
Materials

 | Journal of Renewable Materials |  |
DOI: 10.32604/jrm.2021.016173
ARTICLE
Tannin Nanoparticles (NP99) Enhances the Anticancer Effect of Tamoxifen on ER+ Breast Cancer Cells
1Department of Biology, College of Science, Taif University, Taif, 21944, Saudi Arabia
2Department of Biotechnology, College of Science, Taif University, Taif, 21944, Saudi Arabia
3Department of Physics, Faculty of Science, Alexandria University, Alexandria, Egypt
4ENSTIB-LERMAB, University of Lorraine, Epinal, 88051, France
*Corresponding Author: Aziza M. Hassan. Email: a.hasn@tu.edu.sa
Received: 13 February 2021; Accepted: 07 April 2021
Abstract: Recently, natural substances in the form of nanoparticles are increasingly being used in different field, particularly in medicines to enhance their beneficial effects in treatment and prevention. Cancer cells of the breast (MCF-7) have been chosen to be examined and treated in vitro with conventional drug Tamoxifen (Tam) and tannin nanoparticles extract (NP99) individually or in combination. MTT reagent has been applied to assess the cell viability and propagation percentage, DNA fragmentation and mRNA relative expression of apoptotic genes to study the cell death pathway. The results showed that Tam and tannin NP99 triggered cytotoxic activity towards the MCF-7 cell. They reduce the viability and induced high potent repressive activity on cell proliferation percentage and induced apoptosis as indicated by rising the percentage in DNA fragmentation. Effect of NP99 extract exhibited its effect in a dose and time-varying. The combined treatment of Tam and NP99 were much more efficient than individual drugs. It could be concluded that NP99 is considered a promising natural anticancer agent as a new tool in therapeutic strategies.
Keywords: Condensed tannin; nanoparticles; tamoxifen; MCF-7 cells; anticancer
The breast cancer (BC) is among the world’s most common diseases which lead to death. This is the most prevalent cancer among women, representing 23% of all female cancers worldwide and 14% of cancer incidence with a survival rate of 40–80% five years [1,2]. Recently, the rate of all cancer cases, including BC, has been estimated to nearly double by 2030 [3]. A new study from the Saudi Cancer Registry suggests, BC ranked the 1st among females in 2015 and accounted for 16.7 percent of all Saudi citizens reported, and 30.1 percent of all women recorded cancers at all ages [4]. The treatment of BC includes various methods such as surgery, radiation and chemo-therapy, as well as hormonal therapy or targeted therapy depending on the type of tumor, its biological properties and hormone status [5]. Several studies have proved that nearly 75 percent of any and all BC cases are estrogen receptor-positive (ER+) [6] suggesting a hormonal response, tumor which can be treated with hormone therapy drugs such as Tamoxifen (Tam) which represented as the first line anti-estrogen therapy for BC prevention and treatment [7]. Unfortunately, using Tam in the treatment of ER+ patients has some challenges which limits its success include, stability, solubility, toxicity, resistant cancer cells, delivery to the cancer sites and prospective side effects [8]. Thus, Tam is usually given in conjunction with other medicines to reduce the frequency of drug resistance progression and achieved the efficacy on the targeted cells [9]. Therefore, the progression of Nano-medicine using natural products opens a new file in therapeutic strategies [10].
The benefits of nanotechnologies are due to the unique interactions and properties of the nanoscale range. Scientists and researchers have started to explore natural products in the form of nanoparticles to be used in the treatment of inflammation, cancer, microbial infection, and other diseases [11]. Thus, manufacturing the natural compound in the form of nanoparticles can increase the bioavailability and decrease the effective dose demanded to get the therapeutic effects, ability to target certain tissues or organs [11]. The most important natural product compounds, which can be formed as nanoparticles, are polyphenols such as tannin. This is due to its highly hydrophilic compound that cannot cross biological membranes. The cytotoxic activity of polyphenols on various types of cancer cells and their antioxidant properties were determined previously [12].
Condensed tannin extracts are polyphenolic compounds which have many health-enhancing activities including antimicrobial, antiparasitic, antioxidant and antigenotoxic. In addition to their radical scavenging and complement modulating agents, they have antitumor activity and inhibit enzymes specifically inducing apoptosis in cancer cells [13]. Thus, they are contributing to the advancement of cancer treatments by a greater effectiveness. Based on these facts, this research had the aim of exploring the role of condensed tannin nanoparticles individually or in combination with the conventional drugs Tamoxifen (Tam) to promote apoptosis in human cancer cells of the breast in vitro. Moreover, the molecular mechanisms, by which these agents influence apoptosis and anti-proliferative activity, were examined.
2.1 Materials, Reagent and Drug Used
Penicillin-Streptomycin antibiotic, DMEM media and Bovine serum (FBS); all provided by Gibco, life technologies (USA). Life Technologies Inc. has acquired TRIzol™ reagent (Carlsbad, CA, USA). Gene expression master Mix, (FIREPol®) and MTT were purchased from Bio Basic Inc., Canada. Tam tablet (EBEWE Pharma®-UK) was obtained from the King Abdul-Aziz hospital, Taif (KSA) and a stock solution (10 mg/ml) was prepared by dissolved a pure Tam tablet in absolute ethanol. Other substances used in the assessments were of the greatest possible extent. The condensed (flavonoid) tannin extract used contained a guaranteed minimum of 75% phenols and polyphenols, the rest being oligomeric and monomeric carbohydrates, it was a commercial extract water extracted from the bark of Pine trees (Pinusradiata) supplied by DitecoLtda, Concepcion, Chile.
Breast cell line MCF-7 with a positive estrogen receptor were kindly provided by Dr. Thamer Ahmed F. Bouback, Cell Culture Laboratory, Biology department, Science collage, Abdul-Aziz University, Saudi Arabia. American Tissue Culture and Cells (ATCC) originally got the cell line.
2.1.2 Synthesis of Tannin Extract Nanoparticles (NP99)
The NP99 nanoparticles were synthesized as follows: 50 ml of propylene glycol was diluted in purified distilled water at a ratio of 1:1 then heated to 70°C. Pure warmed water was added to EDTA and pluronic F-127 at 0.5% concentrations each and stirring continuously until the mixture temperature down to 50°C. After the gel process was cooled to 40°C, the NP99 was integrated into the Carbopol gel and mixed uniformly to get a suitable gel. The NP99 was dispersed into the Carbopol gel with the concentration of 1.5% in 2% Carbopol. The NP99 nanoparticles was dissolved in sterilized distilled water (ddH2O) to prepare different concentrations for the determination of their cytotoxic effect against MCF-7 cells, which expressed as IC50 values (Concentration that significantly decreased the viability of cancer treatment cells by 50% when compared with the control sample).
2.2 Experimental Design and Treatment Protocols
The experiment was conducted to examine the effect of either Tam or NP99, individually or in combination, on cancer cells in vitro. The MCF-7 cells were grown and plated at 1 × 105 cells/well using DMEM medium supplemented by bovine serum (10%) and Penicillin-streptomycin (1% each) then cells were actually allowed to stay attached up overnight at optimum conditions [14]. After 24 h in the basal medium, the cells were then treated with a medium containing different treatments and then further incubation for 24, 48 and 72 h. The following treatment groups were included: Group 1: Control untreated MCF-7 cells line which treated the same as other groups of cells without tamoxifen and/or tannins nanoparticles); Group 2: MCF-7 cell line treated with Tam (1 µg/mL); Group 3: Cell line treated with Tam (10 µg/mL) [15], Group 4: Cell line treated with NP99 at IC50 (18 µg/mL), Group 5: Cell line treated with NP99 at 18 µg/mL and Tam at 1 µg/mL and Group 6: Cell line treated with NP99 at 18 µg/mL and Tam at a dose of 10 µg/mL.
2.3.1 Characterization of Tannin NP99 Nanoparticles
The particle size and polydispersity index (PDI) of NP99 nanoparticles were analyzed by dynamic light scattering technique (DLS) with a Zeta-sizer TM 3000E (Malvern Instruments Worcestershire, UK). Each sample was diluted using double distilled water until the appropriate concentration of the particles then it was kept constant to avoid multi scattering events. The particle size, size distribution and zeta potential were measured using a particle size analyzer (Nano-ZS, Malvern Instruments, Ltd., UK).
2.3.2 HPLC Analysis for Tannin NP99
The analysis for Tannin extract nanoparticles was done usage of an Agilent 1260 HPLC series. The separation was completed utilizing a 100 Å pore size C18 column (LI.D. 4.6 mm × 250 mm, 5 μm particle size). The mobile process consisted of (A) water and (B) 0.02 percent tri-floro-acetic acid in acetonitrile at 1 mL/min flow rate. The stage (A) was customized one after another in a direct inclination just as: 0 min (80%); 0–5 min (80%); 5–8 min (40%); 8–12 min (50%); 12–14 min (80%); and monitoring at 280 nm. For each sample solution about 10 μl was injected and the column temperature should be kept at 35°C. The outcomes results of the analysis represented by mean ± standard error (SE).
2.3.3 Recording the Proliferation of MCF-7cells
Using Trypan blue staining, the number of cells was counted. Cells that are alive and proliferating with healthy cell membranes are not colored. Trypan blue is not absorbed by a living cell. As a result, under a microscope, dead cells have a distinct blue colour. The attached cells were washed in PBS after the culture medium was removed. Centrifuge the cells at 2500 rpm for 7 min at 4°C after trypsinization, discard the media, and add 1 ml media. Put 100 µl of the cell suspension in a microcenterfuge tube with 10 µl of the dye “Trypan blue,” then apply 10 µl of the mix suspension to the Heamocytometer slide and test it under a light microscope.
The 3-(4,5-dimethylthiazol-2-yl)2,5-diphenyltetrazolium (MTT) reagent applied to examine the viability of MCF-7cells and the cytotoxicity test was induced after treatment by either NP99 or Tam at different concentrations. The MTT reagent is used to measure the mitochondrial dehydrogenase activity, which forms purple formazan salt crystals. The cells were seeded and treated with NP99 and Tam individually or in combination. After incubation for different times, as mentioned above, the medium was removed and 40 μl L MTT reagent (5 mg/mL) added then incubated for another four hours. After 4 h the MTT solution was taken away and the remaining precipitate was dissolved in 140 μL of dimethyl sulfoxide (DMSO). A plate reader (MR-96A) was used to measure the optical density (OD) of the wells at wavelength 570 nm. The impact of NP99 and TAM on cell viability was determined, and the frequency of inhibition (I percent) was calculated by using equation [16]:
I percent = [A570 (control) − A570 (treated)]/A570 (control) * 100
2.3.5 DNA Fragmentation for Apoptosis
Inter-nucleosome DNA fragmentation is considered a hallmark for the measurement of apoptosis. DNA fragmentation was analyzed from extracted genomic DNA using a Bio-Vision’s DNA Ladder Detection Kit (Bio-Vision, Life Science SOURCE ™, USA) as instructed by the manufacturer. DNA pellet suspended in a buffer then samplesapplied onto a 1.5 percent agarose gel. DNA bands were examined and analyzed under UV radiation. The fragmented DNA quantities were assigned colorimetrically at 575 nm using Diphenylamine (DPA), as described by Burton [17] and modified by Perandones et al. [18].
2.3.6 Gene Expression Analysis
To analyze the expression profiles of related genes to apoptosis in cancer cells exposed to different treatment, the RNA isolated using TRIzole reagent and its integrity was checked by gel electrophoresis. The isolated RNA was subjected to DNase digestion and used in RT-PCR to generate cDNA by using transcription reagents (Maxime RT PreMix) purchased from iNtRON Biotechnology (Korea). The PCR reactions, using a thermal cycler (PXE 0.5 Thermo), were implemented according to Sun et al. [19] using the pairs of primers listed in Tab. 1. The product of PCR amplification runs on a 1.5% agarose gel and envisioned on Trans-illuminator UV. DNA bands were scanned with Gel-Pro program (version 3.1 for Windows 3) to quantify the signal intensities. The proportion between the levels of the target genes-amplification product and GAPDH gene was determined to standardize for initial variance in sample as an endogenous control gene for productivity of the reaction [20].
The SPSS.11 program was applied for all statistical analyses. The significance differences between treated groups set via One Way–ANOVA test followed by Duncan’s multiple tests [21]. The result values are presented through Mean ± SE. If p < 0.05, the finding should have been statistically significant.
3.1 Characterization of Tannin NP99 Nanoparticles
The Transmission Electrone Microscopy (TEM) analysis of the synthesized NP99 nanoparticles showed round shape (Fig. 1A) with an average size of 368.9 nm (Fig. 1B) and zeta potential of −6.97 mV (Fig. 1C).
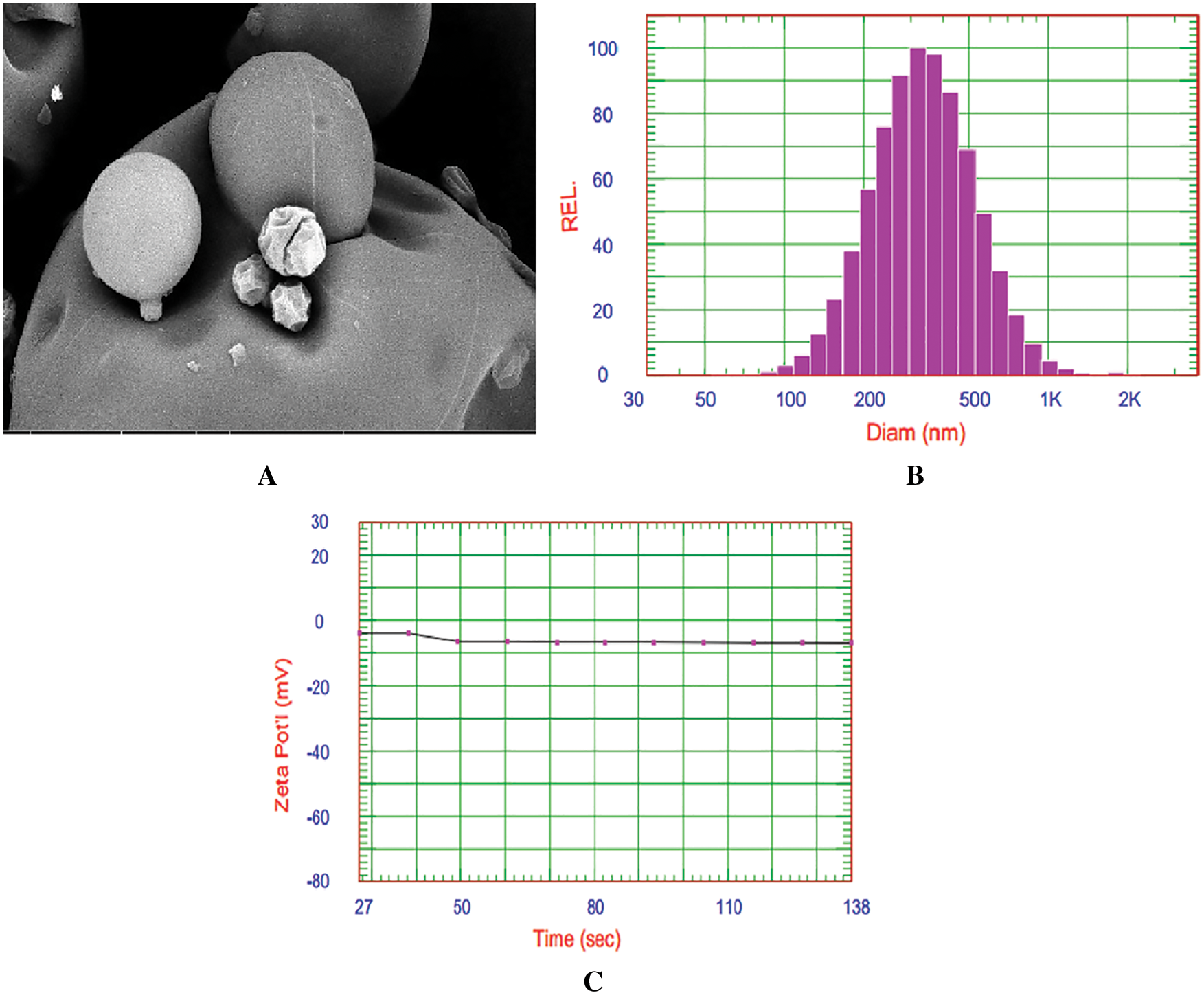
Figure 1: TEM image of NP99 (A), DLS analysis of the synthesized NP99 nanoparticles size distribution (B) and zeta potential (C) of NP99 nanoparticles
Phytochemical screening by HPLC for NP99 identified five major polyphenols namely; gallic acid, catechin, syringic acid, pyrocatechol, naringenin, and ellagic acid in concentrations of 20.02 ± 2.3, 68.67 ± 3.2, 8.24 ± 1.2, 13.14 ± 1.7, 4.95 ± 0.22, and 4.40 ± 0.12 mg/g, respectively (Figs. 2 and 3). Tannins are composed of oligomers whose behavior is dual according to their chemical structure. It is a radical scavenger and achemo-preventive agent as well as it can generate oxidative stress in specific conditions due to its polyphenolic components [22]. On the base of this fact, several reports have confirmed that tannin can be considered as anti-carcinogenic agent. Moreover, other researchers demonstrated that tannin has proapoptotic action by generating reactive oxygen species and by activating endogenous pathways as reported by Chen et al. [23]. The present study data demonstrated that, NP99 extract at 18 μg/ml dose significantly decreased the MCF-7 cells proliferation and viability percentage by about 58% and 73% after treated for either 48 or 72 h.
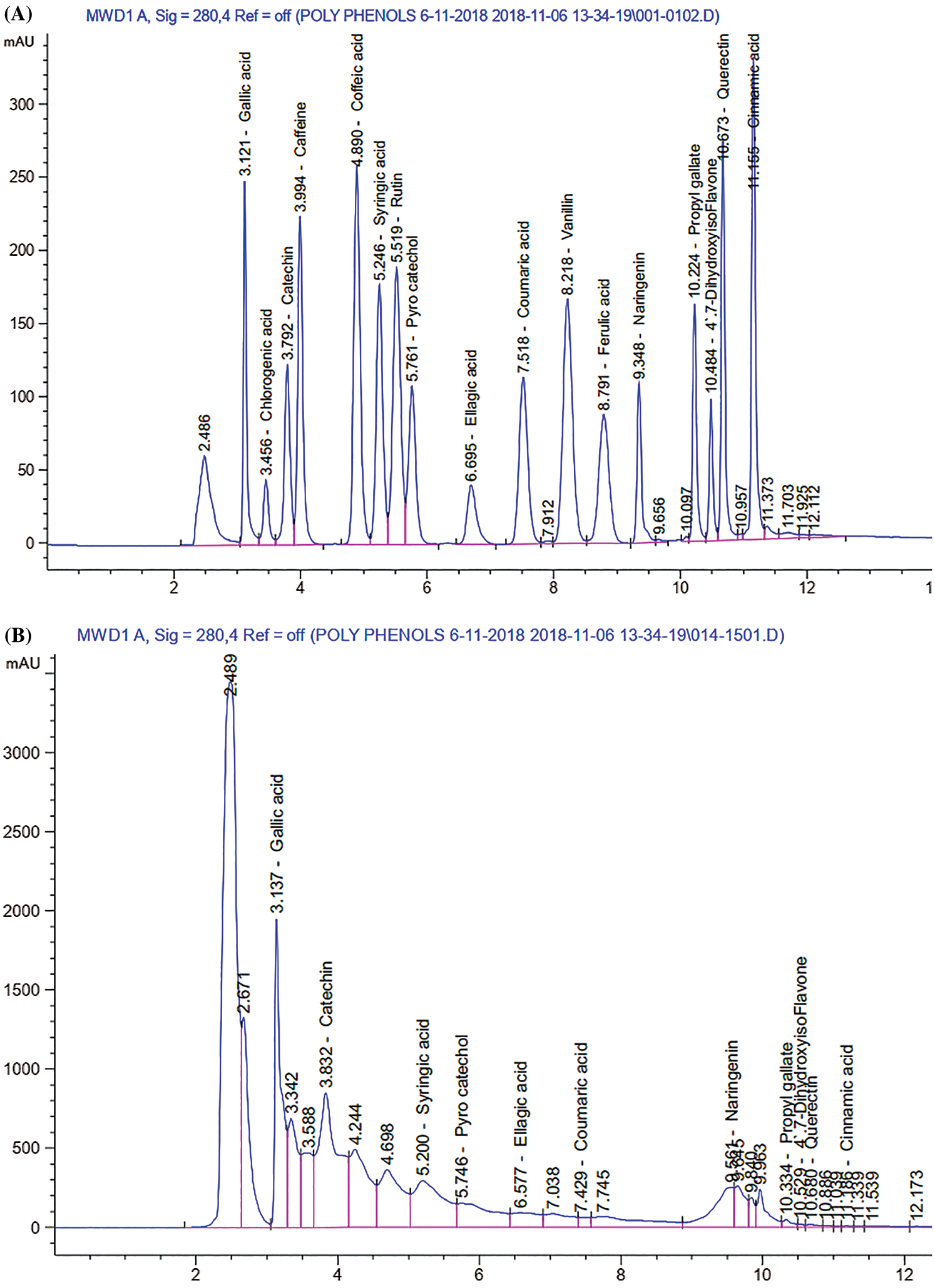
Figure 2: HPLC chromatogram of (A): Standard polyphenols compounds, and (B) Tannin nano-particles represent the different component of polyphenolic contents
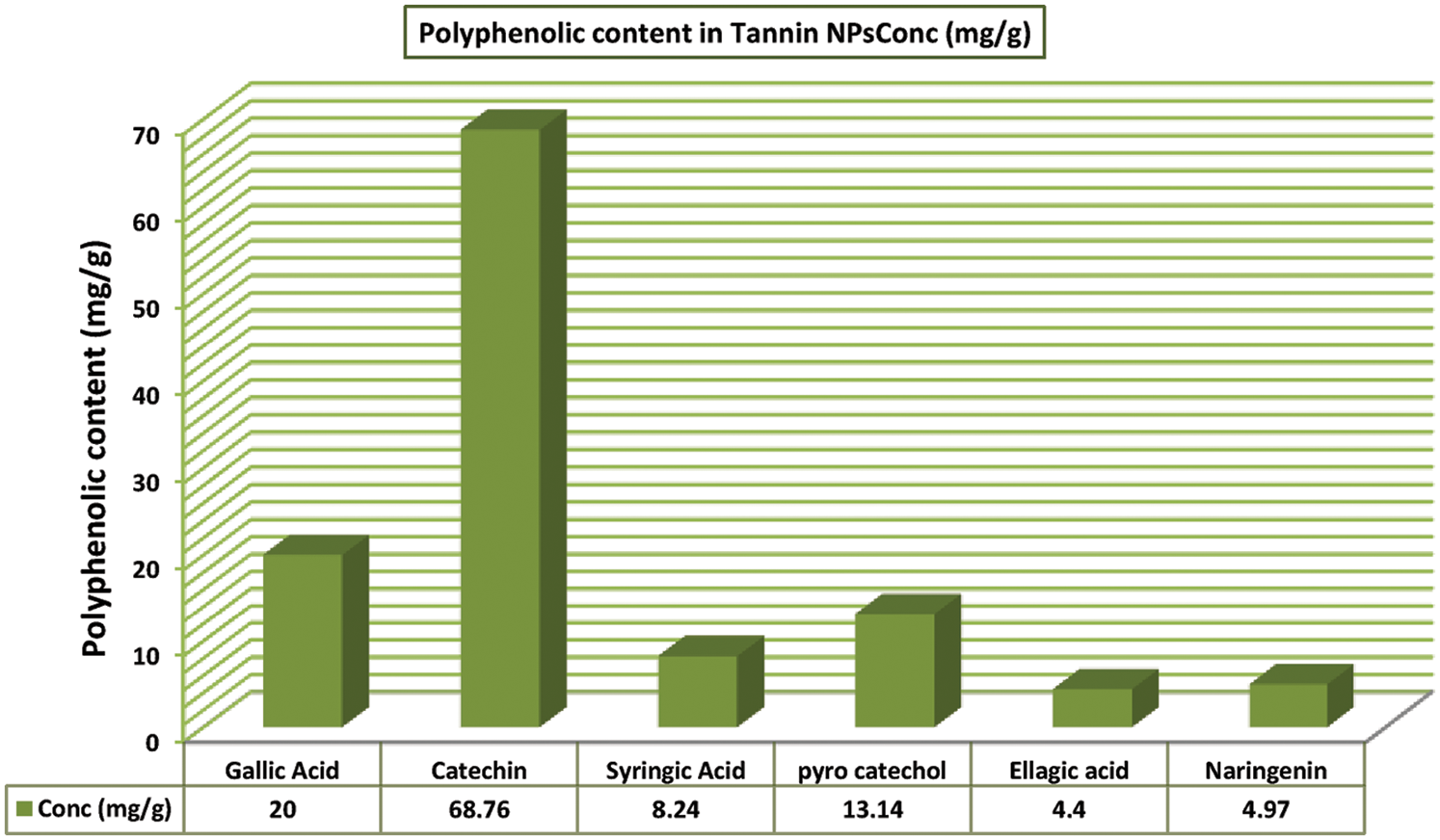
Figure 3: Histogram represents the main polyphenolic components in Tannin nanoparticles (NP99)
3.3 Effects of IC50 of NP99 on the Proliferation and Viability Percentage in MCF-7 Cells
Different concentrations and exposure times were used to examine the cytotoxic effect and IC50 value of tannin nanoparticles (NP99) on MCF-7 cancer cell. The collected data, using Trypan blue staining, are listed in Tab. 2 which revealed that NP99 treatments significantly reduce cell growth in a dose and time- dependent when compared with control cells (Fig. 4). The recorded IC50 values of NP99 were 24, 18 and 12 μg/mL after incubation for 24, 48 and 72 h, respectively. Moreover, the effect of NP99 on the viability of cancer cells was also investigated using MTT assay and the collected data are shown as IC50 (Tab. 2 and Fig. 4). These results showed a significant decrease in the viability of the cells after 24 h at all the tested doses of NP99 except the dose of 6.0 μg/mL.
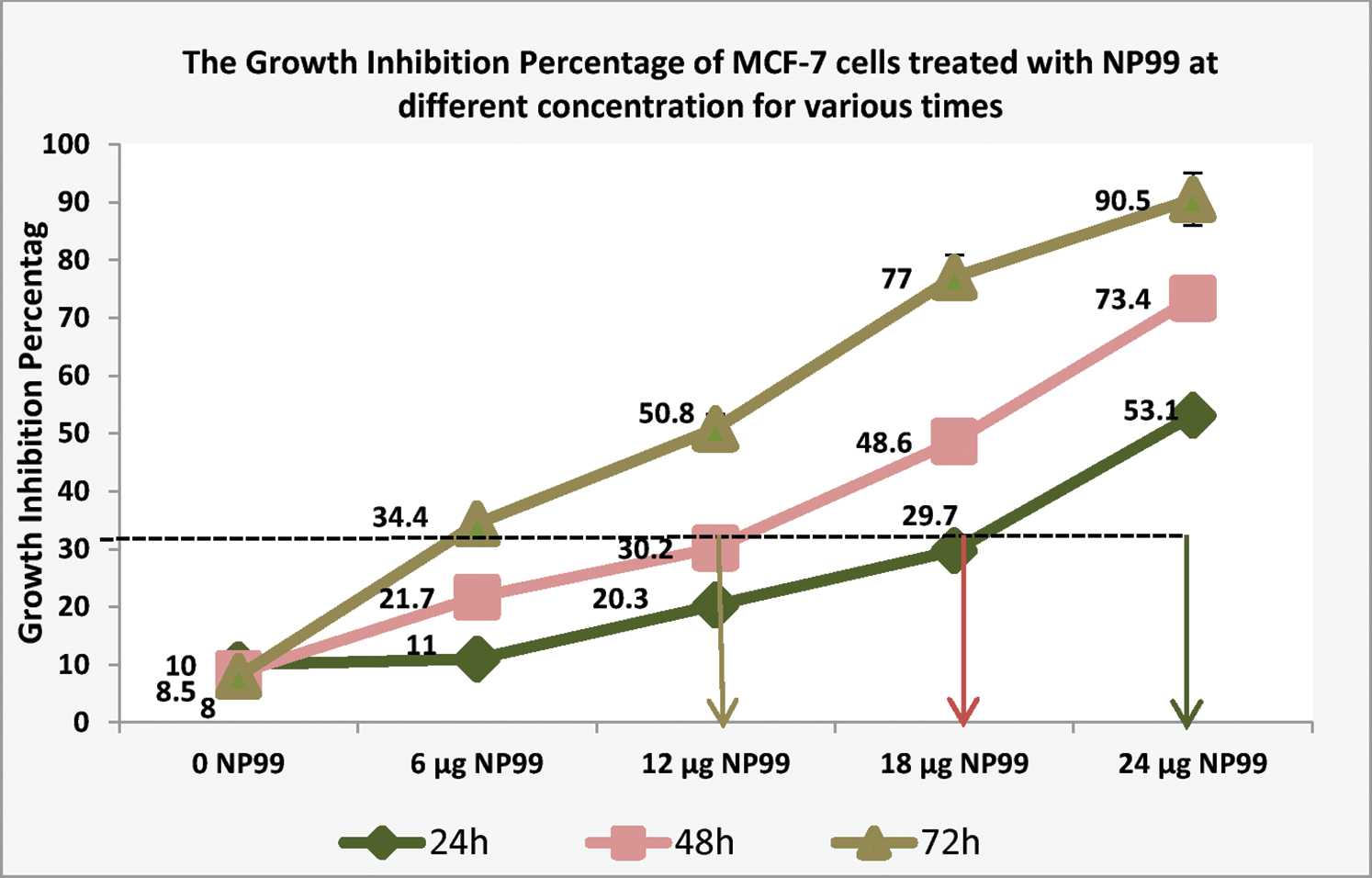
Figure 4: Histogram represents growth inhibition percentage of MCF-7 cells treated with NP99 at different concentration in various times to determine the IC50 of NP99 indicating that it was 12, 18 and 24 µg/mL when NP99 treated MCF-7 for 72, 48 and 24 h, respectively
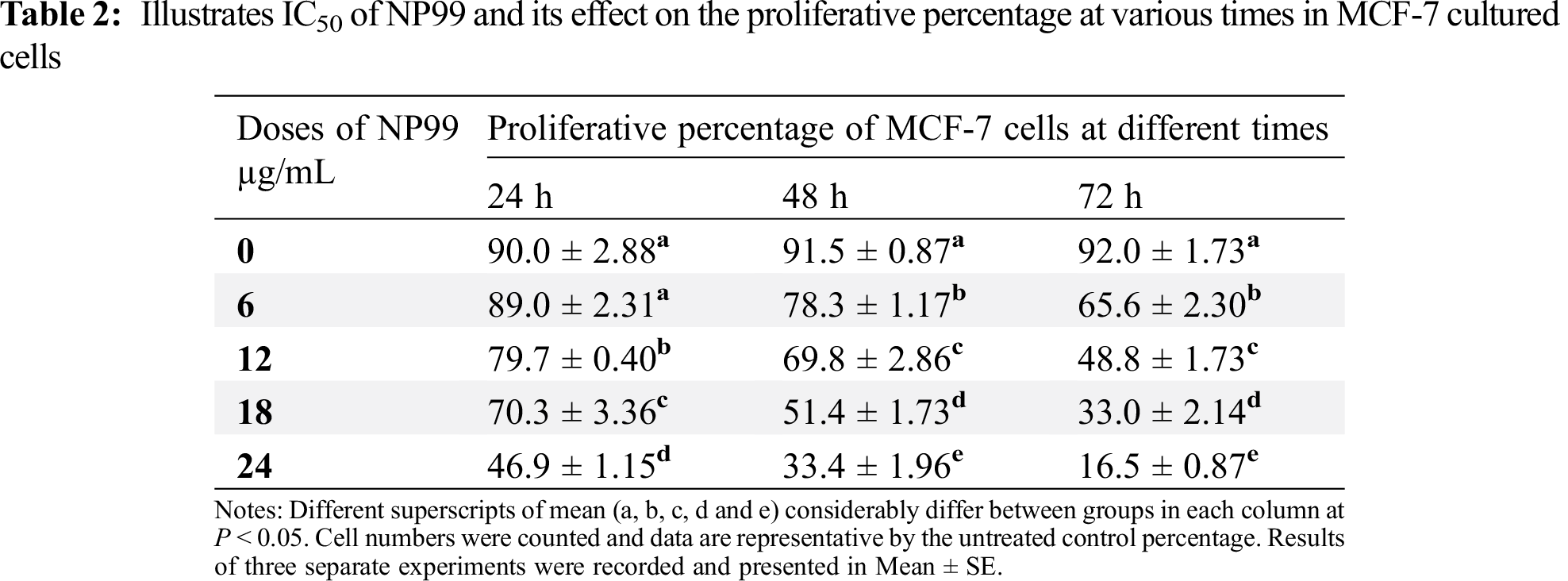
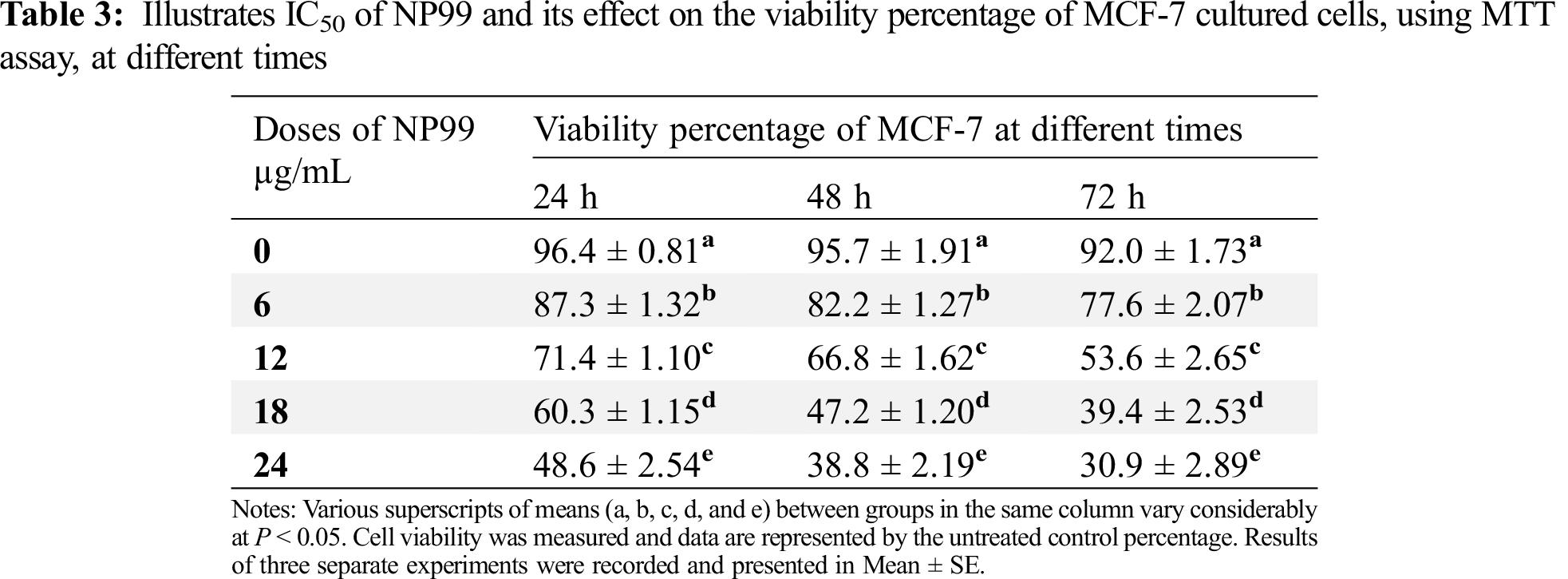
The presented data also showed that the maximum growth inhibition and decreased MCF-7 cells viability were obtained after NP99 treatment for 72 h at a dose of 18 and 24 μg/mL (Tab. 3 and Fig. 5). Therefore, the concentration of 18 μg/mL of NP99 was applied for further investigations.
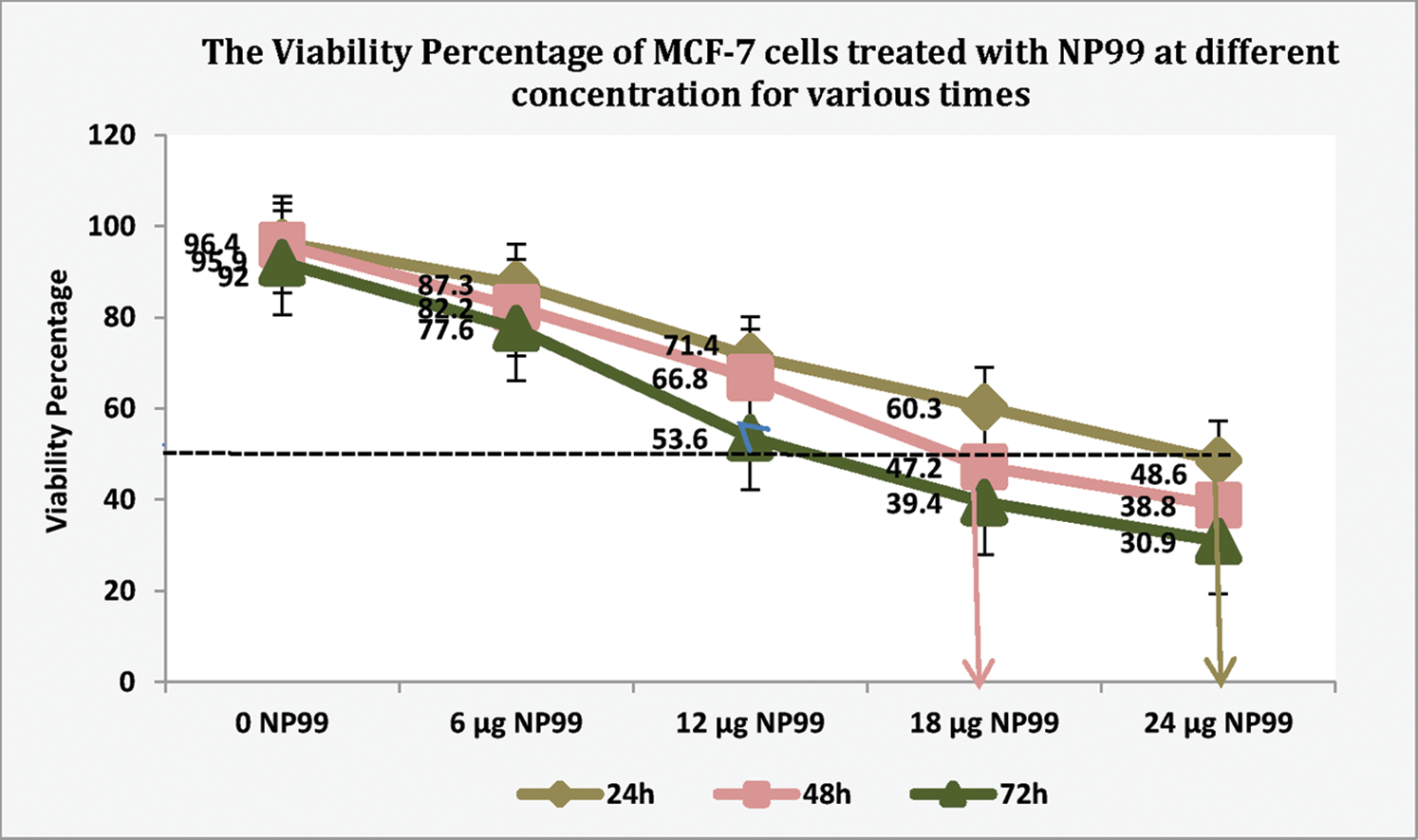
Figure 5: The Viability percentage in MCF-7 cells treated with NP99 for different times at various concentrations to determine the IC50
3.4 Proliferation and Viability Percentage in MCF-7 Cells Treated with Tam and NP99
To study the proliferation and viability effects of Tam drug and NP99; either individually or in combination, on cancer cell, MCF-7 cells were grown and treated with Tam at two different doses; the therapeutic (10 μg/mL) and at 1/10th of the therapeutic dose (1 μg/mL) [15]. Comparing to control group, Tam, either at one of the already mentioned doses, reduced the percentage of MCF-7 cells viability in time and concentration-dependent manner (Tabs. 4 and 5), as well as inhibited the cells proliferation by 30%, 43% and 60% after a treatment lasted for 24, 48 or 72 h, as seen in Tab. 4 and Fig. 6. However, the maximum reduction in cells viability and proliferation were obtained after the cell being treated for 72 h (Figs. 5 and 6).
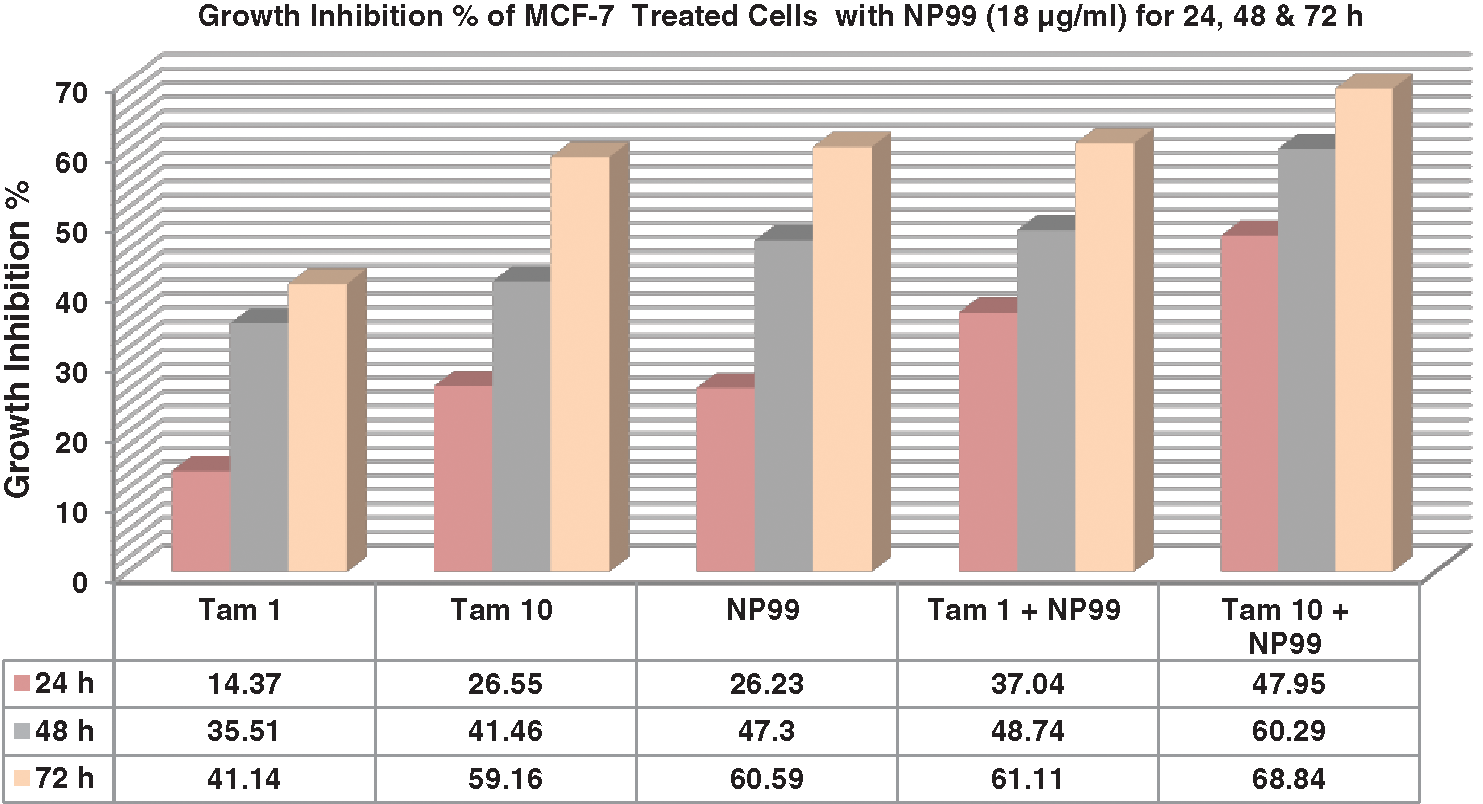
Figure 6: Column chart illustrate the effects of Tam and NP99 treatment on growth inhibition percentage of MCF-7 cultured cells after 24, 48 & 72 h. Results of three separate experiments were recorded and presented in Mean ± SE; Tam: Tamoxifen
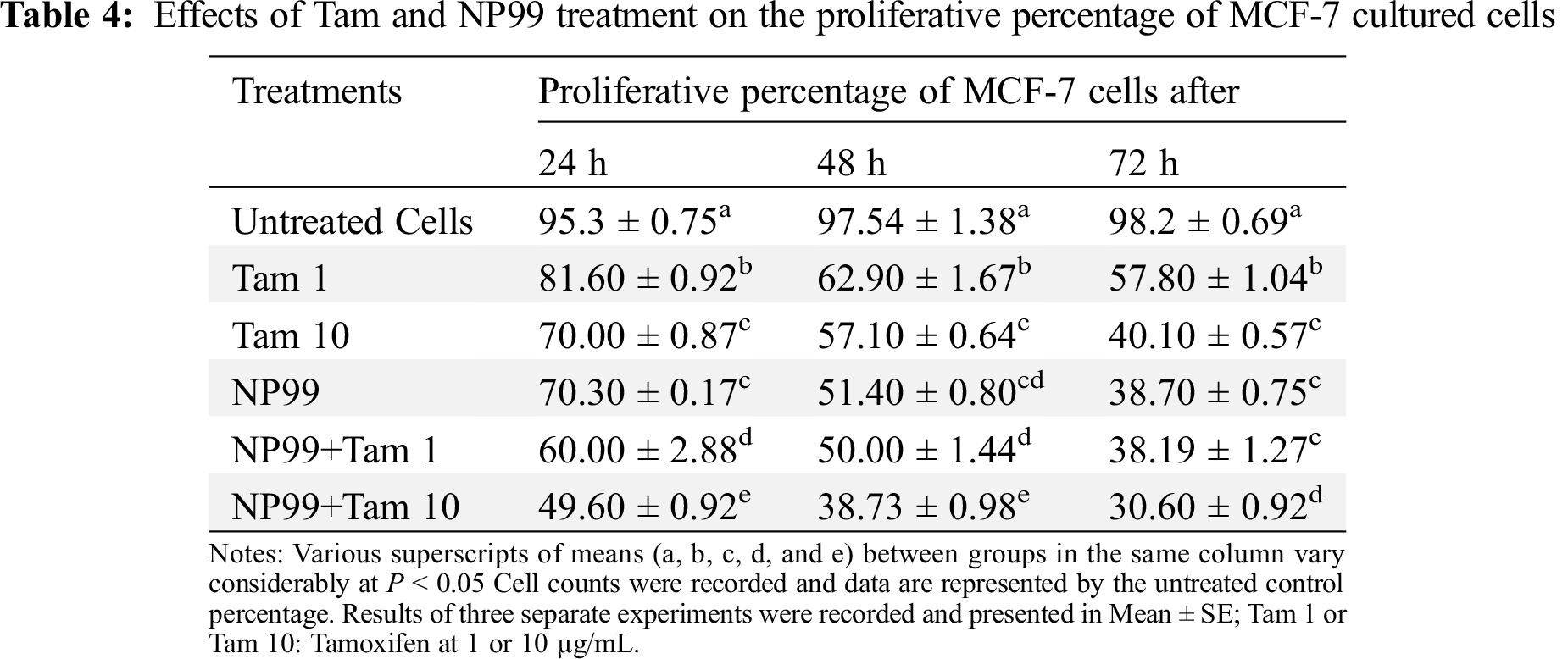
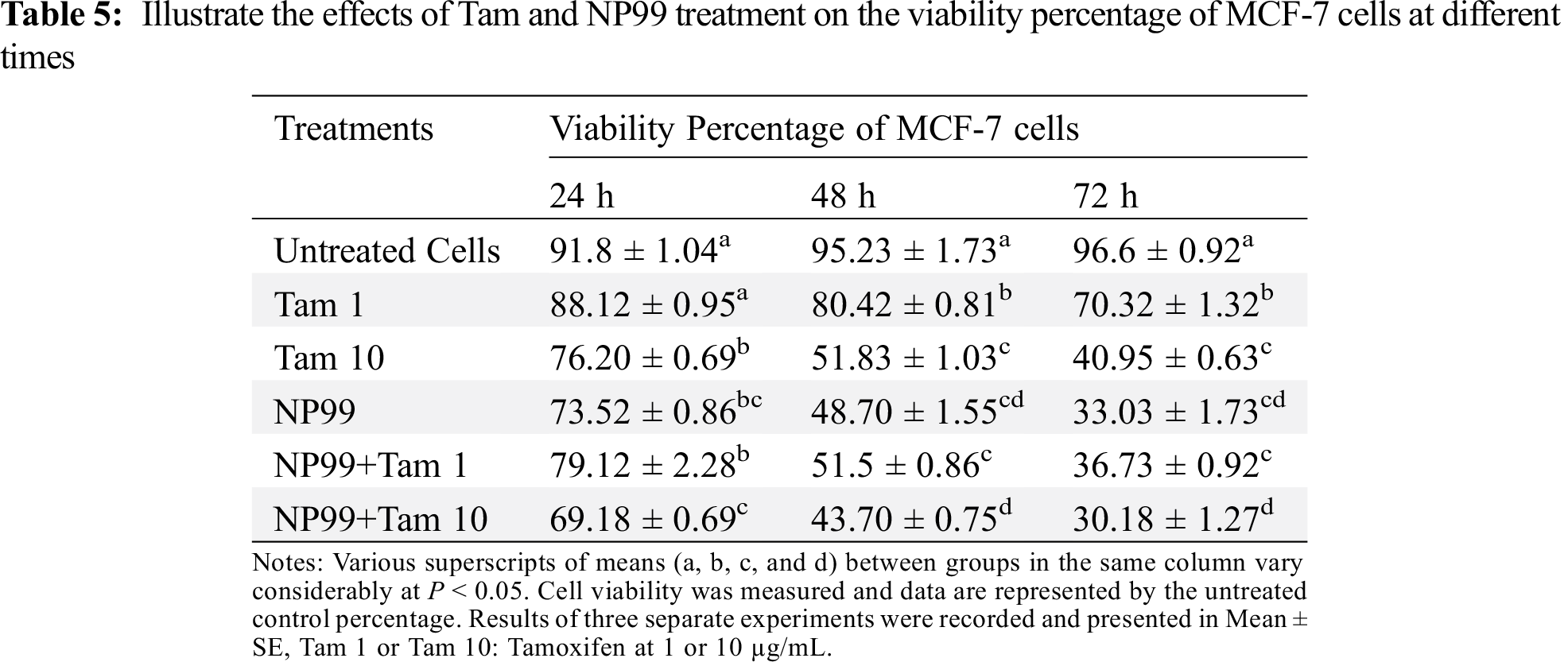
MCF-7 cells also were treated with the NP99 chosen dose 18 μg/mL (IC50value) for 24, 48 and 72 h. These time-dependent treatments significantly increased the inhibition of growth and cells viability (P < 0.05) in a duration based time (Tabs. 4, 5 and Fig. 5). Additionally, combination treatments of Tam and NP99 on the MCF-7 cell count, proliferation and viability showed a more prominent inhibition in the growth of treated cells as presented in Tabs. 4 and 5. These results consistent with those reported by Jordan and colleagues [24] who investigated the inhibitory effects of Tam on the growth of BC cells in vitro [24]. Cosan et al. [25] reported that, tannic acid has anti-cancer and apoptotic activity in their study on one type of BC cells and colon cancer cells which in agreement with our findings.
Another study by Bawadi et al. [26] examined the effects of Tannic acid (hydrolysable tannin, not a polyflavonoid) on three types of cells: breast cancer MCF-7 cell line, triple negative BC cells MDA-MB-231 and normal cells MCF-10A. Those authors demonstrated that, the apoptosis induced in breast epithelial cells via activation of caspase 9 and 7 and MCF-7 cell line was more susceptible to Tannic acid comparing with the other two cell types which is confirmed the results of the present study.
3.5 Morphological Changes in MCF-7 Treated Cells
The treated cancer cells were morphologically different from the untreated one; whereby the untreated MCF-7 cells were irregular polygonal and fusiform grown firmly adherent. The cells were bright and entirely stretched with intact membrane and obvious nuclei (Fig. 7a). Treatments with Tam and NP99, both individual or in combination methods affected the cells morphology distinctly. These changes represent the cells death and can be noticed using inverted microscope that includes blabbing, cell shrinkage and cellular detachment. Additionally, the combination treatment for 72 h induced clearly cytotoxic effects such as shrunken cells and decreased in the cellular crowding and cells number (Fig. 7d). Death of treated cancer cells induced through several linked molecular pathways and cell signaling pathways [27]. In the current investigation, it was found that apoptosis was the suggested mechanism of cells death caused after exposure to NP99 as represented by the previous morphological changes indicated in the cancer cells.
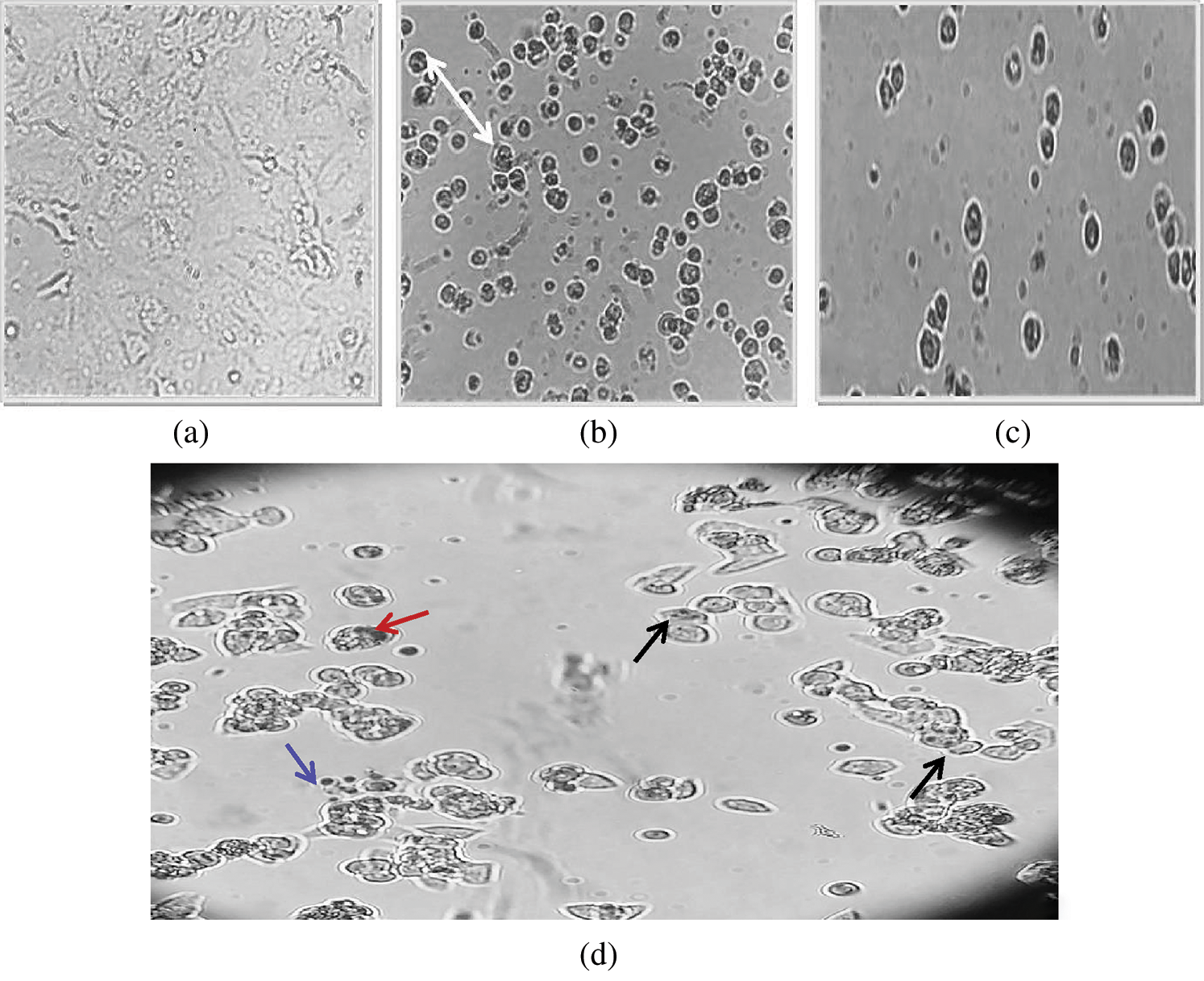
Figure 7: Inverted microscopic photographs of MCF-7 cells untreated (a) and treated with Tam10 (b); NP99 (c) and with Tam 10 & NP99 after 48 h. In (d): Arrow’s head pointed to the various alterations of apoptosis particularly compressed and fragmented nuclei (Red and Black Arrows, respectively), and apoptotic bodies (Blue Arrow)
3.6 DNA Fragmentation Percent Induced in Treated Cancer Cells
Nuclear DNA degraded into pieces by the action of nuclear endonucleases is considered a hallmark of apoptotic cells death. DNA fragmentation assay was applied on MCF-7 cells in all treated groups to detect whether NP99 decreases cells number and changes cell morphology in MCF-7 cells by inducing apoptosis. DNA fragments were detected by either comparing DNA profile on agarose gel electrophoresis that was observed as a ladder pattern (Fig. 8) or by measuring the level of fragmented DNA colorimetrically by diphenylamine reagent. The DNA fragmentation percent was calculated and the results were analysed as presents in Tab. 6 and Fig. 8. However, the level of DNA fragmentation appears more prominent in the combination treatment with Tam 10 μg/mL and NP99.
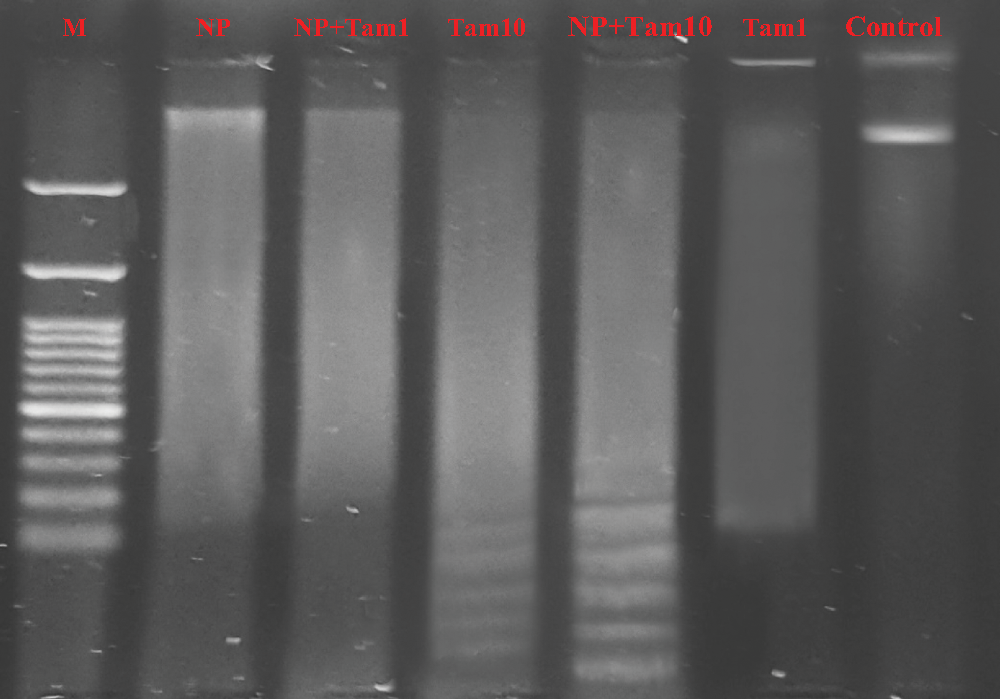
Figure 8: Agarose gel electrophoresis showing DNA profile on agarose gel electrophoresis that was observed as a smear pattern induced in MCF-7 cells treated with different treatment at the indicated doses after 48 h: Lane 1: Control showing non fragmented DNA band; Lane 2 (Tam 1): Effect of Tam 1 μg/mL, with low density smear DNA; Lane 3 (Tam 10 + NP): Effect of Tam 10 μg/mL+ NP9918 μg/mL showing a high density of fragmented DNA with a a ladder pattern. Lane 4 (Tam 10): Effect of Tam 10 μg/mL, Lane 5 (Tam 1 + NP): Effect of Tam 1 μg/mL+ NP99 18 μg/mL, Lane 6 (NP): Effect of NP99 18 μg/mL, and Lane 7 (M): DNA marker
3.7 Expression of Genes Related to the Apoptosis in Treated MCF-7 Cells
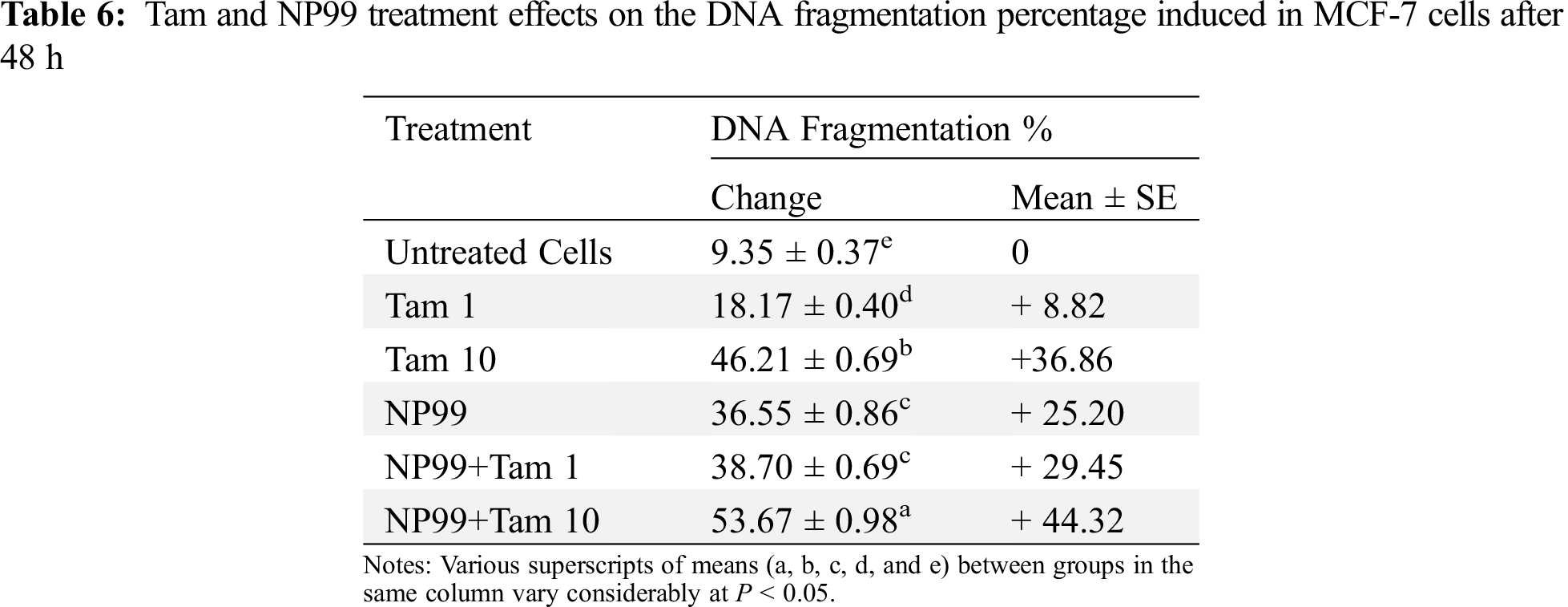
Bcl-2 (B-cell lymphoma 2) and Bax (Bcl-2 associated x) are members of Bcl-2 gene family that act as anti- and pro- apoptotic regulators, respectively. The balance between these two genes controls the cell growth and death. The expression of these genes at the mRNA level in MCF-7 treated cells were examined and analyzed with semi-quantitative PCR. In one hand, the treated cells with Tam (1 μg/mL) showed insignificant result in the Bax transcription level (Fig. 9). On the other hand, MCF-7 cells treated with NP99 and/or Tam 10 μg/mL for 48 h presented marked elevation in Bax and inhibition in the Bcl-2 mRNA expression levels (Figs. 9 and 10) and increased in the Bax mRNA expression level. Consequently, the Bcl-2/Bax ratio was significantly decreased as shown in Fig. 11.
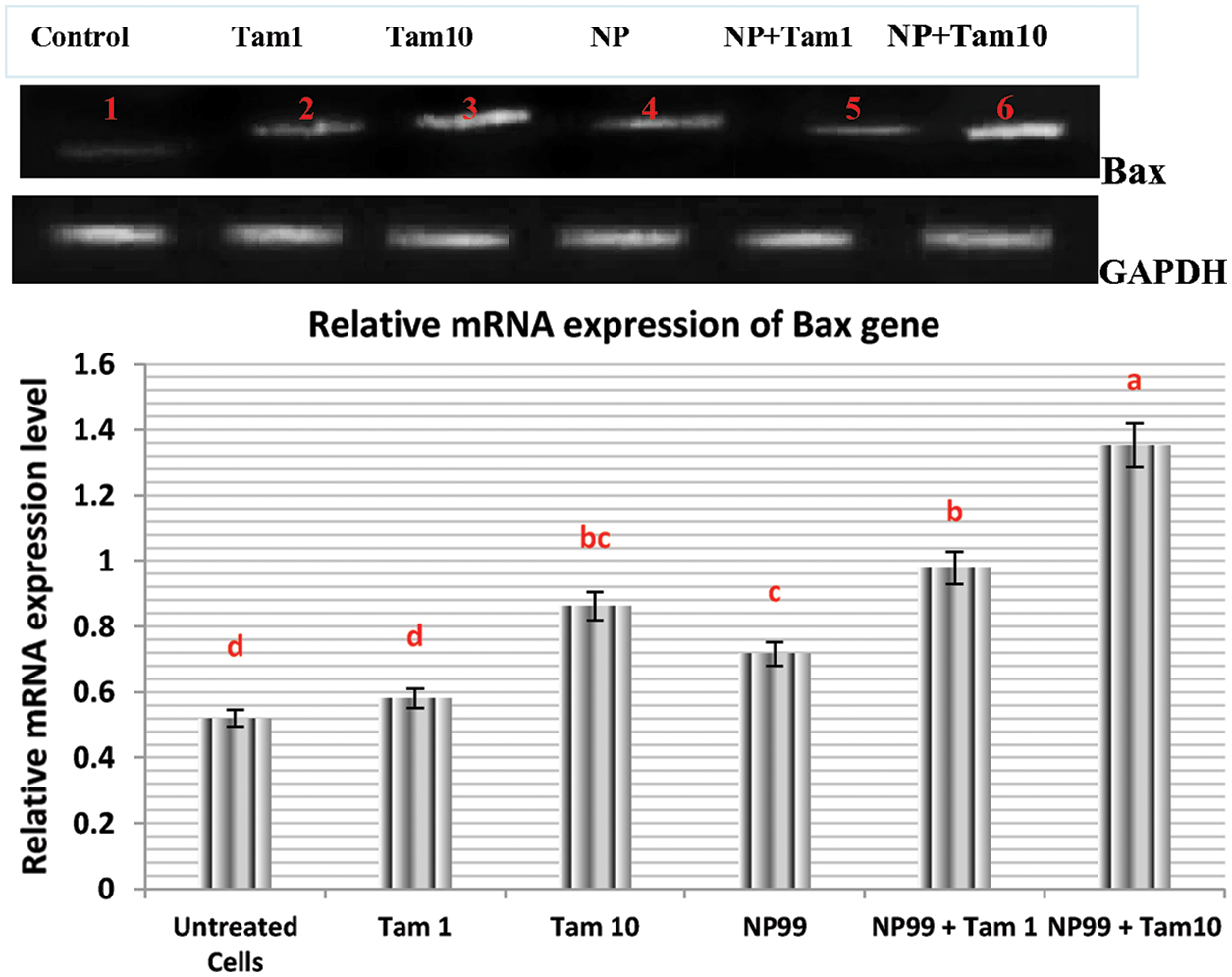
Figure 9: Photograph and histogram showing the effects of Tam and NP99 treatment on the relative transcription level of Bax gene in MCF-7 treated cells for 48 h. Results of three separate experiments were recorded and presented in Mean ± SEM. In photograph the mRNA expression level of Bax apoptotic gene were determined by sq-RT-PCR and the product of PCR amplification runs on a 1.5% agarose gel and envisioned on Trans-illuminator UV. DNA bands were scanned with Gel-Pro program (version 3.1 for Windows 3) to quantify the signal intensitiesin different groups of control untreated and treated MCF. Lane 1: Control untreated MCF-7 cell; Lane (2) Tam 1: Effect of Tam 1 μg/ml; Lane (3) Tam 10: Effect of Tam 10 μg/mL, Lane (4) NP: Effect of NP99 18 μg/mL, Lane (5) Tam 1 + NP: Effect of Tam at 1 μg/mL + NP99 at 18 μg/mL, Lane (6) Tam 10 + NP: Effect of Tam at 10 μg/mL + NP99 18 μg/mL showing a high up-regulation in relative expression of Bax apoptotic regulation gene
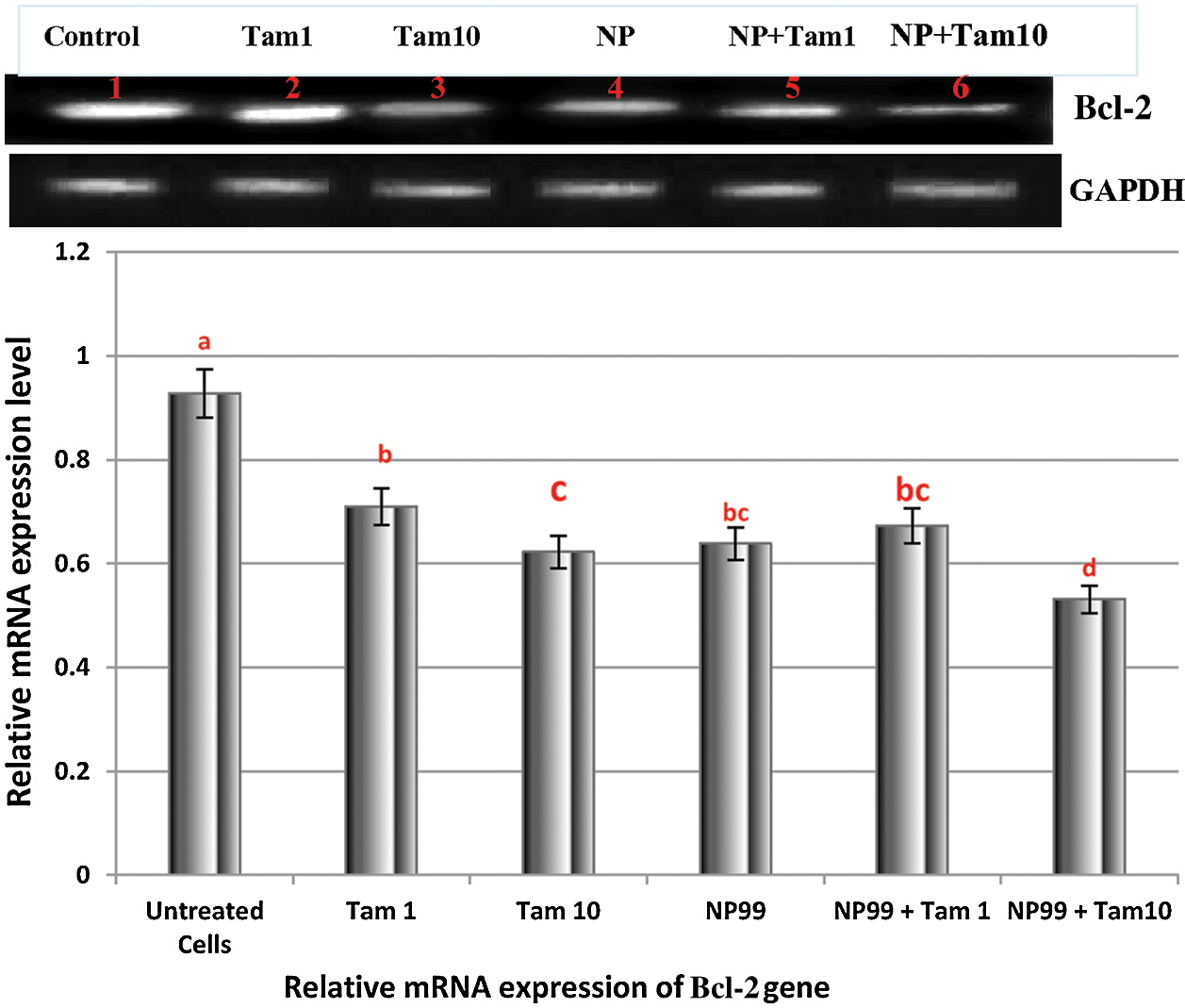
Figure 10: Photograph and histogram showing the effects of Tam and NP99 treatment on the relative transcription level of Bcl-2 gene in MCF-7 treated cells for 48 h. Results of three separate experiments were recorded and presented in Mean ± SEM. In photograph the mRNA expression level of Bcl-2 gene were determined by sq-RT-PCR and the product of PCR amplification runs on a 1.5% agarose gel and envisioned on Trans-illuminator UV. DNA bands were scanned with Gel-Pro program (version 3.1 for Windows 3) to quantify the signal intensities in different groups of control untreated and treated MCF-7 cells. Lane (1): Control untreated MCF-7 cells; Lane (2) Tam 1: Effect of at Tam 1 μg/mL; Lane (3) Tam 10: Effect of Tam at 10 μg/mL, Lane (4) NP: Effect of NP99 at 18 μg/mL, Lane (5) Tam 1 + NP: Effect of Tam 1 μg/mL + NP99 18μg/mL, Lane (6) Tam 10 + NP: Effect of Tam 10 μg/mL + NP99 18 μg/mL showing a lowest down-regulation in relative expression of Bcl-2 gene
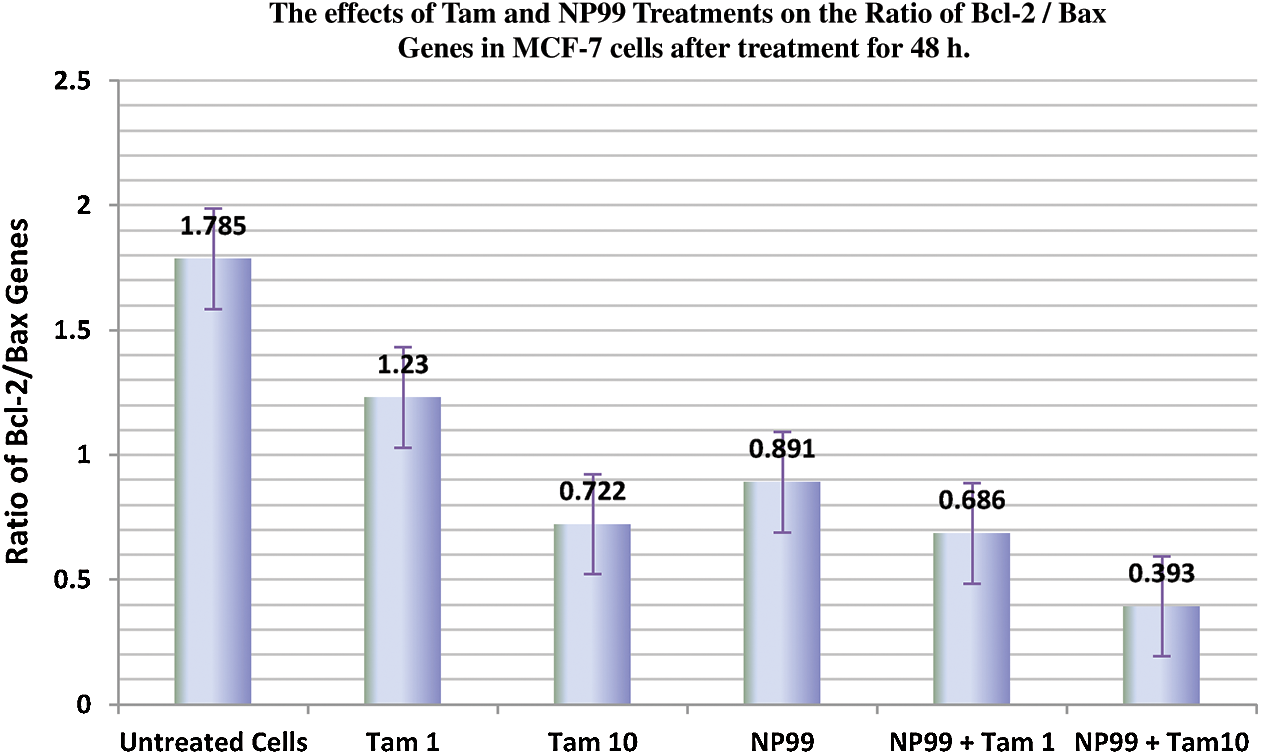
Figure 11: Histogram showing the effects of Tam and NP99 treatments on the relative transcription levels of Bcl-2 and Bax Ratio in MCF-7 cells after treatment for 48 h. The Bcl-2/Bax ratio was significantly decreased in MCF-7 cells treated with Tamoxifen and/or NP99, each individually at target doses, and the group treated with Tam 10 μg/mL + NP99 18 μg/mL showing a lowest down-regulation in the Bcl-2/ Bax ratio
The apoptosis process that controlled by Bcl-2 family genes through inhibiting or stimulating the releasing of cytochrome c from the mitochondria to the cytoplasm. However, such migration of cytochrome C suppressed by Bcl-2 anti-apoptotic gene and accelerated by the Bax proapoptotic gene [28]. The present finding indicated that, Bcl-2 expression was decreased and Bax expression was increased, whereas the ratio of Bcl-2/Bax was decreased significantly in treated breast cancer cells. According to these results, NP99 can be act as down regulator of Bcl-2 gene and up-regulator of Bax gene which may induce apoptosis in MCF-7 cancer cells.
It can be concluded that, tannin nanoparticles, as a natural flavonoids, have a potential anticancer effect against MCF-7 cells at a dose as low as 18 µg along with the therapeutic drug tamoxifen at the lowest dose. The combination between the natural condensed flavonoids tannin extract properties at nanometer sizes showed a synergistic effect with the drug and should be considered in the treatment of cancer. Therefore, long term studies in vitro and in vivo are required to determine the physiological properties of Tannin extract in normal and nanoparticles forms which can establish a new platform in treatment of human breast cancer and/or other types of cancer.
Phytochemical screening for natural condensed flavonoids Tannins extract identified five major polyphenols namely; gallic acid, catechin, syringic acid, pyrocatechol, naringenin, and ellagic acid. The combination between the natural condensed flavonoids tannin extract properties at nanometer sizes showed a synergistic effect with the anticancer drug and should be considered in the treatment of cancer.
Funding Statement: This work was supported by the Deanship of Scientific Research at Taif University, Research Supporting Project No. TURSP-2020/76, Taif University, Taif, Saudi Arabia
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Siegel, R. L., Miller, K. D., Jemal, A. (2017). Cancer statistics. A Cancer Journal for Clinicians, 67(1), 7–30. DOI 10.3322/caac.21387. [Google Scholar] [CrossRef]
2. Al Dossary, R., Alkharsah, K. R., Kussaibi, H. (2018). Prevalence of Mouse Mammary Tumor Virus (MMTV)-like sequences in human breast cancer tissues and adjacent normal breast tissues in Saudi Arabia. BMC Cancer, 18(1), 170. DOI 10.1186/s12885-018-4074-6. [Google Scholar] [CrossRef]
3. Lorenzana-Jiménez, M., Avila, M. E., Figueroa, A., Mendiola-Almaraz, L., Guerrero, G. A. M. et al. (2018). The Mexican mistletoe Struthanthusvenetus (HBK Blume) inhibits proliferation and synergizes antagonistic actions of Tamoxifen and Fulvestrant in breast cancer MCF-7 cells. Journal of Pharmacognosy and Phytotherapy, 10(8), 133–141. DOI 10.5897/JPP2018.0508. [Google Scholar] [CrossRef]
4. Saudi Cancer Registry (2018). Cancer Incidence Report in 2018, pp. 616–992. Saudi Health Council. Kingdom of Saudi Arabia. [Google Scholar]
5. Tang, Y., Wang, Y., Kiani, M. F., Wang, B. (2016). Classification, treatment strategy, and associated drug resistance in breast cancer. Clinical Breast Cancer, 16(5), 335–343. DOI 10.1016/j.clbc.2016.05.012. [Google Scholar] [CrossRef]
6. Kaklamani, V. G., Gradishar, W. J. (2017). Endocrine therapy in the current management of postmenopausal estrogen receptor-positive metastatic breast cancer. The Oncologist, 22(5), 507–517. DOI 10.1634/theoncologist.2015-0464. [Google Scholar] [CrossRef]
7. Wen, C., Wu, L., Fu, L., Zhang, X., Zhou, H. (2016). Berberine enhances the anti-tumor activity of tamoxifen in drug-sensitive MCF-7 and drug-resistant MCF-7/TAM cells. Molecular Medicine Reports, 14(3), 2250–2256. DOI 10.3892/mmr.2016.5490. [Google Scholar] [CrossRef]
8. Glassman, D., Hignett, S., Rehman, S., Linforth, R., Salhab, M. (2017). Adjuvant endocrine therapy for hormone-positive breast cancer, focusing on ovarian suppression and extended treatment: An update. Anticancer Research, 37, 5329–5341. DOI 10.21873/anticanres.11959. [Google Scholar] [CrossRef]
9. Singh, K. S., Singh, S., Lillard, J. W., Singh, R. (2017). Drug delivery approaches for breast cancer. International Journal of Nanomedicine, 12, 6205–6218. DOI 10.2147/IJN.S140325. [Google Scholar] [CrossRef]
10. Khosropanaha, M. H., Dinarvanda, A., Nezhadhosseinia, A. (2016). Analysis of the antiproliferative effects of curcumin and nanocurcumin in mda-mb231 as a breast cancer cell line. Iranian Journal of Pharmaceutical Research, 15(1), 231–239. [Google Scholar]
11. Rizvi, S. A., Saleh, A. M. (2018). Applications of nanoparticle systems in drug delivery technology. Saudi Pharmaceutical Journal, 26(1), 64–70. DOI 10.1016/j.jsps.2017.10.012. [Google Scholar] [CrossRef]
12. Kwon, Y. (2018). Food-derived polyphenols inhibit the growth of ovarian cancer cells irrespective of their ability to induce antioxidant responses. Heliyon, 4(8), e00753. DOI 10.1016/j.heliyon.2018.e00753. [Google Scholar] [CrossRef]
13. Sharma, A., Kaur, M., Katnoria, J. K., Nagpal, A. K. (2018). Polyphenols in food: Cancer prevention and apoptosis induction. Current Medicinal Chemistry, 25(36), 4740–4757. DOI 10.2174/0929867324666171006144208. [Google Scholar] [CrossRef]
14. Walle, T., Ta, N., Kawamori, T., Wen, X., Tsuji, P. A. et al. (2007). Cancer chemo preventive properties of orally bioavailable flavonoids–Methylated vs. unmethylated flavones. Biochemical Pharmacology, 73(9), 1288–1296. DOI 10.1016/j.bcp.2006.12.028. [Google Scholar] [CrossRef]
15. Ichikawa, A., Ando, J., Suda, K. (2008). G1 arrest and expression of cyclin-dependent kinase inhibitors in tamoxifen-treated MCF-7 human breast cancer cells. Human Cell, 21(2), 28–37. DOI 10.1111/j.1749-0774.2008.00048.x. [Google Scholar] [CrossRef]
16. Yu, J., Guo, Q. L., You, Q. D., Zhao, L., Gu, H. Y. (2007). Gambogic acid-induced G2/M phase cell-cycle arrest via disturbing CDK7-mediated phosphorylation of CDC2/p34 in human gastric carcinoma BGC-823 cells. Carcinogenesis, 28(3), 632–638. DOI 10.1093/carcin/bgl168. [Google Scholar] [CrossRef]
17. Burton, K. (1956). A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. The Biochemical Journal, 62(2), 315–323. DOI 10.1042/bj0620315. [Google Scholar] [CrossRef]
18. Perandones, C. E., Illera, V. A., Peckham, D., Stunz, L. L., Ashman, R. F. (1993). Regulation of apoptosis in vitro in mature murine spleen T cells. Journal of Immunology, 151(7), 3521. [Google Scholar]
19. Sun, Y. F., Song, C. K., Viernstein, H., Unger, F., Liang, Z. S. (2013). Apoptosis of human breast cancer cells induced by microencapsulated betulinic acid from sour jujube fruits through the mitochondria transduction pathway. Food Chemistry, 138(2–3), 1998–2007. DOI 10.1016/j.foodchem.2012.10.079. [Google Scholar] [CrossRef]
20. Raben, N., Nichols, R. C., Martiniuk, F., Plotz, P. H. (1996). A model of mRNA splicing in adult lysosomal storage disease (glycogenosis type II). Human Molecular Genetics, 5(7), 99–1001. DOI 10.1093/hmg/5.7.995. [Google Scholar] [CrossRef]
21. Walter, R. A., Duncan, D. B. (1969). A Bayes rule for the symmetric multiple comparison problems. Journal of the American Statistical Association, 64(328), 1484–1503. DOI 10.1080/01621459.1969.10501073. [Google Scholar] [CrossRef]
22. Bouki, E., Dimitriadis, V. K., Kaloyianni, M., Dailianis, S. (2013). Antioxidant and pro-oxidant challenge of tannic acid in mussel hemocytes exposed to cadmium. Marine Environmental Research, 85, 13–20. DOI 10.1016/j.marenvres.2012.12.005. [Google Scholar] [CrossRef]
23. Chen, Y. C., Chien, L. H., Huang, B. M., Chia, Y. C. (2014). Toonasinensis (aqueous leaf extracts) induces apoptosis through the generation of ROS and activation of intrinsic apoptotic pathways in human renal carcinoma cells. Journal of Functional Foods, 7, 362–372. DOI 10.1016/j.jff.2014.01.024. [Google Scholar] [CrossRef]
24. Jordan, V. C. (2014). Tamoxifen as the first targeted long-term adjuvant therapy for breast cancer, Endocrin Related Cancer, 21(3), 235–246. DOI 10.1530/ERC-14-0092. [Google Scholar] [CrossRef]
25. Cosan, D., Soyocak, A., Basaran, A., Degirmenci, I., Gunes, H. V. (2009). The effects of resveratrol and tannic acid on apoptosis in colon adenocarcinoma cell line. Saudi Medical Journal, 30, 191–195. [Google Scholar]
26. Bawadi, H. A., Bansode, R. R., Trappey, A., Truax, R. E., Losso, J. N. (2005). Inhibition of Caco-2 colon, MCF-7 and Hs578T breast, and DU 145 prostatic cancer cell proliferation by water-soluble black bean condensed tannins. Cancer Letters, 218(2), 153–162. DOI 10.1016/j.canlet.2004.06.021. [Google Scholar] [CrossRef]
27. Mense, S. M., Hei, T. K., Ganju, R. K., Bhat, H. K. (2008). Phytoestrogens and breast cancer prevention: Possible mechanisms of action. Environmental Health Perspectives, 116(4), 426–433. DOI 10.1289/ehp.10538. [Google Scholar] [CrossRef]
28. Aranovich, A., Liu, Q., Collins, T., Geng, F., Dixit, S. et al. (2012). Differences in the mechanisms of proapoptotic BH3 proteins binding to Bcl-XL and Bcl-2 quantified in live MCF-7 cells. Molecular Cell. 2012, 45, 754–763. DOI 10.1016/j.molcel.2012.01.030. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |