DOI:10.32604/jrm.2021.011081

| Journal of Renewable Materials DOI:10.32604/jrm.2021.011081 |  |
| Article |
Melt Extrusion of Environmentally Friendly Poly(L-lactic acid)/Sodium Metabisulfite Films for Antimicrobial Packaging Applications
1Department of Materials Engineering, Federal University of São Carlos, São Carlos, Brazil
2Department of Food Engineering, Regional Integrated University of High Uruguay and Missions (URI), Erechim, Brazil
*Corresponding Author: Francys K. V. Moreira. Email: francys@ufscar.br
Received: 13 July 2020; Accepted: 04 September 2020
Abstract: Food packaging materials compounded with antimicrobial additives can substantially diminish the incidence of foodborne diseases. Here, poly(L-lactic acid) (PLA) films containing sodium metabisulfite (NaM) were produced by melt extrusion as an attempt to develop a new biodegradable material with antimicrobial properties for packaging. Life cycle assessment (LCA) simulations revealed that the environmental footprints of the PLA film did not change upon NaM addition, and that NaM is more eco-friendly than silver nanoparticles. The PLA/NaM films with NaM content varying from 0.5 to 5.0 wt.% were characterized by differential scanning calorimetry (DSC), thermogravimetric analysis (TGA), and optical and mechanical properties determinations. The optical properties were sustained after the addition of NaM, but high NaM contents degraded the light transparence of the PLA matrix at some extent. The thermal stability and tensile properties of the PLA film decreased proportionally to the NaM content, while no changes were observed on Tg, Tm and Xc, as determined by DSC. Agar diffusion tests revealed that the PLA/NaM films had no antimicrobial activity on Saccharomyces cerevisiae at 35°C, which was related to the limited migration of NaM from the glassy PLA matrix. The biodegradable PLA films compounded with NaM through melt extrusion display adequate optical, thermal, and mechanical properties to cover most food packaging applications, representing an essential step toward the development of eco-friendly packaging materials that can potentially exhibit an antimicrobially active surface.
Keywords: Biobased packaging; antimicrobial activity; life cycle assessment
Antimicrobial plastic packaging received much attention recently due to its potential for decreasing the incidence of foodborne diseases [1,2]. These innovative technologies have been developed by compounding antimicrobial active substances with polymers used as primary packaging to inhibit microbial growth in foods generally through a contact-migration mechanism [3]. The most used antimicrobial agents include organic salts [3], silver nanoparticles (AgNPs) [4], bacteriocins [5], essential oils [6], and so on. Furthermore, the use of biodegradable polymers in antimicrobial packaging could potentially lead to more eco-friendly packaging systems, and thus a reduced usage of non-biodegradable plastics in the food industry [7].
Poly(lactic acid) (PLA) is a biodegradable polymer with adequate characteristics for food packaging. PLA is a semicrystalline polyester obtained by ring-opening polymerization of lactic acid, which can be produced from corn starch, sugarcane, and other renewable plant-based feedstocks [8]. As a packaging material, PLA exhibits suitable mechanical and gas barrier properties, high optical transparency, in addition to being a thermoplastic [8,9].
Antimicrobial packaging films based on PLA were previously explored by incorporating AgNPs to inhibit the growth of E. coli and S. aureus [10], nisin to kill L. monocytogenes strains [11], nanoclays impregnated with Ag+ ions to inhibit Salmonella [12], and propolis extract to inhibit S. aureus, E. coli and P. aeruginosa [13]. Despite these effective antimicrobial properties, all the films were produced by the solvent casting technique, indicating that the development of antimicrobial PLA-based packaging through melt processing routes is a less explored topic. PLA films containing lactic acid (5 to 10 wt.%) and sodium lactate (15 to 30 wt.%) were developed by the extrusion film-blowing process and found to inhibit the growth of L. monocytogenes. The addition of lactic acid also increased the stiffness of the PLA films, making them less suitable for flexible packaging, for instance [14]. PLA-based composites containing microcrystalline cellulose (MCC) and 1 wt.% AgNPs were prepared by twin-screw extrusion followed by injection molding. The composites presented bactericidal effect against S. aureus and higher tensile strength than the neat PLA [15]. More recently, PLA was extruded with 1% potassium aluminum sulfate dodecahydrate (ALUM) to produce antimicrobial foams. The foams had antimicrobial activity on S. aureus and E. coli due to their porous microstructure, which boosted the ALUM release rate [16].
Sodium metabisulfite (Na2S2O5, NaM) is a food preservative with potential for antimicrobial packaging applications due to its well-known antioxidant properties and wide antimicrobial activity [17]. NaM is a commercially available, water-soluble, cheap additive with various applications in the pharmaceutical and food industries. Furthermore, NaM is approved as a generally recognized as safe (GRAS) additive by the Food and Drug Administration (FDA) at the currently used levels [18]. NaM has been included as a coating additive or SO2-emitter in packaging systems to inhibit fungal and bacterial proliferation in fresh foods [17,19,20]. However, to the best of our knowledge, there is no information regarding the use of NaM in antimicrobial packaging based on melt processed PLA.
Herein, we report on the incorporation of NaM in biodegradable PLA films by melt extrusion aiming at antimicrobial packaging applications. Importantly, the compounding and film casting processes were carried out in a single thermal cycle to minimize thermal degradation effects on PLA and NaM. To extend the sustainable perspective, we first applied the life cycle assessment (LCA) methodology to estimate some environmental footprints (EFPs) of the PLA/NaM films and performed a comparison with their counterparts loaded with AgNPs, which is one of the most used biocidal agents in polymers. The disc diffusion test was used to assess the antimicrobial activity of the PLA/NaM films on Saccharomyces cerevisiae, given the fungicide potential of NaM [21]. By varying the NaM content, the optical, mechanical, and thermal properties of the PLA/NaM films were evaluated, which are of prime interest for food packaging applications.
Poly(L-lactic acid) with ~4% D-isomer content (Ingeo™ Biopolymer 2003D) was acquired from NatureWorks (Blair, Nebraska, USA). The polymer was dried at 60°C for 72 h in an air circulating oven prior to use. Sodium metabisulfite (97%) was purchased from Synth (Diadema, São Paulo, Brazil).
2.2 Melt Extrusion of PLA/NaM Films
The PLA/NaM films were produced by hot-melt extrusion combined with a flake-like masterbach preparation approach. First, 5 g of PLA pellets were vigorously mixed with precise amounts of NaM and were thermo-pressed at 150°C and 3 ton for 1 min. The resulting masterbach was cut into smalls flakes (0.5 × 0.5 cm) and dried overnight at 50°C in an air-circulating oven. The NaM concentration in the masterbatch was varied to result in films with final NaM contents of 0.5 wt.%, 1.0 wt.%, 2.5 wt.% and 5.0 wt.%, after compounding with pure PLA through extrusion.
The extrusion of the PLA/NaM formulations was carried out with a MP19-TC co-rotating twin-screw extruder (L/D = 25, D = 19 mm, B&P Process Equipament and Systems) coupled to a 220 mm-wide rectangular sheet die. The temperature profile was 180–150–150–150–160°C, the screw speed was 160 rpm, and the residence time was 3 min. In a typical run, the PLA pellets and the flake-like PLA/NaM masterbach (mtotal = 500 g) were premixed and fed in the twin-screw extruder at a nominal rate of 0.7 kg h−1 using a volumetric metering feeder. The molten was cast into a monolayer film using a Chillroll 16 stretching machine (AX Plastics). The film was stretched at a constant speed and cooled down with compressed air flow at the die outlet. In addition to the four PLA/NaM films with the abovementioned NaM contents, a pure PLA film (no masterbach addition) was produced and used as a control.
2.3 Life Cycle Assessment (LCA)
The environmental footprints (EFPs) of the PLA/NaM films were estimated through a simplified cradle-to-grave life cycle assessment (LCA) according to ISO 14044 standard [22]. This environmental analysis was extended by performing a comparison of the PLA/NaM films with hypothetical PLA films loaded with silver nanoparticles (AgNPs), as AgNPs are one of the main biocidal additives used in commercial products. The relative contribution of each antimicrobial additive on the overall EFPs of the PLA film was determined. The functional unit was defined as 1 kg of packaging film (no targeted packaging design) in all cases to ensure the comparability of the LCA results.
The process units included in the system boundaries were raw material extraction, antimicrobial production, film production (melt extrusion) and disposal in landfill (Fig. 1). The use phase was also not considered because it has a short duration and provides little chemical emissions to the environment [8,23]. Regarding the antimicrobial production phase, NaM is obtained from the reaction of sulfur dioxide (SO2) with aqueous sodium sulfite (Na2SO3) at low pH, whereas the arc discharge was considered as the process for the AgNPs synthesis due to the high yields obtained, however the reduction of silver salts like AgNO3 is the most popular route for AgNPs synthesis in scientific reports [24]. For the landfill disposal, it was assumed a hypothetical condition in which PLA was fully depolymerized into lactic acid, which further suffered anaerobic biodegradation into CH4 and CO2, whereas NaM was fully hydrolyzed with subsequent release of SO2 and Na+ ions.
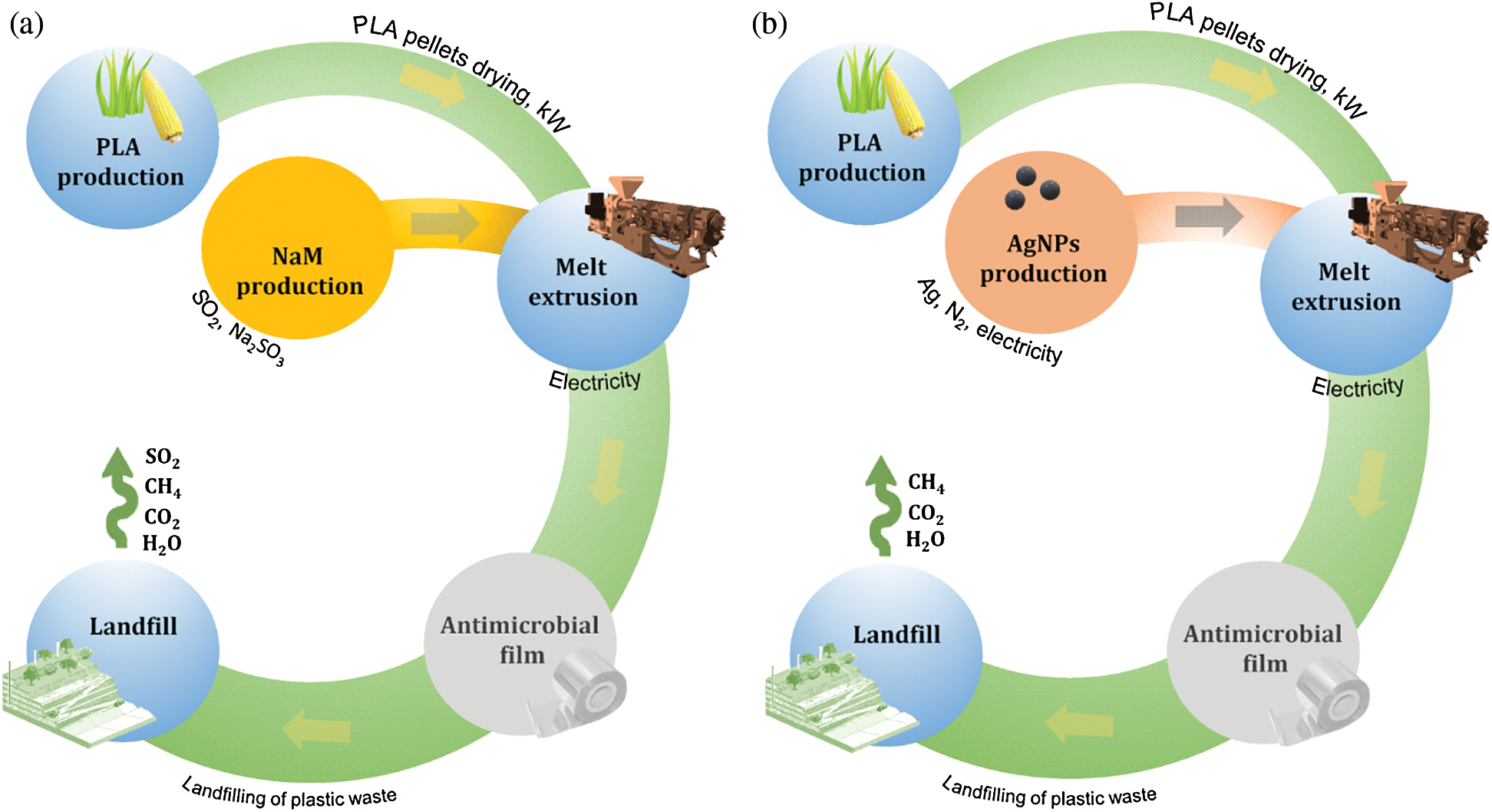
Figure 1: Cradle-to-grave life cycle flowchart of biodegradable PLA films loaded with (a) NaM (b) AgNPs
The cradle-to-grave LCAs were modeled using the OpenLCA 1.9 software (Green Delta, GitHub). The upstream life cycle inventories (LCIs) were completed by associating the inputs and outputs (materials, energy and chemical emissions) of each process unit with the European ecoinvent life cycle inventory dataset (Ecoinvent v3.3). The EFPs were calculated using the impact assessment method International Reference Life Cycle Data System (ILCD) 1.0.8 2016 midpoint (RLCDS, ILCD, EC-JRC). The EFPs were expressed as the results of the following midpoint impact categories: Global warming potential (GWP) in kg CO2 eq, human health impacts from carcinogenic substances (HHCS) in health comparative toxic units or CTUh, and resources depletion (ReD) in kg Sb eq.
Optical parameters (luminous transmittance T%, haze H%, and clarity C%) were determined at 20°C with a Haze-Gard Plus hazemeter (BYK Additives & Instruments) as per ASTM D1003 (2007). The measurements were performed on 50 × 50 cm specimens with six repetitions for each film. Thermogravimetric (TG) and differential thermogravimetric (DTG) curves were recorded with a Q500 thermal analyzer (TA Instruments). Samples (8–10 mg) were placed in a platinum crucible and were heated up to 600°C using heating ramp of 10°C min-1 and synthetic air atmosphere flowing at 60 mL min−1. Tonset was determined from the TG curves as the temperature at which the sample lost 3% of its mass. Tmax was determined from the DTG curves. Dynamic scanning calorimetry (DSC) tests was conducted on a Q2000 calorimeter (TA instruments) using hermetic Al pans, heating rate of 10°C min−1 and N2 atmosphere (50 mL min−1). The samples were first heated from 25°C to 200°C (1° heating scan), then cooled down to 0°C, and heated again to 200°C (2° heating scan). The glass transition temperature (Tg), melting temperature (Tm) and crystallinity degree (Xc) of the samples were determined from the second heating scans [25]. Uniaxial tensile tests were performed on strip-shaped specimens using a universal testing machine Instron 5569 (Instron Corporation) equipped with a 500 N load cell. The tests were conducted using crosshead speed of 10 mm min−1 with the clamps initially separated by 100 mm. All specimens were preconditioned under 54 ± 3 % RH for 48 h prior to testing. Average specimen thickness was previously determined from three random positions in each specimen using a digital micrometer (Mitutoyo Manufacturing, Japan). Tensile strength (σT) and elongation break (εB) were calculated from the stress (σ)—strain (ε) curves. Young’s modulus (E) was obtained through linear regression of σ–ε curves in the limit σ = ε = 0 ([dσ/dε]ε = 0). Tensile properties were determined in quintuplicate.
The in vitro antimicrobial activity of the PLA/NaM films was tested against S. cerevisiae by the agar diffusion test. Film disks were placed on petri dishes containing solidified agar medium previously inoculated with S. cerevisiae. The inoculum concentration was standardized to 108 cells mL−1 with a 0.5 McFarland turbidity standard. Petri dishes containing only the inoculum were also prepared as positive controls. All petri dishes were incubated for 72 h at 25°C. The antimicrobial activity of the samples was evaluated qualitatively by observing the formation of inhibitory zones, microbial growth, and plastic deterioration. All tests were done in duplicate.
Data were subjected to one-way analysis of variance (ANOVA). Mean values were compared using the Tukey’s test with a confidence level of 95% (p < 0.05). Statistical analyses were performed using the software Origin, version 8.0 (Origin Lab, MA, USA).
3.1 Estimation of Environmental Impacts
The potential environmental footprints (EFPs) of the PLA/NaM films were estimated by LCA. The results were compared with the EFPs of hypothetical extruded PLA films loaded with AgNPs to identify possible environmental advantages of NaM as an antimicrobial additive for biodegradable packaging. Fig. 2a presents the total EFP quantities represented by the midpoint categories global warming potential (GWP) or carbon footprint, human health impacts from carcinogenic substances (HHCS) and resources depletion (ReD) for representative samples: pure PLA film, 1.0 wt.% NaM-loaded PLA film and 0.5 wt.% AgNPs-loaded PLA film. The 0.5 wt.% AgNPs content was chosen due to the higher silver antimicrobial efficiency compared to that of NaM.
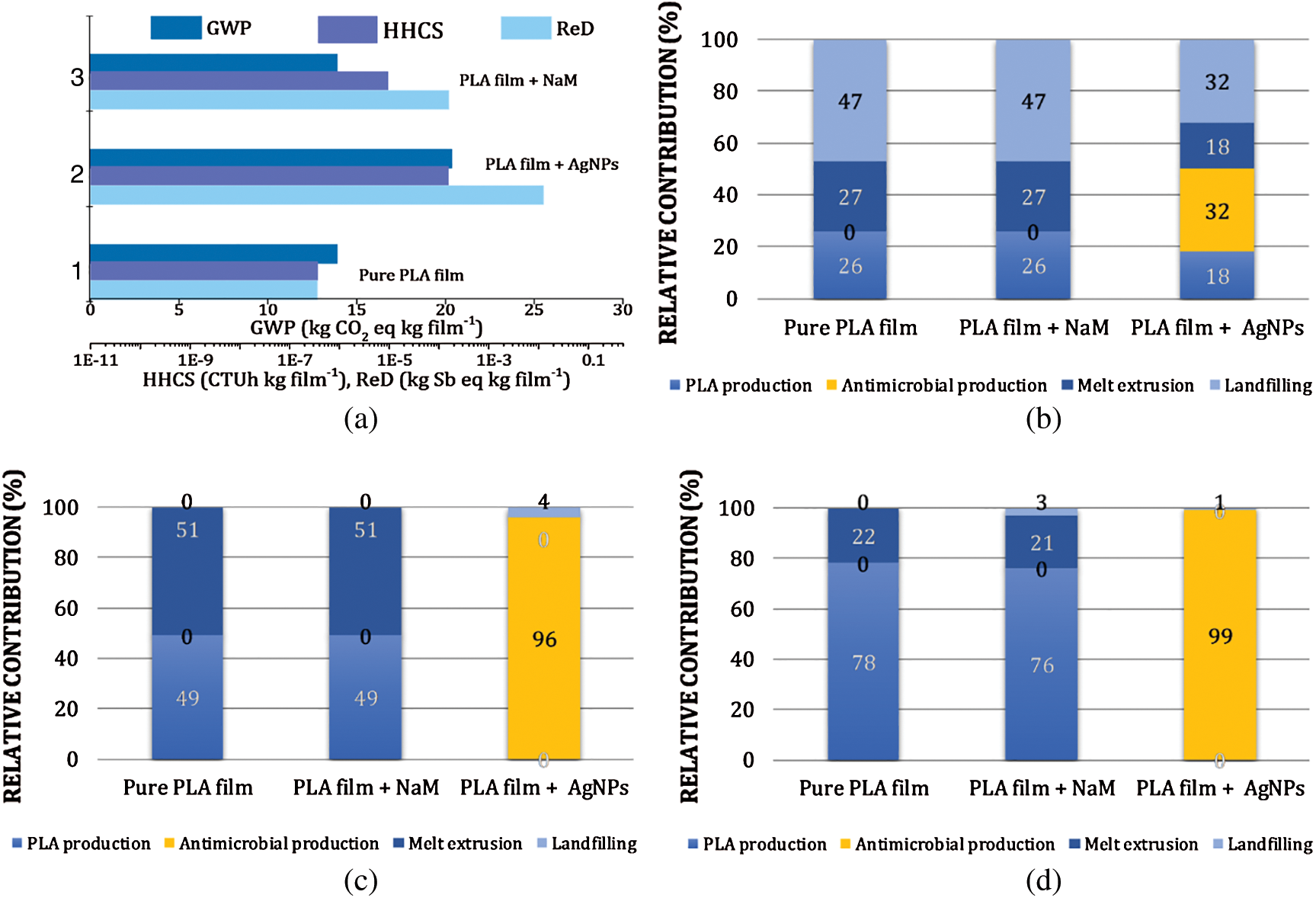
Figure 2: Life cycle assessment (LCA) results of pure PLA film and 1.0 wt.% NaM-loaded PLA film. (a) Total global warming potential (GWP), health impacts from carcinogenic substances (HHCS) and resources depletion (ReD) EFPs. Relative contributions of process units (PLA production, antimicrobial production, melt extrusion and hypothetical landfilling) for total EPFs (b) GWP (c) HHCS and (d) ReD. LCA results of a hypothetical melt extruded PLA film loaded with AgNPs (0.5 wt.%) are included for comparison. Functional unit = 1 kg packaging film
The pure PLA film displayed estimated GWP = 14 kg CO2eq kg film−1, HHCS = 3.6 × 10−7 CTUh kg film−1, and ReD = 3.6 × 10−7 kg Sbeq kg film−1. These results point out that the EFP of the PLA life cycle is more significant in terms of carbon footprint, considering the functional unit (1 kg PLA packaging film) used in the LCA simulations. It can be observed that the addition of NaM at 1.0 wt.% had no effect on the GWP of the PLA film but increased by 1- and 3-times the HHCS and ReD EFPs, respectively. In comparison, if 0.5 wt.% AgNPs are hypothetically compounded with PLA, the EFPs become much higher, with 150%, and 3- and 5-times increases in GWP, HHCS and ReD, respectively. The relative contribution of each life cycle stage (process units) from Fig. 1 are detailed in Figs. 2b–2d.
Fig. 2b revealed that the PLA production, the melt extrusion process, and the hypothetical landfilling accounted for 26%, 27% and 47% of the pure PLA film’s total GWP. The larger contribution of the landfilling stage relies mainly on the CH4 emission from the hypothetical PLA anaerobic biodegradation considered in the LCA. Fig. 2b further revealed that the AgNPs production by arc discharge exceeded the PLA production and landfilling contributions in the case of the 0.5 wt.% AgNPs-loaded PLA film, whereas the NaM production had no contribution or did not change these relative contributions, even considering the SO2 emission in the landfilling stage.
Regarding the HHCS and ReD, the pure PLA and 1.0 wt.% NaM-loaded PLA films displayed similar EFPs (Figs. 2c and 2d, respectively). There was no significant contribution of NaM to the PLA film performance in these impact categories, in opposition to the AgNPs that accounted for nearly 100% of the HHCS and ReD potentials, even at a low content. These results indicate that the negative impact of the silver production chain (upstream processes including mining, refinery, etc.) on human health is potentially higher than that related to NaM. Silver is also much less abundant in the Earth's crusth than any other element necessary to produce NaM.
Hence, the LCA results suggest that the upstream EFPs of the PLA films did not chance due to compounding it with NaM. Further LCA simulations indicated similar GWP, HHCS, and ReD for the NaM contents of 0.5, 1.0, 2.5 and 5.0 wt.% (data not shown). This means that NaM remains as an eco-friendly antimicrobial additive for PLA films even at high contents. The comparison with the 0.5 wt.% AgNPs-loaded PLA film supports this finding.
3.2 Characterization of the PLA/NaM Films
The PLA/NaM films were produced in a twin-screw extruder coupled to a sheet die. This allowed PLA to be compounded with NaM under intense distributive and dispersive flow conditions alongside casting the molten into films in a single thermal processing cycle. Fig. 3 compares the visual aspect of the PLA/NaM films, which were as transparent as the pure PLA film. In particular, the addition of NaM up to 5 wt.% did not affect the glossy and brittle behavior of PLA [8,9].
Figure 3: Visual aspect of melt extruded PLA films with varying NaM content (0–5.0 wt.%)
Tab. 1 lists the optical parameters of the PLA/NaM films. The transmittance of all samples was similar (p > 0.05), with values between 92% and 96%. This indicates that the addition of NaM did not interfere on the overall light transmission through the film matrix. By contrast, the clarity and haze parameters changed as the antimicrobial agent was added to PLA. For example, the film with 0.5 wt.% NaM showed clarity 10% lower than that of the pure PLA film (C = 99.5%), and the lowest value was found for the film with 5 wt.% NaM. Also, the films with 2.5 wt.% and 5 wt.% NaM presented the largest haze values, which were 18% higher than that of the pure film (H = 1.8%). These results suggest that the presence of NaM would not have effect in seeing a product through the PLA film, but high contents of the antimicrobial agent degrade the light transparence of PLA at some extent.
Table 1: Optical properties of extruded PLA/NaM films determined as per ASTM D1003
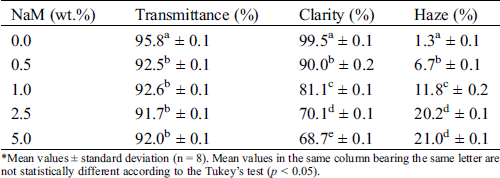
*Mean values ± standard deviation (n = 8). Mean values in the same column bearing the same letter are not statistically different according to the Tukey’s test (p < 0.05).
Thermogravimetric analysis was performed in O2-rich atmosphere (Fig. 4). The TG/DTG curves of the pure PLA film showed a mass loss step (97%) between 297 and 380°C with Tmax at 360°C ascribed to the PLA decomposition [26]. PLA exhibits a high thermal stability, which is suitable for withstanding the melt extrusion process over a broad temperature window. For pure NaM, the TG/DTG curves displayed a single mass loss between 120 and 200°C, with Tmax at 171°C, which is related to the decomposition of NaM (Na2S2O5) into Na2SO3 and gaseous SO2. Considering the reaction stoichiometry, the generated SO2 accounts for 32% of the Na2S2O5 mass, which agrees with the mass loss observed in the TG curve. Yet the 5 wt.% NaM-loaded PLA film had a thermal profile similar to that of the pure PLA film, and this profile was not altered regardless of the NaM content. All thermal parameters extracted from the TG/DTG curves are listed in Tab. 2.
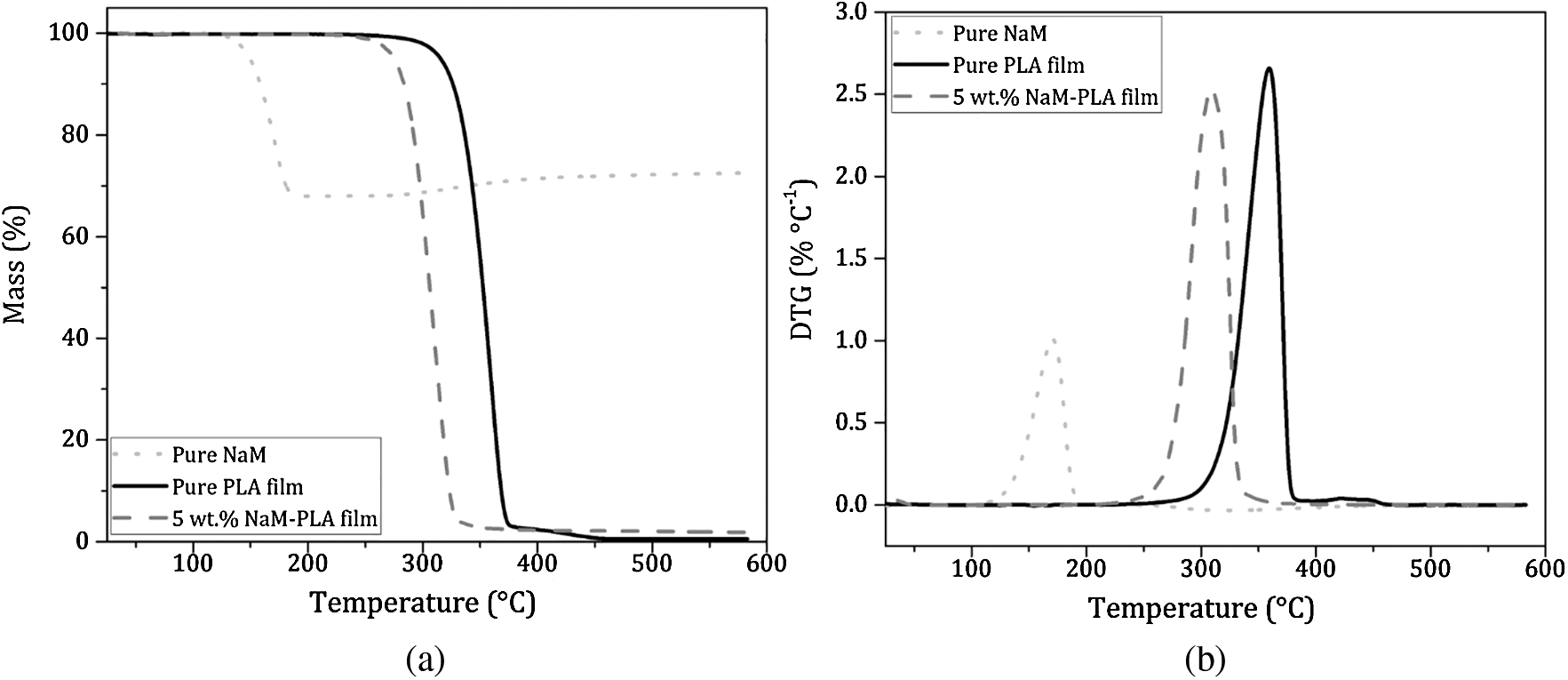
Figure 4: (a) TG curves and (b) DTG curves of pure NaM, pure PLA film and 5 wt.% NaM-loaded PLA film. (Heating rate = 10°C min−1; synthetic air atmosphere = 60 mL min−1)
Table 2: Thermal parameters and crystallinity index (Xc) of extruded PLA/NaM films
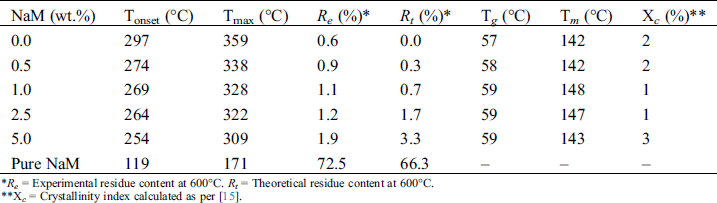
*Re = Experimental residue content at 600°C. Rt = Theoretical residue content at 600°C.
**Xc = Crystallinity index calculated as per [15].
It was possible to ascertain the composition of the PLA/NaM films by comparing the theoretical and experimental residue contents at 600°C. It can be seen that except for the 2.5 wt.% and 5 wt.% NaM-loaded films, the obtained residue contents were close to the nominal values, taking into account the residue left by the PLA matrix (~0.6 %). This indicates that PLA was properly compounded with NaM at the targeted contents through the one-step melt extrusion process. The result also indicates that NaM endured the thermal processing, considering that its Tonset was lower than the used processing window (T = 150–180°C). This can be explained by the fact that the residence time (t = 3 min) was not sufficiently long to induce the thermal decomposition of the antimicrobial agent, and that no other heating step was used to cast the films. Additionally, it can be observed from Tab. 2 that the Tonset and Tmax of the films decreased with increasing the NaM content, suggesting a reduction in the thermal stability of PLA.
The thermal transitions of PLA were assessed by DSC to gain insights into the thermal behavior of the PLA/NaM films. DSC curves relating to the second heating scan are shown in Fig. 5. Overall, it can be seen the glass transition followed by the small endothermic peak of glassy phase relaxation between 40 and 70°C, the cold crystallization event between 100 and 140°C, and the melting peak above 140°C [25]. The glass transition temperature (Tg), melting temperature (Tm), cold-crystallization temperature (Tcc) and crystallinity index (Xc) of the films are provided in Tab. 2.
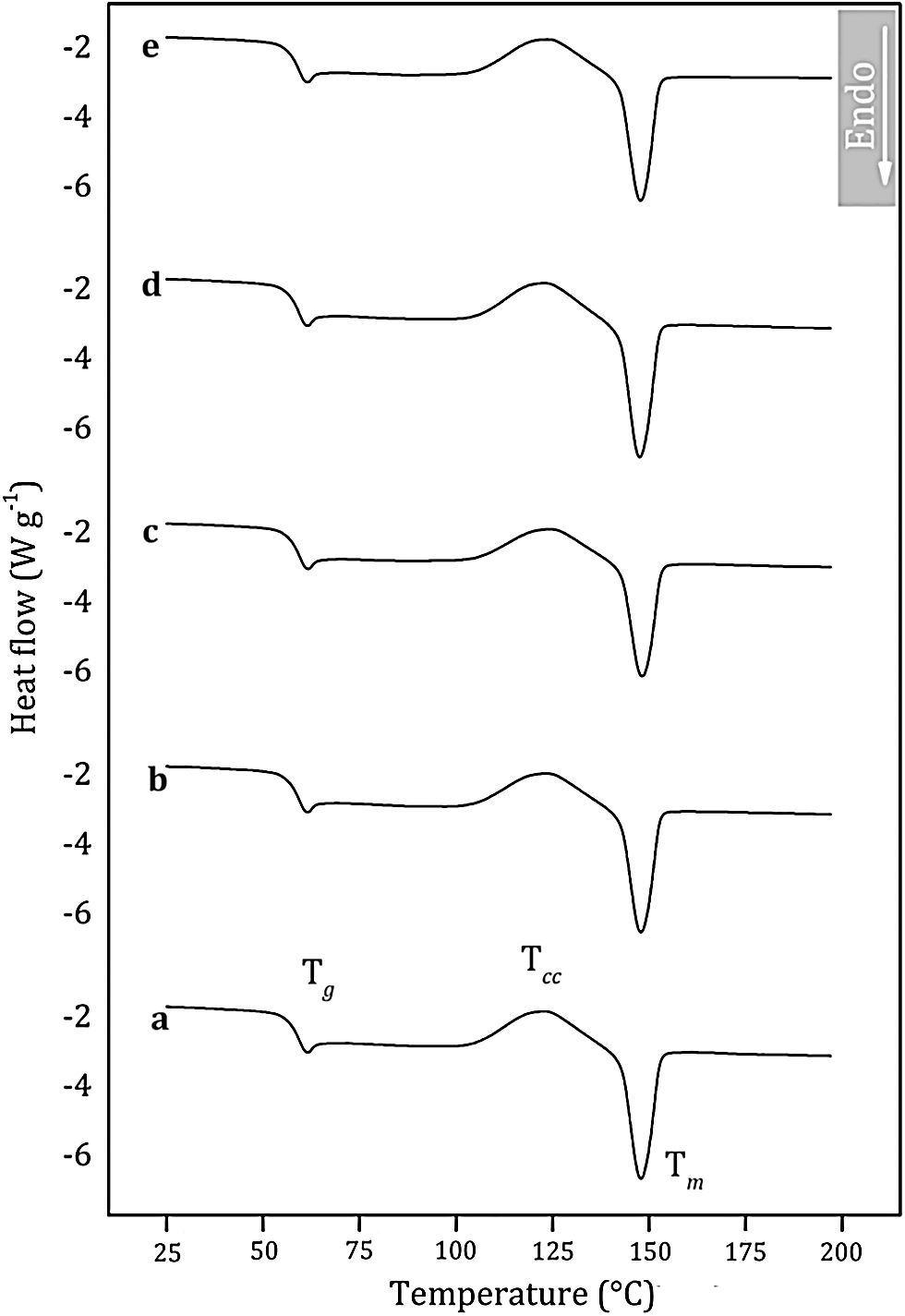
Figure 5: Differential scanning calorimetry (DSC) curves of melt extruded PLA films loaded with (a) 0 wt.% NaM (b) 0.5 wt.% NaM (c) 1.0 wt.% NaM (d) 2.5 wt.% NaM (e) 5.0 wt.% NaM
It is observed that the pure PLA film displayed Tg at 57°C and Tm at 142°C, which agrees with the literature [26–28]. The PLA film is in the glassy state at room temperature, which makes it viable for applications that require rigid packaging materials. It is also noted that the addition of NaM had no significant effect on the thermal transitions of PLA. All films presented similar Tg, and Tm, as shown in Tab. 2. Moreover, the Xc values were low, meaning that the films were predominantly amorphous, and that NaM did not change the crystallization behavior of the PLA matrix. This supports the high optical transparency of the films reported in Tab. 1. All these results indicate that the NaM particles were only finely distributed within the PLA films, but the antimicrobial agent was not dispersed at the molecular level and did not alter the molecular weight of the PLA chains.
Tab. 3 shows the tensile properties (elastic modulus E, tensile strength σT, and elongation at break εB) of the PLA/NaM films. The pure PLA film displayed E = 3.2 GPa, σT = 56 MPa and εB = 3.5%, these values were similar to previously extruded PLA samples [25,27]. σT and εB significantly decreased when NaM was added at 0.5 wt.% (p < 0.05), while E was the same in relation to the pure PLA film (p > 0.05). Similar results were observed for PLA extruded with 10–40 wt.% propolis ethanolic extract [13] and 1 wt.% AgNPs [25]. For the higher NaM contents, σT and E further decreased, reaching the lowest values for the 5 wt.% NaM-added PLA film (p < 0.05). No clear relationship is noted between the high NaM contents and εB. The PLA/NaM films displayed the fragile behavior of pure PLA regardless of the NaM content.
Table 3: Tensile properties of extruded PLA/NaM films determined at 25°C*
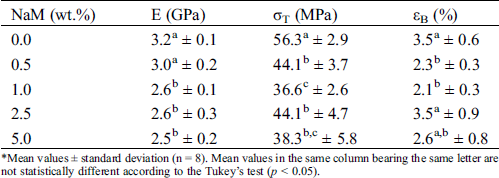
*Mean values ± standard deviation (n = 8). Mean values in the same column bearing the same letter are not statistically different according to the Tukey’s test (p < 0.05).
The reduced tensile properties of the PLA/NaM films may be explained by the fact that the NaM particles acted as stress concentration factors within the glassy PLA matrix. This may be due to the hydrophobicity of PLA, while NaM is a water-soluble salt, thus the miscibility between PLA and NaM was already expected to be low. However, it is worth noticing that the mechanical properties of the PLA/NaM films were still high enough to cover most packaging applications, even for the highest NaM content (5 wt.%). Furthermore, the chemical incompatibility between NaM and PLA allows one to expect that the NaM particles can be released from the PLA films, exerting an antimicrobial effect through migration.
The results of the agar diffusion tests are illustrated in Fig. 6. NaM is also a water-soluble salt, so it readily dissolves into the agar medium, inhibiting microbial growth. The antimicrobial activity of NaM relates to its reducing properties, which decreases the level of oxygen, inhibiting the proliferation of aerobic microorganisms, like S. cerevisiae [29]. NaM also releases sulfite, which is a strong nucleophile agent that reacts with many biomolecules by substitution at electrophilic positions, causing a myriad of potential cell damaging reactions, thereby leading to cell death [30,31]. In contrast, the PLA/NaM films presented no antimicrobial activity against S. cerevisiae, since there was no formation of inhibitory zones for the pure PLA film (Fig. 6a) neither for the 5 wt.% NaM-added PLA film (Fig. 6e). Similarly, Luzi et al. [27] also found no antimicrobial activity for PLA films loaded with 1–3 wt.% ZnO nanoparticles on E. coli and S. aureus.
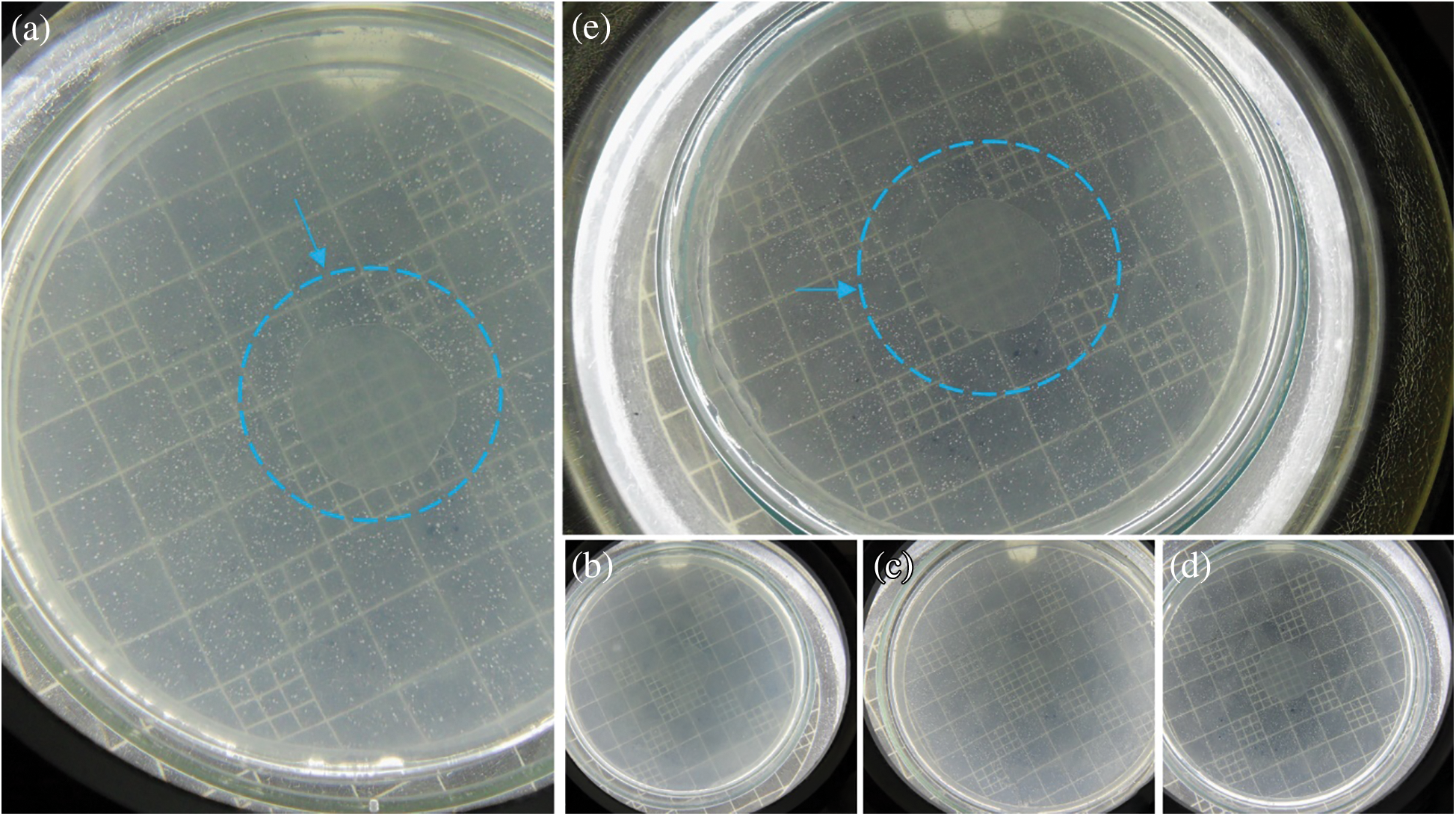
Figure 6: In vitro antimicrobial tests against S. cerevisiae of melt extruded PLA films loaded (a) 0 wt.% NaM, (b) 0.5 wt.% NaM, (c) 1.0 wt.% NaM, (d) 2.5 wt.% NaM, (e) 5.0 wt.% NaM
A possible explanation for the antimicrobial inactivity of the PLA/NaM films is that NaM did not migrate to a large extent from the PLA matrix to the agar medium. This is because the Tg of the films was around 60°C, while the agar diffusion tests were carried out at 25°C (optimal temperatures for microbial growth). Under this condition, the PLA matrix is at the glassy state, thus the migration of NaM though diffusion toward the gel-like agar medium is restricted [32]. Furthermore, the agar diffusion method only indicates antimicrobial effects based on the migration of the antimicrobial agent. There are other methods that evaluate the antimicrobial activity of immobilized antimicrobial agents. For example, Busolo et al. confirmed that the surface of PLA films loaded with Ag+ ions-impregnated nanoclay was activity on Salmonella spp. under dynamic conditions [12]. Li et al. [32] found through the liquid culture test that PLA films loaded with 1 and 5 wt.% TiO2 and 0.5 wt.% AgNPs were effective against E. coli and L. monocytogenes. Thus, considering the high fungicide activity of NaM [21], the PLA/NaM films may exhibit biocidal effect on S. cerevisiae and other microorganisms by contact. Future studies will be conducted to assess the contact-activity surface of the melt extruded PLA/NaM films through antimicrobial surface methods.
An eco-friendly packaging material was successfully developed by melt compounding PLA with NaM, a traditional food preservative. Using a twin-screw extruder coupled to a sheet die allowed for uniform distribution of MNa within the PLA matrix and film formation with no significant thermal degradation. As a result, the PLA/NaM films displayed important physical properties, including tensile strength as high as other synthetic polymer like PP and PE, high optical transparency, and thermal stability up to 250°C, which may be suitable for rigid food packaging applications, such as bowls and standup pouches. Furthermore, the antimicrobial activity of the films was not significant due to the high Tg of the PLA matrix rather than being leveraged by the polarity difference between PLA and NaM. The PLA/NaM films may exhibit an antimicrobially active surface or their migration-dependent antimicrobial performance may be enhanced by further increasing the NaM content above 5 wt.%, providing that the maximum estimated daily SO2 intake (0.2–2 mg of SO2 equivalent per kg body weight per day) is respected [18]. Overall, compounding biodegradable PLA films with GRAS antimicrobials like NaM is an eco-friendly approach for both fundamental studies and practical applications of antimicrobial packaging. This approach can be readily extended to produce antimicrobial food packaging from several other food additives and biobased, compostable polymers.
Acknowledgement: The authors thank DEMa/UFSCar and Embrapa Instrumentation for the support to this work.
Funding Statement: This work received financial support from the MCTIC/CNPq Universal program (Project 437501/2018-3).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Sofi, S. A., Singh, J., Rafiq, S., Ashraf, U., Dar, B. N. et al. (2018). A comprehensive review on antimicrobial packaging and its use in food packaging. Current Nutrition & Food Science, 14(4), 305–312. DOI 10.2174/1573401313666170609095732. [Google Scholar] [CrossRef]
2. Suppakul, P., Miltz, J., Sonneveld, K., Bigger, S. W. (2003). Active packaging technologies with an emphasis on antimicrobial packaging and its applications. Journal of Food Science, 68(2), 408–420. DOI 10.1111/j.1365-2621.2003.tb05687.x. [Google Scholar] [CrossRef]
3. Khaneghah, A. M., Hashemi, S. M. B., Ismail, E., Fracassetti, D., Limbo, S. (2018). Efficacy of antimicrobial agents for food contact applications: Biological activity, incorporation into packaging, and assessment methods: A review. Journal of Food Protection, 81(7), 1142–1156. DOI 10.4315/0362-028X.JFP-17-509. [Google Scholar] [CrossRef]
4. Hoseinnejad, M., Jafari, S. M., Katouzian, I. (2018). Inorganic and metal nanoparticles and their antimicrobial activity in food packaging applications. Critical Reviews in Microbiology, 44(2), 161–181. DOI 10.1080/1040841X.2017.1332001. [Google Scholar] [CrossRef]
5. Silva, C. C. G., Silva, S. P. M., Ribeiro, S. C. (2018). Application of bacteriocins and protective cultures in dairy food preservation. Frontiers in Microbiology, 9, 169. DOI 10.3389/fmicb.2018.00594. [Google Scholar] [CrossRef]
6. Santos, R. R., Andrade, M., De Melo, N. R., Sanches-Silva, A. (2017). Use of essential oils in active food packaging: Recent advances and future trends. Trends in Food Science & Technology, 61, 132–140. DOI 10.1016/j.tifs.2016.11.021. [Google Scholar] [CrossRef]
7. Al-Tayyar, N. A., Youssef, A. M., Al-hindi, R. (2020). Antimicrobial food packaging based on sustainable bio-based materials for reducing foodborne pathogens: A review. Food Chemistry, 310, 125915. DOI 10.1016/j.foodchem.2019.125915. [Google Scholar] [CrossRef]
8. Castro-Aguirre, E., Iñiguez-Franco, F., Samsudin, H., Fang, X., Auras., R. (2016). Poly(lactic acid)—mass production, processing, industrial applications, and end of life. Advanced Drug Delivery Reviews, 107(15), 333–366. DOI 10.1016/j.addr.2016.03.010. [Google Scholar] [CrossRef]
9. Auras, R., Harte, B., Selke, S. (2004). An overview of polylactides as packaging materials. Macromolecular Bioscience, 4(9), 835–864. DOI 10.1002/mabi.200400043. [Google Scholar] [CrossRef]
10. Li, W., Zhang, C., Chi, H., Li, L., Lan, T. et al. (2017). Development of antimicrobial packaging film made from poly(lactic acid) incorporating titanium dioxide and silver nanoparticles. Molecules, 22(7), 1170. DOI 10.3390/molecules22071170. [Google Scholar] [CrossRef]
11. Shiroodi, S. G., Nesaei, S., Ovissipour, M., Al-Qadiri, H. M., Rasco, B. et al. (2016). Biodegradable polymeric films incorporated with nisin: Characterization and efficiency against listeria monocytogenes. Food and Bioprocess Technology, 9(6), 958–969. DOI 10.1007/s11947-016-1684-3. [Google Scholar] [CrossRef]
12. Busolo, M. A., Fernandez, P., Ocio, M. J., Lagaron, J. M. (2010). Novel silver-based nanoclay as an antimicrobial in polylactic acid food packaging coatings. Food Additives & Contaminants: Part A, 27(11), 1617–1626. DOI 10.1080/19440049.2010.506601. [Google Scholar] [CrossRef]
13. Safaei, M., Azad, R. P. (2020). Preparation and characterization of poly-lactic acid-based films containing propolis ethanolic extract to be used in dry meat sausage packaging. Journal of Food Science and Technology, 57(4), 1242–1250. DOI 10.1007/s13197-019-04156-z. [Google Scholar] [CrossRef]
14. Theinsathid, P., Visessanguan, W., Kingchaand, Y., Keeratipibul, S. (2011). Antimicrobial effectiveness of biobased film against Escherichia coli 0157: H7, Listeria monocytogenes and Salmonella typhimurium. Advance Journal of Food Science and Technology, 3(4), 294–302. [Google Scholar]
15. Fortunati, E., Armentano, I., Zhou, Q., Iannoni, A., Saino, E. et al. (2012). Multifunctional bionanocomposite films of poly(lactic acidcellulose nanocrystals and silver nanoparticles. Carbohydrate Polymers, 87(2), 1596–1605. DOI 10.1016/j.carbpol.2011.09.066. [Google Scholar] [CrossRef]
16. Moghaddam, M. A., Stloukal, P., Kucharczyk, P., Tow-Swiatek, A., Garbacz, T. et al. (2019). Microcellular antibacterial polylactide-based systems prepared by additive extrusion with ALUM. Polymers for Advanced Technologies, 30(8), 2100–2108. DOI 10.1002/pat.4643. [Google Scholar] [CrossRef]
17. Ahmadi, F., Lee, Y. H. L., Lee, W. H., Oh, Y. K., Park, K. K. et al. (2018). Preservation of fruit and vegetable discards with sodium metabisulfite. Journal of Environmental Management, 224(15), 113–121. DOI 10.1016/j.jenvman.2018.07.044. [Google Scholar] [CrossRef]
18. Food and Drug Administration. (2015). Select Committee on GRAS Substances (SCOGS) Opinion: Potassium metabisulfite, sodium bisulfite, sodium metabisulfite, sodium sulfite, sulfur dioxide. http://wayback.archive-it.org/7993/20171031061022/https://www.fda.gov/Food/IngredientsPackagingLabeling/GRAS/SCOGS/ucm261009.htm. [Google Scholar]
19. Saito, S., Obenland, D., Xiao, C. L. (2020). Influence of sulfur dioxide-emitting polyethylene packaging on blueberry decay and quality during extended storage. Postharvest Biology and Technology, 160, 111045. DOI 10.1016/j.postharvbio.2019.111045. [Google Scholar] [CrossRef]
20. Arroyo-López, F. N., Bautista-Gallego, J., Durán-Quintana, M. C., Garrido-Fernández, A. (2008). Effects of ascorbic acid, sodium metabisulfite and sodium chloride on freshness retention and microbial growth during the storage of Manzanilla-Aloreña cracked table olives. LWT—Food Science and Technology, 41(4), 551–560. [Google Scholar]
21. Natskoulis, P. I., Lappa, I. K., Panagou, E. Z. (2018). Evaluating the efficacy of turbimetric measurements as a rapid screening technique to assess fungal susceptibility to antimicrobial compounds as exemplified by the use of sodium metabisulfite. Food Research International, 106, 1037–1041. DOI 10.1016/j.foodres.2018.01.058. [Google Scholar] [CrossRef]
22. ISO. Environmental Management. (2016). Life Cycle Assessment—Requirements and Guidelines (ISO14044). Berlin, Germany: DIN Deutsches Institut für Normung e.V. [Google Scholar]
23. Maga, D., Hiebel, M., Thonemann, N. (2019). Life cycle assessment of recycling options for polylactic acid. Resources, Conservation and Recycling, 149, 86–96. DOI 10.1016/j.resconrec.2019.05.018. [Google Scholar] [CrossRef]
24. Slotte, M., Zevenhoven, R. (2017). Energy requirements and life cycle assessment of production and product integration of silver, copper and zinc nanoparticles. Journal of Cleaner Production, 148, 948–957. DOI 10.1016/j.jclepro.2017.01.083. [Google Scholar] [CrossRef]
25. Coppola, B., Cappetti, N., Di Maio, L., Scarfato, P., Incarnato, L. (2018). 3D printing of PLA/clay nanocomposites: Influence of printing temperature on printed samples properties. Materials, 11(10), 1947. DOI 10.3390/ma11101947. [Google Scholar] [CrossRef]
26. Fortunati, E., Armentano, I., Zhou, Q., Iannoni, A., Saino, E. et al. (2012). Multifunctional bionanocomposite films of poly(lactic acidcellulose nanocrystals and silver nanoparticles. Carbohydrate Polymers, 87(2), 1596–1605. DOI 10.1016/j.carbpol.2011.09.066. [Google Scholar] [CrossRef]
27. Dias, P. P., Chinelatto, M. A. (2019). Effect of poly(ε-caprolactone-b-tetrahydrofuran) triblock copolymer concentration on morphological, thermal and mechanical properties of immiscible PLA/PCL blends. Journal of Renewable Materials, 7(2), 132–138. [Google Scholar]
28. Luzi, F., Fortunati, E., Jiménez, A., Puglia, D., Chiralt, A. et al. (2017). PLA nanocomposites reinforced with cellulose nanocrystals from posidonia oceanica and ZnO nanoparticles for packaging application. Journal of Renewable Materials, 5(2), 103–115. DOI 10.7569/JRM.2016.634135. [Google Scholar] [CrossRef]
29. Jay, J. M., Loessner, M. J., Golden, D. A. (2005). Modern Food Microbiology. 7th edition, pp. 790, New York: Springer US. [Google Scholar]
30. Irwin, S. V., Fisher, P., Graham, E., Malek, A., Robidoux, A. (2017). Sulfites inhibit the growth of four species of beneficial gut bacteria at concentrations regarded as safe for food. PLoS One, 12(10), e0186629. DOI 10.1371/journal.pone.0186629. [Google Scholar] [CrossRef]
31. Neta, P., Huie, R. E. (1985). Free-radical chemistry of sulfite. Environmental Health Perspectives, 64, 209–217. DOI 10.1289/ehp.8564209. [Google Scholar] [CrossRef]
32. Li, W., Zhang, C., Chi, H., Li, L., Lan, T. et al. (2017). Development of antimicrobial packaging film made from poly(lactic acid) incorporating titanium dioxide and silver nanoparticles. Molecules, 22(7), 1170. DOI 10.3390/molecules22071170. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |