DOI:10.32604/jrm.2021.012560

| Journal of Renewable Materials DOI:10.32604/jrm.2021.012560 |  |
| Article |
Preparation of Activated Carbon from Durian Rind with Difference Activations and Its Optimization
1Program of Food Process Engineering, Faculty of Agro-Industry, King Mongkut’s Institute of Technology Ladkrabang, 10520, Bangkok, Thailand
2Program of Fermentation in Food industry, Faculty of Agro-Industry, King Mongkut’s Institute of Technology Ladkrabang, 10520, Bangkok, Thailand
3Department of Chemical Engineering, Faculty of Engineering, King Mongkut’s Institute of Technology Ladkrabang, 10520, Bangkok, Thailand
*Corresponding Author: Pornsawan Assawasaengrat. Email: pornsawan.as@kmitl.ac.th
Received: 04 July 2020; Accepted: 23 September 2020
Abstract: Durian rind wastes are an important raw material for activated carbon production due to their renewable sources and low-cost materials. The efficiency of increasing surface area and the quantity of oxygen groups on the surface of activated carbon were studied for the preparation of activated carbon. The preparation of activated carbon has been studied with the different methods as follows: activation by acid, activation by base, hydrothermal and activation by acid, and hydrothermal and activation by base. The results showed that hydrothermal and activation by acid had high iodine number which was chosen to determine the optimum condition for activated carbon preparation. The optimum condition for preparation of durian rind activated carbon was studied by Box-Behnken design. Solid/water ratio, solid/acid ratio and temperature were chosen as the important parameters for achieving the optimum reaction condition. The reaction products were analyzed by iodine number. Based on the results, the optimum condition for preparation of durian rind activated carbon was predicted using RSM. The maximum iodine number of 626.47 mg/g was expected at the optimum condition: solid/water ratio (1:175, g/mL), solid/acid ratio (1:23, g/mL) and temperature (500°C). The preparation of durian rind activated carbon at the optimal condition was carried; the percentages of iodine number achieved (666.73 ± 6 mg/g) were close to the maximum predicted value (666.73 mg/g), thus verifying the model. At the optimum condition, the functional group on surface of durian rind activated carbon was characterized by FT-IR. The result showed that the oxygen content on surface was increased in the form of carbonyl and sulfonyl group.
Keywords: Durian rind; activated carbon; hydrothermal; iodine number
Durian rind is the waste from durian fruit consumption. In durian season, a lot of durian rind waste is produced in Thailand every day. Direct disposal of durian peel will increase the amount of agricultural waste. Therefore, it would be economically attractive if durian peel can be utilized as a raw material to produce an effective activated carbon [1].
Activated carbons are widely used for adsorbing gaseous- and liquid-phase pollutants. In the processing units, they are used for several industrial sectors, such as chemical industries, food- and beverage-processing industries, pharmaceuticals, petroleum, and many manufacturing industries [2–5]. Some of these applications utilize these carbon adsorbents because of their characteristics and surface area.
The high surface area, large porosity, well-developed internal pore structure, and a functional group appear on the surface of AC make it a useful material which has a lot of applications in many areas, but mostly in the environmental area [6,7].
There are two main steps for preparing an activated carbon including carbonization of the raw materials in an inert atmosphere and activation of char in the presence of the appropriate oxidizing agents. By pyrolytic decomposition, most of the non-carbon elements, such as H2 and O2, are removed as the gaseous form in carbonization, giving a carbon with the fixed mass and the structure of rudimentary pore. To increase the diameter of particle pores and to create new pores, the activation step is then used for developing the adsorptive power of the carbonization product [8].
Activation can be conducted by either chemical or physical method. Commonly within the process of chemical activation, carbonization and activation occur at the same time as accommodated by the chemical activating agents, that is, dehydrating agents and oxidants. On the contrary, for physical activation, the precursor carbonization takes place in priority, followed by the activation at high temperatures in the presence of the suitable activating agents such as CO2 or steam. It has been found that the physical activation process usually emerges at a temperature higher than the chemical activation process [9].
The precursor-material impregnation using chemical agents such as FeCl3, ZnCl2, H3PO4, and KOH can restrain the formation of tar and reduce the amount of the volatile-matter evolution also, achieving the high precursor-to-carbon conversion [10–14]. So, the chemical activation process can adjust the evolution of a porous structure. Zinc chloride (ZnCl2), among the chemical activating agents, is the chemical which is the most often applied in the preparation of activated carbon; however, it causes the environmental problems. Furthermore, activated carbons using zinc chloride are not proper in pharmaceutical and food industries due to its feasibility to contaminate products. Another more likeable agent, KOH has been claimed to be the most effective alkali salt for activated carbons production [9]. Nevertheless, acidic activation of activated carbon has been associated with oxide structures, which are part of the chemisorbed oxygen found on carbon surfaces, which have been exposed to air or other oxidizing media [15]. In addition to apply of strong acid oxidizers such as phosphoric acid [16] and sulfuric acid [17], other oxidization agents such as hydrogen peroxide, oxygen, and acetic acid are also used to increase the acidic functional groups [12].
This study aimed to determine the good durian rind activated carbon preparation method and the physical and chemical activation processes of activated carbon derived from durian peel. The response surface methodology (RSM) was used for optimization of durian rind activated carbon preparation parameters including solid/water ratio, solid/acid ratio and temperature.
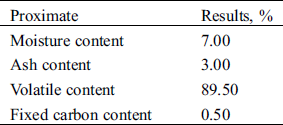
Durian rinds were collected from local markets in Bangkok, Thailand, washed many times with distilled water in order to remove the dust and other inorganic impurities. After that, it was cut into approximately 1 cm × 1 cm size and dried at 60–80°C for 24 hrs to reduce its moisture content. The dried durian rind was grounded in the hammer mill and then stored in desiccators to prevent it from moisture. Potassium hydroxide (KOH; ACS reagent) and sulfuric acid (H2SO4; ACS reagent) were purchased from UNILAB (Thailand) and LAB SCAN (Thailand), respectively. The proximate analysis of durian rind which was conducted according to ASTM standard E 870-82 was given in Tab. 1.
Table 1: Proximate analysis of the durian rind
2.2 Activated Carbon Preparation
Firstly, the durian rind biochar was prepared, which was put into the furnace, then was heat at 400°C for 1 hr. Secondly, the durian rind biochar was activated by the four different methods including activation by acid (A1), activation by base (A2), hydrothermal and activation by acid (A3), and hydrothermal and activation by base (A4).
For the activation by acid and base, the charcoal was chemically activated by H2SO4 or KOH at the several ratios, then washed with DI water until the pH value was 5.5–6.0. The washed charcoal was carbonized in the furnace at the several temperatures for 1 hr.
For hydrothermal and activation by acid and base, the charcoal was immersed in DI water with the several ratios and then transferred to the thermal reactor, which was heated at 110°C for 24 hr. After that, it was activated by H2SO4 or KOH at the several ratios, then washed with DI water until the pH value was 5.5–6.0. The washed charcoal was carbonized in the furnace at the several temperatures for 1 hr.
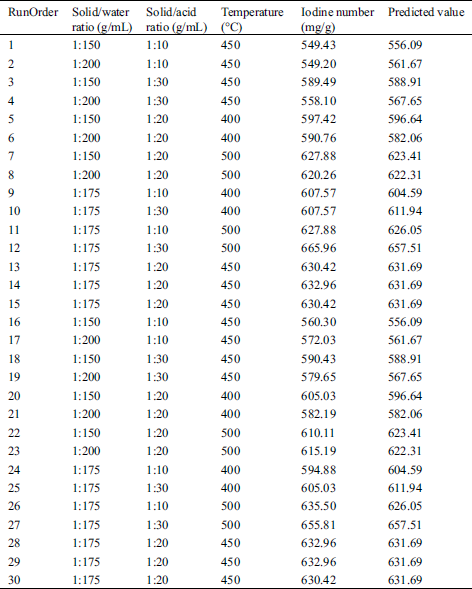
In this study, the optimization of the preparation of durian rind activated carbon was applied by Box-Behnken design (BBD) technique under response surface methodology (RSM), which is a collection of mathematical and statistical techniques for designing experiments, building models, and evaluating the effects of factors. This method allows evaluating not only the main factors affecting the preparation of durian rind activated carbon, but also the interaction between these factors. The complete model is based on the simultaneous variation of 3 factors at 3 levels with 30 experiments. The independent variables reaction, i.e., solid/water ratio, solid/acid ratio, and temperature, were coded with the low level as –1 and the high levels as +1 in BBD and the results with the experimental conditions were shown in Tab. 2. The response measured was the lignin degradation%. The software MINITAB version 16.0 was used for the experimental design, data analysis, quadratic model buildings, and graph plotting.
Table 2: Box-Benhken design of activated carbon preparation using hydrothermal and activation by acid method
The surface area, pore volume and average pore diameter of the activated carbon were determined by nitrogen adsorption-desorption measurements (Autosorb IQ, Quantachrome). The crystalline structure of the durian rind and the activated carbon were characterized by X-ray diffraction (XRD 6100, Shimadzu) using Cu Kα radiation (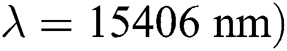 with an anode current of 30 mA and an accelerating voltage of 40 kV. The functional group of the durian rind and the activated carbon were analyzed by Fourier-transform infrared spectroscopy (IRPrestige-21, Shimadzu). The morphological structure of the durian rind and the activated carbon were determined using scanning electron microscopy (Quanta 250, ThermoFisher).
with an anode current of 30 mA and an accelerating voltage of 40 kV. The functional group of the durian rind and the activated carbon were analyzed by Fourier-transform infrared spectroscopy (IRPrestige-21, Shimadzu). The morphological structure of the durian rind and the activated carbon were determined using scanning electron microscopy (Quanta 250, ThermoFisher).
3.1 Activation Preparation Screening
The durian rind biochar was activated by the different methods which were divided into 4 methods including activation by acid (A1), activation by base (A2), hydrothermal and activation by acid (A3), and hydrothermal and activation by base (A4). The iodine number is targeted as to choose the activation method for optimization as shown in Tab. 3. The results showed that the highest values obtained from iodine number for A3 is 198.21 mg/g which reflects the iodine adsorption capacity of A3 in microporous. Slightly lower value of 162.78 mg/g was obtained for A4. Thus, hydrothermal and activation by acid was chosen to optimization.
Table 3: Iodine number of durian rind activated carbon with the different preparation methods

3.2 Response Surface Methodology for the Preparation of Durian Rind Activated Carbon Optimization
The Box-Behnken design (BBD) with three factors at three levels, as well as the results of the preparation of durian rind activated carbon were shown in Tab. 2. An approximate regression model of the preparation of durian rind activated carbon based on the experimental results was evaluated and expressed by the following polynomial equation:
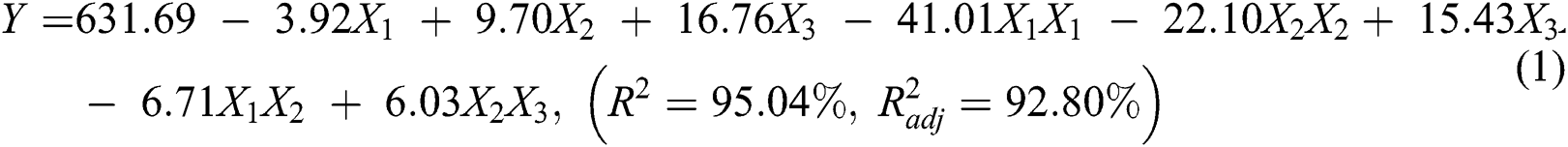
In Eq. (1), Y is the lignin degradation efficiency; X1, X2, and X3 are the corresponding coded variables of solid/water ratio (g/mL), solid/acid ratio (g/mL), and temperature (°C), respectively.
One of the purposes of the experimental design was to allow a simple and reliable model efficient of relating to directly response to the most significant variables. In Eq. (1), a factor was considered significant if the p-value was lower than 0.05, meaning that the probability of noise causing the correlation between a factor and the response is lower than 0.05. The insignificant factors were eliminated using backward elimination, and the significant factors were kept modelling the data.
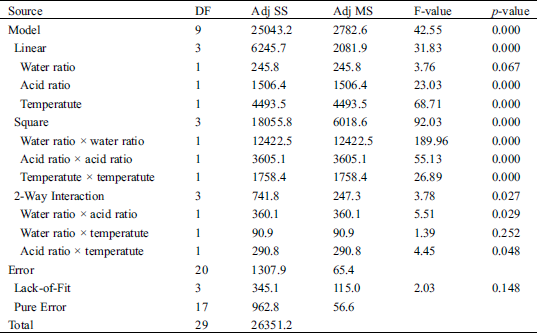
The accuracy of model fit was verified by the coefficient of determination R2, which was 0.9504, indicating that 0.9504 of the variability in the response could be described by the model. The statistical significance of this model equation was assessed by the F-test for ANOVA (Tab. 4). The F-value of 2.03 for the model indicated that the model was significant. There is only a 0.01% chance that the model F-value could take place due to noise. The p-value is also very low (p < 0.05), indicating the significance of the model. Values of “Prob > F” less than 0.05 showed that model terms were significant. The lack of fit p-value of 0.148 is higher than 0.05, indicating that there is no lack of fit in the model at 95% level of significance [18,19].
Table 4: ANOVA of the regression model for durian rind activated carbon preparation using hydrothermal and activation by acid method
Adequacy check of the presented model is an important part of the analysis. Good adequacy can endorse that the approximating model allows an adequate estimation to the real system; however, it may give poor or misleading results [20–23]. The diagnostic plots were applied to approximate the adequacy of regression model fitted as shown in Fig. 1. The actual and the predicted of the preparation of durian rind activated carbon plots are shown in Fig. 1. The actual preparation of durian rind activated carbon is the assessed value for a run, and the predicted value is assessed from the model. In experiment designs, R2 is a measure of the amount of reduction in the variability of the response achieved by using the independent variables in the model. A large value of R2 does not always indicate that the regression model is a good one. On the other hand, R2adj was selected to determine the fit of a regression model as it does not always increase when variables are added. A good agreement was achieved between the predicted preparation of durian rind activated carbon and the experimental values with R2 and R2adj of 0.9504, and 0.9280, respectively, as shown in Fig. 1, which suggested that the proposed model had adequate approximation to the actual value.
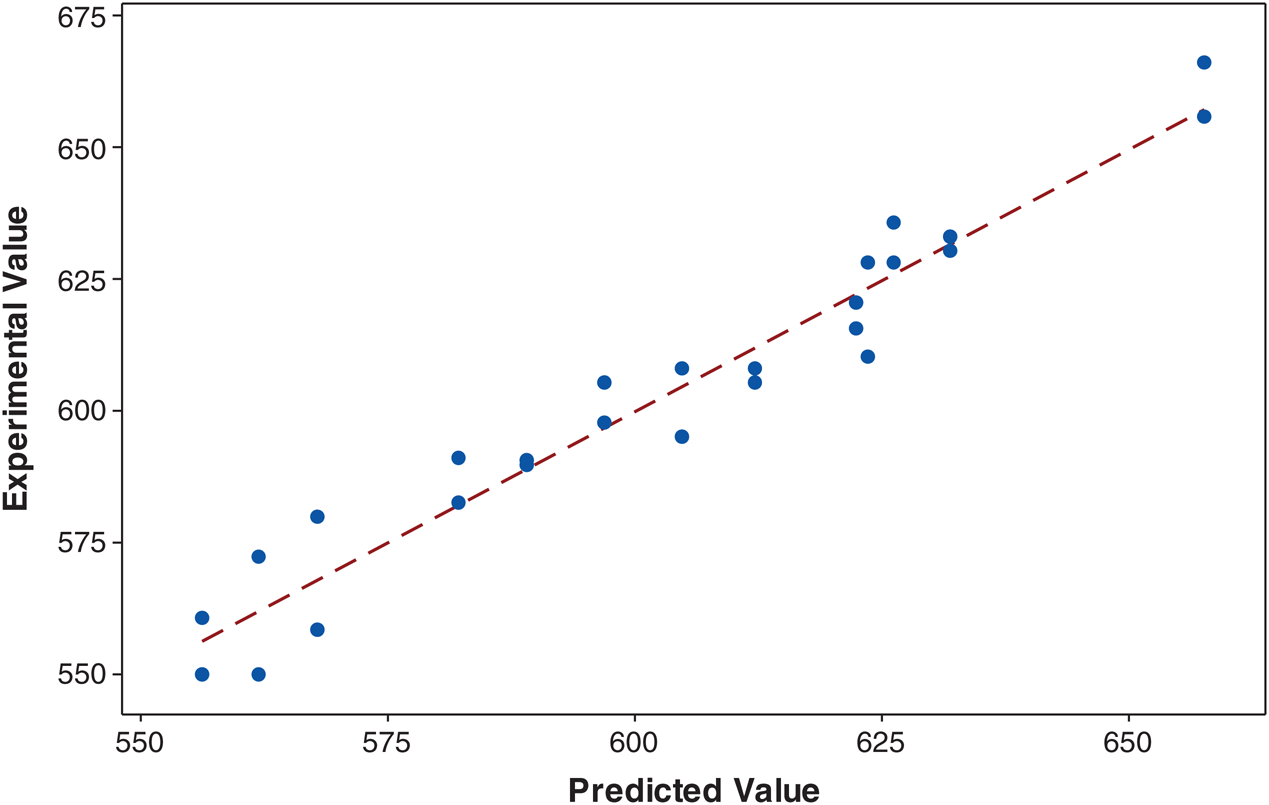
Figure 1: The experimental values (%) plotted against the predicted values (%) derived from model of durian rind activated carbon preparation using hydrothermal and activation by acid method
As shown in Fig. 2, the residual plots were applied to assess that the model satisfied the hypothesis made for ANOVA, which in the response model was examined against four analyses. The normal probability plot (Fig. 2a) demonstrates that the experiments data was distributed on the strength line, implying that the normality hypothesis was satisfied. In Fig. 2b, the residual versus the fit plot scattered randomly. It can be seen that the data appeared to show the relatively constant variance across the predicted values. The histogram chart was exhibited in Fig. 2c suggest that the histogram has structure as the bell overturn. It is suggested that there was no apparent problem of the data with the normal curve. Fig. 2d shows the residual versus order plot suggesting the random scatters fluctuated around the center line (in the range of ± 2). These results indicate that the experiment data was distributed very well. It can be considered that the data has the good accuracy and reliability [23–27].
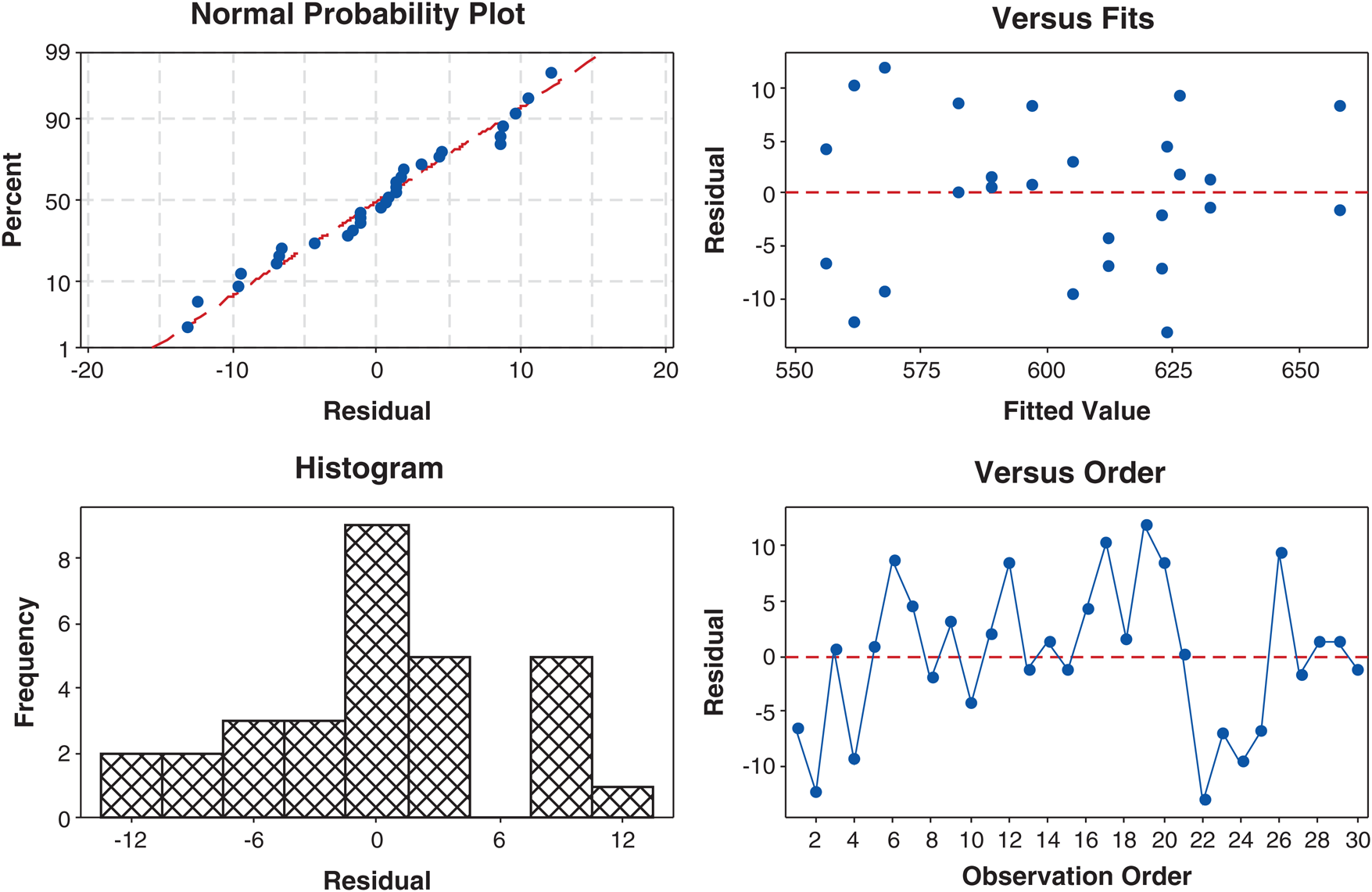
Figure 2: Internal standardized residual plots versus (a) normal probability, (b) fits, (c) histogram, and (d) observation order for iodine number of durian rind activated carbon preparation using hydrothermal and activation by acid method
3.3 Factors Affecting the of the Preparation of Durian Rind Activated Carbon
As shown in Fig. 3a, the effects of solid/water ratio for hydrothermal durian rind biochar at 1:150, 1:175, and 1:200 g/mL on iodine number showed that solid/water ratio effect was insignificant. The efficiency of iodine number increased from 590 to 629 mg/g when the solid/water ratio increased from 1:150 g/mL to 1:175 g/mL, respectively. The iodine number could be enhanced by increasing the solid/water ratio. The carbonized biomass appeared to have been carbonized into the spherical particles. Cui et al. also showed that the hydrothermal treatment did not destroy the underlying morphologies. However, Roman et al. showed that the underlying morphologies of biomass largely remained unaffected by a hydrothermal treatment [16].
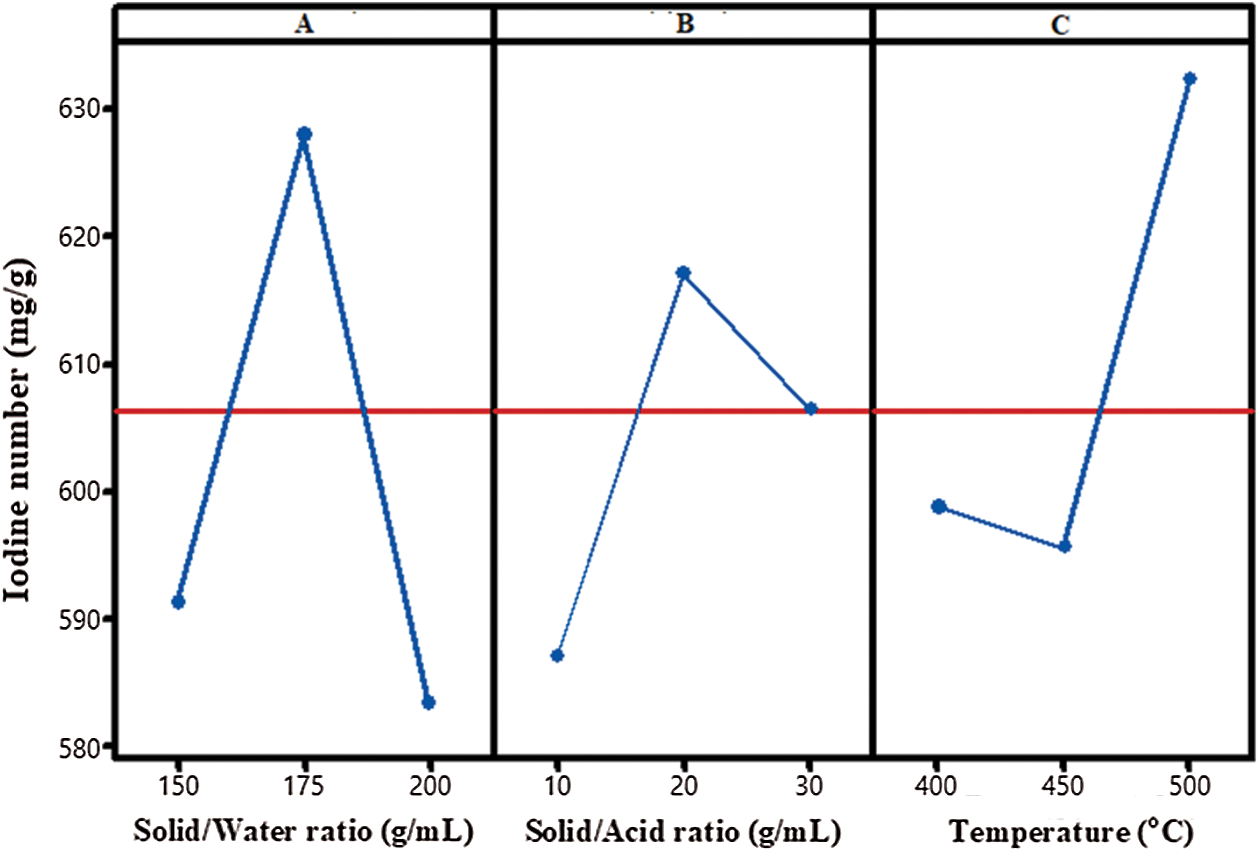
Figure 3: Plot of main effects (a) Solid/Water ratio (b) Solid/Acid ratio and (c) Temperature for iodine number of durian rind activated carbon preparation using hydrothermal and activation by acid method
As shown in Fig. 3b, the solid/acid ratio is another important operating parameter affecting the iodine number in the activated durian rind biochar. The solid/acid ratio was exhibited significant as the main effect in ANOVA analysis, it influenced other parameters. The solid/acid ratio directly affected the surface area and the oxygen content on durian rind activated carbon, which related to the porosity development due to the formation of sulfate linkages such as sulfate and poly-sulfate esters which can help to connect and crosslink the biopolymers. These linkages expand the structure of the durian rind and sulfate compounds retained in the expanded structure and hinder the shrinkage at high-temperature treatment [15]. The solid/acid ratio on iodine number was measured at the different initial solid/acid ratios (1:10, 1:20, and 1:30 g/mL), which demonstrations the mentioned effect on the iodine number (Fig. 3b). When solid/acid ratio was increased from 1:10 to 1:20 g/mL, the iodine number increased from 590 to 620 g/mL. Thus, the oxygen content can be showed in the carbonyl and sulfonyl form. In a study, the highest iodine number was obtained at the solid/acid ratio of 1:20 by hydrothermal and activation by acid method.
The effects of temperature on iodine number of activated carbon preparation are shown in Fig. 3(c). When the temperature was increased from 450 to 500°C, the iodine number was increased between 595 to 630 g/mg. These results related to the increase of heating duration which proved to enhance the development of micropores and the total volume because longer duration has the possibility for generation of sample average pore diameter micropore surface area micropore volume. With the increasing activation temperatures, the total volume of new micropores and mesopores in the carbon was more obtained. When the activation temperature was increased to 500°C, a larger total volume and the micropore volume is observed as compared to activated carbons prepared from 400°C [28].
Fig. 4 presents the response surface modeling in a three-dimensional (3D) representation reflecting the interactive effects of the solid/water ratio and the solid/acid ratio on the iodine number of activated carbons. As can be seen, water possesses a high ionization constant at high temperatures and is responsible for hydrolysis of organics which can further be catalyzed by acids [29–32]. However, the activated carbons synthesized under the same carbonization condition (either N2 atmospheric or vacuum pyrolysis), those treated with HCl solution had greater BET surface areas and pore volumes than those treated with H2O. An increase in these properties of activated carbons treated with HCl solution is likely to be due to the removal of impurities on the surface and/or in the pores. Acid solution is generally used to purify activated carbon after the synthesis [33].
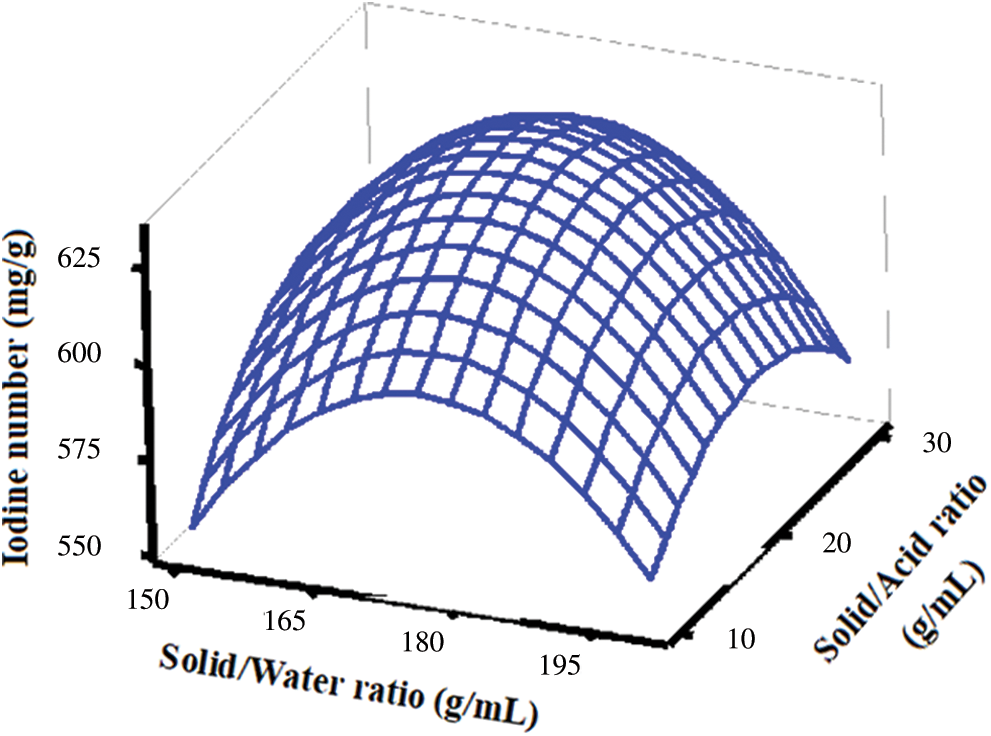
Figure 4: Interaction effects of solid/water ratio and solid/acid ratio for iodine number of durian rind activated carbon preparation using hydrothermal and activation by acid method
3.4 The Optimum Condition and Model Verification
The performance of activated carbon preparation using hydrothermal and activation by acid technique can be evaluated in term of iodine number efficiency, which largely varies with the change and the interactions in variables. Thus, the accommodations between the variables were created and optimized depending on the response from the model for economic motivation. Accordingly, if the iodine number was set at the assumed high iodine number (666.73 mg/g) as the target criteria, the optimum condition of activated carbon preparation was 1:175 g/mL of solid/water ratio 1:23, g/mL of solid/acid ratio and 500°C of temperature. At the optimum condition, the iodine number could be obtained as 666.73 ± 6 mg/g in triplicate actual experiments. The optimum condition showed that the predicted values were closer to the experimental values. Thus, the results confirmed the suitability of prediction model for the iodine number of activated carbons.
3.5 Characterization of Durian Rind Activated Carbon
The A1, A2, A3, and A4 were prepared by the different techniques from Section 3.1. The iodine adsorption method was investigated for studying the microporous in activated carbon [34]. The iodine adsorption capacity of different activated carbons was shown in Tab. 3. The highest values obtained from iodine number for A3 is 198.21 mg/g which reflects the iodine adsorption capacity of A3 in microporous. Slightly lower value of 162.78 mg/g was obtained for A4 since the presence of sulfuric acid promotes the hydrolysis and decomposition of oligomer and monomer to smaller fragments [35].
The BET surface area of the activated carbon (A4) was 331.02 m2/g while the commercial activated carbon (CarboTech) was 900 m2/g [36]. The total pore volumes and pore diameter of the activated carbon (A4) were 0.685 cm3/g and 0.008 µm respectively. The adsorption isotherm of the activated carbon was shown in Fig. 5.
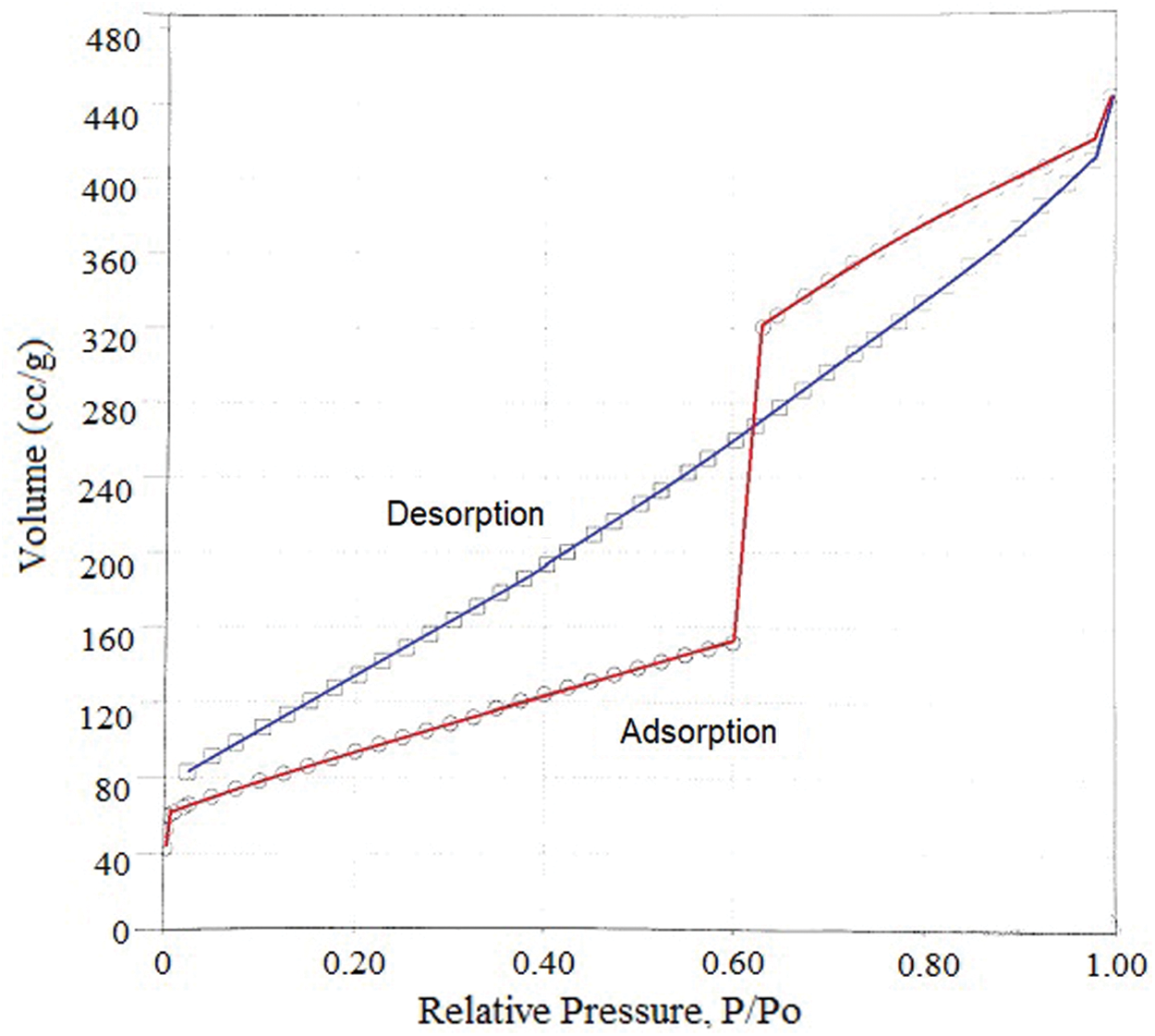
Figure 5: The adsorption isotherm of the activated carbon
The crystallinity of durian rind raw material and activated carbon were studied by XRD. As shown in the Figs. 6a and 6b, both samples are the same pattern. The XRD pattern showed two broad peaks at approximate 24° and 42° attributed to turbostratic carbon or disorder carbon (amorphous carbon) [37].
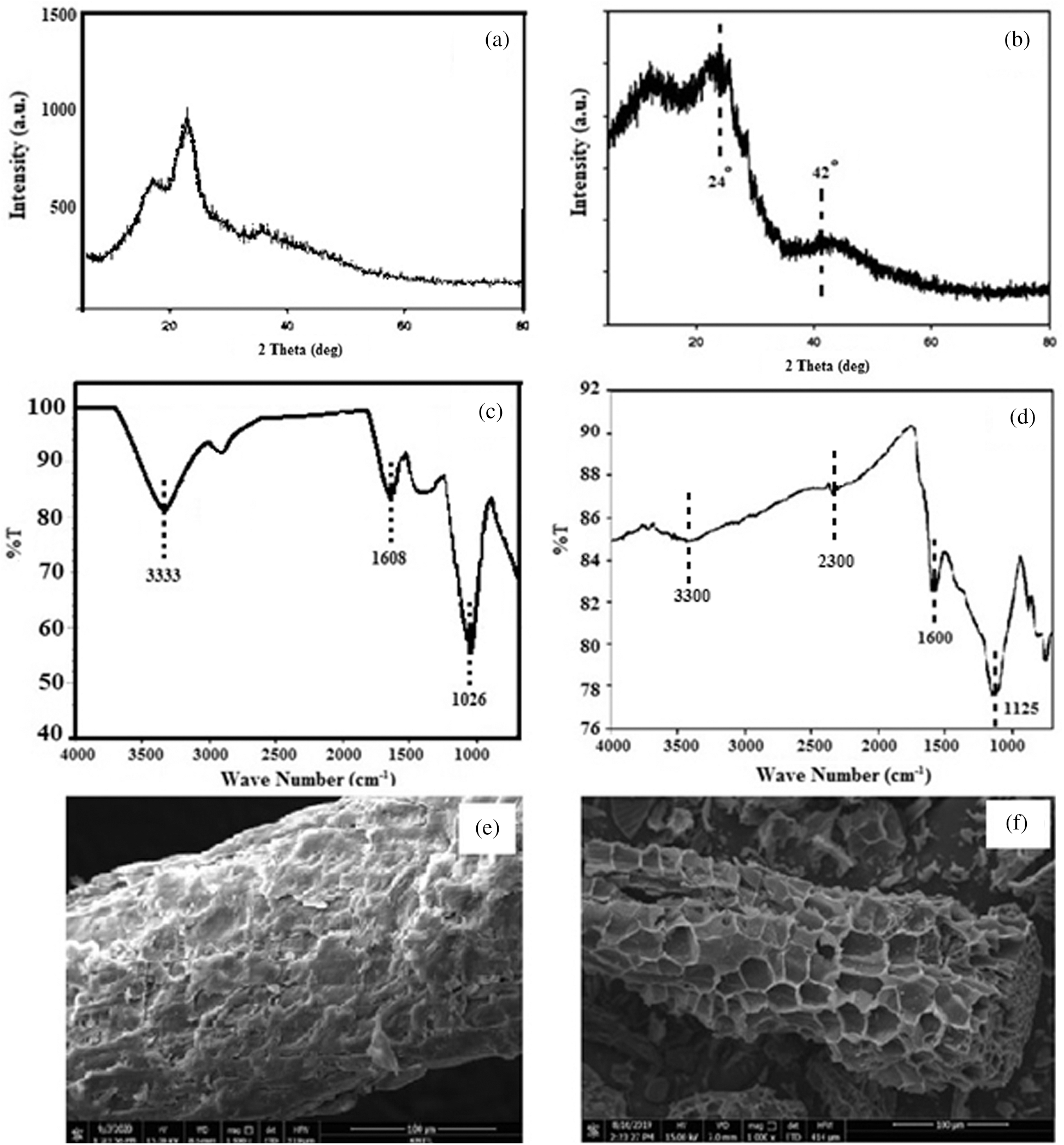
Figure 6: The characterization of durian rind and activated carbon (a) XRD pattern of durian rind, (b) XRD pattern of activated carbon, (c) FTIR spectra of durian rind, (d) FTIR spectra of activated carbon, (e) SEM images of durian rind and (f) SEM images of activated carbon
The most characteristic vibrations were selected by the FTIR spectra of durian rind and activated carbon. As shown in Figs. 6c and 6d, the strong bands at 3300, 1600 and 1125 cm−1 were assigned to the stretching vibration of the hydroxyl group, carbonyl group and sulfonyl group, respectively. However the FTIR spectra of activated carbon located at 2270–2280 cm−1 which was corresponding to isocyanate group of vibration (N=C=O) [38]. The isocyanate group may be occurred from calcination at high temperature in N2 atmosphere.
The morphology of durian rind and activated carbon was shown in Figs. 6e and 6f. After activation, the activated carbon was more porous than durian rind. It can be seen from the SEM images that the external surface of the activated carbon has the various sizes about 20–30 µm.
Durian rind is a good precursor to produce activated carbons with well-developed surface area and functional group. The durian rind biochar was activated by the different methods which were divided into 4 methods including activation by acid, activation by base, hydrothermal and activation by acid, and hydrothermal and activation by base. The hydrothermal and activation by acid was chosen to optimization which is the highest values obtained from iodine number (198.21 mg/g). The response surface methodology based on 3 variables Box-Behnken design was applied to determine the effects of solid/water ratio (ranging 1:150–1:120 mg/g), solid/acid ratio (ranging 1:10–1:30 mg/g) and temperature (ranging 400–600°C). For each response, the coefficients of the hypothesized model were analyzed based on the experimental responses. The analysis of the responses characterizing the surface area development shows, on one hand, the positive effects of solid/acid ratio and temperature on the iodine number and a negative effect of solid/water ratio on these responses. The R2 values of all factors showed a good fit of the models with the experimental data. Based on the four models obtained, the numerical optimization was performed. The optimum condition was confirmed and fitted the experimental data well. In this optimum condition, the activated carbon shown that the functional group on activated carbon surface are oxygen content including the hydroxyl group, carbonyl group and sulfonyl group, respectively.
Funding Statement: The authors gratefully acknowledgement funding of this research (KREF116101) by King Mongkut’s Institute of Technology Ladkrabang Research Fund, Thailand.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Srikhun, S., Hirunpraditkun, S., Nuithitikul, K. (2009). Adsorption of malachite green dye onto activated carbon derived from durian peel. Proceedings of the 7th IASME/WSEAS International Conference on Heat Transfer, Thermal Engineering and Environment, Moscow, Russia, 106–111. DOI 10.1007/s11270-014-2057-z. [Google Scholar] [CrossRef]
2. Sariol, H. C., Peacok, T. M., Yperman, J., Sauvanell, A. B., Carleer, R. et al. (2016). Characterization of granular activated carbons used in rum production by immersion ‘Bubblemetry’ in a pure liquid. Journal of Food Processing & Beverages, 4(2), 10. [Google Scholar]
3. Nunes, A. A., Franca, A. S., Oliveira, L. S. (2009). Activated carbons from waste biomass: An alternative use for biodiesel production solid residues. Bioresource Technology, 100(5), 1786–1792. DOI 10.1016/j.biortech.2008.09.032. [Google Scholar] [CrossRef]
4. Haw, K. G., Bakar, W. A. W. A., Ali, R., Chong, J. F., Kadir, A. A. A. (2010). Catalytic oxidative desulfurization of diesel utilizing hydrogen peroxide and functionalized-activated carbon in a biphasic diesel–acetonitrile system. Fuel Processing Technology, 91(9), 1105–1112. DOI 10.1016/j.fuproc.2010.03.021. [Google Scholar] [CrossRef]
5. Roy, G. M. (1994). Activated carbon applications in the food and pharmaceutical industries. CRC Press. Technomic Publishing Company, Inc. 851 New Holland Avenue, Box 3535 Lancaster, Pennsylvania 17604 U.S.A. [Google Scholar]
6. Figueiredo, J. L., Pereira, M. F. R., Freitas, M. M. A., Orfao, J. J. M. (1999). Modification of the surface chemistry of activated carbons. Carbon, 37(9), 1379–1389. DOI 10.1016/S0008-6223(98)00333-9. [Google Scholar] [CrossRef]
7. Stavropoulos, G. G., Samaras, P., Sakellaropoulos, G. P. (2008). Effect of activated carbons modification on porosity, surface structure and phenol adsorption. Journal of Hazardous Materials, 151(2–3), 414–421. DOI 10.1016/j.jhazmat.2007.06.005. [Google Scholar] [CrossRef]
8. Hu, Z., Srinivasan, M. P., Ni, Y. (2001). Novel activation process for preparing highly microporous and mesoporous activated carbons. Carbon, 39(6), 877–886. DOI 10.1016/S0008-6223(00)00198-6. [Google Scholar] [CrossRef]
9. Ahmadpour, A., Do, D. D. (1997). The preparation of activated carbon from macadamia nutshell by chemical activation. Carbon, 35(12), 1723–1732. DOI 10.1016/S0008-6223(97)00127-9. [Google Scholar] [CrossRef]
10. Girgis, B. S., El-Hendawy, A. N. A. (2002). Porosity development in activated carbons obtained from date pits under chemical activation with phosphoric acid. Microporous and Mesoporous Materials, 52(2), 105–117. DOI 10.1016/S1387-1811(01)00481-4. [Google Scholar] [CrossRef]
11. Tsai, W. T., Chang, C. Y., Lin, M. C., Chien, S. F., Sun, H. F. et al. (2001). Adsorption of acid dye onto activated carbons prepared from agricultural waste bagasse by ZnCl2 activation. Chemosphere, 45(1), 51–58. DOI 10.1016/S0045-6535(01)00016-9. [Google Scholar] [CrossRef]
12. Toles, C. A., Marshall, W. E., Johns, M. M. (1999). Surface functional groups on acid-activated nutshell carbons. Carbon, 37(8), 1207–1214. DOI 10.1016/S0008-6223(98)00315-7. [Google Scholar] [CrossRef]
13. Njoku, V. O., Foo, K. Y., Asif, M., Hameed, B. H. (2014). Preparation of activated carbons from rambutan (Nephelium lappaceum) peel by microwave-induced KOH activation for acid yellow 17 dye adsorption. Chemical Engineering Journal, 250, 198–204. DOI 10.1016/j.cej.2014.03.115. [Google Scholar] [CrossRef]
14. Rashid, R. A., Jawad, A. H., Ishak, M. A. B. M., Kasim, N. N. (2018). FeCl3-activated carbon developed from coconut leaves: Characterization and application for methylene blue removal. Sains Malaysiana, 47(3), 603–610. DOI 10.17576/jsm-2018-4703-22. [Google Scholar] [CrossRef]
15. Ismail, A., Sudrajat, H., Jumbianti, D. (2010). Activated carbon from durian seed by H3PO4 activation: Preparation and pore structure characterization. Indonesian Journal of Chemistry, 10(1), 36–40. DOI 10.22146/ijc.21495. [Google Scholar] [CrossRef]
16. Hao, W., Björkman, E., Lilliestrale, M., Hedin, N. (2014). Activated carbons for water treatment prepared by phosphoric acid activation of hydrothermally treated beer waste. Industrial & Engineering Chemistry Research, 53(40), 15389–15397. DOI 10.1021/ie5004569. [Google Scholar] [CrossRef]
17. Karagöz, S., Tay, T., Ucar, S., Erdem, M. (2008). Activated carbons from waste biomass by sulfuric acid activation and their use on methylene blue adsorption. Bioresource Technology, 99(14), 6214–6222. DOI 10.1016/j.biortech.2007.12.019. [Google Scholar] [CrossRef]
18. Sriprom, P., Neramittagapong, S., Lin, C., Wantala, K., Neramittagapong, A. et al. (2015). Optimizing chemical oxygen demand removal from synthesized wastewater containing lignin by catalytic wet-air oxidation over CuO/Al2O3 catalysts. Journal of the Air & Waste Management Association, 65(7), 828–836. DOI 10.1080/10962247.2015.1023908. [Google Scholar] [CrossRef]
19. Sriprom, P., Lin, C., Neramittagapong, A., Neramittagapong, S. (2016). Investigation of important parameters for lignin degradation using fenton-like reaction via Cu doped on bagasses-MCM-41. In: Parinov, I., Chang, S. H., Topolov, V. (eds.Springer Proceedings in Physics: Vol 175. Advanced Materials. Springer, Azov, Russia, 115–127. DOI 10.1007/978-3-319-26324-3_9. [Google Scholar] [CrossRef]
20. Khataee, A. R., Dehghan, G. (2011). Optimization of biological treatment of a dye solution by macroalgae Cladophora sp. using response surface methodology. Journal of the Taiwan Institute of Chemical Engineers, 42(1), 26–33. DOI 10.1016/j.jtice.2010.03.007. [Google Scholar] [CrossRef]
21. Papadopoulou, K., Kalagona, I. M., Philippoussis, A., Rigas, F. (2013). Optimization of fungal decolorization of azo and anthraquinone dyes via Box-Behnken design. International Biodeterioration & Biodegradation, 77, 31–38. DOI 10.1016/j.ibiod.2012.10.008. [Google Scholar] [CrossRef]
22. Salman, J. M. (2014). Optimization of preparation conditions for activated carbon from palm oil fronds using response surface methodology on removal of pesticides from aqueous solution. Arabian Journal of Chemistry, 7(1), 101–108. DOI 10.1016/j.arabjc.2013.05.033. [Google Scholar] [CrossRef]
23. Sriprom, P., Champa, V., Kitchaiya, P., Assawasaengrat, P. (2018). Optimizing decolorization efficiency of methylene blue by photo-fenton process over fe-diatomite using central composite design. In: Friedl, A., Klemeš, J. J., Radl, S., Varbanov, P. S., Wallek, T. (eds.Computer Aided Chemical Engineering. Elsevier, Graz, Austria, vol. 43, 409–414. DOI 10.1016/B978-0-444-64235-6.50074-7. [Google Scholar] [CrossRef]
24. Sivalingam, S., Sen, S. (2018). Optimization of synthesis parameters and characterization of coal fly ash derived microporous zeolite X. Applied Surface Science, 455, 903–910. DOI 10.1016/j.apsusc.2018.05.222. [Google Scholar] [CrossRef]
25. Ismail, M. N., Aziz, H. A., Ahmad, M. A., Yusoff, N. A. (2015). Optimization of areca catechu fronds as adsorbent for decolorization and cod removal of wastewater through the adsorption process. Sains Malaysiana, 44(11), 1609–1614. [Google Scholar]
26. Uma, D. B., Ho, C. W., Wan Aida, W. M. (2010). Optimization of extraction parameters of total phenolic compounds from henna (Lawsonia Inermis) leaves. Sains Malaysiana, 39(1), 119–128. [Google Scholar]
27. Yap, C. F., Ho, C. W., Wan Aida, W. M., Chan, S. W., Lee, C. Y. et al. (2009). Optimization of extraction conditions of total phenolic compounds from star fruit (Averrhoa Carambola L.) residues. Sains Malaysiana, 38(4), 511–520. [Google Scholar]
28. Jun, T. Y., Arumugam, S. D., Latip, N. H. A., Abdullah, A. M., Latif, P. A. (2010). Effect of activation temperature and heating duration on physical characteristics of activated carbon prepared from agriculture waste. Environment Asia, 3, 143–148. [Google Scholar]
29. Titirici, M. M., White, R. J., Falco, C., Sevilla, M. (2012). Black perspectives for a green future: Hydrothermal carbons for environment protection and energy storage. Energy & Environmental Science, 5(5), 6796–6822. DOI 10.1039/c2ee21166a. [Google Scholar] [CrossRef]
30. Libra, J. A., Ro, K. S., Kammann, C., Funke, A., Berge, N. D. et al. (2011). Hydrothermal carbonization of biomass residuals: A comparative review of the chemistry, processes and applications of wet and dry pyrolysis. Biofuels, 2(1), 71–106. DOI 10.4155/bfs.10.81. [Google Scholar] [CrossRef]
31. Bobleter, O. (1994). Hydrothermal degradation of polymers derived from plants. Progress in Polymer Science, 19(5), 797–841. DOI 10.1016/0079-6700(94)90033-7. [Google Scholar] [CrossRef]
32. Titirici, M. M., Thomas, A., Yu, S. H., Müller, J. O., Antonietti, M. (2007). A direct synthesis of mesoporous carbons with bicontinuous pore morphology from crude plant material by hydrothermal carbonization. Chemistry of Materials, 19(17), 4205–4212. DOI 10.1021/cm0707408. [Google Scholar] [CrossRef]
33. Nuithitikul, K., Srikhun, S., Hirunpraditkoon, S. (2010). Influences of pyrolysis condition and acid treatment on properties of durian peel-based activated carbon. Bioresource Technology, 101(1), 426–429. DOI 10.1016/j.biortech.2009.07.040. [Google Scholar] [CrossRef]
34. Bestani, B., Benderdouche, N., Benstaali, B., Belhakem, M., Addou, A. (2008). Methylene blue and iodine adsorption onto an activated desert plant. Bioresource Technology, 99(17), 8441–8444. DOI 10.1016/j.biortech.2008.02.053. [Google Scholar] [CrossRef]
35. Jain, A., Balasubramanian, R., Srinivasan, M. P. (2016). Hydrothermal conversion of biomass waste to activated carbon with high porosity: A review. Chemical Engineering Journal, 283, 789–805. DOI 10.1016/j.cej.2015.08.014. [Google Scholar] [CrossRef]
36. Abdulsalam J., Mulopo J., Oboirien B., Bada S., Falcon R. (2019). Experimental evaluation of activated carbon derived from South Africa discard coal for natural gas storage. International Journal of Coal Science & Technology, 6(3), 459–477. DOI 10.1007/s40789-019-0262-5. [Google Scholar] [CrossRef]
37. Tsubouchi, N., Xu, C., Ohtsuka, Y. (2003). Carbon crystallization during high-temperature pyrolysis of coals and the enhancement by calcium. Energy & Fuels, 17(5), 1119–1125. DOI 10.1021/ef020265u. [Google Scholar] [CrossRef]
38. Zhang, J., Hori, N., Takemura, A. (2020). Influence of NCO/OH ratio on preparation of four agricultural wastes liquefied polyols based polyurethane foams. Polymer Degradation and Stability, 179, 109256. DOI 10.1016/j.polymdegradstab.2020.109256. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |