

 | Journal of Renewable Materials |  |
DOI: 10.32604/jrm.2021.016786
ARTICLE
Plasma Treatment Induced Chemical Changes of Alkali Lignin to Enhance the Performances of Lignin-Phenol-Formaldehyde Resin Adhesive
1College of Forestry, Guizhou University, Guiyang, 550025, China
2Kaili University, Qiandongnan, 556011, China
3ENSTIB-LERMAB, University of Lorraine, Epinal, 88051, France
4Yunnan Provincial Key Laboratory of Wood Adhesives and Glued Products, Southwest Forestry University, Kunming, 650224, China
*Corresponding Authors: Bengang Zhang. Email: zbg18082968142@163.com; Hong Lei. Email: lfxgirl@163.com
#Zhigang Wu and Sicheng Chen have contributed equally to this work
Received: 26 March 2021; Accepted: 19 April 2021
Abstract: Alkali lignin was processed by plasma and then used in modification of phenol formaldehyde resin in this study. Chemical structural changes of lignin which was processed by plasma as well as bonding strength, tensile property, curing performance and thermal property of the prepared phenol formaldehyde resin which was modified by the plasma processed lignin were analyzed. Results demonstrated that: (1) Alkali lignin was degraded after the plasma processing. The original groups were destroyed, and the aromatic rings collected abundant free radicals and oxygen-containing functional groups like hydroxyls, carbonyls, carboxyls and acyls were introduced into increase the reaction activity of lignin significantly. (2) The introduction of alkali lignin decreased the free formaldehyde content and increased bonding strength and toughness of the prepared phenol formaldehyde resin, especially after the introduction of lignin treated with plasma. (3) The introduction of alkali lignin led to high curing temperature for the prepared phenol formaldehyde resin, but that was reduced by the plasma processed alkali lignin. (4) The introduction of alkali lignin could also increase thermal stability of phenol formaldehyde resin, but that was modified by plasma processed alkali lignin was better than the unprocessed lignin. Based on the results, the plasma processed lignin was used to modify phenol formaldehyde resin, which could increase the strength and toughness of phenol formaldehyde resin significantly.
Keywords: Alkali lignin; plasma; phenol formaldehyde resin; bonding performance; tensile property
Urea formaldehyde resin, phenol formaldehyde resin and melamine formaldehyde resin are adhesives which are used mostly in wood industry at present. The consumption of them accounts for 60%–70% and even higher of total glue consumption in the man-made board industry [1–5]. Among them, phenol formaldehyde resin is widely applied in the production of man-made boards for outdoor use due to its high bonding strength, good durability, weather resistance, water resistance and aging resistance. It is the second major wood adhesive next to the urea formaldehyde resin. Phenol formaldehyde resin have several disadvantages of high curing temperature, long curing time and easy penetration when it is used in the production of man-made boards. Due to the high crosslinking degree and poor toughness, the cured phenol formaldehyde resin can cause poor impact resistance indirectly and easy to cause fractures [6–9]. Recently, the price of phenol rises quickly, which brings a huge pressure on the manufacturing enterprises of man-made boards for outdoor uses. In addition, during synthesis of phenol formaldehyde resin and the use of man-made boards are challenged with releasing of toxic gases [10–14]. These characteristics further restrict the application of phenol formaldehyde resin and hinder the development of man-made board industry. Hence, it has become a focus of attention to seek substitute of phenol.
Biomass-based copolycondensation technology is attracting more and more attentions, which increases the use of renewable raw materials significantly under the premise of resin performances. Most studies focus on the copolycondensation resins of soy protein [15,16], tannin [17,18], starch [19,20] with melamine formaldehyde resin, and some products have begun in industrial production. Lignin is the sole natural aromatic organic raw materials on earth which can be gained from renewable resources, and it has attracted increasing attentions in countries around the whole due to its unique characteristics of non-toxicity, low cost and easy biological decomposition. Lignin is composed of Guaiac-based, lilac-based and para-hydroxyphenyl-based structural units. Its molecules contain methoxyls, phenolic hydroxyls, alcohol hydroxyls, unsaturated double bonds, ether bonds and other functional groups, which provide a foundation for partial substitution of phenol in preparation of phenol formaldehyde resin adhesive [21–24]. So far, there are many studies on lignin modified synthetic resins, such as lignin-epoxy resin [25,26], lignin-polyurethane [27,28] lignin-acrylamide [29,30], and so on. As a waste from pulping and papermaking, alkali lignin is cheap and it brings extremely high economic values to the phenol formaldehyde resin adhesive which is prepared by alkali lignin [23–32].
Alkali lignin was treated with plasma and then used in the preparation of phenol formaldehyde resin. Moreover, chemical structural changes of lignin which was processed by plasma as well as bonding strength, tensile property, curing performance and thermal property of the prepared phenol formaldehyde resin which was modified by the plasma processed lignin were analyzed.
Formaldehyde solution (with a concentration of 37 wt %) was from Sinopharm Chemical Reagent Co., Ltd., China. Alkali lignin, brown powder (pH 10.5 in 50% aqueous solution), Nanjing Dulai Biological Co., Ltd., China. Other chemicals used in this work were also obtained from Sinopharm Chemical Reagent Co., Ltd., China. Poplar veneer with a size of 400 mm (length) × 400 mm (width) × 2 mm (thickness) and moisture content 8%–10%, was bought from Qunyou Wood Co., Ltd., Shandong, China.
2.2 Lignin Processed with Plasma
Dielectric barrier discharge (DBD) is an effective form to generate low-temperature plasma. It can generate low temperature plasmas with a large-volume and high-density in a wide air pressure. DBD is characteristic of no use of vacuum system, simple technology, easy-to-operate equipment, easy control and existence of insulating media between electrodes. It avoids partial discharge or arc discharge which is caused in the corona discharge process. The system has strong reliability and it is easy to realize large-scaled industrial continuous production. Hence, DBD plasma was chosen in this experiment. The alkali lignin was put in a glass container. To assure uniform and full reactions, lignin was paved in the container to increase the exposure area as much as possible. The DBD cold plasma (model: CTP-2000 K; medium: quartz glass; distance between medium: 8 mm) was opened and the power was adjusted to react for 5 min. At the end of reaction, lignin was stored under dry conditions for the next experiment (since plasma processing effect has timeliness, the lignin which was processed by plasma were used and tested in 1 h). The lignin was processed by plasma or not was recorded as PL and L, respectively.
2.3 Preparation of Phenol Formaldehyde Resins
Preparation of phenol formaldehyde resin (n(formaldehyde):n(phenol) = 1.3:1) was prepared accord to our previous study [33,34]. 94 parts of phenol, 20 parts of deionized water, 15.4 parts of formaldehyde, 2 parts NaOH (solid) and 5 parts of alkali lignin or plasma processed alkali lignin were charged into a three-neck 500 ml round-bottom flask equipped with a condenser, thermometer and a magnetic stirrer bar. The temperature was slowly increased to 90°C–92°C and kept for 60 min, and then the mixture was cooled to 45°C and a second 90 parts formaldehyde was added. The temperature was increased to 90°C again. After the water tolerance arrived to 150%, the mixture was then cooled to room temperature to obtain a reddish brown transparent resin. The mixture without adding lignin was recorded as PF. Lignin and plasma treated lignin were used in modification of phenol formaldehyde resin, and were recorded as L-PF and PL-PF, respectively.
2.4 Preparation of Three-Layer Plywood and Testing of Bonding Strength
A three-layer plywood with a size of 400 mm (length) × 400 mm (width) × 5 mm (thickness) was manufactured with the prepared phenol formaldehyde resins. Poplar veneers loaded resin (200 g/m2 for the double-sided), and then rested at room temperature for 15–20 min. The assembled poplar veneers were then put on a single-layer hot press (XLB type from Shanghai Rubber Machinery Plant). A plywood panel was gotten at the hot pressing parameters of 1.5 MPa at 130°C for 4 min. After conditioning at room temperature and relative humidity of 65 ± 5% for 1 day, the plywood was cut into a size of 100 mm (length) × 25 mm (width) × 5 mm (thickness). The wet shear strength in boiling water was measured according to Chinese National Standard (GB/T 17657-2013).
With references to national standards GB/T 6344-2008, the prepared phenol formaldehyde resins were made into “dumb-bell” model as seen in Fig. 1 and then the tensile test was carried out. The difference between the fracture range in the tensile process and the original range was used to calculate the elongation.

Figure 1: Tensile property test model and size
The calculation formula of elongation is:
L is the fracture range of specimens (mm);
L0 is the original range of specimens (mm).
2.6 Fourier Transform Infrared Spectroscopy (FT-IR)
0.001 g power of cured phenol formaldehyde resins was mixed well with 1 g KBr to prepare a pill. And then the pill was tested in a Varian 1000 infrared spectrophotometer USA within the range of 400–4,000 cm−1 with a 4 cm−1 resolution using 32 scans.
2.7 Differential Scanning Calorimetry (DSC)
A Perkin–Elmer differential scanning calorimeter (DSC 204F1, Rodgau, Germany) was used for investigation of thermal analysis. The test was performed from 25°C to 250°C at a heating rate of 10 °C/min. PYRISTM Version 4.0 software (Rodgau, Germany) was used for data treatment.
2.8 Thermogravimetric Analysis (TGA)
A thermogravimetric analyzer (TGA) (NETZSCH; Bavaria, Germany) was used for determine the thermal performance of the samples under N2 from 25°C to 700°C at a heating rate 10 °C/min.
2.9 Scanning Electron Microscopy (SEM)
Fractured surface sections of the cured phenol formaldehyde resins were tested using a Hitachi S-3400 N emission scanning electron microscope (SEM, Tokyo, Japan) operated at 12.5 kV.
FT-IR spectra of lignins are shown in Fig. 2. Lignin belongs to polyphenols and phenylpropane is the major structure of lignin. Lignin is composed of Guaiac-based, lilac-based and para-hydroxyphenyl-based structural units. The absorption peak at 3430.9 cm−1 was caused by the O-H stretching vibration and the absorption peak at 2932.1 cm−1 was caused by stretching vibration of C-H in -CH3 and -CH2 on the side chain of lignin. The peaks at 1602.9 cm−1 and 1511.1 cm−1 were attributed to the skeletal vibration of benzene rings. The peak at 1462.3 cm−1 was caused by the asymmetric bending vibration of C-H on CH3, indicating that lignin had methoxyls. The peak at 1386.3 cm−1 was caused by the stretching vibration of C-H on the benzene ring and the peak at 1272.3 cm−1 was the C-O stretching vibration on the guaiac-based rings. Peaks at 1217.7 cm−1 and 1125.8 cm−1 were caused by C-O stretching vibrations on the syringyl rings. The peak at 1038.7 cm−1 was caused by deformation vibration and out-of-plane bending vibration of C-H in the aromatic rings.

Figure 2: FT-IR curves of lignin
After plasma treatment, the O-H absorption peak at 3430.9 cm−1 was strengthened, while the absorption peaks at 2932.1 cm−1 and 1462.3 cm−1 were weakened significantly. This reflected that phenylpropane units of lignin and groups (-CH2-, -CH3 and -OH) on branched-chains break. Meanwhile, the broken groups reacted to generate new compounds or reacted with nitrogen-containing and oxygen-containing free radicals which were produced during plasma treatment to form oxygen-containing functional groups, such as hydroxy, carbonyl, carboxyl and acyl groups. The increased contents of these polar functional groups made the molecular movement of lignin more actively. The peak at 1272.3 cm−1 moves to 1217.7 cm−1 (this is caused by the increase of lilac-based structural units), indicating that lignin degraded and chemical bonds broke into micro-molecular lignin after plasma processing. This was because under plasma processing, phenolic ortho-position was easier to be attacked by O to generate O free radicals, which could increase the lilac structures. Besides, the unique aromatic ring structures of lignin provided a place for gathering of lone pair electrons. The peak at 1602.9 cm−1 disappeared, while an equicohesive conjugated carbonyl absorption peak was developed at 1641.3 cm−1, indicating that plasma oxidized the hydroxide radicals into conjugated carbonyls. This increased the electron density and strengthened the reaction activity. This result was consistent with the research conclusions of Sahin [35] and Li [36].
In summary, lignin degraded after the plasma processing and the original groups were destroyed. The aromatic rings collected a lot of free radicals and introduced in oxygen-containing functional groups like hydroxy, carbonyl, carboxyl and acyl groups, thus increasing the reaction activity of lignin significantly.
FT-IR spectra of phenol formaldehyde resins are shown in Fig. 3. The strong and wide absorption band at 3430.9 cm−1 was the stretching vibration absorption peak of the associated hydroxyls. There were special C=C stretching vibration peaks of benzene ring skeletons at 1641.3 cm−1 and 1478.2 cm−1. The peak at 1239.3 cm−1 was the C-O stretching vibration peak on phenol rings. The peak at 1152.9 cm−1 was the stretching vibration peak of C-C which connected phenol rings and hydroxymethyl. It was the ether bond between benzene rings at 1011.5 cm−1. Therefore, degraded lignin had structures similar to phenol. It had extremely strong chemical activity with p-hydroxyphenyl diortho and guaiac-based single ortho-vacancy point, and could have substitution or condensation reactions with phenols and formaldehydes.

Figure 3: FT-IR curves of phenol formaldehyde resins
Compared with PF, L-PF and PL-PF had following characteristics: (1) the stretching vibration absorption peak of C=C bond of benzene ring skeleton at 1641.3 cm−1 was weakened, while the absorption peaks at 1239.3 cm−1 and 1375.1 cm−1 were strengthened, which indicated that the phenolic ring had different benzene ring substituted structures by introducing in lignin. (2) The C-O stretching vibration peak on the phenolic ring had a blue shift, which was attributed to the reaction between lignin and hydroxymethyl of phenol formaldehyde resin. This reaction caused a steric hindrance that was difficult to form hydrogen bonds. Due to the inductive effect of active groups on lignin, the electron cloud density of the system increased, accompanied with growths of the force constant and frequency. So, the system made a blue shift. This proved that lignin participated in the synthetic reactions. Generally speaking, FT-IR curves of L-PF, PL-PF and PF showed the similar characteristics, because lignin participated in the synthetic reactions and increased the distance between ring and ring. As a result, the molecular symmetry was decreased and the dipole moment changed more in the vibration process, thus resulting in the weak FT-IR responses.
3.2 Physical and Chemical Properties of Phenol Formaldehyde Resins
Physical and chemical properties of phenol formaldehyde adhesives resins are shown in Tab. 1. Viscosity of unmodified phenol formaldehyde resin was 200 mPa ⋅ s, while the viscosities of L-PF and PL-PF were 420 mPa ⋅ s and 360 mPa ⋅ s, respectively. The viscosities of L-PF and PL-PF were increased significantly after lignin was introduced in. This was because: (1) Lignin might contain a certain amount of impurities that did not participate in synthetic reaction of resins. For example, starch and polysaccharide could increase viscosity of the resin system after they contacted with water. (2) The lignin had relatively high molecular weight and it had electrostatic force and hydrogen bond associations with above impurities, thus increasing viscosity of the resin system. (3) Benzene rings on the molecules of lignin had hydrophobicity and water showed slow affinity to the lignin molecules, thus increasing viscosity of resins. The viscosity of PL-PF was smaller than that of L-PF, because the lignin degraded after the plasma processing and its molecular weight was decreased to some extent.
Table 1: Properties of phenol formaldehyde resins

In addition, the free formaldehyde contents of L-PF and PL-PF were 25.8% and 53.0% which were lower than that of unmodified phenol formaldehyde resin. The free formaldehyde could be decreased significantly by introducing in lignin, indicating that lignin served as the formaldehyde catching agent. Specifically, the free formaldehyde content in phenolic resin which was modified by plasma processed lignin decreased, indicating that the plasma processing could increase activity of lignin, and sites which participated in reaction were increased.
3.3 Bonding Strength of Phenol Formaldehyde Resins
According to the classical synthesis theory of phenol formaldehyde resin [37], the molar ratio between formaldehyde and phenol can influence the degree of crosslinking and polycondensation of resins greatly. When the molar ratio was low, the reaction products were O-hydroxymethylphenol and Para-hydroxymethylphenol. The content of Para-hydroxymethylphenol was the higher, which determined the higher proportion of linear structures of the final condensation and polymerization products. When the molar ratio was high, reaction products were di-hydroxymethyl phenol and tri-hydroxymethyl phenol. Besides, content of the tri-hydroxymethyl phenol increased with the increase of molar ratio. However, there was excessive high content of free formaldehyde when the molar ratio was too high. Hence, the molar ratio was determined n(formaldehyde):n(phenol) = 1.3:1 in this paper.
Results of bonding strength of phenol formaldehyde resins are shown in Fig. 4. The bonding strength of unmodified phenol formaldehyde resin was 1.01 MPa. While that of L-PF and PL-PF were increased by 16.8% and 29.7% to 1.18 MPa and 1.31 MPa. Firstly, it could be confirmed that the introduction of lignin could increase the bonding strength of phenolic resins. The bonding strength of L-PF was increased slightly, which was related with low reaction activity of unprocessed lignin as well as the high viscosity and wettability of phenol formaldehyde resin which was synthesized by unprocessed lignin. In the process of phenol formaldehyde resin modified by lignin, lignin reacted with formaldehyde to generate hydroxymethyl lignin. Subsequently, polycondensation occurred between the hydroxymethyl lignin and between hydroxymethyl-lignin and hydroxymethyl-phenol. There was a competitive relationship between hydroxymethylation of lignin and the hydroxymethylation of phenol. However, the reaction activity of lignin which was processed by plasma was high and the competitiveness of hydroxymethylation of lignin was not weak. There were enough hydroxymethyl lignin formed, which laid a foundation for the follow-up copolycondensation. In addition, the process of LPF bonding plywood can be divided into resinification stage and curing stage. In the resinification, the resin was under solution environment, where chemical reaction was of selectivity. But in the hot-pressing for plywood preparation process (curing stage), moisture was rapidly volatilized, and the curing reaction had no selectivity. So, the bonding strength of L-PF was increased, especially for PL-PF.
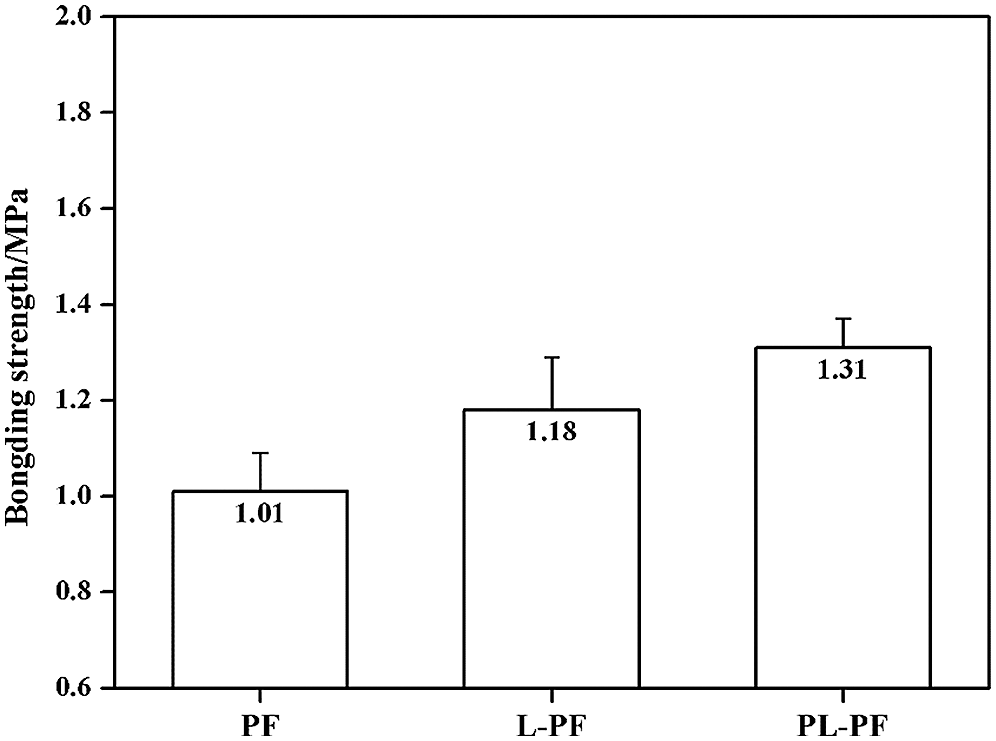
Figure 4: Bonding performance of lignin-phenol-formaldehyde resins
3.4 Tensile Property of Phenol Formaldehyde Resins
The relation curves between tension and deformation of phenol formaldehyde resins are shown in Fig. 5. Due to the high phenolic ring stiffness and short lattice chain, high crosslinking density and large steric hindrance in resin molecules, the phenol formaldehyde resins are difficult to generate strains when they undertake external stresses, so its capacity of absorbing impact energy decreases. Additionally, phenol formaldehyde resins have moisture absorption because of the hydroxymethyl which do not participate in the reaction. The resins generate stresses and fatigues after frequent moisture absorption and desorption, finally becoming brittle [13,14].
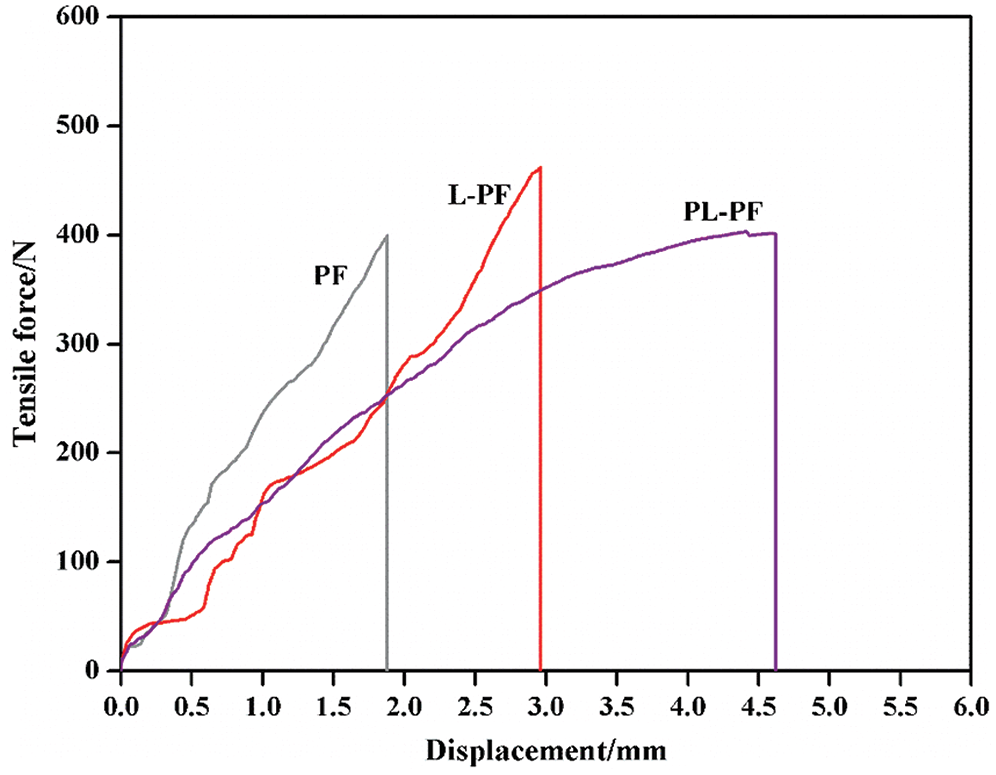
Figure 5: Relationship between tension and deformation of phenol formaldehyde resins
There was no evident yield phenomenon on the tensile failure curve of unmodified phenol formaldehyde resin. This was because the unmodified resin had high strength and brittleness after curing, but the stress had not reached the yield limit and it developed cracks inside and thereby break.
After lignin was introduced in, the resin deformation increased significantly, but the L-PF resin still had no evident yielding phenomenon at fracture, while the PL-PF resin showed evident yielding at fracture. Lignin belongs to a flexible long-chain molecule. Firstly, lignin could be connected with hydroxymethyl of phenolic resin, which could introduce in the flexible segments into spaces of rigid phenolic rings. Lignin had the larger intramolecular deformability than the original molecular chain which was connected by methylene, thus enabling to increase toughness. Secondly, hydroxymethyl which had not reacted in resin was consumed to some extent and the weakening moisture absorption increased toughness indirectly. However, lignin processed by plasma not only had relatively higher activity and more reaction sites, but also showed the higher polycondensation degree with phenolic resin and increased the toughness more compared to the original lignin.
Fracture surface micrographs of the cured phenol formaldehyde resins are shown in Fig. 6. The fracture surface of unmodified phenol formaldehyde resin is smooth, indicating that it had brittle fracture. There were scattered rough textures, concaves and pores on the fracture surface of L-PF resin, showing typical honeycomb morphology. It began to have brittle fractures. There were abundant concaves and pores on the fracture of PL-PF resin. Especially, there were large-scaled wrinkle and wave textures, indicating that resin absorbed a lot of energies and yields in the fracture process. This was typical characteristics of ductile failure. To sum up, lignin could improve the brittleness of phenol formaldehyde resins, especially what the lignin processed by plasma.
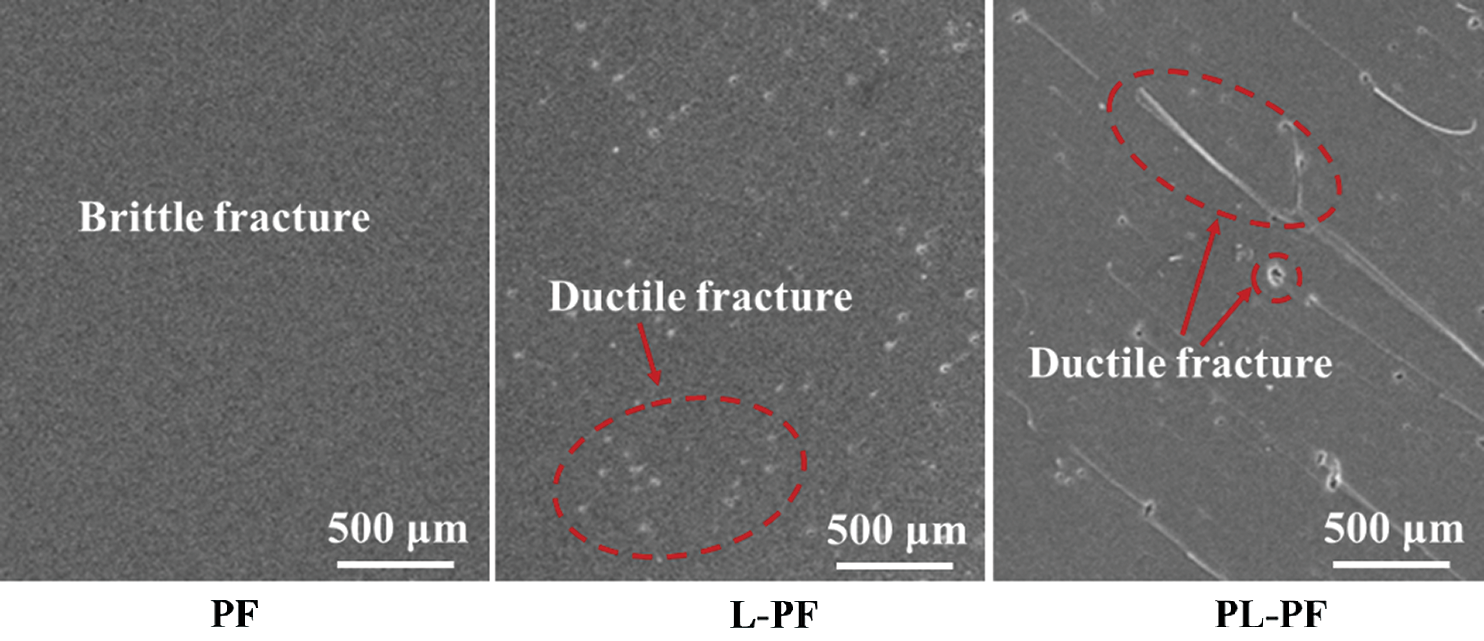
Figure 6: Fracture surface micrographs of the cured phenol formaldehyde resins
3.5 Curing Performance of Phenol Formaldehyde Resins
Curing performance of phenol formaldehyde resins are shown in Fig. 7. The heat releasing peak temperatures of unmodified phenol formaldehyde resin and L-PF were 108.9°C and 137.8°C, and the curing temperature of L-PF was relatively high. So, the hot pressing temperature when preparing the man-made board was relatively high. Combining with the bonding strength results, although L-PF was superior to PF resin in term of bonding strength, it still had disadvantages of high hot pressing temperature and high viscosity. The heat releasing peak temperature of PL-PF was 120.9°C, which was between those between PF and L-PF. It was 16.9°C lower than that of L-PF. This was because activity of lignin was improved after plasma processing and the number of active functional groups was increased. So, it is easier to have condensation reactions in the system.
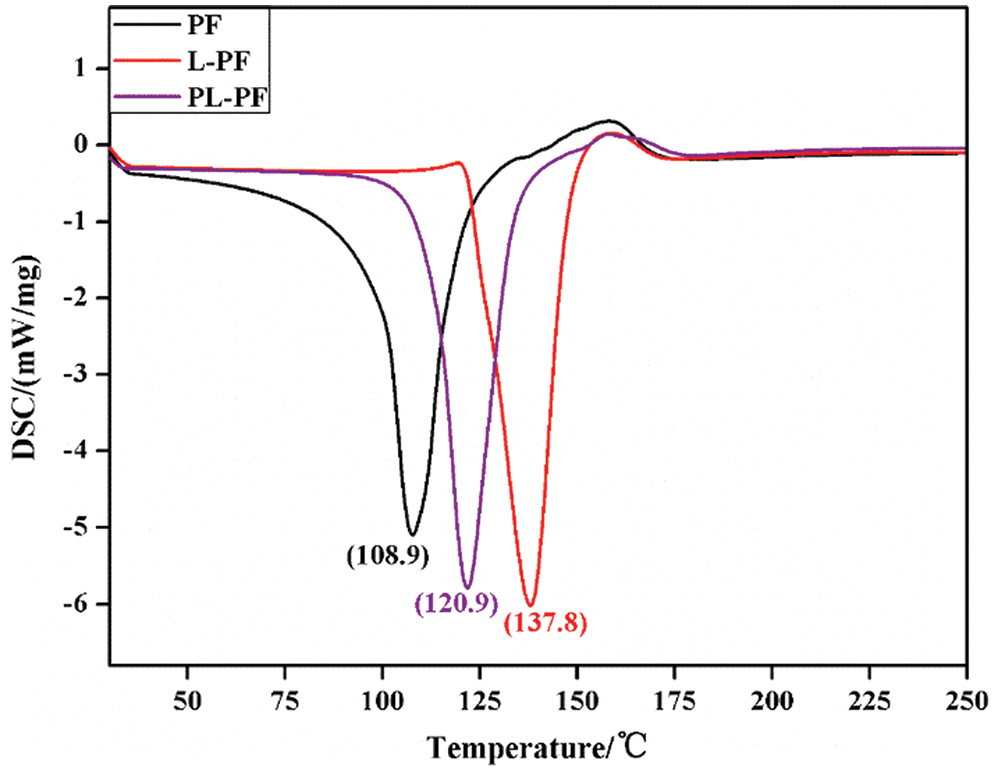
Figure 7: DSC curves of phenol formaldehyde resins
The integral of DSC curves was calculated in Fig. 8. The x-axis was time and the y-axis was integral area. So, the curing degree curves of resins can be gained. Unmodified phenol formaldehyde resin took the shortest time to reach the same curing degree (50%), while that of L-PF was the longest.

Figure 8: Curing degree of phenol formaldehyde resins
3.6 Thermal Property of Phenol Formaldehyde Resins
The TG/DTG curves of resins and parameters are shown in Fig. 9 and Tab. 2. The TG/DTG curves of PF, L-PF and PL-PF were basically consistent and they could be generally divided into three stages. Stage 1: 25–300°C. The mass loss ratios of PF, L-PF and PL-PF were 14.1%, 14.5% and 13.4%, respectively. In this stage, the system mainly generated steam and gases and volatilization of some micromolecular groups which had not participated in the reaction. Stage 2: 300–600°C. The mass loss ratios of PF, L-PF and PL-PF were 19.2%, 16.7% and 16.9%. This was a pyrolysis stage, when skeleton structure of the phenolic resin was destroyed. Oxygen-containing functional groups were degraded by heats to form amorphous carbon and generated volatile gases like H2O, CH4, CO and CO2 [38]. Weight loss of PF was relatively high. Since lignin in the system was mainly decomposed in Stage 1, mass loss ratios of L-PF and PL-PF in Stage 2 were relatively low. Stage 3: 600–700°C. The mass loss ratios of PF, L-PF and PL-PF were 3.9%, 2.7% and 2.8%, respectively. In this stage, degradable substances had been decomposed basically and TG curves tended to be stable, indicating the slow process of weight loss. In this process, carbon-containing substances continueed to dissociate and rearrange, which were developing toward graphitization, and the mass loss ratios were the minimum in three stages.
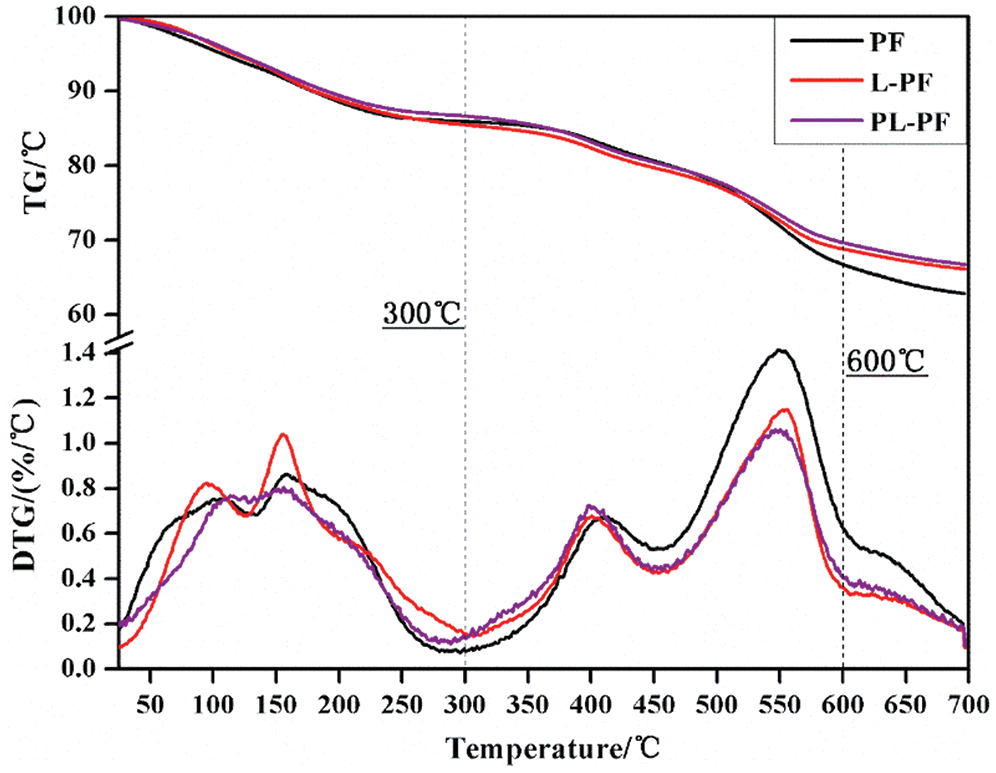
Figure 9: TG and DTG curves of phenol formaldehyde resins
Table 2: TG parameter of phenol formaldehyde resins

The final residual weights of PF, L-PF and PL-PF were 62.8%, 66.1% and 66.9%. In other words, PL-PF has the highest thermal stability, followed by L-PF. This proved that lignin could increase density of crosslinking network structures of the resin and thereby improve thermal stability of resin. The higher activity of lignin brought the higher density of crosslinking network structures and the higher thermal stability.
Alkali lignin was processed by plasma and then used in modification of phenol formaldehyde resin in this study. Results showed that:
(1) Alkali lignin was degraded after the plasma processing. The original groups were destroyed, and the aromatic rings collected abundant free radicals and oxygen-containing functional groups like hydroxyls, carbonyls, carboxyls and acyls were introduced into increase the reaction activity of lignin significantly.
(2) The introduction of alkali lignin decreased the free formaldehyde content and increased bonding strength and toughness of the prepared phenol formaldehyde resin, especially after the introduction of lignin treated with plasma.
(3) The introduction of alkali lignin led to high curing temperature for the prepared phenol formaldehyde resin, but that was reduced by the plasma processed lignin.
(4) The introduction of alkali lignin could also increase thermal stability of phenol formaldehyde resin, but that was modified by plasma processed lignin wasbetter than the unprocessed. Based on the results, the plasma processed lignin was used to modify phenol formaldehyde resin, which could increase the strength and toughness of phenol formaldehyde resin significantly.
Acknowledgement: The authors highly appreciate the program from Science-technology Support Foundation of Guizhou Province of China (Nos. ZK [2021]162 and [2019]2325). The authors also thank the anonymous reviewers for their invaluable comments and suggestions to improve the quality of the paper.
Funding Statement: This work was supported by National Natural Science Foundation of China (No. 31800481), Yunnan Fundamental Research Key Projects (No. 2019FA012), Science-Technology Support Foundation of Guizhou Province of China (Nos. [2019]2308 and [2020]1Y125), Forestry Department Foundation of Guizhou Province of China (No. [2018]13), Cultivation Project of Guizhou University of China (No. [2019]37).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding thepresent study.
1. Xi, X., Pizzi, A., Amirou, S. (2018). Melamine-glyoxal-glutaraldehyde wood panel adhesives without formaldehyde. Polymers, 10(1), 1–18. DOI 10.3390/polym10010022. [Google Scholar] [CrossRef]
2. Deng, S., Pizzi, A., Du, G., Lagel, M., Delmotte, L. et al. (2017). Synthesis, structure characterization and application of melamine-glyoxal adhesive resins. European Journal of Wood and Wood Products, 76(1), 283–296. DOI 10.1007/s00107-017-1184-9. [Google Scholar] [CrossRef]
3. Xu, Q., Sun, Q., Zhao, X., Cao, S., Sun, R. (2017). Study on hydroxymethylation of industrial lignin. Journal of Forestry Engineering, 2(3), 90–96. DOI 10.13360/j.issn.2096-1359.2017.03.015. [Google Scholar] [CrossRef]
4. Wu, Z., Lei, H., Du, G., Cao, M., Xi, X. et al. (2016). Urea-formaldehyde resin prepared with concentrated formaldehyde. Journal of Adhesion Science and Technology, 30(24), 2655–2666. DOI 10.1080/01694243.2016.1193963. [Google Scholar] [CrossRef]
5. Lei, H., Frazier, C. E. (2015). Curing behavior of melamine-urea-formaldehyde (MUF) resin adhesive. International Journal of Adhesion and Adhesives, 62, 40–44. DOI 10.1016/j.ijadhadh.2015.06.013. [Google Scholar] [CrossRef]
6. Du, G., Lei, H., Pizzi, A., Pasch, H. (2008). Synthesis-structure-performance relationship of cocondensed phenol-urea-formaldehyde resins by MALDI-tOF and 13C-nMR. Journal of Applied Polymer Science, 110, 1182–1194. DOI 10.1002/app.28735. [Google Scholar] [CrossRef]
7. Gong, Y., Deng, C., Wu, X. (2013). Research progress of high performance modified phenolic resin. Materials Guide, 2013, 27(11), 83–88. [Google Scholar]
8. Mo, X., Fan, D., Qin, T., Chu, F. (2014). 13C-NMR study on the structure of ZnO-catalyzed phenol-urea-formaldehyde resin during its synthesis process. Journal of Adhesion Science and Technology, 28, 2316–2326. DOI 10.1080/01694243.2014.962787. [Google Scholar] [CrossRef]
9. Pizzi, A., Orovan, E., Cameron, F. A. (1984). The development of weather- and boil-proof phenol-resorcinol-furfural cold-setting adhesives. HolzAlsRoh-Und Werkstoff, 42(12), 467–472. DOI 10.1007/bf02615403. [Google Scholar] [CrossRef]
10. Wang, J., Zhang, Y. F. (2012). Chemical structure and curing characteristics of phenol formaldehyde resins catalyzed with calcium oxide. Polymer-Plastics Technology and Engineering, 51(12), 1213–1217. DOI 10.1080/03602559.2012.696764. [Google Scholar] [CrossRef]
11. Tao, X., Wu, Y., Xu, W., Zhan, X., Zhang, J. (2019). Preparation and characterization of heating floor impregnated by graphene/phenol-formaldehyde resin. Journal of Forestry Engineering, 4(5), 167–173. DOI 10.13360/j.issn.2096-1359.2019.05.024. [Google Scholar] [CrossRef]
12. Wang, J., Laborie, M. P. G., Wolcott, M. P. (2011). Correlation of mechanical and chemical cure development for phenol-formaldehyde resin bonded wood joints. Thermochimica Acta, 513(1–2), 20–25. DOI 10.1016/j.tca.2010.11.001. [Google Scholar] [CrossRef]
13. Grinins, J., Biziks, V., Irbe, I., Rizhikovs, J. (2019). Water related properties of birch wood modified with phenolformaldehyde (PF) resins. Key Engineering Materials, 800, 246–250. DOI 10.4028/www.scientific.net/KEM.800.24. [Google Scholar] [CrossRef]
14. Shams, M. I., Yano, H., Endou, K. (2005). Compressive deformation of wood impregnated with low molecular weight phenol formaldehyde (PF) resin III: Effects of sodium chlorite treatment. Journal of Wood Science, 51(3), 234–238. DOI 10.1007/s10086-004-0638-y. [Google Scholar] [CrossRef]
15. Qu, P., Huang, H., Wu, G., Sun, E., Chang, Z. (2015). Hydrolyzed soy protein isolates modified urea–formaldehyde resins as adhesives and its biodegradability. Journal of Adhesion Science and Technology, 29(21), 2381–2398. DOI 10.1080/01694243.2015.1067176. [Google Scholar] [CrossRef]
16. Gao, Q., Shi, S. Q., Zhang, S., Li, J., Wang, X. et al. (2012). Soybean meal-based adhesive enhanced by MUF resin. Journal of Applied Polymer Science, 125(5), 3676–3681. DOI 10.1002/app.36700. [Google Scholar] [CrossRef]
17. Pizzi, A. (1979). The chemistry and development of tannin/urea-formaldehyde condensates for exterior wood adhesives. Journal of Applied Polymer Science, 23(9), 2777–2792. DOI 10.1002/app.1979.070230922. [Google Scholar] [CrossRef]
18. Moubarik, A., Pizzi, A., Allal, A., Charrier, F., Khoukh, A. et al. (2010). Cornstarch-mimosa tannin-urea formaldehyde resins as adhesives in the particleboard production. Starch-Stärke, 62(3–4), 131–138. DOI 10.1002/star.200900228. [Google Scholar] [CrossRef]
19. Luo, J., Zhang, J., Gao, Q., Mao, A., Li, J. (2019). Toughening and enhancing melamine-urea-formaldehyde resin properties via in situ polymerization of dialdehyde starch and microphase separation. Polymers, 11(7), 1167. DOI 10.3390/polym11071167. [Google Scholar] [CrossRef]
20. Baishya, P., Maji, T. K. (2014). Studies on effects of different cross-linkers on the properties of starch-based wood composites. ACS Sustainable Chemistry & Engineering, 2(7), 1760–1768. DOI 10.1021/sc5002325. [Google Scholar] [CrossRef]
21. Song, G. (2019). The development of catalytic fractionation and conversion of lignocellulosic biomass under lignin-first strategy. Journal of Forestry Engineering, 4(5), 1–10. DOI 10.13360/j.issn.2096-1359.2019.05.001. [Google Scholar] [CrossRef]
22. Sun, G., Sun, H., Liu, Y., Zhao, B., Zhu, N. et al. (2007). Comparative study on the curing kinetics and mechanism of a lignin-based-epoxy/anhydride resin system. Polymer, 2007, 48(1), 330–337. DOI 10.1016/j.polymer.2006.10.047. [Google Scholar] [CrossRef]
23. Huang, C., He, J., Liang, C., Tang, S., Yong, Q. (2019). Progress in applications of high value-added lignin materials. Journal of Forestry Engineering, 4(1), 17–26. DOI 10.13360/j.issn.2096-1359.2019.01.003. [Google Scholar] [CrossRef]
24. Li, F., Xu, Z., Zhang, X., Qin, L., Luo, B. et al. (2020). Enhancement of properties of wood plastic composites by modifying lignin. Journal of Forestry Engineering, 5(5), 45–51. DOI 10.13360/j.issn.2096-1359.201910010. [Google Scholar] [CrossRef]
25. Li, R. J., Gutierrez, J., Chung, Y. L., Frank, C. W., Billington, S. L. et al. (2018). A lignin-epoxy resin derived from biomass as an alternative to formaldehyde-based wood adhesives. Green Chemistry, 20(7), 1459–1466. DOI 10.1039/C7GC03026F. [Google Scholar] [CrossRef]
26. Ferdosian, F., Yuan, Z., Anderson, M., Xu, C. (2014). Synthesis of lignin-based epoxy resins: Optimization of reaction parameters using response surface methodology. RSC Advance, 4(60), 31745–31753. DOI 10.1039/c4ra03978e. [Google Scholar] [CrossRef]
27. Nacas, A. M., Ito, N. M., Sousa, R. R. D., Spinacé, M. A., Dos Santos, D. J. (2016). Effects of NCO:OH ratio on the mechanical properties and chemical structure of kraft lignin–based polyurethane adhesive. The Journal of Adhesion, 93(1–2), 18–29. DOI 10.1080/00218464.2016.1177793. [Google Scholar] [CrossRef]
28. Li, J., Wang, B., Chen, K., Tian, X., Zeng, J. et al. (2017). The use of lignin as cross-linker for polyurethane foam for potential application in adsorbing materials. BioResources, 12(4), 8653–8671. [Google Scholar]
29. Hasan, A., Fatehi, P. (2018). Stability of kaolin dispersion in the presence of lignin-acrylamide polymer. Applied Clay Science, 158, 72–82. DOI 10.1016/j.clay.2018.02.048. [Google Scholar] [CrossRef]
30. Rong, H., Gao, B., Zhao, Y., Sun, S., Yang, Z. et al. (2013). Advanced lignin-acrylamide water treatment agent by pulp and paper industrial sludge: Synthesis, properties and application. Journal of Environmental Sciences, 25(12), 2367–2377. DOI 10.1016/s1001-0742(12)60326-x. [Google Scholar] [CrossRef]
31. Younesi-Kordkheili, H., Pizzi, A. (2016). Properties of plywood panels bonded with ionic liquid-modified lignin–phenol–formaldehyde resin. The Journal of Adhesion, 94(2), 143–154. DOI 10.1080/00218464.2016.1263945. [Google Scholar] [CrossRef]
32. Di, M., Wang, S., Yao, Z. (2017). Research progress in the lignin-based formaldehyde-free wood adhesives. Journal of Forestry Engineering, 2(1), 8–14. DOI 10.13360/j.issn.2096-1359.2017.01.002. [Google Scholar] [CrossRef]
33. Wu, Z., Xi, X., Lei, H., Liang, J., Liao, J. et al. (2019). Study on soy-based adhesives enhanced by phenol formaldehyde cross-linker. Polymers, 11(2), 365. DOI 10.3390/polym11020365. [Google Scholar] [CrossRef]
34. Wu, Z., Chen, S., Liang, J., Li, L., Lei, H. et al. (2020). Properties and synthesis mechanism of lignin-phenol-formaldehyde resin. Transactions of the Chinese Society of Agricultural Engineering, 36(21), 308–315. DOI 10.11975/j.issn.1002-6819.2020.21.037. [Google Scholar] [CrossRef]
35. Sahin, H. T. (2009). Rf-o-2 plasma surface modification of kraft lignin derived from wood pulping. Wood Research, 54(1), 103–112. [Google Scholar]
36. Li, S. (2014). Mechanism of activation lignin with deep eutectic solvents and plasma. Nanjing: Nanjing Forestry University. [Google Scholar]
37. Cao, M., Li, T., Liang, J., Wu, Z., Zhou, X. et al. (2016). A 13C-nMR study on the 1,3-dimethylolurea-phenol co-condensation reaction: A model for amino-phenolic co-condensed resin synthesis. Polymers, 8(11), 391. DOI 10.3390/polym8110391. [Google Scholar] [CrossRef]
38. Sauget, A., Zhou, X., Pizzi, A. (2014). Tannin-resorcinol-formaldehyde resin and flax fiber biocomposites. Journal of Renewable Materials, 2(3), 173–181. DOI 10.7569/JRM.2013.634128. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |