

 | Journal of Renewable Materials |  |
DOI: 10.32604/jrm.2021.015931
ARTICLE
Effect of Mitigating Strength Retrogradation of Alkali Accelerator by the Synergism of Sodium Sulfate and Waste Glass Powder
College of Materials Science and Engineering, Xi’an University of Architecture and Technology, Xi’an, 710055, China
*Corresponding Author: Tingshu He. Email: hetingshu@xauat.edu.cn
Received: 24 January 2021; Accepted: 26 February 2021
Abstract: This work aims to utilize waste glass powder (WGP) as a plementary material to mitigate the strength shrinkage caused by the alkaline accelerator. Waste glass power was used to replace cement by 0%, 10%, and 20% to evaluate waste glass powder on the alkaline accelerator’s strength retrogradation. The results show that the strength improvement effect of unitary glass powder is inconspicuous. Innovative methods have been proposed to use sodium sulfate and waste glass powder synergism, using the activity of amorphous silica in glass powder. Compared with the reference group, the compressive strength of 28d mortar increases by 67% when the sodium sulfate content is 2.5%, and the replacement amount of waste glass powder is 10%. Besides, XRD and SEM analysis of hydration products also confirmed that the synergistic effect of sodium sulfate and waste glass powder could reduce strength inversion. The findings presented in this paper are pivotal for using waste glass to solve the problem of strength inversion caused by the alkaline accelerator.
Keywords: Synergism; waste glass powder; alkaline accelerator; strength inversion
Waste glass has been recognized as municipal solid waste. In China, 20 tons of waste glass will be produced every year, while landfill is usually used to treat this waste [1,2]. Due to the high silicon dioxide composition of waste glass, non-degradable, the landfill method often causes environmental damage and destruction of resources [3]. Therefore, recycling waste glass as aggregates or as a partial replacement cement in the construction industry has become an exciting topic in the construction industry.
The cooling process endows amorphous silica with different pozzolanic properties reactivity [4–8], such as fly ash, slag, silica fume, which can reduce the cement consumption and modify the strength performances of concrete. Meanwhile, it alleviated the environmental hazards caused by many waste glasses and expanded the source of Supplementary Cementitious Materials [9–12]. Relevant literature shows that WG can replace 0%, 5%, 10%, 15%, 20%, and 30% of cement dosage in mortar mixture. The data showed that all replacement levels’ compressive strength was lower than that of the control sample at 28 days of age [13]. Generally, limiting the amount of glass powder to replace cement is less than 30%, reducing the apparent loss of early strength. A 20% cement replacement rate can increase strength and even facilitate expansion. Besides, Compared with ordinary paste, the water content of chemical bond is higher, and the hydration process C3S is accelerated after adding glass powder, that is low water absorption of waste glass powder, increased the effective water-cement ratio of cement, and the contact surface between cement and water is increased.
As the main component of waste glass is silica, sodium, and calcium, which are also the input components of alkali activation. The researchers initially expressed concern that the alkali-silica reaction (ASR) of amorphous silicon in WGP leads to the corresponding degradation. On the contrary, The experimental results show that WGP can be used as an alkaline silicon reaction retardant, prevent ASR expansion, and exhibit a specific mitigation effect on ASR expansion when the WG is ground to a particle size of less than 75 um [14–16]. The particle size cannot reach the expected size, Considering the energy consumption in the grinding process of waste glass. It is essential to select the appropriate reagent to stimulate the activity of waste glass powder. Unfortunately, some research is on the excitation of waste glass powder, unless the waste glass powder in alkali activated concrete. Furthermore, the Alkali accelerator can cause concrete strength back shrinkage at a later stage, which is more evident in cement mortar. This problem always persecutes to a tunnel and underground engineering construction.
Therefore, we innovatively propose to use the synergism of glass powder and activator to solve the problem of strength inversion caused by the alkaline accelerator. In this study, this paper mainly investigates the use of sodium sulfate and waste glass powder to improve the reduced strength caused by the alkali accelerator, aiming to reveal the content of waste glass powder and sodium sulfate and collaborative improvement mechanism. Scanning electron microscopy (SEM), X-ray diffraction (XRD) were implemented.
2 Materials and Experimental Program
The ordinary Portland cement is used in this experiment, complying with Chinese standard GB/T 4131-2014. Tab. 1, show its chemical compositions.
Alkali accelerator is provided by Shaanxi Youbang New Material Technology Co., Ltd., China. Fig. 1 provides granularity distribution, Tab. 1 provides Chemical properties of Cement and WGP.
Table 1: Chemical compositions of cement and waste glass powder
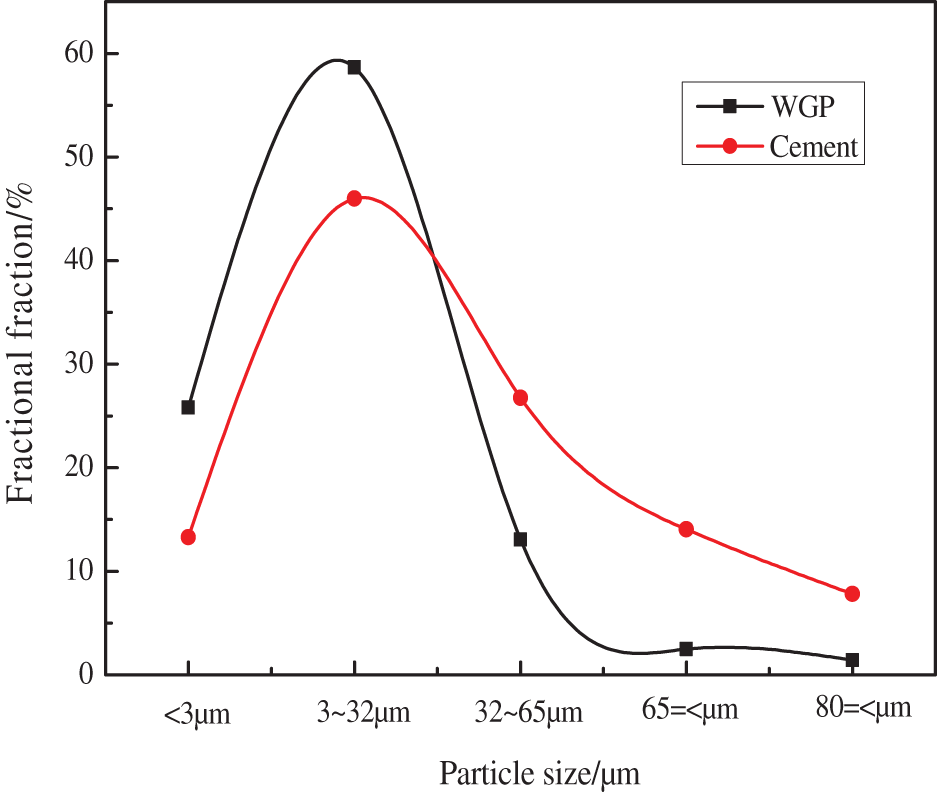
Figure 1: Particle size distribution of wgp and cement
The cement mortar experiment was prepared by fixed the water to-binder ratios of 0.5, using a mix of ordinary portland cement (P.O 42.5), and the mixing amount of alkaline accelerator (reliable content of 50%) is 4% of cement quality. The waste glass powder is replaced by 10% and 20% of the cement quality. To ensure the water-binder ratio remains unchanged, Weight sodium sulfate according to the percentage of the mass of 450 g water. After demolding, the mortar specimens (40 cm × 40 cm × 160 cm) are cured to the strength test’s corresponding age. Tab. 2 shows the mixed design proportions.
Table 2: Mix design proportions
2.2.1 Compressive Strength Test Analysis
The mortar specimens (40 cm × 40 cm × 160 cm) were cast to evaluate compressive strength following the Chinese standard. Since the Alkaline accelerator mainly causes 28d strength inversion, the compressive strength tests were conducted at 1, 3,7, and 28 days to understand the synergism of sodium sulfate and waste glass powder mitigating strength retrogradation of alkali accelerator of this study. The improvement effect is expressed by the relative compressive strength coefficient, Abbreviated as RSC.
CSi: Strength of mortar added with sodium sulfate or sodium sulfate and glass powder.
CS0: Reference specimen
2.2.2 X-Ray Diffraction and SEM Test Analysis
According to the experimental mix proportion, the cement paste blocks were manufactured and cured to the corresponding age. The hydration is then terminated after crushing, dried in vacuum at 40°C, ground to 200 mesh with an agate mortar. The X-ray diffraction pattern measurements of samples were performed using a Rigaku Ultima IV and X-ray radiation source (40 kV and 40 mA). The scanning range was 5°C–70°C, the scan speed was 0.02 °C/s.The hydrate and microstructure were carried out using a scanning electron microscope (SEM) technique of ZEISS.
3.1 Compressive Strength Test Results
Fig. 2 shows the influence of sodium sulfate and waste glass powder on the strength of mortar mixed with the alkaline accelerator. As shown in (a), the mortar’s strength mixed with alkali accelerator is affected by the dosage of sodium sulfate 0%, 0.5%, 1.5%, 2.5%, 3.5%. The results show that sodium sulfate has hardly any effect on the compressive strength of mortar at 1d, 3d, and 7d but significant influence 28d compressive strength. Compared with the blank group (the 28d compressive strength was 15.3 Mpa), the highest compressive strength was 21.7 Mpa, when the content of 2.5% is added. Aluminates may cause this phenomenon will consume gypsum quickly and form hydrated Sulphoaluminate (AFt or AFm) in the pores. Still, the stability of AFt or AFm is related to the sulfate content in the liquid phase. When the content is sufficient, AFt will be formed, conversely AFm be formed. The transformation from AFt to AFm results in the change of compactness (porosity) of mortar, which leads to the change of strength [17]. Another reason may be that the alkaline accelerator increases the alkali-aggregate reaction of mortar, resulting in decreased mortar strength. However, Waste glass powder is an amorphous silica material with pozzolanic properties reactivity. Under identical conditions, the strength of mortar was tested using waste glass powder instead of 10% and 20% cement, respectively. The results are shown in Figs. 2b and 2c, mortar compressive strength had a little effect at 1d, 3d, and 7d, with significant influence at 28d by comparison with CS0W10 CS0W20; the strength was 25.52 MPa and 21.31 MPa, respectively. The synergism of sodium sulfate and waste glass powder can improve the strength inversion caused by the alkaline accelerator. Waste-Glass powder replacement 10% of cement, the 28d strength of the mortar can be improved by adding 0.5%, 1.5%, 2.5%, and 3.5% sodium sulfate, but 20% glass powder is not satisfactory. It may be that the activity of waste glass powder is low; when the number of substitution increases, the adhesive material in the mortar will decrease, the strength decreases. It would seem that in the mixing water containing sulfate, 10% glass powder is relatively economical and safe for mortar mixed with the alkaline accelerator.
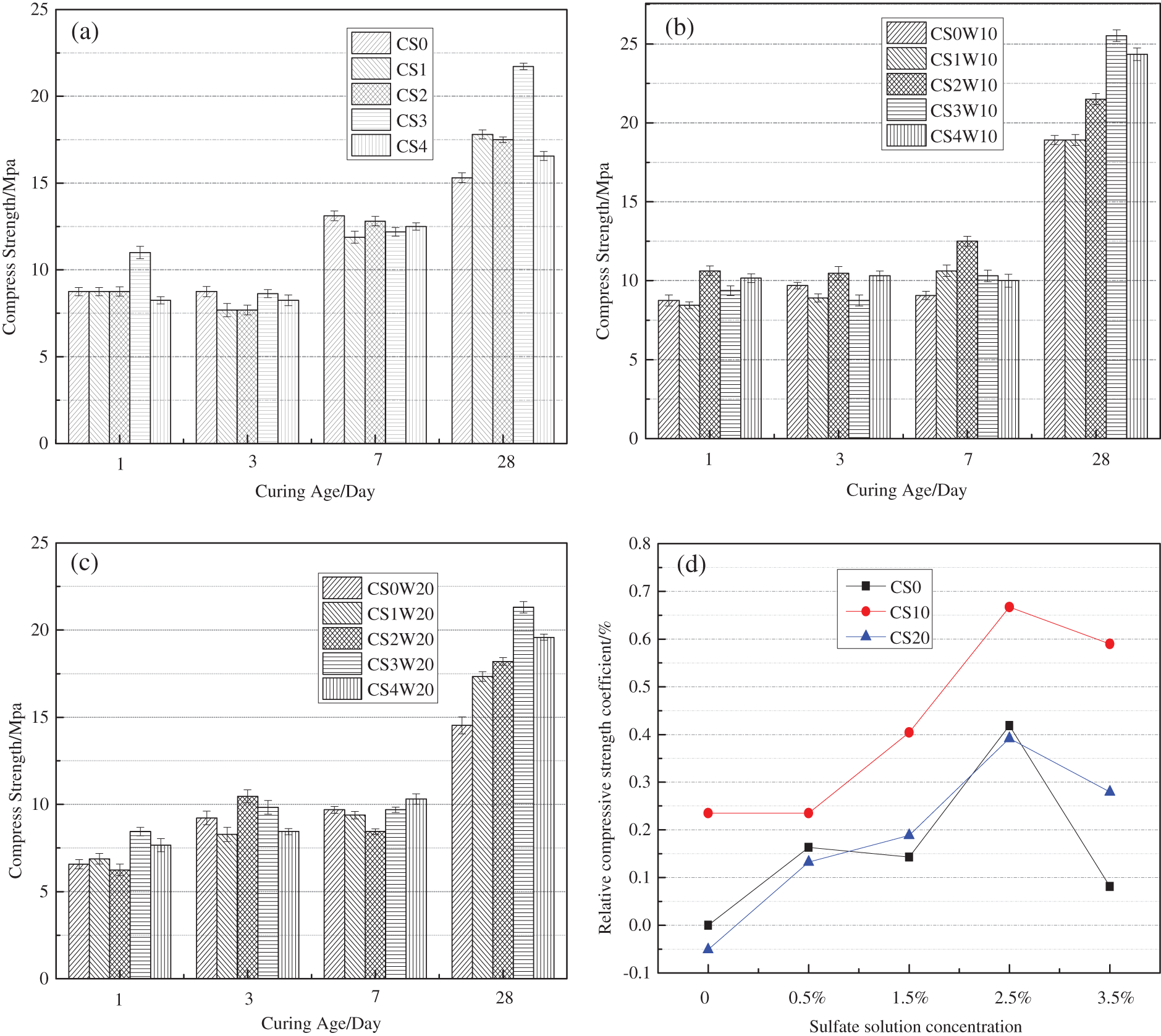
Figure 2: Influence of dosage of sodium sulfate and WGP on mortar compressive strength: (a) Influence of sodium sulfate content on mortar strength. (b) Influence of sodium sulfate and 10% glass powder on the strength of mortar. (c) Influence of sodium sulfate and 20% glass powder on the strength of mortar. (d) Influence of synergistic effect of sodium sulfate and waste glass powder on 28-day strength of mortar
3.2 X-Ray Diffraction Test Results
According to the test results of mortar strength, the 28-day strength of mortar changed significantly. So the hydration products of 28d were mainly analyzed by XRD. The analysis results of CS0, CS0W10, and CS0W20 hydration products are shown in Fig. 3. The relationship between CH peak intensity is as follows: CS0W10 > CS0W20 > CS0; the reason for this phenomenon may be that the amorphous silica in waste glass powder reacts with CH, resulting in the decrease of peak strength. When the presence of sodium sulfate, the regular pattern is consistent with CS3, CS3W10, and CS3W20. Also, compared with CS0W10 and CS3W10, it was found that the intensity of the CH peak of the former is greater than that of the latter. This result is consistent with the compressive strength of CS0W10 less than that of CS3W10 in Fig. 2. As a result of the hydrolysis of sodium sulfate, The formation of OH- leads to the disintegration of the tetrahedral three-dimensional network structure and the fracture of the Si-O bond, which makes glass powder produce more C-S-H by pozzolanic effect [18–21].
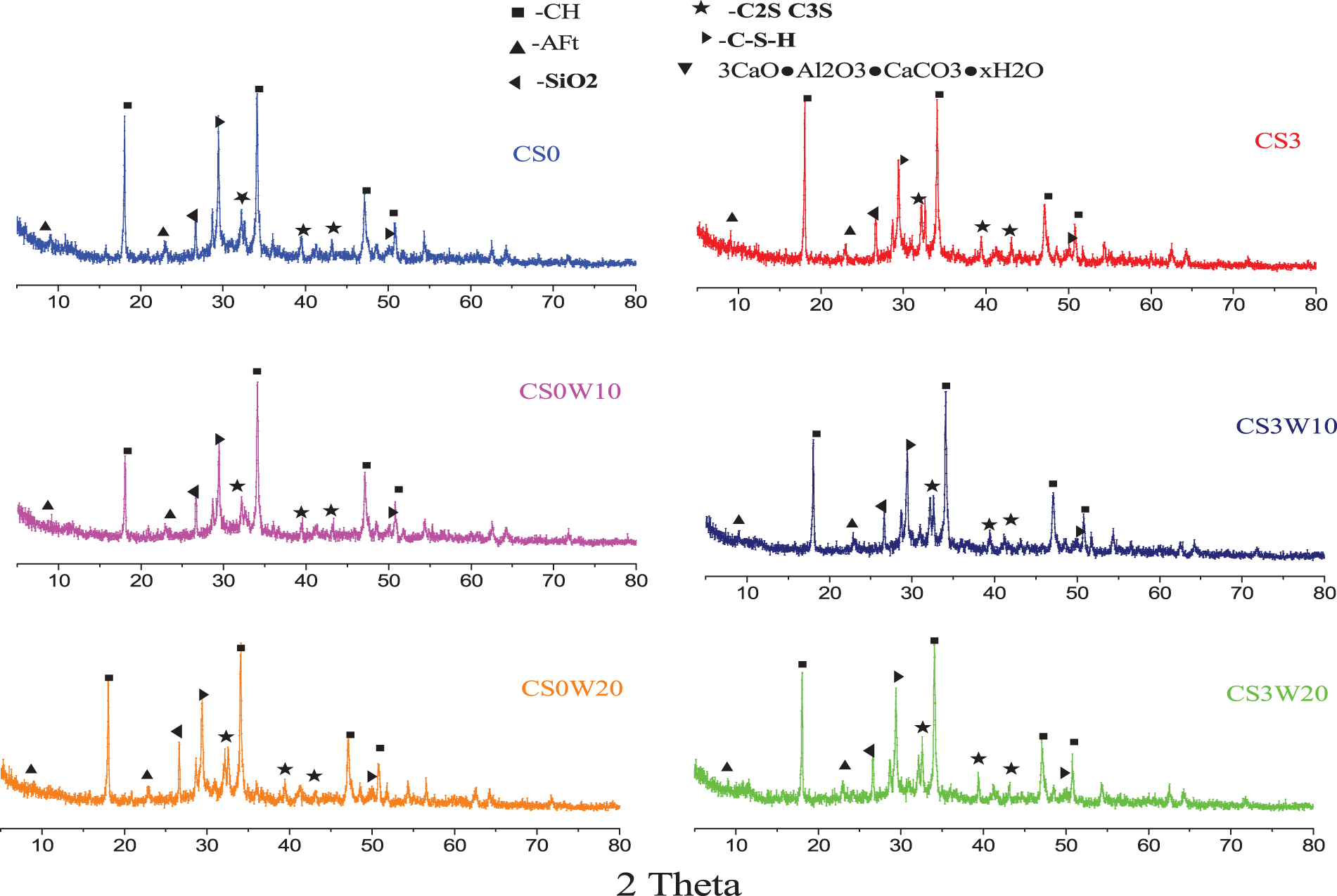
Figure 3: XRD patterns of concrete samples at theage of 28 days
3.3 Scanning Electron Microscope Test results
Fig. 4 shows the SEM images of CS0, CS0W10, CS0W20, CS3, CS3W10 and CS3W20 curing for 28 days. For CS0, CS0W10, and CS0W20, the C-S-H and calcium hydroxide (CH) can be found. But the development of crystal form of CS0W10 and CS0W20 is obviously different from that of CS0, and the main performance is that the crystal shape of glass powder is obvious, and the hydration products are rich. Among them, CS0W10 is the most obvious; the hydration products interweave with each other, showing a more dense state, which is consistent with the compressive strength. It indicates that the waste glass powder and calcium hydroxide (CH) react to produce a large amount of C–S–H (calcium–silicate–hydrate) gels, but this does not mean that the higher the content of glass powder, the more gel it produces. The activity of silica determines the degree of reaction. As shown in CS3, CS3W10, and CS3W20, plenty of C–S–H gels are observed to the phenomenon of layer by layer coverage, which can dedicate to the dense microstructures. By comparing the two groups of experiments, it is not difficult to determine that the glass powder has a better pozzolanic effect under sodium sulfate’s synergistic effect.
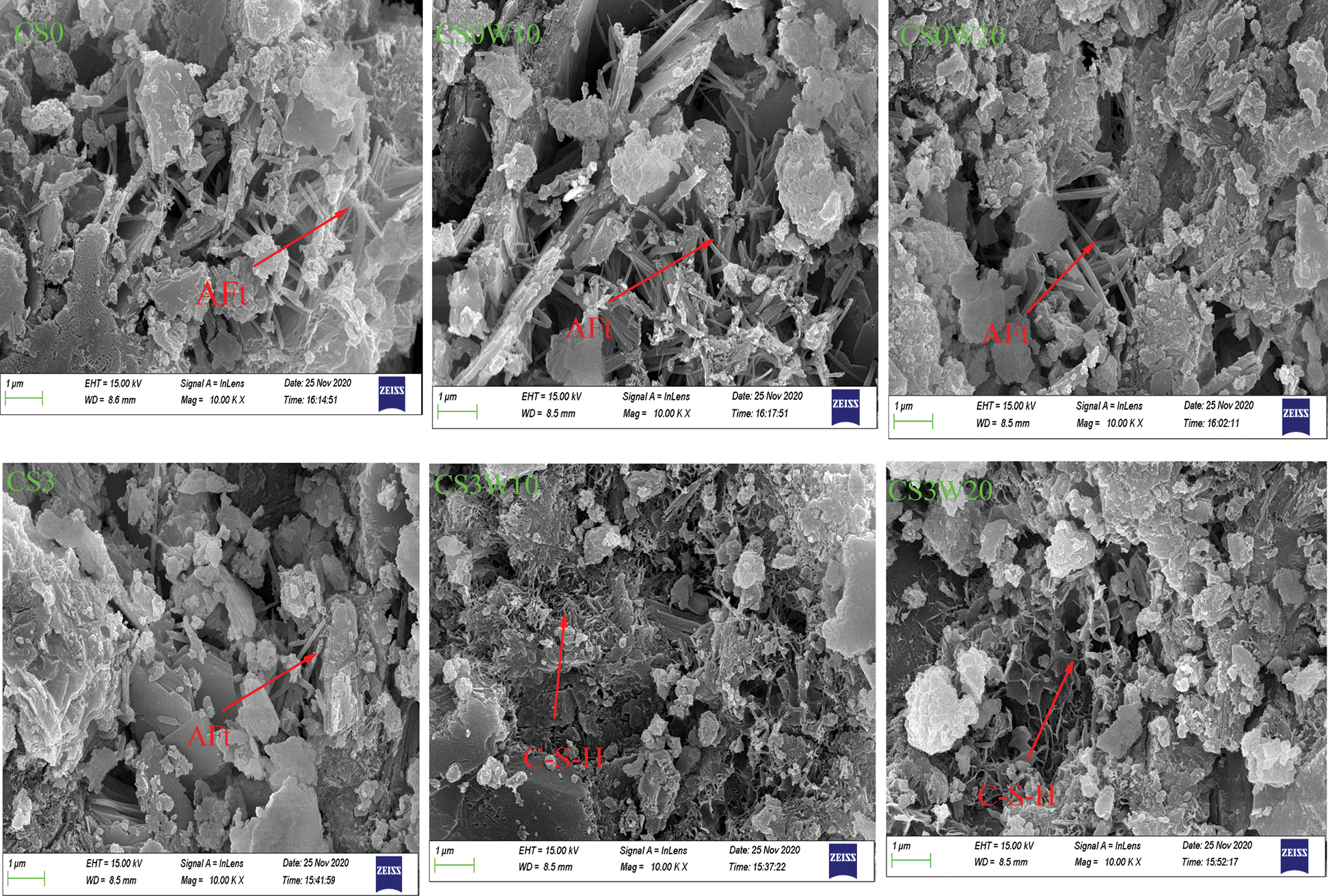
Figure 4: SEM patterns of concrete samples at theage of 28 days
It indicates that the sodium sulfate promotes the pozzolanic reaction of waste glass powder by disintegrating the tetrahedral three-dimensional network structure and the fracture of Si-O bond, making glass powder produce more C-S-H by pozzolanic effect as expressed by equations in Fig. 5.
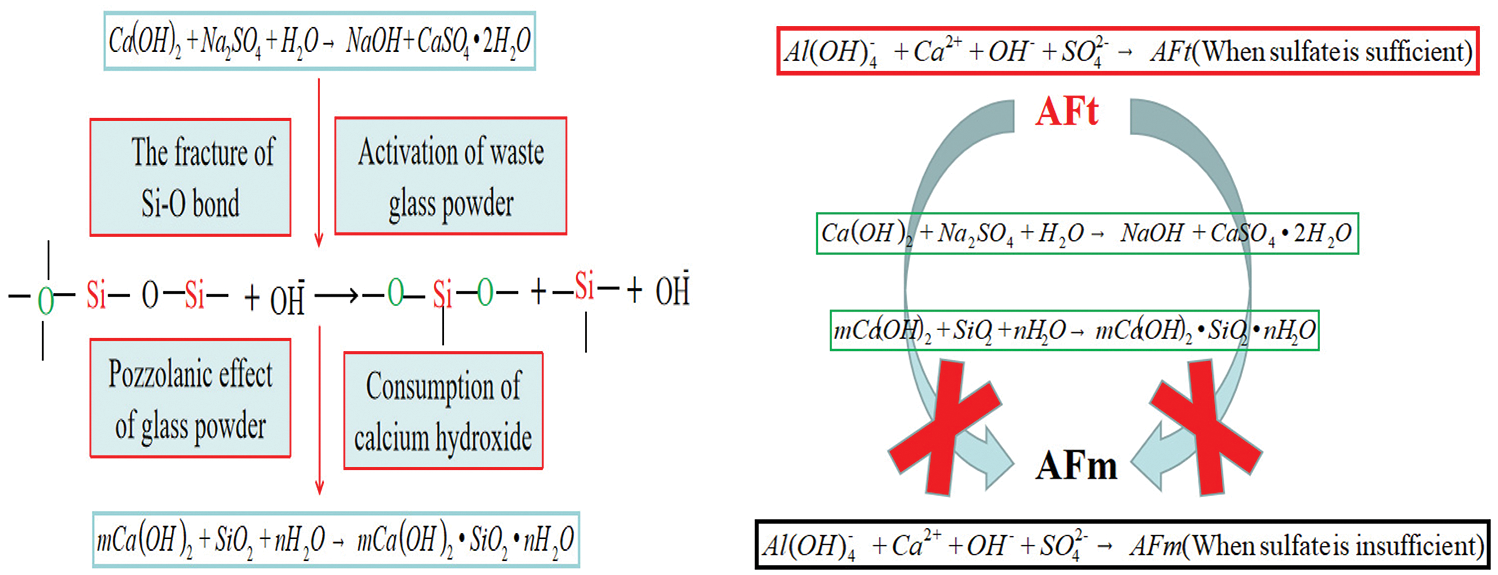
Figure 5: Schematic diagram of synergy between sodium sulfate and waste glass powder
As the density of AFt [1.73 g/cm3 (at 25°C) ]was lower than that of AFM [2.01 g/cm3 (at 25°C)], therefore When the conversion of AFt to AFM, the void ratio of cement paste will increase, the compressive strength will decrease. Therefore, inhibiting the conversion of AFt to AFm is one way to improve the alkaline accelerator’s strength inversion.
The synergism of sodium sulfate and waste glass powder is investigated in this study. Based on the experimental results and discussion, the following conclusions can be drawn up: 1. The addition of waste glass powder or sodium sulfate can improve the 28-day strength retrogradation of mortar caused by the alkaline accelerator. However, it has little effect on the early strength. 2. The improvement effect of single addition of sodium sulfate or waste glass powder is lower than that of sodium sulfate and waste glass powder. When the sodium sulfate content is 2.5%, and the replacement amount of waste glass powder is 10%, The effect is pronounced. 3. The intensity of CH peak decreases obviously by XRD. SEM’s microstructure characterization confirms that the pozzolanic result of waste glass powder is not equal to sodium sulfate and waste glass powder 4. The synergistic effect of sodium sulfate and waste glass powder is mainly reflected in the sodium sulfate promotion of the fracture of silicon-oxygen bond in the glass powder’s three-dimensional network structure. It was making the activity of glass powder and the volcano ash effect, which produces gel and makes the specimen structure denser. This can also be confirmed from SEM.
Acknowledgement: The authors wish to thank Xi’an University of architecture and technology for providing experimental instruments.
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Islam, G. M. S., Rahman, M. H., Kazi, N. (2016). Waste glass powder as partial replacement of cement for sustainable concrete practice. International Journal of Sustainable Built Environment, 6(1), 37–44. [Google Scholar]
2. Wang, Y. B., Li, J. W., He, X. Y., Zheng, Z. Q., Su, Y. et al. (2020). Effects of wet-grinded superfine waste glass on the fresh properties and reaction characteristic of cement pastes. Construction and Building Materials, 247, 118593. [Google Scholar]
3. Naik, T. R. (2008). Sustainability of concrete construction. Practice Periodical on Structural Design and Construction, 13(2), 98–103. DOI 10.1061/(ASCE)1084-0680(2008)13:2(98). [Google Scholar] [CrossRef]
4. Avila-López, U., Almanza-Robles, J. M., Escalante-García, J. I. (2015). Investigation of novel waste glass and limestone binders using statistical methods. Construction and Building Materials, 82(7), 296–303. DOI 10.1016/j.conbuildmat.2015.02.085. [Google Scholar] [CrossRef]
5. Ali, E. E., Al-Tersawy, S. H. (2012). Recycled glass as a partial replacement for fine aggregate in self compacting concrete. Construction and Building Materials, 35(7), 785–791. DOI 10.1016/j.conbuildmat.2012.04.117. [Google Scholar] [CrossRef]
6. Hilton, B., Bawden, K., Winnebeck, K., Chandrasiri, C., Ariyachandra, E. et al. (2019). The functional and environmental performance of mixed cathode ray tubes and recycled glass as partial replacement for cement in concrete. Resources, Conservation & Recyclings, 4(5), 507–517. [Google Scholar]
7. Alwaeli, M., Jacek, G., Marian, N., Jan, P., Małgorzata, G. (2020). Recycle option for metallurgical sludge waste as a partial replacement for natural sand in mortars containing CSA cement to save the environment and natural resources. Journal of Hazardous Materials, 398, 123101. [Google Scholar]
8. Ahmad, S. Y., Xu, A. (2005). Performance of glass powder as a pozzolanic material in concrete: A field trial on concrete slabs. Cement and Concrete Research, 36(3), 457–468. [Google Scholar]
9. Schwarz, N., Neithalath, N. (2008). Influence of a fine glass powder on cement hydration: Comparison to fly ash and modeling the degree of hydration. Cement and Concrete Research, 38(4), 429–436. DOI 10.1016/j.cemconres.2007.12.001. [Google Scholar] [CrossRef]
10. Shi, C. J., Wu, Y. Z., Riefler, C., Wang, H. (2005). Characteristics and pozzolanic reactivity of glass powders. Cement and Concrete Research, 35(5), 987–993. DOI 10.1016/j.cemconres.2004.05.015. [Google Scholar] [CrossRef]
11. Chen, C. H., Huang, R., Wu, J. K., Yang, C. C. (2006). Waste E-glass particles used in cementitious mixtures. Cement and Concrete Research, 36(3), 449–456. DOI 10.1016/j.cemconres.2005.12.010. [Google Scholar] [CrossRef]
12. Schwarz, N., Cam, H., Neithalath, N. (2008). Influence of a fine glass powder on the durability characteristics of concrete and its comparison to fly ash. Cement and Concrete Composites, 30(6), 486–496. DOI 10.1016/j.cemconcomp.2008.02.001. [Google Scholar] [CrossRef]
13. Wang, H. Y., Liang, H. W. (2010). Durability of self-consolidating concrete using waste LCD glass. Construction and Building Materials, 24(6), 1008–1013. DOI 10.1016/j.conbuildmat.2009.11.018. [Google Scholar] [CrossRef]
14. Islam, G. M. S., Rahman, M. H., Kazi, N. (2017). Waste glass powder as partial replacement of cement for sustainable concrete practice. International Journal of Sustainable Built Environment, 6(1), 37–44. DOI 10.1016/j.ijsbe.2016.10.005. [Google Scholar] [CrossRef]
15. Shao, Y. X., Lefort, T., Moras, S., Rodriguez, D. (2000). Studies on concrete containing ground waste glass. Cement and Concrete Research, 30(1), 91–100. DOI 10.1016/S0008-8846(99)00213-6. [Google Scholar] [CrossRef]
16. Du, H. J., Tan, K. H. (2014). Effect of particle size on alkali-silica reaction in recycled glass mortars. Construction and Building Materials, 66, 275–285. DOI 10.1016/j.conbuildmat.2014.05.092. [Google Scholar] [CrossRef]
17. Corinaldesi, V., Gnappi, G., Moriconi, G., Montenero, A. (2005). Reuse of ground waste glass as aggregate for mortars. Waste Management, 25(2), 197–201. DOI 10.1016/j.wasman.2004.12.009. [Google Scholar] [CrossRef]
18. Park, H. G., Sung, S. K. (2008). Influence of a CA mineral-based accelerator on the strength and durability of shotcrete. Cement and Concrete Research, 38(3), 379–385. [Google Scholar]
19. Puertas, F., Torres-Carrasco, M. (2014). Use of glass waste as an activator in the preparation of alkali-activated slag. Mechanical strength and paste characterisation. Cement and Concrete Research, 57(5), 95–104. DOI 10.1016/j.cemconres.2013.12.005. [Google Scholar] [CrossRef]
20. Torres-Carrasco, M., Puertas, F. (2015). Waste glass in the geopolymer preparation: Mechanical and microstructural characterisation. Journal of Cleaner Production, 90, 397–408. [Google Scholar]
21. Ahmad, S. Y., Xu, A. (2006). Performance of glass powder as a pozzolanic materials in concrete: A field trial on concrete slabs. Cement and Concrete Research, 36(3), 457–468. DOI 10.1016/j.cemconres.2005.12.012. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |