(10), 18551–18564. DOI 10.3390/molecules201018551. [Google Scholar] [CrossRef]
55. Asmara, A. P. (2018). A preliminary study of investigating of compound group contained in ethanolic extract of mahagony (Swietenia mahagoni l. Jacq.) seeds related to α−glucosidase inhibition. Journal Natural, 18(2), 49–56. DOI 10.24815/jn.v18i2.9236. [Google Scholar] [CrossRef]
56. Chen, Y. Y., Wang, X. N., Fan, C. Q., Yin, S., Yue, J. M. (2007). Swiemahogins A and B, two novel limonoids from Swietenia mahogany. Tetrahedron Letters, 48(42), 7480–7484. DOI 10.1016/j.tetlet.2007.08.066. [Google Scholar] [CrossRef]
57. Saad, M. M., Iwagawa, T., Doe, M., Nakatani, M. (2003). Swietenialides, novel ring D opened phragmalin limonoid orthoesters from Swietenia mahogani JACQ. Tetrahedron, 59(40), 8027–8033. DOI 10.1016/j.tet.2003.08.033. [Google Scholar] [CrossRef]
58. Kadota, S., Marpaung, L., Kikuchi, T. (1990). Constituents of the seeds of Swietenia mahagoni Jacq. III. Structures of mahonin and secomahoganin. Chemical and Pharmaceutical Bulletin, 38(6), 1495–1500. DOI 10.1248/cpb.38.1495. [Google Scholar] [CrossRef]
59. Bourdy, G., De Walt, S. J., Chavez De Michel, L. R., Roca, A., Deharo, E. et al. (2000). Medicinal plants uses of the Tacana, an Amazonian Bolivian ethnic group. Journal of Ethnopharmacology, 70(2), 87–109. DOI 10.1016/S0378-8741(99)00158-0. [Google Scholar] [CrossRef]
60. Brummitt, R. K. (1992). Vascular plant families and genera. UK: Whitstable Litho, Ltd. [Google Scholar]
61. Yoshimoto, M., Hiraoka, T., Kuwano, H., Kishida, Y. (1970). Four new naphtoquinone derivates from Diospyros spp. Chemical and Pharaceutical Bulletin, 19(4), 851–854. [Google Scholar]
62. Adeniyi, B. A., Robert, M. F., Chai, H., Fong, H. H. S. (2003). In vitro cytotoxicity activity of diosquinone, a naphthoquinone epoxide. Phytotherapy Research, 17(3), 282–284. DOI 10.1002/ptr.1116. [Google Scholar] [CrossRef]
63. Tangmouo, J. G., Meli, A. L., Komguem, J., Kuete, V., Ngninzeko Ngounou, F. et al. (2006). Crassiflorone, a new naphthoquinone from Diospyros crassiflora (Hien). Tetrahedron Letters, 47(18), 3067–3070. DOI 10.1016/j.tetlet.2006.03.006. [Google Scholar] [CrossRef]
64. Brown, A. G., Lovie, J. C., Thomson, R. (1965). Ebenaceae extractives. Part I. Naphthalene derivatives from Macassar Ebony (Diospyros celebica Bakh). Journal of the Chemical Society, 2355–2361. London. DOI 10.1039/JR9650002355. [Google Scholar] [CrossRef]
65. Brown, A. G., Thomson, R. H. (1965). Ebenaceae Extractives. Part II. Naphthaldehydes from Diospyros ebenum Koen, Journal of the Chemical Society, 4292–4295. London. DOI 10.1039/JR9650004292. [Google Scholar] [CrossRef]
66. Fallas, A., Thomson, R. (1968). Ebenaceae extractives. Part III. Binaphthaquinones from Diospyros species. Journal of the Chemical Society, 2279–2282. London. DOI 10.1039/J39680002279. [Google Scholar] [CrossRef]
67. Maiti, B. C., Musgrave, O. C. (1986). Ebenaceae extractives. Part 9. New naphthoquinones and binaphthylquinones from macassar ebony. Journal of the Chemical Society Perkin Transactions, 1, 675–680. DOI 10.1039/p19860000675. [Google Scholar] [CrossRef]
68. Lillie, T., Musgrave, O., Skoyles, D. (1976). Ebenaceae extractives. Part V. New Diospyrin derivatives from Diospyros montana Roxb. Journal of the Chemical Society Perkin Transactions, 1, 2155–2160. DOI 10.1039/p19760002155. [Google Scholar] [CrossRef]
69. Matsushita, Y., Jang, I. C., Imai, T. (2010). Naphthalene derivatives from Diospyros kaki. Journal of Wood Science, 56(5), 418–421. DOI 10.1007/s10086-010-1115-4. [Google Scholar] [CrossRef]
70. Kuroyanagi, M., Yoshihira, K., Natori, S. (1971). Naphthoquinone Derivatives from the Ebenaceae III, Shinanolone from Diospyros japonica SIEB. Chemical and Pharmaceutical Bulletin, 19(11), 2314–2371. DOI 10.1248/cpb.19.2314. [Google Scholar] [CrossRef]
71. Lillie, T. J., Musgrave, O. C., Skoyles, D. (1976). Ebenaceae extractives. Part 6. Ehretione, a Bisnaphtoquinone derived from Plumbagin and 7-Methyljuglone. Journal of the Chemical Society Perkin Transactions, London. 1, 2546. DOI 10.1039/P19760002546. [Google Scholar]
72. Sagar, S., Kaur, M., Minneman, K. P., Bajic, V. B. (2010). Anti-cancer activities of diospyrin, its derivatives and analogues. European Journal of Medicinal Chemistry, 45(9), 3519–3530. DOI 10.1016/j.ejmech.2010.06.021. [Google Scholar] [CrossRef]
73. Sumitriasih, N. L., Ridhay, A., Indriani. (2020). Uji aktivitas antibakteri ekstak n−heksan, etil asetat, dan etanol kulit batang kayu eboni (Diospyros celebicabakh) menggunakann metode difusi. Kovalen, 5(3), 233–239. DOI 10.22487/kovalen.2019.v5.i3.11540. [Google Scholar] [CrossRef]
74. Adzu, B., Amos, S., Muazzam, I., Inyang, U. S., Gamaniel, K. S. (2002). Neuropharmacological screening of Diospyros mespiliformis in mice. Journal of Ethnopharmacology, 83(1–2), 139–143. DOI 10.1016/S0378-8741(02)00249-0. [Google Scholar] [CrossRef]
75. Chen, J., Ni, C., Lou, J., Peng, W. (2018). Molecules and functions of rosewood: Diospyros celebica. Arabian Journal of Chemistry, 11(6), 756–762. DOI 10.1016/j.arabjc.2017.12.033. [Google Scholar] [CrossRef]
76. Ganapaty, S., Thomas, P. S., Fotso, S., Laatsch, H. (2004). Antitermitic quinones from Diospyros sylvatica. Phytochemistry, 65(9), 1265–1271. DOI 10.1016/j.phytochem.2004.03.011. [Google Scholar] [CrossRef]
77. Millar, C. I. (1998). Early evolution of pines. In: Richardson DM (ed.Ecology and biogeography of Pinus, pp. 69–91. Cambridge: Cambridge University Press. [Google Scholar]
78. Wiyono, B., Tachibana, S., Tinambunan, D. (2006). Chemical composition of Indonesian Pinus merkusii turpentine, oils, gum oleoresines and rosin from Sumatra and Java. Pakistan International Journal of Biological Science, 9(1), 7–14. DOI 10.3923/pjbs.2006.7.14. [Google Scholar] [CrossRef]
79. Hardiyanto, E. B., Mada, U. G., Marsoem, S. N., Mada, U. G. (2015). Oleoresin production, turpentine yield and components of Pinus merkusii from various Indeniesian provenances. Journal of Tropical Forest Science, 27, 136–141. [Google Scholar]
80. Song, S. Q. (2009). Oleoresin characteristics and chemical classification of Pinus, pp. 34. China: University of Science and Technology of China Press. [Google Scholar]
81. Masendra, Ashitani, T., Takahashi, K., Lukmandaru, G. (2018). Lipophilic extractives of the inner and outer barks from six different Pinus species grown in Indonesia. Journal of Forestry Research, 29(5), 1329–1336. DOI 10.1007/s11676-017-0545-x. [Google Scholar] [CrossRef]
82. Masendra, Ashitani, T., Takahashi, K., Lukmandaru, G. (2018). Triterpenoids and Steroids from the Bark of Pinus merkusii (Pinaceae). BioResources, 13, 6160–6170. [Google Scholar]
83. Wijayanto, A., Dumarc, S., Gérardin−charbonnier, C. (2015). Phenolic and lipophilic extractives in Pinus merkusii Jungh. et de Vries knots and stemwood. Industrial Crops and Products, 69, 466–471. DOI 10.1016/j.indcrop.2015.02.061. [Google Scholar] [CrossRef]
84. Suwitono, M., Tobing, J., Hutauruk, E. (2018). Uji aktivitas antioksidan ekstrak daun pinus (Pinus merkusii) dengan metode dpph (1−1 Difenil−2−Pikrilhidrazil). Jurnal Sains dan Teknologi, 1(1), 1–6. [Google Scholar]
85. Kurose, K., Okamura, D., Yatagai, M. (2007). Composition of the essential oils from the leaves of nine Pinus species and the cones of three of Pinus species. Flavour and Fragrance Journal, 22(1), 10–20. DOI 10.1002/ffj.1609. [Google Scholar] [CrossRef]
86. Siringo−Ringo, M. (2015). Analisis komponen kimia dan uji aktivitas antibakteri minyak atsiri daun pinus (Pinus merkusii Jungh et de Vries) dari kabupaten Samosir. Sumatra: University of Sumatra Utara Institutional Repository. [Google Scholar]
87. Pangestika, F. W. (2019). Daya antibakteri ekstrak daun red pine (Pinus densiflora) dan green pine (Pinus merkusii) terhadap bakteri Enterococcus faecalis (Thesis). Indonesia: Airlangga University Institutional Repository. [Google Scholar]
88. Tillah, M., Batubara, I., Sari, R. (2017). Antimicrobial and Antioxidant Acitivites of Resins and Essential Oil from Pine (Pinus merkusii, Pinus oocarpa, Pinus insularis) and Agathis (Agathis loranthifolia). In: International conference on mathematics, science, and education. Biosaintifika, Journal of Biology and Biology Education, 9(1), 134–139. DOI 10.15294/biosaintifika.v9i1.8371. [Google Scholar] [CrossRef]
89. Sudjarwo, S. A., Ngadino, Koerniasari, Setiawan, Sudjarwo, G. W. (2017). Larvicidal activity of ethanol leaf extract of Pinus merkusii on Aedes aegypti larvae. Research Journal of Pharmacy and Technology, 10(4), 1011. DOI 10.5958/0974-360X.2017.00182.2. [Google Scholar] [CrossRef]
90. Setiawan, S., Koerniasari, K., Ngadino, N., Sudjarwo, S. A. (2017). Bioinsecticide Effect of Pinus merkusii Tree Bark Extract on Aedes aegypti larvae. Journal of Young Pharmacists, 9(1), 127–130. DOI 10.5530/jyp.2017.9.24. [Google Scholar] [CrossRef]
91. Singh, L., Dixit, P., Srivastava, R. P., Verma, P. C. (2019). Ethnobotany and Pharmacology of Pinus species growing naturally in Indian Himalayas: A plant review. Current Pharmaceutical Biotechnology, 20(15), 1281–1287. DOI 10.2174/1389201020666190819153600. [Google Scholar] [CrossRef]
92. Turner, I. M. (1997). A catalogue of the Vascular Plants of Malaya. Gardens Bulletin Singapore, 47(2), 347–655. [Google Scholar]
93. Harmida, H., Sarno, Yuni, V. F. (2011). Ethnofitomedika study in Lawang Agung Village Mulak Ulu Sub−District Lahat Regency of South Sumatra. Journal of Science Research, 14(1), 42–46. [Google Scholar]
94. Ibrahim, A., Kuncoro, H. (2012). Identification of secondary metabolites and antibacterial activity of sungkai leaf extract (Peronema canescens Jack.) against some pathogenic bacteria. Journal of Tropical Pharmacy and Chemistry, 2(1), 8–18. DOI 10.25026/jtpc.v2i1.43. [Google Scholar] [CrossRef]
95. Rosdiana, N. A. (2014). Fraksi aktif antioksidan dari ekstrak kulit kayu sungkai (Penorema canescens Jack.) (Thesis). Bogor: IPB University. [Google Scholar]
96. Ibrahim, A., Arifuddin, M., Cahyo, W., Widayat, W., Bone, M. (2019). Isolation and characterization endopyhctic fungal isolate from Penorema canescens Jack Leaf and Captosapelta tomentosa Val. K. Heyne Root. Journal of Tropical Pharmacy and Chemistry, 4(5), 215–225. DOI 10.25026/jtpc.v4i5.169. [Google Scholar] [CrossRef]
97. Al’amri, A. F., Herman, Amir, M. (2011). Antioxidant activity of merchant root (coptosapelta tomentosa Valeton K. Heyne) against dpph free radicals (1,1 diphenyl−2−pikril hydrazil) (Bachelor's Thesis). Indonesia: Faculty of Pharmacy Mulawarman University. [Google Scholar]
98. Sotheeswaran, S., Pasuparthy, V. (1993). Distribution of resveratrol oligomers in plants. Phytochemistry, 32(5), 1083–1092. DOI 10.1016/S0031-9422(00)95070-2. [Google Scholar] [CrossRef]
99. Hakim, E. H. (2002). Oligostilbenoid from the Dipterocarpaceae trees. Bulletin of the Indonesian Society of Natural Products Chemistry, 2(1), 1–19. [Google Scholar]
100. Newman, M. F., Burgess, P. F., Whitemore, T. C. (1999). Key of identification to Dipterocarpaceae trees from Sumatra Island. Bogor: Prosea. [Google Scholar]
101. Atun, S. (2006). Oligoresveratrol activity from stem bark of the Hopea mengarawan (Dipterocarpaceae) as hydroxylradical scavenger. Journal Hayati, 13(2), 65–68. DOI 10.1016/S1978-3019(16)30383-7. [Google Scholar] [CrossRef]
102. Rosyidah, K. L., Juliawati, D., Syah, Y. M., Hakim, E. H., Achmad, S. A. et al. (2007). Thimer resveratrol from the stem bark of Shorea parvifolia Dyer. Sains dan Terapan Kimia, 1(1), 47–52.
103. Sahidin, S., Hakim, E. H., Syah, Y. M., Juliawaty, L. D., Achmad, S. A. et al. (2007). Oligomer resveratrol from the stem bark of Shorea assamica Dyer (Dipterocarpaceae) and its citotoxicity. Journal Matematika dan Sains, 12(3), 115–118. [Google Scholar]
104. Noviany, Hadi, S. (2009). The isolation of α −viniferin, a trimer stilbene, from Shorea ovalis Blume. Advances in Natural and Applied Sciences, 3(1), 45–51. [Google Scholar]
105. Muhammad, N., Din, L. B., Sahidin, I. (2012). Acuminatol and other antioxidative resveratrol oligomers from the stem bark of Shorea acuminata. Molecules, 17(8), 9043–9055. DOI 10.3390/molecules17089043. [Google Scholar] [CrossRef]
106. Murthy, K. S. R., Lakshmi, N., Raghu Ramulu, D. (2011). Biological activity and phytochemical screening of the oleoresin of Shorea robusta Gaertn.f. Tropical and Subtropical Agroecosystems, 14, 787–791. [Google Scholar]
107. Zain, W. Z. W. M., Ahmat, N., Nawi, L., Jusoff, K. (2010). Chemical Prospecting of Malaysian Dipterocarpaceae from HSUiTM, Pahang, Malaysia. World Applied Sciences Journal, 8(9), 1050–1055. [Google Scholar]
108. Göltenboth, F., Langenberger, G., Widmann, P. (2006). Special forest ecosystems. Elsevier B.V: Ecology of Insular Southeast Asia. [Google Scholar]
109. The IUCN Red List of Threatened Species. (1998). Asian regional workshop (conservation and suistainable management of trees, Vietnam, August 1996) Eusideroxylon zwageri. https://www.iucnredlist.org/species/31316/9624725. [Google Scholar]
110. Aiso−Sanada, H., Nezu, I., Ishiguri, F. (2020). Basic wood properties of Borneo ironwood (Eusideroxylon zwageri) planted in Sarawak, Malaysia. Tropics, 28(4), 99–103. DOI 10.3759/tropics.MS19-10. [Google Scholar] [CrossRef]
111. Anonymous. 2010). (. Introduction and literature review on ulin: Borneo ironwood (Eusideroxylon zwageri Teijsm and Binn.) (Thesis), 1–46, https://openaccess.leidenuniv.nl/bitstream/handle/1887/18056/01.pdf?sequence=3. [Google Scholar]
112. Zahorka, H. (2006). Blowpipe dart poison in Borneo and the secret of its production: the latex of Antiaris toxicaria; the poison-making procedure; the heat-sensitive main toxic chemical compound, and the lethal effect of the poison. 1–6. Indonesia: Borneo Research Bulletin, https://www.nomadicpixel.com/wp-content/uploads/2015/08/Blowpipe-Dart-Poison-in-Borneo.pdf. [Google Scholar]
113. Hobbs, B. J. J., King, F. E. (1960). The chemistry of extractives from hardwoods Eusiderin, a possible by−product of lignin xynthesis in Eusideroxylon. Journal of Chemical Society, 922, 4732–4738. DOI 10.1039/jr9600004732. [Google Scholar] [CrossRef]
114. Yoosu, S., Namseok, C., Minoru, T. (2009). Two new isomeric lignans from Eusideroxylon zwageri. Chemistry of Natural Compounds, 45(3), 356–359. DOI 10.1007/s10600-009-9346-6. [Google Scholar] [CrossRef]
115. Syamsurizal, A. (2014). Modification of allylic moiety of Eusiderin a to enhance the antifeedant potency. Journal of Natural Science Research, 4(6), 7–10. [Google Scholar]
116. Muhaimin, Syamsurizal, Chaerunisaa, A. Y., Sinaga, M. S. (2016). Eusiderin I from Eusideroxylon zwagery as antifungal agent against plant pathogenic fungus. International Journal of ChemTech Research, 9, 418–424. [Google Scholar]
117. Ping, S. S. 2012). (, Antifungal activities of dichloromethane and methanol extracts from eusideroxylon zwageri and potoxylon melagangai heartwood (ThesisUniversiti Malaysia Sarawak, Malaysia. [Google Scholar]
118. Abdullah, F., Jusoh, I., Assim, Z. (2013). Extractives from eusideroxylon zwageri and potoxylon melagangai against white-rot fungi. Proceedings of the ICNP, Selangor, Malaysia. 4, 2013. DOI 10.2174/2210289201304010166 [Google Scholar] [CrossRef]
119. Ma’ruf, A., Noorhidayah, Atmoko, T. (2011). Medicinal properties of bornean orangutan food plants in gunung beratus protected forest, East Kalimantan, Indonesia, Int. Conf. of Indonesian Forestry Researchers (INAFOR), Bogor, Indonesia. 1–14. [Google Scholar]
120. Arung, E. T., Kusuma, I. W., Christy, E. O. (2009). Evaluation of medicinal plants from Central Kalimantan for antimelanogenesis. Journal Natural Medicine, 63(4), 473–480. DOI 10.1007/s11418-009-0351-7. [Google Scholar] [CrossRef]
121. Kusuma, I. W., Rahmini, Ramadhan, R. (2018). Phytochemicals and antidiabetic activity of Eusideroxylon zwageri stem bark collected from East Kalimantan, Indonesia. IOP Conference Series Earth and Environmental Science, pp. 144. Samarinda, East Kalimantan, Indonesia. DOI 10.1088/1755-1315/144/1/012030. [Google Scholar] [CrossRef]
122. Nintasari, R., Amaliyah, D. M. (2016). Ektraksi zat warna alam dari kayu ulin (Eusideroxylon zwagerikayu secang (Caesalpinia sp) dan kayu mengkudu (Morinda citrifolia) untuk bahan warna kain sasirangan. Jurnal Riset Industri Hasil Hutan, 8(1), 25. DOI 10.24111/jrihh.v8i1.2065. [Google Scholar] [CrossRef]
123. PROSEA (2017). Dipterocarpus, Dryobalanops, Koompassia, Palaquium. Plant resources of South-East Asia, https://uses.plantnet-project.org/e/index.php?title=Category:PROSEA&from=D. [Google Scholar]
124. Batubara, I., Kotsuka, S., Yamauchi, K. (2012). TNF−alpha production inhibitory activity, phenolic, flavonoid, and tannin contents of selected indonesian medicianl plants. Research Journal of Medicinal Plants, 6(6), 406–415. DOI 10.3923/rjmp.2012.406.415. [Google Scholar] [CrossRef]
125. Wong, A. H., Grace, J., Kirton, L. (1998). Termite resistance of malaysian and exotic woods with plantation potential: Field evaluation. In: IRG/WP 98–10289. Maastricht, Netherlands. [Google Scholar]
126. Wong, A. H., Jem, J. M., Lai, J. (2012). Classifying white rot decay resistance of some hardwoods from Sarawak and Peninsular Malaysia and correlation with their tropical in-ground durability. IRG/WP 12-10788, Estoril, Portugal. In: IRG IUFRO Document 2012. [Google Scholar]
127. Martawijaya, A., Kartasujana, I., Kadir, K., Prawira, S. A. (1992). Indonesian Wood Atlas Volume II. Indonesia: Department of Forestry, Forestry Research and Development Agency, Forest Product and Development Centre. [Google Scholar]
128. Kitagawa, I. (1992). Chemical investigation of natrually occurring drug materials. Elucidation of scientific basis for traditional medicines and exploitation of new naturally occurring drugs. Yakugaku Zasshi, 112(1), 1–41. DOI 10.1248/yakushi1947.112.1_1. [Google Scholar] [CrossRef]
129. Kobayashi, M., Mahmud, T., Yoshioka, N. (1996). Indonesian Medicinal Plants. XVIII. Kompasinol A, a New Stilbeno−phenylpropanoid from the bark of Koompassia malaccensis (Fabaceae). Chemical and Pharmaceutical Bulletin, 44(12), 2249–2253. DOI 10.1248/cpb.44.2249. [Google Scholar] [CrossRef]
130. Kuspradini, H., Mitsunaga, T., Ohashi, H. (2009). Antimicrobial activity against Streptococcus sobrinus and glucosyltransferase inhibitory activity of taxifolin and some flavanonol rhamnosides from kempas (Koompassia malaccensis) extracts. Journal of Wood Science, 55(4), 308–313. DOI 10.1007/s10086-009-1026-4. [Google Scholar] [CrossRef]
131. Batubara, I., Kuspradini, H., Mitsunaga, T. (2010). Anti−acne and tyrosinase inhibition properties of taxifolin and some flavanonol rhamnosides from Kempas (Koompassia malaccensis). Wood Research Journal, 1, 45–49. [Google Scholar]
132. Maniglia−ferreira, C., Silva, J. B. A., De Paula, R. C. M., Feitosa, J. P. A., Cortez, D. G. N. et al. (2005). Brazilian gutta−percha points. Part I: Chemical composition and X-ray diffraction analysis. Brazilian Oral Research, 19(3), 193–197. [Google Scholar]
133. Karliati, T., Febrianto, F., Syafii, W., Wahyudi, I., Wistara, I. N. Y. (2014). Gutta−percha−based adhesive for laminated wood production. BioResources, 9(3), 5034–5044. DOI 10.15376/biores.9.3.5034-5044. [Google Scholar] [CrossRef]
134. Heilbron, I., Kennedy, T., Spring, F.S. (1938). The unsaturated centre of the triterpene alcohol lupeol. Journal of the Chemical Society, 329–334. London. DOI 10.1039/JR9380000329. [Google Scholar] [CrossRef]
135. Gunawkbra, S. P., Vijaya, K., Sultanbawa, M., Balasubramaniam, S. (1977). Triterpenoids and steroids of some Sapotaceae and their chemotaxonomic. Significance. Phytochemistry, 16(7), 923–926. DOI 10.1016/S0031-9422(00)86694-7. [Google Scholar] [CrossRef]
136. Ragasa, C. Y., Torres, O. B., Bernardo, L. O., Madrazo, L. D. J., Mandia, E. H. et al. (2015). Chemical constituents of Palaquium luzoniense. Chemistry of Natural Compounds, 51(1), 181–182. DOI 10.1007/s10600-015-1237-4. [Google Scholar] [CrossRef]
137. Kawamura, F., Shaharuddin, N. A., Sulaiman, O., Hashim, R., Ohara, S. (2010). Evaluation on antioxidant activity, antifungal activity and total phenols of 11 selected sommercial Malaysian timber species. Japan Agricultural Research Quarterly, 44(3), 319–324. DOI 10.6090/jarq.44.319. [Google Scholar] [CrossRef]
138. Jemi, R., Syafii, W., Febrianto, F., Hanafi, M. (2012). Aktivitas anti jamur 2,3−dihidroksipentadekanoat dari kayu mahalilis (Palaquium sp.). Jurnal Kimia Terapan Indonesia, 14, 14–19. [Google Scholar]
139. Al−sagheer, N. A., Prasad, A. G. D. (2010). Variation in wood specific gravity, density and moisture content of Dipterocarpus indicus (Bedd). among different populations in Western Ghats of Karnataka, India. Journal of Applied Agricultural Research, 5, 583–599. [Google Scholar]
140. Belardo, L., Lawrence, B. M., Coronel, A., Mata, M. F. (1983). Essential oil of Dipterocarpus grandjflorus blanco: Chemistry and possible source of energy. Transactions of National Academy of Science Technology, 3, 233–241. [Google Scholar]
141. Chatuphonprasert, W., Tatiya−aphiradee, N., Thammawat, S. (2019). Antibacterial and wound healing activity of Dipterocarpus alatus crude extract against methicillin−resistant Staphylococcus aureus−induced superficial skin infection in mice. Journal of Skin and Stem Cell, 6, 1–8. [Google Scholar]
142. Aslam, M. S., Ahmad, M. S., Mamat, A. S. O. H. (2015). A phytochemical, ethnomedicinal and pharmacological review of genus Dipterocarpus. Journal of Pharmacy and Pharmaceutical Sciences, 7, 27–38. [Google Scholar]
143. Satiraphan, M. (2012). Phytochemical screening of two Thai tropical rainforest Dipterocarps: Hopea odorata Roxb. and Dipterocarpus costatus Gaertn,f. HAL, 1–322. France. https://tel.archives-ouvertes.fr/tel-01124074. [Google Scholar]
144. Chen, C. J. C., Jiang, R., Wang, G., Jiao, R. H., Tancharoen, C. et al. (2014). Oligostilbenoids with acetylcholinesterase inhibitory activity from Dipterocarpus alatus. Planta Medica, 80(17), 1641–1646. DOI 10.1055/s-0034-1383194. [Google Scholar] [CrossRef]
145. Chen, W., Vermaak, I., Viljoen, A. (2013). Camphor—A fumigant during the black death and a coveted fragrant wood in ancient egypt and babylon—A review. Molecules, 18(5), 5434–5454. DOI 10.3390/molecules18055434. [Google Scholar] [CrossRef]
146. Ali, R., Koh, M. (1991). Quantification of some components of the extractives of Dryobalanops aromatica obtained from different sources. Journal of Tropical Forest Science, 3, 367–371. [Google Scholar]
147. Wibowo, A., Ahmat, N., Hamzah, A. S., Sulfian, A. S., Ismail, N. H. et al. (2011). Malaysianol A, a new trimer resveratrol oligomer from the stem bark of Dryobalanops aromatica. Fitoterapia, 82(4), 676–681. DOI 10.1016/j.fitote.2011.02.006. [Google Scholar] [CrossRef]
148. Wibowo, A., Ahmat, N., Hamzah, A. S., Latif, F. A., Norizzah, J. S. et al. (2014). Identification and biological activity of secondary metabolites from Dryobalanops beccarii. Phytochemical Letter, 9(1), 117–122. DOI 10.1016/j.phytol.2014.05.001. [Google Scholar] [CrossRef]
149. Wibowo, A., Ahmat, N., Hamzah, A. S., Low, A. L. M., Mohamad, S. A. S. et al. (2012). Malaysianol B, an oligostilbenoid derivative from Dryobalanops lanceolata. Fitoterapia, 83(8), 1569–1575. DOI 10.1016/j.fitote.2012.09.004. [Google Scholar] [CrossRef]
150. Ahmat, N., Wibowo, A., Syed, S. A. (2014). A new symmetrical tetramer oligostilbenoid containing tetrahydrofuran ring from the stem bark of Dryobalanops lanceolata. Journal of Asian Natural Products Research, 6(11), 1099–1107. DOI 10.1080/10286020.2014.938059. [Google Scholar] [CrossRef]
151. Kuspradini, H., Putri, A. S., Sukaton, E., Mitsunaga, T. (2016). Bioactivity of essential oils from leaves of Dryobalanops lanceolata, Cinnamomum burmannii, Cananga odorata, and Scorodocarpus borneensis. Agricultural and Agricultural Sceince Procedia, 9, 411–418. DOI 10.1016/j.aaspro.2016.02.157. [Google Scholar] [CrossRef]
152. Kuspradini, H., Putri, A. S., Mitsunaga, T. (2018). Chemical composition, antibacterial and antioxidant activities of essential oils of Dryobalanops lanceolata Burck. Leaf. Research Journal of Medicinal Plants, 12(1), 19–25. DOI 10.3923/rjmp.2018.19.25. [Google Scholar] [CrossRef]
153. Le, T., Ho, A. S., Mah, S., Wong, T. W., Ong, H. C. et al. (2016). Determination of borneol and other chemical compounds of essential oil of Dryobalanops aromatica exudate from Malaysia. Tropical Journal of Pharmaceutical Research, 15(6), 1293–1297. DOI 10.4314/tjpr.v15i6.23. [Google Scholar] [CrossRef]
154. Krisnawati, H., Varis, E., Kallio, M., Kanninen, M. (2011). Paraserianthes falcataria (L.) Nielsen Ecology, silviculture and productivity. CIFOR, pp. 1–23. Bogor, Indonesia. https://www.cifor.org/publications/pdf_files/Books/BKrisnawati1103.pdf. [Google Scholar]
155. Kaida, R., Kaku, T., Oyadomari, M., Watanabe, T., Hartati, S. et al. (2009). Enzymatic saccharifi cation and ethanol production of Acacia mangium and Paraserianthes falcataria wood, and Elaeis guineensis trunk. Journal of Wood Science, 55(5), 381–386. DOI 10.1007/s10086-009-1038-0. [Google Scholar] [CrossRef]
156. Hussin, M. C., Kasim, J., Yusoff, N. F., Jasmi, N. F., Misfar, S. N. (2014). Effect of tree portion and distance from pith on the basic density, fiber properties and chemical composition of Albizia falcataria wood. International Journal of Latest Reseacrh in Science Technology, 3, 187–191. [Google Scholar]
157. Liswidowati, Karina, M., Syafii, W., Suzuki, S., Umezawa, T. et al. (2001). Isolation of Syringaresinol from Paraserinthes falcataria. Wood Research Institut Kyoto University, 88, 40–41. [Google Scholar]
158. Masruri, M., Pangestin, D. N., Pangesti, P. A., Arini, W. (2019). Reducing environmental effect of bark waste of Sengon (Paraserianthes falcataria L.) by applying as a source of green ingredients to lower glucose−related diseases. IOP Conference Series Earth and Environmental Science, 239, 1–8. DOI 10.1088/1755-1315/239/1/012025. [Google Scholar] [CrossRef]
159. Ari, N., Dumarçay, S., Gérardin, C., Chapuis, H., Santiago-Medina, F. J. et al. (2017). Industrial crops & products characterization of bark extractives of different industrial Indonesian wood species for potential valorization. Industrial Crops and Products, 108, 121–127. DOI 10.1016/j.indcrop.2017.06.034. [Google Scholar] [CrossRef]
160. Febrianto, F., Pranata, A., Septiana, D., Arinana Gumilang, A. et al. (2015). Termite resistance of the less known tropical woods species grown in West Java. Journal Korean Wood Science Technology, 43(2), 248–257. DOI 10.5658/WOOD.2015.43.2.248. [Google Scholar] [CrossRef]
161. Mulyono, N., Adrianus, R. I. O. (2012). Biodegradable Coating from Agathis alba. International Journal of Engineering, Science, and Technology, 4, 4639–4643. [Google Scholar]
162. Jost, T., Foussereau, J. (1989). Contact allergy to Manilla resin, Nomenclature and physico−chemistry of Manilla, kauri, damar and copal resins. Contact Dermatitis, 21(4), 228–238. DOI 10.1111/j.1600-0536.1989.tb03201.x. [Google Scholar] [CrossRef]
163. Taylor, P., Lassak, E. V., Brophy, J. J. (2008). The steam−volatile oil of commercial almaciga resin (agathis philippinensis warb.) from the Philippines. Journal of Essential Oil Bearing Plants, 11(6), 634–637. DOI 10.1080/0972060X.2008.10643679. [Google Scholar] [CrossRef]
164. Resmeiliana, I. (2011). Ciri kimiawi asam resin kopal. Bogor: IPB University. [Google Scholar]
165. Khan, N., Ilyas, M., Rahman, W., Mashima, T., Okigawa, M. et al. (1972). Biflavones from the leaves of Araucaria Bidwillii Hooker and Agathis alba Foxworthy (Araucariaceae). Tetrahedron, 28(23), 5689–5695. DOI 10.1016/S0040-4020(01)88913-4. [Google Scholar] [CrossRef]
166. Mashima, T., Okigawa, M., Kawano, N., Khan, N. U., Ilyas, M. et al. (1970). On the bisflavones in the leaves of Agathis alba Foxworthy. Tetrahedron Letter, 11(3), 2937–2940. DOI 10.1016/S0040-4039(01)98378-9. [Google Scholar] [CrossRef]
167. Khan, N., Ansari, W., Usmani, J., Ilyas, M., Rahman, W. (1971). Biflavonyls of the Aaraucariales. Phytochemistry, 10(9), 2129–2131. DOI 10.1016/S0031-9422(00)97208-X. [Google Scholar] [CrossRef]
168. Gutierrez, R. M., Baculi, R., Pastor, N., Puma−at, T., Balangcod, T. (2013). Antibacterial potential of some medicinal plants of the Cordillera Region. Philippines Indian Journal of Traditional Knowledge, 12(4), 630–637. [Google Scholar]
169. Sirimangkalakitti, N., Dewi, L., Hakim, E. H., Waliana, I. (2019). Naturally occurring biflavonoids with amyloid β aggregation inhibitory activity for development of anti−Alzheimer agents. Bioorganic and Medicinal Chemistry Letters, 29(15), 1994–1997. DOI 10.1016/j.bmcl.2019.05.020. [Google Scholar] [CrossRef]
170. Rain, N., Khozirah, S., Ridzuan, M., Ong, B. K., Rohaya, C. et al. (2007). Antiplasmodial properties of some Malaysian medicinal plants Antiplasmodial properties of some Malaysian medicinal. Tropical Biomedicine, 24(1), 29–35. [Google Scholar]
171. Wong, A. H. H., Ahmad, S. (2000). Variation in infection rates of blue-stain, mould and white rot tropical fungi on mixed light Malaysian woods. In: IRG/WP 00-10334. Kuala Lumpur, Malaysia. [Google Scholar]
172. Meier. (2020). The wood database, Jelutong (Dyera costulata). https://www.wood−database.com/jelutong/. [Google Scholar]
173. Majid, N. M., Islam, M. M., Rauf, R. A. (2012). Evaluation of Jelutong (Dyera cotulata) as a phytoremediator to uptake copper (Cu) from contaminated soils. Ausraliant Journal of Crop Science, 6, 369–374. [Google Scholar]
174. Subhadhirasakul, S., Jankeaw, B., Malinee, A. (2003). Chemical constituents and antioxidative activity of the extracts from Dyera costulata leaves. Songklanakarin Journal of Science and Technology, 25(3), 351–357. [Google Scholar]
175. Mirand, C., Men−olivier, L. L. E., Men, J. L. E., Delaude, C. (1983). Alkaloids of Dyera costulata. Phytochemistry, 22(2), 577–579. DOI 10.1016/0031-9422(83)83050-7. [Google Scholar] [CrossRef]
176. Wong, S. K., Lim, Y. Y., Ling, S. K., Chan, E. W. C. (2014). Caffeoylquinic acids in leaves of selected Apocynaceae species: Their isolation and content. Pharmacognosy Research, 6(1), 67–72. DOI 10.4103/0974-8490.122921. [Google Scholar] [CrossRef]
177. Sakurai, K., Hosomi, K., Arakawa, T., Usawa, M., Ito, Y. et al. (1992). Isolation and characterization of an allergy inhibitor from the Jelutong, Dyera costulata Hook. f. Bioscience, Biotechnology, and Biochemistry, 56(6), 975. DOI 10.1271/bbb.56.975. [Google Scholar] [CrossRef]
178. Norhayati, I., Getha, K., Haffiz, J. M., Ilham, A., Sahira, H. et al. (2013). In vitro antitrypanosomal activity of malaysian plants. Journal of Tropical Forest Science, 25(1), 52–59. [Google Scholar]
179. Mohmod, A. L., Krishnasamy, G., Adenan, M. I. (2015). Review: Malaysian plants with potential in vitro trypanocidal activity. Annals of Phytomedicine, 4, 6–16. [Google Scholar]
180. Wong, S. K., Lim, Y. Y., Abdullah, N. R., Nordin, F. (2011). Assessment of antiproliferative and antiplasmodial activities of five selected Apocynaceae species. BMC Complementary and Alternative Medicine, 11(1), 235. DOI 10.1186/1472-6882-11-3. [Google Scholar] [CrossRef]
181. Wong, S. K., Lim, Y. Y., Abdullah, N., Nordin, F. (2011). Antiproliferative and phytochemicacal analyses of leaf extracts of ten Apocynaceae species. Pharmacognosy Research, 3(2), 100–106. DOI 10.4103/0974-8490.81957. [Google Scholar] [CrossRef]
182. Reanmongkol, W., Subhadhirasakul, S., Pairat, C., Poungsawai, C., Choocare, W. (2002). Antinociceptive activity of Dyera costulata extract in experimental animals. Songklanakarin. Journal of Science and Technology, 24(2), 227–234. [Google Scholar]
183. Orwa. (2009). Anthocephalus cadamba (Roxb.) Miq. Agrofor Database, 40, 1–5. [Google Scholar]
184. Krisnawati, H., Kallio, M., Kanninen, M. (2011). Anthocephalus cadamba. CIFOR, pp. 1–22. Bogor, Indonesia. https://pdfs.semanticscholar.org/a7a1/8ccc08e97178830d33b1bf65b966e728e152.pdf. [Google Scholar]
185. Dubey, A., Nayak, S., Goupale, D. C. (2011). Anthocephalus cadamba: A Review. Pharmacognosy Journal, 2(18), 71–76. DOI 10.1016/S0975-3575(11)80029-5. [Google Scholar] [CrossRef]
186. Gautam, R., Irchhaiya, R., Swarnakar, R. (2012). Anthocephalus cadamba (roxbAn overview. International Journal of Pharmaceutical Research and Development, 4, 169–173. [Google Scholar]
187. Brown, R., Fraser. S. (1974). Anthocephalus alkaloids: Cadambine and 3α-dihydrocadambine. Tetrahedron Letters, 15(23), 1957–1959. DOI 10.1016/S0040-4039(01)82603-4. [Google Scholar] [CrossRef]
188. Banerji, N. (1977). New saponin from stem bark of Anthocephalus cadamba Miq. Indian Journal of Chemistry B, 15, 654–655. [Google Scholar]
189. Kapil, A., Koul, I. B. (1995). Antihepatotoxic effects of chlorogenic acid from Anthocephalus cadamba. Phytotherapy Research, 9(3), 189–193. DOI 10.1002/ptr.2650090307. [Google Scholar] [CrossRef]
190. Sahu, N. P., Koike, K., Jia, Z., Achari, B., Banerjee, S. et al. (1999). Structures of two novel isomeric triterpenoid saponins from Anthocephalus cadamba. Magnetic Resonance in Chemistry, 37, 837–842. DOI 10.1002/(SICI)1097-458X(199911)37:11<837::AID-MRC567>3.0.CO;2-Y. [Google Scholar] [CrossRef]
191. Sahu, N. P., Koike, K., Jia, Z., Banerjee, S., Mondal, N. B. et al. (2000). Triterpene glycosides from the bark of Anthocephalus cadamba. Journal of Chemical Research, 1(1), 22–23. DOI 10.3184/030823400103165536. [Google Scholar] [CrossRef]
192. Chandel, M., Kaur, S., Kumar, S. (2011). Studies on the genoprotective / antioxidant potential of methanol extract of Anthocephalus cadamba (Roxb.) Miq. Journal of Medicinal Plants Research, 5, 4764–4770. [Google Scholar]
193. Alam, M., Subhan, N., Chowdhury, S., Awal, M. A., Mustofa, M. et al. (2011). Anthocephalus cadamba extract shows hypoglycemic effect and eases oxidative stress in alloxan−induced diabetic rats. Brazilian Journal of Pharmacognosy, 21, 155–164. DOI 10.1590/S0102. [Google Scholar] [CrossRef]
194. Acharyya, S., Dash, G. K., Mondal, S. (2010). Studies on glucose lowering efficacy of the Anthocephalus cadamba (roxb.) Miq. Roots. S. International Journal of Pharma and Bio Science, 21(1), 1–9. [Google Scholar]
195. Bachhav, R., Buchake, V., Saudagar, R. (2009). Analgesic and anti-inflammatory activities of Anthocephalus cadamba Roxb. leaves in Wistar Rats. Advances in Pharmalogy and Toxiclogy, 10, 123–130. [Google Scholar]
196. Chandrashekar, K. S., Abinash, B., Subraya, K. (2010). Anti-inflammatory effect of the methanol extract from Anthocephalus cadamba stem bark in animal modelsa. International Journal of Plant Biology, 1(1), 6. DOI 10.4081/pb.2010.e6. [Google Scholar] [CrossRef]
197. Alam, M., Akter, R., Subhan, N., Rahmat, M. M., Majumder, M. M. et al. (2008). Antidiarrhoeal property of the hydroethanolic extract of the fl owering tops of Anthocephalus cadamba. Brazilian Journal of Pharmacognosy, 18, 155–159. [Google Scholar]
198. Umachigi, S., Kumar, G. S., Jayaveera, K. N., Kishore Kumar, D. V., Ashok Kumar, C. K. et al. (2007). Antimicrobial, wound healing and antioxidant activities of Anthocephalus cadamba. African Journal Traditiom Complementary Alternative Medicine, 4(4), 481–487. DOI 10.4314/ajtcam.v4i4.31241. [Google Scholar] [CrossRef]
199. Datar, H., Datar, A. (2016). Antimicrobial activity of Anthoceplalus cadamba and Scirpus kysoor roxb. against food pathogens. International Journal of Current Pharmaceutical Research, 8(4), 13. DOI 10.22159/ijcpr.2016v8i4.15269. [Google Scholar] [CrossRef]
200. Khandelwal, V., Bhatia, A., Goel, A. (2016). Antimicrobial and antioxidant efficacy of aqueous extract of Anthocephalus cadamba Leaves. Journal of Pure Applied Microbiology, 10, 209–216. [Google Scholar]
201. Subhan, N., Hasan, S. M. R., Hossain, M., Akter, R. (2009). Antinociceptive and gastro−protective effect of the ethanolic extract of the flowering top of Anthocephalus cadamba Roxb. Oriental Pharmacy and Experimental Medicine, 9(4), 326–334. DOI 10.3742/OPEM.2009.9.4.326. [Google Scholar] [CrossRef]
202. Alam, M. A., Ghani, A., Subhan, N., Rahman, M. M., Haque, M. S. et al. (2008). Natural product communications antioxidant and membrane stabilizing properties of the flowering tops of Anthocephalus cadamba. Natural Product Communications, 3, 65–70. [Google Scholar]
203. Chandel, M., Sharma, U., Kumar, N., Singh, B., Kaur, S. (2012). Antioxidant activity and identification of bioactive compounds from leaves of Anthocephalus cadamba by ultra-performance liquid chromatography / electrospray ionization quadrupole time of flight mass spectrometry. Asian Pacifis Journal Tropical Medicine, 5(12), 977–985. DOI 10.1016/S1995-7645(12)60186-2. [Google Scholar] [CrossRef]
204. Kumar, D., Tejaswi, C., Rasamalla, S., Mallick, S., Pal, B. C. (2015). Bio-assay guided isolation of anti-cancer compounds from Anthocephalus cadamba Bark Deepak. Natural Product Comunnications, 10(8), 1349–1350. [Google Scholar]
205. National Research Council. (1983). Mangium and other fast−growing acacias for the humid tropics. Washington DC: The National Academies Press. [Google Scholar]
206. Syafii, W., Siregar, Z. (2006). Sifat Kimia dan Dimensi Serat Kayu Mangium (Acacia mangium Willd) dari Tiga Provenans. Journal Tropical Wood Science and Technology, 4, 28–32. [Google Scholar]
207. Krisnawati, H., Kallio, M., Kanninen, M. (2011). Acacia mangium Willd.: Ecology, silviculture and productivity. CIFOR. Bogor, Indonesia. https://play.google.com/books/reader?id=1l_BhXzp3gYC&lr=&printsec=frontcover&pg=GBS.PP1. [Google Scholar]
208. Barry, K. M., Mihara, R., Davies, N. W., Mitsunaga, T., Mohammed, C. L. (2005). Polyphenols in Acacia mangium and Acacia auriculiformis heartwood with reference to heart rot susceptibility. Journal Wood Science, 51(6), 615–621. DOI 10.1007/s10086-005-0707-x. [Google Scholar] [CrossRef]
209. Mihara, R., Barry, K. M., Mohammed, C. L., Mitsunaga, T. (2005). Comparison of antifungal and antioxidant activities of Acacia mangium and A. auriculiformis heartwood extracts. Journal of Chemical Ecology, 31(4), 789–804. DOI 10.1007/s10886-005-3544-x. [Google Scholar] [CrossRef]
210. Kalsom, Y. U., Khairuddin, H. I., Zakri, M. M. (2001). Flavonol glycosides from the leaves of Acacia mangium and related species. Malaysian Journal of Analytical Science, 7, 109–112. [Google Scholar]
211. Lawal, I. O., Uzokwe, N. E., Igboanugo, A. B. I., Adio, A. F., Awosan, E. A. et al. (2010). Ethno medicinal information on collation and identification of some medicinal plants in Research Institutes of South−West Nigeria. African Journal Pharmacy Pharmacology, 4(1), 1–7. [Google Scholar]
212. Chen, X., Xiong, J., He, Q., Wang, F. (2019). Characterization and potential antidiabetic activity of proanthocyanidins from the barks of Acacia mangium and Larix gmelinii. Journal of Chemistry, 2019, 1–9. DOI 10.1155/2019/4793047. [Google Scholar] [CrossRef]
213. Yuniar, I., Darmanto, W., Soegianto, A. (2017). Effect of saponin-Pods Extract Acacia (Acacia mangium) to Hematocrit, Hemoglobin at Tilapia (Oreochromis niloticus). In: Proceeding The 1st IBSC: Towards the Extended Use of Basic Science for Enhancing Health, Environment, Energy and Biotechnology, pp 67–69. Jember, Indonesia. https://jurnal.unej.ac.id/index.php/prosiding/article/view/4138/3831. [Google Scholar]
214. Chibber, S. S., Khera, U. (1978). Dalbinol−a new 12a−hydroxyrotenoid from Dalbergia latifoliaseeds. Phytochemistry, 17(8), 1442–1443. DOI 10.1016/S0031-9422(00)94613-2. [Google Scholar] [CrossRef]
215. Rastogi, R. P., Malhotra, B. N. (1993). Compendium of Indian Medicinal Plants. Lucknow and New Delhi: Central Drug Research Institute, Publications and Information Directorate. [Google Scholar]
216. Thurlough, O., Ceridian, M. O. S., Mary, J. M., Deville, M. X. D. (1981). Lat inone, a phenanthrene−1, 4− Quinone from Dalbergia latifolia. Phytochemistry, 20(5), 1089–1092. DOI 10.1016/0031-9422(81)83033-6. [Google Scholar] [CrossRef]
217. Yadav, S. K., Nagarathna, P. K. M., Yadav, C. K. (2015). Research article of evaluation of immunomodulatory activity of Dalbergia Latifolia on Swis Albino Mice. Journal of Pharmacy and Biological Sciences, 10(1), 58–64. [Google Scholar]
218. Truong, D., Nguyen, D. H., Thuy, N., Ta, N. T. A., Bui, A. V. et al. (2019). Evaluation of the use of different solvents for phytochemical constituents, antioxidants, and in vitro anti-inflammatory activities of severinia buxifolia. Journal of Food Quality, 2019, 1–9. DOI 10.1155/2019/8178294. [Google Scholar] [CrossRef]
219. Saxena, M., Saxena, J., Nema, R., Singh, D., Gupta, A. (2013). Phytochemistry of medicinal plants. Journal Pharmacognosy Phytochemistry, 1(6), 168–182. [Google Scholar]
 | This work is licensed under a Creative Commons Attribution 4.0 International License,, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |