
Materials

 | Journal of Renewable Materials |  |
DOI: 10.32604/jrm.2021.016811
ARTICLE
Chemically Modified Lignin: Correlation between Structure and Biodegradability
1Key Laboratory of Biomaterials of Guangdong Higher Education Institutes, Guangdong Provincial Engineering and Technological Research Center for Drug Carrier Development, Department of Biomedical Engineering, Jinan University, Guangzhou, China
2Hunan Engineering Technology Research Center for Comprehensive Development and Utilization of Biomass Resources, Hunan University of Science and Engineering, Yongzhou, China
*Corresponding Author: Hui Li. Email: october_hui@126.com
Received: 28 March 2021; Accepted: 06 May 2021
Abstract: Lignin is the most abundant heteropolymer based on aromatic subunits in nature. Large quantities of lignin are annually produced from pulping processes and biorefinery industries. Its unclearly defined structure and difficult biodegradation mainly limit its utilization. This work focused on the effect of hydroxylation of lignin on its microbial degradation. Butyloxy carbonyl-modified lignin, and hydroxylated-lignin were synthesized with di-tert-butyl dicarbonate and hydrogen peroxide, respectively, using lignin as raw material. The degradation of the modified-lignins both by P. chrysosporium and B. subtilis were analyzed using UV-vis spectroscopy. Results revealed that the lignin degradation velocity raises with the increase hydroxylation level of lignin. Moreover, FTIR and 1H NMR analysis of the biodegradation products of lignin further indicated that higher content of hydroxyl groups in lignin facilitated the demethylation combined with the aromatic ring cracking in the presence of fungus and bacteria.
Keywords: Lignin; modification; biodegradation; degradation mechanism
Lignin, a naturally occurring aromatic heteropolymer, is one of most abundant renewable biopolymers. Huge amount of lignin generated from numerous pulping processes and biorefinery industries, are usually being discharged as sewage causing environment pollution. For instance, its dark color reduces oxygen availability for aquatic organisms [1–3]. At present, lignin is mainly used for combustion to produce power, however, only 2% of lignin is used as commercial products, such as adhesives and dispersants [4]. Unfortunately, the resistance of lignin to breakdown is one of the major bottlenecks in the process because of its complex chemical structure and high stability of bonds [5]. There are many methods for the separation of lignin, including acid method, alkali method and so on. Alkali lignin, containing more methoxyl groups, less alcoholic hydroxyl groups, numerous guaiacyl and a small quantity of syringyl, is separated with lignin through alkali treatment. It has the same low reactivity as original lignin [6]. In addition, its heterogeneous structure and diversification after separation and fragmentation processes, lead to the difficulty of its commercial applications and reduce the utilization value of lignin [1]. Thus, development of efficient modification technologies of lignin for value-added applications is highly desired in recent years.
Copolymerization of lignin with other polymers is an approach to increasing the commercial potential of industrial lignin [7]. Hydroxyl moieties, acting as the key reactive functional groups in lignin, make lignin relatively polar to have a better compatibility with polar polymers. The lignin possessing higher content of hydroxyls exert more reactivity and yield higher crosslinking density when reacted with other materials [8]. Consequently, the reactivity of lignin can be regulated by modification of its -OH content. However, previous studies focused on the reactivity of lignin during the process of its chemically modification, while neglect its degradation, particularly biodegradation. Nowadays, biological degradation by some microorganisms is becoming a great alternative due to its economical and eco-friendly merits in comparison with traditional physical and chemical depolymerization of lignin [9].
Meanwhile, considering the incompatibility of lignin with other non-polar thermoplastic materials due to its high polar, many scientists tried to modify lignin by capping its hydroxyl groups such as alkylation and acetylation [8]. However, there are few researches on the relationship between biodegradation and structural modification especially the hydroxylation of lignin in recent studies. In our previous work, we have synthesized a new type of modified Butyloxy carbonyl (Boc)-modified lignin (BOC-lignin) with di-tert-butyl dicarbonate ((Boc)2O) to protect the free hydroxyls. However, we found that the presence of Boc groups in lignin significantly inhibited the lignin degradation by white rot fungus Phanerochaete chrysosporium [10]. Therefore, we assume that changing the content of hydroxyl groups in lignin framework might have impacts on its depolymerization by microorganisms. In order to validate this assumption, herein we described the modification of lignin with hydroxylation and explored the biodegradability of hydroxylated product compared with the control experiments of BOC-lignin and unmodified lignin with P. chrysosporium and Bacillus subtilis as well. The present work focused on the relationship between structural modification and biodegradation of lignin, and further discussed its biodegradation mechanism.
Alkali lignin and other common chemicals were purchased from Aladdin (Shanghai, China). Di-tert-butyl dicarbonate (Boc2O) was purchased from Sigma Aldrichh (St Louis, MO, USA). The white rot fungus P. chrysosporium (ATCC 24725) and bacteria B. subtilis (ACCC 11089) were both purchased from Guangdong culture collection center.
2.2 Procedure for Modification of Lignins
Hydroxylated-lignin, namely OH-lignin (Fig. 1A), was prepared according to the reported method [11]. Here, the unsaturated hydrocarbon on -C5 of the benzene ring in lignin is hydroxylated to form aliphatic hydroxyl groups. Briefly, 1.0 g of alkali-lignin was dissolved in 10 ml NaOH solution (1 wt%), and then 30 mg Fe(OH)3 catalyst and 1.1 mL H2O2 solution (30%) were subsequently added. The mixture was stirred at 60°C for 1 h and then was centrifuged to remove the catalyst. The pH was adjusted to 2–3 with 2.0 mol/L HCl to precipitate the hydroxylated lignin. The hydroxyl-modified lignin was obtained with centrifugation and washed with deionized water till the filtrate become neutral. Finally, the lignin was freeze-dried in vacuum for next use.

Figure 1: Preparation and structure of OH-lignin (A) and BOC-lignin (B)
BOC-lignin (Fig. 1B) was synthesized according to previously described protocols [10]. The phenol hydroxyl group on -C1 and alcohol hydroxyl group on -C4 of the benzene ring in lignin are protected by the BOC group. Accordingly, 1.0 g of alkali-lignin was mixed with 5 ml NaOH (2.0 mol/L) and 2.5 ml (Boc)2O. The mixture was stirred in an ice-water bath with the addition of 4-dimethylaminopyridine (DMAP) and then was warmed to room temperature. Then the pH value of the mixture was adjusted to 2–3 with 2.0 mol/L HCl. The modified lignin was isolated by centrifugation and washed thoroughly with deionized water. The product was freeze-dried in vacuum to set aside.
2.3 Strains and Culture Conditions
1. P. chrysosporium was inoculated in malt-agar medium containing (g/L): glucose, 10.0; malt extract, 10.0; peptone, 2.0; yeast extract, 2.0; asparagine, l.0; KH2PO4, 2.0; MgSO4·7H2O, l.0; thiamin-HC1, 0.001 and agar, 20.0 [12]. After 7 days of growth in solid medium at 39°C, the spores on the agar were suspended in the sterile water and adjusted the concentration to 5.0 × 106 spores/ml.
2. B. subtilis was inoculated in minimal salt medium (MSM)-agar medium for 3 days at 30°C. The MSM-agar medium consisted of (g/L): Na2HPO4, 2.4; K2HPO4, 2.0; NH4NO3, 0.1; CaCl2, 0.01; MgSO4, 0.01; peptone, 5.0; glucose, 10.0 and agar, 15.0 [13]. After that the bacterial colonies were transformed into MSM-broth and incubated at 30°C in rotary shaking incubator under aerobic condition overnight until the OD600 of inoculum reached approximately 1.2.
2.4 Biodegradation Experiments
Lignin biodegradation of fungi was carried out in Kirk’s nitrogen limitation cultures in the absence of veratryl alcohol [14]. Lignin (1.0 g/L) was dissolved in dimethyl sulfoxide (DMSO) and filtered to eliminate bacteria before adding to the culture. The final concentration of DMSO in the culture was 0.25%.
Rubber-stoppered Erlenmeyer conical flasks (200 mL) containing 50 ml of Kirk’s nitrogen limitation cultures (pH was adjusted to 4.5 with H3PO4) were inoculated with 5 mL of spore suspension under 100% oxygen. The uninoculated (control) and spores inoculated flakes were incubated at 39°C for 15 days without shaking and flushed with pure oxygen every day.
The bacterial degradation experiment was studied in 100 mL MSM culture as mentioned above containing 500 mg/L lignin in 250-ml Erlenmeyer flasks [13]. 1 ml of bacterial culture was added to each culture, and the pH was adjusted to 7.6. Lignin degradation was carried out at 30°C in rotary shaking incubator (120 rpm) under aerobic condition for 15 days. Uninoculated medium was used as control in all cases.
2.5 Determination of Cell Growth
The growth of P. chrysosporium was measured in term of increase in mycelium weights. Briefly, a certain volume of homogenized culture was collected every day and suction filtered by glass fiber filter. Then the filtered culture was washed three times with PBS and was dried overnight for weighting mycelium dry weights [15]. B. subtilis growth was determined by measuring degraded culture absorbance at 600 nm with spectrophotometer using uninoculated medium as blank.
2.6 UV-Visible Assay for Lignin Degradation
The amount of lignin remaining was determined with UV-visible spectrophotometer (UV-2550, Beijing Persee General Instrument Ltd., Beijing) [14]. Samples (0.5 ml) were taken every 24 h intervals. The cultures were sonicated for 30 s and added in 4.5 ml of 0.55% (w/v) NaOH to precipitate mycelia and protein. Then, the samples were centrifuged to remove insoluble solids. The supernatants were used to determine the content of lignin at 280 nm [16]. Each sample was measured three times.
2.7 Analysis of Fourier Transform Infrared (FTIR) and 1H NMR Spectroscopy
Samples before and after 15 days of biodegradation were sonicated and centrifuged. The supernatants were adjusted pH to 1–2 with HCl and extracted with the equal volume of ethyl acetate for three times. Finally, the ethyl acetate was evaporated from the sample under rotary drying vacuum pump.
Infrared spectrophotometer was used to investigate the changes of functional groups on the surface of lignin after degradation. The sample for the FTIR measurement was prepared by adding a small amount of the resultant sample into KBr to make the pellet within the spectral range of 4000–500 cm−1 using infrared spectrometer (SX2-4-10NP, Yiheng Scientific Instrument Co., Ltd., Shanghai). The 1H NMR spectra of lignin was recorded on Bruker 300 MHz (Bruker Science and Technology, Ltd., Brucker, Switzerland) using d6-DMSO as solvent. Each sample was measured three times. Finally, the aromatic nucleus peak was as a constant peak and set to 1. Then it was used to calculate the ratio of other peaks of protons in lignin.
BOC modified lignin (BOC-lignin) and hydroxylated lignin (OH-lignin) were synthesized with di-tert-butyl dicarbonate ((Boc)2O) and hydrogen peroxide (H2O2), respectively, using alkali lignin as the raw material. The 1H NMR spectra of lignin, BOC-lignin, and OH-lignin are shown in Fig. 2. The peaks area of the aromatic protons of two modified lignins are both decreased, indicating that the changes of structure. Clearly, Fig. 2 shows that -BOC groups on BOC-lignin, and -OCH3 groups which are specific to lignin disappeared on OH-lignin, suggesting that -OCH3 groups of lignin may be cracked by H2O2. However, the peaks of hydroxyl groups were not observed on the 1H NMR spectrum, which may be due to trace amount H2O existed in the deuterated solvent.

Figure 2: 1H NMR spectrum of modified alkali lignins
3.2 Cell Growth with Lignin Addition
The fungal and bacterial cells growth curves during the biodegradation course are shown in Fig. 3. We can see that the growth rate of P. chrysosporium and B. subtilis was both increased in the presence of OH-lignin, whereas, there is no obvious difference in lignin and BOC-lignin. It follows that OH-lignin provides a better carbon source for the growth of bacteria and fungi.

Figure 3: Growth curve of P. chrysosporium (A) and B. subtilis (B) during course of lignin biodegradation
3.3 Lignin Biodegradation Studies
The organisms primarily responsible for biodegradation of lignin are white-rot fungi (especially P. chrysosporium) [17]. Recently, bacteria degradation seems to be more significant because of their higher environmental compatibility and biochemical versatility [16]. Some bacteria species have been reported to have the ability to degrade lignin, such as Bacillus sp. [18]. Here, we selected P. chrysosporium and B. subtilis to explore the effect of hydroxylated modification on lignin degradation by fungi and bacteria.
UV-vis spectrophotometer was used to determine the extent of degradation for different modified lignin samples. The degradation curves are shown in Fig. 4. All microbial treatments after 15 days showed a significant lignin degradation. In P. chrysosporium, an obvious decrease in lignin was observed after a 4-day lag period, coinciding with ligninase activity appeared at the 4th day [12]. Within 15 days, lignin, BOC-lignin and OH-lignin was degraded approximately 23.6%, 17.0%, 30.3%, respectively. And in B. subtilis, lignin was rapidly degraded after 1 day, and approximately 17.3% for lignin, 11.4% for Boc-lignin and 24.6% for OH-lignin within 15 days. There were no obvious changes for the control groups (data was not given). We can see that the OH-lignin was the most degradable lignin among the three forms of lignin both by fungus and bacteria. However, the degradation rate of BOC-lignin, with its hydroxylation level reduced, was the lowest. The result is consistent with the proposed enzyme mediated breakdown oxidative mechanism, which is initiated from hydroxyl groups [19,20].

Figure 4: The UV spectrum of lignins degraded by P. chrysosporium (A) and B. subtilis (B)
3.4 Degradation Products Analysis
Fourier transform infrared spectrometer (FTIR) has been widely used in qualitative or quantitative analysis of lignin due to its advantages of large luminous flux, high resolution, high precision and wide spectral range. Further, to have a better understanding of the biodegradation process in the three lignins, we carried out the degradation products analysis by FTIR. The results are shown in Fig. 5. After lignin was degraded by P. chrysosporium, we can see an obvious decline of the strong absorption peak around 3400 cm−1 and 2900 cm−1 in OH-lignin compared with the other two lignins, which are assigned to the aromatic and aliphatic -OH stretching and the -CH stretching in -CH2-, -CH3 and -CH- groups, respectively, indicating that more methoxy groups may be removed from OH-lignin in the degradation process [21]. In addition, many well-defined peaks in the fingerprint region between 1800 and 600 cm−1 and apparent differences both in the absorbencies and shapes of the bands can be found in Fig. 4. Specifically, the intensities of the absorption bands around 1730 cm−1 decreased obviously, which are due to C=O stretching of acetyl or carboxylic acid, showing that an oxidation process was occurred [22]. This is also a decline around the peak 1600 cm−1, which is assigned to aromatic skeleton vibrations, meaning that the aromatic ring chain was destroyed or replaced [23]. While, a significant reduction was seen around the band 1260 cm−1, which represents the C-O stretching in guaiacyl aromatic methoxyl groups [22]. The absorption peaks of the bonds at their respective corresponding location mentioned above reduced the most in OH-lignin, while the least in BOC-lignin. In conclusion, more demethylation, oxidation and aromatic ring cracking reactions occurred during the lignin degradation process with high hydroxyl content. However, the lignin degradation mechanism by some fungi is different from that of white rot fungi, such as the brown rot fungi, there has been a rise of the phenolic hydroxyl due to the formation of new carbonyl and carboxyl groups, besides, the aromatic ring structure was not destroyed [24]. The results in bacteria B. subtilis were consistent with those in fungi except that the bacteria had a weaker lignin degradation ability. High active radicals are produced via oxidative reactions during the process of lignin degradation by bacteria, facilitating the cracking of the aromatic ring. The C4-ether and Cα-Cβ bonds of lignin are destroyed to give aldehydes as the products. Besides, the radicals have the ability to demethoxylate the rings, producing methanol as the main product [24].

Figure 5: FTIR spectrum of lignin samples before (1) and after P. chrysosporium (2) and B. subtilis (3) degraded: (A) lignin; (B) BOC-lignin; (C) OH-lignin
Additionally, the three lignin samples before and after degraded by P. chrysosporium and B. subtilis were analyzed in d6-DMSO solvent via 1H NMR spectra. The absorption at 1.20, 1.97, 2.50, 3.33, and 4.03 ppm representing ethyl acetate and DMSO was deducted. The characteristic absorption peak of methoxyl group is disturbed by the peak of water, so we have not discussed it here. From Fig. 6, we can see that all kinds of lignin were degraded in the process of biodegradation, and more NMR absorption peaks appeared after fungal degradation. The absorption peaks of protons was summarized in Tab. 1, we can see that after fungi and bacteria degradation, the percentage of protons on the same chemical shift is different, indicating that the degradation level of modified lignins is different. In P. chrysosporium, the proton percentages of aromatic nucleus reduced 53.4%, 37.8%, 35.0% in OH-lignin, lignin, BOC-lignin, respectively, and 31.4%, 16.0%, 16.9% in B. subtilis, proving that more aromatic ring cracking during OH-lignin degradation process. Correspondingly, more aliphatic compounds (2.2-fold, 1.4-fold, 0.5-fold increase in OH-lignin, lignin, BOC-lignin respectively in P. chrysosporium, and 2.2-fold, 0.9-fold, 0.3-fold in B. subtilis) were generated in the process of lignin decay. However, during the degradation process of BOC-lignin, there exerted more phenyl H in side chain and β-O-4 ether bond, which is the most abundant inter-unit linkages in lignin responsible for the connection of the Cβ of one unit and the phenolic hydroxyl of the other [25], indicating that the decayed BOC-lignin maybe repolymerized. Combined with the above FTIR results, we can draw the conclusion that more demethylation, oxidation and aromatic ring fracture chemical reactions were involved in the degradation process of lignin with more hydroxyls.
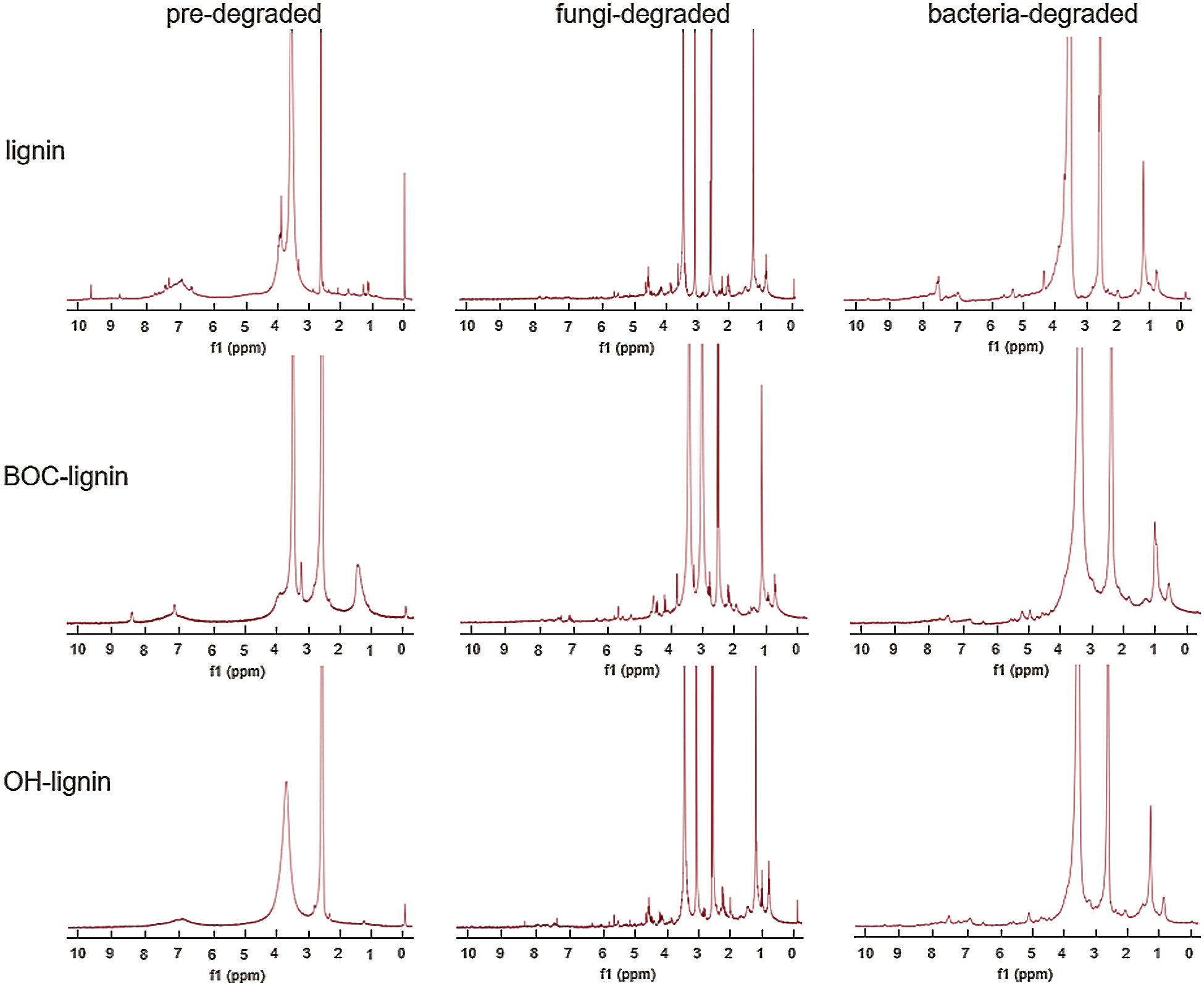
Figure 6: 1H NMR of lignin before and after degradation by fungi and bacteria

Fungi and bacterial catabolic lignin through several pathways, including β-aryl ether degradation, diarylpropane degradation, biphenyl degradation, phenylcoumarane and pinoresinol lignin degradation, oxidative cleavage of protocatechuic acid and so on. Many of these metabolic pathways product vanillin or its oxidation form vanillic acid, and then is converted to protocatechuic acid by demethylation. In white-rot fungi and some bacteria, the metabolic pathways occur primarily via Cα-Cβ oxidative cleavage, catalysing by certain enzymes such as lignin peroxidase in fungi and lignostilbene dioxygenase in bacteria. Protocatechuic acid can be finally degraded by way of oxidative ring cleavage [17].
Boc modified lignin and hydroxylated lignin was synthesized under mild conditions with (Boc)2O and H2O2 respectively to change the hydroxylation level. The effect of hydroxylation level on lignin degradation by microbial was studied here for the first time. We found that the higher content of hydroxyl groups in lignin has prompted the biodegradation of lignin, along with more demethylation, oxidation and aromatic ring cracking reactions during the degradation process both in fungi and bacteria. This finding gives us an important implication to the future research on green plastics with modified lignin that we should develop new methods of lignin modification in order to achieve high microbial degradation efficiency and high versatile utilization value.
Funding Statement: This work was financially supported by the Science and Technology Innovation Program of Hunan Province (Contract Grant No. 2018RS3101).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Laurichesse, S., Avérous, L. (2014). Chemical modification of lignins: Towards biobased polymers. Progress in Polymer Science, 39(7), 1266–1290. DOI 10.1016/j.progpolymsci.2013.11.004. [Google Scholar] [CrossRef]
2. Koljonen, K., Österberg, M., Kleen, M., Fuhrmann, A., Stenius, P. (2004). Precipitation of lignin and extractives on kraft pulp: Effect on surface chemistry, surface morphology and paper strength. Cellulose, 11(2), 209–224. DOI 10.1023/B:CELL.0000025424.90845.c3. [Google Scholar] [CrossRef]
3. Gaete, H., Larrain, A., Bay-Schmith, E., Baeza, J., Rodriguez, J. (2000). Ecotoxicological assessment of two pulp mill effluent, biobio river basin, Chile. Bulletin of Environmental Contamination and Toxicology, 65(2), 183–189. DOI 10.1007/s0012800113. [Google Scholar] [CrossRef]
4. Brebu, M., Cazacu, G., Chirila, O. (2011). Pyrolysis of lignin-a potential method for obtaining chemicals and/or fuels. Cellulose Chemistry and Technology, 45(1), 43–50. DOI 10.2488/jwrs.57.42. [Google Scholar] [CrossRef]
5. Li, H., Song, G. (2019). Ru-catalyzed hydrogenolysis of lignin: Basedependent tunability of monomeric phenols and mechanistic study. Acs Catalysis, 9(5), 4054–4064. DOI 10.1021/acscatal.9b00556. [Google Scholar] [CrossRef]
6. Ye, J., Fang, G., Jin, C. (2012). Hydrogenation of alkali lignin catalyzed by Pd/C. APCBEE Procedia, 3, 53–59. DOI 10.1016/j.apcbee.2012.06.045. [Google Scholar] [CrossRef]
7. Sahoo, S., Seydibeyoglu, M. O., Mohanty, A. K., Misra, M. (2011). Characterization of industrial lignins for their utilization in future value-added applications. Biomass & Bioenergy, 35(10), 4230–4237. DOI 10.1016/j.biombioe.2011.07.009. [Google Scholar] [CrossRef]
8. Duval, A., Lawoko, M. (2014). A review on lignin-based polymeric, micro- and nano-structured materials. Reactive & Functional Polymers, 85, 78–96. DOI 10.1016/j.reactfunctpolym.2014.09.017. [Google Scholar] [CrossRef]
9. Knežević1, A., Stajić1, M., Jovanović, V. M., Kovačević1, V., Ćilerdžić1, J. et al. (2016). Induction of wheat straw delignification by trametes species. Scientific Reports, 6(1), 26529. DOI 10.1038/srep26529. [Google Scholar] [CrossRef]
10. Gu, L., Wang, M., Li, H., Kai, N. T., Li, Y. et al. (2018). Tunable Boc modification of lignin and its impact on microbial degradation rate. ChemRxiv. DOI 10.26434/chemrxiv.7257212.v4. [Google Scholar] [CrossRef]
11. Zhou, W., Chen, F., Zhang, H., Wang, J. (2017). Preparation of a polyhydric aminated lignin and its use in the preparation of polyurethane film. Journal of Wood Chemistry & Technology, 37(4–6), 323–333. DOI 10.1080/02773813.2017.1299185. [Google Scholar] [CrossRef]
12. Tien, M., Kirk, T. K. (1988). Lignin peroxidase of Phanerochaete chrysosporium. Methods in Enzymology, 161(C), 238–249. DOI 10.1016/0076-6879(88)61025-1. [Google Scholar] [CrossRef]
13. Raj, A., Reddy, M. M. K., Chandra, R. (2007). Identification of low molecular weight aromatic compounds by gas chromatography–mass spectrometry (GC–MS) from kraft lignin degradation by three Bacillus sp. International Biodeterioration & Biodegradation, 59(4), 292–296. DOI 10.1016/j.ibiod.2006.09.006. [Google Scholar] [CrossRef]
14. Ulmer, D. C., Leisola, M. S. A., Schmidt, B. H., Fiechter, A. (1983). Rapid degradation of isolated lignins by Phanerochaete chrysosporium. Applied and Environmental Microbiology, 45(6), 1795–1801. DOI 10.1016/0005-2728(84)90100-2. [Google Scholar] [CrossRef]
15. Janshekar, H., Brown, C., Haltmeier, T., Leisola, M., Fiechter, A. (1982). Bioalteration of kraft pine lignin by Phanerochaete chrysosporium. Archives of Microbiology, 132(1), 14–21. DOI 10.1007/BF00690810. [Google Scholar] [CrossRef]
16. Chandra, R., Abhishek, A., Sankhwar, M. (2011). Bacterial decolorization and detoxification of black liquor from rayon grade pulp manufacturing paper industry and detection of their metabolic products. Bioresource Technology, 102(11), 6429–6436. DOI 10.1016/j.biortech.2011.03.048. [Google Scholar] [CrossRef]
17. Bugg, T. D. H., Ahmad, M., Hardiman, E. M., Rahmanpour, R. (2011). Pathways for degradation of lignin in bacteria and fungi. Natural Product Reports, 28(12), 1883–1896. DOI 10.1039/c1np00042j. [Google Scholar] [CrossRef]
18. Abd-Elsalam, H. E., El-Hanafy, A. A. (2009). Lignin biodegradation with ligninolytic bacterial strain and comparison of Bacillus subtilis and Bacillus sp. isolated from Egyptian soil. American-Eurasian American-Eurasian Journal of Agricultural & Environmental Sciences, 5, 39–44. [Google Scholar]
19. Brown, M. E., Chang, M. C. Y. (2014). Exploring bacterial lignin degradation. Current Opinion in Chemical Biology, 19, 1–7. DOI 10.1016/j.cbpa.2013.11.015. [Google Scholar] [CrossRef]
20. Have, R. T., Teunissen, P. J. M. (2001). Oxidative mechanisms involved in lignin degradation by white-rot fungi. Chemical Reviews, 101, 3397–3413. DOI 10.1021/cr000115l. [Google Scholar] [CrossRef]
21. Liu, Y., Hu, T., Wu, Z., Zeng, G., Huang, D. et al. (2014). Study on biodegradation process of lignin by FTIR and DSC. Environmental Science & Pollution Research, 21, 14004–14013. DOI 10.1007/s11356-014-3342-5. [Google Scholar] [CrossRef]
22. Xu, G., Wang, L., Liu, J., Wu, J. (2013). FTIR and XPS analysis of the changes in bamboo chemical structure decayed by white-rot and brown-rot fungi. Applied Surface Science, 280, 799–805. DOI 10.1016/j.apsusc.2013.05.065. [Google Scholar] [CrossRef]
23. Mousavioun, P., George, G. A., Doherty, W. O. S. (2012). Environmental degradation of lignin/poly(hydroxybutyrate) blends. Polymer Degradation and Stability, 97(7), 1114–1122. DOI 10.1016/j.polymdegradstab.2012.04.004. [Google Scholar] [CrossRef]
24. Muhammad, U. K., Birgitte, K. A. (2019). Lignin degradation under anaerobic digestion: Influence of lignin modifications–A review. Biomass and Bioenergy, 128, 105325. DOI 10.1016/j.biombioe.2019.105325. [Google Scholar] [CrossRef]
25. Chakar, F. S., Ragauskas, A. J. (2014). Review of current and future softwood kraft lignin process chemistry. Industrial Crops & Products, 20(2), 131–141. DOI 10.1016/j.indcrop.2004.04.016. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |