

 | Journal of Renewable Materials |  |
DOI: 10.32604/jrm.2021.015400
ARTICLE
Alcoholysis of Waste Polyurethane Rigid Foam and Its Modification with Lignin for Recovery
1College of Materials Science and Engineering, Qiqihar University, Qiqihar, 161006, China
2Shanghai Crane City Polymer Co., Ltd., Shanghai, 201615, China
**Corresponding Author: Xiaohua Gu. Email: gxh218@163.com
Received: 18 December 2020; Accepted: 19 January 2021
Abstract: A bi-component alcoholysis agent containing propylene glycol (PG) and ethanolamine (ETA) was used to catalyst the degradation of the waste polyurethane rigid foam. The oligomer polyols obtained through degradation were used as raw materials to produce recycled polyurethane rigid foam composites with lignin as reinforcing filler. The effect of alcoholysis mass ratio on degradation was investigated by analyzing the viscosity, hydroxyl content and chemical structure of the degradation products. The effect of lignin addition on the properties of regenerated polyurethane rigid foam were investigated by analyzing water absorption rate, compressive strength, porosity, thermal stability, thermal conductivity coefficient, morphology and thermal stability of the recycled polyurethane rigid foam. Results show that different mass ration of PG to ETA significantly affects the degradation of waste polyurethane rigid foam. Besides, only with the addition of appropriate amount of lignin, the regenerated polyurethane rigid foam composites can meet the Chinese national standard “rigid polyurethane foam for building thermal insulation” (GB/T21558-2008). At this point, the composite is with good mechanical and thermal prperties, including high compressive strength, excellent thermal insulation performance, complete cell morphology and good thermal stability.
Keywords: Waste PU rigid foam; degradation; bi-component alcoholysis agent; lignin
In recent years, the production technology of polyurethane has made great progresses [1]. Polyurethane materials, which are widely used in both daily life and industrial production, have gradually become indispensable materials nowadays. However, at present, the production and application of polyurethane materials are facing great challenges [2,3]. Due to the expansion of the global application field of polyurethane, its dosage is increasing, leading to large amounts of wastes in the process of production and use, causing great damages to the environments. Therefore, recycling and reuse of waste polyurethane is an important issue concerning the environment and resources [4]. Moreover, raw materials polyether polyols of polyurethane are mostly from petroleum products. With the gradual consumption of petroleum resources, people are paying more and more attention to look for renewable resources to replace petroleum resources for polyurethane production [5–9]. Lignin mainly exists as industrial waste. More than 90% of lignin can be discharged into water and other environments in the form of pollutants, which not only fails to be effectively utilized, but also causes serious pollution to the environments [10]. Lignin contains a large number of phenolic hydroxyl groups, which leads to strong adsorption of pollutants [11–14]. It also contains aromatic structure, which can enhance the mechanical properties of polymer materials.
The consumption of polyurethane rigid foam accounts for about 25% of the total polyurethane consumption [15], and the recycle of waste polyurethane rigid foam has become an urgent issue [16]. In order to resolve the above problem, we try to recycle the waste polyurethane rigid foam by alcoholysis, and reuse the resultant products for fabrication of high-performanced polyurethane composite rigid foam with lignin. A bi-component alcoholysis agent containing propylene glycol (PG) and ethanolamine (ETA) was used as alcoholysis agent. The optimized bicomponent alcoholysis ratio was determined by analyzing the viscosity, hydroxyl contant and chemical structure of the alcoholysis products [17]. The alcoholysis products were then used as the alternatives of polyether polyols to prepare polyurethane materials. This protocol not only resolved the waste treatment issues, but also reduced the consumption of non-renewable energy such as petroleum, so that the objectives of environmental protection and circular economy could be achieved. In addition, in order to enhance the performance of polyurethane, lignin was added into the regenerated polyurethane matrix to prepare regenerated PU rigid foam composites. The regenerated PU hard foam composites modified by lignin were synthesized by using degradation materials (DM), polyether polyol (4110), lignin (Lignin) and diphenylmethane diisocyanate (MDI). The effects of the addition of lignin on the mechanical properties, water absorption capacity, thermal conductivity, thermal stability, molecular structure and microstructure of the composites were also studied herein.
Waste polyurethane rigid foam was from Zibo, Shandong, China. Propylene glycol (PG) was purchased from Shanghai Fengrui Chemical Co., Ltd., China. Ethanolamine (ETA) was purchased from Shanghai Fengrui Chemical Co., Ltd., China. KOH was purchased from Tianjin Tianli Chemical Reagent Co., Ltd., China. Industrial grade polyether 4110 was purchased from Shandong Vanke New Materials Co., Ltd., China. Industrial grade organic tin was purchased fromShanghai Kono Chemical Co., Ltd., China. Dimethyl silicone oil was purchased from Tianjin Kemio Chemical Co., Ltd., China. Industrial foaming agent 141b was purchased from Wuxi Huachang Chemical Co., Ltd., China. Industrial isocyanate (MDI) was purchased from Yantai Fude Chemical Co., Ltd., China. Industrial lignin was purchased from Aladdin.
2.2 Preparation of Alcoholysis Products of Waste PU Rigid Foam
The waste PU rigid foam was initially crushed into 5 mm to 10 mm sized particles. The bi-component alcoholysis agent (PG as the alcoholysis agent, ETA as the co-alcoholysis agent) and 0.5 wt% (the mass fraction of the total mass of the reaction system) KOH catalyst were mixed in a reaction kettle, which was placed in an electric heating mantle at 80°C with stirring. The crushed waste PU rigid foam was then added into the reaction kettle with the same quality as the bi-component alcoholysis agent. The mixture was heat for 4 h at 160°C for degradation. After cooling to room temperature, the mixture was poured into a plastic cup to obtain the alcoholysis product, which was named as DM. 2:3, 1:1, 3:2 and 2:1 were selected as the mass ratio of PG to ETA alcoholysis agent to investigate the influence of the ratio of different alcoholysis agents on DM.
2.3 Preparation of Lignin Modified Recycled Polyurethane Rigid Foam
In order to enhance the performance of recycled polyurethane rigid foam, a series of reactions by adding lignin with different contents (MLignin = 0%, 5%, 10%, 15%) were performed. The preparation processes were as follows:
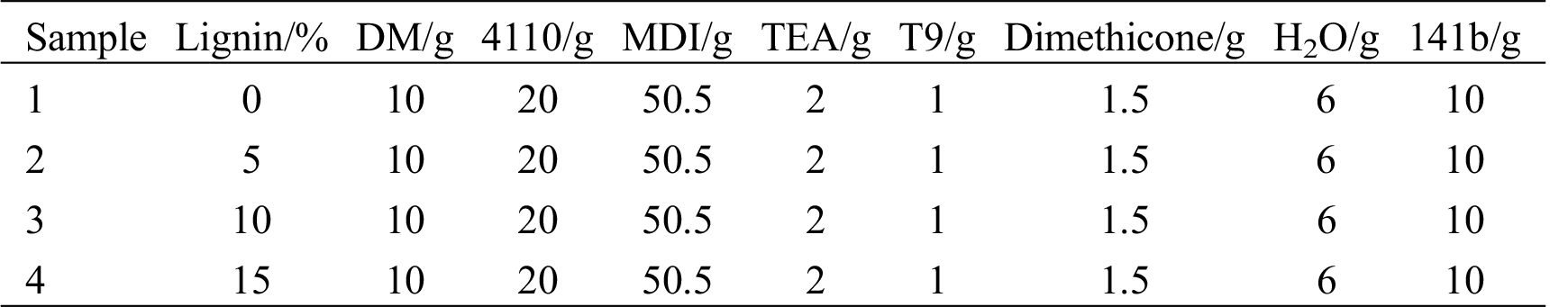
(1) DM prepared under the above optimized conditions was used to replace part of polyether polyol as the raw material for polyurethane production. 10 g of DM, 20 g of polyether polyols (4110), 50.5 g of diphenylmethane diisocyanate (MDI) and certain amount of lignin were placed in a vacuum drying box, followed by vacuum drying and dehydration at 85°C for 2 h. Alcoholysis products, polyether polyol (4110) and lignin were then transferred to a disposable plastic cup, and were stirred by an electric mixer for 5 min. The mixture was finally placed in vacuum dryers for secondary dehydration at 160°C for more than 0.5 h.
(2) After cooling to room temperature, MDI was added into the above plastic cup. The prepolymerization was carried out at 60°C for 20 min under stirring.
(3) The catalyst, surfactant and foaming agent were added into a new disposable plastic cup, and were stirred evenly at room temperature for 2 min. After obvious delamination phenomenon appeared, it was mixed with the prepolymer in the previous step through stirring until 1 cm of filament be pulled out with a glass rod. The foam grew until the foaming reaction was a complished. The porous recycled polyurethane rigid foam composites modified with lignin were recorded as PUDM-Lignins. The synthesis formula was shown in Tab. 1. Fig. 1 indicated the mechanism of the foaming reaction of recycled polyurethane rigid foam.
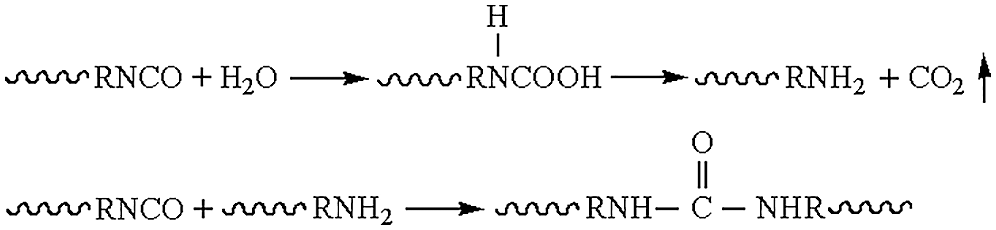
Figure 1: Schematic diagram of foaming reaction principle
Table 1: Preparation of PUDM-Lignin formula with different lignin content
2.4 Performance Test and Structural Characterization
According to the GB/T12008.3-2009 standard, a proper amount of oligomer polyol was placed in a 10 ml conical flask. The hydroxyl content was determined by the ester anhydride method-pyridine.
Viscosity was measured by rotational viscometer DSW-4. Certain amount of degradation materials were put in designated containers. The viscosity was measured at 25°C.
According to GBT 8810-2005 test standard, the water absorption was measured by immersing a 50 mm × 10 mm × 10 mm sample into a tank of water for 5 min. The mass of the sample before and after soaking was weighed, and the water absorption was calculated.
The apparent density of recycled polyurethane rigid foam composites was measured according to GB/T6343-1986. Four samples were taken from each sample and the average value was calculated. After that, the skeleton density of foam was measured by porosity meter 3H-2000PB and finally according to the formula: Porosity = (1 − apparent density of material/skeleton density) × 100%.
The compression strength test refers to the GB8813-2008 test standard. The sample size was 50 mm × 50 mm × 50 mm, and the compression strength was tested by EFS-24RE universal testing machine.
The thermal conductivity was tested according to the standard of QB/T3806-1999. The sample size was 200 mm × 200 mm × 20 mm. The instrument employed was the FEHC-S thermal conductivity tester from Changzhou Huaao instrument Manufacturing Co., Ltd., China.
The chemical structure of foam samples was analyzed by SpectrumOne Fourier transform infrared spectrometer (FTIR) of American PE company. The samples were prepared by KBr tableting method. The test wavelength ranges from 800 cm−1 to 4000 cm−1.
Microstructures of foam samples were observed by scanning electron microscope (SEM) of American model S4300 at an acceleration voltage of 20 kV. The sample was prepared by liquid nitrogen fracturing method. Scale bars of SEM pictures were 200 um.
Thermogravimetric analysis was carried out in a nitrogen atmosphere, using the TGA-101 thermogravimetric analyzer from Nanjing Institute of Mechanical and Electrical Technology. The temperature was increased from room temperature 25°C to 500°C at a rate of 30 °C/min. The gas flow rate was selected as 50 mL/min. Air was used as carrier gas, and alumina was used as the reference object.
3.1 Reaction Mechanism of Alcoholysis and DM Foaming of Waste PU
The alcoholysis mechanism of waste polyurethane rigid foam can be simplified as follows. Under the catalyst of alcoholysis agent and potassium hydroxide, the carbamate bond in polyurethane was broken and coupled with small molecularly alcohol chain to form polyol. The reaction mechanism was shown as formula (1):
where R1 and R2 denote long polymer chains linked to the carbamate bond in polyurethane, and R3 denotes the short molecularly chain in alcoholysis agents.
Because many functional groups were activated in the reaction system during the degradation process. Many side reactions occured. The main side reaction was that the urea group would break to form amine and polyol. The side reaction mechanism was shown as formula (2):
where R1 and R2 denote long polymer chains linked to the urea group in polyurethane, and R3 denotes the short molecularly chain in alcoholysis agents.
Fig. 1 shows the foaming mechanism of recycled polyurethane rigid foam. The reaction mechanism is that isocyanate reacts with water to form carbon dioxide, and the bubbles produced by carbon dioxide are foamed. At the same time, a large amount of heat releases during the reaction. And the foaming agent (141b) will evaporate into gas when heated, thus completing the auxiliary foaming.
3.2 Chemical Structure Analysis of Waste PU Rigid Foam DM
The main purpose of degrading waste polyurethane rigid foam is to recover polyol by using it to prepare recycled polyurethane rigid foam. Therefore, in order to study whether the prepared DM is a polyol, an infrared characterization test was performed. The result was shown in Fig. 2.
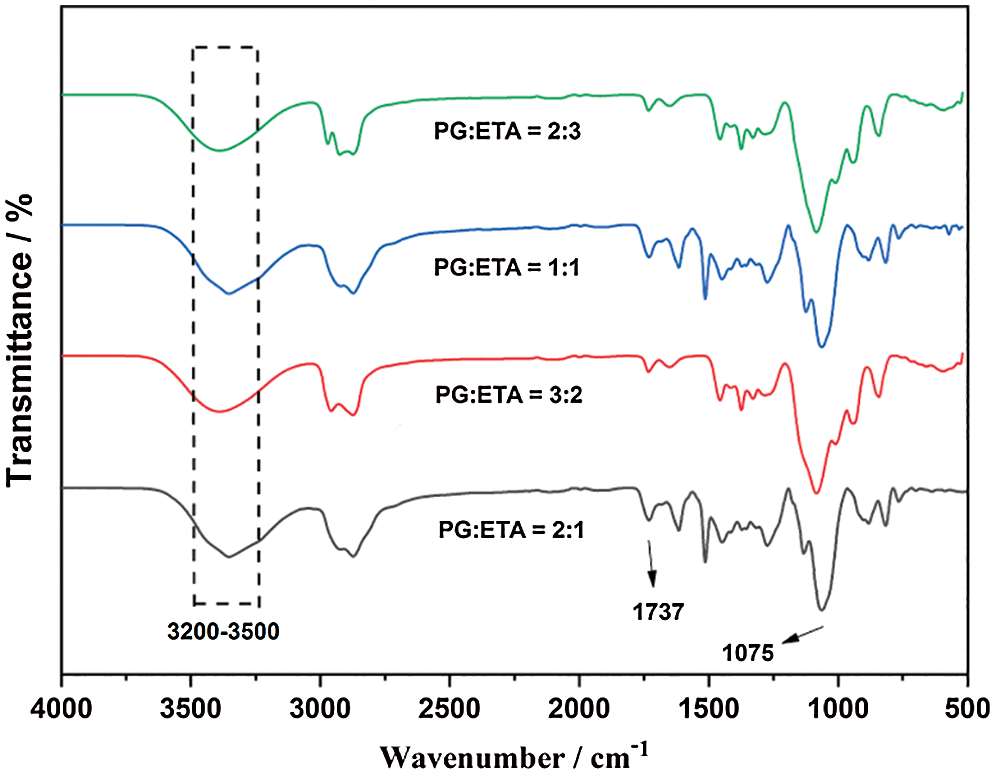
Figure 2: Infrared spectra of DM with different alcoholysis agent ratios
From Fig. 2, a strong characteristic peak near 3200 cm−1–3500 cm−1 was found, which is the stretching vibration peak of alcohol hydroxyl group. A strong characteristic peak near 1737 cm−1 was found, which is a benzene over-frequency peak, while a clear characteristic peak near 1075 cm−1 reflects the polyether polyurethane ether group absorption band. Therefore, it can be proved that the components of the prepared DM are the mixed products of polyether polyols and aromatic polyols. Due to the existence of rigid benzene ring in aromatic polyols, the mechanical properties of recycled polyurethane rigid foams are better than those of pure polyether polyols, indicating that the properties of DM prepared by alcoholysis are better than those of pure polyether polyols in foaming applications.
3.3 Viscosity and Hydroxyl Content Test of Waste PU Rigid Foam DM
The viscosity and hydroxyl content of DM prepared by alcoholysis agent PG and ETA in different proportions were tested. The relationship between the viscosity, hydroxyl content of DM and alcoholysis agent ratio was obtained. The results are shown in Figs. 3 and 4.
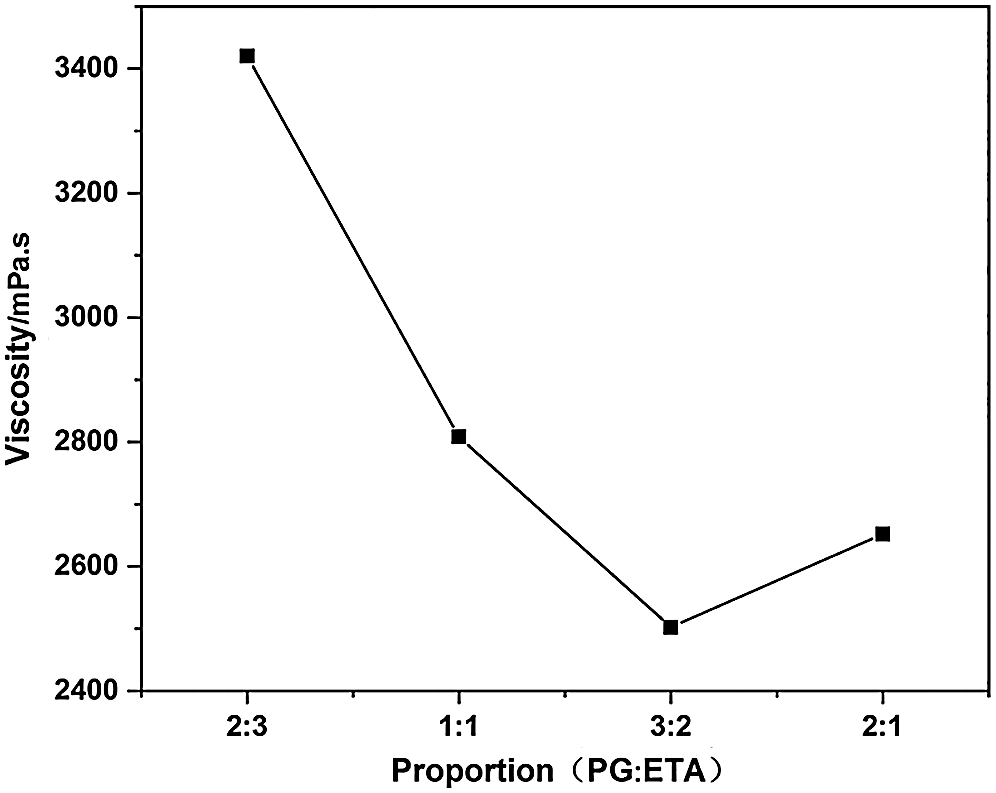
Figure 3: Effect of different proportion of alcoholysis agent on viscosity of DM
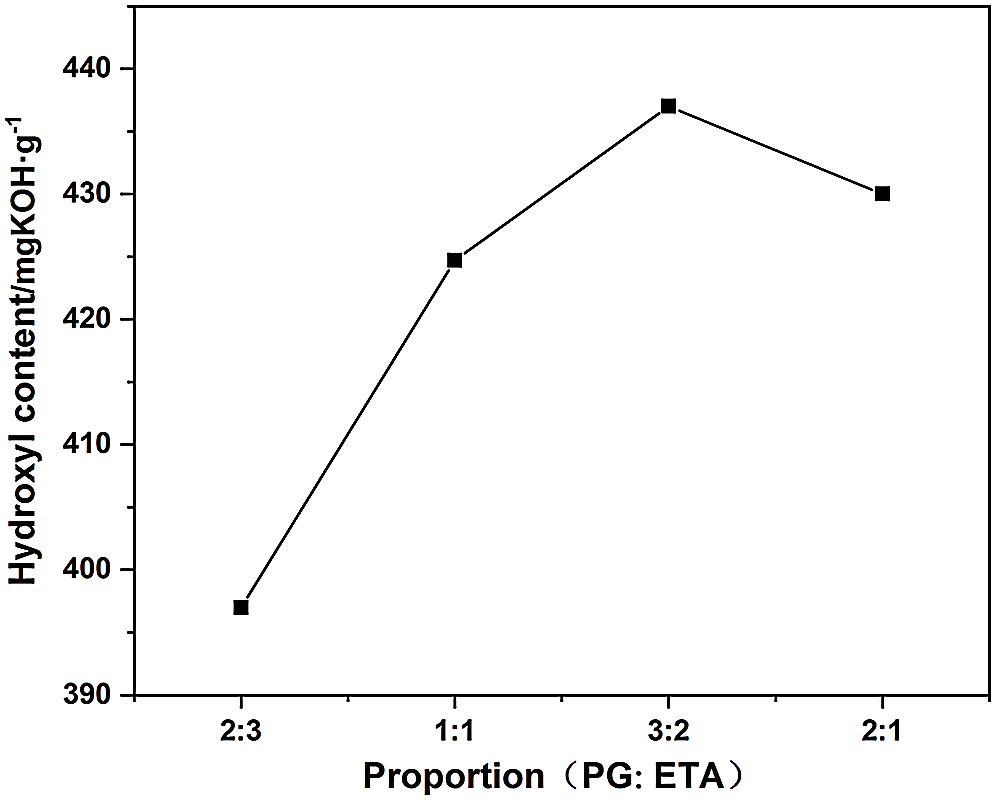
Figure 4: Effect of different proportion of alcoholysis agent on hydroxyl content of DM
It is known that the lower the viscosity of DM obtained after alcoholysis of waste polyurethane, the more thorough the degradation, the higher the hydroxyl content. From Figs. 3 and 4, it can be seen that when m (PG):m (ETA) is less than 3:2, the hydroxyl content of DM increases with the increase of the ratio of PG to ETA. This was because that with the increase of the ratio, the content of PG in the matrix elevated, and the reaction activity of PG was also enhanced, so that the alcoholysis reaction was more thorough. When further increasing the mass ratio of PG to ETA to 2:1, the PG content in the matrix is too high, side reaction occurs during alcoholysis. Urea group breaks to form amine and polyol, which leads to the decrease of hydroxyl content. When mass ratio of PG to ETA eaquals to 3:2, DM prepared by alcoholysis was better than DM prepared by other proportions in terms of both viscosity and hydroxyl content. Thus the optimal bicomponent alcoholysis ratio was 3:2, and DM prepared with this ratio was used to prepare regenerated polyurethane rigid foam.
3.4 Analysis of Mechanical Properties of Different PUDM-Lignins
PUDM-Lignins prepared with different lignin addition amounts were subjected to compression tests. The compression force and compression time of PUDM-Lignin prepared with different lignin addition amounts can be tested. The curve of the compressive strength of the composite with time can be calculated, as shown in Fig. 5.
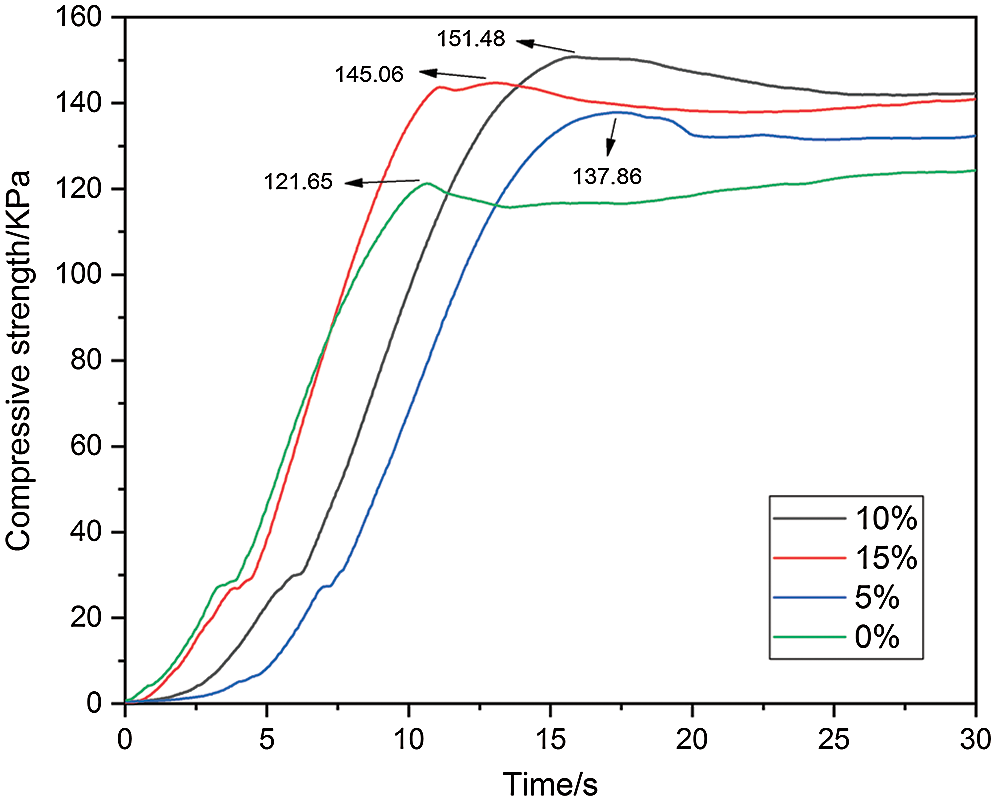
Figure 5: Relationship between compressive strength and time of PUDM-Lignin with different lignin content
As can be seen from Fig. 5, when the lignin content in the composite increases from 0% to 10%, the compressive strength of PUDM-Lignin increases gradually. With the increase of lignin content in the composite, the maximum value reaches 151.48 KPa when the lignin content was 10%, which was 24.5% higher than that without lignin addition. The main reason that lignin can improve the compressive strength of composite materials are as follows. Lignin is a high molecular compound rich in hydroxyl and aromatic groups. Hydroxyl in lignin can react with isocyanate to form a prepolymer. So that the rigid structure of benzene ring in lignin could be introduced into the prepolymer, which can effectively enhance the compressive strength of composite materials. A small amount of lignin fillers can be well dispersed in the PUDM matrix. In addition, hydroxyl groups in the lignin structure could form intermolecular hydrogen bonds with the matrix, enhancing the intermolecular force. So the stress on the PUDM matrix could be transferred to the lignin fillers, delaying the failure process of the materials, thus improving the compressive strength of the materials. This conclusion can prove that the addition of lignin can strengthen and toughen PUDM. Furthermore, because DM is an aromatic polyol and has a rigid structure of benzene ring, the compressive strength (121.65 KPa) of the prepared PUDM is higher than that of waste polyurethane (105.84 KPa) without lignin. The compressive strength (151.48 KPa) of PUDM-Lignin prepared with lignin is obviously higher than that of PUDM prepared without lignin.
With further increase of lignin filler, the compressive strength of PUDM-Lignin decreased. When the addition of lignin was 15%, the compressive strength of the composite decreased to 145.06 KPa. This is because that with the increase of lignin content, the content of PUDM matrix decreases, so that the lignin could not be fully infiltrated by PUDM matrix. Large amounts of lignin aggregated, which hinder the transfer of stress from the PUDM matrix to the lignin, leading to a decrease in the compressive strength of PUDM-Lignin. It is worth noting that if the addition of lignin further increases to more than 15%, the property of lignin will play a leading role in the composites, which will change PUDM-Lignin from a ductile material to a brittle material, resulting in a much lower compressive strength and even difficult foaming.
3.5 Analysis of Thermal Conductivity of Different PUDM-Lignin
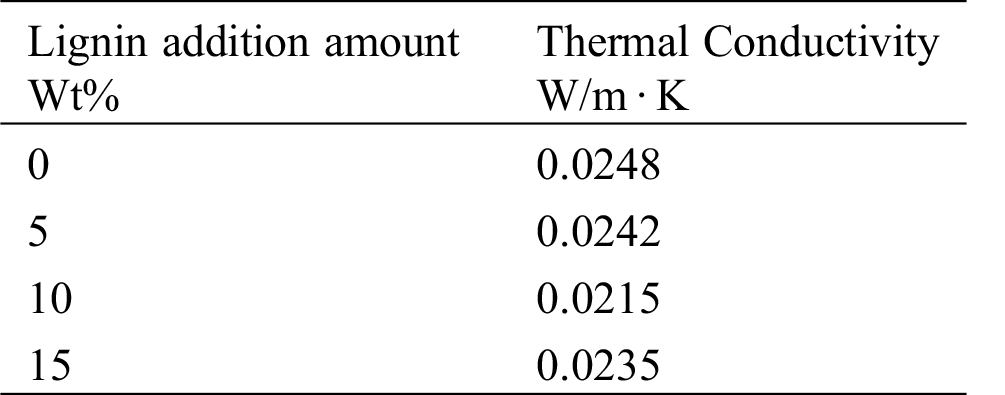
Tab. 2 showed that the addition amounts of lignin did not affect the thermal conductivities of PUDM-Lignins greatly. The thermal conductivity of the prepared recycled PU rigid foam was lower than 0.03 W/(m · K), which met the Chinese national standards of 0.025 W/(m · K)–0.036 W/(m · K) in “Rigid Polyurethane Foam for Building Thermal Insulation” (GB/T44248-2012) [18]. When the addition of lignin was 10%, the value of thermal conductivity was the lowest. This is because that at this point the foam has more completed and highly cross-linked cell structures, leading to a good thermal insulation performance.
Table 2: Thermal conductivity of PUDM-Lignin prepared with different amounts of lignin
3.6 Analysis of Water Absorption Performance of Different PUDM-Lignin
The effect of different amount of lignin on the water absorption of PUDM was studied by measuring the water absorption. PUDM-Lignin was initially cut into a cube-shaped experimental sample with a volume of 1 cm3. The sample was then placed in deionized water for absorption for 5 min, and weighed. The sample was finally put in an oven for drying and dewatering. The water absorption capacity of PUDM-Lignin with different lignin addition was calculated by formula. The results are shown in Fig. 6.
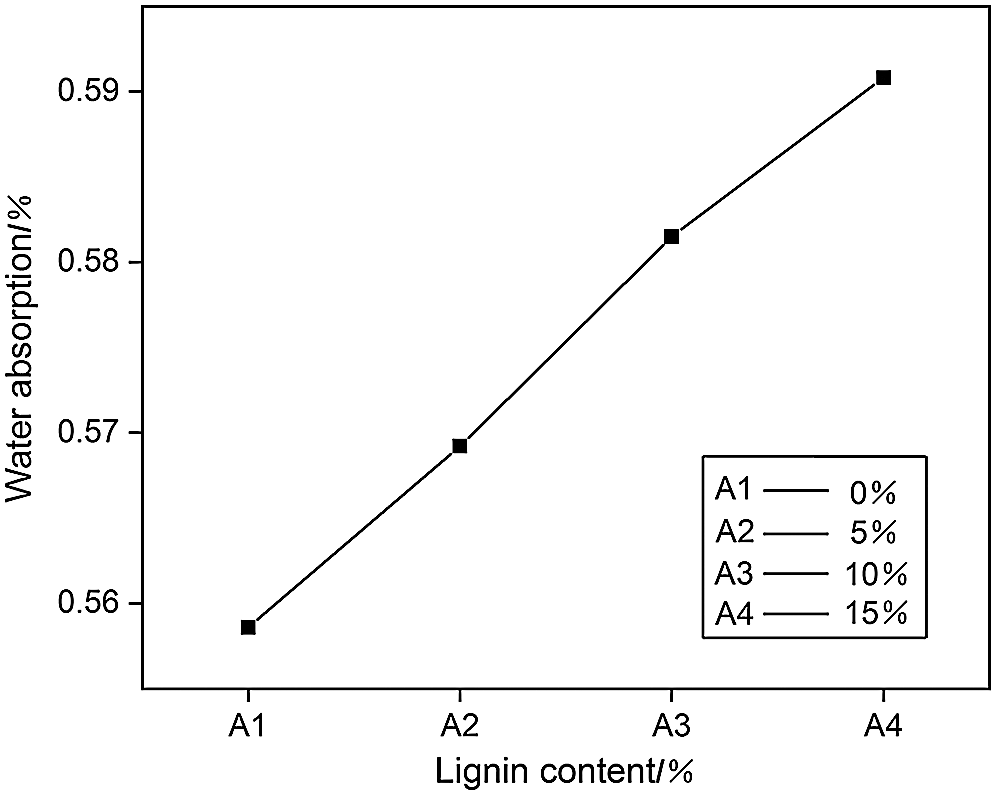
Figure 6: The effect of the amount of lignin added on the water absorption of polyurethane
It can be seen from Fig. 6 that the water absorption of PUDM-Lignin prepared with lignin is significantly higher than that of PUDM-Lignin without lignin. Lignin itself has many hydrophilic groups, such as carboxyl and hydroxyl groups. These hydrophilic groups are the main factors to improve the water absorption of PUDM-Lignin. Therefore, with the increase of lignin content, the water absorption of PUDM-Lignin will also increase.
The loss rate of PUDM-Lignin soaked in deionized water was measured, as shown in Fig. 7. It can be seen that the loss rate of PUDM without lignin was close to 9%, while the loss rate of PUDM-Lignin with lignin was obviously decreased to about 4% and no obvious change was found with the increase of lignin addition. This is because that there are many unreacted small molecules in the PUDM without lignin, which could be dissolved in deionized water after soaking. Therefore, the loss rate of PUDM is high. On the other hand, PUDM-Lignin forms a firm cross-linked network structure between lignin and the matrix, therefore, it is hard to soluble in deionized water, leading to a lower weight loss rate.
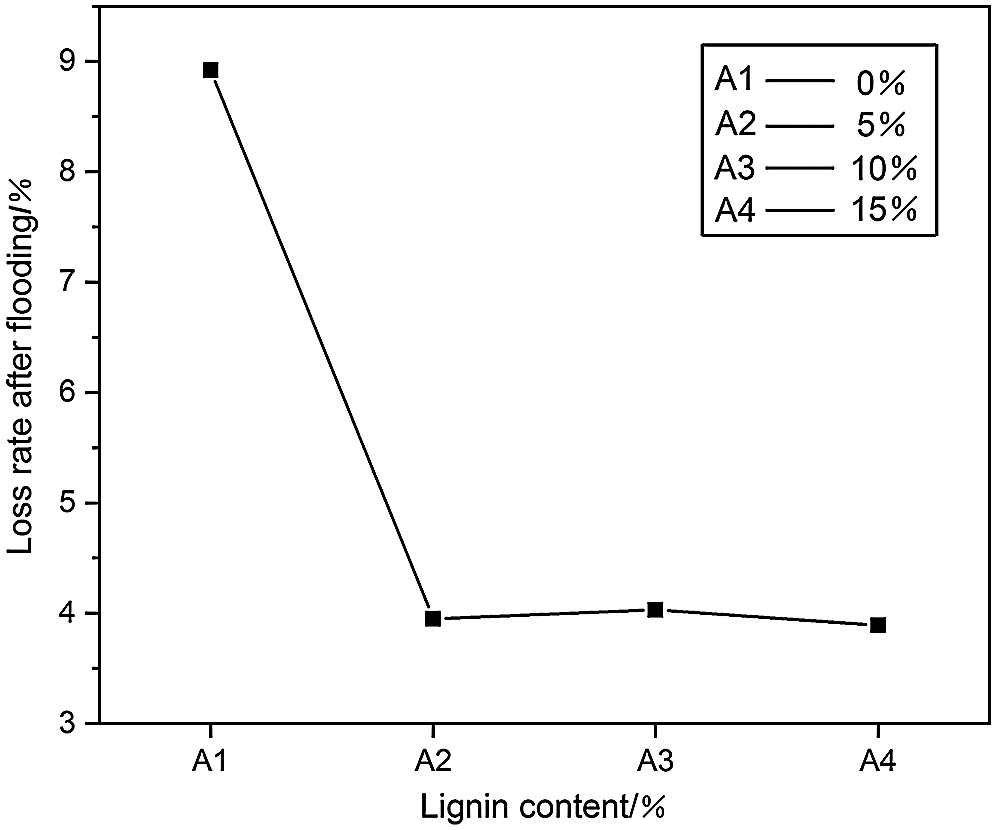
Figure 7: The effect of the amount of lignin added on the loss rate of PUDM-Lignin after water immersion treatment
On the whole, with the addition of lignin, the water absorption of PUDM-Lignin does not increase significantly, but the weight loss rate can be decreased a lot. Therefore, the addition of lignin can improve the waterproof performance of PUDM.
3.7 Measurement of Apparent Density and Porosity of Different PUDM-Lignin
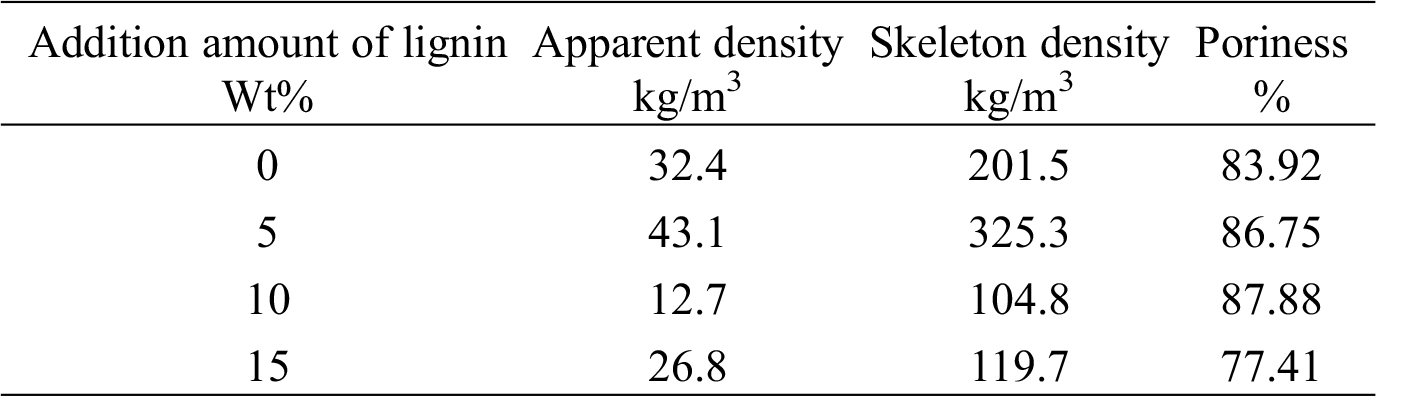
The density of PUDM-Lignin prepared with different lignin addition was measured by pycnometer method, and the measurement results are shown in Tab. 3. The research results show that with the increase of lignin addition, the porosity of foam increases at first and then decreases. When the lignin content is 10%, PUDM has lower density and higher porosity. This is because the addition of appropriate amount of lignin can well disperse in the PUDM matrix and further enhance the skeleton structure of PUDM-Lignin, resulting in better foam initiation and larger foaming volume. As a result, the apparent density of PUDM-Lignin is smaller and the porosity is higher, which makes the prepared PUDM have better structure and uniform cell distribution.
Table 3: Effect of lignin addition on apparent density and porosity of PUDM-Lignin
3.8 Chemical Structure Characterization of Different PUDM-Lignin
Fig. 8 is the FT-IR spectrum of PUDM-Lignin, used to study the structural differences of lignin in composite materials. The infrared spectra of different PUDM-Lignins are roughly the same, but the intensity of some peaks is different. As can be seen from Fig. 8, the wide peak near 3328 cm−1 is the stretching vibration peak of X–H (X=O, N, etc.) in the amino group in the polyurethane structure and the carboxyl and hydroxyl group in the lignin structure. Peaks at 2925 cm−1 and 2872 cm−1 are symmetric and antisymmetric stretching vibration peaks of −CH2, respectively. Peak at 1728 cm−1 is the stretching vibration absorption peak of the ester group (−C=O) in the carbamate group and the stretching vibration characteristic absorption peak of the carbonyl group (C=O) in the lignin [19]. 1523 cm−1 and 1503 cm−1 correspond to the stretching vibration absorption peaks of −NH. 1224 cm−1 corresponds to the C–O–C bond stretching vibration peak. 1108 cm−1 corresponds to the absorption peak of stretching vibration of C–O–C bond [20].
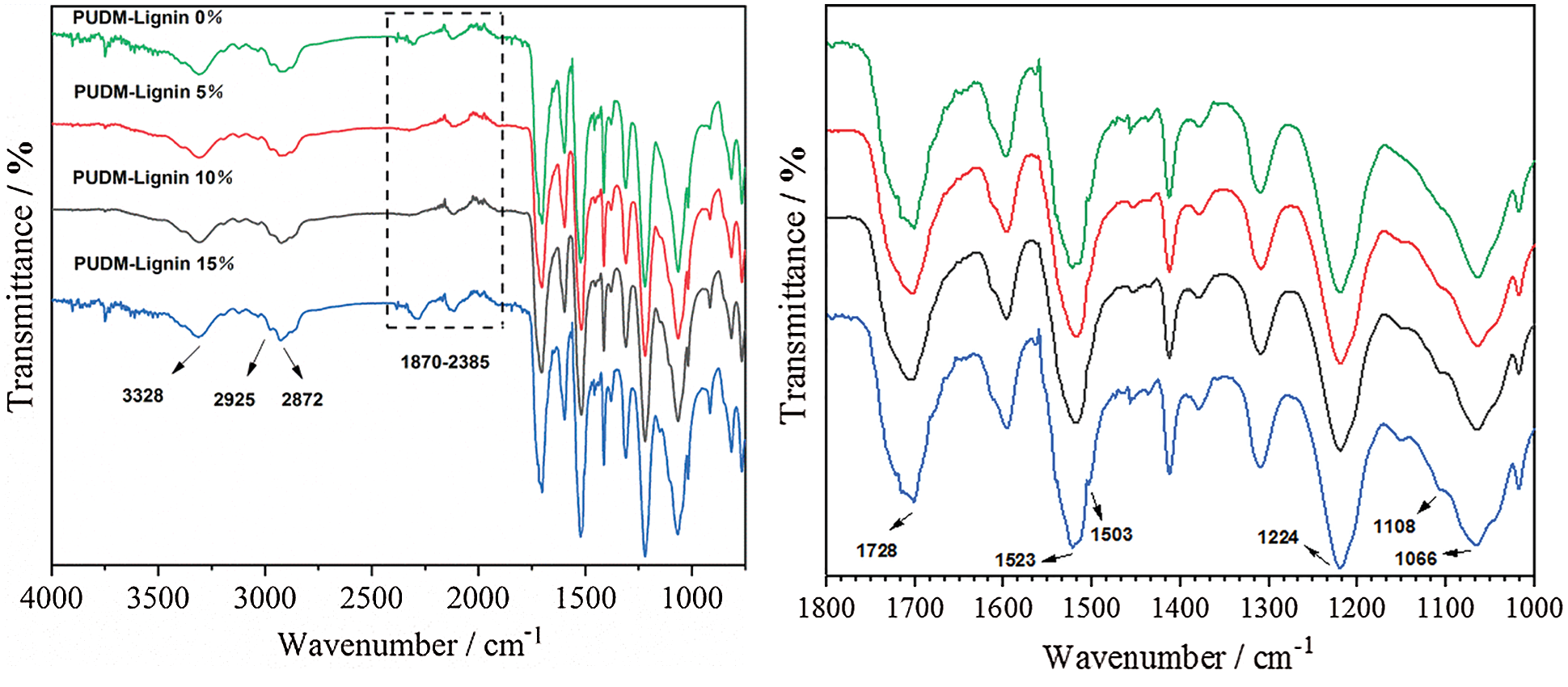
Figure 8: Infrared spectra of PUDM-Lignin prepared with different lignin contents. (The left side of Fig. 8 shows the magnified area with a wavelength range of 4000 cm−1–750 cm−1, and the right side of Fig. 8 shows the magnified area with a wavelength range of 1800 cm−1–1000−1)
Absorption peaks in the range of 1870 cm−1–2385 cm−1 correspond to the residual isocyanate groups (−NCO) indicating that excess MDI in the stage of prepolymerization. The absorption peak near 1066 cm−1 relates to the characteristic absorption peak of lignin [21], and its intensity changes with the lignin content, indicating that that the addition of lignin had a certain effect on the molecular structure of PUDM-Lignin. The above results show that these peaks are consistent with the typical infrared spectra of polyurethane, indicating that PUDM-Lignin is indeed prepared.
3.9 Morphologies of Different PUDM-Lignin Foams
In order to observe the integrity and the microstructure of the foam skeleton, PUDM-Lignin foams prepared with different lignin additions were cut into thin samples. The areas with clear visual field and relatively intact and regular cells were selected for observation. The observation results are shown in Fig. 9.
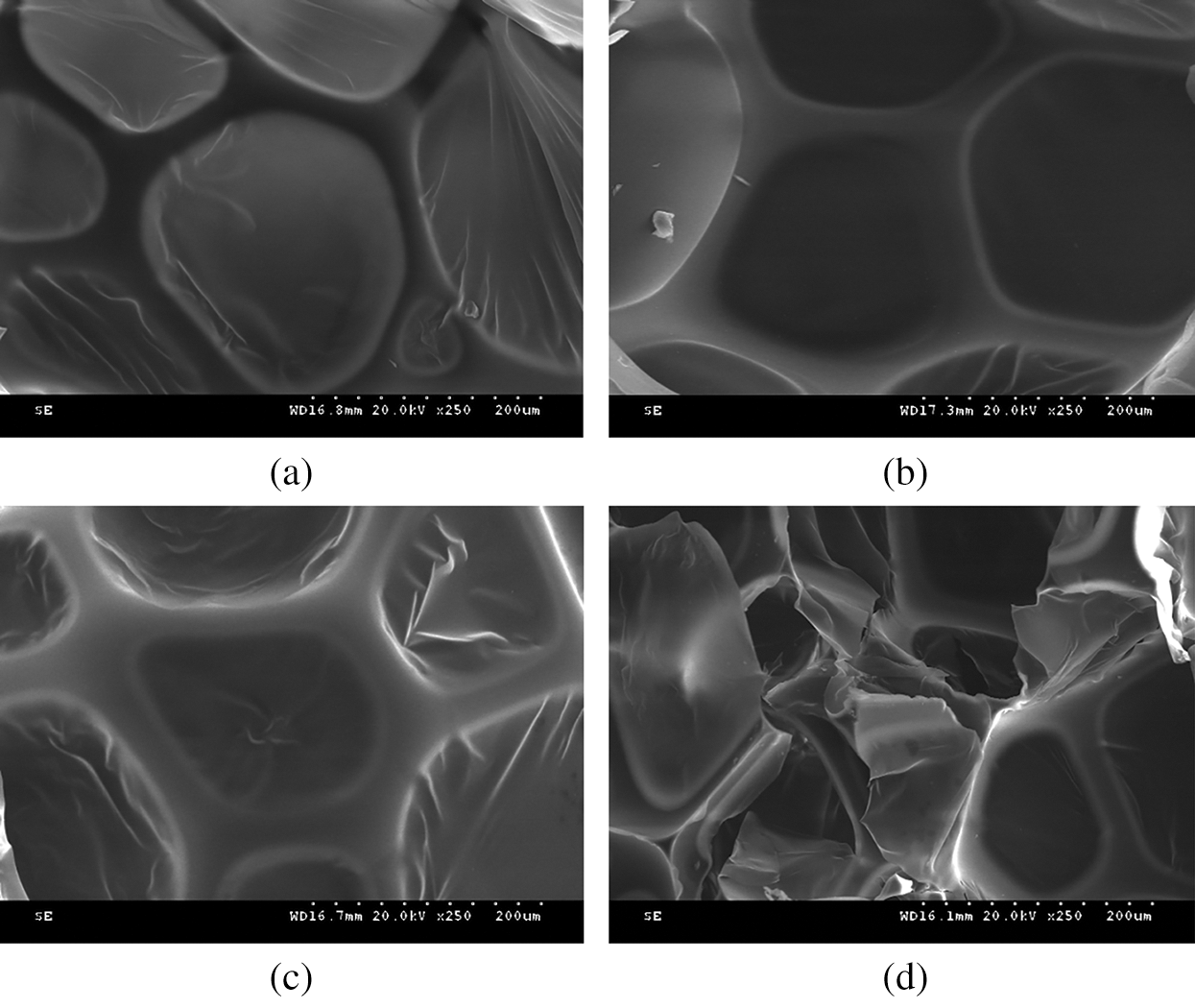
Figure 9: SEM images of PUDM-Lignin prepared with different lignin contents. (Scale bar is 200 um for each picture.) (a) PUDM-Lignin 0% (b) PUDM-Lignin 5% (c) PUDM-Lignin 10% (d) PUDM-Lignin 15%
As shown in Figs. 9a–9c, when the addition of lignin is 0%, 5% and 10%, the cells of PUDM-Lignin foams are relatively intact and regular, and the cell size is relatively uniform in the whole field of vision, indicating that the foam is relatively stable in the initiation process. With the lignin addition of 10%, the cell wall of the foam is the thickest and the most uniform, indicating a higher cross-linking degree of the foam, which can provide a good compressive strength for the foam. The rigid structure of lignin is rich in aromatic groups. Therefore, addition of lignin can effectively enhance the compression modulus of the foam. Besides, it can be found that at 10% of lignin addition, the closed-pore ratio of PU foam is relatively high. The closed-pore ratio is of great significance to the thermal insulation performance of PU foam, which consistent with the thermal insulation results shown in Tab. 2. As shown in Fig. 9d, when the addition of lignin is 15%, the cell structure is incomplete and the pore-closure degree is poor. This is because with the increase of lignin content, the matrix content is relatively low, and lignin cannot be fully infiltrated by the matrix, resulting in the accumulation if lignin in large quantities and breakage of the integrity of the bubble holes. Consequently, the initiation process of the foam is not stable enough, and the shape and structure of the bubble are not good.
3.10 Thermal Stability Analysis of Different PUDM-Lignin
Fig. 10 shows the TG and DTG curves of PUDM-Lignin, which can be used to study the thermal stability of PUDM-Lignin. It can be seen that the thermogravimetric curve mainly consists of two temperature stages, Stage 1 (150°C−295°C) and Stage 2 (300°C−400°C). This is mainly because PUDM-Lignin has both “soft” segments and “hard” segments. The “hard” segment refers to the carboxylic acid groups in the prepolymer, and the “soft” segment refers to the ammonia groups in the prepolymer. The weight loss in the first stage is mainly due to the decomposition of hard segments in PUDM-Lignin and the mutual reaction or detachment of functional groups in lignin molecules. The weight loss in the second stage is mainly due to the degradation of the “soft” segments in PUDM-Lignin.
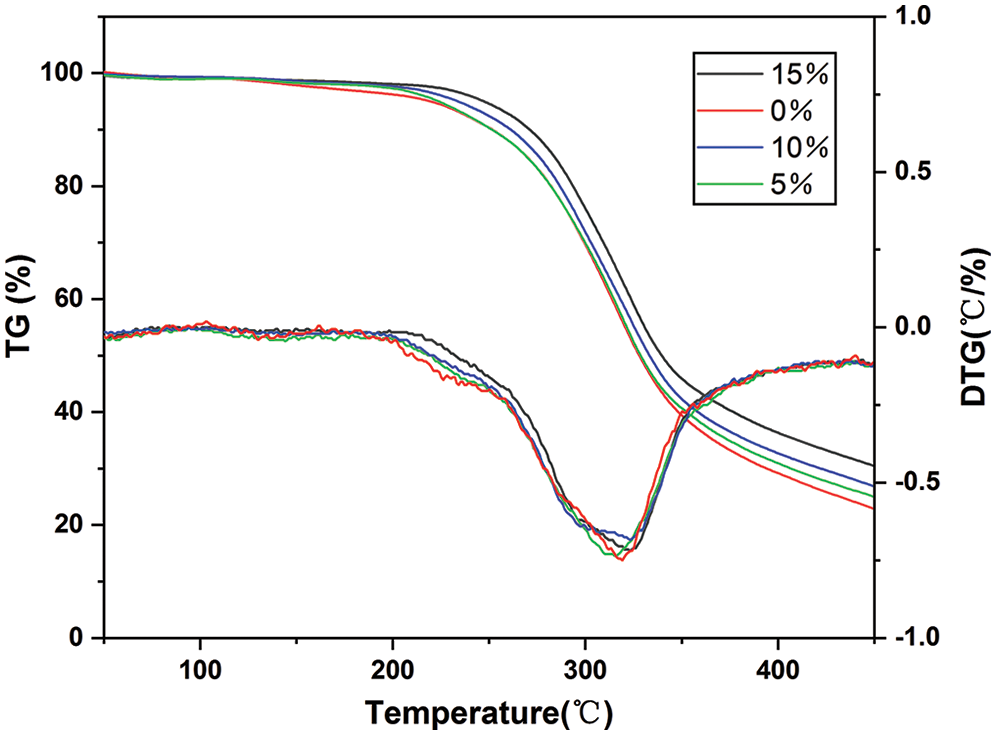
Figure 10: Thermogravimetric curve of PUDM-Lignins prepared with various lignin contents
It can be seen from the first stage (150°C−295°C) that the thermal weight loss rate of the composites decreases with the increase of lignin content. The initial decomposition temperature of PUDM without lignin is 157°C, while the initial decomposition temperatures of PUDM with different lignin contents, 5%, 10% and 15%, are 195°C, 215°C and 242°C, respectively. Therefore, thermal stability of the composite materials added with lignin were improved by the addition of lignin. From the second stage (300°C−400°C), it can be seen that the thermal weight loss temperature of the composites is obviously higher than that of PUDM, and the rate of thermal degradation is also obviously decreased, indicating that the thermal stability of the composites has been improved with the lignin addition. There are two reasons. Firstly, the addition of lignin promotes the crystallization of the irregular “soft” segment of the composite, which makes the soft segments of the composite transforming to hard segments, and increases the cross-linking density of the composite. Secondly, phenolic hydroxyl groups in lignin can capture the free radicals during the degradation, thus reducing the degradation rate [22]. Therefore, the addition of lignin can improve the thermal stability of polyurethane composites.
In this study, the waste PU hard foam was recovered by alcoholysis with a bi-component alcoholysis agent. Recycled polyurethane materials were prepared by using the recovered products as raw materials instead of part of polyether polyols. Through testing and characterization, following conclusions can be obtained.
1. The waste polyurethane was successfully alcoholized into oligomer polyols (DMs), and PUDM-Lignins with excellent properties were successfully prepared from DMs.
2. The optimum mass ratio of PG) to ETA is 3:2 for the waste polyurethane alcoholysis. And the viscosity and hydroxyl content of DMs meet the requirements of replacing polyether polyols for foaming.
When the addition amount of lignin is 10%, the foam cell wall of the prepared PUDM-Lignin is thicker and more uniform, the skeleton geometry is also better. The PUDM-Lignin foam with 10% of lignin has higher compression strength, better thermal insulation property, waterproof and thermal stability compared with that of other composition, At the same time, it has lower apparent density and higher porosity.
3. The hydroxyl group in lignin can react with isocyanate in the polyurethane, introducing the rigid benzene ring of lignin into the prepolymer, thus improving the mechanical properties of PUDM. In addition, the mechanical properties of PUDMs prepared from DMs are better than the original waste polyurethane.
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Ma, M. A., Ma, N. (2017). Chemical recycling and liquefaction of rigid polyurethane foam wastes through microwave assisted glycolysis process. Journal of Macromolecular Science Part A-Pure and Applied Chemistry, 44(6), 613–617. DOI 10.1080/10601320701285003. [Google Scholar] [CrossRef]
2. Murai, M., Ta, T. (2017). Recycling of polyurethane foam refrigerator insulation. Mitsubishi Electric Advance, 10(6), 104–106. DOI 10.1177/0021955X02038004142. [Google Scholar] [CrossRef]
3. Gao, L. P., Zeng, G. Y., Zhou, Y. H., Liu, P., Li, L. et al. (2015). The mechanical properties of lignin-based rigid polyurethane foam nanocomposites are improved. Journal of Thermal Analysis and Calorimetry, 13(120), 131–132. DOI 10.3390/ma8020586. [Google Scholar] [CrossRef]
4. Gai, V. (2017). Chemical degradation of polyurethane. Rubber World, 3(15), 1–31. DOI 10.1002/pen.24466. [Google Scholar] [CrossRef]
5. Detomi, A. C., dos Santos, R. M., Filho, S. L. M. R., Martuscelli, C. C., Panzera, T. H. et al. (2014). Statistical effects of using ceramic particles in glass fibre reinforced composites. Materials & Design, 55(463), 5–8. DOI 10.1016/j.matdes.2013.09.026. [Google Scholar] [CrossRef]
6. Mehl, J. T., Murgasova, R., Dong, X., Hercules, D. M., Nefzger, H. (2000). Murgasova. characterization of polyether and polyester polyurethane soft blocks using MALDI mass. Analytical Chemistry, 72(3), 2490–2498. DOI 10.1021/ac991283k. [Google Scholar] [CrossRef]
7. Renata, M., Ericl, B. (2018). Characterzation of polyeste-polyurethane soft and hard blocks by a cominat ion of MALDI. SEC and Chemical Degradation Macromolecules, 35(2), 8338–8345. DOI 10.1021/ma020879y. [Google Scholar] [CrossRef]
8. Singh, H., Sharma, T. P., Jain, A. K. (2007). Reactivity of the raw materials and their effects on the structure and properties of rigid polyurethane foams. Journal of Applied Polymer Science, 15(106), 1014–1023. DOI 10.1002/(ISSN)1097-4628. [Google Scholar] [CrossRef]
9. Pravakar, M., Du, V. K. (2007). Rigid polyurethane-clay nanocomposite foams: Preparation and properties. Journal of Applied Polymer Science, 2(14), 2802–2809. DOI 10.1177/0731684408096949. [Google Scholar] [CrossRef]
10. Va, B. C., Ai, S. (2016). Processing and mechanical characterization of lightweight polyurethane composites. Journal of Material Science, 16(3), 163–164. DOI 10.1023/A:1023203121299. [Google Scholar] [CrossRef]
11. Mu, M., Ta, T. (2017). Recycling of polyurethane foam refrigerator insulation. Mitsubishi Electric Advance, 10(8), 104–106. DOI 10.1080/09593330.2014.918180. [Google Scholar] [CrossRef]
12. Gao, L. P., Zheng, G. Y., Zhou, Y. H., Wang, L., Li, N. et al. (2015). Improved mechanical properties and fire resistance of lignin-based rigid polyurethane foam nanocomposites. Journal of Thermal Analysis and Calorimetry, 13(120), 1311–1325. DOI 10.1007/S10973-020-10415-5. [Google Scholar] [CrossRef]
13. Murai, M., Takagi, T. (2015). Recycling of polyurethane foam refrigerator insulation. Mitsubishi Electric Advance, 10(3), 77–85. DOI 10.1080/09593330.2014.918180. [Google Scholar] [CrossRef]
14. Behrendt, G., Naber, B. W. (2019). The chemical recycling of poly. Journal of the University of Chemical Technology and Metallurgy, 44(1), 3–23. DOI 10.1016/j.wasman.2020.10.031. [Google Scholar] [CrossRef]
15. Se, S., Han, K., Jing, Y. L., Sang, H. L., Dai, S. L. et al. (2019). Sustainable rigid polyurethane foams based on recycled polyols from chemical recycling of waste polyurethane foams. Journal of Applied Polymer Science, 4(1), 33–35. DOI 10.1002/app.47916. [Google Scholar] [CrossRef]
16. Nikje, M. M., Mohammadi, F. H. A. (2015). Hydrophobisation of mortars containing waste polyurethane foam. Polymer–Plastics Technology and Engineering, 10(2), 25–27. DOI 10.1051/matecconf/201816304006. [Google Scholar] [CrossRef]
17. Molero, C., Lucas, A., Rodriguez, J. F. (2006). Recycled waste black polyurethane sponges for solar vapor generation and distillation. Polymer Degradation Stability, 9(3), 8–9. DOI 10.1016/j.apenergy.2017.08.169. [Google Scholar] [CrossRef]
18. Cao, Y. (2018). Development of green technology for degradation of rigid polyurethane materials. Changchun University of Technology, 10(2), 7–8. DOI 10.1016/j.apenergy.2017.08.169. [Google Scholar] [CrossRef]
19. Britain, J. W., Gemeinhardt, P. G. (2016). Catalysis of the isocyanate-hydroxyl reaction. Mechanics of Materials, 11(3), 207–211. DOI 10.1016/S0300-9440(99)00006-5. [Google Scholar] [CrossRef]
20. Zhang, X. (2017). Industrial application of environmentally friendly titanium-based polyester catalyst. Tianjin University, 10(3), 45–55. DOI 10.3103/S1063455X14040031. [Google Scholar] [CrossRef]
21. Murai, M., Sanou, M. (2017). Glycolysis of rigid polyurethane foam under various reaction conditions. Journal of Cellular Plastics, 39(2), 15–27. DOI 10.1177/002195503031021. [Google Scholar] [CrossRef]
22. Do, J., Sholz, R., Schwa, D. (2017). Effects of high-lignin-loading on thermal, mechanical and mor phological properties of bioplastic composites. Composite Structures, 47(2), 22–24. DOI 10.1016/j.compstruct.2017.12.003. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |